Abstract
Previous research demonstrates that prenatal testosterone exposure increases aggression, possibly through its effects on the structure and function of neural circuits supporting threat detection and emotion regulation. Here we examined associations between regional gray matter volume, trait aggression, and the ratio of the second and fourth digit of the hand (2D:4D ratio) as a putative index of prenatal testosterone exposure in 464 healthy young adult volunteers. Our analyses revealed a significant positive correlation between 2D:4D ratio and gray matter volume of the dorsal anterior cingulate cortex (dACC), a brain region supporting, emotion regulation, conflict monitoring, and behavioral inhibition. Subsequent analyses demonstrated that reduced (i.e., masculinized) gray matter volume in the dACC mediated the relationship between 2D:4D ratio and aggression in women, but not men. Expanding on this gender-specific mediation, additional analyses demonstrated that the shared variance between 2D:4D ratio, dACC gray matter volume, and aggression in women reflected the tendency to engage in cognitive reappraisal of emotionally provocative stimuli. Our results provide novel evidence that 2D:4D ratio is associated with masculinization of dACC gray matter volume, and that this neural phenotype mediates, in part, the expression of trait aggression in women.
1. INTRODUCTION
Exposure to testosterone during critical periods of development can shape aggression in response to perceived social challenge by modulating the structure and function of neural circuits supporting threat detection and emotion regulation (Archer, 2006; Montoya et al., 2012). Testosterone can modulate neuronal structure and function either directly by binding to the androgen receptor thereby leading to its translocation to the nucleus where it functions as a transcription factor or indirectly via aromatization of testosterone to estradiol, and subsequent binding to the estrogen receptor, which also functions as a transcription factor (Arnold and Breedlove, 1985). Animal models have demonstrated that testosterone exposure early in development results in enhanced aggression in adulthood and it has previously been suggested that these behavioral changes emerge through masculinization of sexually dimorphic brain regions involved in aggression (Mann and Svare, 1983; Morris et al., 2004; Saal, 1983).
Although well documented in animal models, attempts to directly assess how prenatal testosterone exposure shapes aggression in humans are impeded by practical considerations. Because it is very difficult to assess variation in the prenatal hormonal milieu, researchers have relied on ‘markers’ of prenatal androgen exposure. Manning and colleagues (Manning et al., 1998) found that the ratio between the second digit and fourth digit of the hand (2D:4D ratio) is sexually dimorphic (higher in women), and subsequent work indicated that this dimorphism emerges by the end of the first trimester of gestation (Malas et al., 2006). Several studies now provide indirect support for the utility of 2D:4D ratio as an index of prenatal androgen concentrations.
First, men and women with congenital adrenal hyperplasia (CAH), a condition leading to hyper-secretion of testosterone, have decreased 2D:4D ratios (Brown et al., 2002; Buck et al., 2003; Ökten et al., 2002; Rivas et al., 2014). Second, men with Klinefelter’s syndrome, a chromosomal abnormality associated with low androgen levels, have higher (i.e., feminized) 2D:4D ratio relative to healthy men (Manning et al., 2013). Third, there is some evidence indicating that 2D:4D ratio is masculinized in women with a male twin relative to women with a female twin, suggesting that hormone transfer from the male co-twin is masculinizing (Hiraishi et al., 2012; van Anders et al., 2006). Additionally, some studies indicate that testosterone concentrations from amniotic fluid is negatively correlated with 2D:4D ratio (Lutchmaya et al., 2004; Ventura et al., 2013).
Perhaps the most compelling evidence for the validity of 2D:4D ratio as a marker of prenatal androgen exposure comes from work in rodents demonstrating that prenatal manipulations of testosterone as well as steroid hormone receptors modulate the development of 2D:4D ratio (Auger et al., 2013; Zheng and Cohn, 2011). Specifically, administration of anti-androgenic compounds results in feminized digit ratios (Auger et al., 2013). Additionally, inactivation of the androgen receptor during prenatal development decreases growth of the 4th digit, leading to a higher (i.e., feminized) 2D:4D ratio, whereas inactivation of the estrogen receptor α increases growth of the 4th digit, leading to a lower (i.e., masculinized) 2D:4D ratio. Similarly, testosterone administration during the prenatal period leads to a lower 2D:4D ratio, while estrogen administration leads to a higher 2D:4D ratio (Zheng and Cohn, 2011). For the purpose of the analyses conducted here we conceptualize 2D:4D ratio as a marker of prenatal androgen exposure. However, it should be noted that 2D:4D ratio can be influenced by multiple factors, including exposure to estradiol (Zheng and Cohn, 2011).
