Abstract
Since natural killer (NK) cells secrete cytotoxic granules and cytokines which can destroy surrounding cells and help shape the subsequent immune response, they must be kept under tight control. Several mechanisms, at different levels, are in place to control NK cell function. In this study, we describe a novel mechanism regulating NK cell function where NK cells acquire ligands for activating receptors from target cells by trogocytosis, rendering the NK cells hyporesponsive. In this model, murine NK cells acquire m157, the murine cytomegalovirus (MCMV)-encoded ligand for the Ly49H activating receptor, from target cells both in vitro and in vivo. Although acquisition of m157 requires cell-to-cell contact, it does not require the expression of the Ly49H receptor by the NK cell. Acquired m157 protein is expressed on the NK cell surface with a glycosylphosphatidylinisotol (GPI)-linkage and interacts with the Ly49H receptor expressed on the NK cell. This interaction results in (1) blocking the Ly49H receptor which prevents the NK cells from recognizing m157 expressing targets and (2) continuous engagement of the Ly49H activating receptor which results in the hyporesponsiveness of the Ly49H+ NK cell to stimulation through other activating receptors. Thus, NK cells acquisition of a ligand for an activation receptor by trogocytosis renders them hyporesponsive. This mechanism, by which mature NK cell function can be altered, has important implications in regards to how NK cells respond to tumors in specific microenvironments as well as the use of expanded NK cells in treating various malignancies.
Introduction
Natural killer (NK) cells play an important role in the initial immune response to viral infection and tumor formation. The NK cell response is mediated through multiple activating and inhibitory receptors expressed on the NK cell surface. A summation of the inhibitory and activating responses is believed to determine if the NK cell will be activated (1, 2). Upon activation, the NK cell releases cytotoxic granules, which results in the killing of the target cell. The NK cell also secretes a number of cytokines that help shape the future adaptive immune response.
With such potentially destructive capability, NK cell function must be tightly controlled. Numerous mechanisms are present to keep NK cells in check and prevent unwanted destruction of nearby cells. These include: (1) the expression of inhibitory receptors on the surface of NK cells that recognize major histocompatibility complex (MHC) class I on the surface of normal cells, preventing them from being killed (3), (2) licensing, education or tuning of the NK cells which results in decreased function of NK cells that fail to express an inhibitory receptor that binds self MHC class I (4–7) and (3) activation receptor mediated NK cell tolerance (8–11). All of these mechanisms represent different levels at which NK cell function can be controlled.
Here we describe trogocytosis as a new additional mechanism of NK cell control using a model system that utilizes the NK cell activating receptor Ly49H and its interaction with the murine cytomegalovirus (MCMV)-encoded protein m157. Approximately half of the NK cells from C57BL/6 mice express the activating receptor Ly49H. The Ly49H receptor signals through the DAP12 adapter protein. This provides a platform for intracellular signal transduction leading to cytokine secretion and degranulation (12–14). The expression of the Ly49H receptor on NK cells in certain strains of mice (C57BL/6) confers resistance to MCMV infection compared to strains that do not express the Ly49H receptor on their NK cells (BALB/c) (15). The Ly49H receptor binds to the MCMV-encoded protein m157, which is expressed on the cell surface as a glycosylphosphatidylinisotol (GPI) linked protein during the early stages of MCMV infection (16). To date, m157 is the only identified ligand for the Ly49H activating receptor and Ly49H is only known to bind m157 (17, 18).
It has been demonstrated that NK cells from humans and mice undergo ligand induced down modulation of the activating receptors on their cell surface, making the NK cell less effective at killing target cells (19, 20). Studies suggest that NK cell receptors acquire ligand that has been shed from the target, or by transfer of patches of membrane in a process known as “trogocytosis” (derived from the Greek word “trogo” meaning to gnaw or nibble) (21–23). Trogocytosis is the intercellular movement of cell surface proteins mediated by the transfer of plasma membrane from one cell to another. Thus, trogocytosis, where NK cells can acquire ligands from target cells, may represent another mechanism by which NK cells can alter their functional capabilities.
When an NK cell comes into contact with a target cell, receptor-ligand interactions do not occur randomly. Instead, an immune synapse (IS) is formed which is an ordered structure that provides a platform for communication between immune cells (24–26). The formation of the IS allows for transfer of molecules from one cell to another. This has been demonstrated in T cells, B cells and NK cells (27–30). The transfer of proteins has been demonstrated to have functional consequences on the recipient cells. For instance, the incubation of NK cells with MHC class I polypeptide-related sequence MIC B (MICB)-expressing targets resulted in the exchange of Natural killer 2, member D (NKG2D) and MICB proteins. This exchange was associated with a reduction in the cytotoxicity of these NK cells upon subsequent encounters with MICB-expressing target cells (19). Another study demonstrated that MICA could be transferred from the target cell to the NK cell and the acquired MICA protein was expressed on the cell surface such that it could interact with the NKG2D receptor and influence the function of other NK cells (31). More recent studies suggest that NK cells that acquire ligands for activating receptors by trogocytosis become viable targets for nearby NK cells (32), providing yet another mechanism of controlling NK cell function once they have been activated.
In this study we demonstrate that NK cells quickly acquire the m157 ligand from target cells and express them on their cell surface as a GPI-linked protein. The acquisition of m157 from the target cells is independent of expression of Ly49H by the NK cell. Cell to cell contact, however, is necessary for transfer of m157 to occur. Upon transfer to the NK cell, m157 can interact with Ly49H expressed on the cell surface of the recipient NK cell. Furthermore, we demonstrate that the acquisition of m157 by NK cells makes them functionally defective both in vitro and in vivo. Specifically, they are less efficient at killing m157 expressing target cells and they are hyporesponsive when stimulated through another activating receptor. We propose a model in which the acquisition of m157 by trogocytosis allows for the interaction of m157 and Ly49H on the NK cell surface. This interaction not only has a blocking effect such that the NK cells can no longer detect m157 on targets, but it also provides a mechanism by which the continuous engagement of the Ly49H receptor with m157 can occur. This results in the NK cells becoming hyporesponsive secondary to the continuous engagement of the activating receptor.
Materials and Methods
Mice and cell lines
The m157-Tg mouse has been previously described (8). The DAP12ki mice, obtained from Eric Vivier, as well as the B6.BxD8 mice, obtained from Wayne Yokoyama, have both been previously described (33, 34). The CD45.1+ mice (B6.SJL-Ptprca Pep3b/BoyJ) were purchased from the Jackson Laboratory. Mice were maintained under specific pathogen-free conditions and used after they reached 8 weeks of age. The Animal Studies Committee at Washington University (St. Louis, MO) reviewed and approved all animal studies. The RMA (Rauscher virus-induced murine T cell lymphoma), RMAm157 (RMA cells that stably express m157), RMA-S (RMA cells that express markedly reduced levels of class-I molecules at the cell surface), BaF (murine pro-B cell line) and BaFm157 (BaF cells that stably express m157) cell lines were generously provided by Wayne Yokoyama.
Generation of mouse Lymphokine activated killer (LAK) cells
Spleens were harvested into single cell suspensions by pulverization through a 70 micron filter as previously described (35). After lysis of red blood cells, cell suspensions were washed in pre-warmed (RT) HBSS/10% FBS and loaded onto a nylon wool column prepared in a 10 ml syringe. Cells were incubated on the column at 37°C for 1 hr. Non-adherent cells were passed through the column after the addition HBSS/10% FBS, collected and grown in T-75 flasks (BD Biosciences) in the presence of IL-2 (8000 U/mL; Proleukin, Chiron). After 4 days, non-adherent cells were removed and flasks were washed with PBS to remove loosely adherent cells. Fresh IL-2 containing media was replenished in the original flasks. Washes were collected with the non-adherent cells, centrifuged and transferred to a second flask with IL-2 containing media. Cells were maintained for an additional 3–4 days before use. This protocol yielded LAK cells in which 60–90% of the cells were NK1.1+, CD3−.
Antibodies
The following antibodies were obtained from EBioscience or Biolegend: APC-PK136 (anti-NK1.1), PerCP-Cy5.5-145-2C11 (anti-CD3), PerCP-Cy5.5-145-1D3 (anti-CD19), Alexa488-XMG1.2 (anti-IFN-γ), PacBlue-XMG1.2 (anti-IFN-γ), FITC-104 (anti-CD45.2), FITC-KH95 (anti-H2-Db) and streptavidin-PE. The 3D10 (anti-Ly49H) and 6H121 (anti-m157) mAbs were purified from hybridomas by the Protein Production and Purification Core Facility of the RDCC and conjugated to biotin using EZ-Link Sulfo-NHS-LC-LC-Biotin (Pierce) according to manufacturer’s protocol. Purified PK136 (anti-NK1.1) was purchased from BioXcell.
In vitro trogocytosis assays
Lymphokine activated killer (LAK) cells (1 × 105) were cocultured in 96-well, flat-bottom plates with either RMA or RMAm157 cells at a 1:1 ratio and harvested at the indicated time points. For studies using fresh NK cells, spleen cell suspensions were prepared as previously described. Splenocytes (1 × 106) were cocultured with RMA, RMAm157, BaF or BaFm157 cells (5 × 105) in 96-well, flat-bottom plates for 2 hr. All cells were harvested, stained for NK1.1, CD3, CD19 and m157 and acquired using a FACS Calibur machine (BD Biosciences). The data were analyzed using FlowJo Software (Tree Star, Inc).
Transwell assays
LAK cells were plated into 24-well cell culture insert companion plates (Corning). RMAm157 cells were plated into transwell inserts (0.4 µm; Corning) atop the LAK cells so that media exchange was allowed but cell-to-cell contact was not. Cells were incubated for 30 min, and LAK cells were harvested from the plates. Cells were stained and assessed for m157 expression on NK1.1+ CD3− cells. Cells were acquired using a FACS Calibur machine (BD Biosciences). Flow cytometry data was analyzed by FlowJo software (Tree Star, Inc.).
