Abstract
Listeria monocytogenes is a Gram-positive, foodborne pathogen responsible for approximately 28% of all food-related deaths each year in the United States. L. monocytogenes infections are linked to the consumption of minimally processed ready-to-eat (RTE) products such as cheese, deli meats, and cold-smoked finfish products. L. monocytogenes is resistant to stresses commonly encountered in the food-processing environment, including low pH, high salinity, oxygen content, and various temperatures. The purpose of this study was to determine if cells habituated at low temperatures would result in cross-protective effects against osmotic stress. We found that cells exposed to refrigerated temperatures prior to a mild salt stress treatment had increased survival in NaCl concentrations of 3%. Additionally, the longer the cells were pre-exposed to cold temperatures, the greater the increase in survival in 3% NaCl. A proteomics analysis was performed in triplicate in order to elucidate mechanisms involved in cold-stress induced cross protection against osmotic stress. Proteins involved in maintenance of the cell wall and cellular processes, such as penicillin binding proteins and osmolyte transporters, and processes involving amino acid metabolism, such as osmolyte synthesis, transport, and lipid biosynthesis, had the greatest increase in expression when cells were exposed to cold temperatures prior to salt. By gaining a better understanding of how this pathogen adapts physiologically to various environmental conditions, improvements can be made in detection and mitigation strategies.
Keywords: Listeria monocytogenes, cold stress, salt stress, cross protection, proteomics
INTRODUCTION
Listeria monocytogenes is a deadly foodborne pathogen of vital importance to public health and the food industry. Infections with L. monocytogenes are more common among the elderly, pregnant women, infants, or immunocompromised individuals and are primarily associated with ingestion of contaminated food products.1 Much care is taken to ensure the safety of ready-to-eat products and proper preservation of these products, including the application of salts and low temperature to improve shelf life.2 However, the psychrophilic and halophilic nature of this pathogen enables it to persist and grow at refrigerated temperatures and moderate salt concentrations.1
It has previously been shown through transcriptome analysis that acclimation of L. monocytogenes to low temperatures induces cross protection to high salt concentrations.3,4 The ability of L. monocytogenes to adapt to osmotic stress has been proposed to be lineage-specific, with lineages I and III exhibiting survival advantages at 37 °C over lineage II strains under osmotic stress.5 The adaptation of L. monocytogenes to cold or osmotic stress revealed an overlap in adaptive mechanisms used by the pathogen, including increased expression genes and proteins related to the transport of compatible solutes (betL, opuC, and gbu operons6–8), alterations in cell membrane fluidity,9 and sequestration and storage of iron by ferritin.10,11 The expression of cold shock proteins (Csps) has also been found to be essential for the growth of L. monocytogenes at both low temperatures and high salt concentrations,4,12 suggesting cold stress induces cross protection against high osmotic conditions. Additionally, osmotic resistance is enhanced in cold temperatures, with resistance being potentially mediated by enhanced expression of peptidoglycan synthesis genes, general stress response genes, and cation transporter genes.3
However, the increase in survival due to cross-protective mechanisms may differ between lineages. A previous study identified that variations exist in the survival of L. monocytogenes and L. innocua following sequential exposure to stressors that were not necessarily related to genetic lineages.13 In this previous study, the genetic lineage II strain EGDe actually had increased survival following sequential exposure to several stressors, including cold temperatures and 6% NaCl. Therefore, the purpose of this current study was to expand upon these previous findings by determining the mechanisms that enhance the adaptation of L. monocytogenes to a minimal osmotic stress (3% NaCl) following exposure to low temperatures utilizing a proteomics-based approach. These mechanisms were found to involve variations in the expression of several proteins associated with cell wall architecture and synthesis. Gaining a better understanding of the mechanisms involved in adaptation to stresses typically encountered in the food-processing environment could potentially aid in the development of mitigation strategies to reduce or eliminate this pathogen in ready-to-eat products.
MATERIALS AND METHODS
Bacterial Growth Conditions and Survival Analysis
The L. monocytogenes strain EGDe (serovar 1/2a) was routinely cultured in brain heart infusion (BHI) medium at 37 °C. Fresh cultures were incubated overnight at 37 °C in BHI broth, after which cultures were diluted 1:100 (~1 × 106 CFU/mL) in 10 mL of BHI (pH 7.4) and incubated at either 4 or 37 °C. A 1 mL aliquot of cells was removed each hour for up to 6 h, pelleted at 10,000 × g for 2 min, and then resuspended in BHI supplemented with 3% NaCl. Cultures were then incubated at 37 °C for 4 h. Aliquots were removed for viable plate analysis hourly following resuspension in media supplemented with 3% NaCl. At least three independent replicates were performed.
Purification of Proteins
Cultures of L. monocytogenes EGDe were incubated for 4 h at either 4 or 37 °C in BHI, after which cells were pelleted by centrifugation at 10,000 × g for 10 min and resuspended in an equal volume of BHI supplemented with 3% NaCl and incubated at 37 °C for another 4 h. Methods previously described by our group were used to isolate proteins after growth at 4 and 37 °C for 4 h and the subsequent exposure to 3% NaCl for 4 h.14,15,16 Briefly, aliquots collected were pelleted by centrifugation at 10,000 × g for 10 min, and resulting pellets were resuspended in 4 mL of a lysis solution [2% Triton X-100, 2.6 mg/mL sodium azide, 0.1 M Tris pH 8.0, and 8 mM phenylmethanesulfonyl fluoride (PMSF)]. Cells were incubated at 37 °C for 20 min with the addition of 20 mg/mL of lysozyme and sonicated (Fisher Scientific Model 100 Sonic Dismembrator, setting 3) for four 30 s pulses on ice, with 1 min cooling between pulses. Following the addition of 85 µg/mL DNase I and 20 µg/mL RNase A, samples were incubated at 37 °C for 30 min and centrifuged at 6,200 × g for 10 min at 10 °C to pellet the cell debris. An equal volume of 50% trichloroacetic acid (TCA) was added to the supernatant of each sample, and proteins were precipitated at −20 °C overnight. The precipitated proteins were pelleted by centrifugation at 6,200 × g for 10 min at 10 °C, washed with ice-cold acetone (Chromosolv for HPLC, Sigma Aldrich), and dried at room temperature.
The proteins were subsequently resuspended in 0.5 mL of solubilization solution (7 M urea, 20 mM Tris-Cl, pH 8.0, 5 mM EDTA, 5 mM MgCl2, 4% CHAPS, 1 mM PMSF) and quantitated using the 2-D Quant Kit (GE Healthcare Life Sciences). An aliquot of 0.1 mg of proteins was precipitated once more with 50% TCA at −20 °C, followed by centrifugation and a wash with ice-cold acetone. Samples were resuspended in 0.1 mL of 100 mM ammonium bicarbonate and 5% acetonitrile, then treated with 5 mM dithiothreitol for 10 min at 65 °C, 10 mM iodoacetamide for 30 min at 30 °C, and finally digested with 2 µg of sequencing-grade trypsin at 37 °C for 16 h. Peptides were desalted using a peptide macrotrap (Michrom Bioresources, Inc.), dried at room temperature, and stored at −80 °C until further processing. Proteins were isolated from three independent experiments.
Protein Analysis
Desalted peptides were resuspended in 250 µL of 5 mM monosodium phosphate in 25% acetonitrile adjusted to a pH of 3 using formic acid and processed using a strong cation exchange (SCX) macrotrap (Michrom Bioresources, Inc.) according to the manufacturer’s instructions. Cleaned samples were dried and resuspended in 40 µL of 2% acetonitrile and 0.1% formic acid; ~50 µg of each sample was transferred to low retention HPLC vials for analysis using mass spectrometry.
