Abstract
The experience of self is unique and pivotal to clinically relevant cognitive and emotional functions. However, well-controlled data on specialized brain regions and functional networks underlying the experience of self remain limited. This functional magnetic resonance imaging study investigated neural activity and connectivity specific to processing one's own face in healthy women by examining neural responses to the pictures of the subjects' own faces in contrast to faces of their own mothers, female friends and strangers during passive viewing, emotional and self-relevance evaluations. The processing of one's own face in comparison to processing of familiar faces revealed significant activity in right anterior insula (AI) and left inferior parietal lobule (IPL), and less activity in right posterior cingulate/precuneus (PCC/PCu) across all tasks. Further, the seed-based correlation analysis of right AI, and left IPL, showed differential functional networks in self and familiar faces contrasts. There were no differences in valence and saliency ratings between self and familiar others. Our preliminary results suggest that the self-experience cued by self-face is processed predominantly by brain regions and related networks that link interoceptive feelings and sense of body ownership to self-awareness and less by regions of higher order functioning such as autobiographical memories.
Keywords: Self awareness, Functional magnetic resonance imaging, Insula, Inferior parietal lobule
1. Introduction
The neural processing of self is regarded as special and distinct from the processing of others, as the experience of self is unique and cannot be completely shared with others. The ability to appreciate the uniqueness and distinctiveness of self from others plays a pivotal role in social relationships, interactions and emotional regulation. Recently, there has been increased interest in understanding the neural basis of self-representation as several neuropsychiatric conditions are associated with disturbances in the subjective experience of self. For example, increased negative self-attribution or self-focus may be related to the development of depression (Grimm et al., 2009), and deficiency in self-monitoring agency and saliency processing may contribute to hallucinations, delusions and the passivity phenomenon in schizophrenia (Spence et al., 1997; Kircher and Leube, 2003). Hence, elucidating the neural correlates of self-processing may have significant clinical implications as these could be used as potential brain markers of self-disturbance in the etiology and treatment outcomes of major psychiatric disorders.
Considerable progress has been made over the last decade in our understanding of the neural mechanisms of self-referential processing in the facial domain. The ability to recognize oneself by recognizing one's own face (mirror self-recognition) has been linked to the capacity for introspection about oneself (self-awareness) and the mental state of others (Theory of Mind) (Gallup, 1970, 1982). Gallup proposed this model based on the observation that chimpanzees and orangutans that live in complex social groups are capable of recognizing themselves in the mirror. However, some view that in non-human animals, mirror self-recognition requires only kinesthetic self-knowledge and does not involve access to other mental states or self-awareness (Mitchell, 1997; Morin, 2006). In previous brain-imaging studies involving human participants, the recognition of one's own face was from pictures and therefore did not require kinesthetic information (Devue and Bredart, 2011). Bruce and Young (1986) proposed a neural model of face recognition that was recently modified by Gobbini and Haxby (2007). According to this revised model, recognizing personally familiar faces including one's own face entails recognition of visual appearance, spontaneous retrieval of biographical information and activation of appropriate emotional response. Based on this model, visual presentation of one's own face is expected to recruit areas associated with self-awareness, knowledge and subjective feelings or emotions. The neural systems for personally familiar face processing or processing of one's own face include visual perceptual brain areas (inferior occipital, fusiform gyri), areas engaged in processing emotional responses (amygdala, insula, caudate) and person knowledge/autobiography (precuneus, posterior cingulate cortex, anterior cingulate cortex, superior temporal cortex, temporo-parietal junction, and anterior temporal area). Based on this neural model of face recognition, it is possible that the neural basis of self-specific processing can be examined in visual perceptual, emotional and cognitive domains by comparing one's own face as a self-specific stimulus with non-self faces.
Using designs in which the subject's own face is contrasted with familiar and unfamiliar faces, several functional imaging studies have examined whether mental or neural representations of self are distinct from representations of others (Kircher et al., 2000; Sugiura et al., 2000; Kircher et al., 2001; Platek et al., 2004; Sugiura et al., 2005; Uddin et al., 2005; Platek et al., 2006; Sugiura et al., 2006; Devue et al., 2007; Platek and Kemp, 2009; Taylor et al., 2009). A meta-analysis of functional magnetic resonance imaging (fMRI) studies of self-face recognition found significant activation in 26 brain regions, including lateral and medial prefrontal, parietal, temporal, occipital areas, anterior and posterior cingulate regions, and subcortical and limbic structures (Platek et al., 2008). Among these brain structures, four areas (left fusiform gyrus, bilateral middle and inferior frontal gyrus, and right precuneus (PCu)) were consistently activated in five or more studies. In a recent meta-analysis, additional brain regions such as the anterior cingulate cortex, insula, fusiform gyrus, and inferior parietal lobule were also reported to be consistently activated in self-recognition in the facial domain (Devue and Bredart, 2011). The specific involvement of perigenual anterior cingulate and insula has been shown in self-specific processing in a descriptive meta-analysis of all studies with with self–aces and other self specific stimuli (Northoff et al., 2011).
There are some methodological limitations in previous imaging studies that need to be addressed. Most previous studies examining self-specific processing have not controlled adequately for general evaluation functions including self-relatedness and affective responses related to personal familiarity (Gillihan and Farah, 2005; Legrand and Ruby, 2009). Hence, the results of previous imaging studies may be confounded by evaluative functions or judgments related to personal familiarity. For example, the cortical midline structures (medial prefrontal cortex, anterior cingulate cortex, precuneus, and posterior cingulate) associated with self-specific stimuli have also been implicated in processing of non-self personally familiar stimuli (Gillihan and Farah, 2005; Northoff et al., 2011). Another methodological issue is that studies using information-processing paradigms with high cognitive load in face-recognition tasks rely predominantly on cognitive or attentional systems (Sugiura et al., 2000, 2005, 2006), and the results of these studies may correspond predominantly to cognitive accounts of self. According to the emerging concept of self in cognitive neuroscience, the unique features of self-specific processing may be related to the subjective experience of self in the experiential domain (Northoff et al., 2006), which is primarily anchored in sensorimotor and homeostatic processing involving interoceptive and exteroceptive functions (Legrand and Ruby, 2009). Hence, to study the neural mechanisms related to self-specific processing in the facial domain, it is critical to use a paradigm that controls for the effects of personal familiarity and evaluative functions on self-relatedness and emotional responses through tasks with low cognitive demand and where face recognition is not explicitly under investigation.
Although previous studies have examined specialized brain regions of self-specific processing, our understanding of functional networks of these regions remains limited. Mapping the functional connectivity of brain regions responsive to one's own face is crucial to study the spatially distributed, domain-specific network of areas that underlie cognitive, emotional and perceptual processing of self. The concurrent activations of multiple brain regions observed during self-related tasks in subtraction paradigms shown in previous studies may not represent a single unified network and could reflect distinct networks (Seeley et al., 2007).
The objective of this study was to examine the distinct neural representations of self and their functional networks in healthy women using one's own face and non-self face conditions (mother, female friend and two female strangers), controlling for the degree of familiarity and for emotional and personal relevance in a paradigm in which self-face recognition was not explicitly under investigation.
2. Methods
Ten healthy, right-handed, female volunteers participated in this study. The participants were between the ages of 20 and 30 years (mean = 25.0, S.D. = 3.4) and had a mean education level of 18 years. To minimize gender variation, only female subjects and gender-matched personally familiar (mother and female friend) and non-familiar controls (older and younger female strangers) were included. The mother and a close non-sexual female friend were chosen as personally familiar others to control for familiarity, person knowledge, emotional responses and self-relatedness.
The Rosenberg self-esteem scale was used to confirm that subjects had self-esteem within the normal range of 15–30 (Rosenberg, 1965) as we predicted that low self-esteem may have an effect on self referential processing. All participants had self-reported stable relationships with their biological mothers since childhood. Furthermore, they received adequate maternal care before the age of 16 years, as determined by the Parental Bonding Instrument (PBI), a retrospective self-report measure of perceived parental bonding before the age of 16 years (Parker et al., 1979). Subjects with a score of 23 and above on the mother care subscale of the PBI were included in the study (Pruessner et al., 2004).
Subjects with a history of early abuse or current conflict in the maternal relationship were excluded. The participants were asked to select the close friend based on length of acquaintance of more than 1 year, frequent interaction (at least once a month), and shared values and activities.
The participants were screened for a history of current or previous serious medical and neurological disorders by self-report and physician-directed medical review of systems. The Structured Clinical Interview for DSM-IV (SCID) (First et al., 1997) was used to rule out current and previous psychiatric illnesses and substance abuse; no subjects had an Axis I diagnosis. Current anxiety and depressive symptoms were measured using Beck's Anxiety Inventory (BAI) and Beck's Depression Inventory (BDI) (Beck et al., 1979, 1988) as anxiety and depressive symptoms may influence neural processing of emotionally relevant stimuli. Right handedness was determined by the Edinburgh Handedness Inventory (Oldfield, 1971). Subjects who were pregnant or had other medical contraindications for magnetic resonance imaging (MRI) were excluded. This study was approved by the institutional review board of Emory University, Atlanta, Georgia, and USA. All participants gave written informed consent before their participation and were compensated 75 USD for their time.
