Abstract
Staphylococcus aureus is an important human pathogen that causes nosocomial and community-acquired infections. One of the most important aspects of staphylococcal infections is biofilm development within the host, which renders the bacterium resistant to the host's immune response and antimicrobial agents. Biofilm development is very complex and involves several regulators that ensure cell survival on surfaces within the extracellular polymeric matrix. Previously, we identified the msaABCR operon as an additional positive regulator of biofilm formation. In this study, we define the regulatory pathway by which msaABCR controls biofilm formation. We demonstrate that the msaABCR operon is a negative regulator of proteases. The control of protease production mediates the processing of the major autolysin, Atl, and thus regulates the rate of autolysis. In the absence of the msaABCR operon, Atl is processed by proteases at a high rate, leading to increased cell death and a defect in biofilm maturation. We conclude that the msaABCR operon plays a key role in maintaining the balance between autolysis and growth within the staphylococcal biofilm.
Keywords: Staphylococcus aureus, proteases, msaABCR, regulation, biofilm, autolysis
msaABCR regulates proteases, cell death and biofilm formation in Staphylococcus aureus.
msaABCR regulates proteases, cell death and biofilm formation in Staphylococcus aureus.
INTRODUCTION
Staphylococcus aureus is a Gram-positive human pathogen that causes nosocomial and community-acquired infections. The increasing prevalence of antibiotic resistance and production of biofilm by S. aureus makes these infections difficult to treat. Indeed, biofilm formation is responsible for the establishment of chronic infections such as osteomyelitis, infective endocarditis, indwelling-medical-device-associated infections and chronic wound infections (Herold et al., 1998; Lowy 1998; Haque et al., 2007). The molecular mechanism of biofilm formation and the associated global regulatory network are still poorly understood. Staphylococcus aureus produces a very well-organized, multilayered, 3D mushroom-shaped biofilm embedded in an extracellular polymeric matrix composed of poly-N-acetylglucosamine (PIA), extracellular DNA (eDNA) and several heterogeneous proteins (Cramton et al., 1999; Whitchurch et al., 2002; Rice et al., 2007; Merino et al., 2009; Houston et al., 2011).
Several studies have determined the genes involved in regulating biofilm development. Transcriptional regulators, including stress response sigma factor B (sigB), staphylococcal accessory regulator A (sarA), the two-component system arlRS and the accessory gene regulator (agr), have been shown to play key roles in the regulation of biofilm development (Kullik, Giachino and Fuchs 1998; Beenken, Blevins and Smeltzer 2003; Boles and Horswill 2008; Tsang et al., 2008). Several operons like icaADBC, dtlABCD, cidABC and psmβ operons also regulate biofilm formation (Heilmann et al., 1996; Gross et al., 2001; Rice et al., 2007; Otto 2013). Other factors, such as secreted proteases, eDNA, major autolysin (Atl) and nucleases, also play major roles in the maintenance and dispersion of biofilms (Qin et al., 2007; Rice et al., 2007; Houston et al., 2011; Kiedrowski et al., 2011; Beenken et al., 2012; Chen et al., 2013).
Despite our current knowledge of the many regulators and factors involved in biofilm development, there is still no consensus on the regulation of biofilm development among the diverse staphylococcal strains nor on the strain-dependent regulation of biofilm development. For instance, several studies have shown that different Staphylococcus strains regulate biofilm formation using various mechanisms such as PIA-dependent, PIA-independent and eDNA-dependent mechanisms (Cramton et al., 1999; Whitchurch et al., 2002; Fitzpatrick, Humphreys and O'Gara 2005; Toledo-Arana et al., 2005; Rice et al., 2007; Mann et al., 2009).
We previously identified a new operon, msaABCR, which includes two non-coding RNAs, msaC and the antisense RNA, msaR, which are essential for the regulation of the msaABCR operon. The msaABCR operon regulates important phenotypes in S. aureus, including biofilm development and virulence (Sahukhal and Elasri 2014). The msaABCR operon also regulates the expression of key global regulators sarA, agr and sigB (Sahukhal and Elasri 2014). The regulatory mechanism of the msaABCR operon is not yet defined. In this study, we show that the msaABCR operon regulates biofilm development by controlling the rate of autolysis. We also show that this operon controls cell death by regulating the rate of processing of the major autolysin Atl by proteases.
METHODS
Bacteria and growth conditions
In this study, we used S. aureus strains USA300_LAC and RN4220 and the Escherichia coli strain, DH5α. The S. aureus strains were grown in tryptic soy broth (TSB) or tryptic soy agar, as appropriate. The E. coli strain was grown in Luria–Bertani broth. Antibiotics (chloramphenicol, 10 μg ml−1; erythromycin, 10 μg ml−1 and ampicillin, 100 μg ml−1) were used as necessary. The strains and plasmid constructs used in this study are listed in Table 1.
Table 1.
