Abstract
Background
Nesfatin-1, a member of the adipokine family, has been detected in synovial fluid (SF) from OA patients. This study aimed to determine whether there is a marked correlation of nesfatin-1 levels in serum and SF of knee OA patients with the disease severity of OA.
Material/Methods
This cross-sectional research enrolled 202 knee OA subjects. The Kellgren-Lawrence grading system was utilized to evaluate the severity of knee OA.
Results
Elevated nesfatin-1 concentrations in serum were found in knee OA patients compared with the controls. Nesfatin-1 concentrations were markedly elevated with increased KL grades. Serum and SF nesfatin-1 concentrations were both significantly associated with the disease severity evaluated by KL grading criteria.
Conclusions
Our investigation indicates a marked association of serum and SF nesfatin-1 concentrations with OA disease severity.
MeSH Keywords: Adipokines, Osteoarthritis, Serum, Severity of Illness Index, Synovial Fluid
Background
Osteoarthritis (OA), one of the most leading causes of disability, is characterized by slow progressive degeneration of articular cartilage, subchondral bone alteration, and variable secondary synovial inflammation [1]. The etiology and pathogenesis of OA remain poorly understood. Various investigations have indicated that intraarticular low-grade inflammation contributes to the occurrence and progression of OA [2]. Recent studies have demonstrated that chondrocytes can produce and/or respond to a number of inflammatory cytokines and chemokines in joint tissues and fluids in individuals with OA [3].
Various radiographic scoring systems, such as OARSI atlas (OARSI), Kellgren-Lawrence (KL) grading scale (KL), and Verbruggen-Veys anatomical phase score (VV), are utilized to evaluate the severity and progression of OA [4]. The specific system for staging OA is controversial. Among those methods, the KL grading system is recommended by the World Health Organization due to its reliability and sensitivity [5]. KL showed higher reliability and better sensitivity, and detected a slightly higher proportion of progression over other grading methods [4]. The KL scale was reported to be a valid and reliable radiographic grading system for assessment of ankle OA and correlates with clinical symptoms [5].
Nesfatin-1, an 82-amino acid peptide, is highly expressed in several regions of the hypothalamus and regulates food intake [6]. In rats, intracerebroventricular nesfatin-1 injection decreases appetite, whereas antibodies against nesfatin-1 injection induces food intake [7]. Nesfatin-1 could promote the release of pro-inflammatory mediators, such as interleukin-8 (IL-8), IL-6, and macrophage inflammatory protein-1α (MIP-1α) in chondrocytes from OA patients [8]. Based on the anti-inflammatory function of nesfatin-1, we hypothesized that nesfatin-1 concentration may be correlated with OA development and progression.
The relationship between nesfatin-1 and the disease severity of knee OA has not been focused on; therefore, we aimed to determine whether there is an association of nesfatin-1 concentrations with the radiological severity of knee OA.
Material and Methods
Patients
A cross-sectional study was performed, including 202 subjects diagnosed with knee OA according to the criteria of the American College of Rheumatology. Exclusion criteria included the following: acute or chronic inflammatory knee disease or rheumatoid arthritis, cancer, systemic or autoimmune diseases, and other chronic diseases, or taking corticosteroids. The control group comprised 118 healthy subjects with a similar age and sex distribution as the patients. The controls all had normal knee radiographs and no previous history of any arthritis. The study protocol was approved by the Human Ethics Review Committee of our hospital and a signed consent form was obtained from each subject.
The disease severity was assessed according to the KL classification. The grading of radiographs was performed by two experienced investigators who were blinded to the source and clinical data of those subjects. The higher grade of the two knees was used for analysis. OA diagnosis was established when KL grade was ≥2 in at least one knee. Controls were defined as having no radiographic knee OA, confirmed by KL grades of 0 for both knees.
