Abstract
Objective
Fibromyalgia (FM) is a disorder characterized by chronic pain and enhanced responses to acute noxious events. However, the sensory systems affected in FM may extend beyond pain itself, as FM patients show reduced tolerance to non-nociceptive sensory stimulation. Characterizing the neural substrates of multisensory hypersensitivity in FM may thus provide important clues about the underlying pathophysiology of the disorder. The aim of this study was to characterize brain responses to non-nociceptive sensory stimulation in FM patients and their relationship to subjective sensory sensitivity and clinical pain severity.
Methods
Functional magnetic resonance imaging (MRI) was used to assess brain response to auditory, visual, and tactile motor stimulation in 35 women with FM and 25 matched controls. Correlation and mediation analyses were performed to establish the relationship between brain responses and 3 types of outcomes: subjective hypersensitivity to daily sensory stimulation, spontaneous pain, and functional disability.
Results
Patients reported increased subjective sensitivity (increased unpleasantness) in response to multisensory stimulation in daily life. Functional MRI revealed that patients showed reduced task-evoked activation in primary/secondary visual and auditory areas and augmented responses in the insula and anterior lingual gyrus. Reduced responses in visual and auditory areas were correlated with subjective sensory hypersensitivity and clinical severity measures.
Conclusion
FM patients showed strong attenuation of brain responses to nonpainful events in early sensory cortices, accompanied by an amplified response at later stages of sensory integration in the insula. These abnormalities are associated with core FM symptoms, suggesting that they may be part of the pathophysiology of the disease.
Fibromyalgia (FM) is a syndrome expressed mainly as chronic somatic symptoms involving enhanced mechanical pain. FM patients are also hypersensitive to experimentally induced pain in other modalities, including thermal, chemical, and electrical stimuli (for example, see refs. 1 and 2), across multiple body sites (3). Accordingly, functional brain imaging studies have shown abnormally enhanced neural responses to a variety of nociceptive stimuli in FM patients (for example, see refs. 4–6).
In addition to greater pain sensitivity, reduced tolerance (augmented unpleasantness) to normatively innocuous auditory, visual, olfactory, and tactile stimuli has been reported in FM patients (7–12), as well as a general preference for lower levels of external stimulation (10). Sensitivity to auditory and tactile stimulation was also significantly augmented in FM in comparison with another chronic pain condition, rheumatoid arthritis (10,12). These observations suggest that a more general multisensory hypersensitivity may characterize the disorder. Recent studies have shown a higher proportion of otoneurologic symptoms in FM patients in the absence of detectable peripheral sensory pathology (for example, see refs. 13 and 14). A study that specifically controlled for peripheral pathology found reduced unpleasantness thresholds for FM patients along a range of auditory stimulus frequencies (14), together with a subclinical hearing loss for specific frequencies. Interestingly, electrical auditory event-related potentials (ERPs) in the brain mostly show reduced amplitudes in FM (for example, see refs. 15–17). Taken together, these findings suggest a 2-stage alteration in sensory processing of auditory stimulation in FM—attenuation of primary sensory responses (reflected in reduced auditory ERPs and hearing symptoms) combined with response amplification at later processing stages (reflected in augmented unpleasantness/reduced tolerance).
Available experimental data give further support to the possibility of a dual functional alteration in FM in response to nonpainful sensory stimulation. As mentioned above, cortical recordings suggest that excitability of primary sensory cortices, and specifically auditory cortex, may be abnormal in FM (7,15–17). A well-established relationship exists between excitability of primary auditory areas and central serotonergic neurotransmission (18). Low serotonin levels have repeatedly been found in FM patients using indirect measures (for example, see ref. 19), and serotonergic pharmacotherapy appears to have therapeutic benefits in FM (20). Evidence in animals and humans shows a robust association between low serotonergic activity and a strong “intensity dependence of auditory evoked potentials (AEPs)” (i.e., the increase of AEP amplitudes as a function of increasing tone intensity) (18,21). Importantly, Carrillo-de-la-Peña and colleagues (7) showed remarkably steeper increases in AEP amplitudes with increasing stimulus intensities in FM patients. Aside from these data suggesting abnormal processing at primary sensory cortices, consistent evidence has been accumulated supporting a role for augmented insula metabolism and response in FM (4–6,22,23). The insula has a role in the multimodal integration of sensory stimuli (24-26) and has been repeatedly associated with feelings of unpleasantness in response to sensory input (24,26,27).
Together, these studies suggest that potential alteration in non-nociceptive sensory processing in FM patients may provide important clues about the underlying pathophysiology and mechanisms of response to treatment. However, to our knowledge, there are no previously published reports of functional magnetic resonance imaging (fMRI) addressing multisensory processing at the neural systems level in FM patients and its relationship with core pain-related symptoms. In the present study we used fMRI to identify alterations in brain responses to nonpainful (auditory, visual, and tactile) sensory stimulation in 35 FM patients compared with 25 healthy controls. We hypothesized that FM patients would show 2 types of changes: 1) reduced responses to non-nociceptive sensory stimulation in early sensory cortices (in accordance with augmented perceptual auditory thresholds, reduced ERPs, and serotonergic dysfunction); and 2) augmented responses in the insula and possibly other areas important for multisensory integration and affect (in accordance with patients’ reduced tolerance to sensory stimulation). These changes might be correlated with subjective measures of hypersensitivity to sensory stimulation in daily life and with core symptoms of FM, including widespread spontaneous pain and functional disability.
SUBJECTS AND METHODS
Ethics statement
This study was conducted according to the principles expressed in the Declaration of Helsinki. The study was approved by the Ethics and Institutional Review Board of the Autonomous University of Barcelona (reference no. SAF2010-19434). All patients and healthy controls provided written informed consent for clinical and fMRI assessment and subsequent analyses.
Subjects
Sixty women participated in the study, including 35 FM patients and 25 healthy controls. Subjects were matched for age (mean ± SD 46.55 ± 5.94 years for patients versus 44.64 ± 5.94 years for healthy controls; t-test = −1.05, P = 0.30), years of education (mean ± SD 14.31 ± 4.56 years versus 15.00 ± 4.98 years, respectively; t-test = 0.54, P = 0.59), and handedness (all right-handed).