Strains and plasmids used in this study
| Strains or plasmids | Relevant characteristics | Source |
|---|---|---|
| Plasmids | ||
| pKOR1 | E. coli– S. aureus shuttle vector, ori(Ts) inducible, secY antisense counterselection, Ampr Cmr | Dr Bae |
| pMOE 399 | pKOR1: Δ msa operon deletion construct | This study |
| pCN34 | pT181-based low copy number E. coli–Staphylococcal shuttle vector | NARSA |
| pMOE 403 | pCN34-msaABCR operon :: msaABCR operon complement | This study |
| pCN58 | pT181-based E.coli–staphylococcal shuttle vector that contains promoterless LuxAB as a reporter gene for promoter-gene fusion. | NARSA |
| pMOE 481 | pCN58-Promoter aur | This study |
| pMOE 482 | pCN58-Promoter ssp | This study |
| pMOE 483 | pCN58-Promoter scp | This study |
| pMOE 498 | pCN58-Promoter spl | This study |
| pMOE 501 | pCN58-Promoter | This study |
| pMOE 448 | E. coli pCN51 vector with pCAD inducible promoter | This study |
| Strains | ||
| RN4220 | Restriction deficient mutant of 8325–4 | NARSA |
| M. luteus | ATCC No. 4698 | Sigma–Aldrich |
| LAC | CA-MRSA USA300 strains | Dr Shaw |
| MOE 401 | LAC :: Δ msaABCR deletion mutant | This study |
| MOE 404 | LAC 401 :: pMOE403 :: msaABCR complement into msaABCR deletion mutant | This study |
| MOE 401/pCN34-vector control | This study | |
| MOE 508 | LAC 13C (Δatl) | Dr Jeffrey Bose /K. Bayles |
| MOE 458 | LAC/proteases | Dr Mark Smeltzer |
| MOE 512 | msaABCR/atl mutant | This study |
| MOE 466 | msaABCR/protease mutant | This study |
| msaABCR complement into msaABCR/atl mutant | This study | |
| MOE 467 | msaABCR complement into msaABCR/protease mutant | This study |
| MOE 535 | LAC :: Δ sarA:kan insertional mutant | This study |
| MOE 532 | LAC :: Δ msaABCR deletion/sarA:kan insertional mutant | This study |
| MOE 533 | msaABCR/sarA:kan mutant with pCN51-sarA vector | This study |
| MOE 536 | sarA:kan mutant with pCN51-sarA vector | This study |
| MOE 545 | msaABCR deletion mutant with pCN51-sarA vector | This study |
| MOE 645 | LAC :: pCN58-Promoteraur | This study |
| MOE 646 | LAC :: pCN58-Promoter scp | This study |
| MOE 647 | LAC :: pCN58-Promoter spl | This study |
| MOE 648 | LAC :: pCN58-Promoter ssp | This study |
| MOE 649 | MOE 401 :: pCN58-Promoter aur | This study |
| MOE 650 | MOE 401 :: pCN58-Promoterscp | This study |
| MOE 651 | MOE 401 :: pCN58-Promoterspl | This study |
| MOE 652 | MOE 401 :: pCN58-Promoterssp | This study |
| MOE 495 | LAC :: pCN58-vector control | This study |
Construction of double-deletion mutants
We used a previously described mutagenesis protocol (Bae and Schneewind 2006; Sahukhal and Elasri 2014) to construct a non-polar, in-frame double-deletion mutant msaABCR/atl and LAC msaABCR/protease. Deletions were verified by end-point and real-time quantitative PCR (qPCR), as described previously (Sahukhal and Elasri 2014).
Biofilm assays
A microtiter biofilm assay was performed as described previously (Sambanthamoorthy et al., 2008; Sahukhal and Elasri 2014). To study the effect of polyanethole sulfonate (PAS) treatment on biofilm formation, the biofilm was grown in biofilm medium in the presence of 500 μg ml−1 PAS, as previously described (Rice et al., 2007). The mean values of three independent experiments, each performed in triplicate, are reported.
Confocal microscopic analysis of the biofilm
Biofilms were grown in 96-well Corning high content imaging microplates, as described previously (Sambanthamoorthy et al., 2008; Sahukhal and Elasri 2014). The biofilm was grown for 48 h with shaking at 150 rpm. Each adherent biofilm was washed three times with sterile phosphate-buffered saline (PBS) and stained with 50 μl of live/dead stain [Syto-9 (1.3 μm) and Toto-3 (2.0 μm)] prepared in PBS, as previously described (Mann et al., 2009). Images of the biofilms were acquired using confocal laser scanning microscopy (Zeiss 510 Meta CLSM) under a 40 × 1.4 oil DIC objective. The Syto-9 stain was excited with an argon laser at 488 nm and the emission band-pass filter used for Syto-9 was 515 ± 15 nm. The Toto-3 stain was excited with an HeNe laser at 633 nm, and emissions were detected with a 680 ± 30 nm filter. Z-stacks were collected at 1.0 μm intervals. Images were processed with the COMSTAT software to quantify the total biomass, biofilm thickness, number of dead cells and amount of eDNA (Heydorn et al., 2000).
Autolysis assay
Autolysis assays were performed as described previously (Mani, Tobin and Jayaswal 1993). To study the effect of PAS on autolysis, the cultures of S. aureus were diluted to an OD600 of 0.05 in TSB containing 1 M NaCl and 500 μg ml−1 of PAS and allowed to grow at 37 °C with shaking until an OD600 of 0.7 was reached. The cells were harvested and the autolysis assay was performed in 0.05 M Tris-Cl (pH 7.2) containing 0.025% Triton X-100. The absorbance (OD580) was measured every 30 min to quantify cell lysis. All the experiments were repeated three times and statistical significance tests (paired t-tests) were performed using the GraphPad software.
Zymographic analysis
Zymographic analyses of the cell-wall-bound and extracellular fractions of murein hydrolases were performed as described previously (Mani, Tobin and Jayaswal 1993). Freeze–thaw extracts, also designated ‘cell-wall-bound autolysins’, were isolated from cells grown to mid exponential phase, as described previously (Mani, Tobin and Jayaswal 1993; Ledala, Wilkinson and Jayaswal 2006). The extracellular autolysins Atl were isolated by the centrifugation of whole-cell cultures grown to late exponential phase. The supernatant was collected and used for zymographic analysis. The enzyme extracts were concentrated using Amicon Ultracel (3K) centrifugal filters. The proteins were quantified with Quick Start™ Bradford reagent.
The zymographic analysis was performed with a 10% polyacrylamide-SDS gel containing 0.2% (wt/vol) crude cell walls from S. aureus (RN4220) or Micrococcus luteus (Bose et al., 2012). The enzyme extracts (3 μg) were mixed with loading buffer and heated for 3 min in a boiling water bath before electrophoresis. After electrophoresis, the gel was washed twice with water and incubated overnight at 37°C in renaturation buffer (25 mM Tris-HCl [pH 8.0], 1% Triton X-100). Lytic activities were observed as clear bands in the opaque gel. The gels were stained with 1% methylene blue in 0.01% KOH before imaging.
Tolerance to lysostaphin, mutanolysin and lysozyme
Overnight-grown cells were diluted to an OD600 of 0.05 in TSB and allowed to grow at 37°C with shaking to an OD600 of 0.7. The cells were harvested, washed twice with ice-cold water and then resuspended in the same volume of 0.05 M Tris-Cl (pH 7.2) containing 2 μg ml−1 lysostaphin, 10 U ml−1 mutanolysin or 10 μg ml−1 lysozyme. The cells were incubated at 37°C with shaking and lysis was calculated from the OD600.