2.1. Stimuli and tasks
The stimulus set for each subject consisted of six pictures. Each subject was asked to provide a full-face digital color head and shoulders photograph of her own face (self), the face of her mother, and the face of a close female friend (age similar to subject), all with a smiling expression and eyes looking forward, and all taken within the past year.
To control for facial emotional expression, we selected a smiling expression because of significant inter-individual variations in processing the affective nature of neutral faces. Two unfamiliar photographs were selected for each subject (one age-matched to mother, one age-matched to friend) from the photographs provided by the other subjects. Images were formatted using Adobe Photoshop CS2 (Adobe Systems, Inc., San Jose, California, USA) to control for size, luminance and background. The visual images were back-projected to a screen attached to a head coil on the scanner, and the subjects viewed the images using a mirror. Subjects were instructed on three tasks (passive viewing, valence and salience) during scanning, while viewing the photos of five faces: self (S), mother (M), close friend (F) and older (O) and younger stranger (Y), intermixed with presentations of a fixation cross.
In the passive viewing task, subjects were instructed simply to view the pictures and provide a button response when a picture was presented. During the valence task, subjects were asked to rate with a button response how pleasant the picture made them feel (1 [least pleasant] to 4 [most pleasant]). For the salience task, participants were asked to rate with a button press how much they were able to relate to the picture (1 [not at all] to 4 [a lot]). Salience and valence tasks were used to control for brain responses associated with salience and valence processing of self-face and personally familiar faces.
2.2. Functional magnetic resonance imaging (fMRI) experimental design
We used a mixed fMRI design in which faces were presented as events, and tasks were presented in blocks. The experiment consisted of four runs: three task blocks with a randomized set of 20 pictures (4 each of self, mother, friend, older stranger, younger stranger) alternating with a fixation cross were presented in each run. Each face was presented for 4 s followed by a visual fixation cross for 10 s. The order of the three task blocks was counterbalanced within each run. Two hundred and forty pictures were presented to each subject. The duration of each block was 280 s, and each run lasted 840 s.
2.2.1. Image acquisition
Imaging was performed at the Emory University School of Medicine using a 3-Tesla Trio MRI scanner (Siemens AG, Erlangen, Germany). The structural magnetic resonance images were acquired by using a T1-weighted high-resolution structural scan (recovery time [TR]/echo time [TE]/flip angle [FA] = 2600 ms/3.93 ms/8°, 176 sagittal slices, 1-mm isotropic resolution) to rule out any structural abnormalities. Functional imaging was done using the Z-SAGA pulse sequence (Simultaneous Acquisition of Gradient-echo and Asymmetric spin-echo for single shot z-shim), which uses a dual-echo echoplanar imaging (EPI) sequence incorporating the z-shim technique to compensate for susceptibility-related signal losses (Heberlein and Hu, 2004). The z-shim gradient was calibrated to optimize signal recovery in the inferior frontal lobe. Pulse sequence parameters were: TR = 2000 ms, TE = 30 ms, FA=90°, 64 × 64 matrix, and 20 slices of 3.44 × 3.44 × 4.00 mm voxels. For each subject, functional data were acquired in four separate runs (described above), with each run acquiring 330 scans.
2.2.2. Image pre-processing and analyses
Functional data were pre-processed using SPM2 (Statistical Parametric Mapping-2) (http://www.fil.ion.ucl.ac.uk/spm) (Wellcome Department of Imaging Neuroscience, London, UK, 2003). Images were realigned, time sliced to the middle slice, re-sampled to obtain a voxel size of 3 × 3 × 3 mm, spatially normalized to the SPM2 EPI template and then spatially smoothed using a Gaussian kernel of 8 mm full width at half maximum (FWHM). Pre-processed images were first analyzed using a general linear model (GLM) (Friston et al., 1995) and then re-analyzed using permutation and the mixed-effect model (the rationale and methodology for permutation and mixed-effect analysis is given in the next section). The neural activity was modeled as a delta function convolved with SPM's canonical hemodynamic response function (HRF) and its temporal derivatives to allow for differences in the onset of the responses across brain regions. A high-pass filter with a cutoff period of 128 s was applied to reduce slow drifts in the signal.
A t-test of contrasts of interest was performed for each subject separately to create individual contrast images, or beta maps. The resultant contrast maps were then submitted to a second level group analysis of random effects. Because there were no a priori hypotheses defining any specific brain areas of activation, a voxel-level statistical threshold of p = 0.001 uncorrected for multiple comparisons with minimal cluster size (k) equal to 10 voxels was used to identify significant brain activations for second level comparisons (Friston et al., 1996). Anatomic labeling of activated clusters was performed using visual inspection and employing the AAL (Automated Anatomical Labeling) SPM toolbox (Tzourio-Mazoyer et al., 2002).
To examine the brain regions of activation during processing of familiarity of one's own face and personally familiar faces (mother and friend), we contrasted one's own face with the younger and older strangers' faces (S>O + Y), and contrasted the mother and friend's faces with the younger and older strangers' faces across all tasks (M+ F>Y + O).
To identify activations and deactivations specific to processing of one's own face, we contrasted one's own face with the mother and friend's faces (i.e. S>M + F), and the mother and friend's face with one's own face (M + F>S) across all tasks combined.
Furthermore, to identify task-dependent activations specific to one's own face condition, we performed the S>M + F contrast analysis for each task (passive, salience, and valence). The brain areas activated after correction for multiple comparisons controlling the family-wise error (FWE) rate at the cluster level (p = 0.05) in both contrasts were considered as significant. In fMRI studies, cluster size inference is reported to be more sensitive than the inference based on voxel intensity (Friston et al., 1996).
The activated clusters (p = 0.05 corrected) from group analyses of self-specific contrast (S>M + F) were then selected as regions of interest (ROIs) for regional signal changes.
Individual percent signal changes in activation from baseline in each ROI were calculated for each of the face types in each task, and all tasks were combined using the SPM2 Marsbar toolbox (Brett et al., 2002), and then averaged across subjects.
2.3. Combined permutation and mixed-effect analysis
The statistical parametric mapping approach using a GLM with multiple comparisons derived from Random Field Theory (RFT) is based on the assumption that data are normally distributed, which is often violated in small sample size (low degrees of freedom). To circumvent this problem, Meriaux et al. (2006) suggested the combined permutation and mixed-effect model for group average analysis in fMRI studies for data sets with low degrees of freedom. Hence, we re-analyzed the data set using the permutation test that uses a mixed-effect decision statistic. We performed the permutation mixed-effect t-test for the group analysis for the contrast S>M + F. The results were set at the threshold for the voxel (p≤0.001 uncorrected with minimum cluster extent = 10) and at the cluster level corrected for multiple comparisons (p<0.05, FWE corrected). Twenty thousand random sign permutations were applied to compute a group statistical map to tabulate the decision statistics; 1024 sign permutations were conducted to correct the second level analyses results. The mixed-effect t-test was conducted using the Distance Toolbox for SPM software.
2.4. Seed based functional connectivity analysis
We used seed-based correlational analysis to examine the functional connectivity of self-face responsive regions. The activated clusters (p = 0.05 corrected) from group analyses of self-specific contrast (S>M + F) were selected as seeds. Functional connectivity analysis was carried out using FEAT (FMRI Expert Analysis Tool V. 5.90), part of FSL 4.0 software (http://www.fmrib.ox.ac.uk/fsl). Functional connectivity maps were generated using the beta-series correlation method proposed by Rissman et al. (2004). The beta-series method was employed due to the event-related design of this study. Contrasts of interest were generated for each and every trial (face picture) of a stimulus for each run.
In total, 45 pictures were displayed (nine of each kind and three per block) and, thus, 45 contrasts of interest were estimated. Each contrast was modeled as the convolution of a delta function with a gamma variate function, with parameters according to Cohen (1997), and subsequently input in a GLM using FEAT. Resulting parameter estimates of a particular face across tasks were sorted to form “condition-specific beta series”. The seed's average beta series was computed for each ROI and then correlated with all other voxels from each condition-specific beta series, using a GLM with one explanatory variable, to form face-ROI Z-statistic functional connectivity maps. This process was repeated for each of the four runs collected per subject. Subject's mean face-ROI Z-score maps of interest were generated and then input in random effect group analyses with a threshold of p<0.01 (uncorrected) at the voxel level to form contrasts of interest.
Changes in functional connectivity due to different faces were estimated by the contrasting of Z-statistic connectivity maps using random-effects group analyses with FEAT. Resulting connectivity maps were normalized to the MNI (Montreal Neurological Institute) standard brain using FSL's registration tool named FLIRT (FMRIB's Linear Image Registration Tool– non-linear registration). The contrasts of interest for each seed were the following: Self>Old + Young: Mother + Friend>Old + Young: and Self>Mother + Friend. From these contrasts, we examined the differential and overlapping functional networks by indirect (S>O + Y: M + F>O + Y) and direct (S>M+F: M + F>S) comparisons. The activations that survived at the cluster level after controlling for multiple comparisons (p = 0.05) were reported. The clusters were corrected for whole brain multiple comparisons to a final p value of 0.05 based on Monte Carlo simulations performed by AlphaSim, part of the AFNI analysis package (http://afni.nimh.nih.gov). Significant clusters of activity were labeled using the Talairach atlas.
