Abstract
The authors measured the effects of bilateral amygdaloid, orbital frontal, or hippocampal lesions on emotional reactivity and passive avoidance in rhesus monkeys (Macaca mulatta). Animals were presented with 8 neutral or 8 aversive objects, each paired with a highly preferred food reward. Shamoperated control animals displayed heightened defensive behaviors and typically would not approach or retrieve the food when paired with a potential predator (coiled rubber snake), 2 conditioned aversive stimuli for laboratory-housed monkeys (a capture net and leather handling gloves), and 1 object displaying a threatening social signal (direct eye contact from a human-like doll). Animals with amygdala lesions, but not hippocampal or orbital frontal lesions, showed less tension-related behaviors and diminished passive avoidance of the rubber snake and its matched neutral item (a coiled piece of hose) relative to control animals. All operated groups displayed normal patterns of behavior toward conditioned and socially aversive objects. These results expand our understanding of how the primate brain evaluates reward and threat, and indicate a highly specialized role for the amygdala in mediating passive avoidance and emotional reactivity to potentially life-threatening stimuli.
Keywords: Macaca mulatta, fear, ibotenic acid, decision making, approach-avoidance conflict
For group-living primates, danger can exist in many forms and survival depends upon efficient behavioral modulation whenever threats are perceived. Natural predators, such as snakes or raptors, prompt an array of fearful and evasive behaviors, along with specific alarm calls in many nonhuman primate species (Cheney & Seyfarth, 1990). Objects or environmental contexts can also trigger fear or anxiety through repeated pairing with aversive consequences (i.e., a hypodermic syringe or a dentist’s office). Danger may also come from members of one’s own social group. Some social signals, such as an angry facial expression in humans (Whalen, 1998) or open-mouthed stare signals in macaque monkeys (Chevalier-Skolnikoff, 1973; van Hooff, 1967), are unequivocal indicators of danger that must be avoided or appeased with appropriate behaviors. An intricate neural network, including the amygdala, hippocampus, ventromedial frontal cortex, and anterior cingulate cortex, appears to link threatening stimuli and contexts to specific consequences, store those memories for future reference, and adapt behavior when contingencies change. Although this fear system has been extensively studied in rodents (Davis, Walker, & Myers, 2003; Maren, 2001; Pare, Quirk, & LeDoux, 2004; Quirk, Garcia, & Gonzalez-Lima, 2006), nonhuman primates, with their remarkable neurological and behavioral similarities to humans, also provide an excellent model organism in which to study the neurobiology of fear and threat avoidance.
Fear-related studies with nonhuman primates have focused primarily on behavioral reactivity or passive avoidance when animals were exposed to aversive stimuli. For example, lesions of the amygdala (Aggleton & Passingham, 1981; Amaral et al., 2003; Izquierdo & Murray, 2004; Kalin, Shelton, Davidson, & Kelley, 2001; Kalin, Shelton, & Davidson, 2004; Mason, Capitanio, Machado, Mendoza, & Amaral et al., 2006; Meunier, Bachevalier, Murray, Málková, & Mishkin, 1999; Prather et al., 2001; Stefanacci, Clark, & Zola, 2003; Zola-Morgan, Squire, Alvarez-Royo, & Clower, 1991), hippocampus (Chudasama, Wright, & Murray, 2008), or the orbital frontal cortex (Butter, Mishkin, & Mirsky, 1968; Butter, Snyder, & McDonald,1970; Butter & Snyder, 1972; Izquierdo, Suda, & Murray, 2005; Kalin, Shelton, & Davidson, 2007; Rudebeck, Buckley, Walton, & Rushworth, 2006) result in blunted fear and avoidance of real or fake snakes. Since snakes may trigger an innate fear in primates (Mineka, Keir, & Price, 1980; Mineka & Öhman, 2002), these results imply that the amygdala, hippocampus and orbital frontal cortex are critical for normal fear and avoidance of natural predators. As noted above, however, danger can present itself in many other forms. Is the same neural network responsible for marshalling fear and avoidance of potentially dangerous social stimuli or objects that have gained aversive connotations through experience? This question has not been directly addressed. In rabbits and rodents, the specific method used to evaluate fear behavior has a large impact on the pattern of results. Some have argued that during the posttraining period, the central nucleus of the amygdala is critical for the expression of both contextual freezing and fear-potentiated startle (McNish Gewirtz, & Davis, 1997), but other studies have found sparing of function after amygdala lesions for inhibitory avoidance conditioned prior to surgery (Kim, Clark, & Thompson, 1995; Parent, Quirarte, Cahill, & McGaugh, 1995). Similarly, neither prenor posttraining lesions of the hippocampus affect fear-potentiated startle to an explicit (light) conditioned stimulus (Heldt, Coover, & Falls,, 2002; Kim & Fanselow, 1992; McNish, Gewirtz, & Davis, 1997, 2000; Phillips & LeDoux, 1992). However, presurgery lesions of the entire hippocampus or temporary inactivation of the ventral hippocampus diminish freezing cued by a tone or context in both trace and delay fear conditioning (Esclassan, Coutureau, Di Scala, & Marchand, 2008). Evidence from the nonhuman primate literature suggests that fear memories conditioned prior to amygdala damage can still potentiate the animal’s startle reflex normally after surgery (Antoniadis, Winslow, Davis, & Amaral, 2007). By contrast, animals with amygdala, but not hippocampal, lesions cannot acquire new potentiated startle reflexes after surgery (Antoniadis et al., 2007). It remains unknown, however, if orbital frontal cortex damage impacts on fear memories acquired before surgery. Reactivity to potentially dangerous social stimuli (such as direct eye contact from humans or other monkeys) has been studied following amygdala, orbital frontal or hippocampal lesions (Izquierdo & Murray, 2004; Kalin & Shelton, 1989; Kalin, Shelton, & Takahashi,, 1991; Kalin et al, 2001; 2004; 2007; Machado & Bachevalier, 2008; Mason et al., 2006; Rudebeck et al., 2006). All three areas appear critical for normal behavioral reactivity to such social stimuli. Therefore, we predicted that amygdala, hippocampal, and orbital frontal lesions would each disrupt fear reactivity and passive avoidance to potentially dangerous social stimuli, but only orbital frontal damage may impact on reactivity to conditioned aversive stimuli.
Recent neuroanatomical studies have also demonstrated that the primate orbital frontal cortex is heterogeneous both in cytoarchitecture and pattern of intrinsic and extrinsic connections (Barbas, 2007a; 2007b; Price, 2007). One prominent view is that the ventral and medial frontal cortex can be divided into two separate, yet partially overlapping, networks (Price, 2007). A “medial network,” including all areas of the medial frontal cortex and the most medial sectors on the orbital surface, has strong connections with the amygdala and autonomic centers in the brain. The “orbital network” includes more lateral areas on the orbital surface and predominantly receives sensory information from all modalities. However, many previous lesion studies investigating the role of the orbital frontal cortex in fear reactivity damaged large portions of the ventromedial frontal lobe (including portions of areas 10, 11, 12, 13 and 14; Butter et al., 1968; 1970; Butter & Snyder, 1972; Izquierdo et al., 2005), or even included areas of the ventrolateral frontal cortex (area 47/12; Rudebeck et al., 2006). It has been known for some time that lesions of discrete orbital and lateral frontal subregions in monkeys produce dissociable impairments in extinction and behavioral adaptation when reward contingencies change (Butter, 1969). Would a different picture of orbital frontal cortex function in fear reactivity and passive avoidance emerge if lesions were mainly confined to areas 11 and 13, rather than also including area 14 and/or 47/12? Although lesion studies have not directly compared the function of specific orbital frontal subregions with respect to fear or anxiety, a recent meta-analysis of 106 human functional neuroimaging experiments indicated that the lateral orbitofrontal cortex (area 47) is most consistently activated in studies of anger perception and expression, whereas fear-related studies most often demonstrate heightened amygdala activity (Murphy, Nimmo-Smith, & Lawrence,, 2003). Areas of the medial orbitofrontal cortex, such as areas 11 and 13, were not reliably activated for fear or anger. These findings predict that lesions of areas 11 and 13 are likely to produce milder impairments, if any, in fear reactivity to potentially dangerous stimuli relative to larger lesions including lateral regions of the orbital frontal cortex, such as 47/12.
Finally, the rodent hippocampus has been well studied with respect to fear (Hobin, Ji, & Maren, 2006; Holt & Maren, 1999; Maren & Fanselow, 1997; Maren, Aharonov, & Fanselow, 1997). By contrast, we know very little about how the primate hippocampus contributes to fear reactivity. Recently, Chudasama and colleagues (2008) demonstrated that neurotoxic lesions of the nonhuman primate hippocampus blunt avoidance and fear reactivity to a fake snake, but not to a toy spider. Would an investigation of how the primate hippocampus contributes to fear and avoidance of a wide range of stimuli confirm or conflict with these previous results? Given that rodents with hippocampal lesions show potentiated startle when exposed to aversive stimuli conditioned prior to surgery (Heldt et al., 2002; Kim & Fanselow, 1992; McNish et al., 1997, 2000; Phillips & LeDoux, 1992) and nonhuman primates with similar lesions demonstrate decreased emotional responsiveness to staring humans (Machado & Bachevalier, 2008), we predicted that fear reactivity and passive avoidance to conditioned aversive stimuli would not be affected by hippocampal lesions, but such measures would be blunted when these animals were exposed to social stimuli.
The present study tested these three hypotheses in a group of young-adult rhesus monkeys (Macaca mulatta). We compared the effects of selective amygdala, hippocampal, or orbital frontal lesions using a common behavioral paradigm that measures fear-related behaviors and passive avoidance when animals are exposed to potentially dangerous objects (Amaral et al., 2003; Chudasama et al., 2008; Izquierdo & Murray, 2004; Izquierdo et al., 2005; Kalin et al., 2001; 2004; 2007; Mason et al., 2006; Mineka et al., 1980; Rudebeck et al., 2006). This task allowed the animals to retrieve a positive food reward presented alone or in close proximity to neutral or aversive stimuli. However, our goal was to extend previous research by exposing the monkeys with amygdala, orbital frontal and hippocampal lesions to three different categories of potentially dangerous visual stimuli presented in the same context. Thus, in addition to objects known to naturally initiate fear and defensive responses, such as snakes, we also included objects that the animals had learned to fear in the laboratory environment (i.e., a hypodermic syringe or a capture net), as well as objects with social features (faces) that could potentially stimulate fearful reactions, such as a doll or mirror reflection. A preliminary report of this work has appeared previously in abstract form (Machado & Bachevalier, 2003).
Method
All procedures were approved by the Animal Care and Use Committee of the University of Texas Health Science Center, Houston and carried out in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals. All efforts were made to minimize the number of animals used, as well as their pain and suffering. No alternatives currently exist for the in vivo techniques described here.
Subjects
Subjects were 24 young-adult male rhesus monkeys (Macaca mulatta), weighing 4.5–6.0 kg and ranging between 3.5 and 4.1 years old at the beginning of testing. Prior to the present study, all animals received pre- and postsurgical assessments of emotional reactivity (Human Intruder Task; Machado and Bachevalier, 2008), social behavior (Machado & Bachevalier, 2006), and reward assessment (Machado & Bachevalier, 2007a; 2007b) and were also tested postoperatively in the Visual Paired Comparison task (Bachevalier & Nemanic, 2008).
Animals were randomly assigned to one of the following five experimental groups, which were balanced with respect to presurgical social dominance rank and age: Sham-operated control (C; n = 6), neurotoxic hippocampal lesion (H-ibo; n = 6), neurotoxic amygdala lesion (A-ibo; n = 6), and neurotoxic orbital frontal lesion (O-ibo; n = 3). Given that the neurotoxic orbital frontal lesions resulted in incomplete damage to this region (see Table 1), the remaining three animals received aspiration orbital frontal cortex lesions (O-asp; n = 3). All animals were raised in semi-naturalistic, outdoor enclosures at the MD Anderson Cancer Center Science Park in Bastrop, Texas. Once being transferred to the laboratory, animals were housed individually and maintained on a 12-hr light/dark cycle. They were fed daily with fresh fruit, vegetables, and high-protein monkey chow (Lab Diet #5045, PMI Nutrition International Inc., Brentwood, MO), and received water ad libitum.