The FM patients were consecutively selected during clinical followup to construct a homogeneous sample showing severe and persistent symptoms. All patients met the American College of Rheumatology 1990 criteria for the classification of FM (28). The mean ± SD duration of illness was 83.63 ± 53.53 months. The mean ± SD number of tender points upon study assessment was 15.86 ± 1.96. The mean ± SD total score on the Fibromyalgia Impact Questionnaire (FIQ) (29) was 66.07 ± 16.05 (maximum score of 100), and the mean ± SD functional capacity score on the FIQ was 4.60 ± 1.86. Patients had a mean ± SD score of 32.07 ± 18.82 (maximum score of 100) for general perception of health according to the 36-item Short-Form health survey (30). Patients’ mean ± SD Hospital Anxiety and Depression Scale (31) ratings were 8.77 ± 4.83 for depression and 11.37 ± 4.13 for anxiety.
Vision and hearing on neurologic examination were normal for all patients and controls. Also, there were no significant differences (P > 0.1) in the proportions of farsightedness (16% of controls, 8.6% of patients; χ2= 0.78, P = 0.38), presbyopia (44% of controls, 45.7% of patients; χ2=0.02, P = 0.90), myopia (32% of controls, 34% of patients; χ2= 0.03, P = 0.85), astigmatism (28% of controls, 14.3% of patients; χ2= 1.71, P = 0.19), cataracts corrected by surgery (4% of controls, 0% of patients; χ2= 1.42, P = 0.23), and reduced corneal humidity (0% of controls, 8.6% of patients; χ2= 2.25, P = 0.13). The proportion of past relevant auditory complications that were functionally asymptomatic at the moment of examination was also similar between groups (8.0% of controls, 8.6% of patients; P = 0.94), and these functionally asymptomatic auditory complications included chronic otitis (2 patients and 2 controls) and mild Ménière syndrome (1 patient).
Patients were allowed to continue with their stable medical treatment (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf) but were required to refrain from taking occasional (rescue) analgesic drugs (i.e., nonsteroidal antiinflammatory drugs [NSAIDs], paracetamol, and tramadol) 72 hours prior to fMRI. We excluded control subjects with relevant medical and neurologic disorders, any form of chronic or acute pain, substance abuse, or history of psychiatric illness. Contraindication to MRI, including pregnancy, was a general exclusion criterion for both groups.
Self-report measures of multisensory sensitivities and pain perception
We used 2 measures to assess for potential hypersensitivity to nonpainful sensory stimulation. The first was the self-rated questionnaire of sound hypersensitivity (adapted Spanish version: test de hipersensibilidad al sonido [THS]; THS-G-ÜF instrument). This measure demonstrates high reliability and internal consistency (Cronbach’s α = 0.90) for measuring subjective distress related to hypersensitivity to sound (32). The second was the Adolescent/Adult Sensory Profile (AASP) (33). We focused on 2 subscales of interest (sensory sensitivity and sensory avoidance) across 2 modalities of interest (visual and tactile processing) to derive a total score for each modality computed as the addition of the subscale scores. We chose these 2 subscales based on their successful prior use in FM patients (12) and their ability to measure subjective hypersensitivity to sensory stimulation. The AASP has been validated in a sample of ~900 adolescents and adults and has been used to examine sensory processing across the age spectrum. Both subscales have shown reasonable internal consistency (Cronbach’s α = 0.66–0.82) (33).
Spontaneous pain was assessed using a 101-point verbal scale. A score of 0 represents no pain and a score of 100 represents the most intense pain imaginable, perceived in the body as a whole or in most of its extension rather than referring to any focal tenderness. Patients were asked to report spontaneous pain ~1 hour before the imaging session. All controls scored “0” for spontaneous pain.
Stimuli and fMRI task description
A block design fMRI paradigm was developed with alternating 30-second periods of rest (no stimulation) and activation (concurrent visual, auditory, and tactile motor stimulation) for a total of 4 rest–activation cycles (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf). Activation periods consisted of the simultaneous presentation of visual stimulation (at a temporal frequency of 3 Hz [equivalent to 6 color reversals per second] with a full-field flashing checkerboard composed of a grid of black and white alternating squares [80 ± 10 lux]) and auditory stimulation (a series of 15 tones of frequencies in the range of 233.1–1,318.5 Hz, presented at a temporal frequency of 3 Hz, with an intensity of 75 ± 5 dB) coupled with a finger-opposition task during which the subject was instructed to touch the tip of the right thumb with the other fingers (from index finger to little finger) (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf).
MRI acquisition
We scanned participants with an Achieva 3.0 TX system (Philips) with an 8-channel phased-array head coil and single-shot echoplanar imaging. Functional sequences consisted of gradient-recalled acquisition in the steady state (repetition time 2.000 msec, time to echo 35 msec, flip angle 90°) within a field of view of 23 cm, a 96 × 69–pixel matrix, and a slice thickness of 4 mm (slice gap 1 mm). Twenty-two slices parallel to the anterior-posterior commissure covered the whole brain. The sequence included 4 additional discarded volumes to allow the magnetization to reach equilibrium.
Image preprocessing
Imaging data were processed using MatLab version 2011b (MathWorks) and statistical parametric mapping (SPM) software (SPM8; The Wellcome Department of Imaging Neuroscience). Preprocessing involved motion correction, spatial normalization, and smoothing using a Gaussian filter (full-width half-maximum kernel of 8 mm). Data were normalized to the standard SPM–echoplanar imaging template and resliced to 2 mm isotropic resolution in Montreal Neurological Institute space. Regarding motion correction, translation and rotation estimates (x, y, z) were less than 2 mm or 2°, respectively, for all participants, and no subjects were excluded because of artifacts or head displacement/rotations.
Statistical analysis
Between-group differences in self-report measures
Two-sample t-tests were computed using SPSS software to assess between-group differences in subjective sensitivity to visual, auditory, and tactile sensory information in daily life as assessed by the AASP and the THS.
Functional MRI brain responses to sensory stimulation
Our analyses aimed to identify between-group differences in brain activation evoked by nonpainful sensory stimulation and their association with measures of clinical severity and subjective sensitivity to stimulation in patients’ daily lives.
For each subject, a primary task regressor was created by convolving the sensory stimulation blocks with a canonical hemodynamic response function. The “off” (rest) condition served as an implicit task baseline. Parameter estimates were calculated at each voxel using the general linear model. A high-pass filter was used to remove low-frequency signal fluctuations (1/128 Hz).
(Sensorimotor stimulation – baseline) contrast images for each participant were carried forward to the group level using the summary statistics approach to random-effects analysis. One-sample t-tests were used to test within-group contrasts, and 2-sample unpaired t-tests were used to test the between-group activation differences (i.e., the [stimulation – baseline] × [FM – control] interaction).
A series of supplementary analyses were performed to assess the influences of head motion, medication, global vascular effects, and time since diagnosis on between-group activation differences. The pattern of between-group differences was maintained overall after controlling for these variables. Medication had limited normalizing effects (i.e., anti-depressants, anxiolytics, and NSAIDs mildly reduced the magnitude of alterations in limited portions of the cerebellum and visual and auditory areas). A complete summary of these analyses and results is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf.