Peptide mass spectrometry was accomplished using an EASY-nLC (Thermo Scientific) high performance liquid chromatography machine (HPLC) coupled with an LTQ Velos (Thermo Scientific) linear ion trap mass spectrometer. The Easy-nLC was configured for reverse phase chromatography using a Hypersil Gold KAPPA C18 column (Thermo Scientific) with a flow rate of 333 nL/min. Peptides were separated for mass spectrometry analysis using an acetonitrile gradient starting at 2% ACN, 0.1% FA and reaching 50% ACN, 0.1% FA in 120 min, followed by a 15 min wash of 95% ACN, 0.1% FA. Column equilibration was handled automatically using the EASY-nLC. The eluate from the HPLC was fed directly to the LTQ Velos for nanospray ionization followed by MS/MS analysis of detected peptides. The LTQ Velos was configured to perform 1 ms scan followed by 20 MS/MS scans of the 20 most intense peaks repeatedly over the 135 min duration of each HPLC run. Dynamic exclusion was enabled with a duration of 5 min, repeat count of 1, and a list length of 500. The collected spectra were subsequently analyzed using the X!tandem search algorithm.17
Raw spectral data from the LTQ Velos were converted to mzML format using the msConvert tool form the ProteoWizard software project because X!tandem cannot read the Thermo raw format directly.18 The FASTA database used for peptide spectrum matching (target database) was the Listeria monocytogenes strain EGD-e RefSeq protein database from the National Center for Biotechnology Information (accession no. 61583). X!tandem was configured to use tryptic cleavage sites with up to two missed cleavages. Precursor and fragment mass tolerance were set to 1000 and 500 ppm, respectively. Four amino acid modifications were included in the database search: single and double oxidation of methionine and both carboxymethylation and carboxamidomethylation of cysteine. A decoy search was also performed using a randomized version of the target database with the same search parameters as above. The search results were filtered using previously described methods.19,20 A decoy score distribution was created and each match from the target database was evaluated as a possible outlier and assigned a probability of being correct. Peptides from the target database were accepted if the probability of being correct was 95% or higher. A list of proteins and identified peptides was generated for each replicate of a given treatment.
Protein Comparison
Protein differential expression between treatments was evaluated on the basis of peptide spectral intensity. The raw spectral data were converted to the MS1 tab delimited format using the MakeMS2 tool available from the MacCoss lab at the University of Washington.21 The intensities for each peptide elution peak were identified from the associated MS1 file using Perl script and summed. For each identified protein, the peptide intensities were combined and organized by experimental replicate. Differential expression was evaluated using Monte Carlo resampling techniques to compare the replicate intensities between treatments. Each comparison used 1 million iterations and was assigned a p-value based on the number of times each test favored one treatment over another (p < 0.05 indicated significance). Functional classifications were assigned based on ListiList categories (http://genolist.pasteur.fr/ListiList/).
RESULTS
Prolonged Exposure to Low Temperatures Increases Resistance to 3% NaCl
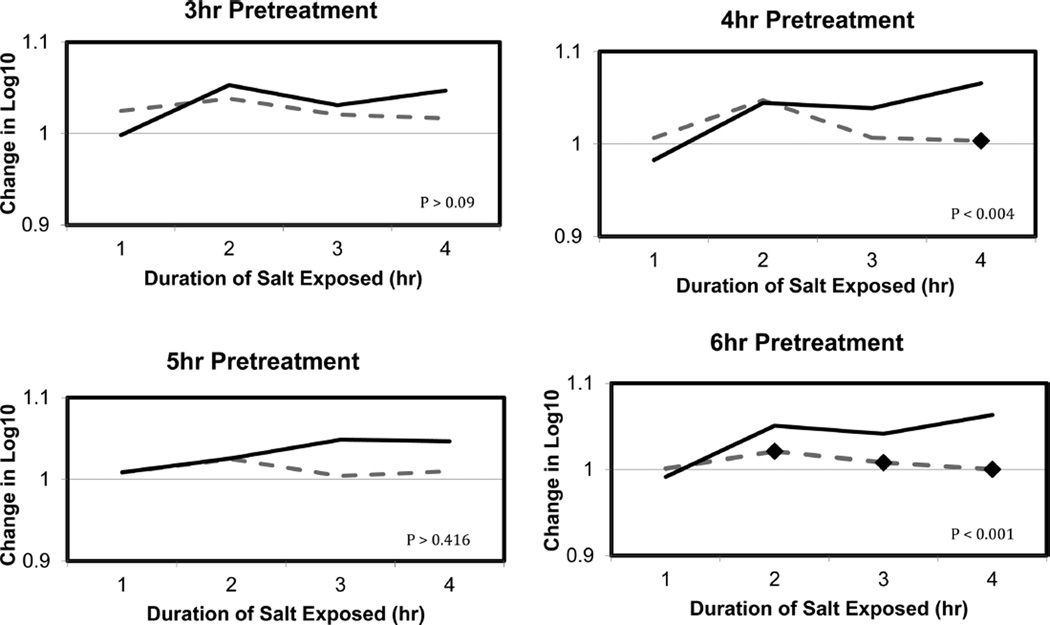
To determine if exposure to cold temperatures could enhance osmotic protection, we analyzed the viability of EGDe after exposure at 4 °C and a subsequent salt exposure. EGDe exposed to cold temperatures had increased resistance to osmotic stress in comparison to cells incubated at 37 °C (Figure 1). Exposure to cold temperatures resulted in a significant increase in the fold change (p < 0.0001) of EGDe’s growth in 3% NaCl within the 6 h time period tested (Figure 1).
Figure 1.
Tolerance to salt increased for EGDe exposed to 4 °C. Fold change in log10 CFU/mL for EGDe grown for 3, 4, 5, or 6 h at either 37 °C (dashed lines) or 4 °C (solid lines) prior to a 4 h treatment in 3% NaCl.
Cold and Osmotic Stress Protein Expression Profile of EGDe
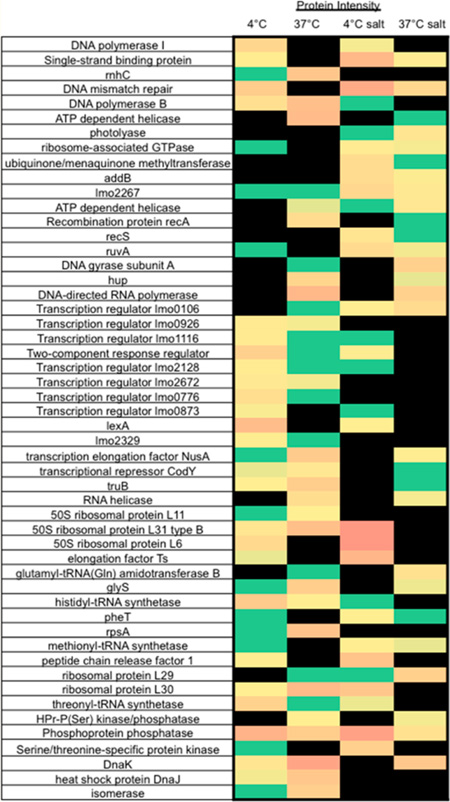
In order to investigate how exposure to cold temperatures can induce cross protection against salt stress, the protein expression of EGDe incubated at either 4 or 37 °C for 4 h prior to exposure to 3% NaCl for 4 h was analyzed by LTQ mass spectrometry in triplicate. ListiList categories were used to classify each of the differentially expressed proteins into functional groups.15,16 A total of 299 significantly differentially expressed proteins were identified.
Differentially Expressed Proteins Associated with Cell Envelope and Cellular Processes (Category 1)
Cell wall associated proteins and proteins involved in membrane bioenergetics that increased in expression following exposure to cold temperatures prior to salt included the invasion associated protein (gi: 16802625) and the H+ transporting ATP synthase chain subunits a and c (gi: 16412019 and 16412022). The cell division protein FtsA (gi: 16411503) also increased in expression, while the cell division proteins FtsZ and FtsW (gi: 16411502 and 16804724) decreased in expression in cold-stressed cells exposed to osmotic stress (Table 1).
Table 1.
