Abstract
Data from cross-sectional and longitudinal studies have illustrated a relationship between short sleep duration (SSD) and weight gain. Individuals with SSD are heavier and gain more weight over time than normal-duration sleepers. This sleep-obesity relationship may have consequences for obesity treatments, as it appears that short sleepers have reduced ability to lose weight. Laboratory-based clinical studies found that experimental sleep restriction affects energy expenditure and intake, possibly providing a mechanistic explanation for the weight gain observed in chronic short sleepers. Specifically, compared to normal sleep duration, sleep restriction increases food intake beyond the energetic costs of increased time spent awake. Reasons for this increased energy intake after sleep restriction are unclear but may include disrupted appetite-regulating hormones, altered brain mechanisms involved in the hedonic aspects of appetite, and/or changes in sleep quality and architecture. Obstructive sleep apnea (OSA) is a disorder at the intersection of sleep and obesity, and the characteristics of the disorder illustrate many of the effects of sleep disturbances on body weight and vice versa. Specifically, while obesity is among the main risk factors for OSA, the disorder itself and its associated disturbances in sleep quality and architecture seem to alter energy balance parameters and may induce further weight gain. Several intervention trials have shown that weight loss is associated with reduced OSA severity. Thus, weight loss may improve sleep, and these improvements may promote further weight loss. Future studies should establish whether increasing sleep duration/improving sleep quality can induce weight loss.
Keywords: body weight, energy balance, sleep
Introduction
Sleep has traditionally been overlooked in the search for modifiable risk factors for obesity. However, since the mid-2000s, there has been increasing evidence relating short sleep duration (SSD) to obesity. In fact, many cross-sectional studies in both adults and children have reported that the risk of obesity is greater in short sleepers (generally those reporting sleeping <7 h/night) than normal sleepers (those reporting sleeping 7–8 h/night) and that there is a greater prevalence of obesity among short than normal sleepers. This has been the topic of several meta-analyses and systematic reviews [1–4] and will not be elaborated on in this report.
Providing further clues that SSD could be a trigger for weight gain and the development of obesity, longitudinal studies have also shown that SSD is associated with greater weight gain than normal sleep. Data from the Zurich Cohort Study showed that sleep duration was a strong predictor of obesity in longitudinal models and there was a trend for the change in sleep duration over time to be negatively associated with the change in body mass index (BMI) [5]. The Nurses’ Health Study also revealed that women who reported sleeping ≤5 h/night had the greatest weight gain over the 16-year follow-up period, followed by those reporting sleeping 6 and 9 h/night. Women who reported sleeping 7 and 8 h/night had the lowest weight gain [6]. In that cohort, short sleepers had an increased risk of developing obesity and having a large weight gain (≥15 kg) compared to normal sleepers. Similar results were obtained in a cohort of older adults followed over 2 years: women who reported sleeping ≤5 h/night had higher odds of gaining ≥5 kg than normal sleepers [7]. Interestingly, this association was not observed in men. A similar increased risk of a 5-kg weight gain was also observed over a 6-year period in short sleepers in the Quebec Family Study, relative to normal sleepers [8]. Conversely, Watanabe et al. [9] reported that SSD increased the odds of developing obesity over a 1-year follow-up period in men but not women, in a large Japanese cohort of working adults.
It is interesting to note that two longitudinal studies that have failed to find a relationship between sleep duration and obesity risk had measured sleep duration with actigraphy [10, 11]. These studies illustrate the inherent flaws associated with self-report of sleep duration. Moreover, longitudinal studies, although providing a better insight into the relationship between sleep and obesity than cross-sectional studies, still do not show causality. This report will focus on intervention studies illustrating the impact of sleep duration and quality on obesity risk. The primary aim will be to describe potential mechanisms by which sleep could play a causal role in the etiology of obesity and studies that have intervened to induce weight loss.
Impact of sleep on weight loss
Given the epidemiological findings of a strong cross-sectional relationship between SSD and obesity, as well as longitudinal associations between SSD and weight gain, it is expected that short sleepers would also have reduced ability to lose weight when embarking on a weight-loss program or that normal sleepers would gain weight if asked to restrict their sleep. Surprisingly, very few studies to date have examined these questions. What’s more, most studies that have assessed the role of sleep duration on weight-loss success or weight gain have not been designed to test whether short sleepers lose less weight than normal sleepers or are less likely to achieve 5% or 10% weight loss over the course of a structured weight-loss program.
A small pilot study by Nedeltcheva et al. [12] was the first published to specifically test the hypothesis that recurrent SSD could impede weight loss during a reduced-calorie diet. Ten overweight and obese men and women were fed at 90% of their resting metabolic rate (RMR) for two 14-day periods, with either 8.5 h time in bed (TIB; 7 h 25 min actual sleep) or 5.5 h TIB (5 h 14 min actual sleep). As food intake was controlled, participants lost a similar amount of weight in both periods. However, the composition of the lost weight differed: 25% of lost weight was fat mass during restricted sleep compared to 55% in the normal sleep condition. Participants also had higher fasting and postprandial respiratory quotient and lower RMR at the end of the sleep restriction weight-loss period compared to normal sleep. The authors concluded that sleep restriction compromised the efficacy of energy-restriction practices for weight loss.
Robertson et al. [13] also performed a short-term intervention study to assess the effects of a 1.5-h sleep restriction on insulin sensitivity and metabolic parameters. Young, normal-weight men either maintained their habitual sleep schedules for 3 weeks or were asked to set their alarm clocks 1.5 h earlier. The authors found a significant week by sleep duration interaction, such that in men who restricted their sleep, body weight was reduced after the first week but returned to baseline during the last week of the intervention. Those who maintained their habitual sleep routine did not have any change in body weight. It is unknown whether the rise back to baseline in the sleep restricted group would have continued to lead to overall weight gain if the study had been prolonged. Nevertheless, this study brings forth interesting questions concerning the time course of metabolic changes during extended mild sleep restriction. However, this was a very small, parallel-arm study with nine and ten participants per group, increasing the chance of bias.