Table 1.
Intended and Unintended Damage for All Experimental Groups
| Hippocampal formation |
Amygdala |
TH |
TF |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W |
| H-ibo-1 | 76.3 | 97.9 | 87.1 | 74.7 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| H-ibo-2 | 75.7 | 81.3 | 78.5 | 61.5 | 0 | 5.9 | 3.0 | 0 | 53.1 | 20.1 | 36.6 | 10.7 | 60.3 | 27.6 | 44.0 | 16.6 |
| H-ibo-3 | 67.5 | 74.1 | 70.8 | 50.0 | 0 | 0 | 0 | 0 | 26.7 | 15.3 | 21.0 | 4.1 | 29.9 | 44.0 | 37.0 | 13.2 |
| H-ibo-4 | 56.2 | 76.2 | 66.2 | 42.8 | 0 | 0 | 0 | 0 | 13.6 | 27.8 | 20.7 | 3.8 | 18.5 | 19.4 | 19.0 | 3.6 |
| H-ibo-5 | 98.8 | 99.3 | 99.1 | 98.1 | 0 | 0 | 0 | 0 | 15.2 | 15.9 | 15.6 | 2.4 | 38.8 | 8.5 | 23.7 | 3.3 |
| H-ibo-6 | 88.8 | 94.8 | 91.8 | 84.2 | 0 | 0 | 0 | 0 | 29.6 | 45.6 | 37.6 | 13.5 | 21.2 | 17.2 | 19.2 | 3.6 |
| X | 77.2 | 87.3 | 82.3 | 68.6 | 0 | 1.0 | 0.5 | 0 | 23.0 | 20.8 | 21.9 | 5.8 | 28.1 | 19.5 | 23.8 | 6.7 |
| Amygdala |
Hippocampal formation |
ERh |
PRh |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W |
| A-ibo-1 | 20.6 | 82.2 | 51.4 | 16.9 | 10.6 | 1.6 | 6.1 | 0.2 | 0 | 1.8 | 0.9 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-2 | 48.9 | 88.1 | 68.5 | 43.1 | 1.2 | 0 | 0.6 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-3 | 27.1 | 73.1 | 50.1 | 19.8 | 15.7 | 13.6 | 14.6 | 2.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-4 | 79.1 | 92.5 | 85.8 | 73.2 | 3.4 | 3.0 | 3.2 | 0.1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-5 | 88.7 | 91.3 | 90.0 | 81.0 | 1.5 | 0.1 | 0.8 | 0 | 0 | 5.5 | 2.8 | 0 | 0 | 0 | 0 | 0 |
| A-ibo-6 | 70.3 | 90 | 80.2 | 63.3 | 21.1 | 10.3 | 15.7 | 2.2 | 0.8 | 0 | 0.4 | 0 | 0.1 | 0 | 0.1 | 0 |
| X | 55.8 | 86.2 | 71.0 | 49.5 | 8.9 | 4.8 | 6.9 | 0.8 | 0.1 | 1.2 | 0.7 | 0.0 | 0 | 0 | 0 | 0 |
| Orbital frontal cortex (Areas 11 & 13) |
Area 12 |
Area 14 |
Ia |
|||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Cases | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W | L | R | Avg | W |
| O-ibo-1 | 37.1 | 19.9 | 28.5 | 7.4 | 2.3 | 0.9 | 1.6 | 0 | 49.0 | 23.8 | 36.4 | 11.7 | 37.0 | 25.3 | 31.2 | 9.4 |
| O-ibo-2 | 33.9 | 37.5 | 35.7 | 12.7 | 28.1 | 41.7 | 34.9 | 11.7 | 8.3 | 5.9 | 7.1 | 0.5 | 28.2 | 34.3 | 31.3 | 9.7 |
| O-ibo-3 | 43.0 | 47.3 | 45.2 | 20.3 | 9.9 | 20.9 | 15.4 | 2.1 | 25.4 | 11.7 | 18.6 | 3.0 | 44.9 | 38.0 | 41.5 | 17.1 |
| X | 38.0 | 34.9 | 36.5 | 13.5 | 13.4 | 21.2 | 17.3 | 4.6 | 27.6 | 13.8 | 20.7 | 5.0 | 36.7 | 32.5 | 34.7 | 12.0 |
| O-asp-1 | 88.4 | 95.3 | 91.9 | 84.2 | 3.9 | 28.9 | 16.4 | 1.1 | 15.2 | 21.7 | 18.5 | 3.3 | 20.6 | 21.1 | 20.9 | 4.3 |
| O-asp-2 | 83.0 | 92.0 | 87.5 | 76.4 | 7.0 | 9.3 | 8.2 | 0.7 | 10.7 | 5.9 | 8.3 | 0.6 | 21.4 | 23.7 | 22.6 | 5.1 |
| O-asp-3 | 90.3 | 87.6 | 89.0 | 79.1 | 4.5 | 10.2 | 7.4 | 0.5 | 0.7 | 0 | 0.4 | 0 | 5.5 | 5.9 | 5.7 | 0.3 |
| X | 87.2 | 91.6 | 89.5 | 79.9 | 5.1 | 16.1 | 10.7 | 0.7 | 8.9 | 9.2 | 9.1 | 1.3 | 15.8 | 16.9 | 16.4 | 3.2 |
Note. Data are the estimated percentage of normal volume that was damaged as assessed from MR images. Areas 11, 12, 13, and 14 = cytoarchitectonic subregions of the macaque frontal lobe as defined by Carmichael and Price (1994); Ia = agranular insular area as defined by Carmichael and Price (1994); ERh = entorhinal cortex and PRh = perirhinal cortex as defined by Amaral and colleagues (Amaral, Insausti, & Cowan, 1987; Insausti, Amaral, Cowan, 1987); L = percentage of damage to the left hemisphere; R = percentage of damage to the right hemisphere; Avg = average of L and R; W=(L × R)/100 [weighted index as defined by Hodos and Bobko (1984)]; X = group mean.
Neuroimaging
MRI procedures have been detailed in four previous studies from our laboratory (Machado & Bachevalier, 2006, 2007b; Nemanic, Alvarado, Price, Jackson, & Bachevalier,, 2002; Nemanic, Alvarado, & Bachevalier, 2004). Briefly, animals were maintained under general anesthesia throughout the scanning procedure. Two scanning sessions were performed with a GE Signa 1.5 Tesla Echo Speed scanner (GE Medical Systems, Milwaukee, WI). The first session occurred 1–3 weeks before surgery, and included one T1-weighted structural scan (1 mm thick) and three Fluid Attenuated Inversion Recovery (FLAIR; 3 mm thick, each offset by 1 mm) scans. The second session occurred 7–10 days after surgery and included the same two MRI series. Presurgical T1-weighted images were used to select stereotaxic coordinates for neurotoxin injections (Saunders Aigner, & Frank, 1990) or to visualize sulcal landmarks for orbital frontal cortex lesions. Postsurgical T1-weighted images were used to quantify the extent of orbital frontal cortex aspiration lesions (Group O-asp). Postsurgical FLAIR images were used to identify localized areas of edema indicative of neurotoxin-induced cell death, and were used to quantify lesion extent for Groups H-ibo, A-ibo and O-ibo (Málková, Lex, Mishkin, & Saunders, 2001; Nemanic et al., 2002).
Surgery
A detailed description of all surgical procedures can be found elsewhere (Machado & Bachevalier, 2006; Nemanic et al., 2002; 2004). Briefly, surgical procedures were performed under deep general anesthesia using aseptic techniques. Vital signs were monitored throughout the surgical procedure until the animal recovered fully from anesthesia. The scalp and connective tissue were incised and gently retracted together with the temporalis muscles. Each group then underwent lesion-specific procedures.
Neurotoxic hippocampal formation lesions were intended to damage all ammonic fields, the dentate gyrus, the prosubiculum, and subiculum. Neurotoxic amygdala lesions were intended to damage all amygdaloid nuclei. For these two operated groups, small bilateral craniotomies were created above the injection sites and slits were cut in the dura bilaterally to allow the needle of the 10 μl Hamilton syringe, held by a Kopf electrode manipulator (David Kopf Instruments, Tujunga, CA), to be lowered to the appropriate injection coordinates. Two Hamilton syringes were filled with ibotenic acid (Biosearch Technologies, Novato, CA, 10 mg/ml in phosphate buffered saline, pH 7.4) and delivered the neurotoxin to each hemisphere simultaneously.
Orbital frontal cortex lesions (both ibotenic and aspiration) were intended to damage those areas of the ventral frontal cortex that are heavily interconnected with the amygdala (Amaral, Price, Pitkänen, & Carmichael, 1992; Ghashghaei, Hilgetag, & Barbas, 2007), namely areas 11 and 13 (as defined by Carmichael & Price, 1994). The bone of the supraorbital ridge was opened, followed by incision and retraction of the dura. The surface landmarks used to approximate areas 11 and 13 were (a) a line joining the anterior tips of the medial and lateral orbital sulci, (b) a line joining the medial bank of the lateral orbital sulcus and the olfactory stria just anterior to its division into the medial and lateral olfactory tracts, posteriorly, (c) the lateral border of the olfactory stria, medially, and (d) the medial bank of the lateral orbital sulcus, laterally. For Group O-ibo, ibotenic acid was injected manually in a 2 mm × 2 mm square matrix within these borders. For animals in Group O-asp, 21- and 23-gauge suckers were used to aspirate the cortical tissue contained within these limits until the white matter beneath could be seen.
For sham lesions, bilateral craniotomies (similar to those used for hippocampal formation or amygdala lesions) were made. For five of the six cases, the dura was cut bilaterally, but no needle penetrations occurred. The remaining animal (case C-1-inj) was prepared to serve as a control animal for one of the hippocampal-operated animals that sustained inadvertent damage to the putamen. Case C-1-inj received ibotenic acid injections into the section of the putamen, which lies dorsal to the posterior one third of the amygdala and the anterior one third of the hippocampal formation.
Following these group-specific procedures, the wound was closed in anatomical layers and the animal was removed from anesthesia. During recovery from surgery, none of the animals displayed any changes in food and water consumption, or arousal state. Reduced locomotor behaviors and weakness of the limbs were temporarily observed in the two cases that sustained additional damage to the ventral putamen (i.e., cases C-1-inj and H-ibo-1, see Machado & Bachevalier, 2006). Because behavioral results of case C-1-inj did not differ from Group C, its data were pooled with those obtained from the other animals in Group C.
MRI-Based Lesion Evaluation
Lesions were evaluated using MRI techniques since all animals used for this study died in the flooding of Tropical Storm Allison in June 2001. These techniques provide an accurate estimate of cell loss following neurotoxic hippocampal lesions in nonhuman primates (Málková et al., 2001; Nemanic et al., 2002) and have been described in detail previously (Machado & Bachevalier, 2006, 2007a, 2007b). Figures 1–4 illustrate a representative case of each lesion type. A full description of lesion extents has been previously reported for all animals (Machado & Bachevalier, 2006), so only a summary will be provided here. Neurotoxic hippocampal lesions (range: 66.2–99.1%; see Table 1 and Figure 1) and neurotoxic amygdala lesions (range: 50.1–90.0%; see Table 1 and Figure 2) were largely as intended, and resulted in only mild to moderate inadvertent damage in adjacent regions (i.e., the pes hippocampus for Group A-ibo, see Figure 2 level + 15, and areas TH/TF for Group H-ibo). Aspiration orbital frontal cortex lesions resulted in damage largely confined to areas 11 and 13 (range: 87.5–91.9%, see Table 1 and Figure 3), as well as the anterior one third of the agranular insular area (5.7–22.6%). By contrast, the three cases with ibotenic acid orbital frontal lesions received damage not only to areas 11 and 13 (range: 28.5–45.2%), but also mild to moderate damage to neighboring area 14 (range: 7.1–36.4%), area 12 (range: 1.6 –34.9%) and more substantial damage to agranular insular area (range: 31.2–41.5%; see Table 1). In addition, the hypersignals seen within the orbital frontal regions were confined mostly to the superficial cortical layers and avoided the deepest layers (see Figure 4), resulting in comparably lower total volume but larger total surface area of damage relative to Group O-asp.
