Summary
To efficiently generate cardiomyocytes from embryonic stem (ES) cells in culture it is essential to identify key regulators of the cardiac lineage and to develop methods to control them. Using a tet-inducible ES cell line to enforce expression of a constitutively activated form of the Notch 4 receptor, we show that signaling through the Notch pathway can efficiently respecify hemangioblasts to a cardiac fate resulting in the generation of populations consisting of more than 60% cardiomyocytes. Microarray analyses revealed that this respecification is mediated, in part, through the coordinated regulation of the BMP and Wnt pathways by Notch signaling. Together, these findings have uncovered a potential novel role for the Notch pathway in cardiac development and in doing so provide a new approach for generating large numbers of cardiac progenitors from ES cells.
ES cells represent a novel and unlimited source of differentiated cells for use in basic biological studies as well as for the development of new therapies for a broad range of degenerative diseases1. Within this context, the directed differentiation of ES cells to the cardiac lineage is of particular interest as readily accessible populations of cardiomyocytes will enable the development of potential new cell based therapies for the treatment of cardiovascular disease, the development of new platforms for drug discovery and toxicology testing, and the development of in vitro models of congenital cardiac abnormalities. In order to establish protocols for the efficient and reproducible generation of any cell population from ES cells, it is essential to understand how the lineage develops in the embryo and to define the signaling pathways and molecular regulators that control its development. The cardiac lineage develops from mesodermal cells that are specified early in embryonic life, shortly following the formation of hematopoietic mesoderm2,3. Analyses of the developmental progression of the mesoderm subpopulations to their respective fates have led to the identification of distinct progenitor populations that display both tissue specific and vascular potential4-10. Within the hematopoietic system this progenitor, known as the hemangioblast, has been defined as a Flk-1+ (KDR+) progenitor that is able to generate cells of the hematopoietic, endothelial and vascular smooth muscle lineages4-6,11-13. A comparable multipotential progenitor has been recently identified for the cardiovascular lineages7-10. This progenitor, which also expresses Flk-1 (KDR), is able to generate progeny of the cardiac, endothelial and vascular smooth muscle lineages7,10.
While the mechanisms controlling the temporal aspects of mesoderm induction are poorly understood, studies with different model systems have shown that both the BMP and Wnt signaling pathways play pivotal roles in the specification of mesoderm to the cardiac lineage14--22. BMP appears to promote cardiac specification14-16, whereas Wnt signaling displays differential stage specific effects, functioning in either an agonistic or antagonistic fashion17-22. Notch signaling has also been shown to influence cardiac development23-25, although its precise role in the regulation of the lineage is not well established. Manipulation of the pathway in different systems points to an inhibitory role in cardiac development. For instance, activation of Notch1 in the heart field of the Xenopus embryo results in decreased expression of cardiac genes23. ES cells deficient in the Notch downstream effector RBP-Jκ generate more cardiomyocytes than wild type counter parts24 whereas ES cells expressing a constitutively active form of the Notch1 receptor display reduced cardiac potential25. While these studies collectively show that Notch signaling is inhibitory to specific stages of the cardiac lineage, the expression patterns of the Notch receptors suggest that this pathway could play additional roles in cardiac development, as most are expressed in the mesoderm of the gastrulating embryo26 as well as in the cardiovascular lineages at different stages26-30.
As Notch signaling has been shown to play a role in lineage specification in many different organisms, we were interested in determining if manipulation of this pathway could influence cardiac development in ES cell cultures. For these studies we focused on Notch4 as its expression is restricted to the endothelial component of the heart30 suggesting that it could function at the level of cardiovascular progenitor development and/or specification to derivative cell types. In this report we show that Notch signaling promotes cardiac development from cardiac mesoderm and is able to respecify Flk-1+ hemangioblasts to a cardiac cell fate. These effects of Notch signaling are stage specific and mediated partially through the activation of BMP signaling and the inhibition of the Wnt pathway. These findings demonstrate a novel role for Notch signaling in the specification of mesoderm to derivative lineages and provide an efficient approach to generate large numbers of cardiovascular cells.
Results
Notch regulates cardiac differentiation from Bry-GFP+/Flk-1− mesoderm
Following 3.0-3.5 days of serum stimulation, the ES cell line containing the green fluorescent protein (GFP) cDNA targeted to the brachyury locus (Bry-GFP) generates three distinct populations based on Flk-1 and GFP expression: Bry-GFP−/Flk-1−, Bry-GFP+/Flk-1− and Bry-GFP+/Flk-1+ (Fig. 1a)11,12. Functional studies have shown that the Bry-GFP+/Flk-1+ population contains hemangioblasts whereas the Bry-GFP+/Flk-1− population (cardiac mesoderm) displays cardiac potential11. When cultured as aggregates in suspension for 24 hours, Bry-GFP+/Flk-1− but not Bry-GFP+/Flk-1+-derived cells express notch4 and the cardiac transcription factor nkx2.5 (Fig. 1b), consistent with the cardiac potential of this fraction. Following 3 days of culture on gelatin coated surfaces in serum-free media, (hereafter referred to as cardiac cultures) the Bry-GFP+/Flk-1−-derived aggregates mature to generate contracting cardiomyocytes.
Figure 1. The role of Notch signaling in cardiac differentiation from EB-derived Bry-GFP+/Flk-1− cardiac mesodermal cells.
(a) Flow cytometric analysis of a day 3.25 population demonstrating the Bry-GFP+/Flk-1+ hemangioblast and the Bry-GFP+/Flk-1− cardiogenic populations. (b) Expression of notch4 and nkx2.5 in one-day-old aggregates of Bry-GFP+/Flk-1+ and Bry-GFP+/Flk-1− cells isolated from day 3.25 EBs. (c-f) Day 3.25 Bry-GFP+/Flk-1− cells derived from the Bry-GFP/Ainv-Notch4 ES cell line isolated by FACS were reaggregated for 24 hours in the presence or absence of Dox or γ-secretase inhibitor. Following the reaggregation step, pools of aggregates were plated for 3-4 days in the cardiac cultures in the presence or absence of Dox or γ-secretase inhibitor (GSI). Populations cultured under the various conditions were analyzed for the presence cTnT+ cells by flow cytometry. The solid line represents cTnT+ cells whereas the shaded area represents control staining with secondary antibody alone. (c) Proportion of cTnT+ cells that develop in the absence or presence of Dox induction. (−Dox/−Dox), non-induced cells; (+Dox/−Dox), Dox added during the reaggregation step; (−Dox/+Dox) Dox added to the cardiac cultures. (d) Expression of cardiac genes, nkx2.5, cardiac α-actin, cardiac mhc, mlc-2a and mlc-2v, in the cells cultured in the presence or absence of Dox. (e) Proportion of cTnT+ cells that developed in the absence of GSI (−GSI/−GSI), or from cells exposed to GSI during the reaggregation step (+GSI/−GSI) or in the cardiac cultures (−GSI/+GSI). (f) Cardiac gene expression of the cells grown in the cardiac cultures in the presence or absence of GSI.