Brain-behavior correlations and mediation analyses
We first identified regions showing significant (stimulation – baseline) × (FM – controls) effects (Figures 1C and D). Then, within these regions, we performed correlation analyses in SPM8 in the patient group to test for linear relationships between (stimulation – baseline) activity and 3 types of outcomes: 1) measures of patients’ clinical impairment (FIQ total and functional scores) and spontaneous pain (numerical rating scale score from 0 to 100) 1 hour before the MRI assessment; 2) subjective reports of hypersensitivity to auditory, visual, and tactile-related stimulation in daily life (measured using the THS and AASP questionnaires described above); and 3) time since diagnosis (which is related to time exposed to the disorder).
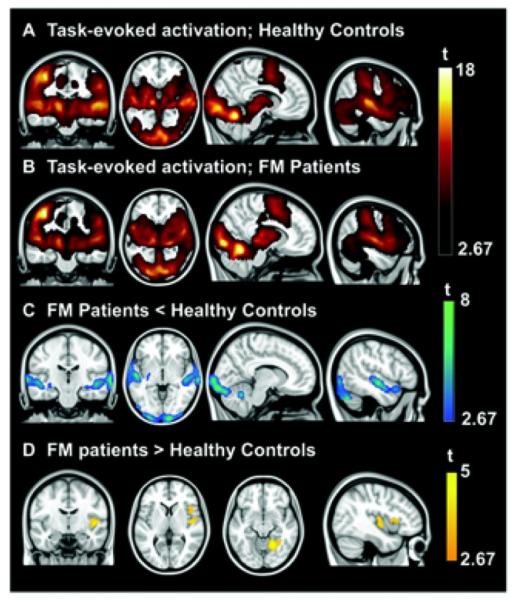
Figure 1.
Regions showing significant task-evoked activation in healthy controls (A) and fibromyalgia (FM) patients (B) and between-group differences in task-evoked activation (C and D). Results are displayed at a corrected threshold Pfamily-wise error < 0.05 estimated using Monte Carlo simulations. t = t-test.
We also tested whether the relationship between enhanced subjective sensory sensitivity and the clinical condition (FM diagnosis present versus absent) was mediated by the magnitude of task-evoked brain responses during the sensory-motor paradigm. The mediation tests several joint hypotheses. Path a tests whether subjective sensory sensitivity predicts (stimulation – baseline) brain activity. Path b tests whether (stimulation – baseline) brain activity predicts FM status (FM versus controls) in a logistic regression, controlling for subjective sensory sensitivity. A test of the path a × b product tests the mediation effect. Thus, the path b effect provides a test of whether brain activity predicts FM status above and beyond subjective sensitivity alone, and the path a × b effect provides a test of whether brain responses explain a significant proportion of the covariation between sensory sensitivity and FM status. The analyses were conducted using the brain mediation toolbox (http://wagerlab.colorado.edu/tools) following an approach that has been used and described extensively in previous work, with bias-corrected, accelerated bootstrap tests and the logistic link function for path b (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf).
Thresholding and multiple comparisons
We used cluster extent-based correction to control the family-wise error (FWE) rate at PFWE < 0.05 across the whole brain (211,062 voxels in gray and white matter). Spatial extent thresholds for all statistical comparisons were determined by 5,000 Monte Carlo simulations using AlphaSim as implemented in the SPM REST toolbox (34). For (stimulation – baseline) and its interaction with (FM – controls), we used a primary cluster-defining threshold of P < 0.005, which resulted in a minimum extent threshold of 321 voxels for cluster level–corrected significance. For correlation analyses, which are less highly powered at the voxel level, we used a primary cluster-defining threshold of P < 0.01 and a reduced brain volume corresponding to the voxels showing significant between-group differences (including [controls > FM] and [FM > controls], 12,777 voxels). The corrected threshold for PFWE< 0.05 was k = 93 voxels.
RESULTS
Behavioral measures
The patient group reported moderate-to-severe spontaneous pain ~1 hour prior to the MRI session (mean ± SD 71.29 ± 15.87 on the numerical rating scale). Compared to controls, FM patients showed significantly higher subjective sensory sensitivity (increased unpleasantness) to acoustic stimulation measured using the THS (mean ± SD 5.16 ± 5.63 versus 16.89 ± 11.32; t-test = −5.28, P < 0.0001) and scores on the visual (mean ± SD 11.56 ± 5.53 versus 21.51 ± 6.26; t-test = −6.37, P < 0.0001) and tactile (mean ± SD 11.81 ± 5.02 versus 18.80 ± 6.20; t-test = −4.71, P < 0.0001) processing subscales of the AASP. The Z-transformed average of subjective sensitivity measures (auditory, visual, and tactile) was likewise significantly greater for the FM group (mean ± SD −0.66 ± 0.48 versus 0.47 ± 0.81; t-test = −6.72, P <0.0001). Table 1 reports strong positive correlations between patients’ clinical severity and subjective hypersensitivity measures.
Table 1.
Bivariate correlations between hypersensitivity to nonpainful stimulation and clinical severity measurements in fibromyalgia patients*
| AASP visual |
AASP tactile |
FIQ total |
FIQ functional |
Spontaneous pain |
|
|---|---|---|---|---|---|
| THS | 0.741† | 0.625† | 0.564‡ | 0.351§ | 0.606† |
| AASP visual | – | 0.480‡ | 0.452‡ | 0.135 | 0.474‡ |
| AASP tactile | – | – | 0.408§ | 0.505‡ | 0.555‡ |
| FIQ total | – | – | – | 0.357§ | 0.616† |
| FIQ functional | – | – | – | – | 0.414‡ |
AASP = Adolescent/Adult Sensory Profile (2 subscales of interest [sensory sensitivity and sensory avoidance] for 2 modalities of interest [visual and tactile processing]); FIQ = Fibromyalgia Impact Questionnaire; THS = self-rating questionnaire on hypersensitivity to sound.
P < 0.001.
P < 0.01.
P < 0.05.
Brain responses to multisensory non-nociceptive stimulation
For both groups, robust activation was observed in primary/secondary auditory cortices, middle temporal gyri, hippocampi, occipital gyri, and cerebellum, primary and secondary somatosensory cortices extending to insular/opercular cortex, and basal ganglia. Other medial and lateral frontal areas were also activated (Figures 1A and B) (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-infibromyalgia-patients-supplement.pdf). These results included sensory areas associated with each component (visual, auditory, and tactile) of the multisensory task.
