Abstract
Virus-like particles (VLPs) can serve as a highly immunogenic vaccine platform for the multivalent display of epitopes from pathogens. We have used bacteriophage VLPs to develop vaccines that target a highly conserved epitope from the Human Papillomavirus (HPV) minor capsid protein, L2. VLPs displaying an L2-peptide from HPV16 elicit antibodies that broadly neutralize infection by HPV types associated with the development of cervical cancer. To broaden the cross-neutralization further, we have developed a strategy to display two different peptides on a single, hybrid VLP in a multivalent, highly immunogenic fashion. In general, hybrid VLPs elicited high-titer antibody responses against both targets, although in one case we observed an immunodominant response against only one of the displayed epitopes. Immunization with hybrid particles elicited antibodies that were able to neutralize heterologous HPV types at higher titers than those elicited by particles displaying one epitope alone, indicating that the hybrid VLP approach may be an effective technique to target epitopes that undergo antigenic variation.
Introduction
Virus-like Particle (VLP) technology is a promising approach for developing new vaccines. VLPs make attractive vaccines because they are non-infectious and present viral antigens in a dense, ordered manner that leads to efficient activation of B cells, resulting in high-titer and long-lasting antibody responses (Buonaguro et al. 2011; Chackerian 2007). VLPs can be used as stand-alone vaccines, but they can also be used as platforms to display practically any antigen in a highly immunogenic, multivalent format (Jegerlehner et al. 2002; Schodel et al. 1994). Linking target antigens, either genetically or chemically, to the surfaces of VLPs causes them to be displayed at high density. This high-density display, in turn, dramatically enhances the ability of linked antigens to induce potent antibody responses.
Chimeric VLPs can be constructed by genetic insertion of a target epitope into a viral structural protein (Pumpens and Grens 2001). Unfortunately, generation of recombinant VLPs can be technically challenging because the effects of peptide insertions into viral structural proteins are notoriously difficult to predict and often lead to protein folding failures (Chackerian 2007; Lua et al. 2014). As a consequence, the engineering of recombinant VLPs in most systems described to date is a largely empirical process of trial and error. However, we have engineered the structural proteins from two related bacteriophages (MS2 and PP7) so that they are dramatically more tolerant of foreign insertions (Caldeira Jdo et al. 2010; Peabody et al. 2008). These bacteriophages encode a single structural protein, coat protein, which self-assembles into a 27nm-diameter icosahedral particle consisting of 90 coat-protein homodimers. While coat protein monomers of MS2 and PP7 are usually intolerant of genetic insertions, fusing two copies of coat protein into one long reading frame, which is possible because the N-terminus of one monomer lies in close physical proximity to the C-terminus of the other monomer, results in a functional protein that is dramatically more thermodynamically stable, and highly tolerant of short peptide insertions at two display sites (the N-terminus and the so-called AB-loop). Recombinant MS2 and PP7 VLPs created using the single-chain dimer display 90 target peptides on the surface of each particle and elicit robust epitope-specific antibody responses upon vaccination (Chackerian et al. 2011; Hunter et al. 2011; Tumban et al. 2011).
Many pathogens have developed strategies to evade immunity by presenting epitopes to the immune system that are antigenically variable, while hiding highly conserved sites that are essential for protein function (Burton et al. 2012). One example is Human Papillomavirus (HPV). Over 150 different strains of HPV have been identified and a subset consisting of 14–20 “high-risk” HPV types causes virtually all cases of cervical cancer (Stanley 2010). VLPs comprised of the HPV major capsid protein, L1, are the basis for the HPV vaccines that are currently available on the market (Kirnbauer et al. 1992; Rose et al. 1993). These vaccines are effective against the two highest risk types, HPV 16 and 18, which account for approximately 70% of cervical cancers cases worldwide (Lehtinen et al. 2012; Munoz et al. 2010). However, antibodies raised against L1 VLPs are largely type-specific, thus the vaccines do not provide protection against other high-risk HPV types. Therefore, there is an impetus to develop more cross-protective HPV vaccines that will provoke immune responses that will protect against more of the high-risk HPV types.
In order to develop a more broadly protective HPV vaccine, we have used a VLP platform approach to target a highly conserved epitope in the HPV minor capsid protein, L2. L2 is essential for the virus life cycle but is normally shielded from immune recognition (Roden et al. 2000). Previous studies have shown that vaccination with recombinant L2 elicits immune responses that protect from papillomavirus infection (Campo et al. 1993; Christensen et al. 1991; Lin et al. 1992) and immunization with epitopes derived from the N-terminal region of L2 can elicit antibodies that broadly inhibit infection by diverse HPV types (Gambhira et al. 2007a; Pastrana et al. 2005; Schellenbacher et al. 2013). In general, the titers of neutralizing antibodies elicited by recombinant L2 vaccination are, unfortunately, lower than those elicited by vaccination with HPV L1 VLPs (Karanam et al. 2009). Further, while anti-L2 antibodies are more cross-protective than anti-L1 antibodies, the breadth of cross-protection has to be sufficient to protect against most, if not all, of the high-risk HPV types (Gambhira et al. 2007b; Tumban et al. 2011). As one solution, we have developed vaccines in which we immunize with a cocktail of VLPs displaying L2 epitopes from different HPV types (Tumban et al. 2011). However, there are obvious manufacturing and cost advantages to using a single antigen that can provoke broadly protective responses.
We hypothesized that one method for broadening protection would be to display multiple L2 epitopes on the surface of a single VLP. We designed a plasmid that encodes two open reading frames of bacteriophage coat protein, each displaying a different epitope. This enabled the production of hybrid VLPs that display two different epitopes on the same particle in the same highly immunogenic display context. We hypothesized that these hybrid particles could elicit antibodies that could bind to both the displayed peptides and other similar targets as well. We found that immunization with VLPs displaying L2 epitopes derived from two different high-risk HPV types induced a broader cross-neutralizing response than immunizing with VLPs targeting one epitope. These hybrid particles may be an effective way to broaden the utility of rationally designed, epitope-based vaccines.