To evaluate the role of Notch signalling in the establishment of the cardiac lineage from the Bry-GFP+/Flk-1− cardiac mesoderm population, we used a tet-inducible ES cell line31 engineered to express a constitutively activated Notch4 receptor, which contains the intracellular domain of Notch4 as well as the membrane-anchored sequence that requires cleavage by the ubiquitous enzyme γ-secretase for activation32. The Notch4-inducible cell line also contains the GFP cDNA targeted to the brachyury locus (hereafter referred to as Bry-GFP/Ainv-Notch4 ES cells; Supplementary Fig. 1 online). In the first set of experiments, Notch signaling was activated by Dox induction in the Bry-GFP+/Flk-1−-derived population or inhibited by the addition of γ-secretase inhibitor (GSI) to the cells either during the 24 hour reaggregation step or during the cardiac culture stage. Induction of Notch4 within the aggregates (+Dox/−Dox) moderately enhanced cardiomyocyte development over the control (−Dox/−Dox) as demonstrated by an increase in the proportion of cardiac troponin-T (cTnT) positive cells (Fig. 1c). When induced at a later stage during the maturation of the population in the cardiac cultures (−Dox/+Dox), Notch4 expression had an opposite effect and led to a reduction in the frequency of cTnT+ cells and in the expression of genes associated with cardiac maturation including cardiac α-actin, cardiac mhc, mlc-2a and mlc-2v (Fig. 1c, d). Addition of GSI to the Bry-GFP+/Flk-1− population during the reaggregation stage (+GSI/−GSI) inhibited the development of cTnT+ contracting cells and the expression of the cardiac genes (Fig. 1e,f). When added to the cardiac cultures (−GSI/+GSI), the Notch inhibitor enhanced cardiac development, as demonstrated by an increased size of the cTnT-expressing population (Fig. 1e). As expected, this population expressed the spectrum of cardiac genes (Fig. 1f). Together, these observations indicate that active Notch signaling in the Bry-GFP+/Flk-1− mesoderm population promotes cardiac development. In addition, they demonstrate that this effect is stage specific as induction of Notch4 expression at a later stage inhibits maturation of the cardiac lineage.
Notch4 respecifies the Flk-1+ hemangioblast population to a cardiac fate
The Bry-GFP+/Flk-1+ population contains hemangioblasts (also known as blast colony forming cells (BL-CFC)) that are characterized by their ability to generate blast colonies comprised of hematopoietic and vascular progenitors in methylcellulose cultures. If isolated and cultured for 2 days as aggregates in serum-containing media, the Bry-GFP+/Flk-1+ cells generate hematopoietic cells as demonstrated by emergence of CD41+ cells (−Dox, Fig. 2a) and the development of hematopoietic progenitors (−Dox, Fig. 2b). Induction of Notch4 in the Bry-GFP+/Flk-1+-derived aggregates for 2 days inhibited development of the CD41+ population and the hematopoietic progenitors indicating a marked reduction in hematopoietic potential (+Dox, Fig. 2a,b). A moderate increase in the proportion of VE-cadherin+ (VE-cad) endothelial cells was observed in the Notch4 induced aggregates (Fig. 2a). Molecular analysis confirmed these findings and revealed dramatically lower expression levels of the hematopoietic specific gene gata-1 and moderately increased levels of genes indicative of endothelial and vascular smooth muscle development, including flk-1, ve-cad, sm22 and pdgfβr (Fig. 2c) in the Notch induced population compared to non-induced controls. Of particular interest was the observation that induction of Notch4 resulted in the upregulation of expression of the cardiac gene nkx2.533 (Fig. 2c) suggesting respecification of these cells to a cardiac fate.
Figure 2. Effects of Notch4 over-expression on the EB-derived Bry-GFP+/Flk-1+ population.
For analyses of hematopoietic potential, day 3.25 Bry-GFP+/Flk-1+ cells were isolated by cell sorting and reaggregated in serum-containing medium in the presence (+Dox) or absence (−Dox) of Dox (1 μg/ml) for 2 days. Following the Dox induction, the aggregates were dissociated and analyzed for hematopoietic potential. (a) Flow cytometric analyses showing the proportion of VE-cad and CD41 positive cells in the aggregates. The solid line represents VE-cad or CD41 positive cells whereas the shaded area represents control staining with secondary antibody alone. (b) Hematopoietic colony forming potential of the aggregate cells. Bars represent the standard error of the mean of the number of colonies from 3 cultures. Ep, primitive erythroid; Ed, definitive erythroid; Mac, macrophage; E/Mac, bipotential erythroid/macrophage. (c) Gene expression analyses of the aggregates. hematopoietic gene: gata-1; endothelial genes: flk-1 and ve-cad; VSM gene: sm22 and pdgfβr; cardiac genes: nkx2.5. (d-e) To test cardiac potential, the Bry-GFP+/Flk-1+ cells were reaggregated in serum-free medium for one day and the resulting aggregates were cultured in cardiac differentiation conditions for 3 days. At this stage, the cells were harvested and subjected to intracellular staining with an antibody to cTnT. (d) Flow cytometric analysis demonstrating the proportion of cTnT+ cells present in cultures generated following different manipulations of Dox. The solid line represents cTnT+ cells whereas the shaded area represents control staining with secondary antibody alone. −Dox/−Dox: non-induced cells, +Dox/−Dox: addition of Dox to the reaggregation culture, +Dox/+Dox: addition of Dox to both the reaggregation and cardiac cultures, +Dox+GSI/−Dox: addition of Dox (0.5 μg/ml) and GSI (5 μM) to the reaggregation culture. (e) The expression of cardiac genes, nkx2.5, cardiac mhc, α-actin, mlc-2a and mlc-2v, in cells from the cardiac cultures. Treatments are indicated on the top of the figure.
When cultured for an additional three days in cardiac conditions, one-day Notch4-induced Bry-GFP+/Flk-1+ aggregates generated extensive areas of contracting cells (Supplementary video 1 online) that expressed cardiac genes (Fig2e +Dox/−Dox). FACS analysis revealed that greater than 60% of the cells in these cultures expressed cTnT (+Dox/−Dox, Fig. 2d) indicating that this population was highly enriched for cardiomyocytes. The control, non-induced population contained no contracting cells and did not express any of the cardiac genes or cTnT (−Dox/−Dox, Fig. 2d,e, Supplementary video 2 online). Induction of the cardiac program within the Bry-GFP+/Flk-1+ population by Notch4 was inhibited by the addition of GSI, indicating that the effect was due to active Notch signaling (+Dox+GSI/−Dox, Fig. 2d,e). If Dox was maintained during the plating of the aggregates in the cardiac cultures, the size of the cTnT-positive population was significantly reduced and the expression of cardiac genes was down regulated (+Dox/+Dox, Fig. 2d,e) demonstrating that the cardiogenic effects were stage specific. Taken together, these findings show that transient activation of the Notch pathway in the hemangioblast population efficiently and rapidly respecifies it to a cardiac fate.
The ability to respecify hemangioblast mesoderm was not unique to Notch4, as enforced expression of activated Notch1 in the Bry-GFP+/Flk-1+ population resulted in the development of contracting cTnT+ populations. (Supplementary Fig. 2 online). Kinetic analyses of EBs indicated that the target population for respecification is transient and restricted to the hemangioblast window of development. Flk-1+ cells isolated from day 3 and day 4 EBs but not those from day 5 upregulated expression of nkx2.5 and generated contracting cells following Dox induction (Fig. 3a,b).
Figure 3. Respecification by Notch signaling is restricted to the hemangioblast stage of EB development.
(a-b) Bry-GFP+/Flk-1+ cells isolated from day 3, 4 and 5 EBs were cultured as aggregates for 24 hours in the presence or absence of Dox. Aggregates from both groups were plated into microtiter wells and monitored for the development of contracting cells or subjected to gene expression analysis. Aggregates were evaluated daily between 3 and 5 days of culture for the presence of contracting cells. (a) The proportion of aggregates that contained contracting cells. (b) Expression of nkx2.5 in the induced (+) and non-induced (−) aggregates from the different populations. (c-f) To test if activation of endogenous Notch signaling can also respecify the hemangioblast population, day 3.25 Flk-1+ cells from the Bry-GFP ES cell line were cultured on OP9 or OP9-DL1 stromal cells in serum-free conditions for 3 days, in the absence or presence of GSI (5 μM). Following this culture step, the cells were harvested, stained with the anti-cTnT antibody and analyzed by flow cytometry. (c) Cells cultured on OP9-DL1 in the absence (−GSI) or (d) presence of inhibitor (+GSI). (e) Cells cultured on OP9 in the absence (−GSI) or (f) presence of inhibitor (+GSI). The black line represents cells stained with cTnT antibody whereas the shaded area represents control staining with secondary antibody alone.