Adoptive transfer experiments
Spleen cell suspensions were generated from CD45.2+ donor mice as described above. Cells were washed with PBS and then resuspended at 250 × 106 cells/ml. Recipient mice (CD45.1+) were injected intravenously in the tail vein with 200 µl of splenocytes (approximately 50×106 total splenocytes). Mice were then harvested at the indicated time points. Donor NK cells were identified as NK1.1+, CD3−, CD19−, CD45.2+.
PI-PLC treatment
After a 30–40 minute co-incubation of NK cells with RMAm157 cells, the cells were harvested, washed and incubated with either one ml of PBS or phosphatidylinositol-specific phospholipase C (PI-PLC) (1.25U/mL; Molecular Probes) diluted in PBS for 20 min on ice. After washing, cells were stained for m157 or Ly49H, as well as NK1.1 and CD3. Cells were acquired using a FACS Calibur machine (BD Biosciences). Flow cytometry data was analyzed by FlowJo software (Tree Star, Inc.).
In vitro killing assay
LAK cells were labeled with 5-(and-6)-carboxyfluorescein diacetate succinimidyl ester (CFSE) (1 uM; Invitrogen) and then cocultured with either RMA or RMAm157 cells at a 1:1 ratio for 30 minutes. CFSE+ LAKs were sort purified by FACSAria II (BD Biosciences) and used as effector cells at 3:1 or 10:1 effector to target ratios. The RMAm157 or RMA-S target cells (1 × 105) were co-cultured with the RMA or RMAm157 “dressed” LAK cells for 2 hr in 96-well U-bottom plates. Cells were stained for NK1.1 and propidium iodide (PI; Sigma). The target cells were identified as NK1.1−, CFSE− and assessed for PI incorporation. The cells were acquired using a FACS Calibur machine (BD Biosciences). Flow cytometry data was analyzed by FlowJo software (Tree Star, Inc.).
IFN-γ assays
Spleen cell suspensions were generated as described above. To coat plates, PK136 mAb (anti-NK1.1) was diluted to 2–4 µg/ml in PBS. One ml of antibody (2–4 µg) was placed in 6 well tissue culture plates (Techno Plastic Product) and incubated at 37°C for 90 minutes. After incubation, the plates were washed with PBS three times prior to use for stimulation assays. For stimulation of NK cells, 1 ml of splenocytes (107 cells/ml in R10) were incubated in 6 well plates coated with anti-NK1.1 mAb for 1/2 hr and then further incubated in the presence of a 833-fold dilution of stock brefeldin A (GolgiPlug, BD Pharmingen) for an additional 6–8 hours. Cells were harvested and stained for the surface markers NK1.1, CD3, CD45.1, and Ly49H. Cells were then fixed and permeabilized using Cytofix/Cytoperm solution (BD Pharmingen) and stained for intracellular IFN-γ. The cells were acquired using a FACS Canto machine (BD Biosciences). Flow cytometry data was analyzed by FlowJo software (Tree Star, Inc.).
Statistical analysis
The data were analyzed with Microsoft Excel X for Mac (Microsoft). Unpaired, two-tailed t test was used to determine statistically significant differences between experimental groups. Error bars in the figures represent the standard error of the mean (SEM).
Results
NK cells rapidly acquire m157 on their cell surface when incubated with m157-expressing target cells, and m157 is expressed on the cell surface as a GPI-linked protein
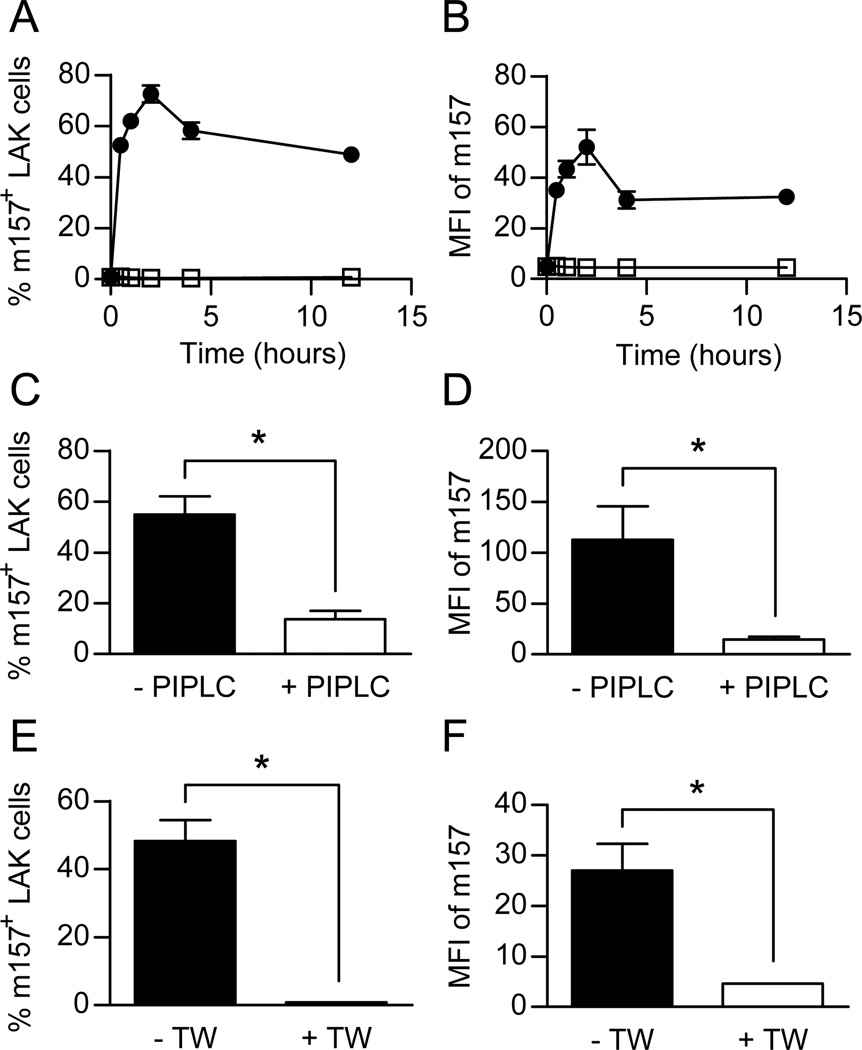
To determine if m157 could be acquired from target cells, IL-2-activated NK cells from C57BL/6 mice were incubated in vitro with RMA or RMAm157 cells. At various time points, the NK cells were assessed by flow cytometry for the presence of m157 on their cell surface using the gating strategy shown in Supplemental Figure 1A. As demonstrated in Figure 1A, the NK cells acquired m157 rapidly after co-culture with RMAm157 with fifty percent of the NK cells expressing the m157 protein on their cell surface by 30 minutes. The percentage of NK cells expressing m157 was maximal by 2 hours. No m157 was detected on the NK cells when they were incubated with RMA cells. We also assessed the mean fluorescence intensity (MFI) of m157 on NK cells incubated with RMAm157 cells. Similar to the increase in the percentage of NK cells that expressed m157, we observed that the MFI of the m157 on NK cells was increased when they were incubated with RMAm157 cells (Fig. 1B). There was no increase in the MFI of m157 when NK cells were incubated with RMA cells.
Figure 1. NK cells rapidly acquire m157 on their cell surface as a GPI linked protein following incubation with m157 expressing target cells in a cell contact dependent manner.
WT IL-2 stimulated NK cells (LAKs) were incubated with RMA (□) or RMAm157 (●) cells and assessed by flow cytometry for the percentage of LAK cells that were positive for m157 on their cell surface (A) and mean fluorescence intensity (MFI) of m157 (B) at various time points (n=3–6 at each time point). WT LAKs were incubated with RMAm157 cells for 2 hours and then treated with PBS as control (n=6) or phosphatidylinositol-specific phospholipase C (PI-PLC) (n=6) and assessed by flow cytometry for percentage of LAKs that were positive for m157 (C) and MFI of m157 (D). WT LAKs were incubated with RMAm157 cells in the presence (n=6) or absence (n=6) of a transwell. The percentage of LAKs expressing m157 (E) as well as MFI of m157 (F) was assessed by flow cytometry after two hours of incubation. All data are presented as the mean+/− SEM. *p<0.005.
The m157 protein is expressed on the surface of MCMV-infected cells as a GPI linked protein (16). To determine if the acquired m157 on “dressed” NK cells was also anchored to the cell surface by GPI-linkage, we treated “dressed” NK cells with phosphatidylinositol-specific phospholipase C (PI-PLC), an enzyme that cleaves GPI-linked proteins. Upon treatment with PI-PLC, the percentage of NK cells that expressed m157 on their cell surface decreased significantly compared to those treated with just phosphate buffer saline (PBS) as a control (Fig. 1C). In addition, the MFI of the m157 on “dressed” NK cells decreased upon treatment with PI-PLC compared to PBS (Fig. 1D). This demonstrates that the m157 present on the “dressed” NK cells is anchored on the cell surface as a GPI-linked protein.
Acquisition of m157 by NK cells requires cell-to-cell contact
To determine if cell-to-cell contact was required for transfer of m157 from target cells to IL-2-activated NK cells, we incubated the NK cells with RMAm157 in the presence or absence of transwells and assessed the percentage of NK cells that acquired m157 as well as the MFI of m157 by flow cytometry. As seen in Figure 1E, in the presence of a transwell, which allows for secreted or shed proteins to cross between cells but does not allow cell contact, acquisition of m157 by NK cells is essentially abolished compared to without the transwell. Furthermore, there was a significant decrease in the MFI of m157 on the “dressed” NK cells in the presence of the transwell (Fig. 1F). This demonstrates that the acquisition of m157 by the NK cells is not due to m157 being secreted or shed by target cells and then picked up by the NK cell, but rather acquisition of the protein by a mechanism that requires cell-to-cell contact.