Figure 1.
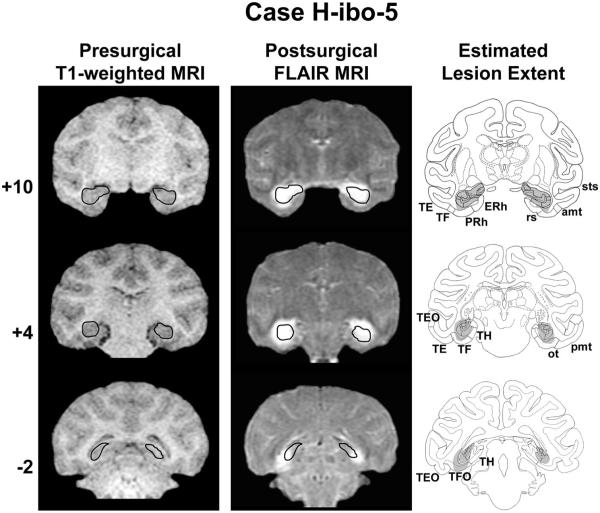
Coronal T1-weighted MR images acquired before surgery, Fluid Attenuated Inversion Recovery (FLAIR) MR images collected approximately 1 week after surgery, and estimated lesion extent in gray (left to right columns, respectively) showing the anterior, middle, and posterior hippocampal formation (top to bottom rows, respectively) for a representative case with a neurotoxic hippocampal formation lesion (H-ibo-5). The ideal extent of the lesion is drawn in black on the presurgical T1-weighted images and then transferred onto the postsurgical FLAIR images for reference. The numerals to the left of each row indicate the distance in millimeters from the interaural plane. amt anterior middle temporal sulcus; ERh = entorhinal cortex; ot = occipitotemporal sulcus; PRh = perirhinal cortex; pmt = posterior middle temporal sulcus; rs = rhinal sulcus; sts = superior temporal sulcus; TE, TEO, TF, TFO and TH = cytoarchitectonic fields described by von Bonin and Bailey (1947). Reprinted with permission of the American Psychological Association.
Figure 4.
Coronal T1-weighted MR images acquired before surgery, Fluid Attenuated Inversion Recovery (FLAIR) MR images collected approximately 1 week after surgery, and estimated lesion extent in gray (left to right columns, respectively) showing the anterior, middle, and posterior orbital frontal cortex (top to bottom rows, respectively) for a representative case with a neurotoxic orbital frontal cortex lesion (O-ibo-3). The ideal extent of the lesion is drawn in black on the presurgical T1-weighted images and then transferred onto the postsurgical FLAIR images for reference. Abbreviations and conventions as in Figure 3. Reprinted with permission of the American Psychological Association.
Figure 2.
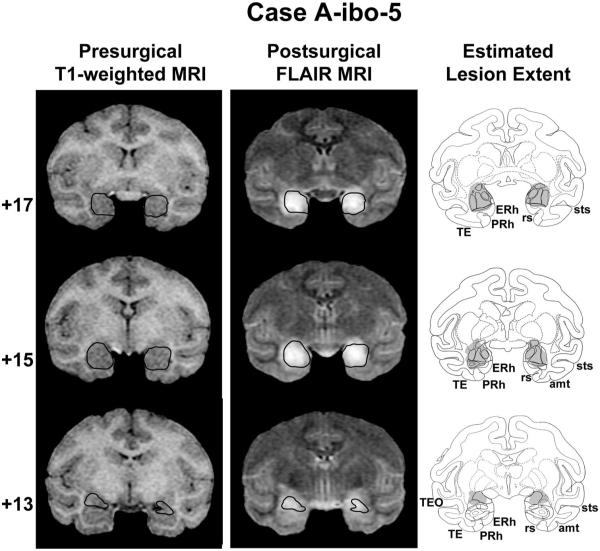
Coronal T1-weighted MR images acquired before surgery, Fluid Attenuated Inversion Recovery (FLAIR) MR images collected approximately 1 week after surgery, and estimated lesion extent in gray (left to right columns, respectively) showing the anterior, middle, and posterior amygdala (top to bottom rows, respectively) for a representative case with a neurotoxic amygdala lesion (A-ibo-5). The ideal extent of the lesion is drawn in black on the presurgical T1-weighted images and then transferred onto the postsurgical FLAIR images for reference. Conventions and abbreviations as in Figure 1. Reprinted with permission of the American Psychological Association.
Figure 3.
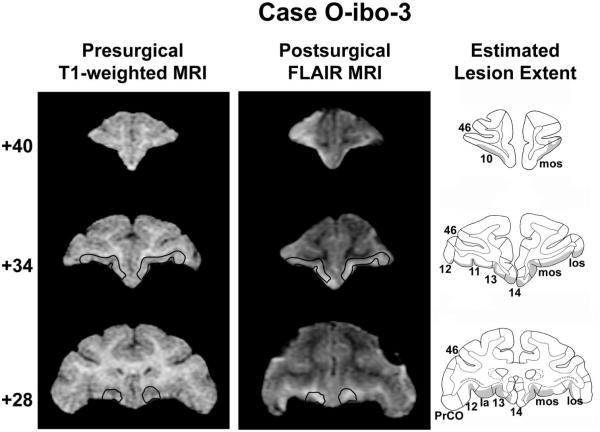
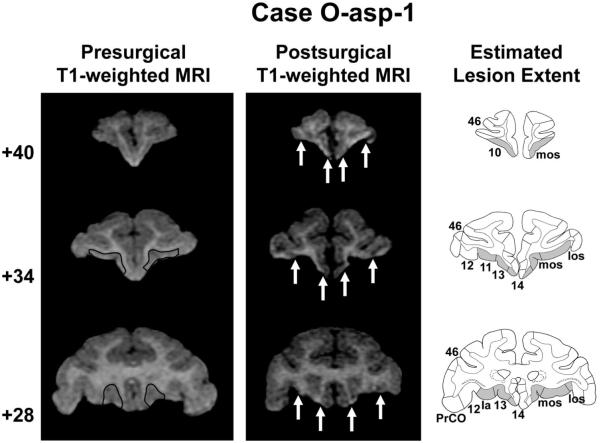
Coronal T1-weighted MR images acquired before surgery, T1-weighted MR images collected after surgery, and estimated lesion extent in gray (left to right columns, respectively) showing the anterior, middle, and posterior orbital frontal cortex (top to bottom rows, respectively) for a representative case with an aspiration orbital frontal cortex lesion (O-asp-1). The ideal extent of the lesion is drawn in black on the presurgical T1-weighted images and arrows in the middle column denote the medial and lateral extent of the lesion in each hemisphere. 10, 11, 12, 13, 14, and 46 = cytoarchitectonic fields of the frontal lobe as described by Carmichael and Price (1994); Ia = agranular insular area; mos = medial orbital sulcus; los = lateral orbital sulcus; PrCO = precentral opercular cortex. Reprinted with permission of the American Psychological Association.
Testing Apparatus and Procedures
Animals were tested in a small stainless steel cage (47 cm wide × 56 cm tall × 47 cm deep) positioned on a rolling base, with a front panel containing vertical bars spaced 6 cm apart. Animals were wheeled to a modified Wisconsin General Testing Apparatus (WGTA) equipped with two opaque, vertically sliding panels: one between the animal and a test tray, and another between the experimenter and the test tray. This tray contained three food wells (one well at center, 16 cm from the animal, two lateral wells located 13 cm on either side, each 2 cm in diameter and 1 cm deep). Only the center well was used for this experiment. All food wells were within arm’s reach for the animals, but only if they were seated in the front half of the test cage. The experimenter controlled each panel by manipulating two weighted pullies, allowing either himself or the animal access to the test tray. The animal could not see the experimenter during a testing session and could not see the objects prior to presentation. All animals had equal and extensive prior experience with the WGTA due to a previous food preference study conducted both before and after surgery (Machado & Bachevalier, 2007b).
Sixteen inanimate stimuli were chosen for this experiment. The emotional valence of these items varied such that eight objects were intended to be aversive or potentially dangerous, whereas the remaining eight were intended to be neutral items of similar size and shape. The aversive items were specifically selected to be either items that the animals innately feared (rubber snake; Mineka et al., 1980; Mineka & Öhman, 2002), items common to the nonhuman primate laboratory that, in our experience with this population, readily elicited fear (hypodermic syringe, capture net, handling gloves, and a pistol-grip water nozzle) or items with a social component (Mr. Potato Head, girl doll, and mirror reflecting monkey image). Social stimuli such as these elicit behavioral expressions of fear, passive avoidance and/or generalized tension since direct eye contact is a highly threatening gesture among macaque monkeys (Chevalier-Skolnikoff, 1973; van Hooff, 1967). The eight aversive items and the eight neutral items with which they were paired are briefly described in Table 2.
Table 2.
Neutral and Aversive Object Pairings
| General category | Neutral object | Aversive object |
|---|---|---|
| Innately Aversive | Coiled Hose1 | Rubber Snake2§ |
| Conditioned Aversive | Mop3 | Capture Net4 |
| Kong Toy5§ | Syringe6§ | |
| Latex Gloves7 | Handling Gloves8 | |
| Lock9§ | Water Nozzle10§ | |
| Socially Aversive | Water Bottle11§ | Girl Doll12§ |
| Beach Picture13 | Mirror14 | |
| Plastic Jug15§ | Mr. Potato Head16§ |
Note.
red garden hose, 0.5 m length, coiled into 20 cm diameter circle.
green and black, 0.5 m length, coiled into a 20 cm diameter circle.
wooden handle (1.5 m length) with cotton rope head (30 cm wide × 30 cm long).
aluminum pole (1.75 m length) with brown nylon netting around a 61 cm diameter hoop, used for nonhuman primate capture in laboratory setting.
red, nonhuman primate enrichment toy, 5.7 cm wide × 5.7 cm deep × 8.9 cm tall, Kong Company, Golden, CO.
60 ml capacity hypodermic, with 24-gauge capped needle, 3 cm diameter × 20 cm tall.
typical white laboratory latex gloves, size XL.
brown leather, used for nonhuman primate physical restraint, 43 cm long × 18 cm wide.
typical nonhuman primate cage lock, brass and stainless steel, 8 cm wide × 2 cm deep × 13 cm tall.
pistol-grip, typical garden usage, 16.5 cm wide × 15.2 cm long × 3.2 cm tall.
typical plastic nonhuman primate water bottle, 1 liter capacity with stainless steel sipper tube, 10 cm wide × 10 cm deep × 30 cm tall.
human-like facial features and hair, 18 cm wide × 15 cm deep × 35 tall while sitting, Zapf Creation, Inc., Orlando, FL.
beach scene in clear plastic frame, 25 cm wide × 1 cm deep × 20 cm tall.
25 cm wide × 1 cm deep × 20 cm tall in black frame.
cylindrical, clear with white lid, 17 cm diameter × 22 cm tall, Rubbermaid, Wooster, OH.
plastic toy with large eyes, 15.2 cm wide × 10.2 cm deep × 20 cm tall, Hasbro, Inc., Pawtucket, RI.
Objects were affixed to 22 cm × 22 cm opaque, black, Plexiglass plaques to prevent the animals from taking the objects into their cage.