Cell Envelope Associated Proteins (ListiList Category 1) with Differential Expression between Treatmentsa
| intensity | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| gi no. | protein | ListiList | 4 °C | 37°C | 4 °C salt |
37 °C salt |
4 to 4 °C salt |
4 to 37 °C |
4 °C salt to 37 °C salt |
37 to 37 °C salt |
| 16802625 | invasion associated protein | 1.1 | 0.0000 | 6.8099 | 6.8360 | 7.1841 | 0.0206 | - | - | - |
| 16409456 | similar to MptC | 1.2 | 6.7586 | 6.7747 | 5.2658 | 4.5423 | NS | NS | - | 0.0079 |
| 16410404 | PTS phosphocarrier protein Hpr | 1.2 | 6.6204 | 7.0356 | 8.2533 | 8.2297 | 0.0327 | - | - | NS |
| 16410405 | PTS enzyme I | 1.2 | 6.2438 | 6.0000 | 6.8722 | 7.6487 | NS | - | 0.0412 | 0.0176 |
| 16410642 | similar to B. subtilis YdgH | 1.2 | 6.9560 | 7.1084 | 7.0663 | 0.0000 | NS | NS | 0.0203 | 0.0019 |
| 16411053 | similar to multidrug-efflux transporter | 1.2 | 7.0510 | 6.3653 | 7.2640 | 6.5801 | NS | NS | 0.0014 | NS |
| 16411174 | similar to PTS lichenan-specific enzyme IIB | 1.2 | 0.0000 | 7.0617 | 0.0000 | 5.7445 | - | 0.0325 | - | 0.0399 |
| 16411454 | similar to PTS mannose-specific enzyme IIC | 1.2 | 6.7404 | 5.9139 | 6.5757 | 0.0000 | NS | - | 0.0122 | - |
| 16411514 | similar to transporter binding protein | 1.2 | 7.1926 | 6.7399 | 7.5163 | 5.8352 | NS | NS | 0.0233 | - |
| 16411654 | similar to FurA | 1.2 | 4.7994 | 0.0000 | 0.0000 | 6.3280 | - | - | 0.0350 | 0.0350 |
| 16411666 | similar to OppA | 1.2 | 6.6197 | 7.6957 | 7.8619 | 7.8669 | 0.0474 | NS | - | NS |
| 16411823 | similar to PTS fructose-specific IIABC | 1.2 | 6.4731 | 7.6951 | 7.9466 | 8.0758 | 0.0450 | 0.0064 | - | NS |
| 16802674 | putative fructose-like permease EIIC subunit 2 | 1.2 | 0.0000 | 0.0000 | 7.2327 | 0.0000 | 0.0108 | - | 0.0108 | - |
| 16802675 | putative fructose-like phosphotransferase EIIB | 1.2 | 0.0000 | 6.4162 | 0.0000 | 0.0000 | - | 0.0231 | - | 0.0230 |
| 16802687 | similar to amino acid transporter | 1.2 | 5.2100 | 0.0000 | 0.0000 | 6.3805 | NS | - | 0.0112 | 0.0111 |
| 16803102 | similar to permease | 1.2 | 6.6757 | 6.5914 | 6.5034 | 0.0000 | NS | NS | <0.0001 | 0.0098 |
| 16803545 | similar to ABC transporter ATP-binding protein | 1.2 | 6.0356 | 5.9003 | 7.0098 | 0.0000 | 0.0169 | - | 0.0076 | - |
| 16804537 | similar to phosphate ABC transporter binding protein | 1.2 | 7.4625 | 5.8699 | 6.1640 | 0.0000 | NS | - | 0.0185 | - |
| 16804720 | similar to cellobiose phosphotransferase enzyme IIB component | 1.2 | 0.0000 | 5.9132 | 7.3123 | 5.6812 | 0.0204 | - | 0.0228 | - |
| 18140806 | similar to PTS system mannose-specific IIAB | 1.2 | 0.0000 | 7.6286 | 7.7607 | 7.9634 | <0.0001 | NS | - | NS |
| 16412018 | similar to H+-transporting AtpG | 1.4 | 0.0000 | 6.7863 | 0.0000 | 0.0000 | - | 0.0294 | - | 0.0294 |
| 16412019 | similar to H+-transporting AtpA | 1.4 | 6.6325 | 7.5308 | 8.3146 | 8.1094 | 0.0266 | 0.0450 | - | NS |
| 16412022 | similar to H+-transporting AtpE | 1.4 | 0.0000 | 0.0000 | 6.6495 | 5.9374 | 0.0134 | - | 0.0461 | - |
| 16802062 | AA3-600 quinol oxidase subunit I QoxB | 1.4 | 6.6654 | 6.5351 | 7.1047 | 0.0000 | NS | - | 0.0246 | - |
| 16804258 | similar to PrsA2 | 1.6 | 5.6330 | 4.6566 | 7.8584 | 7.3901 | 0.0089 | - | - | NS |
| 16411502 | highly similar to FtsZ | 1.7 | 7.0514 | 6.8614 | 6.2807 | 5.9949 | 0.0327 | NS | - | - |
| 16411503 | highly similar to FtsA | 1.7 | 7.4381 | 6.9844 | 6.7043 | 0.0000 | NS | NS | 0.0246 | - |
| 16804724 | similar to FtsW | 1.7 | 7.2647 | 0.0000 | 0.0000 | 5.7918 | 0.0278 | 0.0280 | - | - |
| 16409569 | ActA | 1.8 | 5.5168 | 0.0000 | 7.1229 | 7.7576 | 0.0164 | - | - | NS |
| 16409952 | hypothetical cell wall associated protein | 1.8 | 6.4446 | 0.0000 | 7.8056 | 5.2354 | 0.0098 | - | 0.0074 | - |
| 16802207 | peptidoglycan binding protein | 1.8 | 0.0000 | 5.5687 | 7.0563 | 0.0000 | 0.0140 | - | 0.0141 | - |
| 16803329 | internalin protein, peptidoglycan bound | 1.8 | 6.4058 | 6.1610 | 6.2332 | 0.0000 | NS | - | 0.0191 | - |
| 16411240 | Internalin C | 1.9 | 0.0000 | 0.0000 | 6.7818 | 6.2085 | 0.0448 | - | - | - |
Expression levels with no significant difference between a given treatment are indicated by NS. 4 °C = cells exposed to 4 °C for 4 h; 37 °C = cells exposed to 37°C for 4 h; 4 °C salt = cells exposed to 4 °C for 4 h followed by exposure to salt; 37 °C salt = cells exposed to 37°C followed by exposure to salt.
The acquisition of nutrients, as well as osmoprotectants, from the environment requires proteins involved in binding and transporting substrates. Such proteins that increased in expression when cold-stressed cells were exposed to salt were those similar to the pheromone ABC transporter binding protein OppA (gi: 16411666), phosphotransferase system (PTS) fructose-specific enzyme IIABC component (gi: 16411823), a putative fructose-like permease EIIC subunit 2 (gi: 16802674), PTS mannose-specific enzyme IIAB (gi: 18140806), cellobiose phosphotransferase enzyme IIB (gi: 16804720), and an ABC transporter/ATP-binding protein and permease (gi: 16803545 and 16803102) (Table 1).
Cells exposed to cold stress prior to osmotic stress had an increase in expression of proteins involved in secretion, such as foldase protein PrsA 2 (gi: 16804258). Cell surface proteins, such as ActA (gi: 16409569), InlC (gi: 16411240), a hypothetical cell wall associated protein (gi: 16409952), a peptidoglycan binding protein (gi: 16802207), and a protein similar to internalin (gi: 16803329) also increased in expression.
Differentially Expressed Proteins Associated with Metabolic Pathways (Category 2)
Adaptation to osmotic stress requires a shift in expression of proteins involved in specific metabolic pathways and metabolism of amino acids and nucleic acids. Cells previously exposed to low temperatures prior to osmotic stress increased in expression of proteins involved in fermentation, such as l-lactate dehydrogenase (gi: 16409575), bifunctional acetaldehyde-CoA/alcohol dehydrogenase (gi:16803674), and phosphotransacetylase (gi: 16804142) (Table 2). Numerous glycolytic enzymes also increased in expression, such as enolase (gi: 16411943), glyceraldehyde 3-phosphate dehydrogenase (gi: 16411947), glucose-6-phosphate isomerase (gi: 16804405), phosphoglyceromutase (gi: 16804494), phosphoglycerate kinase (gi: 16804496), and pyruvate formate-lyase (gi: 227478797). The glycolytic enzyme pyruvate carboxylase (gi: 16803112) had reduced expression (Table 2) following subsequent exposure to NaCl.