Laboratory methods
Venous blood samples were drawn from all subjects. Synovial fluid was taken from the most affected knee during hyaluronic acid medication. The blood and SF specimen was then centrifuged and stored at −80°C until examination. A commercially available enzyme-linked immunosorbent assay (Phoenix Pharmaceuticals, Inc., USA) was utilized to examine serum and SF nesfatin-1 concentrations.
Statistical analysis
The results are expressed as means ±SD or median (interquartile range), as appropriate. The Kolmogorov-Smirnov test was used to assess normal distribution. The variables among OA patients and controls were compared using chi-square tests, unpaired t-test, or Mann-Whitney U test, respectively. Kruskal-Wallis test was utilized to compare the difference in nesfatin-1 between knee patients with different KL grades. The correlation of nesfatin-1 with disease severity was assessed by Spearman correlation analysis and multinomial logistic regression analysis. P-values <0.05 were set for the level of statistical significance.
Results
Clinical parameters of OA patients and healthy controls
There were no marked differences in age and sex between these two groups (Table 1).
Table 1.
The characteristics between patients with knee OA and healthy controls.
| Characteristics | knee OA patients (n=202) | Healthy controls (n=118) | P value |
|---|---|---|---|
| Age (years) | 61.01±10.61 | 61.32±7.39 | 0.782 |
| Gender (male/female) | 84/118 | 47/71 | 0.758 |
| Nesfatin-1 in serum (ng/mL) | 1.18 (0.94–1.36) | 0.93 (0.72–1.12) | <0.001 |
| Nesfatin-1 in SF (ng/mL) | 0.39 (0.34–0.47) |
Serum nesfatin-1 concentrations
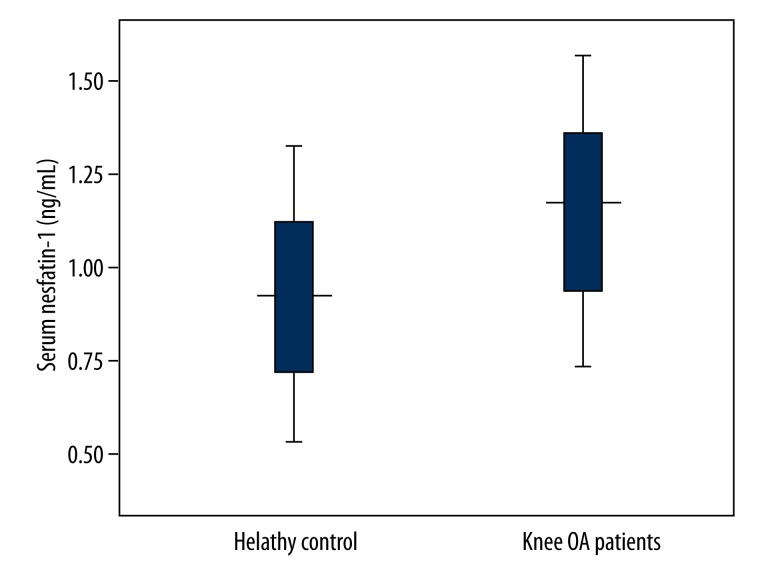
There were relatively higher serum nesfatin-1 concentrations in knee OA subjects than those in the controls (P<0.001) (Table 1, Figure 1).
Figure 1.
Serum nesfatin-1 concentrations in knee OA patients and healthy controls. Knee OA subjects showed relatively higher serum nesfatin-1 concentrations compared with healthy controls (P<0.001).