Compared with healthy controls, the FM group showed reduced task-related activation in primary/secondary auditory cortices, middle temporal gyri, hippocampi, ventral basal ganglia, and inferior occipital gyri (very specifically, BA17 and parts of BA18) extending to the bilateral cerebellum (Table 2 and Figure 1C). Regions of significantly greater activation were found for the patient group in the right insula extending to the opercula and the anterior part of the lingual gyrus contiguous with the parahippocampal gyrus (Table 2 and Figure 1D).
Table 2.
Statistics for the region clusters showing significant between-group differences in task-evoked activation*
| Direction of difference, | |||
|---|---|---|---|
| region | k† | x, y, z‡ | t-test |
| FM patients < healthy | |||
| controls | |||
| Left temporal gyri/basal | 2,528 | −52, −14, −6; | 6.61; |
| ganglia/hippocampus | −70, −30, 6 | 6.63 | |
| Right temporal gyri/basal | 2,733 | 48, −24, −4; | 5.55; |
| ganglia/hippocampus | 74, −24, −2 | 6.23 | |
| Inferior occipital gyri | 6,093 | 22, −88, −20; | 7.92; |
| (BA17, BA18)/ | 0, −100, −14 | 7.14 | |
| cerebellum | |||
| FM patients > healthy | |||
| controls | |||
| Insula | 467 | 46, −8, 0 | 4.00 |
| Anterior lingual gyrus | 956 | 20, −54, −12 | 4.81 |
Statistics correspond to a corrected threshold Pfamily-wise error < 0.05 estimated using Monte Carlo simulations. FM = fibromyalgia. BA = Brodmann Area.
Cluster size (k) is given in 2 × 2 × 2–mm voxels.
Coordinates in Montreal Neurological Institute space.
Associations between clinical severity of FM and task-evoked brain activation
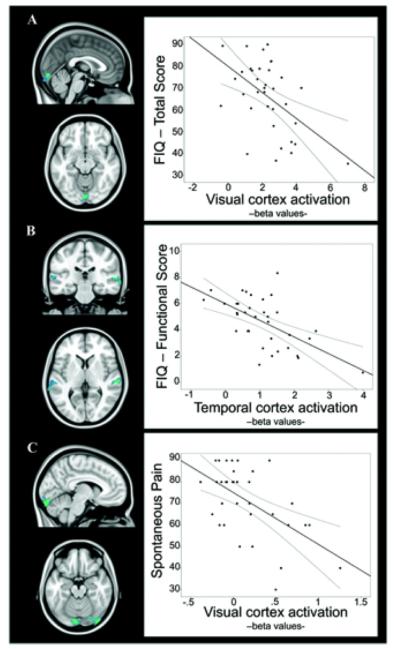
A specific analysis was carried out to assess the correlation between FM clinical severity (total FIQ score, functional FIQ score, and spontaneous pain) and fMRI responses to multisensory stimulation in regions showing significant between-group effects (which are reported in Table 2 and Figure 1). Higher total FIQ and spontaneous pain scores were significantly correlated with lower activation magnitudes in visual areas (Figures 2A and C) (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf). Additionally, the FIQ functional score was significantly negatively correlated with activation in auditory cortices (Figure 2B) (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf).
Figure 2.
Regions for which task-evoked activation was significantly correlated with symptom severity in fibromyalgia patients. A, Correlation between brain activation and Fibromyalgia Impact Questionnaire (FIQ) total score. B, Correlation between brain activation and FIQ functional score (item 1 of the FIQ assessing functional impairment in a variety of daily life activities). C, Correlation between brain activation and spontaneous pain. The plots illustrate the correlation at the peak voxel (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf) for each analysis. Lines above and below the regression line represent the 95% confidence interval of the correlation. Brain map results are displayed at a corrected threshold Pfamily-wise error < 0.05 estimated using Monte Carlo simulations within the mask of regions showing significant between-group effects. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38781/abstract.
Association between subjective hypersensitivity to daily sensory stimulation and task-evoked brain activation in FM
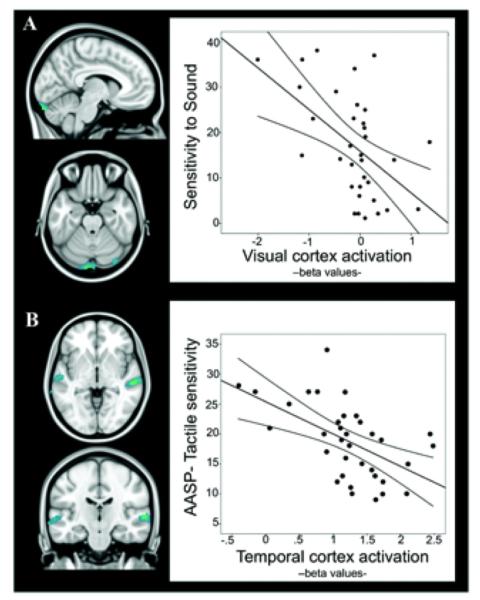
We found a significant negative association between primary visual cortex activation and subjective reports of hypersensitivity to sound and between superior/middle temporal gyri activation and hypersensitivity to tactile stimulation (Figure 3) (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf). Such results suggest that lower activation of primary sensory regions in FM is associated with greater unpleasantness evoked by daily-life acoustic and tactile stimulation.
Figure 3.
Regions for which task-evoked activation was significantly correlated with measures of hypersensitivity to sound (A) and touch (B). The plots illustrate the correlation at the peak voxel (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf) for each analysis. Lines above and below the regression line represent the 95% confidence interval of the correlation. Brain map results are displayed at a corrected threshold Pfamily-wise error < 0.05 estimated using Monte Carlo simulations within the mask of regions showing significant between-group effects. AASP = Adolescent/Adult Sensory Profile. Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38781/abstract.
Consistent with these findings, an exploratory whole-brain mediation analysis (Figure 4) (further information is available at http://wagerlab.colorado.edu/files/papers/Lopez-Sola-fMRI-responses-to-Nonpainful-stimulation-in-fibromyalgia-patients-supplement.pdf) showed that the relationship between enhanced subjective multisensory sensitivity and FM status (FM versus controls) was partially mediated by reductions in task-evoked brain activation within specific visual and auditory areas. These regions were entirely contained within the map of significant between-group effects presented above. The results suggest that attenuated neural responses mostly in early sensory cortices explain some of the enhanced sensory sensitivity (unpleasantness) to multisensory events in FM and thus may contribute to functional impairments in the disease.
Figure 4.