Despite data suggesting that prenatal androgen exposure is associated with 2D:4D ratio, previously reported associations between 2D:4D ratio and sexually dimorphic behaviors in humans have been inconclusive. While individual studies have reported significant negative correlations between 2D:4D ratio and various measures of human aggression in men and women (Bailey and Hurd, 2005; Benderlioglu and Nelson, 2004; Kuepper and Hennig, 2007), a recent meta-analysis observed only a weak negative correlation between such indices of aggression and 2D:4D ratio in men (rs = −.08 to −.11; (Hönekopp and Watson, 2011)). In women, however, left and right 2D:4D ratios were not significantly correlated with aggression (rs = .09 and −.05, respectively; (Hönekopp and Watson, 2011)).
Previous attempts to link underlying differences in biology and emergent behavioral processes have benefited from the use of intermediate neural phenotypes, which can improve statistical power through the modeling of indirect effects while implicating plausible biological mechanisms (Hyde et al., 2011). Building on this prior research, the primary aim of this study was to determine if gray matter volume within circuits supporting threat detection and response regulation mediate relationships between 2D:4D ratio and self-reported trait aggression. Previous research in animals has consistently implicated the hypothalamus, amygdala, hippocampus, and regions of the frontal lobe in generating and regulating aggressive responses (Nelson and Trainor, 2007). Research in human participants has further demonstrated that trait aggression is associated with reduced gray matter in the orbitofrontal cortex (OFC) and anterior cingulate cortex (ACC) (Boes et al., 2008; Emerton et al., 2009). Additionally, the hypothalamus, amygdala, hippocampus, and ACC express aromatase, which is necessary for the conversion of androgens to estradiol and subsequent neural masculinization (Lephart, 1996; MacLusky et al., 1994; McCarthy and Arnold, 2011). Based on this literature, we tested for associations between 2D:4D ratio and gray matter volume within the hypothalamus, amygdala, hippocampus, orbitofrontal cortex, and ACC explored if any resulting associations mediate effects of 2D:4D ratio on self-reported aggression.
Additionally, as this is the first study to assess gray matter correlates of 2D:4D ratio in a relatively large sample, we further tested for relationships between 2D:4D ratio and neural morphology broadly, by conducting exploratory whole-brain analyses. Previous research has reported associations between prenatal testosterone assessed during the second trimester and regional gray matter during adulthood (Lombardo et al., 2012). Specifically, prenatal testosterone exhibited positive correlations with gray matter within the pre- and post-central gyri, temporal parietal junction, and amygdala, and negative correlations with the subgenual ACC, posterior lateral OFC, anterior insula, and middle and superior temporal gyri (Lombardo et al., 2012). Thus, our whole-brain exploratory analyses allow for comparisons between structural correlates of directly measured fetal testosterone exposure and the indirect proxy measure of 2D:4D ratio. Lastly, previous research has noted that right hand 2D:4D ratio may be more sensitive to prenatal sex steroids (Manning et al., 2014), and as such may represent a better candidate for predicting gray matter volume than left hand 2D:4D ratio. We included analyses testing associations between gray matter volume and 2D:4D ratio from both hands to test for such selectivity in our measures and sample.
2. MATERIAL AND METHODS
2.1 Participants
Data for our analyses of interest were derived from 1000 participants who had successfully completed the ongoing Duke Neurogenetics Study (DNS) as of July 10, 2014. The DNS assesses a wide range of behavioral and biological phenotypes as well as genotypes among non-patient, 18–22 year old university students. All participants provided informed consent in accordance with Duke University guidelines prior to participation. The participants were in good general health and free of the following study exclusions: (1) medical diagnoses of cancer, stroke, head injury with loss of consciousness, untreated migraine headaches, diabetes requiring insulin treatment, chronic kidney or liver disease, or lifetime history of psychotic symptoms; (2) use of psychotropic, glucocorticoid, or hypolipidemic medication; and (3) conditions affecting cerebral blood flow and metabolism (e.g. hypertension). As the DNS seeks to establish broad variability in multiple behavioral phenotypes related to psychopathology, we did not exclude participants based on a diagnosis of any past or current DSM-IV Axis I or select Axis II disorders as identified through clinical interviews using the electronic MINI (Sheehan et al., 1998). However, as stated earlier, no subjects were taking psychotropic medication at the time of or at least 10 days prior to study participation. Measurement of 2D:4D ratio was added approximately midway during the DNS, thus our analyses were limited to 464 participants (253 females, mean age=19.63 +/− 1.23 SD) with overlapping 2D:4D ratio, self-reported aggression, and high resolution structural MRI data.