Materials and Methods
Construction of expression plasmids
PCR was used to independently insert the peptides representing the L2 amino acids 17–31 from HPV 1, 18, and 16, as well as the FLAG epitope into the AB-loop of the single-chain dimer version of PP7 coat protein (using the expression vector pET2P7K32) as previously described (Caldeira Jdo et al. 2010; Tumban et al. 2011). Similarly, the L2 sequence representing HPV16 and HPV 31 L2 amino acids 17–31 were cloned onto the amino-terminus of a single-chain dimer version of the MS2 coat protein (using the expression vector pDSP62) by PCR as previously described (Tumban et al. 2012). All constructs were confirmed by sequence analysis.
Construction of the dual expression plasmid
PCR was used to engineer complementary, unique restriction sites bracketing the single-chain dimer expression cassette. As a template for the PCR, we used MS2 or PP7 single-chain dimer expression plasmids containing insertions in the AB loop (PP7) or at the N-terminus (MS2). The plasmid was constructed as shown in Fig. 1A. Briefly, the upstream expression cassette was amplified using a forward primer that contained a BglII restriction site and reverse primer that contained a PstI restriction site. The downstream single-chain dimer was amplified similarly, except that the forward primer included a PstI restriction site and the reverse primer contained an EcoRI site. After amplification, the original plasmid was digested with the BclI (leaving an end compatible with a Bgl II cut end) and EcoRI. The dual expression plasmid was then constructed by three-piece ligation and then confirmed by restriction digest analysis.
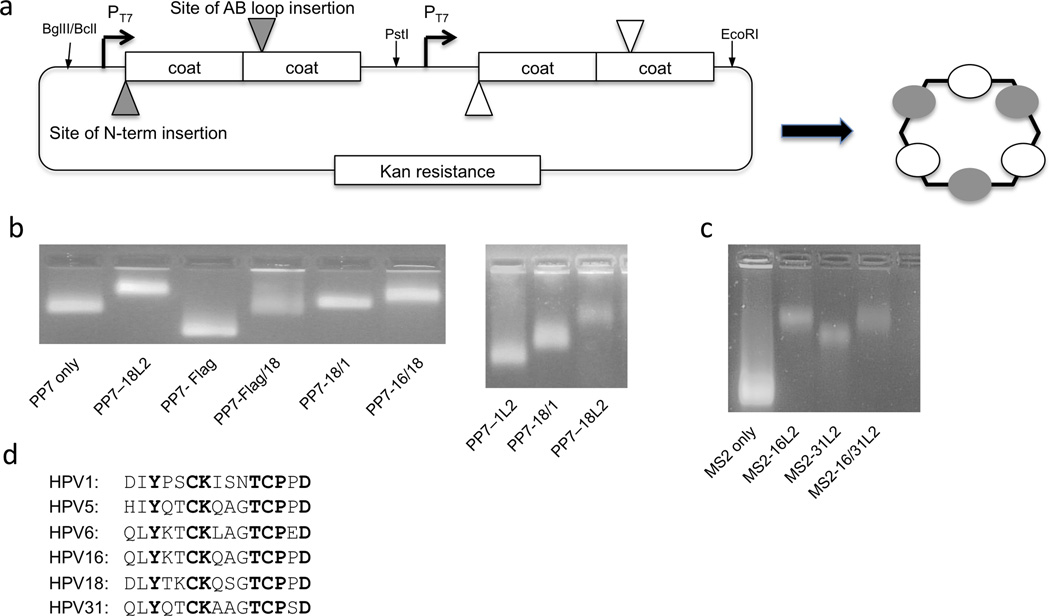
Figure 1.
Design and characterization of hybrid bacteriophage VLPs. A: Design of the hybrid VLP expression plasmid. Peptide targets can be displayed at either the N-terminus or the AB-loop of the coat protein single-chain dimer. Each expression cassette is engineered separately, amplified by PCR, and then plasmids are assembled by three-piece ligation using the restriction sites listed. B: PP7 or C: MS2 VLPs were analyzed using a 1% agarose, non-denaturing gel stained with ethidium bromide (which binds to the genomic material encapsidated by the VLPs). The mobilities of the bands were compared to VLPs of unmodified PP7 or MS2 coat protein. D: An alignment of selected HPV sequences representing L2 aa 17–31 (or the equivalent).
Expression, purification, and characterization of VLPs
Recombinant VLPs were made by transforming C41 cells (Lucigen) with the PP7 or MS2 expression vectors and VLPs were purified from the soluble fraction as previously described (Caldeira Jdo et al. 2010). Intact VLPs were visualized on a 1% agarose gel with ethidium bromide (Invitrogen) and quantified by polyacrylamide gel electrophoresis.
Capture ELISA
ELISA wells were coated with 500 ng/well of RG-1, an anti-L2 antibody that binds the L2 17–31 epitope (provided by Richard Roden) overnight at 4°C (Gambhira et al. 2007b). The wells were then blocked with 0.5% milk in PBS for one hour. Purified recombinant PP7 VLPs were added at 5 µg, 1 µg, or 0.5 µg/well for 2 hours at room temperature. The wells were then probed with a biotinylated anti-FLAG M2 monoclonal antibody (Sigma) diluted 1:2000 for 1 hour at room temperature. Finally, a HRP conjugated streptavidin (Life Technologies) was added, which was diluted to 1:4000 for one hour. ABTS was added as the developer and reactivity was determined by measuring the mean optical density (OD) values at 405 nm.
Immunizations and characterization of antibody responses
All animal work was done in accordance with National Institutes of Health and University of New Mexico guidelines. Groups of Balb/c mice were immunized twice intramuscularly (i.m.) at a two-week interval with 5 g of PP7-L2 (displaying L2 amino acids 17–31 from HPV18, 16, 1, 18/1 or 18/16), or MS2-L2 VLPs 16L2 (displaying L2 amino acids 17–31 from HPV16, 31, or 16/31), or, as negative controls, unmodified MS2 and PP7 VLPs. Vaccine was prepared with incomplete Freund’s adjuvant (IFA). Two weeks after the second immunization, sera were collected and anti-L2 IgG titers were determined by peptide-based ELISA using disulfide-constrained L2 peptides representing amino acids 14–40 from HPV1, 5, 6, 16, and 18 (American Peptide company, as described (Tumban et al. 2011))
HPV pseudovirus (PsV) production and purification
HPV6, 16, 18, 31, 45, and 58 PsVs with encapsidated reporter plasmid (pClucf) encoding both luciferase and green fluorescence protein (GFP) genes were produced in 293TT cells as previously described (Buck et al. 2004; Buck et al. 2005b; Tumban et al. 2011). PsV-infectivity titer was characterized using flow cytometry by determining the fraction of 293TT cells expressing the GFP protein.
