Abstract
To determine the influence of time from injury to surgery on neurological recovery and length of stay (LOS) in an observational cohort of individuals with traumatic spinal cord injury (tSCI), we analyzed the baseline and follow-up motor scores of participants in the Rick Hansen Spinal Cord Injury Registry to specifically assess the effect of an early (less than 24 h from injury) surgical procedure on motor recovery and on LOS. One thousand four hundred and ten patients who sustained acute tSCIs with baseline American Spinal Injury Association Impairment Scale (AIS) grades A, B, C, or D and were treated surgically were analyzed to determine the effect of the timing of surgery (24, 48, or 72 h from injury) on motor recovery and LOS. Depending on the distribution of data, we used different types of generalized linear models, including multiple linear regression, gamma regression, and negative binomial regression. Persons with incomplete AIS B, C, and D injuries from C2 to L2 demonstrated motor recovery improvement of an additional 6.3 motor points (SE=2.8 p<0.03) when they underwent surgical treatment within 24 h from the time of injury, compared with those who had surgery later than 24 h post-injury. This beneficial effect of early surgery on motor recovery was not seen in the patients with AIS A complete SCI. AIS A and B patients who received early surgery experienced shorter hospital LOS. While the issues of when to perform surgery and what specific operation to perform remain controversial, this work provides evidence that for an incomplete acute tSCI in the cervical, thoracic, or thoracolumbar spine, surgery performed within 24 h from injury improves motor neurological recovery. Early surgery also reduces LOS.
Key words: : clinical trials, spinal cord injury, surgical decompression, timing of surgery, translational research
Introduction
Each year, the lives of thousands of persons around the globe are irrevocably altered after being paralyzed by an acute traumatic spinal cord injury (tSCI).1,2 Therapeutic initiatives such as surgical stabilization and decompression, hemodynamic manipulation, and pharmacological interventions are intended to prevent further neurological deterioration while encouraging the functional recovery of injured neurological elements. Proven pharmaceutical treatment options to improve neurologic function and reduce the substantial burden of disability are limited.3 The influence of hemodynamic manipulation on neurological recovery after tSCI also remains uncertain.4,5 The decision to operate rather than to pursue nonsurgical treatment, although still controversial,6 has gained popularity because of patterns of surgical practice, expert opinion, and resource availability that has tended to favor surgery.7 The influence of surgical decompression and stabilization, particularly when it is performed early after the injury, has recently gained some support as an intervention that may benefit neurological recovery.8,9
The identification of effective interventions and acute therapies for persons with acute tSCI remain an important, although elusive, objective because of the dramatic impact that even modest improvements in motor function could have on this patient population.10–12
Over the past four decades, considerable progress in our scientific understanding of SCI has generated numerous therapeutic interventions with very promising results reported in preclinical studies.13,14 Preclinical studies have investigated the influence of time from injury to decompression and have generally shown a beneficial effect of early decompression,15–23 although most of these animal models use slow mechanisms of injury that severely underrepresent the velocity of human injuries. Also, not all preclinical studies have demonstrated the benefits of early surgical decompression.24–26
Surgeons, who often provide acute care for those with tSCI, understand the concept of secondary injury to the spinal cord27 and find it intuitively appealing that some of the early neurological recovery that occurs in many patients may be enhanced or accelerated after surgical decompression and stabilization. Whether or not this neurological recovery simply reflects the natural history of neurological recovery or whether its trajectory of improvement and ultimate magnitude can be influenced by surgical procedure is not fully known. Also difficult to quantify is the potential influence of surgical timing on the occurrence of adverse events and the costs of care as reflected in length of hospital stay. The optimal timing of an operation in promoting motor recovery has considerable health services implications and thus is a question of substantial controversy and relevance to persons with acute tSCI as well as to the health care system and society in general.
Surgical intervention encompasses a combination of either stabilization and/or decompression, both of which are hypothesized to improve the environment around the traumatically injured spinal cord. Performing early decompression and stabilization of these injuries would appear to be a logical intervention that could minimize secondary injury and optimize neurological recovery.
Several clinical trials have focused on the timing of surgical intervention and have produced conflicting results. Fehlings and associates8 demonstrated an increased odds ratio of AIS improvement in traumatic cervical SCI when surgery was performed within 24 h from injury. This study has faced some criticism, mostly because of an imbalance between early and late surgery groups with respect to the initial American Spinal Injury Association Impairment Scale (AIS), although the authors attempted to control for any differences in baseline function using multivariate techniques.28 Several authors have studied raw motor score improvement in tSCI persons undergoing early surgery compared with those experiencing late surgery. While a number of studies have failed to demonstrate an advantage to early surgery,29–34 a prospective Canadian cohort study35 did report a 5 motor score improvement in 22 patients who had surgery within 24 h of injury out of a group of 55 patients. Several other studies have identified a motor score benefit to early surgery, however, these studies have 50 or fewer patients and tend to focus on subpopulations of tSCI such as central cord or thoracolumbar injuries.36–39
In a meta-analysis and systematic review of timing of surgery,9 van Middendorp and colleagues9 reported that patients who underwent early surgery experienced an additional 5.94 motor score improvement when compared to those whose surgery was performed later, although it was not clear whether early surgery occurred within 24 or 72 h. The authors also found that those undergoing early surgery spent almost 10 fewer days in hospital.9 Although early surgery may be more costly (priority operating room/advanced imaging access), the decrease in length of hospital stay may reduce overall costs.40 Hospital length of stay (LOS) may be viewed as an imprecise surrogate measure for costs of hospital care.
Every day, treating physicians are faced with newly injured patients and have to make decisions as to the urgency of transport, priority of investigations, and access to operating room and other hospital resources. The identification of any potentially beneficial or harmful influence of surgical timing in acute tSCI is a critically important issue for persons with new injuries and for health systems that strive to provide timely access to the most appropriate care.
The purpose of this study was to determine the degree to which the time from injury to surgical procedure influences the early motor neurologic recovery after acute tSCI. Our specific objectives were to compare motor recovery based on the International Standards for the Neurological Classification of SCI (ISNCSCI) from the baseline (initial) neurological examination to final neurological follow-up while accounting for the factors that may influence neurological outcome after tSCI. We also investigated inpatient acute hospital LOS as surrogate measure for systems outcomes and costs, and recognized potential confounders of neurological outcome that most previous studies fail to consider.
Characterizing the degree to which the timing of surgical inervention influences patterns of motor recovery in various subgroups of tSCI would allow for a more informed and personalized approach to tailoring therapies, facilitate the precise prioritization of treatment, and provide support for the advocacy for timely access to resources for these persons.
Methods
This prospective observational Canadian multicenter comparative cohort study used data collected in the Rick Hansen Spinal Cord Injury Registry (RHSCIR).41
The subjects recruited for this study formed a longitudinal cohort of consecutive patients recruited at the time of their initial injury to the RHSCIR beginning at one pilot site in 2005 through to the present; the national SCI registry is currently active in 31 sites in 15 Canadian cities. Spine fellowship trained surgeons provide care at each of these sites, many of which are level 1 trauma centers.
The sites all obtained Institutional Research Ethics Board approval to enroll patients and enter data elements based on a priori research questions, some of which are outlined in the article describing the registry.41
Local treating clinicians examined each patient, conducted neurological assessments, and reviewed radiographic images to confirm the diagnosis of acute tSCI.