As an alternate method of activating the Notch pathway in the hemangioblast population, day 3.25 Flk-1+ cells were co-cultured with OP9-Dl1 stromal cells engineered to express the Notch ligand Delta-like 133. Following 3 days of culture, cTnT+ (24%) contracting cells were detected in the cultures indicating that the respecification could be mediated by signaling through endogenous Notch receptors (Fig. 3c). A much lower proportion of cTnT+ cells (8%) developed from Bry-GFP+/Flk-1+ cells cultured with control OP9 cells not expressing Dl1 (Fig. 3e). Cardiomyocyte development on the OP9-Dl1 cells was inhibited by GSI (Fig. 3d), indicating that the effect was mediated by Notch signaling.
Notch respecifies the BL-CFC to a cardiac fate
To determine if the BL-CFC is the target of the Notch4-induced fate change, day 3.25 Flk-1+ cells were cultured in the blast colony assay4 in the presence or absence of Dox. In the absence of Dox, this population generated typical blast colonies that appeared as grape-like clusters of cells. When cultured in the presence of Dox, these cells formed compact colonies of tightly packed cells that were easy to distinguish from the blast colonies (Fig. 4a). The number of these compact colonies was similar to the number of blast cell colonies that developed in the non-induced cultures (Fig. 4b). Addition of GSI together with Dox inhibited the appearance of the compact colonies, indicating that their development was dependent on Notch signaling. Molecular analysis revealed that most of the compact colonies expressed genes indicative of cardiac, endothelial and vascular smooth muscle development (Fig. 4c, left panel). None of these colonies expressed gata-1. As expected, the blast colonies expressed the endothelial genes as well as gata-1 but did not express appreciable levels of the cardiac genes (Fig. 4c, right panel). With extended time in the methylcellulose cultures, some of the compact colonies began contracting indicating maturation of cardiac cells (Supplementary video 3 online). When picked from the methylcellulose cultures and replated in microtiter wells in the cardiac culture conditions, approximately 70% of the compact colonies generated contracting cells. Hemangioblast-derived blast colonies did not give rise to contracting cells under these conditions. Compact colonies grown on glass coverslips for 4 day in the presence of VEGF and basic FGF generated adherent populations that consisted of cTnT+/SMA+ cardiomyocytes, CD31+ endothelial cells, and SMA+/cTnT− vascular smooth muscle (VSM) cells as demonstrated by immunostaining (Fig. 4d). The expression profiles together with the immunostaining analyses strongly suggest that the compact colonies represent colonies of cardiovascular cells.
Figure 4. Effect of Notch4 expression on BL-CFC-derived blast colony development.
Day 3.25 Bry-GFP+/Flk-1+ cells were cultured in the methylcellulose blast colony assay in the presence or absence of Dox. (a) Photograph of blast (upper, −Dox) and compact (lower, +Dox) colonies following 4 days of culture. Original magnification 400x. (b) Number of blast or compact colonies generated in the absence or presence of Dox or in the presence of Dox and GSI. Colonies were scored following 4 days of culture. (c) Gene expression analysis of individual compact and blast colonies. cardiac genes: nkx2.5, cardiac α-actin, and mlc-2a; endothelail genes: flk-1 and ve-cad; VSM gene: sm22; hematopoietic gene: gata-1. Each lane represents a single 7-day old colony. (d) Immunostaining demonstrating the presence of cTnT, CD31, SMA in the adherent outgrowth of a single compact colony. Cells from a 7-day-old compact colony were grown on a glass coverslip for 4 days prior to staining. Green arrows indicate cardiac cells that express both cTnT and SMA; yellow arrows mark endothelial cells expressing CD31; orange arrows mark vascular smooth muscle cells expressing SMA. (Original magnification 200x). (e) Cell-dose response showing the relationship between the number of Bry-GFP+/Flk-1+ cells (X-axis) plated and the numbers of blast and compact colonies (Y-axis) that developed in blast colony cultures in the absence or presence of Dox. Error bars, s.e.m. (f) Photograph of a mixed lineage hematopoietic and cardiac colony (Original magnification 200x). Bry-GFP+/Flk-1+ cells were cultured for 1-2 day in the blast colony assay in the presence of Dox. Following this induction step, the entire contents of the methylcellulose culture was harvested, the developing colonies washed several times, and replated in the same volume in the blast colony assay without Dox. The secondary cultures were supplemented with Epo and IL-3 to support hematopoiesis within the colonies. (g) Gene expression analysis of individual mixed lineage colonies. cardiac genes: cardiac α-actin, and mlc-2a; endothelail genes: flk-1 and ve-cad; VSM gene: sm22 and pdgfβr; hematopoietic gene: gata-1. Each lane represents a single 7 day-old colony.
To establish the clonality of the compact cardiovascular colonies, we performed a cell dose-response experiment, in which different numbers of Bry+/Flk-1+ cells were plated in blast colony conditions in the absence or presence of Dox. As shown in Figure 4e, the relationship between the number of blast or compact colonies and the number of cells plated was linear, with a slope approaching 1, indicating that each colony was generated from a single colony-forming unit. Additionally, the number of compact and blast colonies generated at the different cell concentrations was the same, consistent with the notion that the compact colonies were derived from BL-CFCs.
To further investigate the relationship between the BL-CFCs and the progenitors that generate the compact colonies, we limited the exposure of the Bry+/Flk-1+ cells to Dox to 24-48 hours in an attempt to identify progenitors that were not fully respecified as assessed by their capacity to generate mixed hematopoietic/cardiac colonies. Following induction the developing colonies were removed from the Dox-containing methylcellulose and replated in hemangioblast methylcellulose supplemented with Epo and IL-3 to promote the expansion of any hematopoietic cells. Within 5 days of culture, approximately 20% of the colonies had a mixed morphology with an inner core of cells surrounded by hematopoietic-like cells (Fig. 4e, Supplementary Fig. 3 online). The remaining 80% were compact colonies, indicating that the respecification was almost complete following this period of induction. The cores within some of the mixed colonies began contracting following 7 days of the cultures (Supplementary video 4 online). When picked and replated in the cardiac cultures, approximately half of these mixed colonies generated contracting cells. Molecular analysis of these colonies confirmed the presence of the hematopoietic, endothelial and cardiac lineages (Fig. 4f). Together, these findings strongly support the interpretation that expression of Notch respecifies the fate of the BL-CFC from a progenitor with hematopoietic and vascular potential to one with cardiovascular potential.