3. Results
3.1. Psychological assessment and behavioral data
The PBI scores for mother's care ranged from 25 to 36 (mean = 31.4, S.D. = 3.1). The scores for the self-esteem questionnaire ranged from 15 to 30 (mean = 23, S.D. = 2.2). The anxiety (BAI) scores ranged from 0 to 6 (mean = 2.4, S.D = 2.5), and the depression (BDI) scores ranged from 0 to 6 (mean = 1.7, S.D. = 2.2). Regarding ratings on salience and valence tasks, there was a main effect of face on salience (F4, 45 = 72.40, p<0.0001) and valence ratings (F4, 45 = 35.42, p<0.0001) (Fig. 1). The post hoc analysis showed no significant differences in salience and valence ratings between self and mother (salience, t = 1.48, d.f. = 9, p = 0.171; valence, t = 1.43, d.f. = 9, p = 0.191) and between self and friend (salience, t = −0.243, d.f. = 9, p = 0.813; valence, t = 1.537, d.f. = 9, p = 0.159), whereas salience and valence ratings of self were significantly greater than younger stranger (salience, t = 17.432, d.f. = 9, p = 0.000; valence, t = 8.229, d.f. = 9, p = 0.000) and older stranger (salience, t = 16.976, d.f. = 9, p = 0.000; valence, t = 3.71, d.f. = 9, p = 0.005). Furthermore, the ratings on salience and valence tasks were significantly different between mother and old stranger (salience, t = 13.01, d.f. = 9, p = 0.001; valence, t = 6.27, d.f. = 9, p = 0.001), mother and young stranger (salience, t = 13.18, d.f. = 9, p = 0.001; valence, t = 10.07, p = 0.001), and between friend and old stranger (salience, t = 9.71: d.f. = 9, p = 0.001; valence, t = 7.85, d.f. = 9, p = 0.001), and friend and young stranger (salience, t = 9.84, d.f. = 9, p = 0.001; valence, t = 14.5, d.f. = 9, p = 0.001). In summary, the ratings on salience and valence tasks were significantly different between familiar (self, mother, friend) and unfamiliar faces and were comparable between self and personally familiar faces.
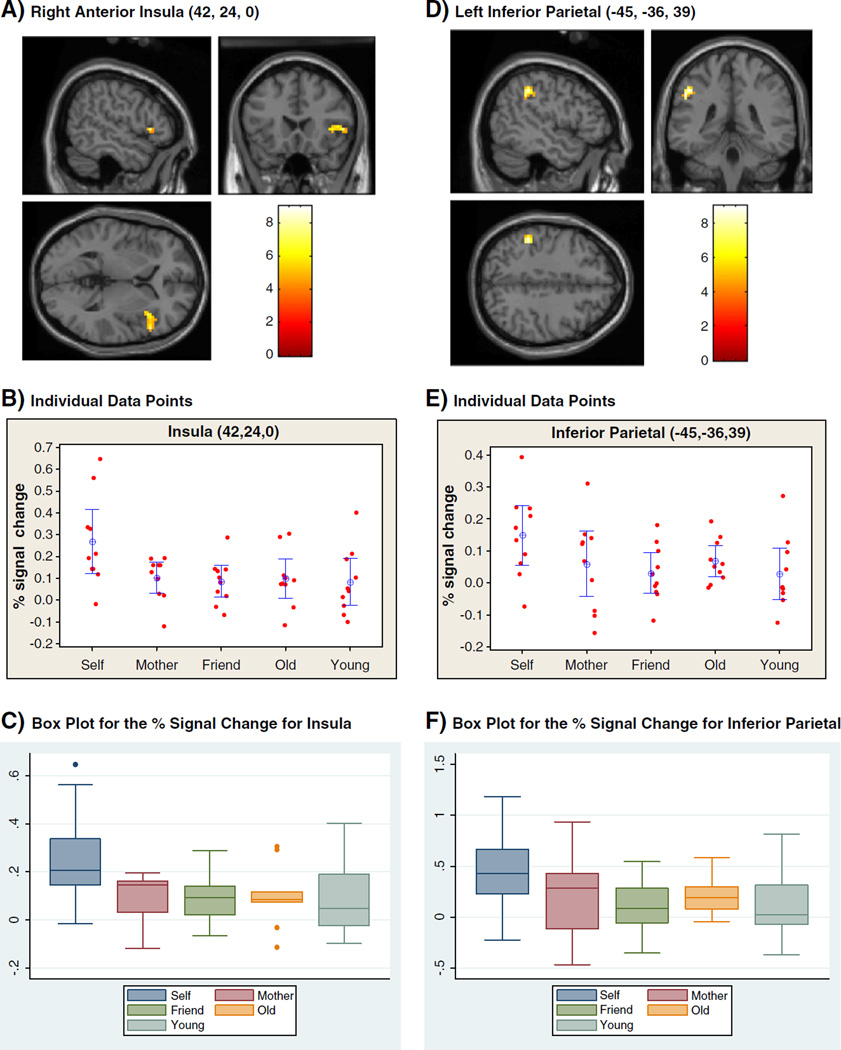
Fig. 1.
A, B, C: Brain activity in the right anterior insula and D, E, F: brain activity in the left inferior parietal in self versus personally familiar contrast (FWE corrected p<0.05 at the cluster level).
3.2. fMRI data
3.2.1. One's own face and personally familiar face processing
Across all tasks, one's own face contrasted with all other faces (S>M + F + O + Y) showed significant activations in anterior insula (AI) of right hemisphere, and inferior parietal lobule (IPL) of left hemisphere (p = 0.05 corrected for multiple comparisons controlling FWE at cluster level). The familiarity processing of one's own face (S>O + Y) was associated with significant activations in the left IPL (p = 0.05 cluster level corrected for FWE) whereas there was no significant activation in personal familiarity face (M + F>O + Y) processing (Table 1). In specific processing of one's own face (S>M+F) across all tasks, only the right AI extending to the inferior frontal region and left IPL showed significant activation (p = 0.05 cluster level corrected for FWE) (Fig. 1A and D). The permutation and mixed-effect analysis also showed significant activation in the left IPL and in the right anterior insula (AI)with the extension to the right inferior frontal region (p = 0.05 cluster level corrected for FWE) (Table 2). Thus, the results derived from RFT/GLM and permutation/mixed-effect analysis were identical, validating our main findings. In the reverse contrast (M + F>S), only the clusters in bilateral precuneus (PCu) and right posterior cingulate cortex (PCC) were significant (p = 0.05 cluster level corrected for FWE) (Table 1). Furthermore, processing of one's own face compared with familiar face processing activated the right cuneus during passive conditions, and right AI and lentiform nucleus during the valence conditions, significant at p = 0.05 after correction for multiple comparisons (Table 3).
Table 1.
Brain regions activated in the contrasts of one's own face, personally familiar and unfamiliar faces (all tasks combined).a
| Brain region (BA) | Hemisphere | MNI coordinates | Cluster size (voxels) |
Z-score | Cluster level p corrected |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| S>F+M+O+Y | |||||||
| Parietal inferior (40) | Left | −48 | −39 | 42 | 39 | 5.33 | 0.051 |
| Parietal superior (7) | Left | −24 | −63 | 51 | 24 | 4.12 | 0.222 |
| Insula | Right | 39 | 9 | 0 | 53 | 4.08 | 0.017 |
| Insula | Right | 42 | 24 | 0 | 53 | 4.04 | 0.017 |
| Occipital middle (19) | Left | −27 | −69 | 27 | 12 | 3.89 | 0.645 |
| Precentral (4) | Left | −48 | −6 | 24 | 17 | 3.86 | 0.424 |
| Precentral (6) | Left | −54 | 3 | 24 | 17 | 3.44 | 0.424 |
| S>Y+O | |||||||
| Parietal inferior (40) | Left | −48 | −39 | 42 | 42 | 4.57 | 0.042 |
| Precentral (4) | Left | −48 | −6 | 24 | 15 | 3.89 | 0.584 |
| Postcentral (6) | Left | −54 | 3 | 24 | 15 | 3.13 | 0.641 |
| M+F>Y+O | |||||||
| Precuneus (7) | Right | 3 | −60 | 27 | 11 | 3.57 | 0.686 |
| S>M+F | |||||||
| Insula | Right | 42 | 24 | 0 | 97 | 4.63 | 0.001 |
| Frontal inferior (45) | Right | 51 | 21 | 3 | 97 | 4.37 | 0.001 |
| Frontal superior (8) | Right | 27 | 0 | 45 | 10 | 3.73 | 0.745 |
| Parietal inferior (40) | Left | −45 | −36 | 39 | 46 | 5.37 | 0.031 |
| Parietal superior (7) | Left | −24 | −63 | 51 | 30 | 4.37 | 0.127 |
| Occipital superior (19) | Left | −24 | −69 | 33 | 19 | 3.9 | 0.745 |
| S<M+F | |||||||
| Posterior cingulate (31) | Right | 9 | −45 | 36 | 62 | 4.34 | 0.009 |
| Precuneus (7) | Right | 6 | 60 | 30 | 62 | 3.81 | 0.009 |
| Precuneus (7) | Left | 0 | −60 | 36 | 62 | 3.55 | 0.009 |
| Temporal superior (38) | Left | −42 | 18 | −18 | 15 | 4.09 | 0.505 |
| Parahippocampus (34) | Left | −9 | −6 | −21 | 24 | 3.89 | 0.222 |
| Hippocampus (34) | Left | −18 | −6 | −15 | 24 | 3.36 | 0.222 |
| Frontal superior (10) | Right | 15 | 66 | 0 | 10 | 3.46 | 0.745 |
S = self, M = mother, F = friend, O = older stranger.