Registry coordinators collected data while the patient was in the acute care setting of each registry site. The registry includes data elements collected during the pre-hospital, acute, and inpatient rehabilitation phase, as well as on discharge into the community and at 1, 2, 5, and 10 years post-injury.
We included only those participants in the RHSCIR who underwent surgery for treatment of their acute tSCI. Patients must have consented to collection of their baseline and follow-up neurological examination results to be included. We excluded anyone with a Glasgow Coma Scale of less than 14 and anyone where timing of surgery and timing of neurological examinations were not specified.
In RHSCIR, a detailed initial or baseline neurological assessment according to ISNCSCI42 is conducted and recorded for all persons with SCI as part of a minimal data set. Coordinators and clinicians at each participating site undergo training on the performance of the ISNCSCI by a team which travels to each site from the central registry site. This ensures the highest possible reliability of the neurological assessments.43 Each ISNCSCI record was processed through a customized algorithm that ensures high quality and consistency of the neurological data. The date and time of injury as well as the date and time of each neurological assessment and surgery are recorded so that the time from injury to initial neurological assessment and the time from injury to surgical procedure can be calculated in hours, as can the time to subsequent neurological assessments.
Neurological assessments are performed at multiple time points, but are recorded in the registry on admission to acute care and again on discharge from acute care or inpatient rehabilitation. We included initial/baseline ISNCSCI assessments that were performed within 168 h of injury. What we described as our “final” ISNCSCI assessments were generally performed between 3 and 6 months after injury. Although neurological improvement may continue to occur beyond a year after injury,44 most neurological recovery is thought to occur within the first 3 months after injury45,46 or possibly up to 6 months.47,48 Pollard and Apple31 have noted that more than 70% of neurological recovery occurs before discharge from rehabilitation. The focus of our analysis was on early neurological recovery.
Factors that we considered potential confounders or effect modifiers based on clinical expertise and the literature include patients' age at injury,49 their sex,50 the Injury Severity Score (ISS),51 the initial AIS grade, (reflecting the severity of the neurological injury) and the neurological level of injury,52 rehabilitation LOS, the time from injury to baseline neurologic examination, and the post-surgery interval to the final neurologic examination.
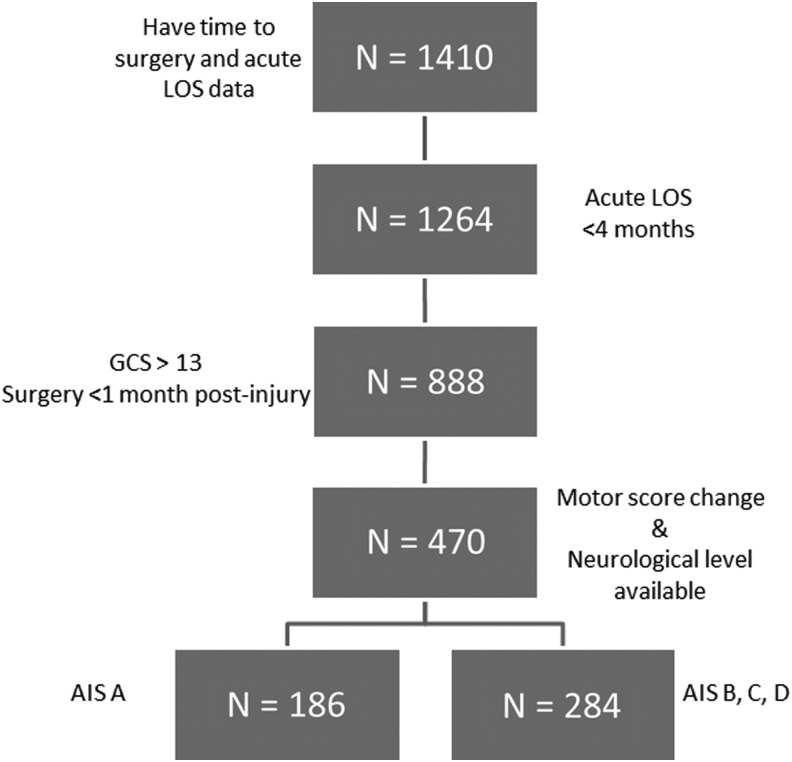
In the majority of patients with acute tSCI, there is a time dependent improvement in motor recovery. This motor recovery may lead to conversion from one AIS grade to another and this may occur within the first hours and even days after injury.46,53 Information bias will occur if the precise time from injury to baseline examination is not accounted for. We included only patients who had a baseline neurological assessment performed before surgical intervention, within 72 h of injury for the analysis on the effect of time to surgery within each AIS group. We performed analyses on the AIS A (n=186) patients separately from AIS B, C, and D patients (n=284; Fig. 1).
FIG. 1.
Participant flowchart. LOS, length of stay; GCS, Glasgow Coma Scale; AIS, American Spinal Injury Association Impairment Scale.
We also investigated the influence of early surgery on the length of acute hospital stay. We selected the time from admission to discharge from an acute care facility as a surrogate for cost and the systems impact of early surgery and analyzed the influence of early surgery on LOS using gamma regression. This analysis was performed on all patients who had time to surgery and acute LOS data, using 4 months as a cut-point based on other research54 and clinical appropriateness (n=1410; Fig. 1).
Statistical methods
All data were tested for normality using histogram graphical analysis and Kolmogorov-Smirnov numerical analysis. Univariate analysis was performed using t tests when data were normally distributed, otherwise the Mann-Whitney U test was used. For categorical variables, we used the chi-square test. Pearson and Spearman correlation was also used for bivariate association. The Kruskal-Wallis test and a one-way analysis of variance were used to model the development of motor score changes for more than two groups. Outliers, namely those data points that showed variability of at least three standard deviations from the mean of the variable of interest, were also removed.55
To evaluate the effect of timing of surgical intervention on change in motor score and acute LOS, we used several different types of generalized linear models, including multiple linear regression, gamma regression, and negative binomial regression. We adjusted for covariates including age, sex, AIS, ISS, and neurological level. In a separate analysis, we compared the effect of surgical timing on rehabilitation LOS and the post-surgery interval to the final neurological examination to ensure there were no differences between groups. AIC (Akaike Information Criteria) and BIC (Bayesian Information Criteria) were used for model selection. Alpha was set at p<0.05. Data were analyzed using SPSS Windows version 21 and SAS 9.2.
Results
From a total population of 1410 patients who met our inclusion criteria, there were 888 patients who had their initial neurological exam performed within 1 week of injury and had surgery performed within the first month after injury (Fig. 1). The participant characteristics are displayed in conventional style in Table 1. The breakdown of participants based on their injury severity and anatomical level of injury as described by Dvorak and associates52 is seen in Table 2.
Table 1.