Notch4 induction up-regulates components of the BMP and Wnt pathways
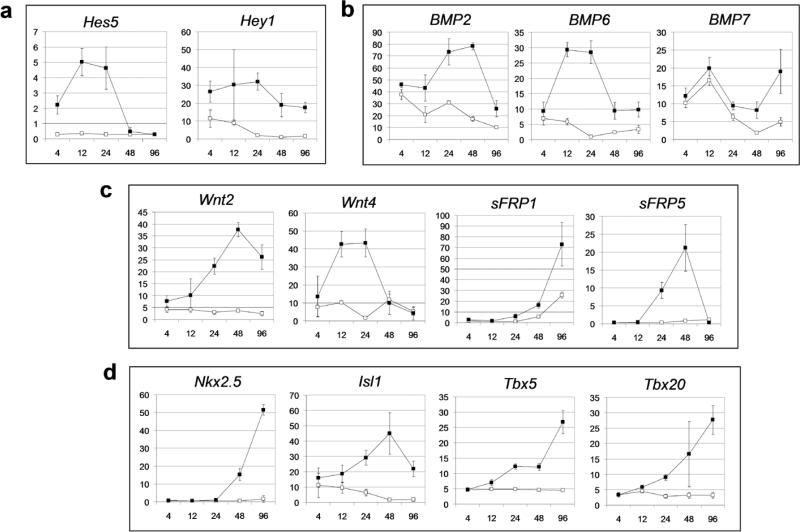
Microarray analyses of induced and non-induced Bry-GFP+/Flk-1+-derived populations at 4, 12, 24, 48, and 96 hours following Dox treatment revealed a rapid upregulation of expression of the Notch target genes Hes5 and Hey1 as well as of specific members of the BMP and Wnt signaling pathways (Fig. 5a). Expression of BMP2, BMP6, BMP7, Wnt2, Wnt4 (β-catenin-independent) and the Wnt inhibitors, sFRP1 and sFRP5 (Fig. 5c) were all upregulated following induction of Notch4. The expression patterns of the other BMP and Wnt family members did not change significantly. The upregulation of expression of transcription factors associated with cardiac development including Nkx2.5, Isl1, Tbx5 and Tbx20 (Fig. 5d) was detected 24 to 36 hours following the onset of expression of the above signaling molecules. The results from the microarray analysis were confirmed by quantitative RT-PCR for a representative number of genes (Supplementary Fig. 4 online). Taken together, these observations suggest that the cardiogenic effects of Notch may be mediated, in part, through the coordinated regulation of BMP and Wnt signaling pathways.
Figure 5. Microarray-based expression analysis of Bry-GFP+/Flk-1+ cells following Dox induction.
Day 3.25 of Bry-GFP+/Flk1+ cells cultured in cardiac differentiation conditions in the absence or presence of Dox were harvested at 4, 12, 24, 48, and 96 hours following induction for gene expression analysis by microarray. X axis: time points, Y axis: normalized expression intensity, average of three biological replicates; open squares: without Dox, closed squares: with Dox. The names of genes are indicated on top of the graphs. Error bars shown are the standard deviation in the normalized intensities determined for the three measurements. (a) Notch targets, (b) BMP family members, (c) genes related to Wnt signalling, (d) genes involved in cardiac induction.
BMP, β-catenin-independent Wnt and sFRP1 promote cardiac differentiation
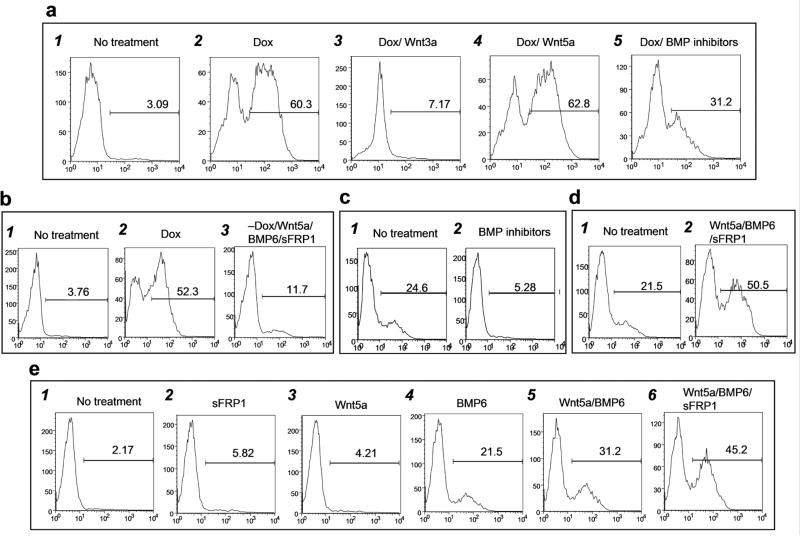
Given the findings from the microarray analysis, we investigated the effects of manipulating BMP and Wnt signaling on cardiac development from both the Bry-GFP+/Flk-1+ and the Bry-GFP+/Flk-1− populations. The addition of Wnt3a to the Notch4-induced Bry-GFP+/Flk-1+ population completely abolished cardiac development, indicating that inhibition of β-catenin-dependent Wnt signaling is required for the Notch mediated cardiac induction (Fig. 6a,3). In contrast, the addition of Wnt5a, a Wnt ligand that signals through a β-catenin-independent pathway, had no effect on the cardiac induction of Notch (Fig. 6a,4). Inhibition of BMP signaling through the addition of BMP receptors 1A and 1B, reduced the size of the Notch-induced cTnT+ population, indicating that BMP plays a role at this stage of cardiac development (Fig. 6a,5). To determine if it is possible to mimic the effects of Notch4 induction by manipulating both the BMP and Wnt pathways, BMP6, Wnt5a (used as an equivalent of β-catenin-independent Wnt4) and the Wnt inhibitor sFRP1 were added either alone or in combination to the non-induced Bry-GFP+/Flk-1+ population. When added individually, BMP6 and Wnt5a displayed little cardiogenic potential over a range of concentrations (not shown). The addition of sFRP1 did induce low levels of cTnT+ cells (not shown). The combination of the factors and the inhibitor did generate a cTnT+ population from the GFP+/Flk-1+ cells, however, the extent of cardiac development was never as large as that observed following Notch4 induction (Fig. 6b). These findings suggest that other factors may be required for the efficient respecification of the hemangioblast mesoderm to a cardiac fate.
Figure 6. Effects of cytokines on the cardiac differentiation from Bry-GFP+/Flk-1+ and Bry-GFP+/Flk-1− cells.
Bry-GFP+/Flk1+ and Bry-GFP+/Flk-1− cells were cultured in cardiac differentiation conditions containing different factors. Cardiac differentiation was monitored by the % cTnT+ cells that developed following 4 days of cultures. The factors added to the cultures are indicated. X axis: cTnT intensity, Y axis: relative cell number (a) Effects of various factors on the respecification of the day 3.25 Bry-GFP+/Flk-1+ population to a cardiac fate. (b) BMP6, Wnt5a, and sFRP1 partially respecified the day 3.25 Bry-GFP+/Flk-1+ cells to cardiomyocytes. (c) BMP signaling is required for cardiac development from day 3.25 Bry-GFP+/Flk-1− cardiac mesoderm. (d) BMP6, Wnt5a, and sFRP1 enhanced the differentiation of the day 3.25 Bry-GFP+/Flk-1− cells to cardiomyocytes. (e) Effects of various factors on cardiac differentiation from day 3.0 Bry-GFP+/Flk-1− cardiac mesoderm
In the next set of experiments, we investigated whether manipulating BMP and Wnt signaling can promote cardiac development from the Bry-GFP+/Flk-1− population. In the absence of any manipulation, day 3.25 EBs-derived cells will generate cardiomyocytes following aggregation and subsequent plating in cardiac cultures. The addition of BMP receptors 1A and 1B completely inhibited the generation of cTnT+ cells, indicating that BMP is required for this stage of cardiac development (Fig. 6c). When added individually, sFRP1, Wnt5a and BMP6 did not significantly increase cardiac development from this population (not shown). However, when added together, these factors substantially enhanced the generation of cTnT+ cells (Fig. 6d). We next evaluated the role of BMP and Wnt signaling on earlier stage (day 3.0) Bry-GFP+/Flk-1− cells that are unable to differentiate to the cardiac lineage without additional induction (Fig. 6e, 1). Neither sFRP1 nor Wnt5a promoted cardiac development from this population (Fig. 6e, 2,3). In contrast, BMP6 did induce the formation of cTnT+ cells (Fig. 6e, 4). The level of cardiomyocyte development induced by BMP6 was enhanced by the addition of Wnt5a (Fig. 6e, 5). As observed with the day 3.25 population, the addition of all three factors induced highest level of cTnT+ cells, resulting in the development of populations consisting of almost 50% cardiac cells (Fig. 6e, 6). These findings demonstrate the factors and inhibitors induced by Notch expression do regulate cardiac development from cardiac mesoderm.