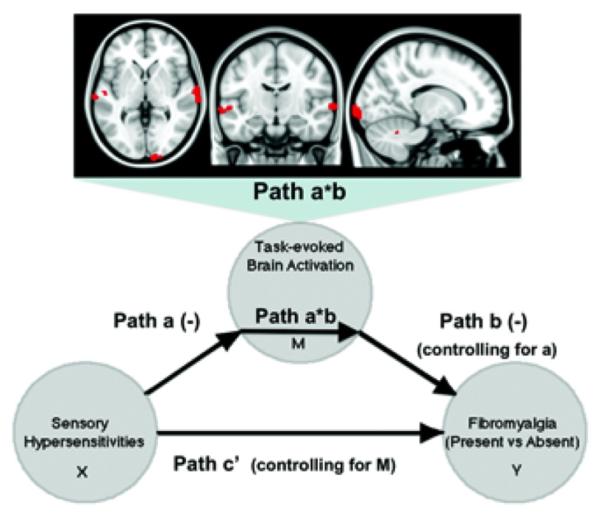
Significant brain regions mediating the relationship between multisensory hypersensitivity and clinical category (fibromyalgia [FM] diagnosis present versus absent). The mediation analysis tests whether the covariance between 2 variables (the predictor X and the predicted outcome Y) can be explained by a third variable (the mediator, M). In this case, path a (−) indicates that subjective sensory sensitivity (X) negatively predicts (stimulation – baseline) brain activity in occipital, temporal, and cerebellar regions (M). Path b (−) shows that (stimulation – baseline) brain activity in such regions (M) negatively predicts FM status (Y) in a logistic regression, controlling for subjective sensory sensitivity. Path a*b corresponds to the product of path a and path b coefficients and shows that response in the brain areas illustrated (top) explains a significant proportion of the covariation between sensory sensitivity and FM status. Path c’ indicates the relationship between X and Y controlling for M. All voxels show 2-tailed P < 0.05 for paths a, b, and a*b (10,000 bootstrapped tests). Color figure can be viewed in the online issue, which is available at http://onlinelibrary.wiley.com/doi/10.1002/art.38781/abstract.
DISCUSSION
This study provides new evidence of a prominent alteration in the central processing of nonpainful multisensory stimulation in FM patients. It extends previous findings by mapping a dual alteration at the cortical level during processing of visual, auditory, and sensorimotor stimulation. Specifically, FM patients showed significant attenuation of brain responses at primary levels of cortical processing in visual and auditory areas. This hypoactivation in sensory areas was accompanied by an amplified response at a later stage of input integration, involving the right insula and the anterior lingual gyrus. Importantly, neural response attenuation was correlated with subjective reports of multisensory hypersensitivity in daily life and core pain-related symptoms in patients, which highlights the functional relevance of the observed brain response alterations.
Importantly, all measures of sensory hypersensitivity were significantly correlated with spontaneous pain in the patient group. Spontaneous pain was also associated with deficient activation in primary/secondary visual cortical areas. Furthermore, the relationship between multisensory hypersensitivities and the clinical category (FM diagnosis present versus absent) was partially mediated by the deficient activation of specific regions of visual and auditory cortices. Overall, these findings suggest that general hypersensitivity to non-painful sensory stimulation and the underlying cortical alteration in sensory processing areas are related to the cardinal symptom of FM (i.e., widespread bodily pain). Importantly, distinct evidence suggests that subjective pain perceptions may emerge from augmented processing of nociceptive signals and/or reduced processing of non-nociceptive sensory information (for example, see refs. 35 and 36), suggesting the importance of imbalances in different forms of sensory processing in mediating pain. Consistent with this evidence, our study confirms that nonpainful primary sensory processing is blunted in FM, although no conclusions can be drawn as to the directionality of the relationship between such functional alteration and pain symptoms.
It has been shown repeatedly that the experience of pain can be reduced by the concurrent processing of nonpainful sensory stimulation (e.g., vibratory and tactile stimulation) (35,37,38). In the context of chronic pain, neurostimulation of peripheral and central loci carrying or processing nonpainful sensory information (e.g., spinal cord stimulation at the dorsal columns) has proven effective in significantly reducing pain symptoms (39,40). Specifically in FM, non-nociceptive sensory neurostimulation interventions such as peripheral nerve stimulation (for example, see ref. 41), transcranial magnetic stimulation, and direct current stimulation (42) have yielded significant pain relief. Interestingly, recent evidence suggests that sensory neurostimulation applied to other central locations (e.g., visual cortex in migraine syndrome) may have similar positive effects on chronic pain symptoms (43). Our findings of reduced cortical activation in primary/secondary visual and auditory cortices, which were associated with subjective pain symptoms, may suggest potential new cortical targets for neurostimulation treatments in FM patients.
The pattern of response attenuation in primary/secondary auditory cortices is congruent with existing evidence of poorer perceptual discrimination of auditory information including subclinical hearing loss for high frequencies (14) without peripheral underlying pathology in FM. It is also consistent with the observed amplitude reduction of ERP components (15–17) in response to auditory stimuli in these patients, and with the observation of lower resting cerebral blood flow in temporal and occipital cortices in FM (44).
We found a paradoxical and intriguing cross-modal effect in the analysis of correlations; for example, hypersensitivity to sounds was correlated with visual cortex hypoactivation in the patient group. There is increasing evidence of relevant cross-sensory interaction at the cortical level (45) and evidence that neural activity in primary sensory areas is modulated by signals from the other primary sensory cortices (46). In this context, the observed correlations may reflect cross-modal attenuation or down-regulation of sensory processes in response to a situation of multisensory hypersensitivity in FM. Alternative explanations may include potential competing effects of attentional resources during concurrent processing of different sensory modalities (47).
FM patients showed task-related hyperactivation in 2 areas, namely, the right insula and the right anterior lingual gyrus. Lingual hyperresponses have been reported for different subject groups showing augmented sensitivity to pain in clinical and genetic studies (for example, see refs. 48 and 49). As predicted, we found enhanced task-evoked responses in the right insula, which were particularly strong in the posterior division.
Such an observation is consistent with recent evidence of augmented insular responses to visual stimulation in FM patients (23). The (posterior) insula region, together with SII in the parietal operculum, has been characterized by its relevant role in sensory integration (24–26), both within the somatosensory modalities (touch and pain) and across sensory modalities (somatosensory, visual, and auditory) (50–52). Congruently, a recent study (24) showed that specifically the posterior aspect of the insula was functionally connected during the resting state and coactivated in a variety of task studies with somatosensory, visual, and auditory sensory areas. Augmented excitatory neurotransmission in the right posterior insula, which was associated with reduced pressure pain thresholds, has been documented in FM (22). Altered functional connectivity between the posterior insula and the so-called default mode network, which is known for its relevance in different aspects of self-referential processing, has also been described in FM and was associated with spontaneous pain (53). Our finding broadens the picture of posterior insula abnormalities in FM by documenting an amplified insula response to nonpainful stimulation in FM patients across sensory modalities.