Table 2.
Intermediary Metabolism Proteins (ListiList Category 2) with differENtial Expression between Treatmentsa
| intensity | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| gi no. | protein | ListiList | 4 °C | 37°C | 4 °C salt |
37 °C salt |
4 to 4 °C salt |
4 to 37 °C | 4 °C salt to 37 °C salt |
37 to 37 ° C salt |
| 16409575 | similar to l-lactate dehydrogenase | 2.1.1 | 6.9612 | 7.3634 | 7.9398 | 7.9802 | 0.0033 | NS | - | NS |
| 16409725 | dihydroxyacetone kinase | 2.1.1 | 0.0000 | 6.6876 | 7.8031 | 0.0000 | - | 0.0020 | - | 0.0020 |
| 16410835 | pyruvate formate-lyase | 2.1.1 | 0.0000 | 6.8677 | 7.7523 | 7.2528 | 0.0498 | 0.0099 | - | NS |
| 16411468 | α-mannosidase | 2.1.1 | 5.7918 | 6.4030 | 6.6137 | 0.0000 | 0.0374 | - | 0.0150 | - |
| 16411565 | similar to 1-phosphofructokinase | 2.1.1 | 0.0000 | 6.1179 | 6.7230 | 0.0000 | 0.0111 | - | 0.0111 | - |
| 16412162 | similar to ribose 5-phosphate epimerase | 2.1.1 | 6.7400 | 6.1520 | 5.1173 | 6.4804 | 0.0086 | NS | - | NS |
| 16802066 | β-glucosidase | 2.1.1 | 7.8661 | 0.0000 | 7.0558 | 0.0000 | NS | NS | 0.0158 | - |
| 16802579 | 6-phospho-β-glucosidase | 2.1.1 | 0.0000 | 0.0000 | 7.0197 | 7.1229 | 0.0030 | - | - | - |
| 16803211 | NADPH-dependent butanol dehydrogenase | 2.1.1 | 6.8034 | 5.9597 | 6.0998 | 0.0000 | 0.0486 | 0.0345 | - | - |
| 16803215 | similar to ethanolamine ammonia-lyase | 2.1.1 | 6.7624 | 5.7603 | 5.5218 | 0.0000 | 0.0258 | 0.0326 | - | - |
| 16803219 | acetaldehyde dehydrogenase/alcohol dehydrogenase | 2.1.1 | 6.9123 | 6.0708 | 7.6642 | 0.0000 | 0.0027 | - | 0.0002 | - |
| 16803416 | 6-phosphogluconate dehydrogenase | 2.1.1 | 6.7825 | 6.6058 | 8.2542 | 7.6424 | 0.0092 | - | 0.0476 | NS |
| 16803610 | pyruvate kinase | 2.1.1 | 6.9556 | 8.1493 | 8.6512 | 8.3909 | 0.0099 | NS | - | NS |
| 16803674 | bifunctional acetaldehyde-CoA/alcohol dehydrogenase | 2.1.1 | 6.3356 | 7.6512 | 8.0228 | 7.7441 | 0.0088 | NS | - | NS |
| 16803907 | pyruvate phosphate dikinase | 2.1.1 | 6.2712 | 6.5881 | 7.3780 | 7.0393 | - | - | - | 0.0363 |
| 16804142 | phosphotransacetylase | 2.1.1 | 6.1527 | 7.4961 | 7.8103 | 7.0716 | 0.0007 | <0.0001 | 0.0048 | 0.0095 |
| 16804374 | fructose-1-phosphate kinase | 2.1.1 | 5.9542 | 6.8914 | 7.0088 | 3.9846 | 0.0445 | NS | 0.0300 | NS |
| 16804701 | sorbitol dehydrogenase | 2.1.1 | 5.9253 | 5.8023 | 7.0092 | 5.1989 | 0.0167 | NS | 0.0105 | - |
| 16804801 | similar to xylose operon regulatory protein and to glucose kinase | 2.1.1 | 0.0000 | 5.5560 | 6.8148 | 5.4736 | 0.0432 | |||
| 16804818 | similar to β-glucosidase | 2.1.1 | 7.3414 | 6.1402 | 7.0027 | 6.9850 | - | 0.0009 | - | 0.0267 |
| 227478797 | pyruvate formate-lyase | 2.1.1 | 4.4422 | 6.5867 | 7.6970 | 7.5296 | 0.0177 | - | - | NS |
| 16409932 | phosphoglycerate mutase | 2.1.2 | 6.7814 | 6.8841 | 6.5850 | 0.0000 | NS | NS | 0.0238 | 0.0171 |
| 16411943 | enolase | 2.1.2 | 0.0000 | 7.8602 | 7.4834 | 8.1384 | 0.0217 | NS | - | NS |
| 16411947 | glyceraldehyde 3-phosphate dehydrogenase | 2.1.2 | 7.2901 | 8.0892 | 8.5794 | 8.3793 | 0.0070 | NS | - | NS |
| 16803112 | pyruvate carboxylase | 2.1.2 | 7.6587 | 7.3676 | 0.0000 | 6.8210 | 0.0167 | NS | - | NS |
| 16804405 | glucose-6-phosphate isomerase | 2.1.2 | 0.0000 | 7.1315 | 7.9954 | 7.4979 | 0.0415 | NS | - | NS |
| 16804494 | phosphoglyceromutase | 2.1.2 | 7.4374 | 7.9937 | 8.4495 | 7.5346 | 0.0005 | NS | 0.0007 | NS |
| 16804496 | phosphoglycerate kinase | 2.1.2 | 4.8207 | 7.7023 | 7.6283 | 7.8756 | 0.0146 | - | - | NS |
| 16410414 | similar to N-acyl-l-amino acid amidohydrolases | 2.2 | 5.0345 | 0.0000 | 5.7760 | 6.8229 | 0.0352 | - | - | - |
| 16411443 | similar to 3-isopropylmalate dehydratase | 2.2 | 7.7082 | 0.0000 | 5.5451 | 0.0000 | 0.0092 | 0.0089 | - | - |
| 16411545 | probable O-sialoglycoprotein endopeptidase Gcp | 2.2 | 7.5966 | 5.1507 | 6.6183 | 6.4445 | NS | NS | - | <0.0001 |
| 16411560 | similar to argininosuccinate synthase | 2.2 | 0.0000 | 6.5992 | 7.5140 | 6.4491 | 0.0149 | <0.0001 | 0.0249 | NS |
| 16411902 | similar to aminotransferase; Fe-S cluster assembly protein | 2.2 | 6.9243 | 7.5226 | 7.6740 | 6.8930 | 0.0495 | NS | 0.0469 | NS |
| 16412034 | threonine synthase | 2.2 | 6.9294 | 7.4119 | 8.3031 | 8.4006 | 0.0187 | NS | - | NS |
| 16412249 | similar to glutamine amidotransferase | 2.2 | 0.0000 | 0.0000 | 7.2733 | 0.0000 | 0.0166 | - | 0.0167 | - |
| 16412325 | phosphoserine aminotransferase | 2.2 | 6.2299 | 0.0000 | 7.4518 | 7.3295 | 0.0076 | - | - | - |
| 16802491 | glutamate decarboxylase | 2.2 | 5.0580 | 6.7632 | 5.0575 | 6.4716 | - | - | 0.0166 | - |
| 16803257 | endo-1,4-β-glucanase and to aminopeptidase | 2.2 | 6.5306 | 7.0107 | 0.0000 | 6.7576 | - | NS | 0.0362 | NS |
| 16803394 | aminopeptidase P | 2.2 | 0.0000 | 0.0000 | 7.4421 | 6.9641 | 0.0093 | - | - | NS |
| 16803660 | dipeptidase PepV | 2.2 | 6.3184 | 7.9841 | 8.1506 | 7.9454 | 0.0091 | 0.0231 | - | NS |
| 16803704 | S-adenosylmethionine synthetase | 2.2 | 0.0000 | 6.7040 | 7.4543 | 6.0209 | 0.0373 | - | 0.0433 | NS |
| 16804022 | dihydroxy-acid dehydratase | 2.2 | 6.7569 | 5.9028 | 0.0000 | 6.5999 | 0.0091 | 0.0249 | 0.0251 | NS |
| 16804026 | 2-isopropylmalate synthase | 2.2 | 6.4251 | 0.0000 | 0.0000 | 7.2174 | 0.0196 | 0.0197 | - | NS |
| 16411289 | highly similar to carbamoyl-phosphate synthetase (catalytic subunit) | 2.3 | 5.8757 | 0.0000 | 7.0540 | 0.0000 | NS | - | 0.0397 | - |
| 16411407 | phosphopentomutase Drm | 2.3 | 5.1172 | 7.4228 | 7.7811 | 6.8321 | 0.0099 | NS | 0.0206 | NS |
| 16802325 | anaerobic ribonucleoside triphosphate reductase | 2.3 | 6.2082 | 6.6751 | 6.8456 | 6.6630 | 0.0156 | - | - | NS |
| 16803877 | dihydroorotase PyrC | 2.3 | 5.5521 | 5.7992 | 6.8805 | 6.2331 | 0.0313 | - | - | - |
| 16804649 | adenylate kinases | 2.3 | 6.5162 | 7.3866 | 7.7739 | 7.6280 | 0.0282 | NS | - | NS |
| 16410787 | similar to branched-chain α-keto acid dehydrogenase E3 subunit | 2.4 | 0.0000 | 6.6942 | 7.1144 | 6.8158 | 0.0216 | 0.0228 | - | NS |
Expression levels with no significant difference between a given treatment are indicated by NS.