Animals were tested over 4 days approximately 10 months after surgery. Two of the eight neutral/aversive stimulus pairs were presented daily, without replication, to measure emotional reactivity and passive avoidance elicited without the influence of experience or habituation. A seedless grape was paired with each of the items to motivate approach. The same food reward was used for all animals since previous food preference testing with these same animals indicated that it was highly preferred both before and after surgery (Machado & Bachevalier, 2007a). A given pair of neutral and aversive objects was presented within a block of four 1-min trials: (a) Baseline Trial—nothing presented on the test tray, (b) Grape Only— grape presented in the center food well, (c) Neutral Item—a neutral item was positioned 2 cm behind the grape in the center food well, and (d) Aversive Item—an aversive item was positioned 2 cm behind the grape. Two four-trial blocks occurred each day, and each trial was separated by a 30-s intertrial interval (ITI). During each trial, animals could take or manipulate the grape and item freely, if present. During the ITI, the opaque panel between the animal and the test tray was lowered, the object and food reward were removed (if present), and the tray was reset for the next trial with the requisite stimuli. The sequence of the four trials within a block occurred either in the order described above (i.e., 1 → 4) or the reverse order (4 → 1). Exposure to each of these two trial sequences was balanced within each group and across test days by Latin Square. To control for circadian effects on animals’ motivation, all testing for this experiment occurred between 10:00 a.m. and noon (i.e., at least 18 hr after their last feeding). Testing order was generated randomly, and that order was counterbalanced within each lesion group.
Behavioral Measures
All trials were videotaped using a Sony Handycam (model CCD-FX710), and these videotapes were subsequently scored by one, previously trained observer (CM) using The Observer Video Pro software package (Noldus, Trienes, Hendriksen, Jansen, & Jansen, 2000) and the behavioral ethogram described in Table 3. Individual behaviors were grouped into more general behavioral categories (also shown in Table 3) for statistical analyses.
Table 3.
Behavioral Ethogram
| Behavior category and specific behavior |
Brief definition |
|---|---|
| Defensive Behaviors | |
| Bark Vocalization | Low-pitched, high-intensity vocalization |
| Cage Aggression | Vigorous shaking of cage walls |
| Crooktail | Tail held in a "?" shape |
| Freezing§ | Rigid, tense, motionless posture except slight head movements |
| Full Threat | Two or more of the following: open-mouth stare, head bobbing, ear flaps, or lunges |
| Mild Threat | One of the following: open-mouth stare, head bobbing, ear flaps, or lunges |
| Object Strike | Aggressive contact with object, including hitting, shoving, or throwing |
| Tooth Grinding | Audible rubbing together of teeth |
| Tension Behaviors | |
| Coo Vocalization | High-pitched, low-intensity "oooooh" vocalization |
| Covert Look | The animal glances at the object from between his legs or under his arm |
| Fear Grimace | Exaggerated grin exposing teeth |
| Motor Stereotypy§ | Repetitive, abnormal motor movements such as bucking, bouncing, or twirling |
| Pacing§ | Repetitive circular pacing around the test cage |
| Scratch§ | Rapid scratching of body with hands or feet |
| Scream Vocalization | High-intensity, high-pitched vocalization |
| Yawn | Open mouth, exposing teeth |
| Body Postures | |
| Bipedal§ | Standing on hind limbs, hands on walls or ceiling |
| Crouch§ | Head on or near floor, front limbs bent |
| Hang§ | Hanging on walls or ceiling, all limbs off floor |
| Sit§ | Sitting with callosities on floor |
| Stand§ | Four-point stance, all on floor |
| Horizontal Position | |
| Back of Cage§ | Animal's head in the back half of the test cage |
| Front of Cage§ | Animal's head in the front half of the test cage |
| Food Selection | |
| Grape Retrieval# | Animal retrieves the food reward and takes it into the holding cage |
| Affiliative Behaviors | |
| Groom Solicitation§ | Shoulder, back, rump, or flank held stationary towards stimulus |
| Grunt Vocalization | Low-pitched, low-intensity bubbly vocalization |
| Lipsmack | Rapid lip movement with pursed lips |
| Mount Solicitation§ | Rump oriented towards stimulus, tail up, and all four legs straight |
| Self-Directed Behaviors | |
| Self-bite§ | Hair plucking, self-biting, or other self-mutilation |
| Self-clasp§ | Abnormal grasping of the torso |
| Self-groom§ | Picking or licking at one's own fur or non-fur body part |
| Self-sex§ | Manual or oral manipulation of one's own genitals |
| Exploratory Behaviors | |
| Cage Oral Exploration§ | Oral manipulation of the test setting (holding cage, test tray, and WGTA walls) |
| Cage Tactile Exploration§ | Manual manipulation of the test setting (holding cage, test tray, and WGTA walls) |
| Food Oral Exploration§ | Oral manipulation of the food reward; using teeth to tear apart food or remove peel |
| Food Tactile Exploration§ | Use of hands to tear apart or pick at food reward |
| Object Oral Exploration§ | Use of mouth to nonaggressively lick or mouth the stimulus object |
| Object Tactile Exploration§ | Use of hands to nonaggressively grab, hold, and explore stimulus object |
| Object Touch§ | Use of hands to lightly touch but not hold or explore the stimulus object |
| Gaze Direction | |
| Look Away§ | Animal's head is pointing in any direction other than forward |
| Look Forward§ | Animal's head is pointing within 45° of the front center of the test cage |
Note. An additional general category of Facial Expressions was also analyzed, which included Lipsmack, Fear Grimace, Mild Threat, and Full Threat. Listed are all behaviors recorded along with brief definitions. All behaviors were analyzed for frequency (total number of occurrences).
Behavior for which total duration was also measured.
Behavior for which the latency was measured relative to when the experimenter raised the Wisconsin General Testing Apparatus (WGTA) panel between the monkey and the test tray.
This ethogram differs from those described in several previous reports from our laboratory (Meunier et al., 1999; Meunier & Bachevalier, 2002; Meunier, Nalwa, & Bachevalier,, 2003; Meunier, Cirilli, Bachevalier, 2006), but closely resembles the ethogram reported recently by Machado and Bachevalier (2008). Specifically, we expanded the number of behaviors in these more recent ethograms to detect subtle differences between each operated group. Several behaviors have also been grouped into different general categories. For example, we previously classified yawns as mildly aggressive, but in the current study this behavior is included in a general category of Tension Behaviors. While the categorization of macaque monkey behavior is inherently subjective, the categories used here were specifically designed to differentiate between behavioral reactivity that typically occurs only in the presence of a threatening stimulus (Defensive Behaviors) and behaviors that convey more generalized fear or anxiety, but do not occur exclusively in the presence of a threatening stimulus (Tension Behaviors).
An animal’s body posture in the test cage can also give an indication of generalized tension. For example, when monkeys are relaxed or engaging in simple exploratory behaviors, they typically sit on their callosities or stand with all four limbs touching the floor. By contrast, when animals exhibit the Tension Behaviors described in Table 3, they may also adopt an atypical posture, such as crouching, hanging, or standing bipedal.
We also attained two different, yet complementary, measurements of passive avoidance for each object presented. Back of Cage Duration measured the total time (in seconds) that the animal spent in the back half of its testing cage during each trial. The Grape Retrieval Latency (GRL) measured the animal’s latency to take the grape into its cage, relative to when the opaque panel was raised at the start of each trial. The GRL, therefore, provides an indication of the animal’s assessment of the reward’s value relative to the stimulus with which it is paired.
Beyond these measures of behavioral reactivity and passive avoidance, a group of common macaque affiliative behaviors (groom and mount solicitations, lip smacking, and grunt vocalizations) was also scored since several objects included in the study had the potential for social engagement (i.e., mirror or girl doll). Heightened frequencies of self-directed and exploratory behaviors have also classically been observed for monkeys with large temporal lobe lesions (Klüver and Bucy, 1939) and were also included here. Finally, we chose to measure how much visual attention the animals devoted to each neutral or aversive object by measuring the animal’s gaze direction (looking within 45° of the test tray center vs. looking elsewhere).
Data Analysis
For all groups, frequency and duration for each individual behavior described in Table 3 were summed within each general category. The distributions of category totals for each object were first assessed for normality using the Shapiro-Wilk test, and inspection of the skewness and kurtosis ratios. From this analysis, total frequency and duration from a majority of the objects were found not to be normally distributed for one or more groups. Therefore, all data were log10(x + 1) transformed prior to statistical analyses, but nontransformed values were used for illustration purposes.
We also performed preliminary data analyses to ascertain if orbital frontal lesion method had any profound effects on the behavioral data, or if any animals consistently refused the food reward when presented alone. To investigate if lesion method impacted differentially on the behavior of Groups O-asp and O-ibo, these two groups were compared for all behavioral categories and all neutral/aversive object pairs using General Linear Model ANOVAs with Group (2) as the between subjects factor and Condition (4; Baseline, Grape Only, Neutral Object, and Aversive Object) as a within subjects factor with repeated measures using the SPSS 12.0 statistical analyses package. For these analyses, data from each of the eight Baseline conditions and eight Grape Only trials were separately averaged. A Huynh-Feldt correction was used to adjust the degrees of freedom if sphericity could not be assumed. No significant ( p < .05) main effects of Group or interactions between Group and Condition were found for any of the behavioral categories described in Table 3 across any of the eight object pairs [frequency data: group effects, .005 < F(1, 4) < 2.6, all p > .18, Group × Condition effects, .01 < F(3, 12) < 2.7, all p > .10; duration data: group effects, .001 < F(1, 4) < 1.5, all p > .30, Group × Condition effects, .16 < F(3, 12) < 1.6 , all p > .25; latency data: group effects .004 < F(1, 4) < 1.2, all p > .33, Group × Condition effects, .029 < F(2, 8) < .755, all p > .50]. Therefore, in the results section below, Groups O-asp and O-ibo are pooled into a single Group O.
Although all animals had shown high preference for grapes during a food preference study conducted earlier (Machado & Bachevalier, 2007a), it was necessary to determine if any animals consistently refused grapes in the current testing context. GRL z-scores were calculated for each animal across the 16 stimuli and an average of the eight Grape Only conditions. Z-scores greater than ± 2 identified animals that significantly deviated from their group’s mean and could therefore be considered outliers. From this assessment, one animal (H-ibo-1) rarely retrieved the food reward during the Grape Only trials and never selected the grape when presented with an object. This animal was therefore omitted from analysis of GRL data, but was included in analysis of the behavioral reactivity data since there was no reason to assume that this animal’s lack of food motivation would affect its emotional reactivity to threatening stimuli.
Following these preliminary assessments, we focused our analysis on Group C to determine if any of the aversive objects in each general category (Innately Aversive, Conditioned Aversive, and Socially Aversive) chosen by the experimenters were ineffective in producing reliable behavioral reactivity and passive avoidance in normal monkeys. Since one object in each series was specifically chosen to be more aversive than its paired neutral item, we predicted that the aversive items would provoke more Defensive Behaviors and Tension Behaviors, more time in the Back of Cage, and increased latency to retrieve the grape (GRL) relative to their paired neutral item, as well as the Grape Only and Baseline conditions. We therefore used one-tailed paired-sample t tests (aversive > neutral, Grape Only and Baseline) to compare these three variables for Group C across the eight neutral-aversive object pairs, the average of eight Grape Only conditions and the average of eight Baseline conditions.
To assess the effect of each lesion, behavioral data for each object series were analyzed across the four experimental groups using repeated-measures ANOVAs. The four experimental groups did not differ in GRL during the Grape Only conditions (one-way ANOVA, p > .10). Therefore, GRL data were analyzed using 4 Group × 2 Condition (neutral object and aversive object only) repeated-measures ANOVAs. Frequency and duration data were analyzed using 4 Group × 4 Condition (Baseline, Grape Only, neutral object, and aversive object) repeated measures ANOVAs. In both repeated measures analyses, significant main effects of Group were investigated further using two-sided Dunnett tests to investigate differences between Group C and the three operated groups and Tukey’s tests when comparing the three operated groups to each other. Main effects of Condition do not provide information regarding differences between the experimental groups, and therefore will not be presented. We employed two-tailed paired-samples t tests and one-way ANOVAs in the post hoc analysis of Group × Condition interactions, since we could not make a priori predictions as to the direction of all lesion effects.