Other studies have examined charts from participants of various weight-loss studies to assess whether sleep duration at baseline plays a role on the achievement of weight loss. Data from three weight-loss studies conducted at Laval University in Quebec City showed that sleep duration was positively associated with loss of body fat, after adjusting for age, sex, baseline BMI, length of the intervention, and change in energy intakes [14]. In that study, sleep quality was inversely associated with loss of body fat, such that those with poor sleep quality had lower body fat loss. Thomson et al. [15] also found that both sleep quality and duration were independent markers of weight-loss success in participants followed over 2 years in a commercial weight-loss program. Significant weight loss was defined as achieving 10% weight loss at 6 months; weight maintenance success was assessed at 12, 18, and 24 months. The Pittsburgh Sleep Quality Index at baseline was used to determine its association with weight-loss success, and at 6 months, to determine whether it predicted weight maintenance. Women who reported SSD and those with fair/poor sleep quality were less likely to achieve significant weight loss. At 6 months, women who reported very good sleep quality or duration >7 h/night had an increased likelihood of successfully maintaining weight loss at 12 and 18 months; those associations were attenuated and no longer significant at 24 months. In addition, poor habitual sleep efficiency (≤85%) was associated with a 38% lower likelihood of successful weight-loss maintenance at 18 months. These results suggest that weight-loss success is more likely if women enter a weight-loss program with good sleep quality and adequate sleep duration and that they have a better chance of maintaining that weight loss than if they have poor sleep quality and SSD.
Data from the LIFE Study, a two-phase trial examining two alternative strategies for weight loss, also showed greater weight-loss success with adequate sleep duration [16]. In phase 1, participants underwent a weight-loss diet based on the Dietary Approaches to Stop Hypertension. Participants who lost at least 4.5 kg in phase 1 moved on to phase 2, which evaluated weight maintenance strategies over 18 months. Participants who reported sleeping 6–8 h/night at baseline had higher rates of eligibility for phase 2 than those reporting sleeping ≤6 h or >8 h/night. The authors proposed that assessing sleep duration at the onset of a weight-loss program could identify individuals who may require additional counseling and resources for weight-loss success. This, however, would need to be tested.
Only one study of this kind has been conducted using data from children. Sallinen et al. [17] performed a retrospective chart review of obese adolescents who participated in a clinical, family-based, weight-loss program to assess whether self-reported sleep duration at entry predicted weight loss at 3 months. More baseline sleep was predictive of greater BMI reductions: a 30-min increase in baseline sleep duration predicted an additional reduction of 0.2 kg/m2 in BMI. Adolescents who had a loss of at least one BMI unit also reported significantly more baseline sleep than those who did not achieve this BMI reduction.
Mechanistic insights: impact of sleep on energy balance
Sleep duration
If SSD plays a causal role in the development of obesity, then it must affect regulators of energy balance. Spiegel and colleagues [18] were among the first to report a SSD-associated decrease in leptin, an adipose tissue-derived hormone signaling satiety and rise in ghrelin, a gut-derived hormone signaling hunger. While this finding was promising on a mechanistic level, subsequent studies have yielded conflicting results, with some reporting no change [19–21] and or an increase [22–25] in leptin after partial sleep restriction. Ghrelin findings are also inconsistent [19–21]. The reasons for the inconsistency in SSD-induced alterations in leptin and ghrelin are unclear but are possibly related to differences in feeding protocol and energy balance state between experiments, both of which are factors that can influence appetite hormone secretion [26]. The timing of the sleep-wake schedule and alterations in sleep architecture, and a role of sex on modulating the effects of sleep curtailment on hormone secretion, may also play a role in the observed discrepancies. Recently, increased leptin and decreased ghrelin in restricted vs. habitual sleep conditions were observed in a study in which participants had ad libitum access to food. Somewhat paradoxically, this observed hormone profile was associated with increased food intake under SSD vs. habitual sleep [27].
While the ad libitum access to food and increased food intake during SSD are likely to have influenced the above observation, the results also importantly suggest that brain mechanisms involved in the reward-driven or hedonic aspects of appetite, as opposed to homeostatic or peripheral hormonal controls of hunger, may influence food intake under restricted sleep conditions. Indeed, using functional magnetic resonance imaging (fMRI), recent work from our laboratory has implicated brain regions involved in motivation, reward, and cognitive processing in the response to experimental sleep restriction [28, 29]. Specifically, relative increases in activity within the orbitofrontal cortex, putamen, nucleus accumbens, thalamus, and insula in response to food stimuli were seen after 5 nights of restricted sleep (4 h TIB/night) compared to habitual sleep (9 h TIB/night) [28]. Moreover, the same partial sleep curtailment manipulation resulted in enhanced activation in the insular cortex, orbitofrontal cortex, and dorsolateral prefrontal cortex in response to unhealthy compared to healthy food stimuli [29].
Regardless of the mechanism, experimental sleep curtailment is shown to induce significant increases in food intake [19, 27, 30–32]. Interestingly, the increased total energy intake after sleep restriction is often characterized by a disproportionate increase in food consumption late in the day [19, 27, 31]. Research should focus not only on the overall increase in energy intake under short sleep but also on the distribution of caloric consumption across the day and the reasons underlying this delay in food intake, as nighttime eating in particular may be associated with increased weight gain [33].
Increased energy intake in response to sleep curtailment beyond the total daily energy that is expended by the combination of metabolic processes and physical activity is a possible means by which SSD can lead to weight gain. It is therefore important to consider how sleep restriction affects energy expenditure (EE). Initial studies [19, 32] reported no effect of sleep duration on total EE when assessed with doubly-labeled water. More recently, whole-room indirect calorimetry has been used to more accurately measure 24-h EE in response to experimental sleep restriction [27, 34, 35]. These studies consistently demonstrated an increase in 24-h EE of ~4–5% after short vs. habitual sleep, which corresponds to an increase of ~90–110 kcal/day. In looking at the distribution of EE across the 24-h day, it is apparent that these increases are mainly the result of the additional hours spent awake during the night [27, 34, 35], suggesting that the increased risk of obesity observed in short sleepers in many epidemiological studies [4] may be caused by a substantially large increase in energy intake that is not offset by the more modest increases in EE associated with reduced sleep duration.
Sleep quality and architecture
In addition to sleep duration, characteristics of sleep, like quality and architecture, can impact energy balance. Rapid eye movement (REM) sleep was found to be negatively associated with overweight in children and adolescents [36]. Slow wave sleep (SWS) was also found to be inversely related to BMI and waist circumference in older adult males [37]. Rutters and colleagues [38] investigated the inter-individual relationships between sleep architecture and energy balance during a 2-day in-lab investigation that included measures of polysomnographic sleep, hunger levels, and a calculated assessment of energy balance. REM sleep duration and energy balance were positively correlated, whereas SWS duration was inversely related to energy balance. This effect may have been mediated by the observed inverse relationship between SWS and hunger [38]. Recent work from our group [39] explored a similar question in the context of experimental sleep curtailment. Negative associations were found between REM sleep duration and hunger and between stage 2 sleep (duration and percent total sleep time) and desire to eat something sweet. Moreover, the proportion of total sleep time spent in stage 2 sleep was negatively associated with total energy intake and that spent in SWS and REM sleep negatively associated with intake of fat and carbohydrate under ad libitum eating conditions [39].