Nesfatin-1 correlated with KL grades
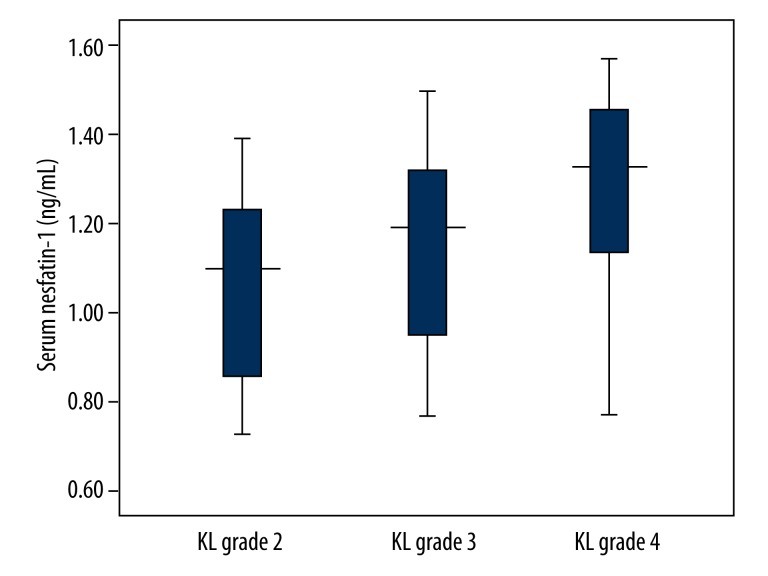
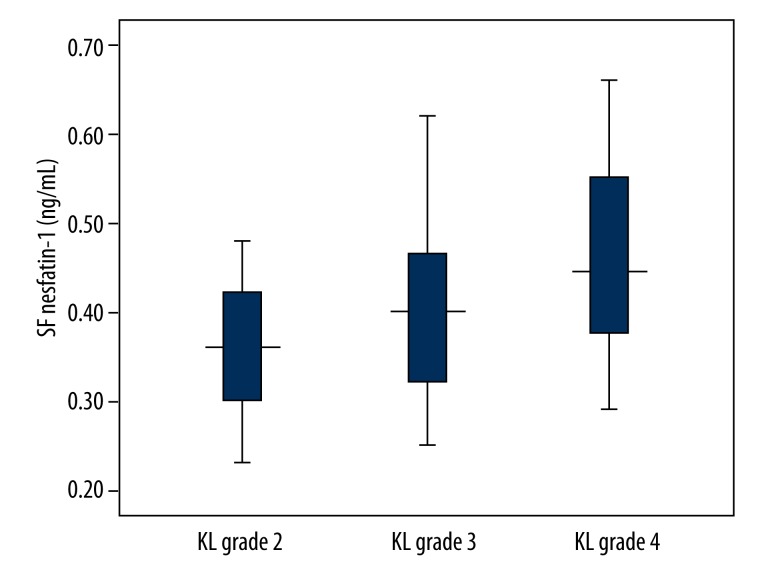
Serum and SF concentrations of nesfatin-1 were elevated with increased KL grades (Table 2, Figures 2 and 3).
Table 2.
Serum and SF levels of nesfatin-1 in knee OA patients with different KL grades.
| Nesfatin-1 (ng/mL) | Grade 2 (n=59) | Grade 3 (n=87) | Grade 4 (n=56) | P value |
|---|---|---|---|---|
| Serum | 1.10 (0.85–1.24)** | 1.19 (0.95–1.33)* | 1.33 (1.13–1.46)*,** | <0.001 |
| SF | 0.36 (0.30–0.42)** | 0.40 (0.32–0.47)* | 0.45 (0.37–0.55)*,** | <0.001 |
P<0.01 vs. KL grade 2;
P<0.01 vs. KL grade 3.
Figure 2.
Serum nesfatin-1 concentrations in knee OA patients with different KL grades. Serum nesfatin-1 concentrations were elevated with increased KL grades.
Figure 3.
SF nesfatin-1 concentrations in knee OA patients with different KL grades. SF nesfatin-1 concentrations were elevated with increased KL grades.
Correlation of KL grades with other variables
We found a significant association of both serum and SF nesfatin-1 concentrations with KL grades (r=0.343, P<0.001 and r=0.419, P<0.001). Multinomial logistic regression analysis also showed a positive association of serum and SF nesfatin-1 concentrations with KL grades (P<0.001 and P<0.001).