Metabolism of alternative carbohydrates is advantageous during stress conditions. Proteins involved in utilization of different sugars were increased in cold-adapted salt-stressed cells and included α-mannosidase (gi: 16411468), phosphofructokinase (gi: 16411565), fructose-1-phosphate kinase (gi: 16804374), sorbitol dehydrogenase (gi: 16804701), glucose kinase (β-glucoside kinase) (gi: 16804801), and 6-phospho-β-glucosidase (gi: 16802579).
Numerous proteins important in the metabolism of nucleic acids increased in expression as well, including a protein similar to phosphopentomutase Drm (gi: 16411407), dihydroorotase PyrC (gi: 16803877), anaerobic ribonucleoside triphosphate reductase (gi: 16802325), and adenylate kinase (gi: 16804649). Cold-adapted salt-stressed cells also had an increased expression of branched-chain α-keto acid dehydrogenase E3 subunit (gi: 16410787), a protein involved in the metabolism of lipids.
Differentially Expressed Proteins Associated with Information Pathways (Category 3) or Other Functions (Category 4)
In order to adapt to stressful environments, bacteria must be able to efficiently repair damaged DNA and synthesize mRNA and proteins. Proteins involved in mismatch repair, homologous recombination, and the SOS response increased in expression in cold-stressed cells when exposed to osmotic shock. These included single-stranded binding protein (gi: 16802093), DNA mismatch repair MutS (gi: 16410832), Holliday junction DNA helicase RuvA (gi: 16410962), and ATP-dependent deoxyribonuclease (gi: 16804306) (Table 3). Proteins involved in base excision repair and nucleotide excision repair decreased in expression in cold-adapted salt-stressed cells: DNA polymerase I (gi: 16410994) and putative DNA polymerase β similar to B. subtilis YshC (gi: 16803271). Proteins involved in transcriptional regulation, such as the SOS response regulator LexA (gi: 16410718) and the transcriptional regulator LacI family (16804167), had a reduced expression in cold-adapted salt-stressed cells (Table 3).
Table 3.
Information Pathway Proteins (ListiList Category 3) and Proteins with Other Functions (ListiList Category 4) with Differential Expression between Treatmentsa
| intensity | p-value | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| gi no. | protein | ListiList | 4 °C | 37°C | 4 °C salt |
37 °C salt |
4 to 4 °C salt |
4 to 37 °C |
4 °C Salt to 37 °C salt |
37 to 37 °C salt |
| 16410994 | DNA polymerase I | 3.1 | 6.8723 | 6.5453 | 5.6347 | 6.5548 | 0.0118 | NS | - | NS |
| 16802093 | similar to single-strand binding protein | 3.1 | 6.0229 | 0.0000 | 7.5454 | 5.9570 | 0.0430 | - | 0.0422 | - |
| 16409964 | DNA photolyase | 3.2 | 0.0000 | 6.6394 | 0.0000 | 6.5368 | - | - | 0.0180 | NS |
| 16410832 | DNA mismatch repair MutS | 3.2 | 7.0763 | 7.0809 | 7.8285 | 6.8644 | 0.0287 | NS | 0.0192 | NS |
| 16803271 | DNA polymerase β, similar B. subtilis YshC | 3.2 | 6.6778 | 7.3774 | 0.0000 | 0.0000 | 0.0057 | 0.0411 | - | - |
| 16410827 | RecA | 3.3 | 6.1232 | 6.7226 | 7.5282 | 0.0000 | - | NS | - | 0.0380 |
| 16410962 | RuvA | 3.3 | 0.0000 | 0.0000 | 6.7651 | 5.5560 | 0.0354 | - | 0.0453 | - |
| 16411395 | RecS | 3.3 | 6.2345 | 6.5578 | 6.5471 | 0.0000 | NS | - | 0.0456 | - |
| 16411738 | ATP-dependent deoxyribonuclease B | 3.3 | 6.3311 | 6.4461 | 6.7314 | 5.8742 | NS | - | 0.0192 | - |
| 16804306 | ATP-dependent deoxyribonuclease A | 3.3 | 0.0000 | 0.0000 | 6.8177 | 6.4622 | 0.0291 | - | - | <0.0001 |
| 16804794 | RecQ | 3.3 | 6.4065 | 4.9159 | 0.0000 | 6.4819 | - | - | 0.0015 | 0.0019 |
| 16410718 | LexA | 3.5.2 | 7.4147 | 7.2348 | 6.2958 | 6.8476 | <0.0001 | NS | - | NS |
| 16804167 | similar to transcription regulator, LacI family | 3.5.2 | 6.4056 | 0.0000 | 0.0000 | 0.0000 | 0.0072 | 0.0072 | - | - |
| 16803697 | elongation factor Ts | 3.5.3 | 5.1279 | 7.7511 | 7.5649 | 7.9317 | 0.0088 | - | - | NS |
| 16412102 | 50S ribosomal protein L30 RpmD | 3.7.1 | 6.2986 | 7.4704 | 7.2308 | 7.6548 | 0.0251 | 0.0001 | - | NS |
| 16412112 | 50S ribosomal protein L29 RpmC | 3.7.1 | 0.0000 | 0.0000 | 0.0000 | 6.9926 | - | - | 0.0319 | 0.0319 |
| 16804586 | 50S ribosomal protein L31 type B RpmE2 | 3.7.1 | 6.5147 | 7.3646 | 8.1881 | 7.7370 | 0.0029 | 0.0221 | - | NS |
| 16804655 | 50S ribosomal protein L6 RplF | 3.7.1 | 6.7922 | 7.7788 | 8.2158 | 7.9844 | 0.0219 | NS | - | NS |
| 16803498 | glycyl-tRNA synthetase β chain | 3.7.2 | 0.0000 | 7.0592 | 5.8591 | 5.2545 | - | - | - | - |
| 16410949 | histidyl-tRNA synthetase | 3.7.2 | 7.1119 | 6.1159 | 0.0000 | 0.0000 | 0.0233 | 0.0389 | - | - |
| 16410988 | threonyl-tRNA synthetase | 3.7.2 | 6.9629 | 0.0000 | 5.2213 | 6.1896 | 0.0374 | 0.0349 | - | - |
| 16411208 | aspartyl/glutamyl-tRNA aminotransferase subunit B | 3.7.2 | 6.6991 | 0.0000 | 6.2265 | 6.6142 | NS | - | NS | 0.0070 |
| 16802223 | methionyl-tRNA synthetase | 3.7.2 | 0.0000 | 5.4281 | 6.1184 | 5.0938 | 0.0039 | - | 0.0089 | - |
| 16803647 | phenylalanyl-tRNA synthetase β subunit | 3.7.2 | 0.0000 | 5.5149 | 6.2381 | 0.0000 | <0.0001 | - | <0.0001 | - |
| 16804581 | peptide chain release factor 1 Prf1 | 3.7.5 | 6.1543 | 7.6104 | 7.2635 | 6.6608 | 0.0153 | NS | NS | NS |
| 16409576 | similar to B. subtilis general stress protein Ctc | 4.1 | 0.0000 | 7.3261 | 6.8294 | 7.7079 | 0.03612 | 0.0109 | NS | NS |
| 16411753 | protein gp20 bacteriophage A118 | 4.3 | 7.3442 | 0.0000 | 6.3662 | 4.9735 | NS | - | 0.0398 | - |
| 16411783 | similar to bacteriophage protein | 4.3 | 6.7270 | 0.0000 | 0.0000 | 6.6206 | 0.0276 | 0.0276 | NS | - |
| 16802169 | similar to bacteriophage minor tail proteins | 4.3 | 6.7460 | 0.0000 | 0.0000 | 6.0269 | 0.0015 | 0.0016 | NS | - |
| 16412311 | tRNA modification GTPase MnmE | 4.5 | 6.4817 | 0.0000 | 7.4959 | 5.5128 | 0.0088 | - | 0.0039 | - |
| 16802166 | antigen A LmaA | 4.5 | 4.7242 | 0.0000 | 6.5650 | 7.2069 | 0.0080 | - | NS | NS |
Expression levels with no significant difference between a given treatment are indicated by NS.