Study Population Characteristics of 888 Persons with Acute Traumatic Spinal Cord Injuries
| Baseline | Follow-up | |
|---|---|---|
| Age: Mean (range) | 45.7 (76) | |
| Male sex: n (%) | 679 (76.5) | |
| Total hospital length of stay (days): mean (SD) | 117 (89.5) | |
| Upper extremity motor score | 32.8 (18.1) | 39.3 (14.2) |
| Lower extremity motor score | 14.2 (18.4) | 22.5 (21) |
| Total motor score (0–100): mean (SD) | 50 (27) | 61.8 (28.1) |
| Baseline neurological examination time (hours post-injury): mean (SD) | 44 (79) | |
| Median | 17 | |
| AIS: n in each grade (%) | ||
| A | 292 (38.8) | |
| B | 90 (12) | |
| C | 138 (18.4) | |
| D | 232 (30.9) | |
| Neurological level of injury: n in each group (%) | ||
| High cervical (C1–C4) | 190 (26.5) | |
| Low cervical (C5–T1) | 257 (35.8) | |
| Thoracic (T2–T10) | 120 (16.7) | |
| Thoracolumbar (T11–L2) | 151 (21.0) | |
| Time from injury to surgery (h): mean (SD) | 60.4 (80) | |
| Median | 33 | |
SD, standard deviation; AIS, American Spinal Injury Association Impairment Scale.
Table 2.
Motor Score Change (Discharge - Baseline) Distribution by Severity and Neurological Level of Injury for the Total Analysis Population (n=888) Who Had Complete Data (n=505, 56.9%)
| Neurological level | ||||||
|---|---|---|---|---|---|---|
| AIS | Change in motor score | High cervical (C1–C4) | Low cervical (C5–T1) | Thoracic (T2–T10) | Thoracolumbar (T11–L2) | p value |
| A (n=196) | Motor score change (SD) | 4.6 (5.9) | 5.3 (6.1) | −0.4 (1.6) | 1.4 (3.9) | <0.001 |
| n | 34 | 51 | 73 | 38 | ||
| B (n=66) | Motor score change (SD) | 36.2 (28.5) | 23.0 (26.2) | 13.8 (16.2) | 8.7 (11.7) | 0.012 |
| n | 15 | 27 | 9 | 15 | ||
| C (n=106) | Motor score change (SD) | 37.9 (18.9) | 36.2 (18.6) | 24.1 (11.6) | 19.0 (13.3) | <0.001 |
| n | 42 | 34 | 7 | 23 | ||
| D (n=137) | Motor score change (SD) | 11.1 (12.5) | 10.9 (12.3) | 10.5 (20.5) | 6.9 (7.4) | 0.443 |
| n | 54 | 54 | 2 | 27 | ||
AIS, American Spinal Injury Association Impairment Scale; SD, standard deviation.
The mean time from injury to surgery was 60 h, with 40% of patients undergoing surgical intervention within 24 h from time of injury and the remaining 60% having a surgical procedure after 24 and up to 168 h after injury.
When the time from injury to operation was analyzed as an independent continuous variable against the improvement in motor score from baseline to follow-up, we found that there was no significant effect identified in the total population of 888 patients. When this entire sample was analyzed specifically for the influence of those patients who had surgical intervention within 24 h from injury, negative binomial regression showed no effect. This was repeated for those with an operation within 48 h of injury and further modeling using negative binomial regression demonstrated no effect. Negative binomial regression was chosen based on the distribution of data and a better “fit” of the data in comparison with other models (correct overdispersion in data and lower AIC and BIC compared with Poisson regression).
By analyzing the motor recovery based on the Canadian classification table for motor recovery,52 it became clear that the AIS A patients demonstrated a significantly different pattern of neurological recovery than did the AIS B, C, and D patients. We therefore chose to analyze the AIS A and the AIS B, C, and D subgroups separately. We also restricted our sample to those who had their baseline neurological assessment performed within 72 h post-injury in an attempt to reduce the potential bias related to the time of the baseline neurological assessment.
The subgroup analysis of AIS A patients revealed that the data did not fit a Gaussian distribution and the best fit was obtained when we performed a negative binomial regression. When the negative binomial regression was performed no significant effect was observed on motor score recovery when the time to operation was analyzed before and after 24 h (Table 3). In this analysis, patients with a lower ISS (less severely injured) were more likely to exhibit greater motor score improvement, as were patients who had anterior column compression or burst fractures as opposed to those with dislocations or distraction/shear type injuries.
Table 3.
Factors Affecting Motor Score Improvement in American Spinal Injury Association Impairment Scale A Patients
| 95% Wald confidence interval | Hypothesis test | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | Std. error | Lower | Upper | Wald chi-square | df | Sig. |
| Intercept | −0.514 | 0.8314 | −2.144 | 1.115 | 0.383 | 1 | 0.536 |
| Age at injury | 0.016 | 0.0097 | −0.003 | 0.035 | 2.673 | 1 | 0.102 |
| Vertebral injury | |||||||
| Compression | 1.515 | 0.7398 | 0.065 | 2.965 | 4.192 | 1 | 0.041 |
| Dislocation | 0.543 | 0.7600 | −0.947 | 2.033 | 0.510 | 1 | 0.475 |
| Shear | 0.669 | 0.6499 | −0.605 | 1.943 | 1.060 | 1 | 0.303 |
| Other | Baseline | - | - | - | - | - | - |
| ISS score | −0.048 | 0.0155 | −0.078 | −0.017 | 9.530 | 1 | 0.002 |
| Neurological level | |||||||
| High cervical (C1–C4) | 2.179 | 0.5109 | 1.177 | 3.180 | 18.187 | 1 | 0.000 |
| Low cervical (C5–T1) | 2.338 | 0.4168 | 1.521 | 3.154 | 31.451 | 1 | 0.000 |
| Thoracic/Thoracolumbar (T2–L2) | Baseline | - | - | - | - | - | - |
| Time from injury to surgery | |||||||
| ≤24h | 0.068 | 0.3533 | −0.625 | 0.760 | 0.037 | 1 | 0.848 |
| >24h | Baseline | - | - | - | - | - | - |
Std. error, standard error; Sig., significance; ISS, Injury Severity Score.
Negative binomial analysis of motor score recovery from baseline to follow-up (dependent variable) with independent variables: age, ISS, injury type, and early (≤24 h) or late (>24 h) surgery for American Spinal Injury Association Impairment Scale A patients. Axial compression injuries (burst fractures), a lower ISS, and a cervical injury were associated with a greater motor score change, but the timing of surgery had no effect.
When we performed a similar analysis of AIS B, C, and D patients, which we grouped together because of their similar patterns of neurological improvement, this cohort of patients revealed a normal distribution. Our analyses demonstrated that patients who had a surgical procedure performed within 24 h of injury (early) had significantly increased motor score improvement than those with surgery performed after 24 h (late) (Table 4). Early surgical intervention resulted in an additional six motor point improvement over that demonstrated by the patients who had surgical procedures later than 24 h after injury (p=0.03).
Table 4.
Factors Affecting Motor Score Improvement in American Spinal Injury Association Impairment Scale B, C, and D
| 95% Wald confidence interval | Hypothesis test | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | Std. error | Lower | Upper | Wald chi-square | df | Sig. |
| Intercept | 0.786 | 6.3278 | −11.616 | 13.189 | 0.015 | 1 | 0.901 |
| Age at injury | −0.142 | 0.0734 | −0.286 | 0.002 | 3.755 | 1 | 0.053 |
| Neurological level | |||||||
| High cervical (C1–C4) | 17.364 | 3.6753 | 10.160 | 24.567 | 22.320 | 1 | 0.000 |
| Low cervical (C5–T1) | 9.459 | 3.5437 | 2.514 | 16.405 | 7.125 | 1 | 0.008 |
| Thoracic (T2–T10) | 7.527 | 6.1510 | −4.529 | 19.583 | 1.497 | 1 | 0.221 |
| Thoracolumbar (T11–L2) | Baseline | - | - | - | - | - | - |
| ISS score | 0.230 | 0.2121 | −0.186 | 0.646 | 1.177 | 1 | 0.278 |
| AIS | |||||||
| B | 6.661 | 3.6831 | −0.558 | 13.879 | 3.271 | 1 | 0.071 |
| C | 22.893 | 3.0928 | 16.831 | 28.954 | 54.788 | 1 | 0.000 |
| D | Baseline | - | - | - | - | 1 | - |
| Time from injury to surgery | |||||||
| ≤24 h | 6.258 | 2.8774 | 0.618 | 11.897 | 4.729 | 1 | 0.030 |
| >24 h | Baseline | - | - | - | - | - | - |
Std. error, standard error; Sig., significance; ISS, Injury Severity Score; AIS, American Spinal Injury Association Impairment Scale.