Cervicovaginal HPV PsV challenge
Prior to challenge, female Balb/c mice were given 3 i.m. immunizations of 5 µg of control VLPs or VLPs displaying one of the L2 epitopes. Two weeks after the last boost, mice were treated with 3 mg of Depo-Provera subcutaneously (Pharmacia Corp). Five days post-Depo-Provera treatment, mice were vaginally challenged with 1.0 × 105 infectious units (IU) of the PsV stock as previously described (Buck et al. 2005a; Roberts et al. 2007). Forty-eight hours post-PsV challenge, mice were vaginally instilled with 0.4 mg of luciferin (Caliper Life Sciences) and imaged with a Caliper IVIS Lumina II (Caliper Life Sciences) as described previously (Tumban et al. 2011).
In vitro L2 neutralization assay
These assays were performed as described in (Day et al. 2012) except that heparin was not added to PsV solutions prior to infection. Following a two-day incubation, the cells were then collected and analyzed by flow cytometry using a Hypercyte autosampler to detect GFP expression as a marker of infection. The dose of PsV used was based on the amount needed to yield 20–40% infection of control pgsa-745 cells. HPV18 PsV stocks were generally poorly infectious, so cells were infected with amounts of HPV18 PsV resulting in ~10% of control cells being infected.
Statistical Methods
All statistical analyses were performed using Graphpad 5.0 Prism software. For antibody titer data, group means were compared by a one-tailed, Mann-Whitney t-tests. ELISA data were analyzed by two-tailed, unpaired t-tests or by two-way ANOVA with Bonferroni multiple comparisons. For statistical analyses of in vivo PsV challenges, data was log-transformed and then analyzed by a Kruskal-Wallis test with Dunn’s multiple Comparisons Test.
Results
Production and characterization of hybrid VLPs
In order to produce hybrid VLPs, we designed a plasmid that contains two identical expression cassettes, each containing a T7 promoter, an open reading frame encoding the single-chain dimer version of either PP7 or MS2, and a transcriptional terminator (Fig. 1A). As a preliminary test of the ability of the PP7 version of this plasmid to produce hybrid VLPs, we engineered one of the coat proteins to display a sequence derived from HPV18 L2 (aa17–31) and the other to display the FLAG epitope. Upon expression, VLPs were purified and then characterized by agarose gel electrophoresis. Because VLPs migrate through the gel due to their overall electrophoretic charge and can be visualized using ethidium bromide by virtue of the RNA that is encapsidated by the particles, this assay can be used to measure charge differences that are conferred by the epitopes that are displayed on the surface of the VLPs. As predicted, the L2/FLAG hybrid particles show a mobility that falls between VLPs that display either the L2 or FLAG peptide alone, suggesting that both peptides are displayed on the surface of the VLPs (Fig. 1B). To confirm this, we performed a sandwich ELISA, in which an anti-L2 monoclonal antibody was used to capture the VLPs, and an anti-FLAG antibody was used as a probe, allowing us to only detect particles that display both peptides on their surfaces. Hybrid HPV/FLAG particles were detected readily using this assay, whereas VLPs displaying either the FLAG epitope or the L2 peptide alone were not, indicating that hybrid particles display both epitopes on their surfaces (Fig. 2A).
Figure 2.
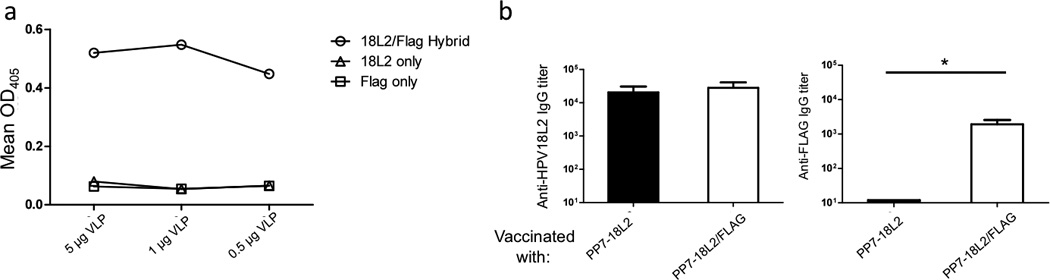
Characterization of PP7-18L2/FLAG hybrid VLPs. A: Hybrid VLPs were characterized via capture ELISA. Different amounts of VLPs were added to wells coated with RG-1, a monoclonal antibody that binds to the aa 17–31 region of L2, and then probed with a biotinylated anti-FLAG antibody followed by HRP-labeled streptavidin. Data points indicate the mean absorption of each well at 405 nm. B: Mice (three per group) were immunized with 5 µg of indicated VLPs twice with two week intervals and sera were collected 2 weeks after the last immunization. Serum anti-L2 peptide or anti-FLAG IgG titers were determined by end-point dilution ELISA using synthetic peptides. Titers indicate the reciprocal of the lowest dilution of serum samples at which reactivity with the immobilized peptide was at least twice that of background. Bars indicated the geometric mean of the group, with error bars indicating standard deviation. Group means were compared by a one-tailed, Mann-Whitney t-test. * indicates p < 0.05.
To assess the immunogenicity of the FLAG/L2 hybrid particles, we immunized mice and then measured the IgG antibody responses against the L2 and FLAG peptides by ELISA (Fig. 2B). Mice immunized with the hybrid particles made high-titer antibodies that bound to both target peptides. In contrast, sera from mice immunized with VLPs displaying only HPV18 L2 peptide only reacted with the 18L2 peptide. Taken together, these data indicate that our dual expression plasmid produces hybrid VLPs displaying both peptides on the surface and can elicit an antibody response against both targets.