Y = younger stranger, BA = Brodmann Area.
Used a statistical threshold of p = 0.001 (uncorrected) with minimal cluster size of 10 voxels, and then the resulting clusters were corrected for whole brain multiple comparisons (family-wise error [FWE] corrected).
Table 2.
Results of the contrast S>M+F (combined tasks) from parametric and permutation/mixed effect analyses.a
| Anatomical location and statistical test |
MNI coordinates | Cluster extent (voxels) |
Cluster level p corrected |
||
|---|---|---|---|---|---|
| X | Y | Z | |||
| Right anterior insula | |||||
| Parametric t test (SPM) | 42 | 24 | 0 | 97 | 0.001 |
| Permutation MFX t test | 45 | 24 | 0 | 43 | 0.012 |
| Left parietal inferior | |||||
| Parametric t test (SPM) | −45 | −36 | 39 | 46 | 0.031 |
| Permutation MFX t test | −48 | −39 | 42 | 55 | 0.007 |
| Frontal inferior | |||||
| Parametric t test (SPM) | 51 | 20 | 3 | 97 | 0.001 |
| Permutation MFX t test | 48 | 18 | 6 | 43 | 0.012 |
| Parietal superior | |||||
| Parametric t test (SPM) | −24 | −63 | 51 | 30 | 0.127 |
| Permutation MFX t test | −24 | −63 | 51 | 16 | 0.180 |
| Frontal superior | |||||
| Parametric t test (SPM) | 27 | 0 | 45 | 10 | 0.745 |
| Permutation MFX t test | 27 | 0 | 45 | 12 | 0.730 |
| Occipital superior | |||||
| Parametric t test (SPM) | −24 | −69 | 33 | 19 | 0.354 |
| Permutation MFX t test | −27 | −69 | 30 | 30 | 0.109 |
S = self, M = mother, F = friend.
Used a statistical threshold of p = 0.001 (uncorrected) with minimal cluster size of 10 voxels, and then the resulting clusters were corrected for whole brain multiple comparisons (family-wise error [FWE] corrected).
Table 3.
Task specific brain activation in self versus personally familiar contrast.a
| Brain region (BA) |
Hemisphere | MNI coordinates |
Cluster size (voxels) |
Z-score | Cluster level p corrected |
||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| Passive | |||||||
| Cuneus (19) | Right | 15 | −81 | 42 | 18 | 3.90 | 0.028 |
| Valence | |||||||
| Insula | Right | 36 | 9 | 0 | 22 | 4.65 | 0.017 |
| Lentiform | Right | 18 | −3 | −3 | 22 | 3.80 | 0.017 |
| nucleus | |||||||
| Salience | |||||||
| Precuneus (7) | Right | 18 | −72 | 42 | 18 | 4.32 | 0.928 |
| Fusiform | Right | 33 | −57 | −12 | 15 | 3.99 | 0.663 |
BA = Broadmann Area.
Used a statistical threshold of p = 0.001 (uncorrected) with minimal cluster size of 10 voxels, and then the resulting clusters were corrected for whole brain multiple comparisons (family-wise error [FWE] corrected).
3.2.2. ROI analysis
Individual percent signal changes in activation from baseline in the right AI (42, 24, 0) and the left IPL (−45, −36, 39) in response to S,M, F, O, Y are shown in Fig. 1B and E. Across tasks, the right AI and the left IPL showed a greater increase in activity with S than with M, F, O, and Y. The paired two-tailed t-test showed significant increases in percent signal change in the right AI between self and mother (t = 2.65, d.f. = 9, p = 0.026), self and friend (t = 2.781, d.f. = 9, p = 0.021), self and older stranger (t = 2.441, d.f. = 9, p = 0.037), and self and younger stranger (t = 2.480, d.f. = 9, p = 0.035). Similarly, there were significant increases in percent signal change in the left IPL between self and mother (t = 2.646, d.f. = 9, p = 0.031), self and friend (t = 3.088, d.f. = 9, p = 0.013), self and older stranger (t = 2.517, d.f. = 9, p = 0.033), and self and younger stranger (t = 3.417, d.f. = 9, p = 0.008).Given the small sample size, we examined the outliers in data for both ROIs using box plots (Fig. 1C and F) and calculated effect sizes (Cohen's d). There was one outlier for right AI for the self-face condition. However, the effect size (Cohen's d values) for the self-condition remained larger, even after excluding the outlier from the analysis (Effect size including the outlier: S>M = 1.02, S>F = 1.12, S>O = 0.99, S>Y = 1.02) (effect size excluding the outlier: S>M = 0.90, S>F = 1.02, S>O = 0.86, S>Y = 0.90), suggesting that outliers did not drive the effect of the self-condition on right AI responses. The effect size for left IPL for the self-condition was medium to large (S>M = 0.66, S>F = 1.08, S>O = 0.79, S>Y = 0.99).
3.3. Functional connectivity of right AI and left IPL
The right AI and left IPL generated from the contrast S>M + F (all tasks combined)were the seeds for functional connectivity analysis. The ROIs, right AI and left IPL, were coordinates of the most significant voxel of each cluster in the MNI standard brain (2 mm × 2 mm × 2 mm) and were considered as the center of each ROI employed in connectivity analyses. The extension and shape of each ROI were defined according to the clusters' volume from the S>M+F analysis, the anatomical structure of the ROI and the assistance of the Talairach atlas. The location of the center and number of voxels of each ROI were: x = 39, y = 9, z = 0 mm, 230 voxels for the right AI; x = −48, y = −39, z = 42 mm, 245 voxels for the left IPL (BA-40).
3.3.1. Right AI-seed correlated networks
During specific processing of one's own face (S>M + F), the right AI was correlated with the left mid-temporal and occipital regions, whereas during the other person-specific processing (M + F>S), the right AI was correlated with left prefrontal regions (Table 4). In familiarity processing of one's own self (S>O + Y), right AI was correlated with right medial parietal, and bilateral middle occipital regions, whereas in personally familiar face processing (M + F>O + Y) the right AI activity was correlated with bilateral parietal, and ACC regions. The left insula, bilateral striatum, left superior temporal and left prefrontal regions were correlated with right AI during both self and person familiar processing.
Table 4.
Right anterior insula seed correlated activity in brain regions.a
| Brain region | Hemisphere | MNI coordinates | Cluster size | Z-score | p-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| S>O+Y | |||||||
| Frontal region | |||||||
| Inferior frontal (46) | Left | −38 | 38 | 6 | 90 | 3.41 | <0.001 |
| Middle frontal (10) | Left | −36 | 40 | 6 | 90 | 2.89 | 0.004 |
| Inferior frontal (45/44) | Left | −42 | 20 | 10 | 83 | 2.93 | 0.003 |
| Parietal region | |||||||
| Precuneus (31) | Right | 30 | −78 | 22 | 287 | 3.31 | 0.001 |
| Cuneus (18) | Right | 26 | −82 | 28 | 287 | 3.14 | 0.002 |
| Temporal region | |||||||
| Middle temporal (37) | Left | −46 | −62 | 2 | 251 | 3.16 | 0.002 |
| Superior temporal (42/41) | Left | −62 | −22 | 10 | 107 | 4.21 | <0.001 |
| Superior temporal (22) | Left | −64 | −32 | 12 | 107 | 2.52 | 0.012 |
| Superior temporal (41) | Right | 34 | −32 | 12 | 91 | 2.78 | 0.006 |
| Occipital region | |||||||
| Middle occipital (19) | Right | 32 | −78 | 22 | 287 | 3.59 | <0.001 |
| Middle occipital (18) | Left | −24 | −88 | 24 | 260 | 4.03 | <0.001 |
| Medial occipital/fusiform (19/37) | Left | −30 | −52 | −2 | 251 | 4.41 | <0.001 |
| Paralimbic/subcortical | |||||||
| Insula (13) | Left | −36 | 24 | 0 | 90 | 2.46 | 0.014 |
| Caudate | Right | 26 | −32 | 14 | 91 | 2.36 | 0.018 |
| Thalamus | Right | 26 | −28 | 12 | 91 | 2.77 | 0.006 |
| Putamen | Left | −26 | 2 | 2 | 157 | 3.3 | 0.001 |
| Claustrum | Left | −28 | 16 | 4 | 157 | 2.79 | 0.005 |
| Insula (13) | Left | −42 | 16 | 10 | 83 | 3.44 | <0.001 |
| M+F>O+Y | |||||||
| Frontal region | |||||||
| Inferior frontal (13/47) | Left | −32 | 16 | 14 | 207 | 3.77 | <0.001 |
| Dorsolateral frontal (6) | Right | 38 | −12 | 36 | 146 | 3.12 | 0.002 |
| Cingulate region | |||||||
| Anterior cingulate (24) | Left | −4 | 30 | −10 | 185 | 3.22 | 0.001 |
| Anterior cingulate (24) | Right | 2 | 22 | −8 | 185 | 3.18 | 0.001 |
| Parietal region | |||||||
| Inferior parietal (40) | Left | −60 | −22 | 14 | 225 | 2.92 | 0.004 |
| Inferior parietal (40) | Right | 32 | −30 | 40 | 146 | 3.53 | <0.001 |
| Superior parietal (3) | Left | −30 | −26 | 42 | 99 | 3.28 | 0.001 |
| Inferior parietal (40) | Left | −42 | −30 | 34 | 99 | 2.74 | 0.006 |
| Temporal region | |||||||
| Transverse temporal (41) | Left | −58 | −22 | 12 | 225 | 4.03 | <0.001 |
| Superior temporal (42) | Left | −54 | −28 | 18 | 225 | 2.87 | 0.004 |
| Paralimbic/subcortical | |||||||
| Insula (13) | Left | −48 | −20 | 14 | 225 | 2.49 | 0.013 |
| Insula (13) | Left | −32 | 16 | 10 | 207 | 3.55 | <0.001 |
| Claustrum | Left | −28 | 12 | 10 | 207 | 3.23 | 0.001 |
| Putamen | Left | −26 | 10 | 2 | 207 | 2.9 | 0.004 |
| Caudate | Right | 6 | 18 | −6 | 185 | 2.83 | 0.005 |
| Caudate | Left | −6 | 16 | −10 | 185 | 2.52 | 0.012 |
| S>M+F | |||||||
| Middle occipital (19) | Left | −40 | −70 | 12 | 165 | 3.73 | <0.001 |
| Middle temporal (37) | Left | −42 | −68 | 12 | 165 | 3.1 | 0.002 |
| M+F>S | |||||||
| Medial frontal (10) | Left | −12 | 56 | 12 | 173 | 3.33 | 0.001 |
| Superior frontal (10) | Left | −18 | 62 | 12 | 173 | 2.6 | 0.009 |
BA = Brodmann Area.