An experimental sleep fragmentation study has further demonstrated a role of sleep quality and architecture in influencing hunger and appetite-regulating hormones [40]. When participants were exposed to sleep fragmentation, via repeated wake-up calls, levels of glucagon-like peptide 1 (a gut-derived hormone signaling satiety) and fullness scores were reduced relative to non-fragmented sleep. Interestingly, experimental sleep fragmentation in this study caused significant reductions in duration of stage 2 and REM sleep compared to the non-fragmented night.
Together, these findings imply that sleep-related alterations in energy balance parameters may not be solely the result of reduced sleep duration alone. Rather, altered sleep architecture – specifically decreases in SWS and REM sleep – likely plays a large role in the development of adverse body weight outcomes by influencing hormone pathways, appetite and hunger.
Obstructive sleep apnea
Obstructive sleep apnea (OSA) is characterized by frequent arousals from sleep and disruptions in sleep quality and architecture. Given the relationships between sleep disturbances, architecture, and energy balance highlighted above, it would be expected that OSA is accompanied by an increased risk of obesity. Indeed, both body fat percentage and BMI are found to be significantly related to the apnea-hypopnea index (AHI), an index of OSA severity [41–43]. Moreover, visceral adiposity [41, 42, 44] is significantly and positively correlated with AHI. As might be expected, increased fat accumulation around the neck and pharyngeal region could lead to narrowing or compression of the upper airway and has been proposed as a cause of OSA [45] and predictor of its severity [46]. OSA patients have a larger parapharyngeal fat pad volume [47], a significant predictor of AHI [45].
While obesity is well-established as a leading risk factor for the development of OSA, recent findings suggest that OSA itself plays a role in promoting further weight gain [48]. Indeed, newly diagnosed OSA patients were demonstrated to have a history of significant weight gain compared to sex, age, and BMI-matched controls over the year prior to diagnosis [49].
Reasons for this reciprocal relationship are uncertain. However, OSA affects various factors influencing the regulation of energy balance, including a disruption of sleep quality and continuity. Specifically, the intermittent hypoxia that occurs in the disorder causes repeated nocturnal arousals from sleep, resulting in sleep fragmentation and a disruption of sleep architecture. Obese individuals with OSA have increased expression of stage 1 sleep and decreased expression of stage 2, REM, and SWS compared to non-OSA obese individuals [50]. As REM sleep is inversely related to hunger levels, and both REM sleep and SWS are inversely related to ad libitum intakes of fat and carbohydrate [39], the particular profile of sleep associated with OSA would indicate propensity towards positive energy balance. Other behavioral, metabolic, or hormonal effects of OSA may further enhance this state, favoring weight gain and/or difficulty losing weight.
Excessive daytime sleepiness is an important consequence of OSA, and is present in 20–40% of patients [51, 52]. A relationship between lack of regular exercise and excessive daytime sleepiness was observed in obese OSA patients [53]. OSA may also affect energy metabolism. RMR [54], sleeping EE [55] and 24-h EE are increased in OSA patients compared to snoring controls [56]. In another study, resting EE was found to be independently and positively associated with AHI even after adjusting for BMI [57] or fat-free mass [58].
These increases in EE in response to OSA may seem paradoxical, as it can be assumed that increased EE could preclude the development of a positive energy balance in these patients. However, we have recently shown that the added thermogenesis associated with increased wake time during SSD is much smaller in magnitude than the increased energy intake observed in response to sleep restriction [35]. This is likely to be the case with OSA [59], although this has not been directly tested. There is some evidence for a dysregulation of appetite-regulating hormones in OSA patients, with increased leptin levels, suggestive of leptin resistance [60–63], and increased ghrelin [62] relative to controls. This aspect of energy balance has not been extensively studied in OSA, although AHI was found to be positively associated with the caloric as well as fat and carbohydrate content of a self-selected meal from a standard hospital menu in adolescents [64]. Food choice and energy intake should be systematically studied in OSA patients to determine how the disorder may influence body weight management.
There is therefore reason to suggest OSA as a risk factor for obesity. This was investigated in a population-based, prospective cohort [65]. A 10% increase in weight predicted a 32% increase in AHI and similar weight loss predicted a 26% decrease in AHI over 4 years of follow-up, after adjusting for sex, smoking, baseline BMI, and age [65].
Somewhat similar results were subsequently reported from the Sleep Heart Health Study [66]. While OSA severity increased with weight gain and decreased with weight loss over the 5 years follow-up, the observed change was greater for weight gain than weight loss. These effects were modulated by sex such that men gaining ≥10 kg had 5.2 times the odds of having a large (>15 events/h) increase in respiratory disturbance index (RDI) compared to weight-stable men whereas the odds in women was 2.6. The impact of weight loss also differed by sex: men losing ≥10 kg had 5.4 times the odds of a large reduction in RDI, whereas this relationship was not significant for women. A similar effect of sex was reported from data from the Cleveland Family Study [67].
Together, these longitudinal studies clearly indicate that increasing prevalence of obesity in the population and within individuals over time can increase the incidence and severity of OSA. Conversely, the findings of these studies also imply that lifestyle interventions, which can successfully reduce body weight can be useful therapeutic options for the management and treatment of OSA.
Relationship between weight change and OSA
Despite the information obtained thus far, it still remains unclear whether SSD and/or sleep disruption as is seen in OSA causes obesity or hinders weight loss. Based on the studies described above, sleep duration and quality at baseline seem to be important factors in an individual’s response to a weight-loss diet. However, because obesity is accompanied by sleep disorders, including a high prevalence of OSA, it is reasonable to hypothesize that obesity leads to sleep disorders, which can then reduce sleep quality and duration. This could lead to further weight gain or difficulty achieving weight loss. Borel et al. [68] showed that patients with visceral obesity and OSA had a smaller decrease in BMI, waist circumference, and fat mass in response to a lifestyle weight-loss intervention compared to those without OSA. The authors suggested that this decreased response to the weight-loss intervention program in OSA patients was due to alterations in sleep quality and/or duration.