Hybrid VLPs elicit more broadly reactive antibodies against HPV L2
Previous studies in our lab showed that immunization with PP7 VLPs displaying an HPV16 L2 sequence (aa17–31) elicited antibodies that were only modestly cross-reactive with L2 sequences from other HPV types (Tumban et al. 2011). To determine if hybrid particles could induce more broadly cross-reactive antibody responses, we produced two hybrid PP7 VLPs that displayed L2 epitopes from two HPV types (HPV18/1 and HPV18/16 VLPs, respectively). The HPV16 and 18 L2 sequences are closely related (13 of the 15 amino acids are identical), whereas the HPV1 and 18 L2 peptides are less so (10 of the 15 amino acids are the same; Fig. 1D). Analysis of the electrophoretic mobility of the L2 hybrid VLPs on an agarose gel suggested that the hybrid VLPs incorporated both L2 peptides (Fig. 1B). Mice were immunized with either PP7-18L2 VLPs or the hybrid VLPs and then antibody binding to peptides representing the L2 sequence from five diverse HPV types was measured by ELISA (Fig. 3A). In agreement with our previous results, the PP7-18L2 anti-serum had strong reactivity to HPV18 L2, moderate reactivity with L2 peptides from HPV5, 6, and 16, and including little to no reactivity with the peptide derived from HPV1, which is the most evolutionarily distant type that we tested (Tumban et al. 2011). Unexpectedly, immunization with the PP7-18/1L2 hybrid VLP elicited antisera that only reacted with the HPV1 peptide. There was little reactivity with the other four peptides, including the HPV18 peptide that was included in the VLP, suggesting that the HPV1 L2 peptide was immunodominant. In stark contrast, the PP7-18/16L2 hybrid VLPs elicited antibodies that bound strongly to both the 16L2 and 18L2 peptides, as well as the other three heterologous L2 peptides that we tested, including HPV1. As a comparison, we also immunized mice with a mixture of VLPs displaying the HPV16 L2 sequence and the 18L2 sequence alone. The hybrid 16/18L2 VLPs elicited higher levels of cross-reactive antibodies than the mixture of VLPs, and only the hybrid VLPs were able to elicit antibody responses that reacted with the HPV1 peptide. The increase in binding to HPV1 and HPV5 L2 peptides, when compared to mice immunized with PP7-18L2 VLPs alone or mice immunized with a mixture of PP7-18L2 and PP7-16L2 VLPs, was statistically significant. These data indicate that more broadly cross-reactive antibodies were elicited by hybrid VLPs than by simply mixing two L2-VLPs together.
Figure 3.
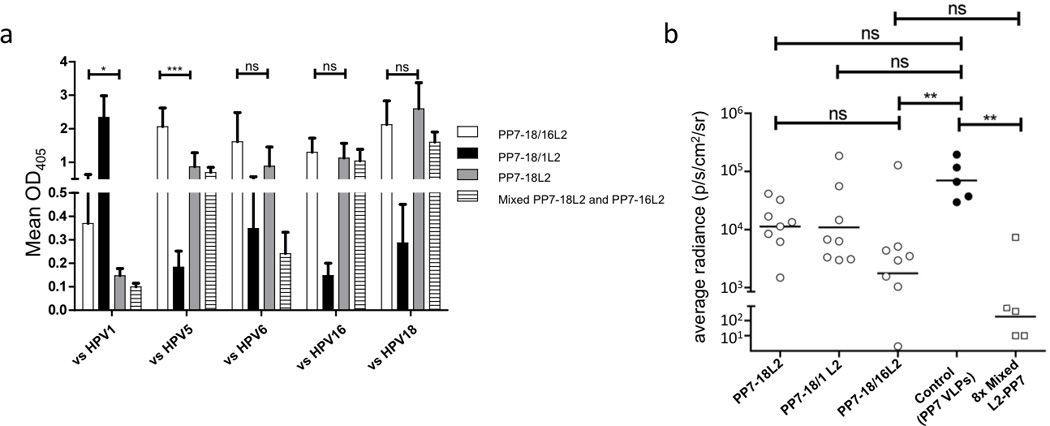
Immunogenicity and in vivo neutralizing activity of hybrid VLPs. A: Groups of eight mice were immunized three times with 5 µg of PP7 VLPs displaying the L2 aa 17–31 peptide from HPV18 alone, HPV18/1 or HPV18/16. Sera collected two weeks after the last immunization was tested for IgG binding to a selection of peptides representing amino acid 14–40 from five HPV types. Sera was diluted at 1:160. Bars indicate group means with standard deviations. Means of PP7-18L2 and PP7-18/16L2 groups were analyzed by two-tailed, unpaired t-tests (vs HPV1 data with Welch’s correction). B: Mice immunized with the indicated VLPs were vaginally challenged with 105 IU of HPV6 PsV encapsidating a luciferase reporter plasmid. Two days after PsV challenge, the mice were vaginally instilled with luciferin and imaged for luminescence. Each data point represents the average radiance for the region of interest (ROI; genital tract) of individual mice; lines representing the geometric mean of each group. The average radiance (p/s/cm2/sr) was calculated by using Living Image 3.2 software. Data were analyzed by a Kruskal-Wallis test with Dunn’s multiple Comparisons Test. * indicates p ≤ 0.05; ** indicates p ≤ 0.01; *** indicates p ≤ 0.001; ns, not significant.
To assess whether hybrid VLPs were capable of providing protection in vivo from challenge with a heterologous HPV type, vaccinated mice were vaginally challenged with a heterologous HPV pseudovirus (HPV6 PsV) encapsidating a luciferase reporter (Fig. 3B). The mice immunized with the PP7-18/16L2 hybrid VLPs were the only group, other than the positive control, that showed a significant reduction (97.5%) in the geometric mean luciferase signal compared to control mice vaccinated with wild-type PP7 VLPs. As a positive control, we also challenged mice that had been immunized with a mixture of eight PP7 VLPs displaying L2 sequences from HPV1, 5, 6, 11, 16, 18, 45 and 58 (Tumban et al. 2011). The geometric mean luciferase signal in this group of mice was only about 10-fold lower than in mice immunized with the PP7-18/16L2 hybrid VLPs, and the difference between groups was not statistically significant.