Used a statistical threshold of p = 0.01 (uncorrected) with minimal cluster size of 10 voxels and then the resulting clusters were corrected for whole brain multiple comparisons. The brain regions listed were significant at p = 0.05 corrected for multiple comparison at cluster level based on Monte Carlo simulations performed by AlphaSim.
3.3.2. Left IPL-seed correlated networks
During processing of one's own face (S>Y + O, S>M + F) activity in the left IPL was correlated with cortical midline structures including bilateral ACC, right PCC/PCu and subcortical and limbic structures (right caudate, thalamus and insula) and bilateral superior temporal regions (Table 5). In personally familiar processing contrasts (M + F>S; M + F>Y + O) no activations survived after corrections for multiple comparisons.
Table 5.
Left inferior parietal seed correlated activity in brain regions.a
| Brain region | Hemisphere | MNI coordinates |
Cluster size | Z-score | p-value | ||
|---|---|---|---|---|---|---|---|
| X | Y | Z | |||||
| S>O+Y | |||||||
| Superior | Right | 66 | −38 | 14 | 237 | 5.46 | <0.001 |
| temporal (22) | |||||||
| Superior | Right | 62 | −42 | 8 | 237 | 3.07 | 0.002 |
| temporal (22) | |||||||
| Superior | Right | 68 | −24 | 14 | 237 | 2.74 | 0.006 |
| temporal (42) | |||||||
| Temporal (41) | Left | −56 | −24 | 10 | 200 | 3.73 | <0.001 |
| Superior | Left | −60 | −24 | 8 | 200 | 3.32 | 0.001 |
| temporal (22) | |||||||
| Temporal (42) | Left | −60 | −32 | 8 | 200 | 3.18 | 0.002 |
| M+F>O+Y | – | – | – | – | – | – | – |
| S>M+F | |||||||
| Cingulate region | |||||||
| Anterior cingulate (32–24) | Right | 6 | 40 | 6 | 95 | 2.71 | 0.007 |
| Anterior cingulate (32–24) | Left | −6 | 38 | 8 | 95 | 2.78 | 0.005 |
| Cingulate gyrus (31) | Right | 10 | −46 | 44 | 83 | 2.76 | 0.006 |
| Paralimbic/subcortical | |||||||
| Caudate | Right | 20 | −6 | 22 | 241 | 3.96 | <0.001 |
| Thalamus | Right | 20 | −12 | 16 | 241 | 3.18 | 0.002 |
| Insula (13) | Right | 38 | −10 | 20 | 241 | 2.78 | 0.005 |
| Parietal region | |||||||
| Cuneus (18) | Left | −4 | −78 | 32 | 239 | 3.47 | 0.001 |
| Precuneus (7) | Right | 6 | −52 | 48 | 83 | 3.29 | 0.001 |
| Precuneus (7) | Left | 0 | −56 | 48 | 83 | 2.96 | 0.003 |
| Precuneus (7) | Left | −10 | −64 | 36 | 82 | 3.4 | 0.001 |
| M+F>S | – | – | – | – | – | – | – |
BA = Brodmann Area.
Used a statistical threshold of p = 0.01 (uncorrected) with minimal cluster size of 10 voxels and then the resulting clusters were corrected for whole brain multiple comparisons. The brain regions listed were significant at p = 0.05 corrected for multiple comparison at cluster level based on Monte Carlo simulations performed by AlphaSim.
4. Discussion
The results demonstrate that processing one's own face involves a unique pattern of brain activation. This pattern of activation was revealed when one's own face was contrasted with personally familiar and with unknown faces, and was distinct from the pattern observed when personally familiar faces were compared with one's own face or with unknown faces. The processing of one's own face compared to personally familiar face processing induced greater activation in the right AI and the left inferior parietal regions and lesser activation in posterior cingulate and PCu. Furthermore, these two brain regions (right AI and left IPL) were differentially correlated with other brain regions during processing of one's own face compared to personally familiar processing, suggesting that these regions and related networks are specific for processing of one's own face, although the regions are functionally heterogeneous and known to participate in both self- and person-related processing (Craig, 2002; Carr et al., 2003; Rizzolatti and Craighers, 2004).
Our finding of stronger activity in the right AI associated with specific processing of one's own face is consistent with previous individual studies and a meta-analysis implicating right AI in the self-/non-self distinction (Kircher et al., 2001; Sugiura et al., 2005; Devue et al., 2007; Devue and Bredart, 2011; Northoff et al., 2011). The insula is implicated in self-related processing such as subjective feeling state, self-awareness, interoceptive awareness (Damasio, 1999; Craig, 2002; Critchley et al., 2004; Critchley, 2005), sense of agency (Farrer and Frith, 2002) and emotional attachment (Bartels and Zeki, 2004; Gobbini et al., 2004; Leibenluft et al., 2004). However, the specific role of the AI in processing one's own face in this study remains speculative. The co-activation of the adjacent right inferior frontal region during processing of one's own face compared with the processing of personally familiar faces extends the literature on the role of right IFG in processing one's own face (Sugiura et al., 2000; Uddin et al., 2005; Devue et al., 2007; Platek et al., 2008), self-awareness (Gallup, 1982; Craig, 2004; Critchley et al., 2004) and saliency processing (Seeley et al., 2007). Right AI activation was more evident during emotional evaluation (see Table 3), and the lentiform nucleus, a brain region implicated in emotional processing of maternal or romantic attachment (Bartels and Zeki, 2004), co-activated with the insula during emotional evaluation, suggesting that right insular activation in response to one's own face may be related to affective responses or the subjective feeling associated with processing of one's own face. Furthermore, processing of one's own face (Table 4: S>M + F; S>O + Y) preferentially involved functional connectivity of the right AI to the occipito-temporal brain regions implicated in early face detection mechanisms from lower level of physical information (Freeman et al., 2010) and PCu implicated in visual imagery and self-related processing (Cavanna and Trimble, 2006), suggesting that right AI activation may be related to the sensory/perceptual level of processing one's own face. In contrast, the observed positive correlations of right AI with the medial and superior frontal regions and lateral parietal 20 regions during personally familiar face processing (Table 4: M + F>S; M + F>Y + O) suggest that right AI involvement in processing familiar persons may be at a cognitive level such as in empathy and imitation (Uddin et al., 2007). The overlapping functional connectivity of the right AI to temporal regions, left anterior insula and striatum observed during both self-and person-related processing (Table 4: S>O + Y; M + F>O + Y) is consistent with previous findings that these networks participate in self- and other-person-related processing such as emotional attachment (Bartels and Zeki, 2004).