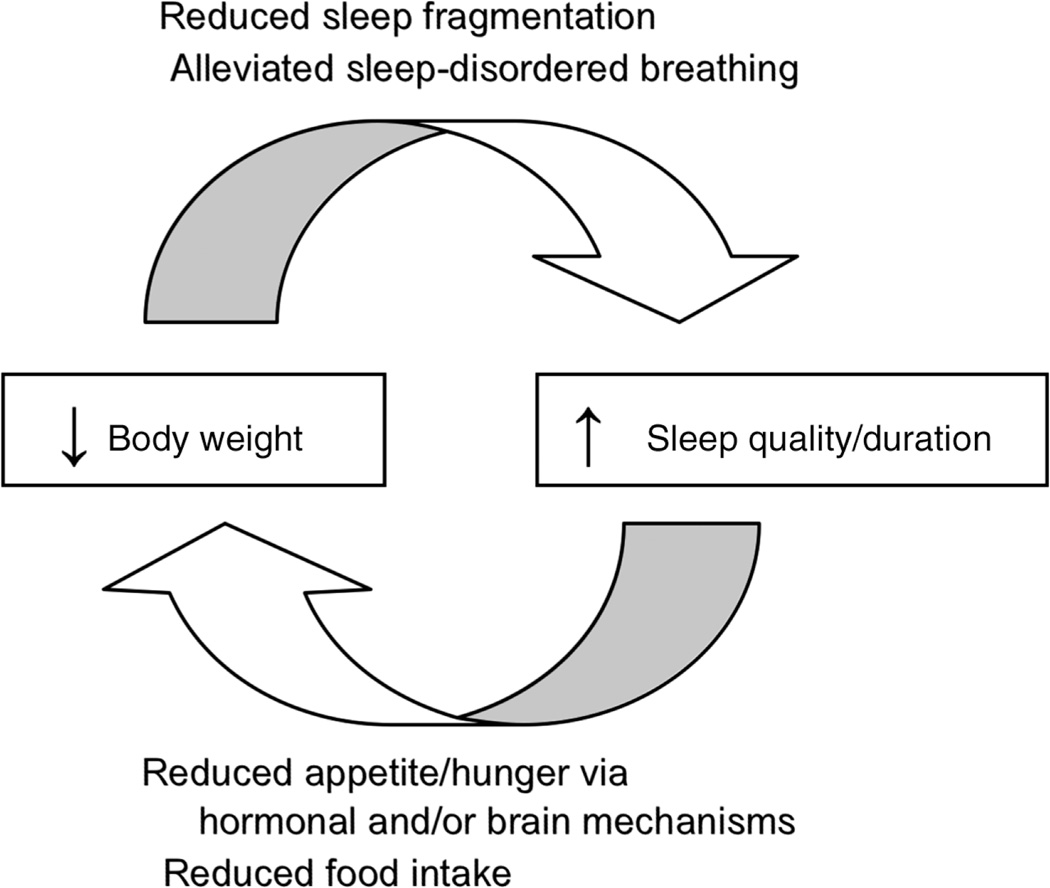
Conversely, weight loss could improve sleep disorders. In such a case then, a reduction in weight could reduce sleep disturbances and sleep-disordered breathing and increase sleep quality and duration. Perhaps this could assist with weight-loss efforts and help individuals achieve further weight loss, propelling a cycle of improved weight and sleep quality/duration (Figure 1).
Figure 1.
Schematic diagram of the proposed reciprocal relationship between weight loss and improved sleep quality/duration. Putative mechanisms by which body weight and sleep quality/duration affect each other are listed in italics, and include (top) reduced sleep fragmentation and alleviation of sleep-disordered breathing in response to weight loss; and (bottom) reduced hunger and food intake via hormonal or brain mechanisms in response to improved sleep quality and duration.
Verhoef et al. [69] attempted to untangle the “chicken-or-the-egg” question of the sleep-obesity paradox and reveal the temporal sequence of changes in sleep and body weight during a weight-loss intervention. Overweight and obese men and women underwent a very-low-calorie diet for 8 weeks followed by 10 months of weight maintenance surveillance. Sleep was assessed by questionnaire at all study visits: before weight loss, after weight loss (8 weeks), and at 3 and 10 months of follow-up. In short and normal sleepers, sleep duration increased with weight-loss: change in sleep duration was negatively correlated with change in BMI. This was also noted at the 3-months follow-up visit but not at 10 months. However, the change in sleep duration during weight loss was not associated with the change in BMI during follow-up. This latter finding suggests that changes in sleep, BMI and fat mass occurred in parallel during weight loss. The authors concluded that sleep duration benefits from weight loss and vice versa, therefore, no conclusions could be drawn on the temporal sequence of the association.
Illustrative of the impact of body weight on sleep disorders, several randomized controlled trials consisting of either diet [70, 71] or diet and exercise [72] have demonstrated the effectiveness of lifestyle interventions in inducing weight loss to improve OSA severity. In a study by Johansson and colleagues [71], obese individuals with at least moderate (AHI>15) OSA at baseline were randomized to a control group or lifestyle intervention consisting of a very-low-energy diet (2.3 MJ/day liquid meals) for 7 weeks, followed by 2 weeks of gradually re-introducing normal food to reach 6.3 MJ/day at week 9. The intervention group had greater reductions in body weight and adiposity measures at week 9 than the control group. The treatment group also had reduced AHI at week 9 compared to the control. Longer-term effects of the intervention were studied in the treatment group participants, who underwent a weight-loss maintenance program to week 52 [73]. Participants in the control group were crossed over to the low-energy intervention phase for 9 weeks and ultimately the weight-loss maintenance program until week 52. At week 52, AHI and body weight were significantly reduced compared to baseline and a 10 kg decrease in weight was associated with a decrease in AHI of 5 events/h.
Tuomilehto and colleagues [70] conducted a prospective randomized controlled trial on obese adults, BMI 28–40 kg/m2, with mild-to-moderate (AHI 5–15 events/h) OSA at baseline. The 1-year lifestyle intervention consisted of a very low calorie diet (600–800 kcal/day) for 12 weeks, followed by a recommendation to reduce fat intake to <30% of total energy for the remainder of the year, combined with counseling sessions. Participants in the control group were provided with general information regarding diet and exercise. The intervention produced greater weight loss than control and differences in AHI between groups. A loss of 5–15 kg body weight was associated with a reduction in AHI of 4 events/h; losing >15 kg was associated with a reduction in AHI of 7 events/h. Any gain in weight was associated with a rise in AHI of 3 events/h. The authors subsequently described the findings of a 4-year post-intervention follow-up, in which no active counseling was provided to either group [74]. The mean change in body weight across the 5-year period was significantly different for participants in the treatment (−5.5 kg) vs. control (+0.6 kg) group as was the change in AHI (−0.8 events/h vs. + 5.0 events/h).