Hybrid VLPs can enhance the cross-neutralizing potential of an already potent L2 immunogen
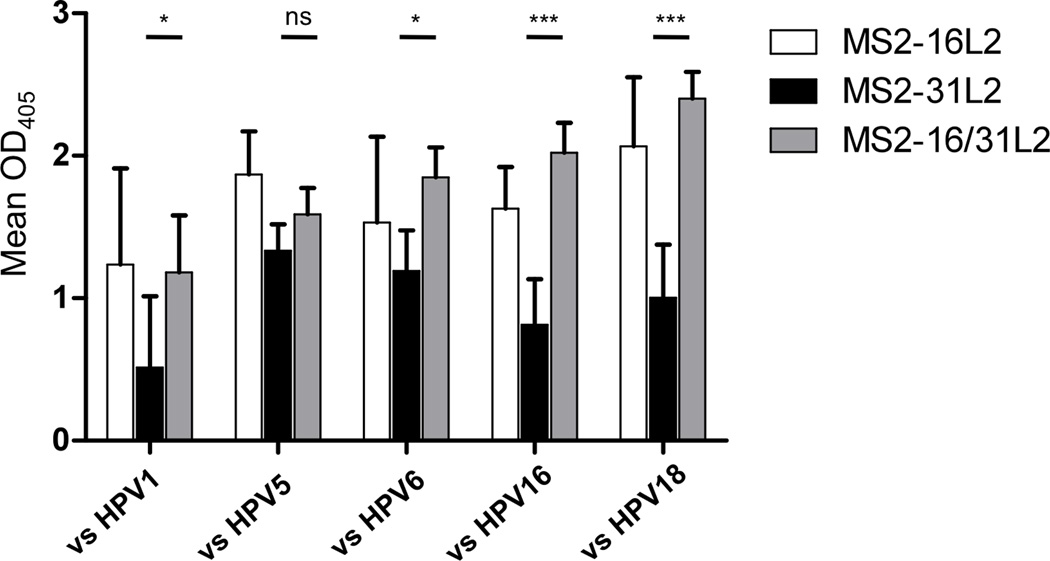
Our lab previously has shown that VLPs that display HPV16 L2 aa17–31 in an unconstrained fashion at the N-terminus of coat protein can elicit broadly cross-reactive antibodies that can provide significant in vivo cross-protection from a panel of eleven diverse HPV PsV types (Tumban et al. 2012). The one outlier was HPV31, which was not as strongly cross-neutralized as other HPV types. The sequence of the HPV16 and 31 L2 epitope only differs by two amino acids, so this result was somewhat surprising. We hypothesized co-display of HPV16 and HPV31 L2 peptides on a hybrid VLP would enhance the already robust cross-protection. To test this, we produced hybrid 16/31 MS2 VLPs (Fig. 1C) and compared the antibody responses in vaccinated mice with mice immunized with either MS2-16L2 or MS2-31L2 VLPs alone, or mice immunized with a mixture of MS2-16L2 and MS2-31L2 VLPs. As shown in Fig. 4, immunization with MS2-16L2 VLPs alone elicited antibody responses with considerable cross-reactivity to a panel of L2 peptides, whereas MS2-31L2 VLPs elicited antibodies with weaker cross-reactivity. Sera from mice immunized with MS2-16/31L2 hybrid VLPs reacted strongly to all the peptides tested, including HPV1 L2, and induced significantly higher cross-reactivity than MS2-31L2 VLPs against four of five L2 peptides tested (Fig. 4). Moreover, co-display of the HPV31 peptide on the hybrid VLPs does not detract from the broad cross-reactivity that we had previously observed.
Figure 4.
Sera from mice immunized with MS2 displaying N-terminal L2 peptides bind to heterologous HPV peptides. Mice (five per group) were immunized three times and the resulting sera was diluted 1:160 and analyzed by ELISA as in Figure 3a. Bars indicate group means and error bars indicate standard deviations. Means of MS2-31L2 and MS2-16/31L2 groups were analyzed by two-way ANOVA with Bonferroni multiple comparisons. * indicates p < 0.05; *** indicates p < 0.001; ns, not significant.
To measure the neutralizing activity of antisera, we utilized a new L2 neutralization assay developed by Day et al that is tailored to measure the neutralization titers developed in response to anti-L2 vaccines (Fig. 5). Previous results using this assay confirmed that immunization with L1-VLPs elicits antibody responses that strongly neutralize the types included in the vaccine but do not cross-neutralize other HPV types (Day et al. 2012). Sera from mice immunized with MS2-16L2 VLPs neutralized all five of the high-risk HPV PsV types that were tested, but the lowest neutralization titer was against HPV31 [in agreement with the in vivo data that we had previously generated (Tumban et al. 2012)]. Serum from mice immunized with MS2-31L2 showed a distinct pattern of neutralization; this serum strongly neutralized the homologous HPV31 PsV, but neutralized the other HPV PsV types at lower titers. Importantly, serum from mice immunized with the either the hybrid VLPs or mixed VLPs neutralized all of the HPV PsV types at high titers. Thus, immunization with hybrid L2-VLPs can enhance the breadth of HPV neutralization without sacrificing the ability to neutralize individual HPV types.
Figure 5.