Discussion
The findings presented in this study indicate that Notch signaling can initiate cardiac lineage specification and demonstrate that it does so in part through the coordinated activation of BMP signaling and the inhibition of the β-catenin-dependent Wnt pathway. The cardiogenic effects of Notch signaling were demonstrated on the normal progression of cardiac mesoderm (Bry-GFP+/Flk-1−) to cardiomyocytes as well as by the dramatic respecification of hemangioblasts to cardiovascular progenitors. These progenitors appear to be similar to the cardiovascular progenitors (cardiovascular colony-forming cell; CV-CFC) that we recently identified6 (Model, Fig. 7). However, their isolation is based on temporal patterns of Flk-1 development that requires two different cell sorting steps, limiting the routine generation of large numbers of such cells. The ability to respecify the Bry-GFP+/Flk-1+ hemangioblast population to a cardiovascular fate through activation of Notch signaling eliminates one sorting step, thereby providing ready access to large numbers of enriched cardiovascular cells for functional studies in vitro and in vivo.
Figure 7.
Model depicting the role of Notch signaling in cardiac differentiation and respecification of the hemangioblast.
Our microarray analysis revealed that Notch4 functions through the activation of BMP signaling and the inhibition of the canonical Wnt pathway. With respect to BMP signaling, our findings are in line with other studies demonstrating that BMP2, BMP6 and BMP7 are expressed in the developing heart35 and that signaling through this pathway is required for cardiac development16,36. The specific induction of Wnt4 and Wnt2 is striking and suggests that they may play early roles in the establishment of the cardiac lineage. Recent studies have shown that Wnt2 can direct cardiac differentiation from mesoderm37,38. Wnt4 is known to function through the β-catenin-independent pathway that has been shown to inhibit the β-catenin-dependent pathway39. The upregulation of Wnt4 together with the Wnt inhibitors sFRP1 and sFRP5 following Notch4 induction may function to rapidly and efficiently inhibit β-catenin-dependent signaling, an event required for cardiac specification of mesoderm17,22. Our functional analyses do indeed show that the combination of factors induced by Notch4, namely BMP, β-catenin-independent Wnt, and the Wnt inhibitor sFRP1 display robust cardiogenic activity on the Bry-GFP+/Flk-1− cardiac mesoderm population.
Our findings appear to be at odds with several studies demonstrating that signaling though the Notch pathway is inhibitory for cardiac development. Rones et al. showed that activation of the Notch pathway in stage 18-19 Xenopus embryos suppressed the expression of genes indicative of cardiac maturation23. Conversely, suppression of Notch signaling in these embryos enhanced the expression of cardiac markers. These effects appear to be at the level of development and maturation of the cardiac lineage rather than on its induction, as the embryos already express both nkx2.5 and gata-4. The role for Notch signaling in cardiac induction that we have described in this study is on populations that represent earlier stages of development that do not yet express nkx2.5 or, in the case of the BL-CFC, on populations undergoing differentiation to other lineages. When induced at later stages in the cardiac cultures, following induction of the cardiac program, Notch4 expression inhibited maturation of the cardiac lineage. These findings are consistent with those demonstrating that RBP-Jκ deficient ES cells or ES cells expressing a RBP-Jκ inhibitory protein exhibit increased cardiomyogenesis compared to wild-type controls24,25. Together, these findings indicate that RBP-Jκ mediated Notch signaling is not required for cardiac specification and negatively regulates specific stages of cardiomyogenesis. Given these observations, it is possible that the cardiogenic effects of Notch signaling described here are mediated through one of the previously described RBP-Jκ-independent pathways40-47. Ongoing experiments are aimed at addressing this possibility.
In summary, the findings reported here define a novel role for Notch signaling at the level of mesoderm specification for the establishment of the cardiac lineage. The rapid and efficient induction of cardiac development following Notch induction in the Flk-1+ hemangioblast mesoderm provides a novel approach for efficiently and reproducibly generating cardiovascular cells from ES cell. This approach provides ready access to cardiovascular progenitors and more mature populations for both in vitro and in vivo functional analysis as well as for developmental biology studies aimed at defining the earliest molecular program involved in the specification of this lineage. Information obtained from such approaches with mouse ES cells will provide important insights into the signaling pathways that regulate the establishment of the cardiac lineage from human ES cells.
Experimental Procedures
ES cell culture and differentiation
ES cells were maintained on irradiated feeders in Dulbecco's Modified Eagle Medium (DMEM) supplemented with 15% fetal calf serum (FCS), 10% ES cell conditioned medium, penicillin, streptomycin, 1.5 × 10−4 M monothioglycerol (MTG; Sigma) and LIF (1% conditioned medium). Prior to induction of differentiation, cells were passaged 2 times on gelatin-coated plates in Iscove Modified Dulbecco Medium (IMDM) containing the same supplements mentioned above to deplete the population of feeder cells. For the generation of EBs, the cells were harvested and cultured in 60 mm low attachment Petri grade dishes (VWR) with IMDM supplemented with 2 mM l-glutamine (Gibco/BRL), 200 μg/mL transferrin (Boehringer Mannheim), 0.5 mM ascorbic acid (Sigma), 4 × 10−4 M MTG plus 15% FCS. For reaggregation cultures to support the differentiation of the hematopoietic and vascular lineages, 3 × 105 Flk-1+ cells/ml were cultured for 2 days in ultra-low attachment 24-well plates (Corning Costar) with the same EB differentiation medium plus 5% Protein-Free Hybridoma Medium-II (PFHM-II, Invitrogen).
Notch4 Inducible ES cells
The activated form of Notch4 cDNA (int-3)27 tagged with hemagglutinin (HA) sequence was obtained from Dr. Kitajewski. The tet-on inducible ES cell line, Ainv18, obtained from Dr. Kyba was further modified by targeting the EGFP cDNA into brachyury locus as described in our earlier study11. The Notch4 cDNA was introduced into the Ainv 18 and the modified Ainv ES cell lines by the approach previously described31. Briefly, the cDNA fragment of the activated form of Notch4 tagged with HA was inserted into the plox plasmid by convenient restriction sites to generate plox-Notch4/HA. Ainv18 and the modified cell line were targeted with plox-Notch4/HA by co-electroporation of 40 μg each of plox-Notch4/HA and the Cre recombinase expression plasmid, pSalk-Cre. Positive clones were screened in ES medium with 300 μg/ml G418 (GIBCO) and isolated to generate inducible cell lines Bry-GFP/Ainv-Notch4. The positive clones were confirmed by immunohistochemistry detecting HA expression after induction.
Flow Cytometry
Dissociated cells were incubated with biotinylated mAbs (against Flk-1, VE-cad, or CD41) in PBS containing 10% FCS on ice for 30 min. The cells were then washed once and incubated with streptavidin-PE-Cy5 (BD Pharmingen) for another 30 min on ice. Following an additional two washes, the cells were analyzed on a FACSCalibur flow cytometer (Becton Dickinson) or sorted on a Moflo cell sorter (Cytomation). For Troponin T or HA staining, cells were fixed in 4% paraformaldehyde (PFA) for 30 min and then incubated in a permeabilizing buffer consisting of PBS with 10% FCS and 0.1% saponin (Sigma) for 10 min. Following fixing and permeabilization, the cells were washed twice and incubated with an anti-Troponin T (unconjugated mouse antibody, Lab Vision) or anti-HA (conjugated with biotin, Covance) antibody for 30 min. After two washes, the cells were incubated with a secondary APC-conjugated goat anti-mouse antibody (for Troponin T antibody) or streptavidin-PE-Cy5 (for biotinylated HA antibody) for 30 min. Finally, the cells were washed twice with permeabilizing buffer and then twice with buffer without saponin.