Statistical significance was set at p < .05 (uncorrected for multiple comparisons) for both the analysis focused on Group C and when all groups were compared. However, given the low number of animals in each experimental group and the heterogeneity of lesion extents, we occasionally report results for which p values fall just above this threshold. Results are identified as marginally significant if their p value is greater than .05 but less than .10. Finally, Pearson product–moment correlation matrices were also generated to determine if the extent of damage to any brain region (intended or unintended) may have significantly influenced the behavioral parameters measured.
Results
Behavior of Sham-Operated Control Animals
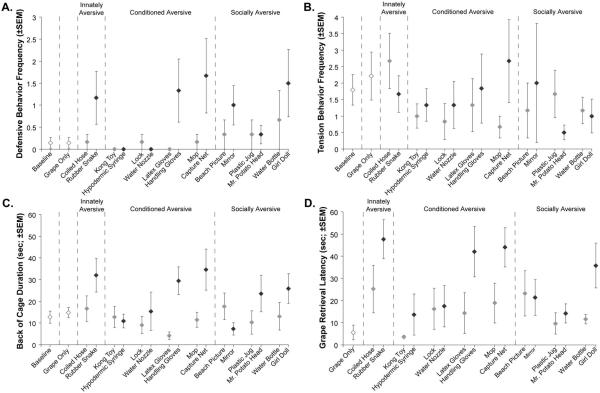
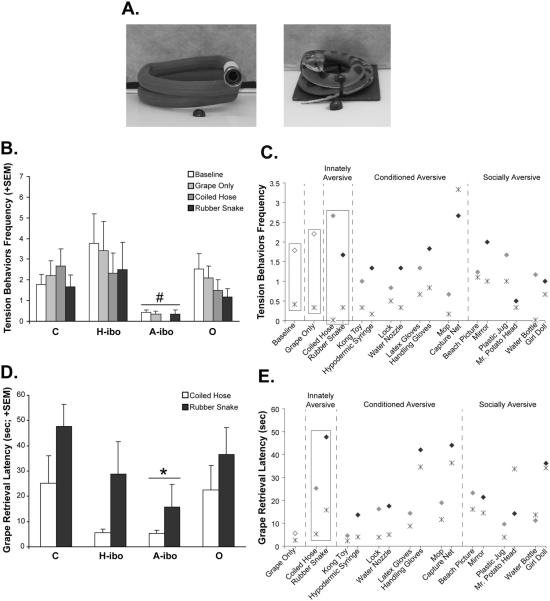
The analysis of Group C alone focused on the most reliable indicators of emotional reactivity (Defensive Behaviors and Tension Behaviors) and passive avoidance (Back of Cage Duration and GRL) in our ethogram. The Innately Aversive object (Rubber Snake) was a highly aversive stimulus for sham-operated animals resulting in heightened Defensive Behaviors relative to the Coiled Hose condition (average: 1.2 with the Rubber Snake vs. 0.2 with the Coiled Hose, see Figure 5A), but this difference did not reach significance (t = 1.464, p = .10, one-tailed). The differences between the Rubber Snake and the Baseline or Grape Only conditions also fell just short of significance (average: 0.1 for both the Baseline and Grape Only conditions, t = 1.909 and 1.882, respectively, both p = .06, one-tailed). Tension Behaviors did not differ between Rubber Snake and either the Coiled Hose, Grape Only, or Baseline conditions (all p > .10; Figure 5B). Sham-operated controls did however spend more time in the Back of Cage when facing the Rubber Snake (32 s; Figure 5C) as compared to the Coiled Hose, Grape Only, and Baseline conditions (t = 2.165, 2.465, and 2.643, respectively, all p < .05, one-tailed). Finally, as shown in Figure 5D, their GRL averaged 47.7 s for the Rubber Snake as compared to 25.2 s for the Coiled Hose (t = 2.184, p < .05, one-tailed) and 5.6 s for the Grape Only condition (t = 6.136, p < .001, one-tailed). It is also noteworthy that Group C found the Coiled Hose stimulus to be mildly aversive relative to the other neutral items in terms of GRL (see Figure 5D). Although this heightened aversion was not significant, it may have been due to the Coiled Hose being positioned on the testing tray in the same way as the Rubber Snake (Figure 6A).
Figure 5.
Average Defensive Behaviors Frequency (A), Tension Behaviors Frequency (B), Back of Cage Duration (C) and Grape Retrieval Latency (GRL; D) for sham-operated control animals in the Baseline and Grape Only conditions, as well as for each of the eight Neutral/Aversive object pairs presented with the food reward. Gray symbols identify the Neutral objects, whereas black symbols denote the Aversive objects. The Baseline condition is not shown for GRL data (D) since grapes were not presented during those trials. Vertical bars represent SEM. Reprinted with permission of the American Psychological Association.
Figure 6.
(A) Photographs of the Coiled Hose and Rubber Snake stimuli (left to right, respectively) as they were seen by the animals. (B) Average frequency of Tension Behaviors displayed by all groups in the Baseline, Grape Only, Coiled Hose and Rubber Snake conditions. (C) Average frequency of Tension Behaviors for Groups C (diamonds) and A-ibo (stars) across all object pairs included in the study; rectangles indicate conditions where Group A-ibo < Group C (p < .05). Gray symbols identify the Neutral objects, whereas black symbols denote the Aversive objects. (D) Average Grape Retrieval Latency (GRL) for all groups during the Coiled Hose and Rubber Snake series. (E) Average GRL data for Groups C and A-ibo across all object pairs included in the study; rectangles highlight the conditions where Group A-ibo < Group C (p < .05). Symbols and color conventions are the same as panel C. Vertical bars in B and D represent the SEM.# p < .05, mean of all conditions relative to Groups C and H-ibo; * p < .05, mean of all conditions relative to Group C. Reprinted with permission of the American Psychological Association.
Of the four Conditioned Aversive objects (Hypodermic Syringe, Water Nozzle, Handling Gloves, and Capture Net), only two triggered aversive reactions in sham-operated controls. For the Capture Net, Group C displayed more Defensive Behaviors (Figure 5A) as compared to the neutral item (Mop, t = 2.087, p < .05), Grape Only (t = 1.853, p = .06) and Baseline (t = 1.853, p = .06) conditions. Heightened Back of Cage Duration (Mop: t = 3.089, Grape Only: t = 2.488, Baseline: t = 2.210, all p < .05, one-tailed; Figure 5C) and a longer GRL (Mop: t = 3.557, Grape Only: t = 6.371, all p < .01, one-tailed; Figure 5D) were also observed for the Capture Net. There was no significant difference in Tension Behaviors between the Capture Net, Mop, Grape Only and Baseline conditions (all p > .10; Figure 5B). The same pattern existed for the Handling Gloves/ Latex Gloves series, but the pairwise differences between conditions were significant only for Back of Cage Duration (Latex Gloves: t = 4.558, Grape Only: t = 2.441, Baseline: t = 2.860, all p < .05, one-tailed; Figure 5C) and for GRL (Latex Gloves: t = 3.233, Grape Only: t = 4.579, all p < .01, one-tailed; Figure 5D) and fell just short of significance for Defensive Behaviors (Latex Glove: t = 1.865, Grape Only and Baseline: t = 1.869, all p = .06, one-tailed; Figure 5A). Significant differences in Defensive Behaviors, Tension Behaviors, Back of Cage Duration, and GRL were not detected between conditions for the other two Conditioned Aversive objects—the Hypodermic Syringe and Water Nozzle.
Since direct eye contact is a threatening social signal among rhesus macaques (van Hooff, 1967; Chevalier-Skolnikoff, 1973), three stimuli (Mirror, Mr. Potato Head, and Girl Doll) were grouped into a Socially Aversive category. Again, there were clear differences in how the control animals reacted to these three social stimuli. For the Girl Doll, sham-operated animals showed heightened Defensive Behaviors (Water Bottle: t = 2.712, Grape Only: t = 2.051, and Baseline: t = 2.071, all p < .05, one-tailed; Figure 5A), spent more time in Back of Cage (Water Bottle: t = 4.54, p < .01, Grape Only: t = 2.002, p .05; Baseline: t = 1.832, p = .06, all one-tailed; Figure 5C), and had longer GRLs (Water Bottle: t = 2.203, p < .05; Grape Only: t = 10.336, p < .001, all one-tailed; Figure 5D). Tension Behaviors did not differ significantly between the Girl Doll and the three other conditions (all p > .10). None of the four parameters differed appreciably across conditions for the Mr. Potato Head/Plastic Jug or Mirror/Beach Picture series.
In summary, sham-operated controls consistently displayed heightened Defensive Behaviors, longer Back of Cage Durations and lengthier GRLs for four aversive objects (Rubber Snake, Capture Net, Handling Gloves, and Girl Doll). These objects, and their paired neutral items, then became our main focus when contrasting the effects of amygdala, hippocampal, or orbital frontal lesions to those of sham lesions.
Emotional Reactivity and Passive Avoidance after Amygdala, Hippocampal, or Orbital Frontal Lesions
The three experimental groups did not differ significantly from the sham-operated group in any parameters measured when they experienced any of the Conditioned Aversive objects or the Socially Aversive objects. There were also no significant correlations between intended or unintended lesion extent and any behavioral variables measured during presentation of these stimuli. By contrast, group differences did emerge for the Innately Aversive object (Rubber Snake) but only for Group A-ibo.
For the Coiled Hose/Rubber Snake series, the four experimental groups differed in the frequency of Tension Behaviors (see Table 3), but not in the frequency of Defensive Behaviors. This difference was revealed by a 4 Group × 4 Condition (Baseline, Grape Only, Coiled Hose and Rubber Snake conditions) repeated measures ANOVA, indicating a significant main effect of Group [F(3, 20) = 4.013, p < .05]. Post hoc comparisons showed that animals with amygdala lesions demonstrated significantly less Tension Behaviors (Figures 6B and 6C) than both sham-operated controls [A-ibo vs. C–Dunnett p < .05] and animals with hippocampal lesions [A-ibo vs. H-ibo–Tukey p < .05]. These differences in Tension Behaviors occurred for all four conditions as reflected by a nonsignificant Group × Condition interaction [F(9, 60) = .374, p > .10]. Group O did not differ appreciably from any other group with regard to Tension Behaviors.
Animals with amygdala lesion also differed from control animals in one measure of passive avoidance (Grape Retrieval Latency; Figures 6D and 6E), but not for Back of Cage Duration. A 4 Group × 2 Condition (Rubber Snake vs. Coiled Hose) repeated measures ANOVA revealed that Group A-ibo displayed a shorter GRL than Group C for both the Rubber Snake and Coiled Hose [Group effect: F(3, 19) = 3.007, p = .06; post hoc: Dunnett Group C vs. Group A-ibo, p < .05]. This group difference was present across the two conditions as revealed by an nonsignificant Group × Condition interaction [F(3, 19) = .323, p > .10]. For Group A-ibo, average GRL across the Coiled Hose and Rubber Snake conditions also correlated negatively with percentage of right hemisphere amygdala damage (r2 = −0.831, p < .05), but not for damage in the left hemisphere (r2 = −0.433, p > .10). It is also interesting to note that, although GRLs for Group A-ibo were typically shorter than those of Group C for all other Conditioned and Socially Aversive stimuli (Figure 6E), these differences did not reach significance (all p > .05). Again, Groups H-ibo and O did not differ appreciably from the sham-operated controls or any other experimental groups in GRL.