2.2 Anthropometric & Self-Report Measures
2.2.1 Aggression
The Buss-Perry Aggression Questionnaire (BPAQ) was used to assess variation in aggressive behavior (Buss and Perry, 1992). Research suggests that the BPAQ is internally consistent, and exhibits high test-retest reliability. We utilized total scores which reflect the sum of all subscales. Self-reported aggression was highly consistent across individuals (Cronbach’s α = .93).
2.2.2 Digit Ratio Measurement
Participants placed their hands, palm side down, onto the bed of a Canon CanoScan LiDE 110 color image scanner. 2D:4D was measured using NIH ImageJ software (http://imagej.nih.gov/ij/) by determining the length of the second (2D) and fourth (4D) digits of the hands, from the basal crease (where the finger meets the hand) to the fingertip. Two independent raters measured all of the digits, and achieved excellent inter-rater reliability (Pearson rs: right hand = .93; left hand = .93). Intra-class correlations for 2D:4D ratios were high (left 2D:4D ratio = .915, p < .001, right 2D:4D ratio = .933, p < .001).
2.2.3 Typical Use of Cognitive Reappraisal
The Cognitive Reappraisal subscale of the Emotional Reappraisal Questionnaire (Gross and John, 2003) was used in exploratory analyses of a possible psychological mechanism supporting the observed shared variance between 2D:4D ratio, gray matter volume, and aggression. The summed score for the six reappraisal items (e.g., “When I want to feel more positive emotion, I change the way I’m thinking about the situation”) was used specifically, because unlike suppression, which is also measured by the Emotional Reappraisal Questionnaire, cognitive reappraisal has been associated with both the function and structure of the dACC (Giuliani et al., 2011; Ochsner and Gross, 2005).
2.4 Assessment of Regional Gray Matter Volume
2.4.1 Structural MRI Data Acquisition
Each participant was scanned using a research-dedicated GE MR750 3T scanner equipped with high-power high-duty-cycle 50-mT/m gradients at 200 T/m/s slew rate, and an eight-channel head coil for parallel imaging at high bandwidth up to 1MHz at the Duke-UNC Brain Imaging and Analysis Center. For optimized voxel-based morphometry (VBM), high-resolution T1-weighted images were obtained after functional imaging (3D Ax FSPGR BRAVO sequence: TR, 8.148 s; TE, 3.22 ms; 162 sagittal slices; flip angle, 12°; FOV, 240 mm; matrix 256 × 256; slice thickness, 1 mm with no gap).
2.4.2 Voxel-based Morphometry
Regional gray matter volumes from T1-weighted FSPGR images were determined using the VBM8 toolbox (version 369 http://dbm.neuro.uni-jena.de/vbm/) within SPM8. Individual FSPGR images were segmented into gray, white, and CSF images using an adaptive Maximum a Posterior technique, Partial Volume Estimation, and an optimized block-wise non-local means denoising filter and classical Markov Random Field approach. Resulting gray matter images were normalized to a canonical gray matter template using affine transformations. Subsequently, gray matter voxel values were multiplied by the Jacobian matrix parameters derived from normalization so that between subject variability at the voxel level accounted for individual differences in total gray matter volume. Normalized gray matter images were then smoothed with a 12 mm FWHM kernel.