Colony assays
The blast and hematopoietic colony assays were performed as described4. Dox was added at 0.5 μg/ml to induce Notch4 expression and γ-secretase inhibitor X (L685,458, Calbiochem) at 5 μM to block Notch signaling in the blast colony culture. To generate mixed hemangioblast/cardiac colonies, blast colony growth was initiated in standard blast colony cultures containing Dox for 24 hours. The developing colonies were then washed from with methylcellulose with IMDM containing 10% FCS to remove Dox. The colonies were recultured in blast colony methylcellulose supplemented with Erythropoietin (2 U/ml) and IL-3 (1% conditioned medium). Mixed colonies containing an inner cardiac core surrounded by outer hematopoietic cells were picked for analysis at day 7.
Cardiac Assay
Sorted cells were reaggregated for 24 hours in StemPro-34 serum-free medium (Invitrogen) containing 2 mM l-glutamine (GIBCO/BRL), transferrin (200 μg/ml), 0.5 mM ascorbic acid and 4.5 × 10−4 M MTG at 1 × 105 cells per ml in ultra-low-attachment 24-well plates (Costar). Single aggregates or pools of aggregates were replated in gelatin-coated 96- or 24-well plates containing StemPro with 2 mM l-glutamine for cardiac culture. VEGF at 5 ng/ml and basic FGF 30 ng/ml were added in the cardiac assay only for Bry-GFP+/Flk1− but not for Bry-GFP+/Flk1+ cell differentiation. Following 2 to 4 days of culture the proportion of aggregates containing contracting cells was scored and the number of Troponin T-positive cells was evaluated by flow cytometric analyses. For the aggregated and cardiac cultures, doxycycline (Dox) was used at 0.5 μg/ml and γ-secretase inhibitor X at 5 μM (dissolved in DMSO). The same concentration of DMSO was added to the control cultures. Medium was changed every 2 days to provide fresh Dox and inhibitor if necessary.
Gene expression analysis
Gene expression analyses of colonies or small amount of mRNA was performed by polyA+ global amplification polymerase chain reaction (PCR) as previously described48,49. Amplified PCR products were resolved on agarose gels and transferred to a Zeta-probe GT membrane (Bio-Rad). Genes of interest were then probed by 32P randomly primed cDNA fragments (Ready-to-Go Labeling; Pharmacia) corresponding to the 3’ regions of the genes. For gene-specific PCR, total RNA was extracted from cells using the RNeasy mini-kit (Qiagen). One microgram total RNA was used to generate cDNAs by reverse transcription using the Omniscript RT kit (Qiagen) with random hexamer and then the cDNAs were subjected to PCR. The sequences of the cDNA fragments for probing the 3’-regions of the genes and the primer sequences for gene-specific PCR are available upon request. Real-time quantitative PCR was performed on a MasterCycler EP RealPlex (Eppendorf). Experiments were done in triplicate using Platinum SYBR GreenER qPCR SuperMix (Invitrogen). All annealing reactions were carried out at 60 °C.
Immunohistochemistry
Cell aggregates or colonies were plated on gelatin-coated coverslips and cultured for 3-4 days in StemPro with 2 mM l-glutamine. Cells cultured on coverslips were fixed in 4% paraformaldehyde for 30 min, washed twice in PBS and permeabilized in 0.5% soponin/PBS for 10 min. Cells attached to the coverslips were incubated with a primary antibody for 2 hours at 37 °C. After 3 washes, the cells on coverslips were incubated with a secondary antibody for 1 hour in the dark. Finally, the coverslips were washed 3 times and then inverted onto a drop of DAPI (Vector Laboratories). Fluorescence was visualized using a Leica DMRA2 fluorescence microscope (Wetzlar) and recorded using a digital Hamamatsu CCD camera. The following antibodies were used for staining: anti-mouse CD31 from BD Biosciences Pharmingen, anti-mouse troponin T and anti-mouse smooth muscle actin (SMA) from NeoMarkers. The Cy2-, Cy3- and Cy5-conjugated secondary antibodies were purchased from Jackson ImmunoResearch.
Cell culture on Dll-1 expressing stromal cells
The OP9-DL1 cell line was a gift from Dr. Zuniga-Pflucker40. The OP9-DL1 cells were cultured in a 24-well plate and irradiated before use. Day 3.25 EB-derived Flk-1+ cells (3×104 per well) were seeded onto OP9 cells in the same medium used for the cardiac cultures. γ-Secretase inhibitor X (dissolved in DMSO) at 5 μM or a corresponding volume of DMSO was included in the cultures. Medium was changed everyday to supply fresh inhibitor. After 3 days of culture, the cells were harvested and subjected to flow cytometric analysis to determine the number of cTnT+ cells.
Microarray analyses
mRNA expression was analyzed by CodeLink Microarray per manufacturer's instructions (CodeLink Express Assay Reagent Kit; GE Healthcare). Briefly, one microgram of total RNA from each sample was reverse-transcribed into cDNA using T7-(dT)24 primers, and biotinylated cRNA prepared from this cDNA template by in vitro transcription. Ten micrograms of fragmented, biotinylated cRNA was hybridized to each CodeLink Mouse Whole Genome Array for 18 hours at 37°C. Afterwards, arrays were washed in 75 mM Tris-HCL, pH 7.6, 113 mM NaCl, 0.0375% Tween-20 for 1 hour at 46°, then stained with a 1:500 dilution of streptavidin-Alexa 647 (Molecular Probes) for 30 min at room temperature. Following the staining, arrays were washed three times, 5 min each, at room temperature with 0.1M Tris-HCL, pH 7.6, 0.15 M NaCl, 0.05% Tween-20, then once with 0.1X SSC/0.05% Tween for 30 sec, then dried in a centrifuge. Processed arrays were scanned using a GenePix 4000B Scanner and GenePixPro v4 software (Axon Instruments). Images were analyzed using CodeLink Expression Analysis Software, and the raw intensity data exported into GeneSpring GX (Agilent Life Sciences), and raw intensity signals for each array were median normalized. Because some CodeLink probes were improperly annotated as to their intended target, refinement of gene-to-probe associations was accomplished by analysis using VistaGen's Fred™ knowledgebase which maps the genomic coordinates of probes with that of the exons of genes and provides various bioinformatics analytical and functional genomics tools. All genomic coordinates on the mouse genome build 36 were determined using BLAST. Invalid probes, such as the ones that target multiple genes or intergenic regions on the genome, were removed from subsequent analyses. The data from three experiments were averaged. One-way ANOVA (p <0.05) was used to generate a set of probes with statistically significant differences between dox− and dox+ treatments, using the Benjamini and Hochberg false discovery rate for multiple testing correction; the genes shown in Figure 5 are members of this ANOVA-qualified probe set.
Cardiac induction by cytokines and inhibitors
The culture procedures to test the effects of cytokines and inhibitors on the cardiac differentiation from both Bry-GFP+/Flk-1− and Bry-GFP+/Flk-1+ populations were the same as mentioned in the cardiac assay. To test the effects of Wnt3a and Wnt5a on the Dox-induced cardiomyogenesis, factors were added (150 ng/ml) individually to Dox to Bry-GFP+/Flk-1+ cells. Dox was maintained in culture for 24 hours and Wnt for 48 hours. To block BMP signaling, BMPR-1A and -1B were added (each at 1500 ng/ml) to the Bry-GFP+/Flk-1+ cells and maintained until cells were harvested. To recapitulate the cardiogenic effects of Notch4, BMP6 (0.1 and 1 ng/ml for Bry-GFP+/Flk-1+ and Bry-GFP+/Flk-1− cells, respectively) and Wnt5a (150 ng/ml) were added directly to the sorted cells, whereas the addition of sFRP1 (500 ng/ml) was delayed for 18 hours according to the expression patterns observed in our microarray analysis. The cytokines and inhibitor were removed from the culture at 48 hours. Factors and inhibitors were tested at different concentrations. Optimal concentrations were used for the studies.