RNA isolation and real-time qPCR
Total RNA for the RT-qPCR was isolated from cells using a Qiagen RNeasy Maxi column (Qiagen), and RT-qPCR was done as described previously (Sambanthamoorthy, Smeltzer and Elasri 2006). Analysis of expression of each gene was done based on at least three independent experiments. Two-fold or higher changes in gene expression were considered significant. All the primers used for RT-qPCR are listed in Table S1 (Supporting Information).
Construction of protease promoter–luxAB fusions and luciferase assays
The E. coli–staphylococcal shuttle vector pCN58 was used in this study to analyze transcriptional fusions (Charpentier et al., 2004). The promoter regions of four different protease genes [aureolysin (aur), staphopain (scp), cysteine (ssp) and serine (spl)] were fused to luxAB, as previously described (Mootz et al., 2013; Sahukhal and Elasri 2014). To study the promoter–luciferase activity, the bacterial cells (5 ml) were harvested at different optical densities (OD600 of 0.7, 1.5 and 4.0) representing the early, mid and late exponential growth phases, respectively. Promoter activity was also measured from 24, 48 and 72 h biofilms. Relative luminescence units were measured as described in (Sahukhal and Elasri 2014). The promoterless version of the reporter gene plasmid (pCN58) was used as a negative control.
RESULTS
Deletion of the msaABCR operon causes a defect in biofilm development
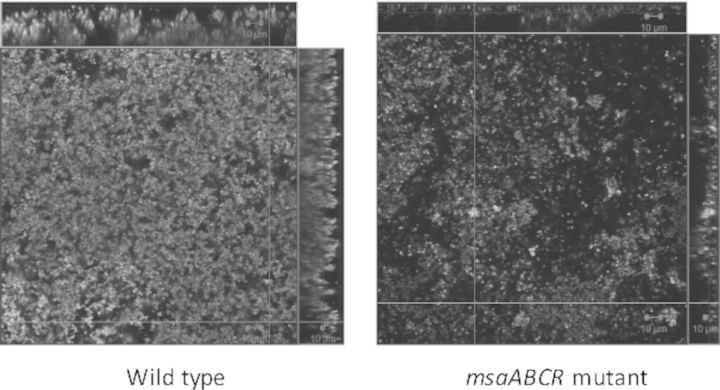
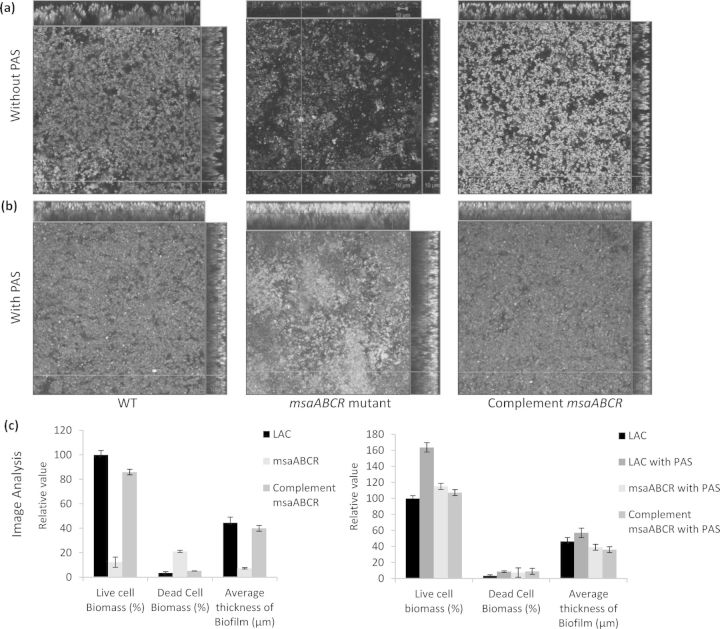
We have previously shown that the deletion of the msaABCR operon in S. aureus leads to a defect in biofilm formation (Sahukhal and Elasri 2014). In this study, we investigated the mechanism behind this phenotype. We examined the biofilm formation of the msaABCR deletion mutant using confocal microscopy after staining with live/dead stain, Syto-9 and Toto-3. Syto-9 stains live cells green, whereas Toto-3 stains dead cells and eDNA red (Fig. 1a). We observed an increase in localized cell death within the biofilm of the deletion mutant relative to that of the wild-type or the complemented mutant. The mutant biofilm also lacked mature biofilm towers. We also analyzed the Z-stack confocal images with the COMSTAT image analysis software, and found that the biofilm of the msaABCR deletion mutant was relatively thin (7 μm, compared with 44 μm in the wild type) and dispersed, with a live cell biomass of only 12% compared with that of the wild-type biofilm, which was set to 100%. COMSTAT image analysis also showed the presence of more dead cells and eDNA in the msaABCR mutant biofilm (21%) relative to their levels in the wild-type biofilm (3%) (Fig. 1c). The complemented mutant showed a phenotype similar to the wild-type phenotype (Fig. 1a). These results suggest that the defective biofilm of the msaABCR mutant is attributable to an increased rate of cell death.
Figure 1.
CLSM images and image analysis of the biofilm. Biofilm was grown upto 48 h and stained with Syto-9 (live cells, green) and Toto-3 (dead cells and eDNA, red). Panel (a) wild-type S. aureus USA300 LAC, msaABCR deletion mutant and msaABCR complement, respectively. Panel (b) CLSM image of biofilm of wild-type S. aureus USA300 LAC, msaABCR deletion mutant and msaABCR complement, respectively, grown in presence of 500 μg ml−1 PAS. Panel (c) COMSTAT image analysis of biofilm (a) and (b). These images are representative of three independent experiments. Scale bar represents 10 μm.