Foster and colleagues [72] conducted a randomized controlled trial on middle-aged to older adults with type 2 diabetes, with BMI>25 kg/m2 (or ≥27 kg/m2 if taking insulin), and AHI≥5 events/h. The intensive lifestyle intervention of reduced-calorie diet and an exercise regimen resulted in significantly larger weight loss and reductions in waist and neck circumferences compared to control, diabetes education group after 1 year. Also, the intensive lifestyle intervention group had a reduction in AHI of 5.4 events/h whereas the control group had an increase in AHI of 4.2 events/h. The change in AHI was significantly related to the change in body weight. Participants were followed for an additional 3 years [75]. During follow-up, weight loss remained significantly greater in the treatment vs. the control group and reductions in AHI persisted. Taken together, these three randomized controlled trials strongly suggest that weight loss induced by lifestyle modifications consisting of diet and exercise can be an effective means of reducing OSA severity.
Expert opinion
It is currently unclear how sleep duration and quality impact body weight. It is possible that poor sleep characteristics cause weight gain/obesity, or hinder weight loss. Conversely, weight gain/obesity could cause alterations in sleep characteristics, which may further influence body weight. Despite strong epidemiological evidence for associations between SSD/poor sleep quality and obesity, and increasing clinical evidence for a causal effect of sleep on weight status, studies are lacking to clearly show that SSD/poor sleep quality leads to actual changes in body weight. A similar pattern of evidence exists for sleep-disordered breathing and obesity. Once it is clearly shown that SSD/poor sleep quality can cause weight gain, then addressing the reverse question of whether increasing sleep duration/improving sleep quality can improve weight loss will be warranted. Information from such studies would then lead to public health messages about appropriate sleep for weight management.
Outlook
We are aware that clinical trials are under way to assess whether extending sleep duration improves weight status in obese short sleepers. Results from such studies will prompt additional questions on the mechanism by which extending sleep can improve body weight control. To date, data are mixed regarding the impact of sleep duration on hormonal regulation of food intake. We hope that future studies will have clarified these questions.
Highlights.
-
–
Short sleepers are heavier and gain more weight over time than normal sleepers.
-
–
Short sleep hinders weight loss efforts compared to normal sleep duration.
-
–
Sleep restriction increases food intake beyond the additional energetic costs of added wake time.
-
–
Sleep architecture, particularly time spent in stage 2 and REM sleep, is related to energy balance parameters.
-
–
Weight loss improves OSA.
-
–
Future studies are needed to establish whether restricting sleep or inducing poor sleep quality hinder weight-loss efforts or cause weight gain.
Acknowledgments
This was funded in part by R01 HL091352 (St-Onge), T32 DK007559 (Shechter), P30 DK26687 (NYONRC).
Footnotes
Copyright of Hormone Molecular Biology & Clinical Investigation is the property of De Gruyter and its content may not be copied or emailed to multiple sites or posted to a listserv without the copyright holder's express written permission. However, users may print, download, or email articles for individual use.
Contributor Information
Marie-Pierre St-Onge, Email: ms2554@columbia.edu, PhD, FAHA, 1090 Amsterdam Avenue, suite 14D, New York, NY 10025, USA, Phone: +212-523-3564, Fax +212-523-3571.
Ari Shechter, New York Obesity Nutrition Research Center, St. Luke’s/Roosevelt Hospital and Institute of Human Nutrition, College of Physicians and Surgeons, Columbia University, New York, NY, USA.
References
- 1.Chen X, Beydoun MA, Wang Y. Is sleep duration associated with childhood obesity? A systematic review and meta-analysis. Obesity (Silver Spring) 2008;16:265–274. doi: 10.1038/oby.2007.63. [DOI] [PubMed] [Google Scholar]
- 2.Keith SW, Redden DT, Katzmarzyk PT, Boggiano MM, Hanlon EC, Benca RM, Ruden D, Pietrobelli A, Barger JL, Fontaine KR, Wang C, Aronne LJ, Wright SM, Baskin M, Dhurandhar NV, Lijoi MC, Grilo CM, Deluca M, Westfall AO, Allison DB. Putative contributors to the secular increase in obesity: exploring the roads less traveled. Int J Obes (Lond) 2006;30:1585–1594. doi: 10.1038/sj.ijo.0803326. [DOI] [PubMed] [Google Scholar]
- 3.Liu J, Zhang A, Li L. Sleep duration and overweight/obesity in children: review and implications for pediatric nursing. J Spec Pediatr Nurs. 2012;17:193–204. doi: 10.1111/j.1744-6155.2012.00332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Patel SR, Hu FB. Short sleep duration and weight gain: a systematic review. Obesity (Silver Spring) 2008;16:643–653. doi: 10.1038/oby.2007.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hasler G, Buysse DJ, Klaghofer R, Gamma A, Ajdacic V, Eich D, Rossler W, Angst J. The association between short sleep duration and obesity in young adults: a 13-year prospective study. Sleep. 2004;27:661–666. doi: 10.1093/sleep/27.4.661. [DOI] [PubMed] [Google Scholar]
- 6.Patel SR, Malhotra A, White DP, Gottlieb DJ, Hu FB. Association between reduced sleep and weight gain in women. Am J Epidemiol. 2006;164:947–954. doi: 10.1093/aje/kwj280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lopez-Garcia E, Faubel R, Leon-Munoz L, Zuluaga MC, Banegas JR, Rodriguez-Artalejo F. Sleep duration, general and abdominal obesity, and weight change among the older adult population of Spain. Am J Clin Nutr. 2008;87:310–316. doi: 10.1093/ajcn/87.2.310. [DOI] [PubMed] [Google Scholar]