Discussion
The current study, although closely related to several previous reports (Amaral et al., 2003; Chudasama et al., 2008; Izquierdo & Murray, 2004; Izquierdo et al., 2005; Kalin et al., 2001; 2004; 2007; Mason et al., 2006; Mineka et al., 1980), was the first to simultaneously study emotional reactivity and passive avoidance in monkeys with selective amygdala, orbital frontal, or hippocampal lesions using a wide range of aversive stimuli. Sham-operated control animals typically showed heightened defensive behaviors, physically avoided and refused to take a highly preferred food during trials with a potential predator (coiled rubber snake), two conditioned aversive stimuli (a nonhuman primate capture net and a pair of nonhuman primate handling gloves), and one socially aversive object (a human-like doll displaying direct eye contact). We found that all operated groups were able to display appropriate emotional reactivity and passive avoidance when confronted with the conditioned aversive and socially aversive stimuli. By contrast, animals with amygdala lesions, but not hippocampal or orbital frontal lesions, showed blunted tension-related behaviors and diminished passive avoidance when exposed to a potential predator. Each of these results will be discussed further in the following sections.
Preserved Reactivity and Avoidance Toward Conditioned and Socially Aversive Stimuli
Animals with amygdala, orbital frontal or hippocampal lesions demonstrated normal passive avoidance and emotional behaviors toward several different types of potentially dangerous stimuli, regardless of size (e.g., large capture net or small hypodermic syringe; see Table 2). Neither amygdala, orbital frontal, nor hippocampal lesions altered animals’ normal fear of two conditioned aversive stimuli (a nonhuman primate capture net and leather handling gloves). It is likely that our animals learned to fear such items while living in seminaturalistic or laboratory settings prior to their lesion. Although such exposures were not explicitly documented, capture with nets and physical restraint with thick, leather gloves are common handling procedures for nonhuman primates. It is possible that during these exposures, our animals learned that capture nets and leather handling gloves resulted in aversive consequences and should be avoided. Even after amygdala, orbital frontal, or hippocampal lesions, animals could show normal levels of tension-related or defensive behaviors (see Table 3) and were able to refrain from approaching and reaching toward a preferred food in close proximity to these items like sham-operated animals did, presumably because they were still able to remember the association between the visual properties of these stimuli and their aversive consequences. One could also argue that each of our conditioned aversive items is normally experienced while being held by a human. However, in the testing context used here, they were simply placed on a testing tray and perhaps that difference diminished their aversive connotations. While this is a valid point, our results are consistent with a recent study in monkeys that found intact potentiated startle reflexes following amygdala lesions when animals were exposed to stimuli conditioned prior to surgery (Antoniadis et al., 2007). Our results are also consistent with rodent studies of inhibitory avoidance and fear conditioning as assessed by potentiated startle. Posttraining amygdala lesions do not impair animals’ ability to avoid entering a compartment previously paired with aversive consequences (Parent et al., 1995). Similarly, posttraining hippocampal lesions also do not affect potentiated startle reflexes to explicit stimuli conditioned prior to surgery (Heldt et al., 2002; Kim & Fanselow, 1992; McNish et al., 1997, 2000; Phillips & LeDoux, 1992). The effects of posttraining orbitofrontal cortex lesions in rodents have not, to our knowledge, been investigated with respect to behavioral inhibition in response to aversive stimuli. However, functional neuroimaging studies with humans indicate that the amygdala and anterior cingulate cortex, but not the orbitofrontal cortex, show elevated activity during the acquisition and expression of conditioned fear (Buchel Morris, Dolan, & Friston, 1998; LaBar, Gatenby, Gore, LeDoux, & Phelps, 1998). By contrast, the orbitofrontal cortex, along with the amygdala, demonstrates elevated activity during extinction of conditioned fear learning (Gottfried & Dolan, 2004). Each of these examples lend support to the results generated here, further indicating that neither the amygdala, hippocampal formation, nor the orbital frontal cortex are solely responsible for the normal expression of fear or passive avoidance once aversive consequences are learned, especially if contingencies remain consistent.
Besides conditioned aversive stimuli, the current study also assessed emotional reactivity and avoidance of three stimuli that had a social component (i.e., a girl doll, a small mirror, and a child’s toy with atypical facial features). Similar to the conditioned aversive objects, and contrary to our hypothesis, none of the lesions had any effect on emotional reactivity or passive avoidance of these stimuli. This finding appears to be at odds with several previous nonhuman primate studies that examined reactivity to stimuli of a social nature. Butter and colleagues (1970) exposed monkeys with ventral frontal cortex lesions to a toy “troll” doll with distorted facial features (see Figure 1 in Butter et al., 1970). The operated animals displayed blunted aggressive behaviors toward the doll, and this impairment was long lasting. The different findings between this study and the current experiment may have been due largely to the size of lesion. The lesions performed by Butter and colleagues (1970) incorporated the entire ventral frontal cortex, including not only areas 11 and 13, but also areas 14 and 47/12 (see Figure 3 in Butter et al., 1970). The orbital frontal lesions in the current study were largely confined to areas 11, 13 and the anterior agranular insular area, but largely spared areas 14 and 47/12. Reactivity to social stimuli has more commonly been studied with nonhuman primates using the Human Intruder paradigm (Izquierdo & Murray, 2004; Kalin & Shelton, 1989; Kalin et al., 1991; 2001; 2004; 2007; Machado & Bachevalier, 2008; Mason et al., 2006), which compares emotional reactivity to an unfamiliar human displaying either direct eye contact or averted gaze. In this context, amygdala damage typically results in diminished defensive freezing and increased time at the front of the test cage (Kalin et al., 2001; 2004; Machado & Bachevalier, 2008; Mason et al., 2006). Orbital frontal lesions produce a similar pattern of behavioral deficits (Kalin et al., 2007; Machado & Bachevalier, 2008), along with an increase in mild aggression (a category including frowning, ears back, and yawning; Izquierdo et al., 2005) and decreases in two types of dominance displays, called tooth grinding and cage shaking (Machado & Bachevalier, 2008). We also previously reported that amygdala or orbital frontal cortex lesions changed how the same animals studied here respond to threatening and affiliative social cues from familiar conspecifics (Machado & Bachevalier, 2006). The results from the current study did not corroborate any of these previous findings regarding emotional reactivity to social stimuli, but are similar to findings recently described by Rudebeck and colleagues (2006). This group found no difference between animals with ventromedial frontal lobe damage (including areas 11, 13, 45, and 47/12 in their entirety, along with portions of areas 8, 9, 10, and 46) and unoperated controls in willingness to retrieve a food reward positioned in front of a video monitor displaying other monkeys. One explanation for these incongruent results may relate to a difference between how the primate brain interprets the meaning of real versus inanimate social stimuli. Interpreting the meaning or threat level of an inanimate girl doll or a video presentation of another monkey may be comparatively less intensive than the same assessment for an actual, unfamiliar human intruder or multiple social partners. The latter cognitive appraisal may be more dependent upon the amygdala, hippocampal formation and orbital frontal cortex, but future studies using both real and inanimate social stimuli are required to directly test this hypothesis.
It is unclear, however, what neural regions could be supporting these intact fear and avoidance abilities in our operated animals. There have been recent indications in the rat literature that the anterior cingulate cortex may be important for the storage of fear memories (Frankland, Bontempi, Talton, Kaczmarek, & Silva, 2004; Han et al., 2003; Santini, Ge Ren Peña de Ortiz, & Quirk, 2004). Rudebeck and colleagues (2006) also recently demonstrated that monkeys with lesions confined to the anterior cingulate gyrus, but not ventromedial frontal cortex or anterior cingulate sulcus, result in less passive avoidance and fewer behavioral responses when exposed to social stimuli (staring human and video clips of other monkeys). It is therefore possible that animals with orbital frontal cortex lesions are able to use their intact anterior cingulate cortex, amygdala, and medial temporal lobe memory system (i.e., hippocampus, entorhinal cortex, perirhinal cortex, and parahippocampal gyrus) to mediate normal avoidance of familiar aversive stimuli. By contrast, normal fear and avoidance for animals with amygdala or hippocampal lesions could be mediated by the anterior cingulate cortex and their largely intact perirhinal and entorhinal cortices. The latter two cortical regions are known to be critical for the formation and maintenance of associations between visual stimuli and their motivational significance (Liu, Murray, & Richmond, 2000; Liu & Richmond, 2000). Lesions of these cortical areas in monkeys also result in attenuated affiliation and approach, as well as enhanced defense in an approach/ avoidance task similar to that described here (Chudasama et al., 2008; Meunier & Bachevalier, 2002; Meunier et al., 2006).
The Amygdala and Fear of Predatory Stimuli
Relative to normal animals, those with amygdala lesions, but not orbital frontal or hippocampal lesions, displayed significantly less passive avoidance of a predator-like stimulus (coiled rubber snake), and a similarly coiled piece of hose. This blunted avoidance was negatively correlated with damage to the right amygdala only, which was likely due to more complete lesions in this hemisphere (see Table 1) rather than contradicting a previous study that found no laterality effect in emotional processing for macaques (Izquierdo & Murray, 2004). Animals with amygdala lesions also showed far less Tension Behaviors (see Table 3) than sham-operated controls and animals with hippocampal lesions throughout the entire Baseline/Grape Only/Coiled Hose/Rubber Snake series. Although the blunted passive avoidance and generalized tension demonstrated by animals with amygdala lesions transcended the other conditions in this series (Baseline, Grape Only, and Coiled Hose), this pattern of behavior did not reach significance for any other conditioned or socially aversive object series. One interpretation of these differing results across categories of aversive stimuli is that the amygdala is a particularly important neural structure for mediating normal tension and passive avoidance when faced with predatory stimuli, but less involved in mediating such behavior for other potentially dangerous conditioned or socially aversive stimuli. This conclusion is in accord with other recently published results for the amygdala generated under similar conditions (Amaral et al., 2003; Izquierdo & Murray, 2004; Kalin et al., 2001; 2004; Meunier et al., 1999; Meunier & Bachevalier, 2002). Blunted aversion to a snake or predator-like stimulus is one of the most consistent observations reported across laboratories for macaques with amygdala lesions, and is likely not due to inadvertent damage to the subjacent entorhinal and perirhinal cortices (Meunier & Bachevalier, 2002; Meunier et al., 2006). Our experiment went a step beyond previous investigations by exposing animals to a variety of aversive items to ascertain the limits of blunted fear and passive avoidance following amygdala lesions. Beyond decreased fear of a predator-type stimulus, animals with amygdala lesions were largely normal in their emotional reactions and passive avoidance of potentially dangerous stimuli. It appears as though the amygdala has been tuned through natural selection to facilitate tension-related behaviors and passive avoidance in response to stimuli that pose a direct and consistent threat to survival (Izquierdo & Murray, 2004; Mineka & Öhman, 2002). This idea is also compatible with rodent studies, since amygdala lesions abolish freezing in the presence of cat odor (Blanchard, Canteras, Markham, Pentkowski, & Blanchard,, 2005; Li, Maglinao, & Takahashi, 2004). While our data lend support to this idea, additional studies are certainly required, perhaps with more than just one potentially life-threatening or predatory stimulus.
As mentioned above, animals with hippocampal lesions demonstrated normal avoidance of the rubber snake. This result appears to be in direct contrast with previous rodent and nonhuman primate studies. Rodents with ventral but not dorsal hippocampus lesions demonstrate reduced fear to cat odor (Blanchard et al., 2005). However, this discrepancy could be due to differences between species and modalities used to cue fear behaviors. Chudasama and colleagues (2008) also recently reported blunted snake fear in monkeys with neurotoxic hippocampal lesions using similar methods to ours, but presented the fake snake five times, each on separate days. In that study, diminished snake fear relative to control monkeys became apparent for animals with hippocampal lesions on the third, fourth, and fifth exposures to the fake snake. In fact, similar to our results, animals with hippocampal lesions reported by Chudasama and colleagues (2008) showed normal levels of snake fear on their first exposure to this stimulus (see Figure 2A in Chudasama et al., 2008). The culmination of these two studies indicate that the hippocampal formation may not be critical for initial fear reactivity to potential predators, but could be more important for potentiating fear and passive avoidance over time or with regard to context (Chudasama et al., 2008).