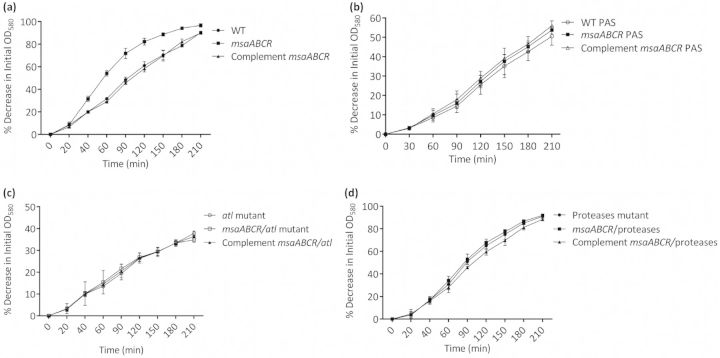
We confirmed these findings by measuring the rate of autolysis of the mutant during planktonic growth and found that in the presence of Triton X-100, the msaABCR mutant is lysed at a higher rate (20–25%) than is the wild-type or complemented mutant (Fig. 2a). The treatment of the strains with PAS, a cell lysis inhibitor, provided further support for the role of cell death in the msaABCR operon defect (Fig. 1b and Fig. 2b). These findings indicate that the msaABCR operon is involved in the regulation of autolysis, which might be responsible for the biofilm defect in this mutant.
Figure 2.
Triton-X-100-induced autolysis assay. (a) Rates of autolysis of wild-type S. aureus USA300 LAC, msaABCR deletion mutant and msaABCR complement. (b) Rate of autolysis measured in presence of 500 μg ml−1 PAS. Wild-type S. aureus USA300 LAC, msaABCR deletion mutant and msaABCR complement. (c) Rates of autolysis of atl mutant, msaABCR/atl double mutant and msaABCR complement in msaABCR/atl double mutant. (d) Rates of autolysis of protease mutant, msaABCR/protease double mutant and msaABCR complement in msaABCR/protease double mutant. The experiments were repeated at least three times. A paired t-test was used in the statistical analysis with the GraphPad software (p < 0.0001).
Biofilm developmental defect in the msaABCR mutant is mediated by increased processing of the major autolysin, Atl
We examined the mechanism involved in the increased cell death in the msaABCR mutant. We tested the susceptibility of whole cell-wall fractions to lysostaphin, mutanolysin and lysozyme extracted from the msaABCR mutant. We found no significant difference between the msaABCR mutant and the wild type, suggesting that cell-wall perturbation is not responsible for the increased autolysis of the mutant. We also measured the expression of all known murein hydrolase genes (atl, lytM, lytM, sle1, lytX, lytY and lytZ), and regulators of autolysis, the cidABC and lgrAB operon genes. We found no significant change in the expression of these genes in the mutant relative to that in the wild type (Table S2, Supporting Information). These findings indicate that the role of the msaABCR operon in autolysis is not attributable to cell-wall perturbation or the regulation of genes known to control cell death.
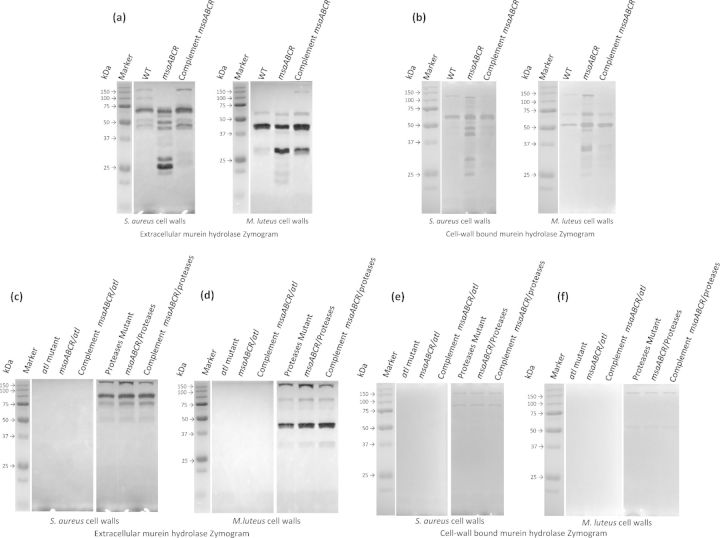
We have previously shown that the deletion of msaABCR increases protease activity (Sahukhal and Elasri 2014). Other studies have shown that murein hydrolases (e.g. Atl) are targeted by proteases, which thus affect the rate of autolysis in S. aureus (Horsburgh et al., 2002; Biswas et al., 2006; Rice et al., 2007; Lauderdale et al., 2009). Because the msaABCR operon does not regulate the expression of murein hydrolase genes, we used zymography to investigate its role in the protease-mediated processing of the murein hydrolases. We measured the activity of the murein hydrolases (cell-wall-bound and extracellular fractions) using whole cells of M. luteus and S. aureus (RN4220) as substrates. Micrococcus luteus cells and S. aureus cell-wall substrates were used to determine the various glucosaminidase (GL)-specific and amidase (AM)-specific activities, respectively, as previously described (Wadstrom and Hisatsune 1970; Oshida et al., 1995; Bose et al., 2012). The msaABCR deletion mutant showed a significantly altered pattern of AM and GL activities relative to those of the wild type and the complemented mutant (Fig. 3a and b). In the zymogram produced with S. aureus as the cell substrate, the msaABCR deletion mutant showed a significantly higher number of bands corresponding to AMs than the wild type in both the cell-wall-bound and extracellular fractions. When M. luteus cells were used as the substrate, we observed a similar pattern of activity for the GLs in both the cell-wall-bound and extracellular fractions (Fig. 3a and b).
Figure 3.
Extracellular and cell-wall bound murein hydrolase zymogram. Staphylococcus aureus cell wall and M. luteus cell-wall substrates were used in the zymogram to determine AM and GL classes of murein hydrolases. (a) Extracellular murein hydrolase zymogram of wild-type S. aureus USA300 LAC, msaABCR deletion mutant and msaABCR complement. (b) Cell-wall bound murein hydrolase zymogram of wild-type S. aureus USA300 LAC, msaABCR deletion mutant and msaABCR complement. (c and d) Extracellular murein hydrolase zymogram of atl mutant, msaABCR/atl double mutant, msaABCR complement in msaABCR/atl double mutant, protease mutant, msaABCR/protease double mutant and msaABCR complement in msaABCR/protease double mutant, respectively. (e and f) Cell-wall bound murein hydrolase zymogram of atl mutant, msaABCR/atl double mutant, msaABCR complement in msaABCR/atl double mutant, protease mutant, msaABCR/protease double mutant and msaABCR complement in msaABCR/protease double mutant, respectively.