The left IPL is thought to contribute to a sense of agency of action (Ruby and Decety, 2001; Farrer and Frith, 2002), and its functional connection with the right IPL constitutes the mirror neuron system (Aziz-Zadeh et al., 2006) implicated in imitation, empathy and self–other distinctions (Kircher et al., 2001; Carr et al., 2003; Avenanti et al., 2005; Iacoboni, 2005; Platek et al., 2006). Although previous studies suggested an association between the right IPL and self–other distinction (Uddin et al., 2005; Platek et al., 2006; Sugiura et al., 2006; Uddin et al., 2007), our results are consistent with the studies implicating the left IPL in recognition of one's own face (Kircher et al., 2001) and relevant to other self-related processing such as imagined self-movement (Ruby and Decety, 2001), self-reflection (Kjaer et al., 2002), and self-agency of action (Farrer and Frith, 2002). In addition, the left IPL is an important brain region in the overlapping networks hypothesized to be common for self–others domain processing, such as default mode network, Theory of Mind (TOM) and mirror neurons (Uddin et al., 2007; Spreng et al., 2009). During processing of one's own face, the left IPL correlated with bitemporal brain regions implicated in TOM (Gallagher and Frith, 2003) and cortical midline structures implicated in self-relatedness (Schneider et al., 2008), suggesting that in the self-others distinction, the left IPL selectively interacts with brain regions that are crucial for self-referential processing. The PCu and the adjacent PCC, which constitute the posterior component of cortical midline structures, are involved in the processing of self-related tasks, personal familiarity, visuospatial imagery and autobiographical memories (Shah et al., 2001; Addis et al., 2004; Gilboa et al., 2004; Lou et al., 2004; Cavanna and Trimble, 2006; Ramasubbu et al., 2007). However, the role of the PCu in processing one's own face remains unclear as both increased (Kircher et al., 2000, 2001; Sugiura et al., 2000, 2006) and decreased activation of PCu (Uddin et al., 2005; Devue et al., 2007) have been reported during the recognition of one's own face. Since our study found a pattern of decreased activation in the PCu and PCC that was associated with reduced activation in neural structures subserving the autobiographical memory system (Maddock et al., 2001; Addis et al., 2004; Gilboa et al., 2004; Cavanna and Trimble, 2006), it is reasonable to suggest that processing one's own face as a self-referential or familiar stimulus does not require the involvement of autobiographical encoding and retrieval in contrast to processing personally familiar other faces. The differential participation of autobiographical memory and self-awareness systems in processing one's own face and non-self personal familiarity processing is consistent with the documented dissociation of these two functions in Alzheimer disease and amnesic syndrome (Rankin et al., 2005; Rosenbaum et al., 2005).
The observed task-specific activations during processing one's own face compared to non-self personally familiar faces suggest that specific brain regions are involved in processing perceptual, cognitive (self-relatedness) and affective components of self (Table 3). Increased activity in the cuneus during passive viewing of one's own face suggests that self-/non-self distinction can occur at the level of visuospatial processing (Taylor et al., 2009). The observed differential brain activations during valence (right AI, lentiform nucleus) and salience (precuneus, fusiform) evaluations of one's own face are in agreement with previous studies that showed that brain regions subserving these two functions can be dissociated using functional magnetic resonance imaging (Moran et al., 2006; Northoff et al., 2009). Although these studies reported an association between self-relatedness or self-relevance and neural activity in MPFC, we found no activations in this brain region for self-specific processing during the evaluation of self-relatedness, suggesting that the MPFC region is not specific to self-processing. Instead, this region may be involved in self-referential processing that is common for both self and familiarity processing. Although activity in precuneus and fusiform did not survive correction for statistical threshold, their participation in self-specific salience operations was consistent with previous reports implicating their role in processing of one's own face (Cavanna and Trimble, 2006; Sugiura et al., 2005). Future studies should address whether these regions discriminate between self-relatedness specific to self stimuli and self-relatedness to non-self personally familiar stimuli. The increased activity in right AI during valence evaluations of one's own face versus personally familiar faces could be related to emotional responses or autonomic arousal associated with self-face processing. Although Morita et al. (2008) demonstrated that AI activation during the recognition of one's own face was related to autonomic arousal and not modulated by embarrassment ratings for an individual's own face, it remains to be investigated whether AI activation related to the processing of one's own face depends on the type of emotional response (positive versus negative). The valence-related activity in lentiform nucleus, a brain region involved in the reward pathway, shows that reward may be linked with self-specific processing (Northoff and Hayes, 2011). In summary, the self-/non-self distinction occurs at the right AI and left IPL, which emerged as specific brain regions across all tasks, and the brain regions that showed task-dependent activations may involve in processing specific components of self.
Our results are consistent with the tripartite neural model of self-processing proposed by Northoff et al. (2006). The first level is sensory processing of interoceptive signals cued by self-specific facial stimuli. In this respect, the right AI activation and its functional correlation with left lateral occipito-temporal regions in response to one's own face suggest processing of interoceptive signals cued by exteroceptive visual stimulus of one's own face. At a second level the sensory information about the self is processed for self-referential information. The activation of left IPL and its functional connectivity with cortical midline structures and subcortical limbic structures including right insula during specific processing of one's own face reflect the integration of visuospatial processing and interoceptive signals into the sense of body ownership. This is in agreement with the literature that IPL integrates visuospatial and abstract cognitive information into a signal of behavioral salience (Gottlieb, 2007). The third level, higher order cognitive processing of autobiographical memories, was required for processing non-self familiar faces more so than for self face. Therefore, in processing of one's own face, the neural components and networks integral for self-awareness of the physical state which refer to protoself or physical self (Damasio, 1999; Panksepp, 2003) are crucial, whereas in non-self personally familiar processing, the neural components that represent person knowledge which refer to autobiographical self or narrative self (Damasio, 1999; Gallagher, 2000) appear to dominate. In the context of this tripartite model, our findings suggest that the distinction between one's own face and personally familiar faces occurs as a prereflective function at a sensory/physical level (right AI, left IPL) of self-referential processing that presupposes the knowledge of self which is processed at a higher cortical level (PFC, PCu).
There are several shortcomings in the current study. The sample was of small size and limited to women only. Hence, the results should be considered preliminary requiring a larger sample that includes both sexes. However, accounting the limitations of parametric analysis related to small sample size, we re-analyzed the data using the combined permutation and mixed-effect method sensitive for small sample size and replicated the main findings of parametric tests (RFT/GLM) showing significant activations in two primary areas (right AI and left IPL) at cluster level after correction for multiple comparisons (clusters with the corrected p value <0.05), validating the key findings of this study. To further minimize the risk of false positives, we reported and discussed only the clusters that were significant at p < 0.05 after corrected for multiple comparisons in both simple contrast and functional connectivity analyses.
The self and non-self contrasts are not well controlled for age-related facial features (S>M + F; S>O + Y) due to the design of the study. However, the functional relevance of right AI and left IPL activation during processing of one's own face could not be explained by differences in facial characteristics between self and non-self conditions.
Given that the pictures were provided by subjects, context-related autobiographical memory retrieval and low level visual processing were not perfectly controlled, though we took measures to control for size, luminance and background of all images. The observed differential results for self and personally familiar others suggest, however, that the impact of context-specific variations on our results was minimal.
We used the saliency and valence tasks primarily to control for explicit self-relevant evaluations between one's own face and personally familiar faces, but we did not explore the effect of each task on brain activity associated with self-relevant processing, face-task interactions and task–brain activity correlations due to small sample size. Future studies should examine the functional specificity of sub-networks that underlie various aspects of self (physical self, minimal self and narrative or autobiographical self) (Damasio, 1999; Gallagher, 2000) supported by appropriate behavioral measures as we did not include these assessments.
Despite these limitations, this is the first study examining neural activity and functional connectivity specific to processing of one's own face using a paradigm that is not cognitively demanding and adequately controlling for familiarity, saliency and valence evaluations. In summary, our findings suggest that self-face is processed as a self-relevant stimulus predominately at the sensory and perceptual level by subjective feelings and physical awareness, whereas non-self personally familiar faces are processed as self-relevant stimuli at a cognitive level. Self-processing in the facial domain may therefore be physically and functionally distinct from person-related processing. Our results showing the importance of AI and IPL neural systems in self-/non-self distinction have clinical relevance. The recent neuroscience literature have proposed novel neural models of AI and IPL dysfunctions for schizophrenia and autism (Torrey, 2007; Williams, 2008; Uddin and Menon, 2009; Wylie and Tregellas, 2010) and provide a theoretical framework for subsequent empirical work in this area.
Acknowledgments
This work was supported by a Faculty Sabbatical Fellowship grant to R. Ramasubbu, MD, from the University of Calgary. The authors thank faculty and staff of the Department of Psychiatry and Behavioral Sciences at Emory University School of Medicine for the strategic, technical and collegial support.