It is worth mentioning that the pattern of brain response alteration did not show a general association with time since diagnosis (an approximation for time exposed to the disorder). Time since diagnosis showed a very limited effect on auditory task–evoked activation, such that the shorter the exposure to the disorder the less the activation of specific temporal regions. This suggests that patients’ response attenuation in temporal cortices may be stronger in earlier stages of the disorder. The effects of medication followed the same direction (medication was associated with greater activation in regions showing response attenuation in FM) and were restricted to very localized clusters. Between-group task-evoked activation differences were also maintained after controlling for medication status.
The present study is limited in that it does not provide comparison with another clinical pain condition. Our task was designed to provide a naturalistic presentation of multimodal sensory stimulation, and therefore our approach does not isolate the net contribution of each stimulation modality to the observed results. This may be particularly relevant for the tactile task component, which involved self-generated finger movements and thus additional brain activity in the motor system. Between-group differences in task activation were found for auditory and visual areas, but not for the somatosensory cortex. The lack of significant results at this level could be related to the more complex nature of the sensorimotor task. Future research aimed at isolating modality-specific abnormalities in FM is needed for each sensory domain and particularly for the somatosensory system using pure tactile stimuli. Our study is also limited in that we did not register unpleasantness scores evoked by the task despite measuring subjective sensitivity to daily sensory stimulation using validated measures.
Finally, fMRI is limited in that it does not provide an absolute measure of neural activity; therefore, we cannot rule out an effect of potential between-group differences in baseline levels of neural activity on the observed results. This may be particularly pertinent in case auditory stimulation related to the scanner background noise (even if attenuated using MRI-compatible headphones) had exerted different effects in the 2 study groups. However, considering that the pattern of task-related hyporesponses in FM was also remarkably present in visual areas, it is rather unlikely that different responses to scanner background noise were mostly responsible for the pattern of between-group effects.
This imaging study is the first to map central alterations of the neural response to non-nociceptive multisensory stimulation in FM. It provides evidence of a 2-fold central alteration involving attenuation of primary cortical processing of sensory signals and amplified response at a level of multimodal sensory integration. The findings may help to reconcile the paradoxical observation in FM patients of augmented subjective unpleasantness to sensory stimulation co-occurring with poorer perceptual discrimination and reduced ERP responses. Considering the robust between-group effects and the relationship between primary sensory processing and monoaminergic neurotransmission, the fMRI paradigm used in this study stands as a suitable candidate to assess the neural mechanisms underlying treatment effects in FM.
Acknowledgments
Supported in part by the Ministry of Science and Innovation of Spain (grant SAF2010-19434), the NIH (grant R01-DA-035484 to Dr. Wager), and the Agency of University and Research Funding Management of the Catalonia Government AGAUR (Research Groups SGR 2009/718, 1435, and 1450). Dr. López-Solá’s work was supported by a Beatriu de Pinós-A Postdoctoral Fellowship (2010_BP_A_00136) from the Government of Catalunya. Dr. Garcia-Fontanals’ work was supported by a Personal Investigador en Formación (PIF) grant from the Autonomous University of Barcelona. Ms Blanco-Hinojo’s work was supported by the Predoctoral Training Grant Research Programme (PFIS grant FI10/00387) from the Carlos III Health Institute. Dr. Harrison’s work was supported by the National Health and Medical Research Council of Australia (NHMRC Clinical Career Development award 628509).
Footnotes
AUTHOR CONTRIBUTIONS All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Drs. López-Solá and Deus had full access to all of the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. López-Solá, Pujol, Garcia-Fontanals, Garcia-Blanco, Poca-Dias, Harrison, Monfort, Garcia-Fructuoso, Deus.
Acquisition of data. López-Solá, Pujol, Garcia-Fontanals, Blanco-Hinojo, Garcia-Blanco, Poca-Dias, Harrison, Contreras-Rodríguez, Garcia-Fructuoso, Deus.
Analysis and interpretation of data. López-Solá, Pujol, Wager, Garcia-Fontanals, Harrison, Monfort.
REFERENCES
- 1.Kosek E, Ekholm J, Hansson P. Sensory dysfunction in fibromyalgia patients with implications for pathogenic mechanisms. Pain. 1996;68:375–83. doi: 10.1016/s0304-3959(96)03188-0. [DOI] [PubMed] [Google Scholar]
- 2.Lautenbacher S, Rollman GB, McCain GA. Multi-method assessment of experimental and clinical pain in patients with fibromyalgia. Pain. 1994;59:45–53. doi: 10.1016/0304-3959(94)90046-9. [DOI] [PubMed] [Google Scholar]
- 3.Gibson SJ, Littlejohn GO, Gorman MM, Helme RD, Granges G. Altered heat pain thresholds and cerebral event-related potentials following painful CO2 laser stimulation in subjects with fibromyalgia syndrome. Pain. 1994;58:185–93. doi: 10.1016/0304-3959(94)90198-8. [DOI] [PubMed] [Google Scholar]
- 4.Cook DB, Lange G, Ciccone DS, Liu WC, Steffener J, Natelson BH. Functional imaging of pain in patients with primary fibromyalgia. J Rheumatol. 2004;31:364–78. [PubMed] [Google Scholar]
- 5.Gracely RH, Petzke F, Wolf JM, Clauw DJ. Functional magnetic resonance imaging evidence of augmented pain processing in fibromyalgia. Arthritis Rheum. 2002;46:1333–43. doi: 10.1002/art.10225. [DOI] [PubMed] [Google Scholar]