Overall, the msaABCR mutant showed more processing of murein hydrolases, evident from the absence of high-molecular-weight bands and the presence of several additional low-molecular-weight bands in the zymogram relative to those in the wild-type zymogram (Fig. 3a and b).
The high-molecular-weight murein hydrolase bands correspond to the major autolysin (Atl) and its derivatives, whereas the low-molecular-weight murein hydrolase bands may have arisen from lytM, Sle1, LytN or LytH. Because we found no significant differences in the transcription levels of all known murein hydrolase genes between the wild type and the mutant, these findings suggest that the bands produced by the msaABCR mutant result from the increased processing of Atl. To investigate this possibility, we generated an msaABCR/atl double mutant. The double mutant showed increased production of extracellular proteases. However, this mutant showed no murein hydrolase activity in any of the fractions tested (Fig. 3c–f). The msaABCR/atl mutant also showed no increase in autolysis relative to that in the wild type (Fig. 2c). These findings support the conclusion that the observed murein hydrolase activity of the msaABCR mutant is primarily attributable to Atl processing.
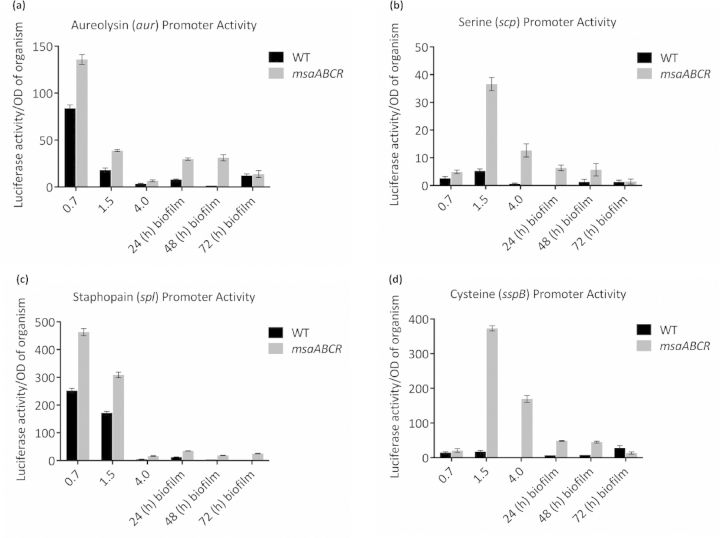
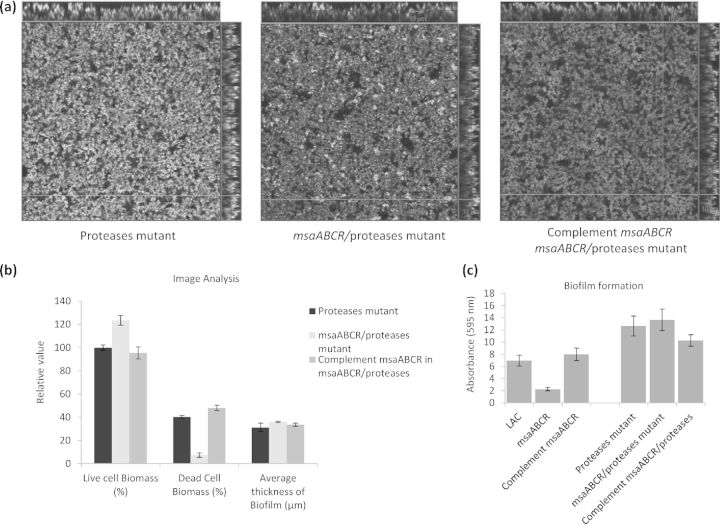
To determine the contribution of proteases to this process, we fused the promoters of the genes (aur, scp, ssp and spl) encoding four major extracellular proteases to luxAB (Mootz et al., 2013; Sahukhal and Elasri 2014). The luciferase assay of the msaABCR deletion mutant showed a significantly higher positive signal (production of light) relative to that produced by the wild type under all the growth conditions tested (early, mid and late exponential phases and biofilm) (Fig. 4). This confirms the role of msaABCR in regulating the expression of the protease genes. We introduced the msaABCR deletion into an LAC protease knockout strain (Beenken et al., 2010; Zielinska et al., 2012) and measured its murein hydrolase activity, autolysis and biofilm formation. This mutant showed no evidence of high-molecular-weight murein hydrolase processing (Fig. 3c–f). Based on previous studies, the two bands produced by this mutant are presumed to correspond to Atl (Bose et al., 2012; Grilo et al., 2014). The msaABCR/protease mutant showed no increase in autolysis activity or any defect in biofilm formation (Figs 2d and 5). These results indicate that the msaABCR biofilm defect is mediated by the overproduction of proteases, which leads to the increased processing of murein hydrolases and increased cell death.
Figure 4.
Promoter activities of protease genes. The activities of the protease promoters were measured in the wild-type and msaABCR deletion mutant. Luciferase activity (luxAB) was measured in three planktonic growth phases (early, mid and late exponential phases) and the biofilm. The vector pCN58 containing luxAB without a promoter was used as the negative control. (a) aureolysin (aur) promoter activity. (b) serine (scp) promoter activity. (c) staphopain (spl) promoter activity. (d) cysteine (sspB) promoter activity. The results are the means of three independent experiments, and each measurement was made in triplicate. Standard error bars are shown.
Figure 5.
CLSM image and biofilm assay of msaABCR/protease double mutant. (a) CLSM images of protease mutant, msaABCR/protease double mutants and complement msaABCR/protease double mutants. (b) COMSTAT image analysis of CLSM images. (c) Microtiter biofilm assay of wild-types and double mutants. The results are representative of three independent experiments. Scale bar represents 10 μm.