References
- Addis DR, McIntosh AR, Moscovitch M, Crawley AP, McAndrews MP. Characterizing spatial and temporal features of autobiographical memory retrieval networks: a partial least squares approach. NeuroImage. 2004;23:1460–1471. doi: 10.1016/j.neuroimage.2004.08.007. [DOI] [PubMed] [Google Scholar]
- Avenanti A, Bueti D, Galati G, Aglioti SM. Transcranial magnetic stimulation highlights the sensorimotor side of empathy for pain. Nature Neuroscience. 2005;8:955–960. doi: 10.1038/nn1481. [DOI] [PubMed] [Google Scholar]
- Aziz-Zadeh L, Koski L, Zaidel E, Mazziotta J, Iacoboni M. Lateralization of the human mirror neuron system. Journal of Neuroscience. 2006;26:2965–2970. doi: 10.1523/JNEUROSCI.2921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartels A, Zeki S. The neural correlates of maternal and romantic love. NeuroImage. 2004;21:1155–1166. doi: 10.1016/j.neuroimage.2003.11.003. [DOI] [PubMed] [Google Scholar]
- Beck AT, Rush AJ, Shaw B. Cognitive Therapy of Depression. New York: Guilford Press; 1979. [Google Scholar]
- Beck AT, Epstein N, Brown G. An inventory for measuring clinical anxiety psychometric properties. Journal of Consulting and Clinical Psychology. 1988;56:893–897. doi: 10.1037//0022-006x.56.6.893. [DOI] [PubMed] [Google Scholar]
- Brett M, Anton J, Valabregue R. Region of interest analysis using an SPM toolbox. NeuroImage. 2002;16:497. [Google Scholar]
- Bruce V, Young A. Understanding face recognition. British Journal of Psychology. 1986;77:305–327. doi: 10.1111/j.2044-8295.1986.tb02199.x. [DOI] [PubMed] [Google Scholar]
- Carr L, Iacoboni M, Dubeau MC, Mazziotta JC, Lenzi GL. Neural mechanism of empathy in humans: a relay from neural systems for imitation to limbic area. Proceedings of the National Academy of Sciences USA. 2003;100:5497–5502. doi: 10.1073/pnas.0935845100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavanna AE, Trimble MR. The precuneus: a review of its functional anatomy and behavioural correlates. Brain. 2006;129:564–583. doi: 10.1093/brain/awl004. [DOI] [PubMed] [Google Scholar]
- Cohen MS. Parametric analysis of fMRI data using linear systems methods. Neuroimage. 1997;6:93–103. doi: 10.1006/nimg.1997.0278. [DOI] [PubMed] [Google Scholar]
- Craig AD. How do you feel? Interoception: the sense of the psychological condition of the body. Neuroscience. 2002;3:655–666. doi: 10.1038/nrn894. [DOI] [PubMed] [Google Scholar]
- Craig AD. Human feelings: why are some more aware than others? Trends in Cognitive Neuroscience. 2004;8:239–241. doi: 10.1016/j.tics.2004.04.004. [DOI] [PubMed] [Google Scholar]
- Critchley HD, Wiens S, Robshtein P, Ohman A, Dolan RJ. Neural systems supporting interoceptive awareness. Nature Neuroscience. 2004;7:189–195. doi: 10.1038/nn1176. [DOI] [PubMed] [Google Scholar]
- Critchley HD. Neural mechanisms of autonomic, affective, and cognitive integration. Journal of Comparative Neurology. 2005;493:154–166. doi: 10.1002/cne.20749. [DOI] [PubMed] [Google Scholar]
- Damasio A. Body and Emotion in the Making of Consciousness. New York: Harcourt Brace; 1999. The Feeling of What Happens. [Google Scholar]
- Devue C, Collette F, Balteau E, Degueldre C, Luxen A, Maquet P, Brédart S. Here I am: the cortical correlates of visual self recognition. Brain Research. 2007;1143:169–182. doi: 10.1016/j.brainres.2007.01.055. [DOI] [PubMed] [Google Scholar]
- Devue C, Bredart S. The neural correlates of visual self-recognition. Consciousness and Cognition. 2011;20:40–51. doi: 10.1016/j.concog.2010.09.007. [DOI] [PubMed] [Google Scholar]
- Farrer C, Frith CD. Experiencing oneself vs. another person as being the cause of an action: the neural correlates of the experience of agency. NeuroImage. 2002;15:596–603. doi: 10.1006/nimg.2001.1009. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbons M. Biometrics Research Department. New York: New York State Psychiatric Institute; 1997. Structured Clinical Interview for DSM-IV Axis I Disorders. [Google Scholar]
- Freeman JB, Ambady N, Holcomb PJ. The face-sensitive N170 encodes social category information. NeuroReport. 2010;21:24–28. doi: 10.1097/WNR.0b013e3283320d54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston KJ, Holmes AP, Worslay KJ. Statistical parametric maps in functional imaging: a general linear approach. Human Brain Mapping. 1995;2:189–210. [Google Scholar]
- Friston KJ, Holmes AP, Poline JB, Price CJ, Frith CD. Detecting activations in PET and fMRI: levels of inference and power. NeuroImage. 1996;40:223–235. doi: 10.1006/nimg.1996.0074. [DOI] [PubMed] [Google Scholar]
- Gallagher S. Philosophical conception of the self: implication for cognitive science. Trends in Cognitive Science. 2000;4:14–21. doi: 10.1016/s1364-6613(99)01417-5. [DOI] [PubMed] [Google Scholar]
- Gallagher HL, Frith CD. Functional imaging of “theory of mind”. Trends in Cognitive Science. 2003;7:77–83. doi: 10.1016/s1364-6613(02)00025-6. [DOI] [PubMed] [Google Scholar]
- Gallup GG. Chimpanzees. Self-recognition. Science. 1970;167 86. 30. [Google Scholar]
- Gallup GG. Self awareness and the emergence of mind in primates. American Journal of Primatology. 1982;2:237–248. doi: 10.1002/ajp.1350020302. [DOI] [PubMed] [Google Scholar]
- Gilboa A, Winocur G, Grady CL, Hevenor SJ, Moscovich M. Remembering our past: functional neuroanatomy of recollection of recent and very remote personal events. Cerebral Cortex. 2004;14:1214–1225. doi: 10.1093/cercor/bhh082. [DOI] [PubMed] [Google Scholar]
- Gillihan SJ, Farah MJ. Is self special? A critical review of evidence from experimental psychology and cognitive neuroscience. Psychological Bulletin. 2005;131:76–97. doi: 10.1037/0033-2909.131.1.76. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Haxby JV. Neural systems for recognition of familiar faces. Neuropsychologia. 2007;45:32–41. doi: 10.1016/j.neuropsychologia.2006.04.015. [DOI] [PubMed] [Google Scholar]
- Gobbini MI, Liebenluft E, Santiago N, Haxby JV. Social and emotional attachment in the neural representation of faces. NeuroImage. 2004;22:1628–1635. doi: 10.1016/j.neuroimage.2004.03.049. [DOI] [PubMed] [Google Scholar]
- Gottlieb J. From thought to action: the parietal cortex as a bridge between perception, action, and cognition. Neuron. 2007;53:9–16. doi: 10.1016/j.neuron.2006.12.009. [DOI] [PubMed] [Google Scholar]
- Grimm S, Jutta E, Boesiger P, Schuepbach D, Daniel H, Boeker H, Northoff G. Increased self-focus in major depressive disorder is related to neural abnormalities in subcortical–cortical midline structures. Human Brain Mapping. 2009;30:2617–2627. doi: 10.1002/hbm.20693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heberlein KA, Hu X. Simultaneous acquisition of gradient-echo and asymmetric spin-echo for single-short Z-SAGA. Magnetic Resonance in Medicine. 2004;51:212–216. doi: 10.1002/mrm.10680. [DOI] [PubMed] [Google Scholar]
- Iacoboni M. Neural mechanisms of imitation. Current Opinions in Neurobiology. 2005;15:632–637. doi: 10.1016/j.conb.2005.10.010. [DOI] [PubMed] [Google Scholar]
- Kjaer TW, Nowak M, Lou HC. Reflective self-awareness and conscious states: PET evidence for a common midline parietofrontal core. NeuroImage. 2002;17:1080–1086. [PubMed] [Google Scholar]
- Kircher TTJ, Leube DT. Self-consciousness, self agency and schizophrenia. Consciousness and Cognition. 2003;12:656–669. doi: 10.1016/s1053-8100(03)00071-0. [DOI] [PubMed] [Google Scholar]
- Kircher T, Senior C, Phillips ML, Benson PJ, Bullmore ET, Brammer M, Simmons A, Williams SC, Bartels M, David AS. Towards a functional neuroanatomy of self-processing effects of faces and words. Cognitive Brain Research. 2000;10:133–144. doi: 10.1016/s0926-6410(00)00036-7. [DOI] [PubMed] [Google Scholar]
- Kircher T, Senior C, Phillips M, Rabe-Hesketh S, Benson PJ, Bullmore ET, Brammer M, Simmons A, Bartels M, David AS. Recognizing one's own face. Cognition. 2001;78:1–15. doi: 10.1016/s0010-0277(00)00104-9. [DOI] [PubMed] [Google Scholar]
- Legrand D, Ruby P. What is self specific? Theoretical investigation and critical review of neuroimaging results. Psychological Review. 2009;116:252–282. doi: 10.1037/a0014172. [DOI] [PubMed] [Google Scholar]
- Leibenluft E, Gobbini MI, Harrison T. Mothers' neural activation in response to pictures of their children and other children. Biological Psychiatry. 2004;56:225–232. doi: 10.1016/j.biopsych.2004.05.017. [DOI] [PubMed] [Google Scholar]
- Lou HC, Luber BM, Crupain M, Keenan JP, Nowak M, Kjaer TW. Parietal cortex and representation of the mental self. Proceedings of the National Academy of Sciences USA. 2004;101:6827–6832. doi: 10.1073/pnas.0400049101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maddock RJ, Garrett AS, Buonocore MH. Remembering familiar people: the posterior cingulated cortex and autobiographical memory retrieval. Neuroscience. 2001;104:667–676. doi: 10.1016/s0306-4522(01)00108-7. [DOI] [PubMed] [Google Scholar]
- Meriaux S, Roche A, Dehaene-Lambertz G, Thirion B, Poline JB. Combined permutation test and mixed-effect model for group average analysis in fMRI. Human Brain Mapping. 2006;27:402–410. doi: 10.1002/hbm.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moran JM, Macrae CN, Heathertone TF, Wyland CL, Kelly WM. Journal of Cognitive Neuroscience. 2006;18:1586–1594. doi: 10.1162/jocn.2006.18.9.1586. [DOI] [PubMed] [Google Scholar]