Supplementary Material
Acknowledgments
We would like to thank members of the Keller laboratory for discussions and critical reading of the manuscript, Stefan Irion for providing the Bry-GFP/Ainv ES cell line. This work was supported by NIH grants R01 HL71800, R01 HL 48834.
Footnotes
Author Contributions
V.C.C. and G.K conceived the experiments and V.C.C. designed experimental details. R.S. and D.J. performed microarray analyses. X.C. generated Notch1-inducible ES cell line and participated in experimental design. V.C.C. performed all remaining experiments. The manuscript was written by V.C.C. and G.K.
The microarray datasets for Notch4-induced Flk-1+ cells are available with accession number GSE12425 in GEO (Gene Expression Omnibus) of NCBI.
References
- 1.Murry CE, Keller G. Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic developmentm. Cell. 2008;132:661–80. doi: 10.1016/j.cell.2008.02.008. [DOI] [PubMed] [Google Scholar]
- 2.Parameswaran M, Tam PP. Regionalisation of cell fate and morphogenetic movement of the mesoderm during mouse gastrulation. Dev. Genet. 1995;17:16–28. doi: 10.1002/dvg.1020170104. [DOI] [PubMed] [Google Scholar]
- 3.Kinder SJ, Tsang TE, Quinlan GA, Hadjantonakis AK, Nagy A, Tam PP. The orderly allocation of mesodermal cells to the extraembryonic structures and the anteroposterior axis during gastrulation of the mouse embryo. Development. 1999;126:4691–701. doi: 10.1242/dev.126.21.4691. [DOI] [PubMed] [Google Scholar]
- 4.Choi K, Kennedy M, Kazarov A, Papadimitriou JC, Keller G. A common precursor for hematopoietic and endothelial cells. Development. 1998;125:725–732. doi: 10.1242/dev.125.4.725. [DOI] [PubMed] [Google Scholar]
- 5.Huber TL, Kouskoff V, Fehling HJ, Palis J, Keller G. Haemangioblast commitment is initiated in the primitive streak of the mouse embryo. Nature. 2004;432:625–30. doi: 10.1038/nature03122. [DOI] [PubMed] [Google Scholar]
- 6.Kennedy M, D'Souza SL, Lynch-Kattman M, Schwantz S, Keller G. Development of the hemangioblast defines the onset of hematopoiesis in human ES cell differentiation cultures. Blood. 2007;109:2679–87. doi: 10.1182/blood-2006-09-047704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kattman SJ, Huber TL, Keller GM. Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev. Cell. 2006;11:723–732. doi: 10.1016/j.devcel.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 8.Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, Chen Y, Qyang Y, Bu L, Sasaki M, Martin-Puig S, Sun Y, Evans SM, Laugwitz KL, Chien KR. Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell. 2006;127:1151–65. doi: 10.1016/j.cell.2006.10.029. [DOI] [PubMed] [Google Scholar]
- 9.Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, Schultheiss TM, Orkin SH. Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell. 2006;127:1137–50. doi: 10.1016/j.cell.2006.10.028. [DOI] [PubMed] [Google Scholar]
- 10.Yang L, Soonpaa MH, Adler ED, Roepke TK, Kattman SJ, Kennedy M, Henckaerts E, Bonham K, Abbott GW, Linden RM, Field LJ, Keller GM. Human cardiovascular progenitor cells develop from a KDR+ embryonic-stem-cell-derived population. Nature. 2008;453:524–8. doi: 10.1038/nature06894. [DOI] [PubMed] [Google Scholar]
- 11.Fehling HJ, Lacaud G, Kubo A, Kennedy M, Robertson S, Keller G, Kouskoff V. Tracking mesoderm induction and its specification to the hemangioblast during embryonic stem cell differentiation. Development. 2003;130:4217–4227. doi: 10.1242/dev.00589. [DOI] [PubMed] [Google Scholar]
- 12.Kouskoff V, Lacaud Gl, Schwantz S, Fehling HJ, Keller G. Sequential development of hematopoietic and cardiac mesoderm during embryonic stem cell differentiation. Proc. Natl. Acad. Sci. USA. 2005;102:13170–13175. doi: 10.1073/pnas.0501672102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ema M, Takahashi S, Rossant J. Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood. 2006;107:111–117. doi: 10.1182/blood-2005-05-1970. [DOI] [PubMed] [Google Scholar]
- 14.Schultheiss T, Burch J, Lassar A. A role for bone morphogenetic proteins in the induction of cardiac myogenesis. Genes Dev. 1997;11:451–462. doi: 10.1101/gad.11.4.451. [DOI] [PubMed] [Google Scholar]
- 15.Andreé B, Duprez D, Vorbusch B, Arnold H, Brand T. BMP-2 induces ectopic expression of cardiac lineage markers and interferes with somite formation in chicken embryos. Mech. Dev. 1998;70:119–131. doi: 10.1016/s0925-4773(97)00186-x. [DOI] [PubMed] [Google Scholar]
- 16.Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]
- 17.Marvin MJ, Di Rocco G, Gardiner A, Bush SM, Lassar AB. Inhibition of Wnt activity induces heart formation from posterior mesoderm. Genes Dev. 2001;15:316–327. doi: 10.1101/gad.855501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nostro MC, Cheng X, Keller GM, Gadue P. Wnt, activin, and BMP signaling regulate distinct stages in the developmental pathway from embryonic stem cells to blood. Cell Stem Cell. 2007;2:60–71. doi: 10.1016/j.stem.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ueno S, Weidinger G, Osugi T, Kohn AD, Golob JL, Pabon L, Reinecke H, Moon RT, Murry CE. Biphasic role for Wnt/beta-catenin signaling in cardiac specification in zebrafish and embryonic stem cells. Proc. Natl. Acad. Sci. USA. 2007;104:9685–90. doi: 10.1073/pnas.0702859104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Naito AT, Shiojima I, Akazawa H, Hidaka K, Morisaki T, Kikuchi A, Komuro I. Developmental stage-specific biphasic roles of Wnt/beta-catenin signaling in cardiomyogenesis and hematopoiesis. Proc. Natl. Acad. Sci. USA. 2006;103:19812–17. doi: 10.1073/pnas.0605768103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Qyang Y, et al. The renewal and differentiation of Isl1+ cardiovascular progenitors are controlled by wnt/β-catenin pathway. Cell Stem Cell. 2007;1:165–179. doi: 10.1016/j.stem.2007.05.018. [DOI] [PubMed] [Google Scholar]
- 22.Schneider VA, Mercola M. Wnt antagonism initiates cardiogenesis in Xenopus laevis. Genes Dev. 2001;15:304–15. doi: 10.1101/gad.855601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Rones MS, McLaughlin KA, Raffin M, Mercola M. Serrate and Notch specify cell fates in the heart field by suppressing cardiomyogenesis. Development. 2000;127:3865–76. doi: 10.1242/dev.127.17.3865. [DOI] [PubMed] [Google Scholar]