DISCUSSION
The msaABCR operon is essential for biofilm formation in S. aureus (Sahukhal and Elasri 2014). In this study, we set out to identify the mechanism underlying the regulatory role of msaABCR in biofilm formation. We have shown that the deletion of msaABCR results in the excessive production of proteases, leading to increased processing of the major autolysin, Atl. This in turn leads to uncontrolled cell death, which contributes to the biofilm defect in the msaABCR mutant. Controlled cell death and the controlled release of eDNA are important in the formation of a robust and mature biofilm in staphylococci and other organisms, including Pseudomonas aeruginosa, Streptococcus intermedius, S. mutans and Enterococcus faecalis. Many studies have shown that controlled cell death and the release of eDNA enhance biofilm formation, however, unregulated cell death may have detrimental effects on the biofilm (Wen, Baker and Burne 2006; Bayles 2007; Rice et al., 2007; Boles et al., 2010; Qamar and Golemi-Kotra 2012; Bitoun et al., 2013; Chan et al., 2013). Our findings are supported by several studies that have shown that excessive protease activity and autolysis results in biofilm instability and a lack of maturation, which reduces the growth rate within the biofilm and upsets the balance between its growth and detachment (Wen, Baker and Burne 2006; Boles et al., 2010; Qamar and Golemi-Kotra 2012; Bitoun et al., 2013; Chan et al., 2013).
Several processes have been implicated in autolysis, including cell-wall perturbation, increased activity or expression of murein hydrolases, and the regulation of holin and antiholin expression (Perkins 1980; Wang et al., 1992; Groicher et al., 2000; Rice et al., 2003; Bayles 2007; Qin et al., 2007). The msaABCR operon regulates autolysis by controlling the processing of Atl by proteases. We found no evidence of the involvement of msaABCR in cell-wall perturbation or in regulating the expression of murein hydrolases, including Atl and the cidABC/lrgAB system. Previous studies have shown that Atl proprotein (134 kDa) contains an AM–GL peptide that is attached to a propeptide (Bose et al., 2012; Grilo et al., 2014). The proprotein is cleaved to yield a 117-kDa AM–GL peptide. This peptide is further processed to produce various AM intermediates (100, 81, 70 and 63 kDa) and GL intermediates. The GL intermediates include a 55-kDa peptide and two products that are slightly less than 55 kDa and lack the repeated domain. The 63-kDa Atl fragment corresponds to the mature AM band, with two repeat domains (AM-R1-R2), and the fragments slightly less than 63 kDa correspond to AM, with one repeat domain (AM-R1) and AM without any repeated domain. The high-molecular-weight bands of 134 and 117 kDa both showed AM and GL activity, whereas the lower-molecular-weight bands (100, 81, 70, 63, 48.75 and 30 kDa) showed AM-specific activity. The 55 kDa and other smaller bands (40.5 kDa and less) showed GM-specific activity. These patterns reflect the sequential order of Atl processing (Bose et al., 2012; Grilo et al., 2014). Our findings show that Atl is the main murein hydrolase regulated by msaABCR. Further support for this conclusion was obtained by examining the msaABCR/atl double mutant, which showed a complete lack of all the processing products described above (Fig. 3c–f).
We have previously shown that the msaABCR operon regulates protease production (Sahukhal and Elasri 2014). In this study, we linked the increase in proteases production to increased processing of Atl (Fig. 3c–f). We have shown that the msaABCR operon controls the expression of four extracellular proteases (Aur, Scp, Ssp and Spl) in various growth phases, including biofilm (Fig. 4). Atl is processed collaboratively by the serine protease Ssp and the cysteine protease Spl (Rice et al., 2001). These proteases modulate the activity, stability and translocation of Atl, and affect the autolysis and biofilm development of S. aureus (Rice et al., 2001; Biswas et al., 2006; Thomas et al., 2008; Lauderdale et al., 2009; Chen et al., 2013; Grilo et al., 2014).
We conclude that the msaABCR operon plays a key role in maintaining the balance between autolysis and growth within a biofilm. The msaABCR operon achieves this balance by controlling the expression of the proteases that process the major autolysin, Atl. The environmental signals to which this operon responds are still unknown. Other regulators have been shown to control biofilm development via proteases. For instance, sarA mutants are biofilm negative because of their increased production of proteases (Beenken et al., 2010; Zielinska et al., 2012). Similarly, SigB also regulates biofilm formation via an agr/protease-dependent pathway (Lauderdale et al., 2009). We plan to investigate the relationship between msaABCR and these global regulators.
SUPPLEMENTARY DATA
Supplementary data is available at FEMSLE online.
Acknowledgments
The authors are grateful to Dr Lindsey N. Shaw for sharing S. aureus strain USA300 LAC and to Dr Taeok Bae for providing the plasmid pKOR1. We are also grateful to Dr Jeffrey L. Bose and Dr Kenneth W. Bayles for sharing S. aureus strain LAC 13C (atl) and to Dr Mark S. Smeltzer for the protease mutant LAC strains.
FUNDING
This work was funded by the National Institutes of Health (grant 1R15AI099922, to MOE) and by the Mississippi INBRE, with an Institutional Development Award (IDeA) of the National Institute of General Medical Sciences (grant number P20GM103476).
Conflict of interest statement. None declared.