Acquisition of m157 by NK cells is independent of the Ly49H receptor
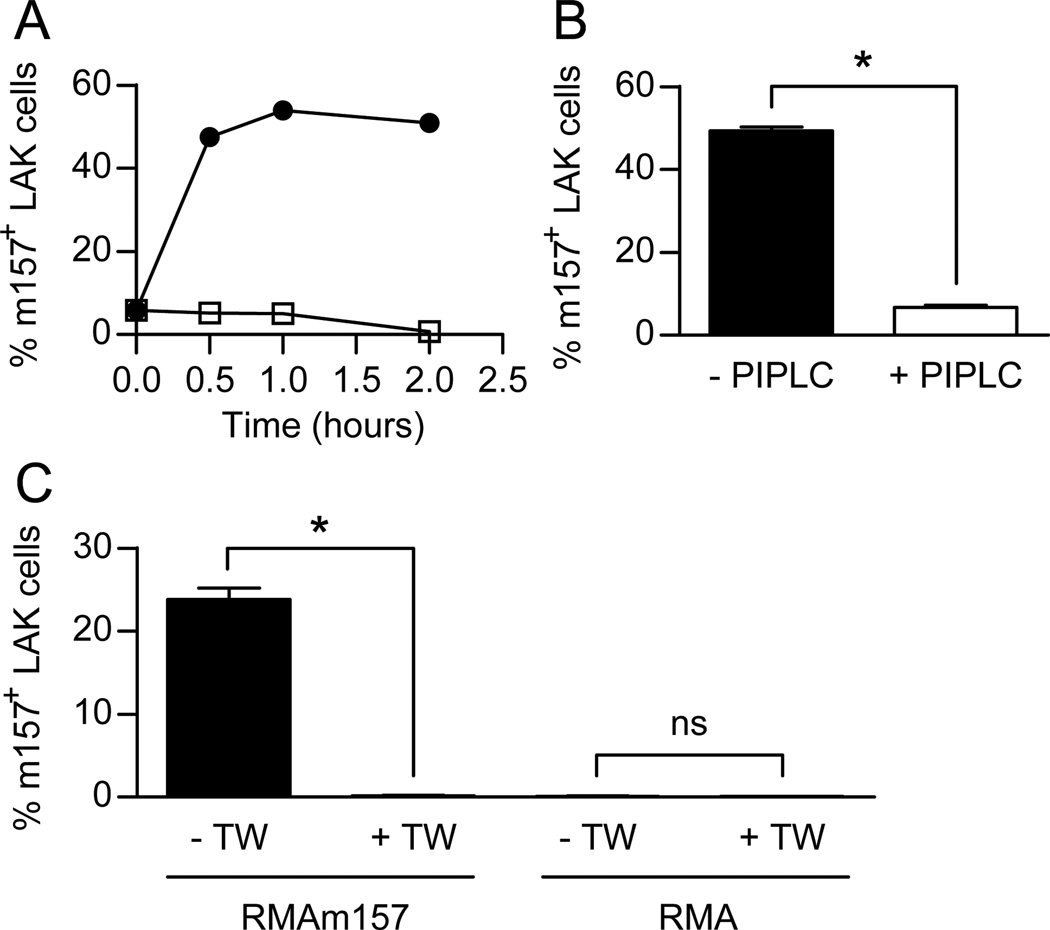
To determine if the Ly49H receptor is required for the acquisition of m157 onto the NK cells, we used IL-2-activated NK cells that were isolated from B6.BxD8 mice. These mice, as previously described, are identical to C57BL/6 mice except that their NK cells do not express the Ly49H receptor (34). The NK cells from the B6.BxD8 mice, unlike WT NK cells, did not express Ly49H (Supplemental Figure 1B). Similar to WT NK cells, upon incubation with RMAm157 cells we observed rapid accumulation of m157 on the surface of these NK cells. This was not seen when the NK cells were incubated with RMA cells (Fig. 2A). In addition, the transferred m157 on the cell surface of B6.BxD8 NK cells was attached by GPI linkage, as the percentage of NK cells expressing m157 as well as the MFI of the m157 on the “dressed” NK cells was significantly decreased upon treatment with PI-PLC (Fig. 2B and data not shown). Finally, the acquisition of m157 onto the NK cells required cell-to-cell contact as the incubation in transwells did not allow for the transfer of m157 (Fig. 2C). Of note, there was no acquisition of m157 by NK cells in the presence or absence of the transwell when RMA cells were the target (Fig. 2C). Thus, trogocytosis of m157 by NK cells does not require the Ly49H receptor and transfer in the absence of the Ly49H receptor still results in the m157 protein being anchored to the membrane of the recipient NK cell by a GPI-linkage. In addition to m157, NK cells were able to acquire other membrane bound proteins including CD45.2 and H2-Db (Supplemental Figure 2), suggesting that membrane transfer was taking place resulting in the transfer of multiple membrane-associated proteins. The acquisition of m157 was greater than CD45.2, both of which were greater than H2-Db. This suggests that the amount of protein acquired by the NK cells corresponds to levels of the proteins expressed on the target cell.
Figure 2. Acquisition of m157 by NK cells is independent of the Ly49H receptor.
(A) IL-2 stimulated NK cells (LAKs) from B6.BxD8 mice were incubated with RMA (□) or RMAm157 (●) and assessed by flow cytometry for the percentage of NK cells that were positive for m157 on their cell surface at various time points (n=3 for each time point). (B) B6.BxD8 LAKs were incubated with RMAm157 cells for 2 hours in the presence (n=3) or absence (n=3) of PI-PLC. The percentage of LAKs expressing m157 was assessed by flow cytometry (C) B6.BxD8 LAKs were incubated for 2 hours with RMA or RMAm157 cells in the presence (n=4–6) or absence (n=4–6) of a transwell. The percentage of LAK cells expressing m157 was assessed by flow cytometry. All data are presented as the mean+/− SEM. *p<0.0005.
Acquired m157 can interact with Ly49H on the NK cell surface
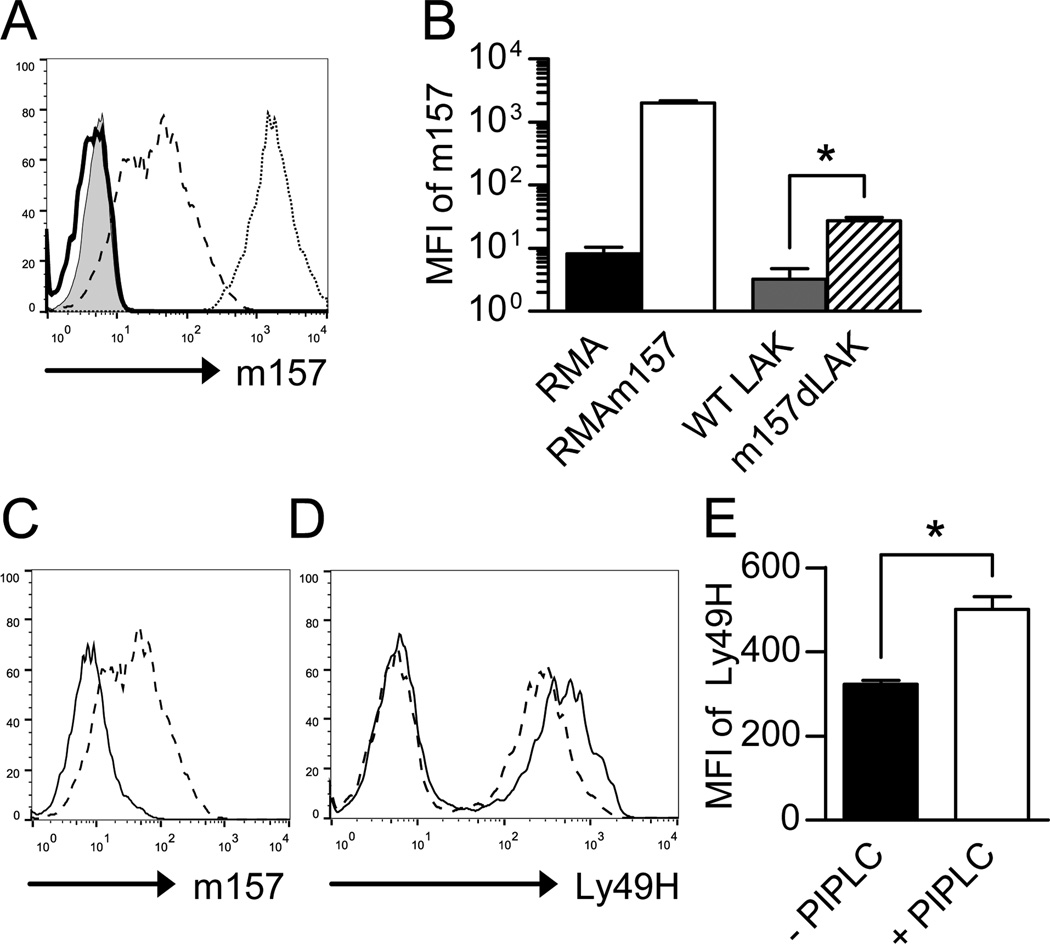
To determine if acquired m157 interacts with the Ly49H receptor expressed on the cell surface of IL-2-activated NK cells, we used a flow cytometry-based cell surface staining approach. The engagement of Ly49H with m157 decreases the detectable level of Ly49H on the cell surface of NK cells when assessed by flow cytometry (8, 35). This could be secondary to down modulation of the receptor in response to engagement of ligand or the fact that the m157/Ly49H interaction prevents efficient binding of the 3D10 mAb to Ly49H. If Ly49H interacts with m157 on the cell surface, we hypothesized that removal of acquired m157 would result in higher detectable levels of Ly49H on the NK cell. WT IL-2-activated NK cells were incubated with RMAm157 cells and then sorted and assessed for cell surface m157 levels. The MFI of m157 on the sorted “dressed” NK cells (MFI ~ 28) was approximately seventy fold lower than on RMAm157 cells (MFI ~ 2000), suggesting that although the NK cells are acquiring the m157 protein, it is not at the level expressed on the target cells (Figs. 3A and B).