- 8.Chaput JP, Despres JP, Bouchard C, Tremblay A. The association between sleep duration and weight gain in adults: a 6-year prospective study from the Quebec Family Study. Sleep. 2008;31:517–523. doi: 10.1093/sleep/31.4.517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watanabe M, Kikuchi H, Tanaka K, Takahashi M. Association of short sleep duration with weight gain and obesity at 1-year follow-up: a large-scale prospective study. Sleep. 2010;33:161–167. doi: 10.1093/sleep/33.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lauderdale DS, Knutson KL, Rathouz PJ, Yan LL, Hulley SB, Liu K. Cross-sectional and longitudinal associations between objectively measured sleep duration and body mass index: the CARDIA Sleep Study. Am J Epidemiol. 2009;170:805–813. doi: 10.1093/aje/kwp230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Appelhans BM, Janssen I, Cursio JF, Matthews KA, Hall M, Gold EB, Burns JW, Kravitz HM. Sleep duration and weight change in midlife women: the SWAN sleep study. Obesity (Silver Spring) 2013;21:77–84. doi: 10.1002/oby.20251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nedeltcheva AV, Kilkus JM, Imperial J, Schoeller DA, Penev PD. Insufficient sleep undermines dietary efforts to reduce adiposity. Ann Intern Med. 2010;153:435–441. doi: 10.1059/0003-4819-153-7-201010050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Robertson MD, Russell-Jones D, Umpleby AM, Dijk DJ. Effects of three weeks of mild sleep restriction implemented in the home environment on multiple metabolic and endocrine markers in healthy young men. Metabolism. 2013;62:204–211. doi: 10.1016/j.metabol.2012.07.016. [DOI] [PubMed] [Google Scholar]
- 14.Chaput JP, Tremblay A. Sleeping habits predict the magnitude of fat loss in adults exposed to moderate caloric restriction. Obes Facts. 2012;5:561–566. doi: 10.1159/000342054. [DOI] [PubMed] [Google Scholar]
- 15.Thomson CA, Morrow KL, Flatt SW, Wertheim BC, Perfect MM, Ravia JJ, Sherwood NE, Karanja N, Rock CL. Relationship between sleep quality and quantity and weight loss in women participating in a weight-loss intervention trial. Obesity (Silver Spring) 2012;20:1419–1425. doi: 10.1038/oby.2012.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Elder CR, Gullion CM, Funk KL, Debar LL, Lindberg NM, Stevens VJ. Impact of sleep, screen time, depression and stress on weight change in the intensive weight loss phase of the LIFE study. Int J Obes (Lond) 2012;36:86–92. doi: 10.1038/ijo.2011.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sallinen BJ, Hassan F, Olszewski A, Maupin A, Hoban TF, Chervin RD, Woolford SJ. Longer weekly sleep duration predicts greater 3-month BMI reduction among obese adolescents attending a clinical multidisciplinary weight management program. Obes Facts. 2013;6:239–246. doi: 10.1159/000351819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Spiegel K, Leproult R, L’Hermite-Baleriaux M, Copinschi G, Penev PD, Van Cauter E. Leptin levels are dependent on sleep duration: relationships with sympathovagal balance, carbohydrate regulation, cortisol, and thyrotropin. J Clin Endocrinol Metab. 2004;89:5762–5771. doi: 10.1210/jc.2004-1003. [DOI] [PubMed] [Google Scholar]
- 19.Nedeltcheva AV, Kilkus JM, Imperial J, Kasza K, Schoeller DA, Penev PD. Sleep curtailment is accompanied by increased intake of calories from snacks. Am J Clin Nutr. 2009;89:126–133. doi: 10.3945/ajcn.2008.26574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schmid SM, Hallschmid M, Jauch-Chara K, Wilms B, Benedict C, Lehnert H, Born J, Schultes B. Short-term sleep loss decreases physical activity under free-living conditions but does not increase food intake under time-deprived laboratory conditions in healthy men. Am J Clin Nutr. 2009;90:1476–1482. doi: 10.3945/ajcn.2009.27984. [DOI] [PubMed] [Google Scholar]
- 21.St-Onge MP, O’Keeffe M, Roberts AL, RoyChoudhury A, Laferrere B. Short sleep duration, glucose dysregulation and hormonal regulation of appetite in men and women. Sleep. 2012;35:1503–1510. doi: 10.5665/sleep.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Omisade A, Buxton OM, Rusak B. Impact of acute sleep restriction on cortisol and leptin levels in young women. Physiol Behav. 2010;99:651–656. doi: 10.1016/j.physbeh.2010.01.028. [DOI] [PubMed] [Google Scholar]
- 23.Reynolds AC, Dorrian J, Liu PY, Van Dongen HP, Wittert GA, Harmer LJ, Banks S. Impact of five nights of sleep restriction on glucose metabolism, leptin and testosterone in young adult men. PLoS One. 2012;7:e41218. doi: 10.1371/journal.pone.0041218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Simpson NS, Banks S, Dinges DF. Sleep restriction is associated with increased morning plasma leptin concentrations, especially in women. Biol Res Nurs. 2010;12:47–53. doi: 10.1177/1099800410366301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.van Leeuwen WM, Hublin C, Sallinen M, Harma M, Hirvonen A, Porkka-Heiskanen T. Prolonged sleep restriction affects glucose metabolism in healthy young men. Int J Endocrinol. 2010;2010:108641. doi: 10.1155/2010/108641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.St-Onge MP. The role of sleep duration in the regulation of energy balance: effects on energy intakes and expenditure. J Clin Sleep Med. 2013;9:73–80. doi: 10.5664/jcsm.2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Markwald RR, Melanson EL, Smith MR, Higgins J, Perreault L, Eckel RH, Wright KP., Jr Impact of insufficient sleep on total daily energy expenditure, food intake, and weight gain. Proc Natl Acad Sci USA. 2013;110:5695–5700. doi: 10.1073/pnas.1216951110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.St-Onge MP, McReynolds A, Trivedi ZB, Roberts AL, Sy M, Hirsch J. Sleep restriction leads to increased activation of brain regions sensitive to food stimuli. Am J Clin Nutr. 2012;95:818–824. doi: 10.3945/ajcn.111.027383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.St-Onge MP, Wolfe S, Sy M, Shechter A, Hirsch J. Sleep restriction increases the neuronal response to unhealthy food in normal-weight individuals. Int J Obes (Lond) 2013 doi: 10.1038/ijo.2013.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Brondel L, Romer MA, Nougues PM, Touyarou P, Davenne D. Acute partial sleep deprivation increases food intake in healthy men. Am J Clin Nutr. 2010;91:1550–1559. doi: 10.3945/ajcn.2009.28523. [DOI] [PubMed] [Google Scholar]