REFERENCES
- Bae T, Schneewind O. Allelic replacement in Staphylococcus aureus with inducible counter-selection. Plasmid. 2006;55:58–63. doi: 10.1016/j.plasmid.2005.05.005. [DOI] [PubMed] [Google Scholar]
- Bayles KW. The biological role of death and lysis in biofilm development. Nat Rev Microbiol. 2007;5:721–6. doi: 10.1038/nrmicro1743. [DOI] [PubMed] [Google Scholar]
- Beenken KE, Blevins JS, Smeltzer MS. Mutation of sarA in Staphylococcus aureus limits biofilm formation. Infect Immun. 2003;71:4206–11. doi: 10.1128/IAI.71.7.4206-4211.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken KE, Mrak LN, Griffin LM, et al. Epistatic relationships between sarA and agr in Staphylococcus aureus biofilm formation. PLoS One. 2010;5:e10790. doi: 10.1371/journal.pone.0010790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beenken KE, Spencer H, Griffin LM, et al. Impact of extracellular nuclease production on the biofilm phenotype of Staphylococcus aureus under in vitro and in vivo conditions. Infect Immun. 2012;80:1634–8. doi: 10.1128/IAI.06134-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Biswas R, Voggu L, Simon UK, et al. Activity of the major staphylococcal autolysin Atl. FEMS Microbiol Lett. 2006;259:260–8. doi: 10.1111/j.1574-6968.2006.00281.x. [DOI] [PubMed] [Google Scholar]
- Bitoun JP, Liao S, McKey BA, et al. Psr is involved in regulation of glucan production, and double deficiency of BrpA and Psr is lethal in Streptococcus mutans. Microbiology. 2013;159:493–506. doi: 10.1099/mic.0.063032-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Horswill AR. Agr-mediated dispersal of Staphylococcus aureus biofilms. PLoS Pathog. 2008;4:e1000052. doi: 10.1371/journal.ppat.1000052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boles BR, Thoendel M, Roth AJ, et al. Identification of genes involved in polysaccharide-independent Staphylococcus aureus biofilm formation. PLoS One. 2010;5:e10146. doi: 10.1371/journal.pone.0010146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bose JL, Lehman MK, Fey PD, et al. Contribution of the Staphylococcus aureus Atl AM and GL Murein hydrolase activities in cell division, autolysis, and biofilm formation. PLoS One. 2012;7:e42244. doi: 10.1371/journal.pone.0042244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan YG, Frankel MB, Dengler V, et al. Staphylococcus aureus mutants lacking the LytR-CpsA-Psr family of enzymes release cell wall teichoic acids into the extracellular medium. J Bacteriol. 2013;195:4650–9. doi: 10.1128/JB.00544-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charpentier E, Anton AI, Barry P, et al. Novel cassette-based shuttle vector system for gram-positive bacteria. Appl Environ Microb. 2004;70:6076–85. doi: 10.1128/AEM.70.10.6076-6085.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C, Krishnan V, Macon K, et al. Secreted proteases control autolysin-mediated biofilm growth of Staphylococcus aureus. J Biol Chem. 2013;288:29440–52. doi: 10.1074/jbc.M113.502039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cramton SE, Gerke C, Schnell NF, et al. The intercellular adhesion (ica) locus is present in Staphylococcus aureus and is required for biofilm formation. Infect Immun. 1999;67:5427–33. doi: 10.1128/iai.67.10.5427-5433.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzpatrick F, Humphreys H, O'Gara JP. Evidence for icaADBC-independent biofilm development mechanism in methicillin-resistant Staphylococcus aureus clinical isolates. J Clin Microbiol. 2005;43:1973–6. doi: 10.1128/JCM.43.4.1973-1976.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grilo IR, Ludovice AM, Tomasz A, et al. The glucosaminidase domain of Atl — the major Staphylococcus aureus autolysin — has DNA-binding activity. MicrobiologyOpen. 2014;3:247–56. doi: 10.1002/mbo3.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Groicher KH, Firek BA, Fujimoto DF, et al. The Staphylococcus aureus lrgAB operon modulates murein hydrolase activity and penicillin tolerance. J Bacteriol. 2000;182:1794–801. doi: 10.1128/jb.182.7.1794-1801.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross M, Cramton SE, Gotz F, et al. Key role of teichoic acid net charge in Staphylococcus aureus colonization of artificial surfaces. Infect Immun. 2001;69:3423–6. doi: 10.1128/IAI.69.5.3423-3426.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haque NZ, Davis SL, Manierski CL, et al. Infective endocarditis caused by USA300 methicillin-resistant Staphylococcus aureus (MRSA) Int J Antimicrob Ag. 2007;30:72–7. doi: 10.1016/j.ijantimicag.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Heilmann C, Gerke C, Perdreau-Remington F, et al. Characterization of Tn917 insertion mutants of Staphylococcus epidermidis affected in biofilm formation. Infect Immun. 1996;64:277–82. doi: 10.1128/iai.64.1.277-282.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herold BC, Immergluck LC, Maranan MC, et al. Community-acquired methicillin-resistant Staphylococcus aureus in children with no identified predisposing risk. Jama. 1998;279:593–8. doi: 10.1001/jama.279.8.593. [DOI] [PubMed] [Google Scholar]
- Heydorn A, Nielsen AT, Hentzer M, et al. Quantification of biofilm structures by the novel computer program COMSTAT. Microbiology. 2000;146:2395–407. doi: 10.1099/00221287-146-10-2395. [DOI] [PubMed] [Google Scholar]
- Horsburgh MJ, Aish JL, White IJ, et al. sigmaB modulates virulence determinant expression and stress resistance: characterization of a functional rsbU strain derived from Staphylococcus aureus 8325–4. J Bacteriol. 2002;184:5457–67. doi: 10.1128/JB.184.19.5457-5467.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston P, Rowe SE, Pozzi C, et al. Essential role for the major autolysin in the fibronectin-binding protein-mediated Staphylococcus aureus biofilm phenotype. Infect Immun. 2011;79:1153–65. doi: 10.1128/IAI.00364-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiedrowski MR, Kavanaugh JS, Malone CL, et al. Nuclease modulates biofilm formation in community-associated methicillin-resistant Staphylococcus aureus. PLoS One. 2011;6:e26714. doi: 10.1371/journal.pone.0026714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kullik I, Giachino P, Fuchs T. Deletion of the alternative sigma factor sigmaB in Staphylococcus aureus reveals its function as a global regulator of virulence genes. J Bacteriol. 1998;180:4814–20. doi: 10.1128/jb.180.18.4814-4820.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauderdale KJ, Boles BR, Cheung AL, et al. Interconnections between Sigma B, agr, and proteolytic activity in Staphylococcus aureus biofilm maturation. Infect Immun. 2009;77:1623–35. doi: 10.1128/IAI.01036-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ledala N, Wilkinson BJ, Jayaswal RK. Effects of oxacillin and tetracycline on autolysis, autolysin processing and atl transcription in Staphylococcus aureus. Int J Antimicrob Ag. 2006;27:518–24. doi: 10.1016/j.ijantimicag.2006.03.008. [DOI] [PubMed] [Google Scholar]