Multiple linear regression analysis of motor score improvement from baseline to follow-up (dependent variable) with independent variables: age, ISS, injury type, and early (≤24 h) or late (>24 h) surgery for AIS B, C, and D patients. Having a cervical injury and having surgery within 24 h from time of injury were associated with a greater motor score change. Age at injury, ISS score, and injury type were not associated with motor score change.
AIS A and B patients who had early surgical intervention demonstrated a reduced acute LOS (AIS A 7.5 d, AIS B 12.8 d; Table 5), while AIS C and D patients demonstrated a similar trend that did not reach statistical significance.
Table 5.
Gamma Regression for Acute Length of Stay in American Spinal Injury Association Impairment Scale A and B Patients
| 95% Wald confidence interval | Hypothesis test | ||||||
|---|---|---|---|---|---|---|---|
| Parameter | B | Std. error | Lower | Upper | Wald chi-square | df | Sig. |
| AIS A | |||||||
| Intercept | 3.143 | 0.2106 | 2.731 | 3.556 | 222.893 | 1 | 0.000 |
| Age at injury | 0.002 | 0.0018 | −0.002 | 0.006 | 1.226 | 1 | 0.268 |
| Sex | |||||||
| Female | −0.065 | 0.0761 | −0.214 | 0.084 | 0.726 | 1 | 0.394 |
| Male | Baseline | - | - | - | - | - | - |
| Neurological level | |||||||
| High cervical (C1–C4) | 0.732 | 0.1133 | 0.510 | 0.954 | 41.776 | 1 | 0.000 |
| Low cervical (C5–T1) | 0.480 | 0.1050 | 0.274 | 0.686 | 20.917 | 1 | 0.000 |
| Thoracic (T2–T10) | 0.126 | 0.0951 | −0.060 | 0.313 | 1.762 | 1 | 0.184 |
| Thoracolumbar (T11–L2) | Baseline | - | - | - | - | - | - |
| ISS score | 0.006 | 0.0027 | 0.001 | 0.011 | 4.811 | 1 | 0.028 |
| Vertebral injury | |||||||
| Compression | 0.079 | 0.1723 | −0.259 | 0.416 | 0.209 | 1 | 0.648 |
| Dislocation | 0.065 | 0.1747 | −0.277 | 0.407 | 0.138 | 1 | 0.710 |
| Shear | 0.052 | 0.1622 | −0.266 | 0.370 | 0.102 | 1 | 0.749 |
| Other | Baseline | - | - | - | - | - | - |
| Surgery time | |||||||
| ≤24 h | −0.181 | 0.0624 | −0.303 | −0.059 | 8.397 | 1 | 0.004 |
| >24 h | Baseline | - | - | - | - | - | - |
| AIS B | |||||||
| Intercept | 3.936 | 0.3895 | 3.172 | 4.699 | 102.087 | 1 | 0.000 |
| Age at injury | 0.005 | 0.0035 | −0.002 | 0.011 | 1.668 | 1 | 0.197 |
| Sex | |||||||
| Female | 0.040 | 0.1426 | −0.239 | 0.320 | 0.080 | 1 | 0.777 |
| Male | Baseline | - | - | - | - | - | - |
| Neurological level | |||||||
| High cervical (C1–C4) | 0.411 | 0.1785 | 0.061 | 0.761 | 5.307 | 1 | 0.021 |
| Low cervical (C5–T1) | 0.198 | 0.1660 | −0.127 | 0.524 | 1.426 | 1 | 0.232 |
| Thoracic (T2–T10) | −0.063 | 0.2107 | −0.476 | 0.350 | 0.088 | 1 | 0.766 |
| Thoracolumbar (T11–L2) | Baseline | - | - | - | - | - | - |
| ISS score | 0.003 | 0.0070 | −0.011 | 0.017 | 0.193 | 1 | 0.660 |
| Vertebral injury | |||||||
| Compression | −0.588 | 0.2913 | −1.159 | −0.017 | 4.072 | 1 | 0.044 |
| Dislocation | −0.552 | 0.3000 | −1.140 | 0.036 | 3.389 | 1 | 0.066 |
| Shear | −0.414 | 0.2841 | −0.970 | 0.143 | 2.119 | 1 | 0.145 |
| Other | Baseline | - | - | - | - | - | - |
| Surgery time | |||||||
| ≤24 h | −0.358 | 0.1185 | −0.590 | −0.126 | 9.127 | 1 | 0.003 |
| >24 h | Baseline | - | - | - | - | - | - |
Std. error, standard error; Sig., significance; ISS, Injury Severity Score.
Finally, we compared the time from operation (date of operation+1 d) to the final neurological examination and length of rehabilitation between the early and late surgery groups in duration of mobilization and rehabilitation and there were no significant effects.
Discussion
We studied the influence of time from injury to surgical stabilization and decompression (time to surgery) in an observational cohort of adult patients with acute tSCI with injuries between C1 and L2. We specifically questioned whether there was an improvement in the magnitude of motor score recovery in patients with tSCI who underwent early surgical intervention compared with those who had their operation performed later. We used clinically relevant time intervals for analyzing the time from injury to surgical procedure, including 24, 48, and 72 h. We hypothesized that the effect of timing of the procedure on neurologic recovery would be influenced by the initial severity of the injury and its anatomical location.
We failed to demonstrate variation in motor recovery with early and late surgical intervention when evaluating the entire sample of AIS A, B, C, and D patients, and we also did not observe an effect in the subgroup of patients who had an initial severity of AIS A.
We did, however, observe that surgical procedures performed within 24 h from the time of injury had a positive influence on motor score neurological recovery in AIS B, C, and D (incomplete) patients. These neurologically incomplete patients recovered an average of 6 motor points more when their operation was performed within 24 h of their injury compared with those who had their operation performed more than 24 h from the time of injury.
The largest clinical trials in SCI have each recruited between 500 and 750 patients.56,57 Data from registries that recruit patients on entry into rehabilitation do not have direct access to accurate acute (early) neurological assessments, with baseline neurological assessment often performed several weeks after the injury.58–60
The literature that does demonstrate that time to surgery has an effect on motor recovery tends to demonstrate this in small populations (fewer than 50 participants) and often in incomplete SCIs such as central cord syndrome.35–39 The strongest evidence to date of a surgical timing influence on motor score recovery comes from the meta-analysis performed by van Middendorp and colleagues.61 These authors describe the available literature as being plagued by significant publication bias and lacking “robustness.” Interestingly, the conclusion by van Middendorp and associates61 of an overall 5.94 motor point improvement with early surgery is very similar to our finding of a six-point improvement in neurologically incomplete patients who had surgery within 24 h of their injury, although the analysis by van Middendorp and colleagues61 did not distinguish the AIS A patients from those with incomplete injuries.