There was an increased expression of proteins involved in translation and ribosome assembly in cold-adapted salt-stressed cells, such as the elongation factor Ts (gi: 16803697), peptide chain release factor 1 (gi: 16804581), 50S ribosomal protein L30 (gi: 16412102), 50S ribosomal protein L31 type B (gi: 16804586), and 50S ribosomal protein L6 (gi: 16804655). In cold-adapted salt-stressed cells, the methionyl-tRNA-synthetase (gi: 16802223) and phenylalanyl-tRNA synthetase β subunit (gi: 16803647) increased in expression, while the histidyl-tRNA synthetase (gi: 16410949) and threonyl-tRNA synthetase (gi: 16410988) decreased in expression. Salt-stressed cells that received no cold pretreatment had an increased expression of aspartyl/glutamyl-tRNA synthetase subunit B (gi: 16411208) and a reduced expression of glycyl-tRNA synthetase β chain (gi: 16803498).
There was an increase in the expression of the general stress response protein Ctc (similar to Bacillus subtilis) (gi: 16409576) and tRNA modification GTPase MnmE (gi: 16412311) in cold-exposed salt-stressed cells. In cold-adapted salt-stressed cells phage related proteins, such as gp20 bacteriophage A118 (gi: 16411753) and antigen A (gi: 16802166), increased in expression, while proteins similar to a bacteriophage protein (gi: 16411783) and a bacteriophage minor tail protein (16802169) had reduced expression.
DISCUSSION
Previous studies have examined the mechanisms L. monocytogenes use to adapt to growth at low temperatures or when exposed to osmotic stress.6–8 It has recently been proposed that exposure to cold stress may induce cross protection against subsequent exposure to salt stress,4 yet the mechanisms that allow for this protection have not been fully elucidated. L. monocytogenes is routinely subjected to multiple stresses within the food-processing environment either concomitantly or sequentially; it is important to discern the mechanisms this organism uses to withstand these conditions in order to eliminate its presence. Therefore, the purpose of this study was to examine the relationship between mechanisms involved in survival in cold temperatures in providing cross protection against osmotic stress. A concentration of 3% NaCl was selected as this is typically the minimum concentration that is used as brine in the food industry, particularly in the smoked finfish industry. A low concentration of NaCl was therefore used in this study to characterize the initial response to this condition while also minimizing cell death.
Though our time course was limited to 6 h of pretreatment to cold temperatures, it did appear as though the longer cells were exposed to cold temperatures, the higher the degree of cross protection against osmotic stress, therefore indicating that exposure to low temperatures may induce cross protection against osmotic stress in L. monocytogenes. To examine the physiological response, the variation in the expression of the proteome was examined for cells preconditioned at either 4 or 37 °C for 4 h prior to exposure to the minimal stress of 3% NaCl for an additional 4 h; this time point was chosen because this was when cells that had received a cold-stress pretreatment began to exhibit an increase in viability following osmotic stress. Adaptation to stressful conditions requires energy in the form of ATP, and the increase in the expression of the H+-transporting ATP synthase chain proteins indicates that the oxidative phosphorylation pathway is active. Increased expression of enzymes involved in oxidative phosphorylation is a common component of the stress-adaptation response of L. monocytogenes. The need for increased uptake of carbohydrates and production of ATP are required to fuel the high-energy demands of the cell for repair of damaged DNA, proteins, and lipids.
Another mechanism used by bacteria to adapt to growth at low temperatures and salt stress is the accumulation of osmolytes from the surrounding environment. Transporters, such as ABC transporter OppA, are involved in the uptake of oligopeptides as a means of accumulating proline, isoleucine, and valine to serve as cryoprotectants or osmolytes.22,23 The fact that OppA is required for growth at low temperatures and its potential role in the uptake of osmolytes strongly suggests that it is involved in cross protection of cold-stressed L. monocytogenes against osmotic stress.
The increased expression of PTS proteins involved in uptake of fructose, mannose, and cellobiose in cold-exposed salt-stressed cells suggests their importance in energy production or as osmoprotectants.8,24 This is interesting considering recent evidence that suggests that salt-stressed L. monocytogenes cells have reduced cell growth as a result of decreased expression of PTS genes. The decreased expression of PTS enzyme II cytoplasmic components associated with the uptake of glucose, fructose, mannose, and cellobiose were found to be dependent on the concentration of NaCl.25 This is congruent with the reduced expression of PTS enzyme II components responsible for the uptake of mannose and fructose in cells exposed to salt stress without a cold pretreatment. Exposure of L. monocytogenes to low temperatures has previously been shown to induce the expression of PTS-associated proteins, indicating their need for production of complex macromolecules and energy.7,8
There was an increase in the expression of proteins secreted by the Sec system (invasion associated protein (Iap) or autolysin, OppA, and enolase).26 Iap has an important role in the degradation of cell wall components and aids in the invasion of nonphagocytic cells. Mutants exhibiting a rough phenotype have reduced expression of Iap but are capable of directional gliding motility toward uncolonized areas of agar media.27 This phenotype could provide L. monocytogenes a competitive advantage in the environment, particularly those associated with food-processing, allowing for increased acquisition of nutrients and colonization of numerous niches in processing plants.
There was an increase in expression of proteins involved in pyruvate metabolism, which implies that the culture was exposed to oxygen-limited conditions and may in part be due to the cultures being incubated statically. Under hypoxic conditions, glucose and other sugars are processed by fermentation and the pentose phosphate pathways. Two enzymes important in the non-oxidative branch of the pentose phosphate pathway were expressed in the cold-adapted salt-stressed cells. The increased expression of 6-phosphogluconate dehydrogenase indicates 6-phosphogluconate is converted into ribulose-5-phosphate, CO2, and NADPH. Given that BHI is a complex medium, carbohydrates other than glucose are available to be transported into the cell and metabolized. Turnover of glycoproteins such as mannose glycopeptides and 2-O-α-mannosyl-glycerate (α-MG) by α-mannosidase allows release of α-d-mannose residues that can be transported into the cell by PTS mannose-specific enzyme IIABC components and PTS fructose-specific enzyme IIABC, respectively, and converted into d-mannose-6-phosphate and shunted into glycolysis. It has previously been shown α-MG serves as an important compatible solute in response to osmotic stress in algae, cyanobacteria, aerobic heterotrophic bacteria, thermohalophilic bacteria, and hyperthermophilic archaea.28–30 Most mesophilic bacteria utilize neutrally charged compatible solutes, while thermophilic eubacteria and archaea utilize negatively charged compatible solutes. It would be interesting to determine if L. monocytogenes is capable of utilizing α-MG as a carbon source or as a compatible solute under temperature or osmotic stress conditions.