- 24.Schroeder T, Fraser ST, Ogawa M, Nishikawa S, Oka C, Bornkamm GW, Nishikawa S, Honjo T, Just U. Recombination signal sequence-binding protein Jkappa alters mesodermal cell fate decisions by suppressing cardiomyogenesis. Proc. Natl. Acad. Sci. USA. 2003;100:4018–4023. doi: 10.1073/pnas.0438008100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Nemir M, Croquelois A, Pedrazzini T, Radtke F. Induction of Cardiogenesis in embryonic stem cells via downregulation of Notch1 signaling. Circ. Res. 2006;98:1471–8. doi: 10.1161/01.RES.0000226497.52052.2a. [DOI] [PubMed] [Google Scholar]
- 26.Williams R, Lendahl U, Lardelli M. Complementary and combinatorial patterns of Notch gene family expression during early mouse development. Mech Dev. 1995;53:357–68. doi: 10.1016/0925-4773(95)00451-3. [DOI] [PubMed] [Google Scholar]
- 27.Uyttendaele H, Marazzi G, Wu G, Yan Q, Sassoon D, Kitajewski J. Notch4/int-3, a mammary proto-oncogene, is an endothelial cell-specific mammalian Notch gene. Development. 1996;122:2251–2259. doi: 10.1242/dev.122.7.2251. [DOI] [PubMed] [Google Scholar]
- 28.Shirayoshi Y, Yuasa Y, Suzuki T, Sugaya K, Kawase E, Ikemura T, Nakatsuji N. Proto-oncogene of int-3, a mouse Notch homologue, is expressed in endothelial cells during early embryogenesis. Genes Cells. 1997;2:213–24. doi: 10.1046/j.1365-2443.1997.d01-310.x. [DOI] [PubMed] [Google Scholar]
- 29.Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J. Clin. Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Loomes KM, Taichman DB, Glover CL, Williams PT, Markowitz JE, Piccoli DA, Baldwin HS, Oakey RJ. Characterization of Notch receptor expression in the developing mammalian heart and liver. Am. J. Med. Genet. 2002;112:181–9. doi: 10.1002/ajmg.10592. [DOI] [PubMed] [Google Scholar]
- 31.Kyba M, Perlingeiro RC, Daley GQ. HoxB4 confers definitive lymphoid-myeloid engraftment potential on embryonic stem cell and yolk sac hematopoietic progenitors. Cell. 2002;109:29–37. doi: 10.1016/s0092-8674(02)00680-3. [DOI] [PubMed] [Google Scholar]
- 32.Das I, Craig C, Funahash Y, Jung KM, Kim TW, Byers R, Weng AP, Kutok JL, Aster JC, Kitajewski J. Notch oncoproteins depend on gamma-secretase/presenilin activity for processing and function. J. Biol. Chem. 2004;279:30771–30780. doi: 10.1074/jbc.M309252200. [DOI] [PubMed] [Google Scholar]
- 33.Lints TJ, Parsons LM, Hartley L, Lyons I, Harvey RP. Nkx-2.5: a novel murine homeobox gene expressed in early heart progenitor cells and their myogenic descendants. Development. 1993;119:419–431. doi: 10.1242/dev.119.2.419. [DOI] [PubMed] [Google Scholar]
- 34.Schmitt TM, de Pooter RF, Gronski MA, Cho SK, Ohashi PS, Zuniga-Pflucker JC. Induction of T cell development and establishment of T cell competence from embryonic stem cells differentiated in vitro. Nat. Immunol. 2004;5:410–417. doi: 10.1038/ni1055. [DOI] [PubMed] [Google Scholar]
- 35.Dudley T, Robertson EJ. Overlapping expression domains of bone morphogenetic protein family members potentially account for limited tissue defects in BMP7 deficient embryos. Dev. Dyn. 1997;208:349–362. doi: 10.1002/(SICI)1097-0177(199703)208:3<349::AID-AJA6>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 36.Kim RY, Robertson EJ, Solloway MJ. Bmp6 and Bmp7 are required for cushion formation and septation in the developing mouse heart. Dev. Biol. 2001;235:449–66. doi: 10.1006/dbio.2001.0284. [DOI] [PubMed] [Google Scholar]
- 37.Wang H, Gilner JB, Bautch VL, Wang DZ, Wainwright BJ, Kirby SL, Patterson C. Wnt2 coordinates the commitment of mesoderm to hematopoietic, endothelial, and cardiac lineages in embryoid bodies. J. Biol. Chem. 2007;282:782–91. doi: 10.1074/jbc.M606610200. [DOI] [PubMed] [Google Scholar]
- 38.Alexandrovich A, Arno M, Patient RK, Shah AM, Pizzey JA, Brewer AC. Wnt2 is a direct downstream target of GATA6 during early cardiogenesis. Mech. Dev. 2006;123:297–311. doi: 10.1016/j.mod.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 39.Pandur P, Lasche M, Eisenberg LM, Kuhl M. Wnt-11 activation of a non-canonical Wnt signalling pathway is required for cardiogenesis. Nature. 2002;418:636–641. doi: 10.1038/nature00921. [DOI] [PubMed] [Google Scholar]
- 40.Shawber C, Nofziger D, Hsieh JJ, Lindsell C, Bogler O, Hayward D, Weinmaster G. Notch signaling inhibits muscle cell differentiation through a CBF1-independent pathway. Development. 1996;122:3765–73. doi: 10.1242/dev.122.12.3765. [DOI] [PubMed] [Google Scholar]
- 41.Nofziger D, Miyamoto A, Lyons KM, Weinmaster G. Notch signaling imposes two distinct blocks in the differentiation of C2C12 myoblasts. Development. 1999;126:1689–702. doi: 10.1242/dev.126.8.1689. [DOI] [PubMed] [Google Scholar]
- 42.Wilson-Rawls J, Molkentin JD, Black BL, Olson EN. Activated notch inhibits myogenic activity of the MADS-Box transcription factor myocyte enhancer factor. 2C. Mol. Cell. Biol. 1999;19:2853–2862. doi: 10.1128/mcb.19.4.2853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Guan E, Wang J, Laborda J, Norcross M, Baeuerle PA, Hoffman T. T cell leukemia-associated human Notch/TAN-1 has I B-like activity and physically interacts with NF- B proteins in T cells. J. Exp. Med. 1996;183:2025–2032. [Google Scholar]
- 44.Axelrod JD, Matsuno K, Artavanis-Tsakonas S, Perrimon N. Interaction between Wingless and Notch signaling pathways mediated by Dishevelled. Science. 1996;271:1826–1832. doi: 10.1126/science.271.5257.1826. [DOI] [PubMed] [Google Scholar]
- 45.Blokzijl A, Dahlqvist C, Reissmann E, Falk A, Moliner A, Lendahl U, Ibanez CF. Cross-talk between the Notch and TGF-beta signaling pathways mediated by interaction of the Notch intracellular domain with Smad3. J. Cell. Biol. 2003;163:723–728. doi: 10.1083/jcb.200305112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Dahlqvist C, Blokzijl A, Chapman G, Falk A, Dannaeus K, Ibanez CF, Lendahl U. Functional Notch signaling is required for BMP4-induced inhibition of myogenic differentiation. Development. 2003;130:6089–6099. doi: 10.1242/dev.00834. [DOI] [PubMed] [Google Scholar]
- 47.Sun Y, Lowther W, Kato K, Bianco C, Kenney N, Strizzi L, Raafat D, Hirota M, Khan NI, Bargo S, Jones B, Salomon D, Callahan R. Notch4 intracellular domain binding to Smad3 and inhibition of the TGF-beta signaling. Oncogene. 2005;24:5365–5374. doi: 10.1038/sj.onc.1208528. [DOI] [PubMed] [Google Scholar]
- 48.Brady G, Barbara M, Iscove NN. Representative in vitro cDNA amplification from individual hematopoietic cells and colonies. Methods Mol. Cell Biol. 1990;2:17–25. [Google Scholar]
- 49.Robertson SM, Kennedy M, Shannon JM, Keller G. A transitional stage in the commitment of mesoderm to hematopoiesis requiring the transcription factor SCL/tal-1. Development. 2000;127:2447–2459. doi: 10.1242/dev.127.11.2447. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.