Figure 3. Acquired m157 can interact with Ly49H on the NK cell surface.
WT IL-2 stimulated NK cells (LAKs) were incubated with RMA m157 cells for 30 minutes and sorted to isolate LAK cells. (A) Representative histogram of RMA cells (tinted histogram), RMAm157 cells (open histogram with dotted line), WT LAKs (open histogram with solid line) and sorted m157 “dressed” LAKs (open histogram with dashed line) that were assessed for m157 levels on the cell surface by flow cytometry. (B) MFI of m157 on RMA cells (black, n=6), RMAm157 cells (white, n=9), WT LAKs (gray, n=3) and sorted m157 “dressed” LAKs (hatched, n=3). *p<0.005. (C) Representative histogram depicting sorted m157 “dressed” LAKs that were assessed for m157 levels in the presence (open histogram) and absence (dashed histogram) of PI-PLC. (D) Representative histogram depicting sorted m157 “dressed” LAKs that were assessed for Ly49H levels in the presence (open histogram) and absence (dashed histogram) of PI-PLC. (E) MFI of Ly49H on Ly49H+ sorted “dressed” LAKs in the absence (black, n=6) or presence (white, n=6) of PI-PLC. All data are presented as the mean+/− SEM. *p<0.0005.
The sorted m157 “dressed” NK cells were also assessed for cell surface m157 levels in the presence and absence of PI-PLC (Fig. 3C). As shown before, incubation with PI-PLC decreased the level of m157 on the surface of the m157 “dressed” NK cells. We assessed the same cells for Ly49H receptor levels on the cell surface. Consistent with the hypothesis that m157 interacts with Ly49H on the cell surface, treatment with PI-PLC (which decreased m157 levels on the cell surface through cleavage of its GPI linkage), increased the level of Ly49H detected on the Ly49H+ NK cells (Figs. 3D and E). This suggests that the m157 acquired via trogocytosis can interact with the Ly49H receptor on the NK cell surface.
Acquisition of m157 occurs in vivo with fresh NK cells
The previous experiments described in this study used lymphokine activated killer (LAK) cells, which are NK cells that have been grown in culture for one week in media containing IL-2. Although this enriches and amplifies the number of NK1.1+, CD3− cells for experimental purposes, LAK cells are functionally different from fresh NK cells. To determine if fresh NK cells can acquire m157 from target cells, we incubated splenocytes from non-Tg mice with RMA or RMAm157 cells (Fig 4A). At various time points, we assessed for the presence of m157 on the NK cell surface by flow cytometry. Similar to the LAK cells, fresh NK cells were able to acquire m157 at a rapid rate when incubated with RMAm157. They did not acquire m157 when incubated with RMA cells. Similar to the increase in the percentage of NK cells that expressed m157, we observed that the MFI of the m157 on the fresh NK cells was increased when they were incubated with RMAm157 cells (data not shown). To determine if target cell type had an impact on trogocytosis, we incubated splenocytes from WT mice with BaFm157 cells and assessed for the acquisition of m157 onto the cell surface of NK cells after two hours of incubation by flow cytometry. Similar to incubation with RMAm157 targets, fresh NK cells acquired m157 when incubated with BaFm157 cells but not the parental BaF cell line (Fig. 4B). To assess if signaling through the Ly49H receptor was necessary for acquisition of m157, we incubated splenocytes from DAP12ki and B6.BxD8 mice with the target cells. The DAP12ki mice, which have been previously described, contain mutations in the tyrosine residues within DAP12 that prevent phosphorylation and thus prevent signaling through the Ly49H receptor (33). As shown in figure 4B, natural killer cells from both the B6.BxD8 and DAP12ki mice were able to acquire m157 on their cell surface from the different target cells, demonstrating that neither signaling through the receptor, nor the receptor itself, was required for trogocytosis of m157.
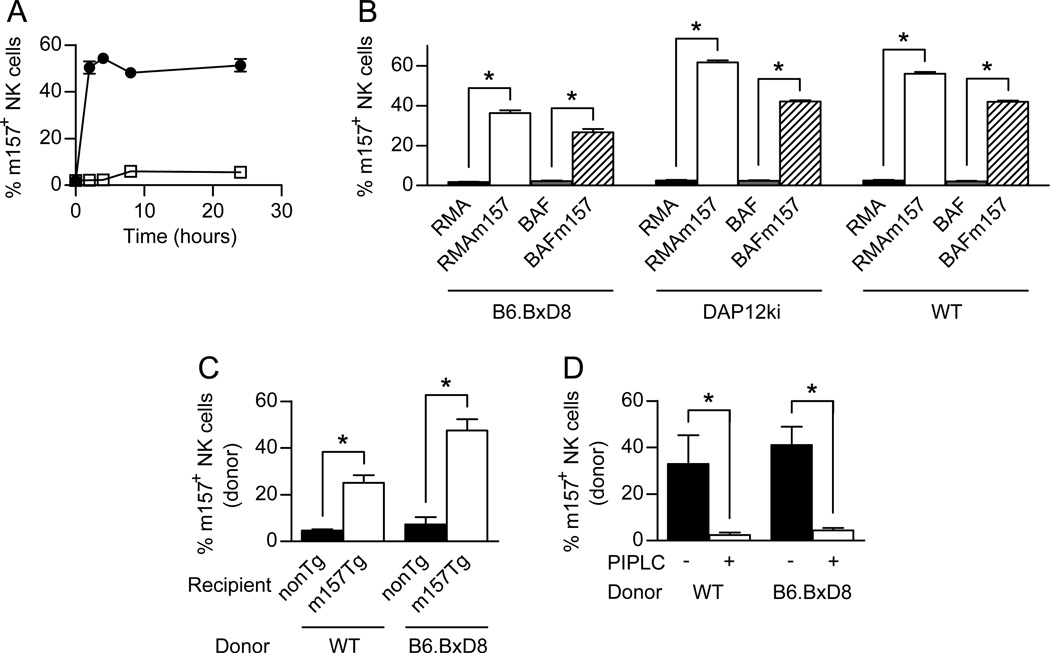
Figure 4. Acquisition of m157 occurs with fresh NK cells and in vivo.
(A) WT splenocytes were incubated with RMA (□) or RMAm157 (●) cells and assessed by flow cytometry for the percentage of NK (NK1.1+, CD3−) cells that were positive for m157 on their cell surface at various time points. (B) Splenocytes from B6.BxD8, DAP12ki or WT mice were incubated with RMA (black), RMAm157 (white), BaF (gray) or BaFm157 (hatched) cells for 2 hours and assessed by flow cytometry for the percentage of NK (NK1.1+, CD3−) cells that were positive for m157 on their cell surface (n= 6 for all groups except for WT with RMA or RMAm157 where n=3). (C) Adoptive transfer of 50 million WT or B6.BxD8 donor splenocytes into non-Tg (black) and m157-Tg (white) recipient mice. Donor NK cells were assessed by flow cytometry for the acquisition of m157 on their cell surface (n=5–17). (D) WT and B6.BxD8 donor NK cells were obtained 24 hours post transfer into m157-Tg recipient mice (n=4–5). The percentage of NK cells expressing m157 was assessed by flow cytometry before (black) and after treatment with PI-PLC (white). All data are presented as the mean+/− SEM. *p<0.005.
To determine if our in vitro findings also apply in vivo, we adoptively transferred splenocytes from WT and B6.BxD8 mice into m157-Tg or non-Tg mice and assessed by flow cytometry for the acquisition of m157 by the donor NK cells 24 hours post transfer. As can be seen in Figure 4C, adoptively transferred NK cells from both WT and B6.BxD8 mice acquired m157 upon transfer into m157-Tg recipients but not non-Tg recipients. This was demonstrated by the increase in the percentage of donor NK cells that express m157. In addition, both WT and B6.BxD8 donor NK cells showed increases in the MFI of m157 24 hours after transfer into m157-Tg mice (data not shown). This was not seen when the NK cells were transferred into non-Tg mice.
We also addressed if the m157 was anchored by a GPI linkage on the membrane of the donor NK cells in vivo. Treatment of donor NK cells from m157-Tg mice with PI-PLC resulted in a decrease in the percentage of donor NK cells that expressed the m157 protein on their cell surface, regardless of whether the donor NK cells were from a WT or B6.BxD8 background (Fig. 4D). In addition, the MFI of m157 on the “dressed” NK cells was also decreased in the presence of PI-PLC (data not shown) regardless of whether the donor NK cells were from a WT or B6.BxD8 background. Together the data demonstrate that NK cells can acquire m157 from target cells in vivo and the m157 is anchored to the cell surface by a GPI linkage. In addition, the acquisition of m157 by NK cells in vivo is independent of Ly49H expression.