Regarding the orbital frontal cortex, our observation of normal avoidance of a rubber snake also appears somewhat different from three previous reports. First, ventral frontal lobe damage reduces aggression (Butter et al., 1970) and passive avoidance (Kalin et al., 2007; Rudebeck et al., 2006) in monkeys confronted by a moving snake model or a real snake. The discrepancy between these studies and the current results could again be due to a difference in how animate and inanimate stimuli are processed by the primate brain, rather than due to differences in lesion size. While the lesions studied by Butter and colleagues (1970) and Rudebeck and colleagues (2006) incorporated orbital frontal and ventrolateral prefrontal cortex, the lesions studied by Kalin and colleagues (2007) were nearly identical to those in the current study (largely confined to areas 11 and 13, but also damaged a small, medial portion of area 12). In studies that used an inanimate, rubber snake to provoke fear, as in the current study, aspiration orbital frontal lesions have also been reported to reduce food retrieval latency and fear behaviors (Izquierdo et al., 2005; Kalin et al., 2007). Animals in these two previous studies experienced the same rubber snake stimulus multiple times, either within the same day or across days. Since Kalin and colleagues (2007) only provided an average across all rubber snake presentations, it remains unclear if their operated animals differed from controls on the first rubber snake presentation. The study by Izquierdo and colleagues (2005) only found lower food retrieval latencies relative to control animals on the third, fourth and fifth presentations (see Figure 2A in Izquierdo et al., 2005). On the first snake presentation, animals with orbital frontal lesions showed the same high level of snake avoidance as normal animals, which is again consistent with our findings. Similarly, Rudebeck and colleagues (2006) also found that animals with extensive ventromedial frontal damage did not differ from unoperated controls in passive avoidance when exposed to a rubber snake. It is reasonable to conclude that the contribution of the orbital frontal cortex and the hippocampal formation to fear of predatory stimuli may differ from that of the amygdala. Initial fear and avoidance of predators may be largely driven by the amygdala, but maintaining such a behavioral pattern over time and across contexts may require the concerted action of the orbital frontal cortex and hippocampal formation. Future studies with nonhuman primates, perhaps using functional neuroimaging techniques, are certainly needed to disentangle these issues regarding animate/inanimate stimuli and how defensive and avoidant behaviors are maintained through time and across experience.
Concluding Comments
The results presented here suggest that the neural mechanisms engaged when primates defend themselves are largely dependent on the specific stimulus encountered and how many times that stimulus has been experienced. Initial reactions to a potential predator (i.e., a coiled snake) appear to be largely dependent upon the integrity of the amygdala. Fear and avoidance to other stimuli that have gained their aversive connotations through experience may be mediated by a more diffuse neural network including, but not limited to, the amygdala, hippocampal formation, orbital frontal cortex, perirhinal cortex, entorhinal cortex, and/or anterior cingulate cortex. Further systematic exploration of the complex neural network that mediates fear reactivity in humans and nonhuman primates is certainly needed and will greatly advance the development of new and more effective treatments for anxiety disorders in humans.
Acknowledgments
This work was supported by grants from the National Institutes of Mental Health (MH-58846), and the National Institute of Child Health and Human Development (HD-35471) to Dr. Bachevalier, and by a National Research Service Award Predoctoral Fellowship from the National Institutes of Mental Health (MH-63577) to Dr. Machado. The data described here served as partial fulfillment of the requirements for the PhD degree from The University of Texas Graduate School of Biomedical Sciences at Houston to Dr. Machado. We thank the University of Texas Health Science Center at Houston veterinary and animal husbandry staff for expert animal care, Roger E. Price and Belinda Rivera for the care and handling of animals during the MR imaging procedures, Edward F. Jackson for assistance in neuroimaging techniques, and David Lane for statistical advice and guidance in data analysis. We also extend thanks to the two anonymous reviewers who offered valuable comments on an earlier version of this paper. Christopher Machado is now at The M.I.N.D. Institute, University of California, Davis, 2805 50th Street, Room 1411, Sacramento, CA 95817. Jocelyne Bachevalier and Andy Kazama are now at the Yerkes National Primate Research Center, Emory University, 954 Gatewood Rd., Atlanta, GA 30329.
References
- Aggleton JP, Passingham RE. Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta) Journal of Comparative and Physiological Psychology. 1981;95:961–977. doi: 10.1037/h0077848. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Capitanio JP, Jourdain M, Mason WA, Mendoza SP, Prather M. The amygdala: Is it an essential component of the neural network for social cognition? Neuropsychologia. 2003;41:235–240. doi: 10.1016/s0028-3932(02)00154-9. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Insausti R, Cowan WM. The entorhinal cortex of the monkey: I. Cytoarchitectonic organization. Journal of Comparative Neurology. 1987;264:326–355. doi: 10.1002/cne.902640305. [DOI] [PubMed] [Google Scholar]
- Amaral DG, Price JL, Pitkänen A, Carmichael ST. Anatomical organization of the primate amygdaloid complex. In: Aggleton JP, editor. The amygdala: Neurobiological aspects of emotion, memory, and mental dysfunction. Wiley; New York: 1992. pp. 1–66. [Google Scholar]
- Antoniadis EA, Winslow JT, Davis M, Amaral DG. Role of the primate amygdala in fear-potentiated startle: Effects of chronic lesions in the rhesus monkey. Journal of Neuroscience. 2007;27:7386–7396. doi: 10.1523/JNEUROSCI.5643-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bachevalier J, Nemanic S. Memory for spatial location and object-place association are differently processed by the hippocampal formation, parahippocampal areas TH/TF and perirhinal cortex. Hippocampus. 2008;18:64–80. doi: 10.1002/hipo.20369. [DOI] [PubMed] [Google Scholar]
- Barbas H. Flow of information for emotions through temporal and orbitofrontal pathways. Journal of Anatomy. 2007a;211:237–249. doi: 10.1111/j.1469-7580.2007.00777.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H. Specialized elements of orbitofrontal cortex in primates. Annals of the New York Academy of Sciences. 2007b;1121:10–32. doi: 10.1196/annals.1401.015. [DOI] [PubMed] [Google Scholar]
- Blanchard DC, Canteras NS, Markham CM, Pentkowski NS, Blanchard RJ. Lesions of structures showing FOS expression to cat presentation: Effects on responsivity to a cat, cat odor, and nonpredator threat. Neuroscience and Biobehavioral Reviews. 2005;29:1243–1253. doi: 10.1016/j.neubiorev.2005.04.019. [DOI] [PubMed] [Google Scholar]
- Buchel C, Morris J, Dolan RJ, Friston KJ. Brain systems mediating aversive conditioning: An event-related fMRI study. Neuron. 1998;20:947–957. doi: 10.1016/s0896-6273(00)80476-6. [DOI] [PubMed] [Google Scholar]
- Butter CM. Perseveration in extinction and in discrimination reversal tasks following selective frontal ablations in Macaca mulatta. Physiology and Behavior. 1969;4:163–171. [Google Scholar]
- Butter CM, Mishkin M, Mirsky AF. Emotional responses toward humans in monkeys with selective frontal lesions. Physiology and Behavior. 1968;3:213–215. [Google Scholar]
- Butter CM, Snyder DR. Alterations in aversive and aggressive behaviors following orbital frontal lesions in rhesus monkeys. Acta Neurobiologiae Experimentalis (Warsaw) 1972;32:525–565. [PubMed] [Google Scholar]
- Butter CM, Snyder DR, McDonald JA. Effects of orbital frontal lesions on aversive and aggressive behaviors in rhesus monkeys. Journal of Comparative and Physiological Psychology. 1970;72:132–144. doi: 10.1037/h0029303. [DOI] [PubMed] [Google Scholar]
- Carmichael ST, Price JL. Architectonic subdivision of the orbital and medial prefrontal cortex in the macaque monkey. Journal of Comparative Neurology. 1994;346:366–402. doi: 10.1002/cne.903460305. [DOI] [PubMed] [Google Scholar]
- Cheney DL, Seyfarth RM. How monkeys see the world. The University of Chicago Press; Chicago: 1990. [Google Scholar]
- Chevalier-Skolnikoff S. Facial expression of emotion in nonhuman primates. In: Ekman P, editor. Darwin and facial expression: A century of research in review. Academic Press; New York: 1973. pp. 11–89. [Google Scholar]
- Chudasama Y, Wright KS, Murray EA. Hippocampal lesions in rhesus monkeys disrupt emotional responses but not reinforcer devaluation effects. Biological Psychiatry. 2008;63:1084–1091. doi: 10.1016/j.biopsych.2007.11.012. [DOI] [PubMed] [Google Scholar]
- Davis M, Walker DL, Myers KM. Role of the amygdala in fear extinction measured with potentiated startle. Annals of the New York Academy of Sciences. 2003;985:218–232. doi: 10.1111/j.1749-6632.2003.tb07084.x. [DOI] [PubMed] [Google Scholar]
- Esclassan F, Coutureau E, Di Scala G, Marchand AR. Differential contribution of dorsal and ventral hippocampus to trace and delay fear conditioning. Hippocampus. 2008 doi: 10.1002/hipo.20473. DOI: 10.10002/hipo.20473. [DOI] [PubMed] [Google Scholar]
- Frankland PW, Bontempi B, Talton LE, Kaczmarek L, Silva AJ. The involvement of the anterior cingulate cortex in remote contextual fear memory. Science. 2004;304:881–883. doi: 10.1126/science.1094804. [DOI] [PubMed] [Google Scholar]
- Ghashghaei HT, Hilgetag CC, Barbas H. Sequence of information processing for emotions based on the anatomic dialogue between prefrontal cortex and amygdala. Neuroimage. 2007;34:905–923. doi: 10.1016/j.neuroimage.2006.09.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried JA, Dolan RJ. Human orbitofrontal cortex mediates extinction learning while accessing conditioned representations of value. Nature Neuroscience. 2004;7:1145–1153. doi: 10.1038/nn1314. [DOI] [PubMed] [Google Scholar]
- Han CJ, O’Tuathaigh CM, van Trigt L, Quinn JJ, Fanselow MS, Mongeau R, et al. Trace but not delay fear conditioning requires attention and the anterior cingulate cortex. Proceedings of the National Academy of Sciences, USA. 2003;100:13087–13092. doi: 10.1073/pnas.2132313100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heldt SA, Coover GD, Falls WA. Posttraining but not pretraining lesions of the hippocampus interfere with feature-negative discrimination of fear-potentiated startle. Hippocampus. 2002;12:774–786. doi: 10.1002/hipo.10033. [DOI] [PubMed] [Google Scholar]
- Hobin JA, Ji J, Maren S. Ventral hippocampal muscimol disrupts context-specific fear memory retrieval after extinction in rats. Hippocampus. 2006;16:174–182. doi: 10.1002/hipo.20144. [DOI] [PubMed] [Google Scholar]
- Hodos W, Bobko P. A weighted index of bilateral brain lesions. Journal of Neuroscience Methods. 1984;12:43–47. doi: 10.1016/0165-0270(84)90046-3. [DOI] [PubMed] [Google Scholar]
- Holt W, Maren S. Muscimol inactivation of the dorsal hippocampus impairs contextual retrieval of fear memory. Journal of Neuroscience. 1999;19:9054–9062. doi: 10.1523/JNEUROSCI.19-20-09054.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Insausti R, Amaral DG, Cowan WM. The entorhinal cortex of the monkey: II. Cortical afferents. Journal of Comparative Neurology. 1987;264:356–395. doi: 10.1002/cne.902640306. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Murray EA. Combined unilateral lesions of the amygdala and orbital prefrontal cortex impair affective processing in rhesus monkeys. Journal of Neurophysiology. 2004;91:2023–2039. doi: 10.1152/jn.00968.2003. [DOI] [PubMed] [Google Scholar]