- Lowy FD. Staphylococcus aureus infections. New Engl J Med. 1998;339:520–32. doi: 10.1056/NEJM199808203390806. [DOI] [PubMed] [Google Scholar]
- Mani N, Tobin P, Jayaswal RK. Isolation and characterization of autolysis-defective mutants of Staphylococcus aureus created by Tn917-lacZ mutagenesis. J Bacteriol. 1993;175:1493–9. doi: 10.1128/jb.175.5.1493-1499.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mann EE, Rice KC, Boles BR, et al. Modulation of eDNA release and degradation affects Staphylococcus aureus biofilm maturation. PLoS One. 2009;4:e5822. doi: 10.1371/journal.pone.0005822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino N, Toledo-Arana A, Vergara-Irigaray M, et al. Protein A-mediated multicellular behavior in Staphylococcus aureus. J Bacteriol. 2009;191:832–43. doi: 10.1128/JB.01222-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mootz JM, Malone CL, Shaw LN, et al. Staphopains modulate Staphylococcus aureus biofilm integrity. Infect Immun. 2013;81:3227–38. doi: 10.1128/IAI.00377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oshida T, Sugai M, Komatsuzawa H, et al. A Staphylococcus aureus autolysin that has an N-acetylmuramoyl-L-alanine amidase domain and an endo-beta-N-acetylglucosaminidase domain: cloning, sequence analysis, and characterization. P Natl Acad Sci USA. 1995;92:285–9. doi: 10.1073/pnas.92.1.285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otto M. Staphylococcal infections: mechanisms of biofilm maturation and detachment as critical determinants of pathogenicity. Annu Rev Med. 2013;64:175–88. doi: 10.1146/annurev-med-042711-140023. [DOI] [PubMed] [Google Scholar]
- Perkins HR. The bacterial autolysins. In: Rogers HJ, Perkins HJ, Ward JB, editors. Microbial Cell Walls. London, UK: Chapman and Hall; 1980. pp. 437–56. [Google Scholar]
- Qamar A, Golemi-Kotra D. Dual roles of FmtA in Staphylococcus aureus cell wall biosynthesis and autolysis. Antimicrob Agents Ch. 2012;56:3797–805. doi: 10.1128/AAC.00187-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Z, Ou Y, Yang L, et al. Role of autolysin-mediated DNA release in biofilm formation of Staphylococcus epidermidis. Microbiology. 2007;153:2083–92. doi: 10.1099/mic.0.2007/006031-0. [DOI] [PubMed] [Google Scholar]
- Rice K, Peralta R, Bast D, et al. Description of staphylococcus serine protease (ssp) operon in Staphylococcus aureus and nonpolar inactivation of sspA-encoded serine protease. Infect Immun. 2001;69:159–69. doi: 10.1128/IAI.69.1.159-169.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KC, Firek BA, Nelson JB, et al. The Staphylococcus aureus cidAB operon: evaluation of its role in regulation of murein hydrolase activity and penicillin tolerance. J Bacteriol. 2003;185:2635–43. doi: 10.1128/JB.185.8.2635-2643.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rice KC, Mann EE, Endres JL, et al. The cidA murein hydrolase regulator contributes to DNA release and biofilm development in Staphylococcus aureus. P Natl Acad Sci USA. 2007;104:8113–8. doi: 10.1073/pnas.0610226104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sahukhal GS, Elasri MO. Identification and characterization of an operon, msaABCR, that controls virulence and biofilm development in Staphylococcus aureus. BMC Microbiol. 2014;14:154. doi: 10.1186/1471-2180-14-154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambanthamoorthy K, Schwartz A, Nagarajan V, et al. The Role of msa in Staphylococcus aureus Biofilm Formation. BMC Microbiol. 2008;8:221. doi: 10.1186/1471-2180-8-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sambanthamoorthy K, Smeltzer MS, Elasri MO. Identification and characterization of msa (SA1233), a gene involved in expression of SarA and several virulence factors in Staphylococcus aureus. Microbiology. 2006;152:2559–72. doi: 10.1099/mic.0.29071-0. [DOI] [PubMed] [Google Scholar]
- Thomas VC, Thurlow LR, Boyle D, et al. Regulation of autolysis-dependent extracellular DNA release by Enterococcus faecalis extracellular proteases influences biofilm development. J Bacteriol. 2008;190:5690–8. doi: 10.1128/JB.00314-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toledo-Arana A, Merino N, Vergara-Irigaray M, et al. Staphylococcus aureus develops an alternative, ica-independent biofilm in the absence of the arlRS two-component system. J Bacteriol. 2005;187:5318–29. doi: 10.1128/JB.187.15.5318-5329.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsang LH, Cassat JE, Shaw LN, et al. Factors contributing to the biofilm-deficient phenotype of Staphylococcus aureus sarA mutants. PLoS One. 2008;3:e3361. doi: 10.1371/journal.pone.0003361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wadstrom T, Hisatsune K. Bacteriolytic enzymes from Staphylococcus aureus. Specificity of ction of endo-beta-N-acetylglucosaminidase. Biochem J. 1970;120:735–44. doi: 10.1042/bj1200735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Mani N, Pattee PA, et al. Analysis of a peptidoglycan hydrolase gene from Staphylococcus aureus NCTC 8325. J Bacteriol. 1992;174:6303–6. doi: 10.1128/jb.174.19.6303-6306.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wen ZT, Baker HV, Burne RA. Influence of BrpA on critical virulence attributes of Streptococcus mutans. J Bacteriol. 2006;188:2983–92. doi: 10.1128/JB.188.8.2983-2992.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitchurch CB, Tolker-Nielsen T, Ragas PC, et al. Extracellular DNA required for bacterial biofilm formation. Science. 2002;295:1487. doi: 10.1126/science.295.5559.1487. [DOI] [PubMed] [Google Scholar]
- Zielinska AK, Beenken KE, Mrak LN, et al. sarA-mediated repression of protease production plays a key role in the pathogenesis of Staphylococcus aureus USA300 isolates. Mol Microbiol. 2012;86:1183–96. doi: 10.1111/mmi.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.