“Dressed” NK cells are defective at killing m157-expressing target cells
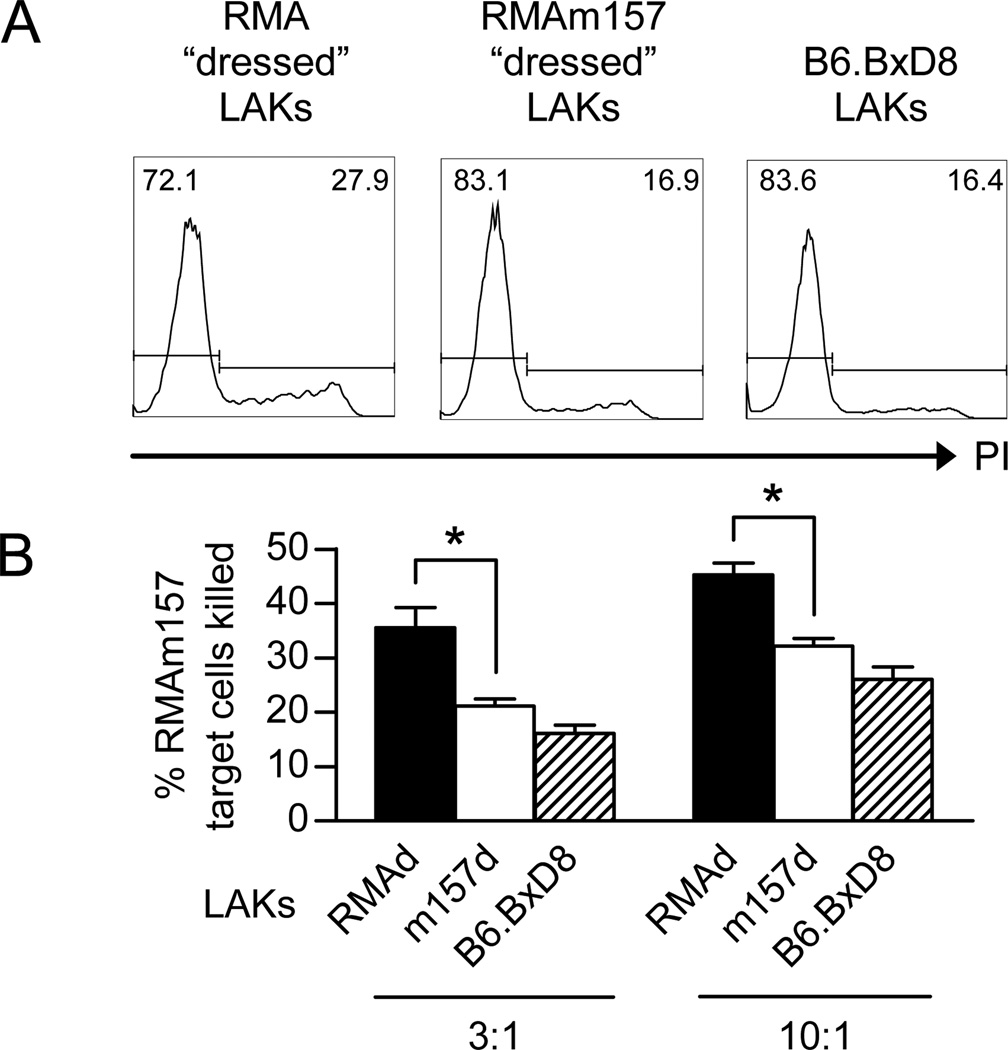
To establish if the acquisition of m157 onto NK cells resulted in functional defects, we assessed killing of m157 expressing target cells. Following incubation of IL-2-activated NK cells with RMA or RMAm157 cells, the NK cells were sorted from the target cells. The sorted RMA-“dressed” and sorted RMAm157-“dressed” NK cells, as well as NK cells from B6.BxD8 mice were incubated with RMAm157 target cells at 3:1 and 10:1 ratios. Death of the target cells was determined by propidium iodide incorporation into dead cells using flow cytometry (Fig. 5A). At both ratios, the RMAm157-“dressed” NK cells were significantly worse at killing RMAm157 cells as compared to RMA-“dressed” NK cells, though both were more effective than NK cells from the B6.BxD8 mice (Fig 5B). Background levels of cell death were noted when RMA cells were used as the target, which were similar to the B6.BxD8 killing of RMAm157 cells (data not shown). In addition, we assessed if co-culture with RMA-m157 would impact IL-2-activated NK cell killing of other tumor targets. Sorted RMAm157-“dressed” IL-2-activated NK cells killed RMA-S targets as well as, if not better, than sorted RMA-“dressed” IL-2-activated NK cells (Supplemental Figure 3). This demonstrates that m157 “dressed” NK cells are less effective at killing m157-expressing target cells than non-m157 “dressed” NK cells and the defect is specific for m157 expressing targets.
Figure 5. The m157-dressed NK cells are defective at killing m157-expressing targets.
WT IL-2 stimulated NK cells (LAKs) were incubated with RMA or RMAm157 cells and then sorted to obtain “dressed” LAKs. The sorted LAKs were used as effector cells at 3:1 and 10:1 E:T ratios. (A) Representative histogram to evaluate killing of RMAm157 target cells based on propidium iodide (PI) incorporation assessed by flow cytometry. The numbers in the histogram are the percentage of cells present in the PI+ and PI− gates. (B) Percentage of RMAm157 target cells killed at two different E:T ratios. Effector cells were sorted RMA- “dressed” LAKs (RMAd; black, n=6), sorted RMAm157-“dressed” LAKs (m157d; white, n=12) or unsorted B6.BxD8 LAKs (B6.BxD8; hatched, n=9). All data are presented as the mean+/− SEM. *p<0.0005.
Expression of the Ly49H receptor is required to induce NK cell hyporesponsiveness
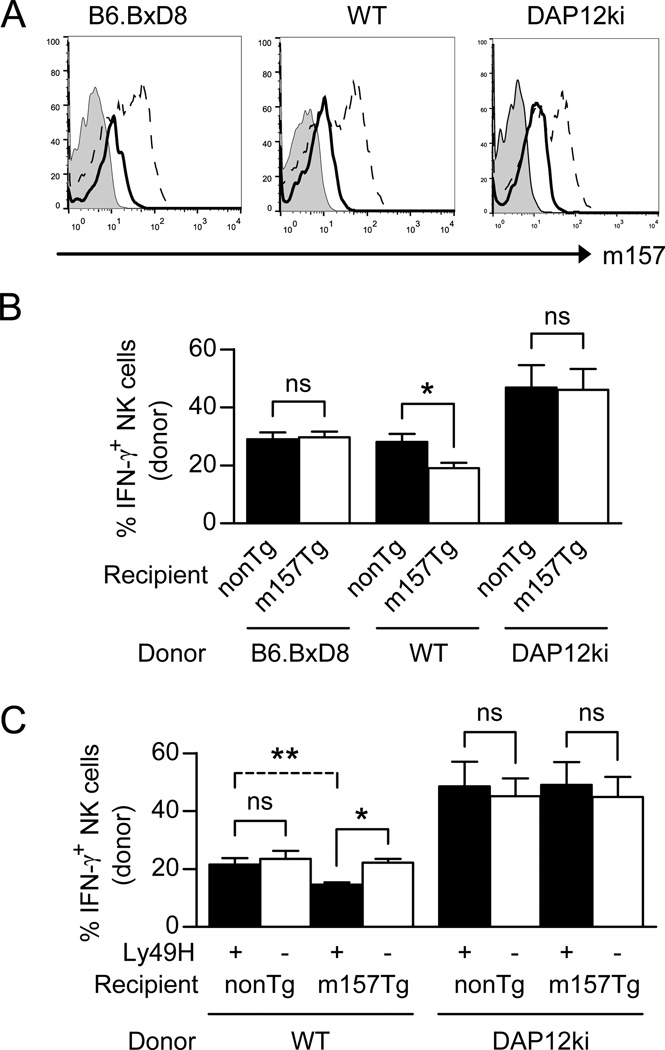
Although Ly49H is not necessary for the acquisition of m157 from target cells, we wanted to determine if the receptor was required for NK cell defects in the production of IFN-γ upon stimulation through the NK1.1 activating receptor. We injected m157-Tg or non-Tg recipient mice with either non-Tg WT donor splenocytes, non-Tg B6.BxD8 donor splenocytes or non-Tg DAP12ki splenocytes. Three days post injection, splenocytes were harvested and stimulated through the NK1.1 receptor and the donor NK cells were assessed for IFN-γ production. The WT, B6.BxD8 and DAP12ki donor NK cells all acquired m157 on their cell surface when injected into m157-Tg recipient mice (Fig. 6A). Of note, the level of m157 expression on the surface of “dressed,” donor NK cells was less than levels seen on the surface of NK cells from m157-Tg mice (Fig 6A). We observed decreased IFN-γ production by donor WT NK cells that had been injected into m157-Tg recipients as compared to non-Tg recipients (Fig 6B). However, although both donor non-Tg B6.BxD8 and DAP12ki NK cells acquired m157 when injected into an m157-Tg recipient, they produced similar levels of IFN-γ as those injected into a non-Tg recipient (Fig. 6B). In addition, we separated the NK cell populations from WT and DAP12ki donors into Ly49H+ and Ly49H− NK cells and assessed IFN-γ production from these groups individually upon transfer into non-Tg or m157-Tg recipient mice. Similar to the bulk NK cell population, we only observed IFN-γ production defects in the Ly49H+ NK cell population upon transfer into m157-Tg mice (Fig. 6C). Our data demonstrate that NK cells that interact with m157 through the Ly49H receptor on their cell surface are least effective at producing IFN-γ upon stimulation through the NK1.1 receptor.
Figure 6. Expression of the Ly49H receptor is required to induce NK cell hyporesponsiveness.
(A) Representative histograms displaying m157 expression, as assessed by flow cytometry, on donor NK cells from either B6.BxD8, WT or DAP12ki mice injected into non-Tg recipient (tinted histogram) or into m157-Tg recipient mice (open histogram with solid line). The expression of m157 on NK cells from m157-Tg recipient mice is also shown (open histogram with dashed line). (B) B6.BxD8, WT, or DAP12ki splenocytes were injected into WT (black) or m157-Tg (white) recipient mice (n=5–6). Splenocytes were isolated at 2–3 days post transfer and assessed for IFN-γ production by donor NK cells following stimulation through the NK1.1 receptor. All data are presented as the mean+/− SEM. *p<0.02, ns=not significant. (C) WT or DAP12ki splenocytes were injected into WT or m157-Tg recipient mice (n=5–6). Splenocytes were isolated at 2–3 days post transfer and assessed for IFN-γ production by donor Ly49H+ NK cells (black) or donor Ly49H− NK cells (white) following stimulation through the NK1.1 receptor. All data are presented as the mean+/− SEM.*p<0.0005, **p<0.005, ns=not significant.