- Morin A. Levels of consciousness and self awareness: a comparison and integration of various neurocognitive views. Consciousness and Cognition. 2006;15:358–371. doi: 10.1016/j.concog.2005.09.006. [DOI] [PubMed] [Google Scholar]
- Morita T, Itakura S, Saito DN, Nakashita S, Harada T, Kochiyama T, Sadato N. The role of the right prefrontal cortex in self evaluation of the face: a functional magnetic resonance imaging study. Journal of Cognitive Neuroscience. 2008;20:342–355. doi: 10.1162/jocn.2008.20024. [DOI] [PubMed] [Google Scholar]
- Mitchell RW. Kinesthetic-visual matching and the self concept as explanations of mirror-self recognition. Journal for the Theory of Social Behaviour. 1997;27:18–39. [Google Scholar]
- Northoff G, Heinzel A, de Greck M, Bermpohl F, Dobrowolny H, Panksepp J. Self-referential processing in our brain — a meta-analysis of imaging studies on the self. NeuroImage. 2006;31:440–457. doi: 10.1016/j.neuroimage.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Northoff G, Schneider F, Rotte M, Matthiae C, Tempelmann C, Wiebking C, Bermpohl F, Heinzel A, Danos P, Heinze H, Bogerts B, Walter M, Panksepp J. Differential parametric modulation of self relatedness and emotions in different brain regions. Human Brain Mapping. 2009;30:369–382. doi: 10.1002/hbm.20510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Northoff G, Qin P, Feinberg TE. Brain imaging of the self— conceptual, anatomical and methodological issues. Consciousness and Cognition. 2011;20:52–63. doi: 10.1016/j.concog.2010.09.011. [DOI] [PubMed] [Google Scholar]
- Northoff G, Hayes DJ. Is our self nothing but reward? Biological Psychiatry. 2011;69:1019–1025. doi: 10.1016/j.biopsych.2010.12.014. [DOI] [PubMed] [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness. The Edinburgh Inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Panksepp J. At the interface of the affective, behavioral and cognitive neurosciences decoding the emotional feelings of the brain. Brain and Cognition. 2003;52:4–14. doi: 10.1016/s0278-2626(03)00003-4. [DOI] [PubMed] [Google Scholar]
- Parker G, Tupling H, Brown LB. A Parental Bonding Instrument. British Journal of Medical Psychology. 1979;52:1–10. [Google Scholar]
- Platek SM, Keenan JP, Gallup GC, Jr, Mohamed FB. Where am I? The neurological correlates of self and other. Cognitive Brain Research. 2004;19:114–122. doi: 10.1016/j.cogbrainres.2003.11.014. [DOI] [PubMed] [Google Scholar]
- Platek SM, Loughead JW, Gur RC, Busch S, Ruparel K, Phend N, Panyavin IS, Langleben DD. Neural substrates for functionally discriminating self-face from personally familiar faces. Human Brain Mapping. 2006;27:91–98. doi: 10.1002/hbm.20168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platek SM, Wathne K, Tierney NG, Thomson JW. Neural correlates of self-face recognition: an effect-location meta-analysis. Brain Research. 2008;1232:173–184. doi: 10.1016/j.brainres.2008.07.010. [DOI] [PubMed] [Google Scholar]
- Platek SM, Kemp SM. Is family special to the brain? An event-related fMRI study of familiar, familial, and self face recognition. Neuropsychologia. 2009;47:849–858. doi: 10.1016/j.neuropsychologia.2008.12.027. [DOI] [PubMed] [Google Scholar]
- Pruessner JC, Champagne F, Meaney MJ. Dopamine release in response to a psychological stress in humans and its relationship to early life maternal care: a positron emission tomography study using raclopride. Neuroscience. 2004;24:2825–2831. doi: 10.1523/JNEUROSCI.3422-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramasubbu R, Masalovich S, Peltier S, Holtzheimer PE, Heim C, Mayberg HS. Neural representation of maternal face processing: a functional magnetic resonance imaging study. Canadian Journal of Psychiatry. 2007;52:726–734. doi: 10.1177/070674370705201107. [DOI] [PubMed] [Google Scholar]
- Rankin KP, Baldwin E, Pace-Savitsky C, Kramer JH, Miller BL. Self awareness and personality change in dementia. Journal of Neurology, Neurosurgery and Psychiatry. 2005;76:632–639. doi: 10.1136/jnnp.2004.042879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rissman J, Gazzaley A, D'Esposito M. Measuring functional connectivity during distinct stages of a cognitive task. Neuroimage. 2004;23:752–763. doi: 10.1016/j.neuroimage.2004.06.035. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Craighers L. The mirror neuron system. Annual Review of Neurosciences. 2004;27:169–192. doi: 10.1146/annurev.neuro.27.070203.144230. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. Society and the Adolescent Self-image. Princeton, NJ: Princeton University Press; 1965. [Google Scholar]
- Rosenbaum RS, Kohler S, Schacter DL, Moscovitch M, Westmacott R, Black SE, Gao F, Tulving E. The case of K.C.: contributions of a memory-impaired person to memory theory. Neuropsychologia. 2005;43:989–1021. doi: 10.1016/j.neuropsychologia.2004.10.007. [DOI] [PubMed] [Google Scholar]
- Ruby P, Decety J. Effects of subjectivity perspective taking during simulation of action. A PET investigation of agency. Nature Neuroscience. 2001;4:546–550. doi: 10.1038/87510. [DOI] [PubMed] [Google Scholar]
- Schneider F, Bermpohl F, Heinzel M, et al. The resting brain and our self: self-relatedness modulates resting state neural activity in cortical midline structures. Neuroscience. 2008;157:120–131. doi: 10.1016/j.neuroscience.2008.08.014. [DOI] [PubMed] [Google Scholar]
- Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H, Reiss AL, Greicius MD. Dissociable intrinsic connectivity networks for salience processing and executive control. Journal of Neuroscience. 2007;27:2349–2356. doi: 10.1523/JNEUROSCI.5587-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah NJ, Marshall JC, Zafiris O, Schwab A, Zilles K, Markowitsch HJ, Fink GR. The neural correlates of person familiarity. A functional magnetic resonance imaging study with clinical implications. Brain. 2001;124:804–815. doi: 10.1093/brain/124.4.804. [DOI] [PubMed] [Google Scholar]
- Spence SA, Brooks DJ, Hirsch SR, Liddle PF, Meehan J, Grasby PM. A PET study of voluntary movement in schizophrenic patients experiencing passivity phenomena (delusion of alien control) Brain. 1997;120:1997–2011. doi: 10.1093/brain/120.11.1997. [DOI] [PubMed] [Google Scholar]
- Spreng RN, Mar RA, Kim ASN. The common neural basis of autobiographical memory, prospection, navigation, theory of mind and default mode: a quantitative meta-analysis. Journal of Cognitive Neuroscience. 2009;21:489–510. doi: 10.1162/jocn.2008.21029. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Kawashima R, Nakamura K, Okada K, Kato T, Nakamura A, Hatano K, Itoh K, Kojima S, Fukuda H. Passive and active recognition of one's own face. NeuroImage. 2000;11:36–48. doi: 10.1006/nimg.1999.0519. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Watanabe J, Maeda Y, Fukuda H, Kawashima R. Cortical mechanisms of visual self-recognition. NeuroImage. 2005;24:143–149. doi: 10.1016/j.neuroimage.2004.07.063. [DOI] [PubMed] [Google Scholar]
- Sugiura M, Sassa Y, Jeong B, Miura N, Akitsuki Y, Horie K, Sato S, Kawashima R. Multiple brain networks for visual self recognition with different sensitivity for motion and body part. NeuroImage. 2006;32:1905–1917. doi: 10.1016/j.neuroimage.2006.05.026. [DOI] [PubMed] [Google Scholar]
- Taylor MJ, Arsalidou M, Bayless SJ, Morris D, Evans JW, Barbeau EJ. Neural correlates of personally familiar faces: parents, partner and own faces. Human Brain Mapping. 2009;30:2008–2020. doi: 10.1002/hbm.20646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Torrey EF. Schizophrenia and the inferior parietal lobule. Schizophrenia Research. 2007;97:215–225. doi: 10.1016/j.schres.2007.08.023. [DOI] [PubMed] [Google Scholar]
- Tzourio-Mazoyer N, Landeau B, Papathanassiou D. Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single subject brain. NeuroImage. 2002;15:273–289. doi: 10.1006/nimg.2001.0978. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Kaplan JT, Molnar-Szakaccs I, Zaidel E, Iacoboni M. Self-face recognition activates a frontoparietal “mirror” network in the right hemisphere: an event-related fMRI study. NeuroImage. 2005;25:926–935. doi: 10.1016/j.neuroimage.2004.12.018. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Iacoboni M, Lange C, Keenan JP. The self and social cognition: the role of cortical midline structures and mirror neurons. Trends in Cognitive Science. 2007;11:153–157. doi: 10.1016/j.tics.2007.01.001. [DOI] [PubMed] [Google Scholar]
- Uddin LQ, Menon V. The anterior insula and autism: under-connected and under-examined. Neuroscience and Behavioral Reviews. 2009;33:1198–1203. doi: 10.1016/j.neubiorev.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams JHG. Self–others relations in social development and autism: multiple roles for mirror neurons and other brain bases. Autism Research. 2008;1:73–90. doi: 10.1002/aur.15. [DOI] [PubMed] [Google Scholar]
- Wylie KP, Tregellas JR. The role of the insula in schizophrenia. Schizophrenia Research. 2010;123:93–104. doi: 10.1016/j.schres.2010.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]