- 31.Spaeth AM, Dinges DF, Goel N. Effects of experimental sleep restriction on weight gain, caloric intake and meal timing in healthy adults. Sleep. 2013;36:981–990. doi: 10.5665/sleep.2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.St-Onge MP, Roberts AL, Chen J, Kelleman M, O’Keeffe M, RoyChoudhury A, Jones PJ. Short sleep duration increases energy intakes but does not change energy expenditure in normal-weight individuals. Am J Clin Nutr. 2011;94:410–416. doi: 10.3945/ajcn.111.013904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Gluck ME, Venti CA, Salbe AD, Krakoff J. Nighttime eating: commonly observed and related to weight gain in an inpatient food intake study. Am J Clin Nutr. 2008;88:900–905. doi: 10.1093/ajcn/88.4.900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klingenberg L, Chaput JP, Holmback U, Jennum P, Astrup A, Sjodin A. Sleep restriction is not associated with a positive energy balance in adolescent boys. Am J Clin Nutr. 2012;96:240–248. doi: 10.3945/ajcn.112.038638. [DOI] [PubMed] [Google Scholar]
- 35.Shechter A, Rising R, Albu JB, St-Onge MP. Experimental sleep curtailment causes wake-dependent increases in 24-h energy expenditure as measured by whole-room indirect calorimetry. Am J Clin Nutr. 2013;98:1433–1439. doi: 10.3945/ajcn.113.069427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Liu X, Forbes EE, Ryan ND, Rofey D, Hannon TS, Dahl RE. Rapid eye movement sleep in relation to overweight in children and adolescents. Arch Gen Psychiatry. 2008;65:924–932. doi: 10.1001/archpsyc.65.8.924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Rao MN, Blackwell T, Redline S, Stefanick ML, Ancoli-Israel S, Stone KL Osteoporotic Fractures in Men Study G. Association between sleep architecture and measures of body composition. Sleep. 2009;32:483–490. doi: 10.1093/sleep/32.4.483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rutters F, Gonnissen HK, Hursel R, Lemmens SG, Martens EA, Westerterp-Plantenga MS. Distinct associations between energy balance and the sleep characteristics slow wave sleep and rapid eye movement sleep. Int J Obes (Lond) 2012;36:1346–1352. doi: 10.1038/ijo.2011.250. [DOI] [PubMed] [Google Scholar]
- 39.Shechter A, O’Keeffe M, Roberts AL, Zammit GK, RoyChoudhury A, St-Onge MP. Alterations in sleep architecture in response to experimental sleep curtailment are associated with signs of positive energy balance. Am J Physiol Regul Integr Comp Physiol. 2012;303:R883–R889. doi: 10.1152/ajpregu.00222.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gonnissen HK, Hursel R, Rutters F, Martens EA, Westerterp-Plantenga MS. Effects of sleep fragmentation on appetite and related hormone concentrations over 24 h in healthy men. Br J Nutr. 2012;8:1–9. doi: 10.1017/S0007114512001894. [DOI] [PubMed] [Google Scholar]
- 41.Schafer H, Pauleit D, Sudhop T, Gouni-Berthold I, Ewig S, Berthold HK. Body fat distribution, serum leptin, and cardiovascular risk factors in men with obstructive sleep apnea. Chest. 2002;122:829–839. doi: 10.1378/chest.122.3.829. [DOI] [PubMed] [Google Scholar]
- 42.Ogretmenoglu O, Suslu AE, Yucel OT, Onerci TM, Sahin A. Body fat composition: a predictive factor for obstructive sleep apnea. Laryngoscope. 2005;115:1493–1498. doi: 10.1097/01.mlg.0000172204.82314.c3. [DOI] [PubMed] [Google Scholar]
- 43.Lovin S, Bercea R, Cojocaru C, Rusu G, Mihaescu T. Body composition in obstructive sleep apneahypopnea syndrome bio-impedance reflects the severity of sleep apnea. Multidiscip Respir Med. 2010;5:44–49. doi: 10.1186/2049-6958-5-1-44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Shinohara E, Kihara S, Yamashita S, Yamane M, Nishida M, Arai T, Kotani K, Nakamura T, Takemura K, Matsuzawa Y. Visceral fat accumulation as an important risk factor for obstructive sleep apnoea syndrome in obese subjects. J Int Med. 1997;241:11–18. doi: 10.1046/j.1365-2796.1997.63889000.x. [DOI] [PubMed] [Google Scholar]
- 45.Shelton KE, Woodson H, Gay S, Suratt PM. Pharyngeal fat in obstructive sleep apnea. Am Rev Respir Dis. 1993;148:462–466. doi: 10.1164/ajrccm/148.2.462. [DOI] [PubMed] [Google Scholar]
- 46.Dixon JB, Schachter LM, O’Brien PE. Predicting sleep apnea and excessive day sleepiness in the severely obese: indicators for polysomnography. Chest. 2003;123:1134–1141. doi: 10.1378/chest.123.4.1134. [DOI] [PubMed] [Google Scholar]
- 47.Schwab RJ, Pasirstein M, Pierson R, Mackley A, Hachadoorian R, Arens R, Maislin G, Pack AI. Identification of upper airway anatomic risk factors for obstructive sleep apnea with volumetric magnetic resonance imaging. Am J Respir Crit Care Med. 2003;168:522–530. doi: 10.1164/rccm.200208-866OC. [DOI] [PubMed] [Google Scholar]
- 48.Romero-Corral A, Caples SM, Lopez-Jimenez F, Somers VK. Interactions between obesity and obstructive sleep apnea: implications for treatment. Chest. 2010;137:711–719. doi: 10.1378/chest.09-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Phillips BG, Hisel TM, Kato M, Pesek CA, Dyken ME, Narkiewicz K, Somers VK. Recent weight gain in patients with newly diagnosed obstructive sleep apnea. J Hypertens. 1999;17:1297–1300. doi: 10.1097/00004872-199917090-00009. [DOI] [PubMed] [Google Scholar]
- 50.Vgontzas AN, Tan TL, Bixler EO, Martin LF, Shubert D, Kales A. Sleep apnea and sleep disruption in obese patients. Arch Intern Med. 1994;154:1705–1711. [PubMed] [Google Scholar]
- 51.Kapur VK, Baldwin CM, Resnick HE, Gottlieb DJ, Nieto FJ. Sleepiness in patients with moderate to severe sleep-disordered breathing. Sleep. 2005;28:472–477. doi: 10.1093/sleep/28.4.472. [DOI] [PubMed] [Google Scholar]
- 52.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–1235. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 53.Basta M, Lin HM, Pejovic S, Sarrigiannidis A, Bixler E, Vgontzas AN. Lack of regular exercise, depression, and degree of apnea are predictors of excessive daytime sleepiness in patients with sleep apnea: sex differences. J Clin Sleep Med. 2008;4:19–25. [PMC free article] [PubMed] [Google Scholar]
- 54.Ucok K, Aycicek A, Sezer M, Fidan F, Akgun L, Akkaya M, Unlu M. Resting metabolic rate and anthropometric measurements in male sleep apnea patients. Intern Med. 2011;50:833–838. doi: 10.2169/internalmedicine.50.4779. [DOI] [PubMed] [Google Scholar]