Discussion
Continuous engagement of activating receptors on NK cells renders them hyporesponsive when stimulated through other activating receptors, a process which occurs both in vitro and in vivo (10, 11, 35–37). We have demonstrated that Ly49H+ NK cells from WT mice, upon transfer into an m157-Tg environment, become hyporesponsive when stimulated through other activating receptors (35). It remained unclear how the Ly49H receptor on transferred NK cells remained continuously engaged with m157, resulting in a hyporesponsive donor NK cell.
In this study, we have provided evidence that NK cells can acquire ligands for activating receptors (in this case m157) on their cell surface upon incubation with cells expressing the ligand. The acquisition occurred quickly in vitro with both lymphokine activated killer (LAK) cells as well as fresh NK cells and required cell-to-cell contact. The acquired m157 was anchored to the surface of the NK cell by GPI linkage. The acquisition of m157, however, did not require signaling through the Ly49H receptor. In fact, it did not even require the expression of the Ly49H receptor by the NK cell. Transfer of the m157 also occurred in vivo, as we observed transfer of m157 onto donor NK cells upon adoptive transfer of donor splenocytes into m157-Tg recipients but not WT recipients.
Though it is clear that m157 can be acquired by NK cells in the absence of the Ly49H receptor, the in vitro data using fresh NK cells suggests that NK cells from B6.BxD8 mice acquire less m157 than WT NK cells (Fig 4B). However, upon injection in vivo, we observe that B6.BxD8 NK cells acquire more m157 (Fig 4C). We believe that the in vivo interaction represents a more natural interaction for the NK cell and its target than in vitro studies where cell densities can play a major role. One explanation as to why B6.BxD8 NK cells appear to acquire more m157 in vivo could be that in WT mice, Ly49H interacts with the acquired m157 and prevents binding of the 6H121 (anti-m157) mAb, thus limiting detection of m157 on the cell surface. In B6.BxD8 NK cells, the interaction of acquired m157 with Ly49H does not occur, so interactions with the anti-m157 mAb are not hindered, allowing for better detection of m157 on the cell surface. The in vivo pattern of staining suggests that acquired m157 interacts with the Ly49H receptor on the NK cell surface.
Upon acquisition of m157 by the NK cells, we demonstrated that the Ly49H receptor (when present) can interact with the acquired protein. More importantly, we demonstrated that the interaction of the acquired m157 with the Ly49H receptor resulted in impaired function of the Ly49H+ NK cells, likely by two mechanisms: (1) blocking the Ly49H receptor from recognizing other targets and (2) continuous engagement of the Ly49H receptor which imparts a functional defect in IFN-γ production upon stimulation through the NK1.1 receptor. The acquisition of ligand appears to be yet another mechanism to control NK cell function following activation.
Of note, cis interactions of MHC class I with receptors on the NK cells surface have been shown to alter NK cell function. For example, the interaction of MHC class I (H2-Dd) with inhibitory receptors (Ly49A) on the NK cell surface have been documented and appear to alter NK cell function (38–40). In the absence of H2-Dd expression on the NK cell surface, Ly49A is efficiently recruited to the NK-cell synapse by the H2-Dd expressed in trans on target cells. However, Ly49A at the NK-cell synapse is reduced when NK cells express both H2-Dd and Ly49A (40). The co-expression of Ly49A and H2-Dd on the NK-cell membrane is believed to sequester the Ly49A receptors, restricting the pool of Ly49A available for functional interaction with MHC class I ligands on target cells. This results in decreased inhibitory capacity of Ly49A in the context of H2-Dd expression. In addition, cis interactions of Ly49C receptor with MHC class I have been shown to block the ability of the Ly49C receptor to bind a mutant form of m157 making mice susceptible to MCMV expressing the mutant m157 (41). Finally, studies suggest that the “education” or “licensing” of NK cells requires both cis and trans recognition of MHC class I molecules (42).
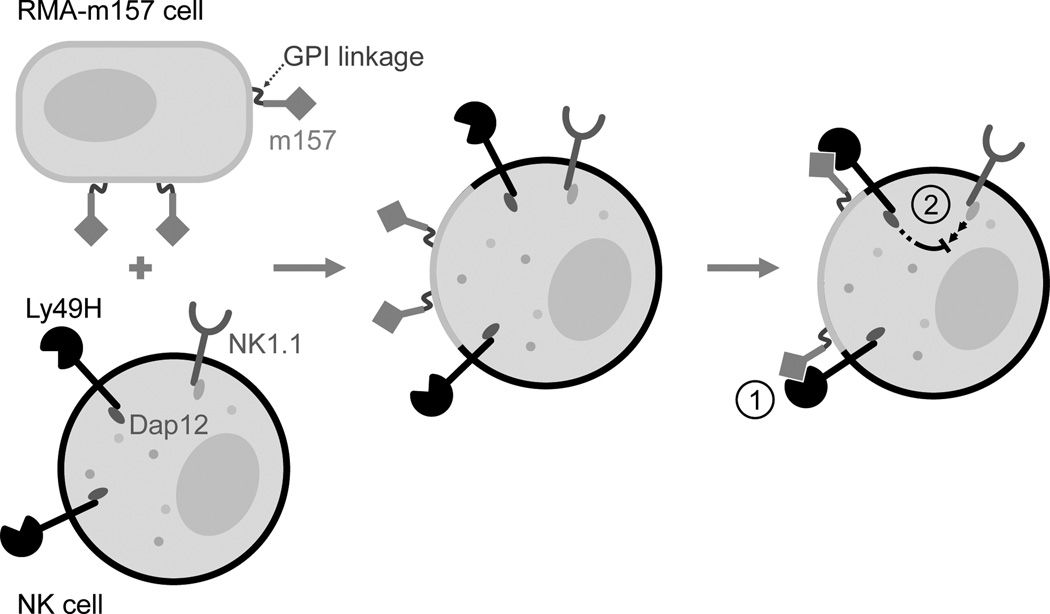
Although the Ly49H receptor is not required for the acquisition of m157, when the receptor is present on the NK cell it appears to be able to interact on the cell surface with the acquired m157. This is suggested by: (1) the increase in detectable levels of Ly49H on the cell surface of m157 “dressed” NK cells upon treatment with PI-PLC (which removes m157 from the cell surface) and (2) the blocking of the ability of m157 “dressed” NK cells to kill RMAm157 targets. The ability of the Ly49H to interact with the acquired m157 in cis would provide an explanation of how continuous engagement of the Ly49H receptor could take place on NK cells, particularly in adoptive transfer experiments where we observe that donor WT NK cells acquire m157 on their cell surface as well as a hyporesponsive phenotype upon transfer into an m157-Tg mouse. Regardless of whether cis or trans interactions are taking place, continuous engagement of Ly49H and m157 results in a hyporesponsive NK cell. We propose a model in which m157, acquired via trogocytosis from the target cell, interacts with Ly49H on the same NK cell, resulting in blocking as well as continuous engagement of the receptor (Fig 7).
Figure 7. Model for trogocytosis mediated hyporesponsiveness of NK cells.
The m157 acquired by the NK cells is expressed on its cell surface as a GPI-linked protein. The interaction of m157 with Ly49H results in (1) blocking of the Ly49H receptor on the NK cell which prevents its recognition of m157 expressing targets and (2) provides a mechanism for the continuous engagement of the Ly49H activating receptor which results in the induction of a hyporesponsiveness of the NK cell to stimulation through other activating receptors.
Our finding that the Ly49H receptor is not necessary for the acquisition of m157 from target cells is novel and differs from a recent paper showing that trogocytosis of Rae-1 by NK cells was decreased if the NKG2D receptor was blocked with antibody or if signaling through the NKG2D receptor was disrupted by using NK cells that did not express adaptor molecules for the receptor (32). In our system, this was not the case, as trogocytosis of m157 was still observed by B6.BxD8 NK cells (where the Ly49H receptor is absent) and by DAP12ki NK cells (where signaling through the receptor is disrupted). The fact that NK cells from B6.BxD8 mice can still acquire m157 from target cells suggest that receptors and ligands other than Ly49H and m157 may be responsible for the formation of the IS between the NK cell and targets. Alternatively, it is possible that an IS does not need to form in order for trogocytosis to occur. Regardless of which receptors are responsible for forming an IS between the NK cell and target or if the formation of an IS is important at all, the expression of Ly49H is not necessary for the acquisition of m157 by the NK cell.
The fact that acquisition of m157 occurs on the surface of NK cells in the absence of the Ly49H receptor also demonstrates that the acquisition of m157 by NK cells was not the result of a trans interaction (between the Ly49H on the NK cell and m157 on the target cell) where the NK cell “rips” the m157 off of the target cell. In addition, it also suggests that the exchange of membrane proteins likely takes place between NK cells and target cells under normal conditions. However, functional consequences to the NK cell, in our experiments, required the presence of the Ly49H receptor on the NK cell. It is possible that a number of NK cells are involved in trogocytosis of membrane and associated proteins from different cells, but display no functional consequence because receptors for the ligands that are acquired are not present on the recipient NK cell. In addition, the amount of ligand taken up on the NK cell membrane is one to two log fold lower than is seen on the target cell, and in order for functional consequences to occur the receptor must be present at a high enough level on the NK cell to be able to engage the acquired ligand. Thus, it is possible that trogocytosis may function to decrease NK cell immune responses in situations where ligands from target cells are taken up in large enough quantities by NK cells expressing an activating receptor which can bind the ligand.