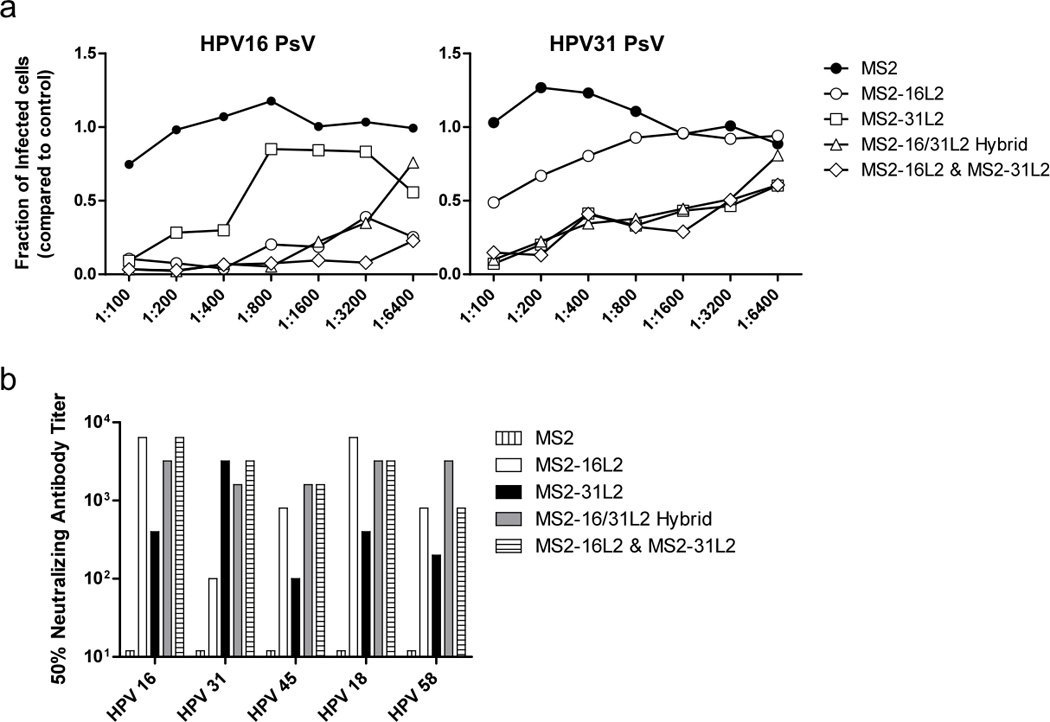
In vitro neutralization of HPV PsV by hybrid MS2 VLP immune sera. A: Sera from groups of 5 mice immunized with the indicated VLPs were pooled and tested for neutralization against (Left) HPV16 PsV at ID40 (Infectious Dose of PsV that results in 40% of control cells being infected) and (Right) HPV31 PsV at ID20 at the indicated dilutions. HPV PsV were incubated on deposited ECM in 96-well plates and treated with furin-conditioned media. HPV PsV was then incubated with pooled dilutions of sera for 6 hours, after which pgsa-745 cells were added. Infected cells were measured by GFP expression, quantified using a Hypercyt autosampling flow cytometer. Data points indicate the relative amount of infected cells in sera treated wells compared to wells with no sera added. B: Pooled sera from mice immunized with the indicated VLPs was tested for neutralization against five high-risk HPV PsV types. HPV45 and 58 PsV were tested at ID20. HPV18 PsV was added at ID10 due to low infectivity. The bars represent the highest titer at which the PsV was neutralized greater than 50% compared to control (no sera) wells.
Discussion
The strong antibody responses elicited by peptide epitopes displayed on VLPs results from the dense, repetitive manner in which the antigen is displayed to B cells (Bachmann and Zinkernagel 1997). This multivalent display allows for increased cross-linking of B cell receptors, strong B cell activation, and enhanced antibody production. By displaying two related HPV-derived peptides in highly immunogenic context on the surface of a single hybrid VLP, we hoped to take advantage of these avidity effects to activate B cells that could produce antibodies that reacted with a broad spectrum of HPV L2-derived peptides and had enhanced neutralizing activity against diverse HPV types (Chan and Brink 2012). We anticipated three possible outcomes at the onset of this study: (1) hybrid VLPs would elicit an immunodominant response against only one of the two epitopes, (2) the VLPs would elicit antibodies that against both epitopes, but the response would not be any different from immunizing with a mixture VLPs displaying each peptide separately, or (3) the VLPs would elicit a broadly-reactive response that would recognize other similar epitopes as well. Our results indicated that all three of these outcomes are possible depending on which peptides are being displayed on the hybrid VLPs.
Immunization with hybrid PP7 VLPs displaying 18/1 L2 elicited high titer antibody responses against HPV1 L2, but largely failed to elicit antibodies that bound to four other L2 peptides, including the HPV18 peptide that was displayed on the VLPs. There are several possible explanations for this result. First, it is possible that these hybrid VLPs preferentially incorporated the HPV1 L2-displaying coat protein. Although we cannot absolutely rule out this possibility, analysis of the mobility of the 18/1 hybrid VLP on an agarose gel showed that the hybrid VLPs displayed an electrophoretic mobility that was midway in between VLPs displaying only the HPV1 or HPV18 L2 peptides, suggesting that both peptides are displayed on the VLPs (Fig 1B right). Second, it is possible that the HPV1 L2 peptide is much more immunogenic than the HPV18 peptide. Previous studies of the immune response to this region of HPV L2 being displayed on PP7 VLPs did not show that PP7-1L2 VLPs elicit higher antibody titers than PP7 VLPs displaying other L2 sequences (Tumban et al. 2011). Moreover, co-immunization with a mixture of eight L2-displaying PP7 VLPs elicited balanced responses against our panel of L2 peptides (Tumban et al. 2011). Third, it is possible that Balb/c mice preferentially responded to the HPV1 peptide due to an increased frequency of precursor B cells specific for the unique elements in the HPV1 L2 peptide (i.e. immunodominance). Interestingly, when we immunized a different strain of mice (C57BL/6) with hybrid 18/1L2 VLPs we also observed immunodominance of the HPV1 L2 peptide (data not shown). Thus, these data indicate that epitope immunodominance is a potential consequence when immunizing with hybrid particles. This potential consequence will need to be carefully evaluated when considering the use of hybrid antigens.
When two highly related L2 peptides were displayed on the same hybrid VLPs, more broadly reactive antibody responses were generated. Both the PP7-18/16L2 and MS2-16/31L2 hybrid particles elicited more cross-reactive IgG responses when compared to that elicited by VLPs displaying only one of the targets. Hybrid VLPs also elicited more broadly neutralizing antibodies than when we simply immunized with mixtures of VLPs, indicating that there are distinct B cell responses to the hybrid particles. In a recent study Nieto and colleagues displayed two L2 peptides (from HPV16 and 31) at two separate display sites on adeno-associated VLPs (AAVLPs) (Nieto et al. 2012). These VLPs elicited strongly neutralizing antibodies, but antibody responses against one of the peptides (HPV31) was somewhat weaker, suggesting that one of the display sites on the AAVLP was less exposed to the immune system. One of the advantages to the approach that we describe is that both targeted peptides are displayed in the same highly immunogenic structural context and spatial arrangement that we think is critical for induction of high-titer strongly neutralizing antibodies against HPV (Tumban et al. 2012).