Fehlings and coworkers8 concluded that early surgery resulted in a greater chance of improving by two AIS grades, but, interestingly, not by one AIS grade, nor did they analyze or report on motor score improvement. In the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS) study, the patients in the early surgery group were on average 4 years younger and had a larger proportion of patients with AIS A and B than in the late surgery group. Both of these imbalances favor two AIS grade improvement in the early surgery group because AIS C and D patients are unlikely to improve by two AIS grades. There has therefore been some criticism of the STASCIS data.28
We acknowledge that our current study does not report on long term or final neurological outcome; however, that was not our intent. Our goal was to study the acute and subacute motor improvement that occurs between injury and 6 months post-injury and analyze the influence of early surgical intervention on this motor score recovery. We contend that it is unlikely that further follow-up would alter these ultimate conclusions. Our study may be criticized in that complete neurological assessments were not collected at 1 or 2 years after injury or at prescribed time points after injury. This is a function of the incremental process of initiating a national registry, first at several pilot sites and subsequently in other cities and provinces. We also acknowledge the differences in rehabilitation LOS and post-surgery interval to final neurological examination; it is unclear what effect these differences have on change in motor score, which will be an area of future research.
Our study population is collected from all of the specialty spine centers across Canada as well as several smaller provincial trauma centers, thus contributing to the generalizability of our conclusions and the minimizing of selection bias. The reliability and accuracy of our neurological assessments performed in more than 31 sites by multiple examiners may also be regarded as a potential concern. When a site is recruited to join the RHSCIR, a central team of nurses and physiotherapists traveled to the site to ensure appropriate training in the performance of the ISNCSCI standards. This training has been demonstrated to improve validity and reliability of examinations.62 In addition, we have developed an algorithm to standardize the reporting for the ISNCSCI, and the algorithm was used to check all the ISNCSCI examination results in RHSCIR.
We acknowledge the peculiarities of analyzing the ISNCSCI and the difficulties inherent in fitting this data to a Gaussian or normal distribution. We have used negative binomial regression techniques as the “best fit” for some of our distributions. Unlike other authors who have chosen to analyze the AIS as a primary outcome,8,57 we have chosen to analyze motor score recovery. We believe that the risks of losing some valuable information in the transformation of raw motor score data into binary, multimodal, or ordinal data are substantial. We understand that transforming the motor score recovery data into AIS or other outcomes may be preferred from a clinical relevance point of view, as well as from a statistical manipulation standpoint. We think that the analysis of motor scores provides more granularity and enables us to better understand the details of the process of recovery. We would like to clarify that negative binomial regression can be used with nonnegative numbers; we changed negative numbers in the outcome to zero (motor score at discharge minus motor score at admission). The amount of change in motor score improvement was very small (13.6 vs. 13.8) and did not alter the results.
A potential bias in this study is to attribute improvement in motor score recovery to surgical intervention when it may simply be because of the natural history of incomplete SCI (type I error). From the time of the baseline neurological assessment to the time of operation, motor improvement can occur, and this improvement could be mistakenly attributed to the influence of the operation. Alternatively, the improvement may have occurred independent of the operation. Because all of our patients had surgical intervention, we are not able to comment on the influence of the operation itself on the natural history of neurological recovery.
We identified no significant differences between the early and late surgery groups in the post-surgery interval until the final neurological examination, suggesting that the groups had an equal opportunity to benefit from rehabilitation/natural recovery. As persons with incomplete SCI were shown to have a larger anticipated amount of motor score recovery, preferentially selecting incomplete participants for early surgical intervention is a potential source of bias. In other work, however, we have demonstrated that those with incomplete injuries are more likely to be observed by surgeons over having early operation (or acute surgical treatment at all), counter to this potential source of bias.63
We also acknowledge the influence of spinal shock on baseline neurological assessments. In our attempts to determine whether or not the timing of surgical intervention is a factor in motor recovery other considerations need to be controlled for to determine the independent effect of operation. We attempted to control for all known confounders and interactions in our multivariate analyses. The purpose of our research was not to promote early surgical intervention but to analyze whether early operation is a significant factor in overall recovery.
Bias related to the timing of the baseline neurological assessment has been the most difficult variable to assess and control for. We are not aware of any studies that report on the rate of neurological motor recovery in the first hours or days after tSCI. It is possible that those patients who had their first ISNCSCI examination performed within the first 24 h and those performed greater than 24 h had different rates of motor recovery and this could bias our results. We performed a sensitivity analysis by restricting our inclusion to those patients who had their baseline neurological assessment within 24 h from time of injury. This analysis resulted in a similar effect on motor improvement with respect to early and late operation. Further analyses are being performed as part of a separate study to specifically analyze the influence of time of baseline neurological assessment as a potential source of type II error and bias.
We have not established the functional significance of a six point motor score difference, and further study is needed to determine the distribution and clinical significance of the change in motor scores that we have reported. The influence of multiple segments improving one or two points may be minimal on a patient's functional status. Nonetheless, given the devastating nature of acute SCI and the paucity of effective neurorestorative treatments, any true and measurable improvement in neurologic function is worth noting.
This study does not address specifically what was actually done at the time of surgery, and whether stabilization or stabilization with concomitant direct or indirect spinal cord decompression was performed. Nor does it address the issue of the efficacy or timing of closed reduction before operation. We also have not specifically verified the degree of pre-operative spinal cord compression or the effectiveness of surgical intervention in fully relieving that compression or effectively stabilizing the spinal column. Across our Canadian RHSCIR sites, there is relative uniformity in surgical philosophy from a surgical training and surgical capability point of view.
We demonstrated a reduced acute hospital LOS in AIS A (7.5 d) and B (12.8 d) patients who had early operation. There are other potential benefits to early operation such as lower rates of complications and shortened intensive care unit stay. Our study population all had surgical intervention within a week of injury and some of the findings related to LOS and complications are found in articles where late surgical intervention is performed beyond 1 week after injury. We plan further analyses in this regard.
When the spinal cord is exposed to varying intensities of injury and when it subsequently displays a time dependent natural recovery, it is not surprising that neurological recovery is influenced by a number of variables. Authors have demonstrated that the severity of the initial neurological injury and the anatomic region of the injury are strong predictors of neurological recovery.45,52,62,64–66 Others have identified patients' age and sex as a predictor of recovery.51 Our current study has provided evidence that the time from injury to operation influences motor recovery in neurologically incomplete (B, C, and D) patients but not in all complete (AIS A) patients.
Studies that are currently in the planning or early recruitment stages should report on the proportion of patients who underwent surgical intervention within 24 and beyond 24 h from injury particularly when they are including incomplete SCI among their participants.
Our study proposes that incomplete AIS B, C, and D patients with acute tSCI in the cervical, thoracic, and thoracolumbar spine would benefit from surgical intervention performed within 24 h from time to injury, with an average improvement in motor score of six points over those who undergo surgical intervention later than 24 h. This effect was not seen when the entire population was analyzed (including the complete AIS A patients).