- Izquierdo A, Suda RK, Murray EA. Comparison of the effects of bilateral orbital prefrontal cortex lesions and amygdala lesions on emotional responses in rhesus monkeys. Journal of Neuroscience. 2005;25:8534–8542. doi: 10.1523/JNEUROSCI.1232-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE. Defensive behaviors in infant rhesus monkeys: Environmental cues and neurochemical regulation. Science. 1989;243:1718–1721. doi: 10.1126/science.2564702. [DOI] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. The role of the central nucleus of the amygdala in mediating fear and anxiety in the primate. Journal of Neuroscience. 2004;24:5506–5515. doi: 10.1523/JNEUROSCI.0292-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ. Role of the primate orbitofrontal cortex in mediating anxious temperament. Biological Psychiatry. 2007;62:1134–1139. doi: 10.1016/j.biopsych.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Davidson RJ, Kelley AE. The primate amygdala mediates acute fear but not the behavioral and physiological components of anxious temperament. Journal of Neuroscience. 2001;21:2067–2074. doi: 10.1523/JNEUROSCI.21-06-02067.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalin NH, Shelton SE, Takahashi LK. Defensive behaviors in infant rhesus monkeys: Ontogeny and context-dependent selective expression. Child Development. 1991;62:1175–1183. [PubMed] [Google Scholar]
- Kim JJ, Clark RE, Thompson RF. Hippocampectomy impairs the memory of recently, but not remotely, acquired trace eyeblink conditioned responses. Behavioral Neuroscience. 1995;109:195–203. doi: 10.1037//0735-7044.109.2.195. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Fanselow MS. Modality specific retrograde amnesia of fear. Science. 1992;256:675–677. doi: 10.1126/science.1585183. [DOI] [PubMed] [Google Scholar]
- Klüver H, Bucy PC. Preliminary analysis of functions of the temporal lobes in monkeys. Archives of Neurology and Psychiatry. 1939;42:979–1000. doi: 10.1176/jnp.9.4.606. [DOI] [PubMed] [Google Scholar]
- LaBar KS, Gatenby JC, Gore JC, LeDoux JE, Phelps EA. Human amygdala activation during conditioned fear acquisition and extinction: A mixed-trial fMRI study. Neuron. 1998;20:937–945. doi: 10.1016/s0896-6273(00)80475-4. [DOI] [PubMed] [Google Scholar]
- Li CI, Maglinao TL, Takahashi LK. Medial amygdala modulation of predator odor-induced unconditioned fear in the rat. Behavioral Neuroscience. 2004;118:324–332. doi: 10.1037/0735-7044.118.2.324. [DOI] [PubMed] [Google Scholar]
- Liu Z, Murray EA, Richmond BJ. Learning motivational significance of visual cues for reward schedules requires rhinal cortex. Nature Neuroscience. 2000;3:1307–1315. doi: 10.1038/81841. [DOI] [PubMed] [Google Scholar]
- Liu Z, Richmond BJ. Response differences in monkey TE and perirhinal cortex: Stimulus association related to reward schedules. Journal of Neurophysiology. 2000;83:1677–1692. doi: 10.1152/jn.2000.83.3.1677. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The impact of selective amygdala, orbital frontal cortex or hippocampal formation lesions on established social relationships in monkeys. Behavioral Neuroscience. 2006;120:761–786. doi: 10.1037/0735-7044.120.4.761. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Measuring reward assessment in a semi-naturalistic context: The effects of selective amygdala, orbital frontal or hippocampal lesions. Neuroscience. 2007a;148:599–611. doi: 10.1016/j.neuroscience.2007.06.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. The effects of selective amygdala, orbital frontal cortex or hippocampal formation lesions on reward assessment in nonhuman primates. European Journal of Neuroscience. 2007b;25:2885–2904. doi: 10.1111/j.1460-9568.2007.05525.x. [DOI] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Behavioral and hormonal reactivity to threat: Effects of selective amygdala, hippocampal or orbital frontal lesions in monkeys. Psychoneuroendocrinology. 2008;33:926–941. doi: 10.1016/j.psyneuen.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machado CJ, Bachevalier J. Impact of amygdaloid, hippocampal and orbital frontal cortex lesion on approach/avoidance responses in monkeys; Annual Meeting Society for Neuroscience; 2003. Abstracts 29. [Google Scholar]
- Málková L, Lex CK, Mishkin M, Saunders RC. MRI-Based evaluation of locus and extent of neurotoxic lesions in monkeys. Hippocampus. 2001;11:361–370. doi: 10.1002/hipo.1050. [DOI] [PubMed] [Google Scholar]
- Maren S. Neurobiology of Pavlovian fear conditioning. Annual Review of Neuroscience. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- Maren S, Aharonov G, Fanselow MS. Neurotoxic lesions of the dorsal hippocampus and Pavlovian fear conditioning in rats. Behavioural Brain Research. 1997;88:261–274. doi: 10.1016/s0166-4328(97)00088-0. [DOI] [PubMed] [Google Scholar]
- Maren S, Fanselow MS. Electrolytic lesions of the fimbria/ fornix, dorsal hippocampus, or entorhinal cortex produce anterograde deficits in contextual fear conditioning in rats. Neurobiology of Learning and Memory. 1997;67:142–149. doi: 10.1006/nlme.1996.3752. [DOI] [PubMed] [Google Scholar]
- Mason WA, Capitanio JP, Machado CJ, Mendoza SP, Amaral DG. Amygdalectomy and responsiveness to novelty in rhesus monkeys: Generality and individual consistency of effects. Emotion. 2006;6:73–81. doi: 10.1037/1528-3542.6.1.73. [DOI] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. Evidence of contextual fear after lesions of the hippocampus: A disruption of freezing but not fear-potentiated startle. Journal of Neuroscience. 1997;17:9353–9360. doi: 10.1523/JNEUROSCI.17-23-09353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNish KA, Gewirtz JC, Davis M. Disruption of contextual freezing, but not contextual blocking of fear-potentiated startle, after lesions of the dorsal hippocampus. Behavioral Neuroscience. 2000;114:64–76. doi: 10.1037//0735-7044.114.1.64. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J. Comparison of emotional responses in monkeys with rhinal cortex or amygdala lesions. Emotion. 2002;2:147–161. doi: 10.1037/1528-3542.2.2.147. [DOI] [PubMed] [Google Scholar]
- Meunier M, Bachevalier J, Murray EA, Málková L, Mishkin M. Effects of aspiration versus neurotoxic lesions of the amygdala on emotional responses in monkeys. European Journal of Neuroscience. 1999;11:4403–4418. doi: 10.1046/j.1460-9568.1999.00854.x. [DOI] [PubMed] [Google Scholar]
- Meunier M, Cirilli L, Bachevalier J. Responses to affective stimuli in monkeys with entorhinal or perirhinal cortex lesions. Journal of Neuroscience. 2006;26:7718–7722. doi: 10.1523/JNEUROSCI.1949-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meunier M, Nalwa V, Bachevalier J. Reactions to familiar and novel objects in infant monkeys with neonatal temporal lesions. Hippocampus. 2003;13:489–493. doi: 10.1002/hipo.10143. [DOI] [PubMed] [Google Scholar]
- Mineka S, Keir R, rice V. Fear of snakes in wild- and laboratory-reared rhesus monkeys (Macaca mulatta) Animal Learning and Behavior. 1980;8:653–663. [Google Scholar]
- Mineka S, Öhman A. Phobias and preparedness: The selective, automatic, and encapsulated nature of fear. Biological Psychiatry. 2002;52:927–937. doi: 10.1016/s0006-3223(02)01669-4. [DOI] [PubMed] [Google Scholar]
- Murphy FC, Nimmo-Smith I, Lawrence AD. Functional neuroanatomy of emotions: A meta-analysis. Cognitive, Affective and Behavioral Neuroscience. 2003;3:207–233. doi: 10.3758/cabn.3.3.207. [DOI] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Bachevalier J. The hippocampal/ parahippocampal regions and recognition memory: Insights from visual paired comparison versus object-delayed nonmatching in monkeys. Journal of Neuroscience. 2004;24:2013–2026. doi: 10.1523/JNEUROSCI.3763-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nemanic S, Alvarado MC, Price RE, Jackson EF, Bachevalier J. Assessment of locus and extent of neurotoxic lesions in monkeys using neuroimaging techniques: A replication. Journal of Neuroscience Methods. 2002;121:199–209. doi: 10.1016/s0165-0270(02)00264-9. [DOI] [PubMed] [Google Scholar]
- Noldus LP, Trienes RJ, Hendriksen AH, Jansen H, Jansen RG. The Observer Video-Pro: New software for the collection, management, and presentation of time-structured data from videotapes and digital media files. Behavior Research Methods, Instruments and Computers. 2000;32:197–206. doi: 10.3758/bf03200802. [DOI] [PubMed] [Google Scholar]
- Pare D, Quirk GJ, LeDoux JE. New vistas on amygdala networks in conditioned fear. Journal of Neurophysiology. 2004;92:1–9. doi: 10.1152/jn.00153.2004. [DOI] [PubMed] [Google Scholar]
- Parent MB, Quirarte GL, Cahill L, McGaugh JL. Spared retention of inhibitory avoidance learning after posttraining amygdala lesions. Behavioral Neuroscience. 1995;109:803–807. doi: 10.1037//0735-7044.109.4.803. [DOI] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contributions of amygdala and hippocampus to cued and contextual fear conditioning. Behavioral Neuroscience. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Prather MD, Lavenex P, Mauldin-Jourdain ML, Mason WA, Capitanio JP, Mendoza SP, et al. Increased social fear and decreased fear of objects in monkeys with neonatal amygdala lesions. Neuroscience. 2001;106:653–658. doi: 10.1016/s0306-4522(01)00445-6. [DOI] [PubMed] [Google Scholar]
- Price JL. Definition of the orbital cortex in relation to specific connections with limbic and visceral structures and other cortical regions. Annals of the New York Academy of Sciences. 2007;1121:54–71. doi: 10.1196/annals.1401.008. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biological Psychiatry. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Rudebeck PH, Buckley MJ, Walton ME, Rushworth MF. A role for the macaque anterior cingulate gyrus in social valuation. Science. 2006;313:1310–1312. doi: 10.1126/science.1128197. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Peña de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. Journal of Neuroscience. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saunders RC, Aigner TG, Frank JA. Magnetic resonance imaging of the rhesus monkey brain: Use for stereotactic neurosurgery. Experimental Brain Research. 1990;81:443–446. doi: 10.1007/BF00228139. [DOI] [PubMed] [Google Scholar]
- Stefanacci L, Clark RE, Zola SM. Selective neurotoxic amygdala lesions in monkeys disrupt reactivity to food and object stimuli and have limited effects on memory. Behavioral Neuroscience. 2003;117:1029–1043. doi: 10.1037/0735-7044.117.5.1029. [DOI] [PubMed] [Google Scholar]
- van Hooff J. The facial displays of the catarrhine monkeys and apes. In: Morris D, editor. Primate ethology. Aldine Publishing Company; Chicago: 1967. pp. 7–69. [Google Scholar]
- von Bonin G, Bailey P. The brain map. In: von Bonin G, Bailey P, editors. The neocortex of Macaca mulatta. The University of Illinois Press; Urbana, IL: 1947. pp. 57–79. [Google Scholar]
- Whalen PJ. Fear, vigilance, and ambiguity: Initial neuroimaging studies of the human amygdala. Current Directions in Psychological Science. 1998;7:177–188. [Google Scholar]
- Zola-Morgan S, Squire LR, Alvarez-Royo P, Clower RP. Independence of memory functions and emotional behavior: Separate contributions of the hippocampal formation and the amygdala. Hippocampus. 1991;1:207–220. doi: 10.1002/hipo.450010208. [DOI] [PubMed] [Google Scholar]