An important metabolite utilized by L. monocytogenes cold-stressed cells is fructose.31 Cold-exposed salt-stressed cells had an increased expression of PTS fructose-specific IIABC, fructose-like permease EIIC subunit, and fructose-1-phosphate kinase responsible for the uptake of d-fructose along with its shunting into central carbon metabolism. Metabolism of sugar alcohols such as d-sorbitol by sorbitol dehydrogenase results in the production of d-fructose (shunted into glycolysis as previously mentioned) and NADH. Sorbitol could possibly serve as an osmoprotectant in Listeria given its roles as such in various species of archaea, bacteria, plants, and fungi.32,33 Previous examination of the metabolic profile of cold-adapted L. monocytogenes cells indicate a decrease in expression of sorbitol and sorbitol-6-phosphate in comparison to cells grown at 37 °C.31 Conversion of sorbitol into fructose by sorbitol dehydrogenase could explain the reduced amount of sorbitol and higher level of fructose present in cold-stressed cells. Cellobiose PTS enzyme IIB component and β-glucoside kinase are involved in the transport of the disaccharide cellobiose into the cell, phosphorylated, and directed toward central carbon metabolism.
The increase in expression of proteins involved in the synthesis of precursors for nucleic acids and the overexpression of double-strand break repair proteins supports the conclusion that exposure of cells to salt stress induces some type of DNA damage.34 One possible mechanism for repair of double-strand breaks is through the tethering of DNA fragments by DNA polymerase X (B. subtilis YshC) as a means of preserving chromosomal integrity. This mechanism of salt -induced DNA damage has been shown to occur in eukaryotic cells.35,36 The increased expression of the RecBCD homologue in B. subtilis AddAB, RuvA, SSB, and RecA suggest homologous recombination is involved in repair of double stranded DNA damage.37 Increased expression of SufB supports the need for iron clusters because the nuclease domain of AddAB contains an iron-sulfur cluster that is required for proper binding and processing of broken DNA.38
Adaptation to stressful conditions requires proper synthesis of mRNA and proteins, increased stability of proteins, proper folding, and subsequent secretion of response proteins. Transcription elongation factor GreA is required for the resumption of elongation following transcriptional arrest.22 Translation elongation factors EF-TU and -G have been shown to possess chaperone-like functions by aiding in proper protein folding and interacting with improperly folded proteins.39,40 General stress protein Ctc in L. monocytogenes may interact with ribosomes as a sensor for osmotic stress.41 It has also been shown to be required for growth in media in the absence of any osmoprotectants.6,42
Numerous physiological mechanisms are involved in cold-stress-induced cross protection against osmotic stress in L. monocytogenes. Expression of proteins involved in uptake of a variety of organic molecules aids in shunting available nutrients toward central carbon metabolism and the accumulation or synthesis of compatible solutes. Synthesis of fatty acids from amino acid precursors is needed to adjust membrane fluidity and stabilize the integrity of the cell. DNA repair mechanisms are also required to repair damaged DNA. A better understanding of the physiological responses of this pathogen to adaptation to various strategies in place in the food-processing environment will hopefully aid in implementing feasible listericidal strategies.
Supplementary Material
ACKNOWLEDGMENTS
We would like to thank John Brooks and Mark Lawrence for their helpful discussions concerning this study. This project was funded through the Department of Biological Sciences at Mississippi State University.
Footnotes
ASSOCIATED CONTENT
S Supporting Information
This material is available free of charge via the Internet at http://pubs.acs.org.
The authors declare no competing financial interest.
REFERENCES
- 1.Farber JM, Peterkin PI. Listeria monocytogenes, a food-borne pathogen. Microbiol. Rev. 1991;55(3):476–511. doi: 10.1128/mr.55.3.476-511.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Desmond E. Reducing salt: A challenge for the meat industry. Meat Sci. 2006;74(1):188–196. doi: 10.1016/j.meatsci.2006.04.014. [DOI] [PubMed] [Google Scholar]
- 3.Bergholz TM, Bowen B, Wiedmann M, Boor KJ. Listeria monocytogenes shows temperature-dependent and-independent responses to salt stress, including responses that induce cross-protection against other stresses. Appl. Environ. Microbiol. 2012;78(8):2602–2612. doi: 10.1128/AEM.07658-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schmid B, Klumpp J, Raimann E, Loessner MJ, Stephan R, Tasara T. Role of cold shock proteins in growth of Listeria monocytogenes under cold and osmotic stress conditions. Appl. Environ. Microbiol. 2009;75(6):1621–1627. doi: 10.1128/AEM.02154-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bergholz TM, den Bakker HC, Fortes ED, Boor KJ, Wiedmann M. Salt stress phenotypes in Listeria monocytogenes vary by genetic lineage and temperature. Foodborne Pathog. Dis. 2010;7(12):1537–1549. doi: 10.1089/fpd.2010.0624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Duche O, Tremoulet F, Namane A, Labadie J. A proteomic analysis of the salt stress response of Listeria monocytogenes. FEMS Microbiol. Lett. 2002;215(2):183–188. doi: 10.1111/j.1574-6968.2002.tb11389.x. [DOI] [PubMed] [Google Scholar]
- 7.Cacace G, Mazzeo MF, Sorrentino A, Spada V, Malorni A, Siciliano RA. Proteomics for the elucidation of cold adaptation mechanisms in Listeria monocytogenes. J. Proteomics. 2010;73(10):2021–2030. doi: 10.1016/j.jprot.2010.06.011. [DOI] [PubMed] [Google Scholar]
- 8.Chan YC, Raengpradub S, Boor KJ, Wiedmann M. Microarray-based characterization of the Listeria monocytogenes cold regulon in log- and stationary-phase cells. Appl. Environ. Microbiol. 2007;73(20):6484–6498. doi: 10.1128/AEM.00897-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Annous BA, Becker LA, Bayles DO, Labeda DP, Wilkinson BJ. Critical role of anteiso-C15:0 fatty acid in the growth of Listeria monocytogenes at low temperatures. Appl. Environ. Microbiol. 1997;63(10):3887–3894. doi: 10.1128/aem.63.10.3887-3894.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Dussurget O, Dumas E, Archambaud C, Chafsey I, Chambon C, Hébraud M, Cossart P. Listeria monocytogenes ferritin protects against multiple stresses and is required for virulence. FEMS Microbiol. Lett. 2005;250(2):253–261. doi: 10.1016/j.femsle.2005.07.015. [DOI] [PubMed] [Google Scholar]
- 11.Olsen KN, Larsen MH, Gahan CG, Kallipolitis B, Wolf XA, Rea R, Hill C, Ingmer H. The Dps-like protein Fri of Listeria monocytogenes promotes stress tolerance and intracellular multiplication in macrophage-like cells. Microbiology. 2005;151(Pt 3):925–933. doi: 10.1099/mic.0.27552-0. [DOI] [PubMed] [Google Scholar]
- 12.Graumann PL, Marahiel MA. Cold shock proteins CspB and CspC are major stationary-phase-induced proteins in Bacillus subtilis. Arch. Microbiol. 1999;171(2):135–138. doi: 10.1007/s002030050690. [DOI] [PubMed] [Google Scholar]
- 13.Pittman JR, Schmidt TB, Corzo A, Callaway TR, Carroll JA, Donaldson JR. Effect of stressors on the viability of Listeria during an in-vitro cold-smoking process. Agric. Food Anal. Bacteriol. 2012;2:195–208. [Google Scholar]