During the process of immune surveillance, NK cells form an IS where they contact the target cell. It has been demonstrated that upon IS formation, activating receptors can undergo ligand-induced down-modulation as a form of negative feedback regulation. NK cells have been shown to acquire a number of proteins from target cells following formation of the immunological synapse. These include ligands for the NKG2D receptor MICA, MICB, Rae-1, and the chemokine receptor CCR7 (19, 29, 32, 43). The acquisition of ligands from target cells can have a profound effect on the function of the NK cell, including down regulation or blocking of the activating receptor. Such alterations can make the NK cell less efficient at killing targets recognized by the down regulated or blocked receptor (19). In addition, acquisition of chemokine receptors (CCRs) can alter cell trafficking; acquisition of CCR7 by NK cells has been described, resulting in increased homing to the lymph node in athymic nude mice (43). In fact, it has been suggested that trogocytosis could be a method to transiently modify expanded NK cells without using gene transfer in adoptive immunotherapy for cancer.
NK cells can also acquire immunomodulatory molecules from tumor cells that alter NK cell function. For example, it has been demonstrated that HLA-G1, commonly expressed by tumor cells in vivo and whose expression on tumor cells confers resistance to killing in vitro, can be acquired by NK cells through trogocytosis. NK cells that acquired HLA-G1 stopped proliferating, were no longer cytotoxic, and were capable of inhibiting cytotoxic functions of other NK cells (44). This suggests that NK cells can acquire proteins from the cell surface of tumor cells resulting in modification of their function.
It has also been shown that interaction of NK cells with cancer cells results in the down-modulation of NKG2D. This has been shown with cervical cancer models where NKG2D was down-modulated, as well as with melanoma models where NKG2D as well as other activating receptors including NKp30 and NKp44 were down-modulated (45–47). The down-modulation of the receptors was associated with impairment of NK cell mediated cytolytic activity. A more recent study described the induction of NKG2D ligands on the surface of healthy myeloid cells by lactate dehydrogenase-5 secreted from glioblastoma cells. This resulted in the down-modulation of the NKG2D receptor on the NK cells as well as their impairment in killing the tumor (48). Trogocytosis of ligands from target cells to NK cells in tumor specific environments could provide an explanation for how the receptors are down modulated as well as how the NK cells become impaired.
Recent studies also suggest that the ligands acquired by NK cells by trogocytosis make them targets for other NK cells in a process termed “fratricide.” Fratricide of NK cells appears to provide another level of control of NK cells (32). Clearly NK cells have evolved a number of ways to control their function. Most likely this is a way to limit collateral damage during NK cell function. In addition to allowing for fratricide, our model suggests that ligand acquired on the NK cells by trogocytosis binds in cis to the receptor resulting in (1) blocking the receptor from recognizing target cells and (2) allows for the continuous engagement of the receptor, resulting in a hyporesponsive NK cell.
Clearly NK cell trogocytosis of ligands from target cells can profoundly alter NK cell function. This has important implications regarding how NK cells respond to tumors. NK cells may acquire, through trogocytosis, tumor-expressed ligands for NK cell activating receptors, impairing the NK cells’ ability to destroy the tumor and possibly allowing for tumor growth and metastasis. In addition, trogocytosis of ligands may play a role in the use of expanded NK cells in immunotherapy for cancer. Modulation of NK cell function by trogocytosis could provide both helpful and harmful effects in regards to tumor immunosurveillance. More work needs to be done to understand the situations in which trogocytosis occurs, which proteins can be transferred by trogocytosis, and the signaling mechanisms that take place that render the NK cell dysfunctional following trogocytosis. Such understanding might make it possible to better utilize NK cells in future immunotherapy protocols.
Supplementary Material
Acknowledgements
We would like to thank Tony French for generously providing us with IL-2. The B6.BxD8 and DAP12ki mice were provided by Wayne Yokoyama and Eric Vivier respectively. The authors would like to thank Deborah Lenschow, Rodney Newberry, Elena Tonc and Matt Ciorba for their critical review of the manuscript.
This work was supported by National Institutes of Health grant R01 AI089870 (to S.K.T) and T32DK007130 (to C.E. M.).
Abbreviations used in this article
- WT
wild type
- Tg
transgenic
- IS
immune synapse
- LAK
lymphokine activated killer
Footnotes
Disclosures:
The authors have no financial conflicts of interest.
References
- 1.Long EO, Kim HS, Liu D, Peterson ME, Rajagopalan S. Controlling natural killer cell responses: integration of signals for activation and inhibition. Annu. Rev. Immunol. 2013;31:227–258. doi: 10.1146/annurev-immunol-020711-075005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lanier LL. Up on the tightrope: natural killer cell activation and inhibition. Nat. Immunol. 2008;9:495–502. doi: 10.1038/ni1581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Karre K. NK cells, MHC class I molecules and the missing self. Scand. J. Immunol. 2002;55:221–228. doi: 10.1046/j.1365-3083.2002.01053.x. [DOI] [PubMed] [Google Scholar]
- 4.Kim S, Poursine-Laurent J, Truscott SM, Lybarger L, Song YJ, Yang L, French AR, Sunwoo JB, Lemieux S, Hansen TH, Yokoyama WM. Licensing of natural killer cells by host major histocompatibility complex class I molecules. Nature. 2005;436:709–713. doi: 10.1038/nature03847. [DOI] [PubMed] [Google Scholar]
- 5.Shifrin N, Raulet DH, Ardolino M. NK cell self tolerance, responsiveness and missing self recognition. Semin. Immunol. 2014;26:138–144. doi: 10.1016/j.smim.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Brodin P, Lakshmikanth T, Johansson S, Karre K, Hoglund P. The strength of inhibitory input during education quantitatively tunes the functional responsiveness of individual natural killer cells. Blood. 2009;113:2434–2441. doi: 10.1182/blood-2008-05-156836. [DOI] [PubMed] [Google Scholar]
- 7.Andrews DM, Sullivan LC, Baschuk N, Chan CJ, Berry R, Cotterell CL, Lin J, Halse H, Watt SV, Poursine-Laurent J, Wang CR, Scalzo AA, Yokoyama WM, Rossjohn J, Brooks AG, Smyth MJ. Recognition of the nonclassical MHC class I molecule H2-M3 by the receptor Ly49A regulates the licensing and activation of NK cells. Nat. Immunol. 2012;13:1171–1177. doi: 10.1038/ni.2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tripathy SK, Keyel PA, Yang L, Pingel JT, Cheng TP, Schneeberger A, Yokoyama WM. Continuous engagement of a self-specific activation receptor induces NK cell tolerance. J. Exp. Med. 2008;205:1829–1841. doi: 10.1084/jem.20072446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun JC, Lanier LL. Tolerance of NK cells encountering their viral ligand during development. J. Exp. Med. 2008;205:1819–1828. doi: 10.1084/jem.20072448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oppenheim DE, Roberts SJ, Clarke SL, Filler R, Lewis JM, Tigelaar RE, Girardi M, Hayday AC. Sustained localized expression of ligand for the activating NKG2D receptor impairs natural cytotoxicity in vivo and reduces tumor immunosurveillance. Nat. Immunol. 2005;6:928–937. doi: 10.1038/ni1239. [DOI] [PubMed] [Google Scholar]
- 11.Wiemann K, Mittrucker HW, Feger U, Welte SA, Yokoyama WM, Spies T, Rammensee HG, Steinle A. Systemic NKG2D down-regulation impairs NK and CD8 T cell responses in vivo. J. Immunol. 2005;175:720–729. doi: 10.4049/jimmunol.175.2.720. [DOI] [PubMed] [Google Scholar]
- 12.Smith KM, Wu J, Bakker AB, Phillips JH, Lanier LL. Ly-49D and Ly-49H associate with mouse DAP12 and form activating receptors. J. Immunol. 1998;161:7–10. [PubMed] [Google Scholar]
- 13.Bakker AB, Hoek RM, Cerwenka A, Blom B, Lucian L, McNeil T, Murray R, Phillips LH, Sedgwick JD, Lanier LL. DAP12-deficient mice fail to develop autoimmunity due to impaired antigen priming. Immunity. 2000;13:345–353. doi: 10.1016/s1074-7613(00)00034-0. [DOI] [PubMed] [Google Scholar]
- 14.Gosselin P, Mason LH, Willette-Brown J, Ortaldo JR, McVicar DW, Anderson SK. Induction of DAP12 phosphorylation, calcium mobilization, and cytokine secretion by Ly49H. J. Leukoc. Biol. 1999;66:165–171. doi: 10.1002/jlb.66.1.165. [DOI] [PubMed] [Google Scholar]
- 15.Brown MG, Dokun AO, Heusel JW, Smith HR, Beckman DL, Blattenberger EA, Dubbelde CE, Stone LR, Scalzo AA, Yokoyama WM. Vital involvement of a natural killer cell activation receptor in resistance to viral infection. Science. 2001;292:934–937. doi: 10.1126/science.1060042. [DOI] [PubMed] [Google Scholar]