- 55.Lin CC, Chang KC, Lee KS. Effects of treatment by laser-assisted uvuloplasty on sleep energy expenditure in obstructive sleep apnea patients. Metabolism. 2002;51:622–627. doi: 10.1053/meta.2002.31969. [DOI] [PubMed] [Google Scholar]
- 56.Stenlof K, Grunstein R, Hedner J, Sjostrom L. Energy expenditure in obstructive sleep apnea: effects of treatment with continuous positive airway pressure. Am J Physiol. 1996;271(6 Pt 1):E1036–E1043. doi: 10.1152/ajpendo.1996.271.6.E1036. [DOI] [PubMed] [Google Scholar]
- 57.Kezirian EJ, Kirisoglu CE, Riley RW, Chang E, Guilleminault C, Powell NB. Resting energy expenditure in adults with sleep disordered breathing. Arch Otolaryngol Head Neck Surg. 2008;134:1270–1275. doi: 10.1001/archotol.134.12.1270. [DOI] [PubMed] [Google Scholar]
- 58.de Jonge L, Zhao X, Mattingly MS, Zuber SM, Piaggi P, Csako G, Cizza G Group NSES. Poor sleep quality and sleep apnea are associated with higher resting energy expenditure in obese individuals with short sleep duration. J Clin Endocrinol Metab. 2012;97:2881–2889. doi: 10.1210/jc.2011-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Penev PD. Update on energy homeostasis and insufficient sleep. J Clin Endocrinol Metab. 2012;97:1792–1801. doi: 10.1210/jc.2012-1067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Phillips BG, Kato M, Narkiewicz K, Choe I, Somers VK. Increases in leptin levels, sympathetic drive, and weight gain in obstructive sleep apnea. Am J Physiol Heart Circ Physiol. 2000;279:H234–H237. doi: 10.1152/ajpheart.2000.279.1.H234. [DOI] [PubMed] [Google Scholar]
- 61.Ip MS, Lam KS, Ho C, Tsang KW, Lam W. Serum leptin and vascular risk factors in obstructive sleep apnea. Chest. 2000;118:580–586. doi: 10.1378/chest.118.3.580. [DOI] [PubMed] [Google Scholar]
- 62.Harsch IA, Konturek PC, Koebnick C, Kuehnlein PP, Fuchs FS, Pour Schahin S, Wiest GH, Hahn EG, Lohmann T, Ficker JH. Leptin and ghrelin levels in patients with obstructive sleep apnoea: effect of CPAP treatment. Eur Respir J. 2003;22:251–257. doi: 10.1183/09031936.03.00010103. [DOI] [PubMed] [Google Scholar]
- 63.Ulukavak Ciftci T, Kokturk O, Bukan N, Bilgihan A. Leptin and ghrelin levels in patients with obstructive sleep apnea syndrome. Respiration. 2005;72:395–401. doi: 10.1159/000086254. [DOI] [PubMed] [Google Scholar]
- 64.Beebe DW, Miller N, Kirk S, Daniels SR, Amin R. The association between obstructive sleep apnea and dietary choices among obese individuals during middle to late childhood. Sleep Med. 2011;12:797–799. doi: 10.1016/j.sleep.2010.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. J Am Med Assoc. 2000;284:3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 66.Newman AB, Foster G, Givelber R, Nieto FJ, Redline S, Young T. Progression and regression of sleep-disordered breathing with changes in weight: the Sleep Heart Health Study. Arch Intern Med. 2005;165:2408–2413. doi: 10.1001/archinte.165.20.2408. [DOI] [PubMed] [Google Scholar]
- 67.Redline S, Schluchter MD, Larkin EK, Tishler PV. Predictors of longitudinal change in sleep-disordered breathing in a nonclinic population. Sleep. 2003;26:703–709. doi: 10.1093/sleep/26.6.703. [DOI] [PubMed] [Google Scholar]
- 68.Borel AL, Leblanc X, Almeras N, Tremblay A, Bergeron J, Poirier P, Despres JP, Series F. Sleep apnoea attenuates the effects of a lifestyle intervention programme in men with visceral obesity. Thorax. 2012;67:735–741. doi: 10.1136/thoraxjnl-2011-201001. [DOI] [PubMed] [Google Scholar]
- 69.Verhoef SP, Camps SG, Gonnissen HK, Westerterp KR, Westerterp-Plantenga MS. Concomitant changes in sleep duration and body weight and body composition during weight loss and 3-mo weight maintenance. Am J Clin Nutr. 2013;98:25–31. doi: 10.3945/ajcn.112.054650. [DOI] [PubMed] [Google Scholar]
- 70.Tuomilehto HP, Seppa JM, Partinen MM, Peltonen M, Gylling H, Tuomilehto JO, Vanninen EJ, Kokkarinen J, Sahlman JK, Martikainen T, Soini EJ, Randell J, Tukiainen H, Uusitupa M Kuopio Sleep Apnea Group. Lifestyle intervention with weight reduction: first-line treatment in mild obstructive sleep apnea. Am J Respir Crit Care Med. 2009;179:320–327. doi: 10.1164/rccm.200805-669OC. [DOI] [PubMed] [Google Scholar]
- 71.Johansson K, Neovius M, Lagerros YT, Harlid R, Rossner S, Granath F, Hemmingsson E. Effect of a very low energy diet on moderate and severe obstructive sleep apnoea in obese men: a randomised controlled trial. Br Med J. 2009;339:b4609. doi: 10.1136/bmj.b4609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Foster GD, Borradaile KE, Sanders MH, Millman R, Zammit G, Newman AB, Wadden TA, Kelley D, Wing RR, Pi-Sunyer FX, Reboussin D, Kuna ST. A randomized study on the effect of weight loss on obstructive sleep apnea among obese patients with type 2 diabetes: the Sleep AHEAD study. Arch Intern Med. 2009;169:1619–1626. doi: 10.1001/archinternmed.2009.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Johansson K, Hemmingsson E, Harlid R, Trolle Lagerros Y, Granath F, Rossner S, Neovius M. Longer term effects of very low energy diet on obstructive sleep apnoea in cohort derived from randomised controlled trial: prospective observational follow-up study. Br Med J. 2011;342:d3017. doi: 10.1136/bmj.d3017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Tuomilehto H, Seppa J, Uusitupa M, Tuomilehto J, Gylling H Kuopio Sleep Apnea Group. Weight reduction and increased physical activity to prevent the progression of obstructive sleep apnea: A 4-year observational postintervention follow-up of a randomized clinical trial. [corrected] J Am Med Assoc Intern Med. 2013;173(10):929–930. doi: 10.1001/jamainternmed.2013.389. [DOI] [PubMed] [Google Scholar]
- 75.Kuna ST, Reboussin DM, Borradaile KE, Sanders MH, Millman RP, Zammit G, Newman AB, Wadden TA, Jakicic JM, Wing RR, Pi-Sunyer FX, Foster GD Sleep AHEAD Research Group of the Look AHEAD Research Group. Long-term effect of weight loss on obstructive sleep apnea severity in obese patients with type 2 diabetes. Sleep. 2013;36:641–649A. doi: 10.5665/sleep.2618. [DOI] [PMC free article] [PubMed] [Google Scholar]