In this study we targeted a single vulnerable neutralizing epitope from HPV that shows a limited degree of sequence heterogeneity. Although our study focused on HPV, there are many pathogens that frustrate vaccination efforts due to antigenic variation and could potentially be targeted using the hybrid VLP approach. Similarly, we have shown that hybrid VLPs can also elicit strong antibody responses against two unrelated epitopes (i.e 18L2 and FLAG). This feature may be useful for targeting pathogens where more polyclonal antibody responses are required. In the context of displaying unrelated peptide epitopes, we think that it is unlikely that hybrid particles will elicit qualitatively distinct antibody responses than co-immunization with two VLPs. However, there are certain manufacturing advantages to using a single hybrid VLP as opposed to a mixture of individual VLPs. Taken together, the use of hybrid VLPs expands the capabilities of an already useful platform for vaccine design.
Acknowledgements
We thank Julianne Peabody for assistance with animal studies and Bruce Edwards for assistance with the Hypercyte autosampler. L1/L2 expression and reporter plasmids were generously provided by Chris Buck, Susana Pang, John Schiller, Martin Müller, and Tadahito Kanda. We would also like to thank members of the University of New Mexico Interdisciplinary HPV Prevention Center for helpful discussions. This study was supported by a grant from the US National Institutes of Health (U19 AI084081) to the University of New Mexico Sexually Transmitted Infections Cooperative Research Center (STI-CRC). MT was funded by a T32 training grant award by the US National Institutes of Health (T32 AI007538) to the University of New Mexico Infectious Disease and Immunity Program. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- Bachmann MF, Zinkernagel RM. Neutralizing antiviral B cell responses. Annu Rev Immunol. 1997;15:235–270. doi: 10.1146/annurev.immunol.15.1.235. [DOI] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Efficient intracellular assembly of papillomaviral vectors. J Virol. 2004;78(2):751–757. doi: 10.1128/JVI.78.2.751-757.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buck CB, Pastrana DV, Lowy DR, Schiller JT. Generation of HPV pseudovirions using transfection and their use in neutralization assays. Methods Mol Med. 2005a;119:445–462. doi: 10.1385/1-59259-982-6:445. [DOI] [PubMed] [Google Scholar]
- Buck CB, Thompson CD, Pang YY, Lowy DR, Schiller JT. Maturation of papillomavirus capsids. J Virol. 2005b;79(5):2839–2846. doi: 10.1128/JVI.79.5.2839-2846.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buonaguro L, Tagliamonte M, Tornesello ML, Buonaguro FM. Developments in virus-like particle-based vaccines for infectious diseases and cancer. Expert Rev Vaccines. 2011;10(11):1569–1583. doi: 10.1586/erv.11.135. [DOI] [PubMed] [Google Scholar]
- Burton DR, Poignard P, Stanfield RL, Wilson IA. Broadly Neutralizing Antibodies Present New Prospects to Counter Highly Antigenically Diverse Viruses. Science. 2012;337(6091):183–186. doi: 10.1126/science.1225416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caldeira Jdo C, Medford A, Kines RC, Lino CA, Schiller JT, Chackerian B, Peabody DS. Immunogenic display of diverse peptides, including a broadly cross-type neutralizing human papillomavirus L2 epitope, on virus-like particles of the RNA bacteriophage PP7. Vaccine. 2010;28(27):4384–4393. doi: 10.1016/j.vaccine.2010.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campo MS, Grindlay GJ, O'Neil BW, Chandrachud LM, McGarvie GM, Jarrett WF. Prophylactic and therapeutic vaccination against a mucosal papillomavirus. J Gen Virol. 1993;74(Pt 6):945–953. doi: 10.1099/0022-1317-74-6-945. [DOI] [PubMed] [Google Scholar]
- Chackerian B. Virus-like particles: flexible platforms for vaccine development. Expert Rev Vaccines. 2007;6(3):381–390. doi: 10.1586/14760584.6.3.381. [DOI] [PubMed] [Google Scholar]
- Chackerian B, Caldeira Jdo C, Peabody J, Peabody DS. Peptide epitope identification by affinity selection on bacteriophage MS2 virus-like particles. J Mol Biol. 2011;409(2):225–237. doi: 10.1016/j.jmb.2011.03.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan TD, Brink R. Affinity-based selection and the germinal center response. Immunol Rev. 2012;247(1):11–23. doi: 10.1111/j.1600-065X.2012.01118.x. [DOI] [PubMed] [Google Scholar]
- Christensen ND, Kreider JW, Kan NC, DiAngelo SL. The open reading frame L2 of cottontail rabbit papillomavirus contains antibody-inducing neutralizing epitopes. Virology. 1991;181(2):572–579. doi: 10.1016/0042-6822(91)90890-n. [DOI] [PubMed] [Google Scholar]
- Day PM, Pang YY, Kines RC, Thompson CD, Lowy DR, Schiller JT. A human papillomavirus (HPV) in vitro neutralization assay that recapitulates the in vitro process of infection provides a sensitive measure of HPV L2 infection-inhibiting antibodies. Clin Vaccine Immunol. 2012;19(7):1075–1082. doi: 10.1128/CVI.00139-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Jagu S, Karanam B, Gravitt PE, Culp TD, Christensen ND, Roden RB. Protection of rabbits against challenge with rabbit papillomaviruses by immunization with the N terminus of human papillomavirus type 16 minor capsid antigen L2. J Virol. 2007a;81(21):11585–11592. doi: 10.1128/JVI.01577-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gambhira R, Karanam B, Jagu S, Roberts JN, Buck CB, Bossis I, Alphs H, Culp T, Christensen ND, Roden RB. A protective and broadly cross-neutralizing epitope of human papillomavirus L2. J Virol. 2007b;81(24):13927–13931. doi: 10.1128/JVI.00936-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter Z, Tumban E, Dziduszko A, Chackerian B. Aerosol delivery of virus-like particles to the genital tract induces local and systemic antibody responses. Vaccine. 2011;29(28):4584–4592. doi: 10.1016/j.vaccine.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jegerlehner A, Tissot A, Lechner F, Sebbel P, Erdmann I, Kundig T, Bachi T, Storni T, Jennings G, Pumpens P, et al. A molecular assembly system that renders antigens of choice highly repetitive for induction of protective B cell responses. Vaccine. 2002;20(25–26):3104–3112. doi: 10.1016/s0264-410x(02)00266-9. [DOI] [PubMed] [Google Scholar]