Contributor Information
Collaborators: The RHSCIR Network
Acknowledgments
We would like to thank the RHSCIR network and all the participating local RHSCIR sites: GF Strong Rehabilitation Centre, Vancouver General Hospital, Foothills Hospital, Glenrose Rehabilitation Hospital, Royal Alexandra Hospital, University of Alberta Hospital, Royal University Hospital, Saskatoon City Hospital, Winnipeg Health Sciences Centre, Toronto Western Hospital, Toronto Rehabilitation Institute, St. Michael's Hospital, Sunnybrook Health Sciences Centre, Hamilton General Hospital, Hamilton Health Sciences Regional Rehabilitation Centre, Victoria Hospital (London), University Hospital (London), Parkwood Hospital (London), The Ottawa Hospital Rehabilitation Centre, The Ottawa Hospital Civic Campus, Hôpital de l'Enfant Jésus, Institut de réadaptation en déficience physique de Québec, Centré de réadaptation Lucie-Bruneau, Institut de réadaptation Gingras-Lindsay-de-Montréal, Hôpital du Sacré Cœur de Montréal, Nova Scotia Rehabilitation Centre, QEII Health Sciences Centre, Saint John Regional Hospital, Stan Cassidy Centre for Rehabilitation, St. John's Health Sciences Centre, and L.A. Miller Rehabilitation Centre.
The Rick Hansen Spinal Cord Injury Registry and this work are supported by funding from the Rick Hansen Institute, Health Canada, Western Economic Diversification Canada, and the Governments of Alberta, British Columbia, Manitoba, and Ontario.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Wyndaele M., and Wyndaele J.J. (2006). Incidence, prevalence and epidemiology of spinal cord injury: what learns a worldwide literature survey? Spinal Cord 44, 523–529 [DOI] [PubMed] [Google Scholar]
- 2.Devivo M.J. (2012). Epidemiology of traumatic spinal cord injury: trends and future implications. Spinal Cord 50, 365–372 [DOI] [PubMed] [Google Scholar]
- 3.Tator C.H., Hashimoto R., Raich A., Norvell D., Fehlings M.G., Harrop J.S., Guest J., Aarabi B., and Grossman R.G. (2012). Translational potential of preclinical trials of neuroprotection through pharmacotherapy for spinal cord injury. J. Neurosurg. Spine 17, 1 Suppl 1, 157–229 [DOI] [PubMed] [Google Scholar]
- 4.Casha S., and Christie S. (2011). A systematic review of intensive cardiopulmonary management after spinal cord injury. J. Neurotrauma 28, 1479–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kong C.Y., Hosseini A.M., Belanger L.M., Ronco J.J., Paquette S.J., Boyd M.C., Dea N., Street J., Fisher C.G., Dvorak M.F., and Kwon B.K. (2013). A prospective evaluation of hemodynamic management in acute spinal cord injury patients. Spinal Cord 51, 466–471 [DOI] [PubMed] [Google Scholar]
- 6.Katoh S.S., el Masry W.S., Jaffray D.D., McCall I.W., Eisenstein S.M., Pringle R.G., Pullicino V., and Ikata T. (1996). Neurologic outcome in conservatively treated patients with incomplete closed traumatic cervical spinal cord injuries. Spine 21, 2345–2351 [DOI] [PubMed] [Google Scholar]
- 7.Fisher C.G., Noonan V.K., and Dvorak M.F. (2006). Changing face of spine trauma care in North America. Spine 31, Suppl 11, S2–S8, S36 [DOI] [PubMed] [Google Scholar]
- 8.Fehlings M.G., Vaccaro A., Wilson J.R., Singh A., Cadotte D., Harrop J.S., Aarabi B., Shaffrey C., Dvorak M., Fisher C., Arnold P., Massicotte E.M., Lewis S., and Rampersaud R. (2012). Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). PLoS One 7, e32037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.van Middendorp J.J., Hosman A.J., and Doi S.A. (2013). The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J. Neurotrauma 30, 1781–1794 [DOI] [PubMed] [Google Scholar]
- 10.Furlan J.C., Noonan V., Singh A., and Fehlings M.G. (2011). Assessment of impairment in patients with acute traumatic spinal cord injury: a systematic review of the literature. (2011). J. Neurotrauma 28, 1445–1477 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Furlan J.C., Noonan V., Singh A., and Fehlings M.G. (2011). Assessment of disability in patients with acute traumatic spinal cord injury: a systematic review of the literature. J. Neurotrauma 28, 1413–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Steeves J.D., Lammertse D.P., Kramer J.L., Kleitman N., Kalsi-Ryan S., Jones L., Curt A., Blight A.R., and Anderson K.D. (2012). Outcome measures for acute/subacute cervical sensorimotor complete (AIS-A) spinal cord injury during a phase 2 clinical trial. Top. Spinal Cord Inj. Rehabil. 18, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kwon B.K., Okon E.B., Plunet W., Baptiste D., Fouad K., Hillyer J., Weaver L.C., Fehlings M.G., and Tetzlaff W. (2011). A systematic review of directly applied biologic therapies for acute spinal cord injury. J. Neurotrauma. 28, 1589–1610 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kwon B.K., Okon E., Hillyer J., Mann C., Baptiste D., Weaver L.C., and Fehlings M.G., Tetzlaff W. (2011). A systematic review of non-invasive pharmacologic neuroprotective treatments for acute spinal cord injury. J. Neurotrauma 28, 1545–1588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kobrine A.I., Evans D.E., and Rizzoli H.V. (1979). Experimental acute balloon compression of the spinal cord. Factors affecting disappearance and return of the spinal evoked response. J. Neurosurg 51, 841–845 [DOI] [PubMed] [Google Scholar]
- 16.Dolan E.J., Tator C.H., and Endrenyi L. (1980). The value of decompression for acute experimental spinal cord compression injury. J. Neurosurg 53, 749–755 [DOI] [PubMed] [Google Scholar]
- 17.Guha A., Tator C.H., Endrenyi L., and Piper I. (1987). Decompression of the spinal cord improves recovery after acute experimental spinal cord compression injury. Paraplegia 25, 324–339 [DOI] [PubMed] [Google Scholar]
- 18.Nystrom B., and Berglund J.E. (1988). Spinal cord restitution following compression injuries in rats. Acta Neurol. Scand. 78, 467–472 [DOI] [PubMed] [Google Scholar]
- 19.Zhang Y., Hillered L., Olsson Y., and Holtz A. (1993). Time course of energy perturbation after compression trauma to the spinal cord: an experimental study in the rat using microdialysis. Surg. Neurol. 39, 297–304 [DOI] [PubMed] [Google Scholar]
- 20.Delamarter R.B., Sherman J., and Carr J.B. (1995). Pathophysiology of spinal cord injury. Recovery after immediate and delayed decompression. J. Bone Joint Surg. Am. 77, 1042–1049 [DOI] [PubMed] [Google Scholar]