- 16.Tripathy SK, Smith HR, Holroyd EA, Pingel JT, Yokoyama WM. Expression of m157, a murine cytomegalovirus-encoded putative major histocompatibility class I (MHC-I)-like protein, is independent of viral regulation of host MHC-I. J. Virol. 2006;80:545–550. doi: 10.1128/JVI.80.1.545-550.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Smith HR, Heusel JW, Mehta IK, Kim S, Dorner BG, Naidenko OV, Iizuka K, Furukawa H, Beckman DL, Pingel JT, Scalzo AA, Fremont DH, Yokoyama WM. Recognition of a virus-encoded ligand by a natural killer cell activation receptor. Proc. Natl. Acad. Sci. U S A. 2002;99:8826–8831. doi: 10.1073/pnas.092258599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Arase H, Mocarski ES, Campbell AE, Hill AB, Lanier LL. Direct recognition of cytomegalovirus by activating and inhibitory NK cell receptors. Science. 2002;296:1323–1326. doi: 10.1126/science.1070884. [DOI] [PubMed] [Google Scholar]
- 19.Roda-Navarro P, Vales-Gomez M, Chisholm SE, Reyburn HT. Transfer of NKG2D and MICB at the cytotoxic NK cell immune synapse correlates with a reduction in NK cell cytotoxic function. Proc. Natl. Acad. Sci. U S A. 2006;103:11258–11263. doi: 10.1073/pnas.0600721103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roda-Navarro P, Reyburn HT. The traffic of the NKG2D/Dap10 receptor complex during natural killer (NK) cell activation. J. Biol. Chem. 2009;284:16463–16472. doi: 10.1074/jbc.M808561200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Joly E, Hudrisier D. What is trogocytosis and what is its purpose? Nat. Immunol. 2003;4:815. doi: 10.1038/ni0903-815. [DOI] [PubMed] [Google Scholar]
- 22.Nolting A, Dugast AS, Rihn S, Luteijn R, Carrington MF, Kane K, Jost S, Toth I, Nagami E, Faetkenheuer G, Hartmann P, Altfeld M, Alter G. MHC class I chain-related protein A shedding in chronic HIV-1 infection is associated with profound NK cell dysfunction. Virology. 2010;406:12–20. doi: 10.1016/j.virol.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Salih HR, Holdenrieder S, Steinle A. Soluble NKG2D ligands: prevalence, release, and functional impact. Front. Biosci. 2008;13:3448–3456. doi: 10.2741/2939. [DOI] [PubMed] [Google Scholar]
- 24.Roda-Navarro P. Assembly and function of the natural killer cell immune synapse. Front. Biosci. (Landmark Ed) 2009;14:621–633. doi: 10.2741/3268. [DOI] [PubMed] [Google Scholar]
- 25.Schleinitz N, March ME, Long EO. Recruitment of activation receptors at inhibitory NK cell immune synapses. PLoS One. 2008;3:e3278. doi: 10.1371/journal.pone.0003278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Orange JS. The lytic NK cell immunological synapse and sequential steps in its formation. Adv. Exp. Med. Biol. 2007;601:225–233. doi: 10.1007/978-0-387-72005-0_23. [DOI] [PubMed] [Google Scholar]
- 27.Caumartin J, Lemaoult J, Carosella ED. Intercellular exchanges of membrane patches (trogocytosis) highlight the next level of immune plasticity. Transpl. Immunol. 2006;17:20–22. doi: 10.1016/j.trim.2006.09.032. [DOI] [PubMed] [Google Scholar]
- 28.Daubeuf S, Aucher A, Bordier C, Salles A, Serre L, Gaibelet G, Faye JC, Favre G, Joly E, Hudrisier D. Preferential transfer of certain plasma membrane proteins onto T and B cells by trogocytosis. PLoS One. 2010;5:e8716. doi: 10.1371/journal.pone.0008716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Roda-Navarro P, Reyburn HT. Intercellular protein transfer at the NK cell immune synapse: mechanisms and physiological significance. FASEB J. 2007;21:1636–1646. doi: 10.1096/fj.06-7488rev. [DOI] [PubMed] [Google Scholar]
- 30.Dhainaut M, Moser M. Regulation of Immune Reactivity by Intercellular Transfer. Front. Immunol. 2014;5:112. doi: 10.3389/fimmu.2014.00112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.McCann FE, Eissmann P, Onfelt B, Leung R, Davis DM. The activating NKG2D ligand MHC class I-related chain A transfers from target cells to NK cells in a manner that allows functional consequences. J. Immunol. 2007;178:3418–3426. doi: 10.4049/jimmunol.178.6.3418. [DOI] [PubMed] [Google Scholar]
- 32.Nakamura K, Nakayama M, Kawano M, Amagai R, Ishii T, Harigae H, Ogasawara K. Fratricide of natural killer cells dressed with tumor-derived NKG2D ligand. Proc. Natl. Acad. Sci. U S A. 2013;110:9421–9426. doi: 10.1073/pnas.1300140110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sjolin H, Tomasello E, Mousavi-Jazi M, Bartolazzi A, Karre K, Vivier E, Cerboni C. Pivotal role of KARAP/DAP12 adaptor molecule in the natural killer cell-mediated resistance to murine cytomegalovirus infection. J. Exp. Med. 2002;195:825–834. doi: 10.1084/jem.20011427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cheng TP, French AR, Plougastel BF, Pingel JT, Orihuela MM, Buller ML, Yokoyama WM. Ly49h is necessary for genetic resistance to murine cytomegalovirus. Immunogenetics. 2008;60:565–573. doi: 10.1007/s00251-008-0313-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bolanos FD, Tripathy SK. Activation receptor-induced tolerance of mature NK cells in vivo requires signaling through the receptor and is reversible. J. Immunol. 2011;186:2765–2771. doi: 10.4049/jimmunol.1003046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mazumdar B, Bolanos FD, Tripathy SK. Viral infection transiently reverses activation receptor-mediated NK cell hyporesponsiveness in an MHC class I-independent mechanism. Eur. J. Immunol. 2013;43:1345–1355. doi: 10.1002/eji.201243215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Coudert JD, Scarpellino L, Gros F, Vivier E, Held W. Sustained NKG2D engagement induces cross-tolerance of multiple distinct NK cell activation pathways. Blood. 2008;111:3571–3578. doi: 10.1182/blood-2007-07-100057. [DOI] [PubMed] [Google Scholar]
- 38.Andersson KE, Williams GS, Davis DM, Hoglund P. Quantifying the reduction in accessibility of the inhibitory NK cell receptor Ly49A caused by binding MHC class I proteins in cis. Eur. J. Immunol. 2007;37:516–527. doi: 10.1002/eji.200636693. [DOI] [PubMed] [Google Scholar]
- 39.Doucey MA, Scarpellino L, Zimmer J, Guillaume P, Luescher IF, Bron C, Held W. Cis association of Ly49A with MHC class I restricts natural killer cell inhibition. Nat. Immunol. 2004;5:328–336. doi: 10.1038/ni1043. [DOI] [PubMed] [Google Scholar]
- 40.Back J, Chalifour A, Scarpellino L, Held W. Stable masking by H-2Dd cis ligand limits Ly49A relocalization to the site of NK cell/target cell contact. Proc. Natl. Acad. Sci. U S A. 2007;104:3978–3983. doi: 10.1073/pnas.0607418104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Forbes CA, Scalzo AA, Degli-Esposti MA, Coudert JD. Ly49C-dependent control of MCMV Infection by NK cells is cis-regulated by MHC Class I molecules. PLoS Pathog. 2014;10:e1004161. doi: 10.1371/journal.ppat.1004161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bessoles S, Angelov GS, Back J, Leclercq G, Vivier E, Held W. Education of murine NK cells requires both cis and trans recognition of MHC class I molecules. J. Immunol. 2013;191:5044–5051. doi: 10.4049/jimmunol.1301971. [DOI] [PubMed] [Google Scholar]
- 43.Somanchi SS, Somanchi A, Cooper LJ, Lee DA. Engineering lymph node homing of ex vivo-expanded human natural killer cells via trogocytosis of the chemokine receptor CCR7. Blood. 2012;119:5164–5172. doi: 10.1182/blood-2011-11-389924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Caumartin J, Favier B, Daouya M, Guillard C, Moreau P, Carosella ED, LeMaoult J. Trogocytosis-based generation of suppressive NK cells. EMBO J. 2007;26:1423–1433. doi: 10.1038/sj.emboj.7601570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Pietra G, Manzini C, Rivara S, Vitale M, Cantoni C, Petretto A, Balsamo M, Conte R, Benelli R, Minghelli S, Solari N, Gualco M, Queirolo P, Moretta L, Mingari MC. Melanoma cells inhibit natural killer cell function by modulating the expression of activating receptors and cytolytic activity. Cancer Res. 2012;72:1407–1415. doi: 10.1158/0008-5472.CAN-11-2544. [DOI] [PubMed] [Google Scholar]
- 46.Garcia-Iglesias T, Del Toro-Arreola A, Albarran-Somoza B, Del Toro-Arreola S, Sanchez-Hernandez PE, Ramirez-Duenas MG, Balderas-Pena LM, Bravo-Cuellar A, Ortiz-Lazareno PC, Daneri-Navarro A. Low NKp30, NKp46 and NKG2D expression and reduced cytotoxic activity on NK cells in cervical cancer and precursor lesions. BMC Cancer. 2009;9:186. doi: 10.1186/1471-2407-9-186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jimenez-Perez MI, Jave-Suarez LF, Ortiz-Lazareno PC, Bravo-Cuellar A, Gonzalez-Ramella O, Aguilar-Lemarroy A, Hernandez-Flores G, Pereira-Suarez AL, Daneri-Navarro A, del Toro-Arreola S. Cervical cancer cell lines expressing NKG2D-ligands are able to down-modulate the NKG2D receptor on NKL cells with functional implications. BMC Immunol. 2012;13:7. doi: 10.1186/1471-2172-13-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Crane CA, Austgen K, Haberthur K, Hofmann C, Moyes KW, Avanesyan L, Fong L, Campbell MJ, Cooper S, Oakes SA, Parsa AT, Lanier LL. Immune evasion mediated by tumor-derived lactate dehydrogenase induction of NKG2D ligands on myeloid cells in glioblastoma patients. Proc. Natl. Acad. Sci. U S A. 2014;111:12823–12828. doi: 10.1073/pnas.1413933111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.