- Karanam B, Jagu S, Huh WK, Roden RB. Developing vaccines against minor capsid antigen L2 to prevent papillomavirus infection. Immunol Cell Biol. 2009;87(4):287–299. doi: 10.1038/icb.2009.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirnbauer R, Booy F, Cheng N, Lowy DR, Schiller JT. Papillomavirus L1 major capsid protein self-assembles into virus-like particles that are highly immunogenic. Proc Natl Acad Sci U S A. 1992;89(24):12180–12184. doi: 10.1073/pnas.89.24.12180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehtinen M, Paavonen J, Wheeler CM, Jaisamrarn U, Garland SM, Castellsague X, Skinner SR, Apter D, Naud P, Salmeron J, et al. Overall efficacy of HPV-16/18 AS04-adjuvanted vaccine against grade 3 or greater cervical intraepithelial neoplasia: 4-year end-of-study analysis of the randomised, double-blind PATRICIA trial. Lancet Oncol. 2012;13(1):89–99. doi: 10.1016/S1470-2045(11)70286-8. [DOI] [PubMed] [Google Scholar]
- Lin YL, Borenstein LA, Selvakumar R, Ahmed R, Wettstein FO. Effective vaccination against papilloma development by immunization with L1 or L2 structural protein of cottontail rabbit papillomavirus. Virology. 1992;187(2):612–619. doi: 10.1016/0042-6822(92)90463-y. [DOI] [PubMed] [Google Scholar]
- Lua LH, Connors NK, Sainsbury F, Chuan YP, Wibowo N, Middelberg AP. Bioengineering virus-like particles as vaccines. Biotechnol Bioeng. 2014;111(3):425–440. doi: 10.1002/bit.25159. [DOI] [PubMed] [Google Scholar]
- Munoz N, Kjaer SK, Sigurdsson K, Iversen OE, Hernandez-Avila M, Wheeler CM, Perez G, Brown DR, Koutsky LA, Tay EH, et al. Impact of human papillomavirus (HPV)-6/11/16/18 vaccine on all HPV-associated genital diseases in young women. J Natl Cancer Inst. 2010;102(5):325–339. doi: 10.1093/jnci/djp534. [DOI] [PubMed] [Google Scholar]
- Nieto K, Weghofer M, Sehr P, Ritter M, Sedlmeier S, Karanam B, Seitz H, Muller M, Kellner M, Horer M, et al. Development of AAVLP(HPV16/31L2) particles as broadly protective HPV vaccine candidate. PLoS One. 2012;7(6):e39741. doi: 10.1371/journal.pone.0039741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastrana DV, Gambhira R, Buck CB, Pang YY, Thompson CD, Culp TD, Christensen ND, Lowy DR, Schiller JT, Roden RB. Cross-neutralization of cutaneous and mucosal Papillomavirus types with anti-sera to the amino terminus of L2. Virology. 2005;337(2):365–372. doi: 10.1016/j.virol.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Peabody DS, Manifold-Wheeler B, Medford A, Jordan SK, do Carmo Caldeira J, Chackerian B. Immunogenic display of diverse peptides on virus-like particles of RNA phage MS2. J Mol Biol. 2008;380(1):252–263. doi: 10.1016/j.jmb.2008.04.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pumpens P, Grens E. HBV core particles as a carrier for B cell/T cell epitopes. Intervirology. 2001;44(2–3):98–114. doi: 10.1159/000050037. [DOI] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, Choyke PL, Lowy DR, Schiller JT. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13(7):857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Roden RB, Yutzy WHt, Fallon R, Inglis S, Lowy DR, Schiller JT. Minor capsid protein of human genital papillomaviruses contains subdominant, cross-neutralizing epitopes. Virology. 2000;270(2):254–257. doi: 10.1006/viro.2000.0272. [DOI] [PubMed] [Google Scholar]
- Rose RC, Bonnez W, Reichman RC, Garcea RL. Expression of human papillomavirus type 11 L1 protein in insect cells: in vivo and in vitro assembly of viruslike particles. J Virol. 1993;67(4):1936–1944. doi: 10.1128/jvi.67.4.1936-1944.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schellenbacher C, Kwak K, Fink D, Shafti-Keramat S, Huber B, Jindra C, Faust H, Dillner J, Roden RB, Kirnbauer R. Efficacy of RG1-VLP Vaccination against Infections with Genital and Cutaneous Human Papillomaviruses. J Invest Dermatol. 2013 doi: 10.1038/jid.2013.253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schodel F, Wirtz R, Peterson D, Hughes J, Warren R, Sadoff J, Milich D. Immunity to malaria elicited by hybrid hepatitis B virus core particles carrying circumsporozoite protein epitopes. Journal of Experimental Medicine. 1994;180(3):1037–1046. doi: 10.1084/jem.180.3.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanley M. Pathology and epidemiology of HPV infection in females. Gynecol Oncol. 2010;117(2) Suppl:S5–S10. doi: 10.1016/j.ygyno.2010.01.024. [DOI] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Peabody DS, Chackerian B. A pan-HPV vaccine based on bacteriophage PP7 VLPs displaying broadly cross-neutralizing epitopes from the HPV minor capsid protein, L2. PLoS One. 2011;6(8):e23310. doi: 10.1371/journal.pone.0023310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tumban E, Peabody J, Tyler M, Peabody DS, Chackerian B. VLPs displaying a single L2 epitope induce broadly cross-neutralizing antibodies against human papillomavirus. PLoS One. 2012;7(11):e49751. doi: 10.1371/journal.pone.0049751. [DOI] [PMC free article] [PubMed] [Google Scholar]