Discussion
Adipose tissue produces many metabolically important factors, not only cytokines such as IL-1 and tumor necrosis factor-α (TNF-α), but also adipokines such as leptin, adiponectin, resistin, and visfatin [9]. Recently, various adipokines secreted from adipose tissues were also demonstrated to play important roles in the pathogenesis of cartilage destruction and OA [10]. These cytokines and adipokines are both demonstrated to be involved in the mechanism of pathogenesis of OA. Nesfatin-1, a newly discovered adipokine, is associated with obesity and metabolic syndrome [11]. Hence, nesfatin-1 concentrations are speculated to be correlated with OA development. We performed this study and demonstrated that serum and SF nesfatin-1 concentrations were associated with knee OA development. This is supported by Jiang et al’s investigation which suggested increased serum and chondrocyte levels of nesfatin-1 in OA patients [12]. In addition to the above finding, we also demonstrated the correlation of serum and SF nesfatin-1 concentrations with OA disease severity.
Traditionally, osteoarthritis is seen as a non-inflammatory disease. However, recent studies have found that inflammatory synovitis is a common process involved in chondrocyte destruction and subsequent knee OA development [13]. It is reported that 60% of joints from osteoarthritis patients showed a clear impression of synovial inflammation during joint replacement [14]. Osteocartilaginous components could promote chondrocyte inflammatory response and consequent release of pro-inflammatory cytokines and proteases [15]. Significantly elevated production of proinflammatory cytokines were found in cartilage, synovial membrane, and subchondral bone in OA patients, which points to a potential link of inflammation with the development and progression of joint-degrading changes in OA patients [16].
The exact mechanism or role of nesfatin-1 in OA development and progression is unknown. Nesfatin-1 gene expressions were found in different tissues, including cartilage, osteophytes, synovium, meniscus, and fat pad samples of OA subjects. We speculated it is partly related to the inflammatory role of nesfatin-1 [12]. This is supported by several studies. In murine chondrocytes, nesfatin-1 expression was elevated during the differentiation of chondrocytes [8]. Nesfatin-1 promoted IL-6 and MIP-1α secretion in both human and murine chondrocytes [8]. Lipopolysaccharides (LPS), mediators inducing peripheral inflammatory response, could increase the proportion of nesfatin-1 neurons in rat brain [17]. Furthermore, a marked correlation was found between synovial nesfatin-1 and IL-18, and CRP [12]. Leivo-Korpela et al. demonstrated that nesfatin-1 correlated positively with IL-6, TNF-α, and IL-8 [18]. Those proinflammatory cytokines mentioned above are all part in the pathogenesis of knee OA. It seems that nesfatin-1 is correlated with occurrence and progression of OA through inducing proinflammatory cytokines production.
The limitations of the present study should be considered. First, a cross-sectional design was used in this study. Further prospective investigation could provide more supportive data. Second, nesfatin-1 concentrations in SF from healthy controls were not investigated in the current study.
Conclusions
In short, nesfatin-1 concentrations in serum and SF were closely correlated with disease occurrence and severity of knee OA. Further basic research is needed to investigate the exact role in the mechanism of OA.