- 6.Pujol J, Lopez-Sola M, Ortiz H, Vilanova JC, Harrison BJ, Yucel M, et al. Mapping brain response to pain in fibromyalgia patients using temporal analysis of FMRI. PLoS One. 2009;4:e5224. doi: 10.1371/journal.pone.0005224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carrillo-de-la-Pena MT, Vallet M, Perez MI, Gomez-Perretta C. Intensity dependence of auditory-evoked cortical potentials in fibromyalgia patients: a test of the generalized hypervigilance hypothesis. J Pain. 2006;7:480–7. doi: 10.1016/j.jpain.2006.01.452. [DOI] [PubMed] [Google Scholar]
- 8.Geisser ME, Glass JM, Rajcevska LD, Clauw DJ, Williams DA, Kileny PR, et al. A psychophysical study of auditory and pressure sensitivity in patients with fibromyalgia and healthy controls. J Pain. 2008;9:417–22. doi: 10.1016/j.jpain.2007.12.006. [DOI] [PubMed] [Google Scholar]
- 9.Hollins M, Harper D, Gallagher S, Owings EW, Lim PF, Miller V, et al. Perceived intensity and unpleasantness of cutaneous and auditory stimuli: an evaluation of the generalized hypervigilance hypothesis. Pain. 2009;141:215–21. doi: 10.1016/j.pain.2008.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.McDermid AJ, Rollman GB, McCain GA. Generalized hypervigilance in fibromyalgia: evidence of perceptual amplification. Pain. 1996;66:133–44. doi: 10.1016/0304-3959(96)03059-x. [DOI] [PubMed] [Google Scholar]
- 11.Waylonis GW, Heck W. Fibromyalgia syndrome: new associations. Am J Phys Med Rehabil. 1992;71:343–8. doi: 10.1097/00002060-199212000-00006. [DOI] [PubMed] [Google Scholar]
- 12.Wilbarger JL, Cook DB. Multisensory hypersensitivity in women with fibromyalgia: implications for well being and intervention. Arch Phys Med Rehabil. 2011;92:653–6. doi: 10.1016/j.apmr.2010.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Bayazit YA, Gursoy S, Ozer E, Karakurum G, Madenci E. Neurotologic manifestations of the fibromyalgia syndrome. J Neurol Sci. 2002;196:77–80. doi: 10.1016/s0022-510x(02)00032-1. [DOI] [PubMed] [Google Scholar]
- 14.Dohrenbusch R, Sodhi H, Lamprecht J, Genth E. Fibromyalgia as a disorder of perceptual organization? An analysis of acoustic stimulus processing in patients with widespread pain. Z Rheumatol. 1997;56:334–41. doi: 10.1007/s003930050047. [DOI] [PubMed] [Google Scholar]
- 15.Alanoglu E, Ulas UH, Ozdag F, Odabasi Z, Cakci A, Vural O. Auditory event-related brain potentials in fibromyalgia syndrome. Rheumatol Int. 2005;25:345–9. doi: 10.1007/s00296-004-0443-3. [DOI] [PubMed] [Google Scholar]
- 16.Montoya P, Sitges C, Garcia-Herrera M, Rodriguez-Cotes A, Izquierdo R, Truyols M, et al. Reduced brain habituation to somatosensory stimulation in patients with fibromyalgia. Arthritis Rheum. 2006;54:1995–2003. doi: 10.1002/art.21910. [DOI] [PubMed] [Google Scholar]
- 17.Ozgocmen S, Yoldas T, Kamanli A, Yildizhan H, Yigiter R, Ardicoglu O. Auditory P300 event related potentials and serotonin reuptake inhibitor treatment in patients with fibromyalgia. Ann Rheum Dis. 2003;62:551–5. doi: 10.1136/ard.62.6.551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hegerl U, Gallinat J, Juckel G. Event-related potentials: do they reflect central serotonergic neurotransmission and do they predict clinical response to serotonin agonists? J Affect Disord. 2001;62:93–100. doi: 10.1016/s0165-0327(00)00353-0. [DOI] [PubMed] [Google Scholar]
- 19.Russell IJ, Vaeroy H, Javors M, Nyberg F. Cerebrospinal fluid biogenic amine metabolites in fibromyalgia/fibrositis syndrome and rheumatoid arthritis. Arthritis Rheum. 1992;35:550–6. doi: 10.1002/art.1780350509. [DOI] [PubMed] [Google Scholar]
- 20.Hauser W, Wolfe F, Tolle T, Uceyler N, Sommer C. The role of antidepressants in the management of fibromyalgia syndrome: a systematic review and meta-analysis. CNS Drugs. 2012;26:297–307. doi: 10.2165/11598970-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 21.Juckel G, Molnar M, Hegerl U, Csepe V, Karmos G. Auditory-evoked potentials as indicator of brain serotonergic activity: first evidence in behaving cats. Biol Psychiatry. 1997;41:1181–95. doi: 10.1016/s0006-3223(96)00240-5. [DOI] [PubMed] [Google Scholar]
- 22.Harris RE, Sundgren PC, Craig AD, Kirshenbaum E, Sen A, Napadow V, et al. Elevated insular glutamate in fibromyalgia is associated with experimental pain. Arthritis Rheum. 2009;60:3146–52. doi: 10.1002/art.24849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ichesco E, Kairys A, Chang E, Ramirez G, Clauw DJ, Harris RE, et al. Presented at the Society for Neuroscience Annual Meeting: Neuroscience 2013. San Diego, California: Nov 9–13, 2013. Further evidence for sensory hypersensitivity in fibromyalgia: sensitivity to visual stimuli and response to pregabalin [poster] [Google Scholar]
- 24.Chang LJ, Yarkoni T, Khaw MW, Sanfey AG. Decoding the role of the insula in human cognition: functional parcellation and large-scale reverse inference. Cereb Cortex. 2013;23:739–49. doi: 10.1093/cercor/bhs065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ethofer T, Pourtois G, Wildgruber D. Investigating audiovisual integration of emotional signals in the human brain. Prog Brain Res. 2006;156:345–61. doi: 10.1016/S0079-6123(06)56019-4. [DOI] [PubMed] [Google Scholar]
- 26.Kurth F, Zilles K, Fox PT, Laird AR, Eickhoff SB. A link between the systems: functional differentiation and integration within the human insula revealed by meta-analysis. Brain Struct Funct. 2010;214:519–34. doi: 10.1007/s00429-010-0255-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chapman HA, Anderson AK. Understanding disgust. Ann N Y Acad Sci. 2012;1251:62–76. doi: 10.1111/j.1749-6632.2011.06369.x. [DOI] [PubMed] [Google Scholar]
- 28.Wolfe F, Smythe HA, Yunus MB, Bennett RM, Bombardier C, Goldenberg DL, et al. The American College of Rheumatology 1990 criteria for the classification of fibromyalgia: report of the Multicenter Criteria Committee. Arthritis Rheum. 1990;33:160–72. doi: 10.1002/art.1780330203. [DOI] [PubMed] [Google Scholar]