- 21.Carlson G.D., Minato Y., Okada A., Gorden C.D., Warden K.E., Barbeau J.M., Biro C.l., Bahnuik E., Bohlman H.H., and Lamanna J.C. (1997). Early time-dependent decompression for spinal cord injury: vascular mechanisms of recovery. J. Neurotrauma 14, 951–962 [DOI] [PubMed] [Google Scholar]
- 22.Dimar J.R., Carreon L.Y., Riina J., Schwartz D.G., and Harris M.B. (2010). Early versus late stabilization of the spine in the polytrauma patient. Spine 35, Suppl 21, S187–S192 [DOI] [PubMed] [Google Scholar]
- 23.Rabinowitz R.S., Eck J.C., Harper C.M., Jr., Larson D.R., Jimenez M.A., Parisi J.E., Friedman J.A., Yaszemski M.J., and Currier B.L. (2008). Urgent surgical decompression compared to methylprednisolone for the treatment of acute spinal cord injury: a randomized prospective study in beagle dogs. Spine 33, 2260–2268 [DOI] [PubMed] [Google Scholar]
- 24.Croft T.J., Brodkey J.S., and Nulsen F.E. (1972). Reversible spinal cord trauma: a model for electrical monitoring of spinal cord function. J. Neurosurg. 36, 402–406 [DOI] [PubMed] [Google Scholar]
- 25.Thienprasit P., Bantli H., Bloedel J.R., and Chou S.N. (1975). Effect of delayed local cooling on experimental spinal cord injury. J. Neurosurg. 42, 150–154 [DOI] [PubMed] [Google Scholar]
- 26.Aki T., and Toya S. (1984). Experimental study on changes of the spinal-evoked potential and circulatory dynamics following spinal cord compression and decompression. Spine 9, 800–809 [DOI] [PubMed] [Google Scholar]
- 27.Tator C.H., and Fehlings M.G. (1991). Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J. Neurosurg. 75, 15–26 [DOI] [PubMed] [Google Scholar]
- 28.van Middendorp J.J. (2012). Letter to the editor regarding: Early versus delayed decompression for traumatic cervical spinal cord injury: results of the Surgical Timing in Acute Spinal Cord Injury Study (STASCIS). Spine J. 12, 540. [DOI] [PubMed] [Google Scholar]
- 29.Vaccaro A.R., Daugherty R.J., Sheehan T.P., Dante S.J., Cotler J.M., Balderston R.A., Herbison G.J., and Northrup B.E. (1997). Neurologic outcome of early versus late surgery for cervical spinal cord injury. Spine 22, 2609–2613 [DOI] [PubMed] [Google Scholar]
- 30.McKinley W., Meade M.A., Kirshblum S., and Barnard B. (2004). Outcomes of early surgical management versus late or no surgical intervention after acute spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1818–1825 [DOI] [PubMed] [Google Scholar]
- 31.Pollard M.E., and Apple D.F. (2003). Factors associated with improved neurologic outcomes in patients with incomplete tetraplegia. Spine 28, 33–39 [DOI] [PubMed] [Google Scholar]
- 32.Duh M.S., Shepard M.J., Wilberger J.E., and Bracken M.B. (1994). The effectiveness of surgery on the treatment of acute spinal cord injury and its relation to pharmacological treatment. Neurosurgery 35, 240–249 [DOI] [PubMed] [Google Scholar]
- 33.Levi L., Wolf A., Rigamonti D., Ragheb J., Mirvis S., and Robinson W.L. (1991). Anterior decompression in cervical spine trauma: does the timing of surgery affect the outcome? Neurosurgery 29, 216–222 [PubMed] [Google Scholar]
- 34.Anderson D.G.Sayadipour A, Limthongkul W, Martin ND, Vaccaro A, and Harrop JS. (2012). Traumatic central cord syndrome: neurologic recovery after surgical management. Am. J.Orthop. 41, E104–E1081–E1085 [PubMed] [Google Scholar]
- 35.Wilson J.R., Singh A., Craven C., Verrier M.C., Drew B., Ahn H., Ford M., and Fehlings M.G. (2012). Early versus late surgery for traumatic spinal cord injury: the results of a prospective Canadian cohort study. Spinal Cord 50, 840–843 [DOI] [PubMed] [Google Scholar]
- 36.Chen L., Yang H., Yang T., Xu Y., Bao Z., and Tang T. (2009). Effectiveness of surgical treatment for traumatic central cord syndrome. J. Neurosurg. Spine 10, 3–8 [DOI] [PubMed] [Google Scholar]
- 37.Guest J., Eleraky M.A., Apostolides P.J., Dickman C.A., and Sonntag V.K. (2002). Traumatic central cord syndrome: results of surgical management. J. Neurosurg. 97, Suppl 1, 25–32 [DOI] [PubMed] [Google Scholar]
- 38.Mirza S.K., Krengel W.F., III, Chapman J.R., Anderson P.A., Bailey J.C., Grady M.S., and Yuan H.A. (1999). Early versus delayed surgery for acute cervical spinal cord injury. Clin. Orthop. Relat. Res. 359, 104–114 [DOI] [PubMed] [Google Scholar]
- 39.Clohisy J.C., Akbarnia B.A., Bucholz R.D., Burkus J.K., and Backer R.J. (1992). Neurologic recovery associated with anterior decompression of spine fractures at the thoracolumbar junction (T12-L1). Spine 17, Suppl 8, S325–S330 [DOI] [PubMed] [Google Scholar]
- 40.Mac-Thiong J.M., Feldman D.E., Thompson C., Bourassa-Moreau E., and Parent S. (2012). Does timing of surgery affect hospitalization costs and length of stay for acute care following a traumatic spinal cord injury? J. Neurotrauma 29, 2816–2822 [DOI] [PubMed] [Google Scholar]
- 41.Noonan V.K., Kwon B.K., Soril L., Fehlings M.G., Hurlbert R.J., Townson A., Johnson M., and Dvorak M.F.; RHSCIR Network. (2012). The Rick Hansen Spinal Cord Injury Registry (RHSCIR): a national patient-registry. Spinal Cord 50, 22–27 [DOI] [PubMed] [Google Scholar]
- 42.Kirshblum S.C., Burns S.P., Biering-Sorensen F., Donovan W., Graves D.E., Jha A., Johansen M., Jones L., Krassioukov A., Mulcahey M.J., Schmidt-Read M., and Waring W. (2011). International standards for neurological classification of spinal cord injury (revised 2011). J. Spinal Cord Med. 34, 535–546 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schuld C., Wiese J., Franz S., Putz C., Stierle I., Smoor I., Weidner N.; EMSCI Study Group, Rupp R. (2012). Effect of formal training in scaling, scoring and classification of the International Standards for Neurological Classification of Spinal Cord Injury. Spinal Cord 51, 282–288 [DOI] [PubMed] [Google Scholar]
- 44.Kirshblum S., Millis S., McKinley W., and Tulsky D. (2004). Late neurologic recovery after traumatic spinal cord injury. Arch. Phys. Med. Rehabil. 85, 1811–1817 [DOI] [PubMed] [Google Scholar]
- 45.Fawcett J.W., Curt A., Steeves J.D., Coleman W.P., Tuszynski M.H., Lammertse D., Bartlett P.F., Blight A.R., Dietz V., Ditunno J., Dobkin B.H., Havton L.A., Ellaway P.H., Fehlings M.G., Privat A., Grossman R., Guest J.D., Kleitman N., Nakamura M., Gaviria M., and Short D. (2006). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP panel: spontaneous recovery after spinal cord injury and statistical power needed for therapeutic clinical trials. Spinal Cord 45, 190–205 [DOI] [PubMed] [Google Scholar]