Footnotes
Source of support: Departmental sources
References
- 1.Pascale V, Pascale W, Lavanga V, et al. L-arginine, asymmetric dimethylarginine, and symmetric dimethylarginine in plasma and synovial fluid of patients with knee osteoarthritis. Med Sci Monit. 2013;19:1057–62. doi: 10.12659/MSM.889275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Bonnet CS, Walsh DA. Osteoarthritis, angiogenesis and inflammation. Rheumatology (Oxford) 2005;44:7–16. doi: 10.1093/rheumatology/keh344. [DOI] [PubMed] [Google Scholar]
- 3.Goldring MB, Otero M, Tsuchimochi K, et al. Defining the roles of inflammatory and anabolic cytokines in cartilage metabolism. Ann Rheum Dis. 2008;67:iii75–82. doi: 10.1136/ard.2008.098764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bijsterbosch J, Haugen IK, Malines C, et al. Reliability, sensitivity to change and feasibility of three radiographic scoring methods for hand osteoarthritis. Ann Rheum Dis. 2011;70(8):1465–67. doi: 10.1136/ard.2010.143479. [DOI] [PubMed] [Google Scholar]
- 5.Holzer N, Salvo D, Marijnissen AC, et al. Radiographic evaluation of posttraumatic osteoarthritis of the ankle: the Kellgren-Lawrence scale is reliable and correlates with clinical symptoms. Osteoarthritis Cartilage. 2014 doi: 10.1016/j.joca.2014.11.010. pii: S1063-4584(14)01332-6. [DOI] [PubMed] [Google Scholar]
- 6.Oh-I S, Shimizu H, Satoh T, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 7.Shimizu H, Oh-I S, Hashimoto K, et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–71. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 8.Scotece M, Conde J, Abella V, et al. NUCB2/nesfatin-1: a new adipokine expressed in human and murine chondrocytes with pro-inflammatory properties, an in vitro study. J Orthop Res. 2014;32:653–60. doi: 10.1002/jor.22585. [DOI] [PubMed] [Google Scholar]
- 9.Kershaw EE, Flier JS. Adipose tissue as an endocrine organ. J Clin Endocrinol Metab. 2004;89:2548–56. doi: 10.1210/jc.2004-0395. [DOI] [PubMed] [Google Scholar]
- 10.Hu PF, Bao JP, Wu LD. The emerging role of adipokines in osteoarthritis: a narrative review. Mol Biol Rep. 2011;38:873–78. doi: 10.1007/s11033-010-0179-y. [DOI] [PubMed] [Google Scholar]
- 11.Özsavcí D, Erşahin M, Şener A, et al. The novel function of nesfatin-1 as an anti-inflammatory and antiapoptotic peptide in subarachnoid hemorrhage-induced oxidative brain damage in rats. Neurosurgery. 2011;68:1699–708. doi: 10.1227/NEU.0b013e318210f258. discussion 1708. [DOI] [PubMed] [Google Scholar]
- 12.Jiang L, Bao J, Zhou X, et al. Increased serum levels and chondrocyte expression of nesfatin-1 in patients with osteoarthritis and its relation with BMI, hsCRP, and IL-18. Mediators Inflamm. 2013;2013:631251. doi: 10.1155/2013/631251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Felson DT. Clinical practice. Osteoarthritis of the knee. N Engl J Med. 2006;354:841–48. doi: 10.1056/NEJMcp051726. [DOI] [PubMed] [Google Scholar]
- 14.Cooke TD. Immune pathology in polyarticular osteoarthritis. Clin Orthop Relat Res. 1986;213:41–49. [PubMed] [Google Scholar]
- 15.Pelletier JP, Martel-Pelletier J, Abramson SB. Osteoarthritis, an inflammatory disease: potential implication for the selection of new therapeutic targets. Arthritis Rheum. 2001;44:1237–47. doi: 10.1002/1529-0131(200106)44:6<1237::AID-ART214>3.0.CO;2-F. [DOI] [PubMed] [Google Scholar]
- 16.Kapoor M, Martel-Pelletier J, Lajeunesse D, et al. Role of proinflammatory cytokines in the pathophysiology of osteoarthritis. Nat Rev Rheumatol. 2011;7:33–42. doi: 10.1038/nrrheum.2010.196. [DOI] [PubMed] [Google Scholar]
- 17.Bonnet MS, Pecchi E, Trouslard J, et al. Central nesfatin-1-expressing neurons are sensitive to peripheral inflammatory stimulus. J Neuroinflammation. 2009;6:27. doi: 10.1186/1742-2094-6-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Leivo-Korpela S, Lehtimäki L, Hämälainen M, et al. Adipokines NUCB2/nesfatin-1 and visfatin as novel inflammatory factors in chronic obstructive pulmonary disease. Mediators Inflamm. 2014;2014:232167. doi: 10.1155/2014/232167. [DOI] [PMC free article] [PubMed] [Google Scholar]