- 29.Burckhardt CS, Clark SR, Bennett RM. The Fibromyalgia Impact Questionnaire: development and validation. J Rheumatol. 1991;18:728–34. [PubMed] [Google Scholar]
- 30.Ware JE, Jr, Snow KK, Kosinski M, Gandek B. SF-36 health survey: manual and interpretation guide. The Health Institute, New England Medical Center; Boston: 1993. [Google Scholar]
- 31.Zigmond AS, Snaith RP. The Hospital Anxiety and Depression Scale. Acta Psychiatr Scand. 1983;67:361–70. doi: 10.1111/j.1600-0447.1983.tb09716.x. [DOI] [PubMed] [Google Scholar]
- 32.Herraiz C, de los Santos G, Diges I, Diez R, Aparicio JM. Assessment of hyperacusis: the self-rating questionnaire on hyper-sensitivity to sound. Acta Otorrinolaringol Esp. 2006;57:303–6. doi: 10.1016/s0001-6519(06)78716-7. In Spanish. [DOI] [PubMed] [Google Scholar]
- 33.Brown C, Tollefson N, Dunn W, Cromwell R, Filion D. The Adult Sensory Profile: measuring patterns of sensory processing. Am J Occup Ther. 2001;55:75–82. doi: 10.5014/ajot.55.1.75. [DOI] [PubMed] [Google Scholar]
- 34.Song XW, Dong ZY, Long XY, Li SF, Zuo XN, Zhu CZ, et al. REST: a toolkit for resting-state functional magnetic resonance imaging data processing. PLoS One. 2011;6:e25031. doi: 10.1371/journal.pone.0025031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Melzack R, Wall PD, Weisz AZ. Masking and metacontrast phenomena in the skin sensory system. Exp Neurol. 1963;8:12. [Google Scholar]
- 36.Yague JG, Foffani G, Aguilar J. Cortical hyperexcitability in response to preserved spinothalamic inputs immediately after spinal cord hemisection. Exp Neurol. 2011;227:252–63. doi: 10.1016/j.expneurol.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 37.Higgens JD, Tursky B, Schwartz GE. Shock-elicited pain and its reduction by concurrent tactile stimulation. Science. 1971;172:866–7. doi: 10.1126/science.172.3985.866. [DOI] [PubMed] [Google Scholar]
- 38.Yarnitsky D, Kunin M, Brik R, Sprecher E. Vibration reduces thermal pain in adjacent dermatomes. Pain. 1997;69:75–7. doi: 10.1016/s0304-3959(96)03250-2. [DOI] [PubMed] [Google Scholar]
- 39.Rasskazoff SY, Slavin KV. An update on peripheral nerve stimulation. J Neurosurg Sci. 2012;56:279–85. [PubMed] [Google Scholar]
- 40.Taylor RS, Desai MJ, Rigoard P, Taylor RJ. Predictors of pain relief following spinal cord stimulation in chronic back and leg pain and failed back surgery syndrome: a systematic review and meta-regression analysis. Pain Pract. 2014;14:489–505. doi: 10.1111/papr.12095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dailey DL, Rakel BA, Vance CG, Liebano RE, Amrit AS, Bush HM, et al. Transcutaneous electrical nerve stimulation reduces pain, fatigue and hyperalgesia while restoring central inhibition in primary fibromyalgia. Pain. 2013;154:2554–62. doi: 10.1016/j.pain.2013.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Marlow NM, Bonilha HS, Short EB. Efficacy of transcranial direct current stimulation and repetitive transcranial magnetic stimulation for treating fibromyalgia syndrome: a systematic review. Pain Pract. 2013;13:131–45. doi: 10.1111/j.1533-2500.2012.00562.x. [DOI] [PubMed] [Google Scholar]
- 43.Vigano A, D’Elia TS, Sava SL, Auve M, De Pasqua V, Colosimo A, et al. Transcranial Direct Current Stimulation (tDCS) of the visual cortex: a proof-of-concept study based on interictal electrophysiological abnormalities in migraine. J Headache Pain. 2013;14:23. doi: 10.1186/1129-2377-14-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wik G, Fischer H, Bragee B, Kristianson M, Fredrikson M. Retrosplenial cortical activation in the fibromyalgia syndrome. Neuroreport. 2003;14:619–21. doi: 10.1097/00001756-200303240-00019. [DOI] [PubMed] [Google Scholar]
- 45.Klemen J, Chambers CD. Current perspectives and methods in studying neural mechanisms of multisensory interactions. Neurosci Biobehav Rev. 2012;36:111–33. doi: 10.1016/j.neubiorev.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 46.Martuzzi R, Murray MM, Michel CM, Thiran JP, Maeder PP, Clarke S, et al. Multisensory interactions within human primary cortices revealed by BOLD dynamics. Cereb Cortex. 2007;17:1672–9. doi: 10.1093/cercor/bhl077. [DOI] [PubMed] [Google Scholar]
- 47.Johnson JA, Zatorre RJ. Attention to simultaneous unrelated auditory and visual events: behavioral and neural correlates. Cereb Cortex. 2005;15:1609–20. doi: 10.1093/cercor/bhi039. [DOI] [PubMed] [Google Scholar]
- 48.Albuquerque RJ, de Leeuw R, Carlson CR, Okeson JP, Miller CS, Andersen AH. Cerebral activation during thermal stimulation of patients who have burning mouth disorder: an fMRI study. Pain. 2006;122:223–34. doi: 10.1016/j.pain.2006.01.020. [DOI] [PubMed] [Google Scholar]
- 49.Loggia ML, Jensen K, Gollub RL, Wasan AD, Edwards RR, Kong J. The catechol-O-methyltransferase (COMT) val158met polymorphism affects brain responses to repeated painful stimuli. PLoS One. 2011;6:e27764. doi: 10.1371/journal.pone.0027764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lewis JW, Van Essen DC. Corticocortical connections of visual, sensorimotor, and multimodal processing areas in the parietal lobe of the macaque monkey. J Comp Neurol. 2000;428:112–37. doi: 10.1002/1096-9861(20001204)428:1<112::aid-cne8>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 51.Meredith MA, Allman BL, Keniston LP, Clemo HR. Auditory influences on non-auditory cortices. Hear Res. 2009;258:64–71. doi: 10.1016/j.heares.2009.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Treede RD, Apkarian AV, Bromm B, Greenspan JD, Lenz FA. Cortical representation of pain: functional characterization of nociceptive areas near the lateral sulcus. Pain. 2000;87:113–9. doi: 10.1016/S0304-3959(00)00350-X. [DOI] [PubMed] [Google Scholar]
- 53.Napadow V, LaCount L, Park K, As-Sanie S, Clauw DJ, Harris RE. Intrinsic brain connectivity in fibromyalgia is associated with chronic pain intensity. Arthritis Rheum. 2010;62:2545–55. doi: 10.1002/art.27497. [DOI] [PMC free article] [PubMed] [Google Scholar]