- 46.Steeves J.D., Kramer J.K., Fawcett J.W., Cragg J., Lammertse D.P., Blight A.R., Marino R.J., Ditunno J.F., Jr., Coleman W.P., Geisler F.H., Guest J., Jones L., Burns S., Schubert M., van Hedel H.J., Curt A.; EMSCI Study Group. (2010). Extent of spontaneous motor recovery after traumatic cervical sensorimotor complete spinal cord injury. Spinal Cord 49, 257–265 [DOI] [PubMed] [Google Scholar]
- 47.Waters R.L., Adkins R.H., Yakura J.S., and Sie I. (1993). Motor and sensory recovery following complete tetraplegia. Arch. Phys. Med. Rehabil. 74, 242–247 [PubMed] [Google Scholar]
- 48.Burns A.S., and Ditunno J.F. (2001). Establishing prognosis and maximizing functional outcomes after spinal cord injury: a review of current and future directions in rehabilitation management. Spine 26, Suppl 24, S137–S145 [DOI] [PubMed] [Google Scholar]
- 49.Hagen E.M., Aarli J.A., Gronning M. (2005). The clinical significance of spinal cord injuries in patients older than 60 years of age. Acta Neurol. Scand. 112, 42–47 [DOI] [PubMed] [Google Scholar]
- 50.Furlan J.C., Krassioukov A.V., and Fehlings M.G. (2005). The effects of gender on clinical and neurological outcomes after acute cervical spinal cord injury. J. Neurotrauma 22, 368–381 [DOI] [PubMed] [Google Scholar]
- 51.Al-Habib A.F., Attabib N., Ball J., Bajammal S., Casha S., and Hurlbert R.J. (2011). Clinical predictors of recovery after blunt spinal cord trauma: systematic review. J. Neurotrauma 28:1431–1443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Dvorak M.F., Noonan V., Fallah N., Fisher C.G., Rivers C.S., Ahn H., Tsai E.C., Linassi A.G., Christie S., Attabib N., Hurlbert R.J., Fourney D.R., Johnson M.G., Fehlings M.G., Drew B., Bailey C., Paquet J., Parent S., Townson A., Ho C., Craven B.C., Gagnon D., Tsui D., Fox R., Mac-Thiong J.M., and Kwon B.K. (2014). Minimizing errors in acute traumatic spinal cord injury trials by acknowledging the heterogeneity of spinal cord anatomy and injury severity: An observational Canadian cohort analysis. J. Neurotrauma 31;1540–1547 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Steeves J.D., Lammertse D., Curt A., Fawcet,t J.W., Tuszynski M.H., Ditunno J.F., Ellaway P.H., Fehlings M.G., Guest J.D., Kleitman N., Bartlett P.F., Blight A.R., Dietz V., Dobkin B.H., Grossman R., Short D., Nakamura M., Coleman W.P., Gaviria M., and Privat A.; International Campaign for Cures of Spinal Cord Injury Paralysis. (2006). Guidelines for the conduct of clinical trials for spinal cord injury (SCI) as developed by the ICCP panel: clinical trial outcome measures. Spinal Cord 45, 206–221 [DOI] [PubMed] [Google Scholar]
- 54.Selassie A.W., Varma A., and Saunders L.L. (2011). Current trends in venous thromboembolism among persons hospitalized with acute traumatic spinal cord injury: does early access to rehabilitation matter? Arch. Phys. Med. Rehabil. 92, 1534–1541 [DOI] [PubMed] [Google Scholar]
- 55.Barnett V., and Lewis T. (1994). Outliers in Statistical Data. 3rd ed. John Wiley & Sons: Chichester [Google Scholar]
- 56.Bracken M.B., Shepard M.J., Collins W.F., Holford T.R., Young W., Baskin D.S., Eisenberg H.M., Flamm E., Leo-Summers L., Maroon J., Marshall L.F., Perot P.L., Jr., Piepmeier J., Sonntag V.K., Wagner F.C., Wilberger J.E., and Winn H.R. (1990). A randomized, controlled trial of methylprednisolone or naloxone in the treatment of acute spinal-cord injury: results of the Second National Acute Spinal Cord Injury Study. N. Engl. J. Med. 322, 1405–1411 [DOI] [PubMed] [Google Scholar]
- 57.Geisler F.H., Coleman W.P., Grieco G., and Poonian D.; Sygen Study Group. (2001). The Sygen multicenter acute spinal cord injury study. Spine 26, Suppl 24, S87–S98 [DOI] [PubMed] [Google Scholar]
- 58.Marino R.J., Ditunno J.F., Jr., Donovan W.H., and Maynard F., Jr. (1999). Neurologic recovery after traumatic spinal cord injury: data from the Model Spinal Cord Injury Systems. Arch. Phys. Med. Rehabil. 80, 1391–1396 [DOI] [PubMed] [Google Scholar]
- 59.Kramer J.L., Lammertse D.P., Schubert M., Curt A., and Steeves J.D. (2012). Relationship between motor recovery and independence after sensorimotor-complete cervical spinal cord injury. Neurorehabil. Neural. Repair 26, 1064–1071 [DOI] [PubMed] [Google Scholar]
- 60.van Middendorp J.J., Hosman A.J., Pouw M.H., and Van de Meent H. (2009). ASIA impairment scale conversion in traumatic SCI: is it related with the ability to walk? A descriptive comparison with functional ambulation outcome measures in 273 patients. Spinal Cord 47, 555–560 [DOI] [PubMed] [Google Scholar]
- 61.van Middendorp J.J., Hosman A., and Doi S.A. (2013). The effects of the timing of spinal surgery after traumatic spinal cord injury: a systematic review and meta-analysis. J. Neurotrauma 30, 1781–1794 [DOI] [PubMed] [Google Scholar]
- 62.Chafetz R.S., Vogel L.C., Betz R.R., Gaughan J.P., and Mulcahey M.J. (2008). International standards for neurological classification of spinal cord injury: training effect on accurate classification. J. Spinal Cord Med. 31, 538–542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Drew B., Fehlings M.G., Paquet J., Ahn H., Attabib A., Bailey C.S., Christie S.D., Duggal N., Finkelstein J., Fourney D.R., Hurlbert R.J., Johnson M.G., Kwon B.K., Parent S., Tsai E.C., Dvorak M., Noonan V.K., Rivers C.S., and Shen T., RHSCIR Network. (2014). Current surgical practice for traumatic spinal cord injury in Canada. Can. J. Surg. 57, Suppl 3, S31 Abstract [Google Scholar]
- 64.Coleman W.P., and Geisler F.H. (2004). Injury severity as primary predictor of outcome in acute spinal cord injury: retrospective results from a large multicenter clinical trial. Spine J. 4,373–378 [DOI] [PubMed] [Google Scholar]
- 65.Zariffa J., Kramer J.L., Fawcett J.W., Lammertse D.P., Blight A.R., Guest J., Jones L., Burns S., Schubert M., Bolliger M., Curt A., and Steeves J.D. (2011). Characterization of neurological recovery following traumatic sensorimotor complete thoracic spinal cord injury. Spinal Cord 49, 463–471 [DOI] [PubMed] [Google Scholar]
- 66.Tuszynski M.H., Steeves J.D., Fawcett J.W., Lammertse D., Kalichman M., Rask C., Curt A., Ditunno J.F., Fehlings M.G., Guest J.D., Ellaway P.H., Kleitman N., Bartlett P.F., Blight A.R., Dietz V., Dobkin B.H., Grossman R., and Privat A.; International Campaign for the Cures of Spinal Cord Injury Paralysis. (2007). Guidelines for the conduct of clinical trials for spinal cord injury as developed by the ICCP Panel: clinical trial inclusion/exclusion criteria and ethics. Spinal Cord 45, 222–231 [DOI] [PubMed] [Google Scholar]