- 14.Payne A, Schmidt TB, Nanduri B, Pendarvis K, Pittman JR, Thornton JA, Grissett J, Donaldson JR. Proteomic analysis of the response of Listeria monocytogenes to bile salts under anaerobic conditions. J. Med. Microbiol. 2013;62(Pt 1):25–35. doi: 10.1099/jmm.0.049742-0. [DOI] [PubMed] [Google Scholar]
- 15.Donaldson JR, Nanduri B, Burgess SC, Lawrence ML. Comparative proteomic analysis of Listeria monocytogenes strains F2365 and EGD. Appl. Environ. Microbiol. 2009;75(2):366–373. doi: 10.1128/AEM.01847-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Donaldson JR, Nanduri B, Pittman JR, Givaruangsawat S, Burgess SC, Lawrence ML. Proteomic expression profiles of virulent and avirulent strains of Listeria monocytogenes isolated from macrophages. J. Proteomics. 2011;74(10):1906–1917. doi: 10.1016/j.jprot.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 17.Craig R, Beavis RC. TANDEM: matching proteins with tandem mass spectra. Bioinformatics. 2004;20(9):1466–1467. doi: 10.1093/bioinformatics/bth092. [DOI] [PubMed] [Google Scholar]
- 18.Kessner D, Chambers M, Burke R, Agus D, Mallick P. ProteoWizard: open source software for rapid proteomics tools development. Bioinformatics. 2008;24(21):2534–2536. doi: 10.1093/bioinformatics/btn323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rousseeuw PJ, Driessen KV. A fast algorithm for the minimum covariance determinant estimator. Technometrics. 1998;41:212–223. [Google Scholar]
- 20.Filzmoser P, Garrett RG, Reimann C. Multivariate outlier detection in exploration geochemistry. Comput. Geosci. 2005;31(5):79–587. [Google Scholar]
- 21.McDonald WH, Tabb DL, Sadygov RG, MacCoss MJ, Venable J, Graumann J, Johnson JR, Cociorva D, Yates JR. MS1, MS2, and SQT—three unified, compact, and easily parsed file formats for the storage of shotgun proteomic spectra and identifications. Rapid Commun. Mass Spectrom. 2004;18(18):2162–2168. doi: 10.1002/rcm.1603. [DOI] [PubMed] [Google Scholar]
- 22.Juven BJ, Barefoot SF, Pierson MD, McCaskill LH, Smith B. Growth and survival of Listeria monocytogenes in vacuum-packaged ground beef inoculated with Lactobacillus alimentarius FloraCarn L-2. J. Food Prot. 1998;61(5):551–556. doi: 10.4315/0362-028x-61.5.551. [DOI] [PubMed] [Google Scholar]
- 23.Borezee E, Pellegrini E, Berche P. OppA of Listeria monocytogenes, an oligopeptide-binding protein required for bacterial growth at low temperature and involved in intracellular survival. Infect. Immun. 2000;68(12):7069–7077. doi: 10.1128/iai.68.12.7069-7077.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Gouffi K, Pica N, Pichereau V, Blanco C. Disaccharides as a new class of nonaccumulated osmoprotectants for Sinorhizobium meliloti. Appl. Environ. Microbiol. 1999;65(4):1491–1500. doi: 10.1128/aem.65.4.1491-1500.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bae D, Liu C, Zhang T, Jones M, Peterson SN, Wang C. Global gene expression of Listeria monocytogenes to salt stress. J. Food Prot. 2012;75(5):906–912. doi: 10.4315/0362-028X.JFP-11-282. [DOI] [PubMed] [Google Scholar]
- 26.Lenz LL, Mohammadi S, Geissler A, Portnoy DA. SecA2-dependent secretion of autolytic enzymes promotes Listeria monocytogenes pathogenesis. Proc. Natl. Acad. Sci. U.S.A. 2003;100(21):12432–12437. doi: 10.1073/pnas.2133653100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lenz LL, Portnoy DA. Identification of a second Listeria secA gene associated with protein secretion and the rough phenotype. Mol. Microbiol. 2002;45(4):1043–1056. doi: 10.1046/j.1365-2958.2002.03072.x. [DOI] [PubMed] [Google Scholar]
- 28.Neves C, da Costa MS, Santos H. Compatible solutes of the hyperthermophile Palaeococcus ferrophilus: osmoadaptation and thermoadaptation in the order thermococcales. Appl. Environ. Microbiol. 2005;71(12):8091–8098. doi: 10.1128/AEM.71.12.8091-8098.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martins LO, Santos H. Accumulation of mannosylglycerate and di-myo-inositol-phosphate by Pyrococcus f uriosus in response to salinity and temperature. Appl. Environ. Microbiol. 1995;61(9):3299–3303. doi: 10.1128/aem.61.9.3299-3303.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nunes OC, Manaia CM, Da Costa MS, Santos H. Compatible solutes in the thermophilic bacteria Rhodothermus marinus and Thermus thermophilus. Appl. Environ. Microbiol. 1995;61(6):2351–2357. doi: 10.1128/aem.61.6.2351-2357.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh AK, Ulanov AV, Li Z, Jayaswal RK, Wilkinson BJ. Metabolomes of the psychrotolerant bacterium Listeria monocytogenes 10403S grown at 37 °C and 8 °C. Int. J. Food Microbiol. 2011;148(2):107–114. doi: 10.1016/j.ijfoodmicro.2011.05.008. [DOI] [PubMed] [Google Scholar]
- 32.Empadinhas N, Da Costa MS. Diversity, distribution and biosynthesis of compatible solutes in prokaryotes. Contrib. Sci. 2010:95–105. [Google Scholar]
- 33.Brown A, Simpson JR. Water relations of sugar-tolerant yeasts: the role of intracellular polyols. J. Gen. Microbiol. 1972;72(3):589–591. doi: 10.1099/00221287-72-3-589. [DOI] [PubMed] [Google Scholar]
- 34.Kültz D, Chakravarty D. Hyperosmolality in the form of elevated NaCl but not urea causes DNA damage in murine kidney cells. Proc. Natl. Acad. Sci. U.S.A. 2001;98(4):1999–2004. doi: 10.1073/pnas.98.4.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dmitrieva NI, Celeste A, Nussenzweig A, Burg MB. Ku86 preserves chromatin integrity in cells adapted to high NaCl. Proc. Natl. Acad. Sci. U.S.A. 2005;102(30):10730–10735. doi: 10.1073/pnas.0504870102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Baños B, Lázaro JM, Villar L, Salas M, de Vega M. Characterization of a Bacillus subtilis 64-kDa DNA polymerase X potentially involved in DNA repair. J. Mol. Biol. 2008;384(5):1019–1028. doi: 10.1016/j.jmb.2008.09.081. [DOI] [PubMed] [Google Scholar]
- 37.Yeeles JT, Dillingham MS. A dual-nuclease mechanism for DNA break processing by AddAB-type helicase-nucleases. J. Mol. Biol. 2007;371(1):66–78. doi: 10.1016/j.jmb.2007.05.053. [DOI] [PubMed] [Google Scholar]
- 38.Yeeles JT, Cammack R, Dillingham MS. An iron-sulfur cluster is essential for the binding of broken DNA by AddAB-type helicase-nucleases. J. Biol. Chem. 2009;284(12):7746–7755. doi: 10.1074/jbc.M808526200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Caldas TD, El Yaagoubi A, Richarme G. Chaperone properties of bacterial elongation factor EF-Tu. J. Biol. Chem. 1998;273(19):11478–11482. doi: 10.1074/jbc.273.19.11478. [DOI] [PubMed] [Google Scholar]
- 40.Caldas T, Laalami S, Richarme G. Chaperone properties of bacterial elongation factor EF-G and initiation factor IF2. J. Biol. Chem. 2000;275(2):855–860. doi: 10.1074/jbc.275.2.855. [DOI] [PubMed] [Google Scholar]
- 41.Gardan R, Duche O, Leroy-Setrin S, Labadie J. Role of ctc from Listeria monocytogenes in osmotolerance. Appl. Environ. Microbiol. 2003;69(1):154–161. doi: 10.1128/AEM.69.1.154-161.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Volker U, Engelmann S, Maul B, Riethdorf S, Volker A, Schmid R, Mach H, Hecker M. Analysis of the induction of general stress proteins of Bacillus subtilis. Microbiology. 1994;140(Pt 4):741–752. doi: 10.1099/00221287-140-4-741. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.