2.4.3 Regions of Interest and Gray Matter Extraction
Primary analyses were limited to the following a priori regions of interest (ROIs): bilateral amygdala, hippocampus, and ACC, which were selected from the Automatic Anatomical Labeling (AAL) toolbox of SPM8, and bilateral hypothalamus and OFC (Brodmann Area 11), which were selected from the Brodmann Area toolbox of SPM8. In order to assess associations between 2D:4D ratio and gray matter volume within these ROIs, single-subject gray matter images were entered into a second-level multiple regression analysis within SPM8. To ensure that results were unique to 2D:4D ratio, we controlled for age and gender by entering these variables as regressors of no interest into the multiple regression model. The analysis was thresholded at p < 0.05, FWE corrected across the total search volume (17,442 voxels; 58,866.75 mm3) of all a priori regions of interest. Secondary exploratory analyses were conducted across the entire volume of the brain, in line with previous research (Lombardo et al., 2012) thresholded at p < 0.05, FWE across the total search volume (515,158 voxels; 1,738,658.25 mm3).
2.5 Statistical Analyses
Extracted gray matter volumes from clusters within our a priori ROIs exhibiting significant associations with 2D:4D ratio were used in a subsequent regression analyses with self-reported aggression using SPSS v21. Extracted gray matter volumes from ROI analyses were subsequently entered into mediation and moderation analyses conducted using the PROCESS macro (Hayes, 2012). Statistical significance for all indirect effects was determined using bootstrapped 95% confidence intervals consistent with published guidelines (Efron, 1987; Preacher et al., 2007). Though parameter estimates for indirect effects are not the results of bootstrapping, we do report them to give a general measure of effect size. BPAQ total scores were winsorized to reduce the influence of extreme values.
3. RESULTS
3.1 Gender Differences
Consistent with prior reports, gender was associated with 2D:4D ratio and self-reported aggression. Gender predicted 2D:4D ratio (right hand: T=4.36, p < 0.001; left hand: T=4.51, p < 0.001) such that men (right hand: M=.9594 +/− .029 SD; left hand: M=.9503 +/− .033 SD) had lower ratios than women (right hand: M=.9722 +/− .034 SD; left hand: M=.9650 +/− .034 SD). Gender predicted BPAQ total scores (T=3.60, p < 0.001) such that men (M=63.27 +/− 17.32 SD) had higher self-reported trait aggression than women (M=57.36 +/− .18.04 SD).
3.2 Voxel-based Morphometry
3.2.1 Region of Interest Analyses
Neither left nor right hand 2D:4D ratio were significantly correlated with individual differences in BPAQ Total Scores after controlling for gender and age. There also was no signification interaction between 2D:4D ratios and gender predicting BPAQ Total Scores (all p’s > 0.1).
Right hand 2D:4D ratio was positively correlated with gray matter volume within a dorsal ACC (dACC) cluster (x=−8, y=39, z=22; T=4.01; p<.05 FWE; 97 voxels) after controlling for gender and age (Figure 1). No significant correlations were observed between right hand 2D:4D ratio and any other ROIs even when using a 4 mm FWHM kernel to better assess gray matter volumes of our subcortical regions of interest. There were no significant correlations between left hand 2D:4D ratio and gray matter volume within any ROI at corrected thresholds. No significant interaction between 2D:4D ratios and gender was observed within any ROI at corrected thresholds.
Figure 1.
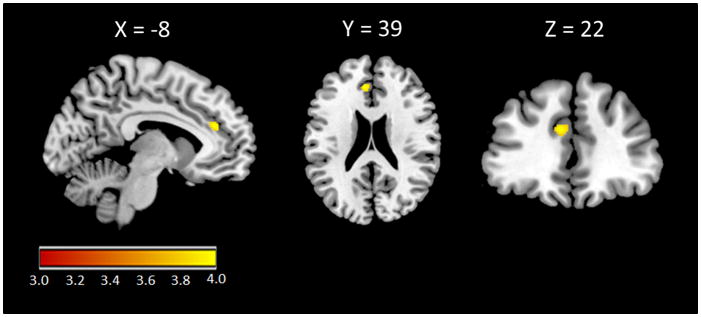
Right hand 2D:4D ratio and gray matter volumes in our regions of interest. Statistical parametric map illustrating the cluster within the dorsal anterior cingulate (dACC) exhibiting a significant positive association with 2D:4D ratio when controlling for age and gender. (MNI coordinates: x=−8, y=39, z=22; T=4.01; p<.05 FWE; 97 voxels).
3.2.2 Whole Brain Analyses
Neither left nor right hand 2D:4D ratio predicted gray matter volume when correcting for multiple comparisons across the entire brain. Further, we observed no signification interaction between 2D:4D ratios and gender predicting gray matter volume at whole brain thresholds.
3.3 Gender Moderated Mediation
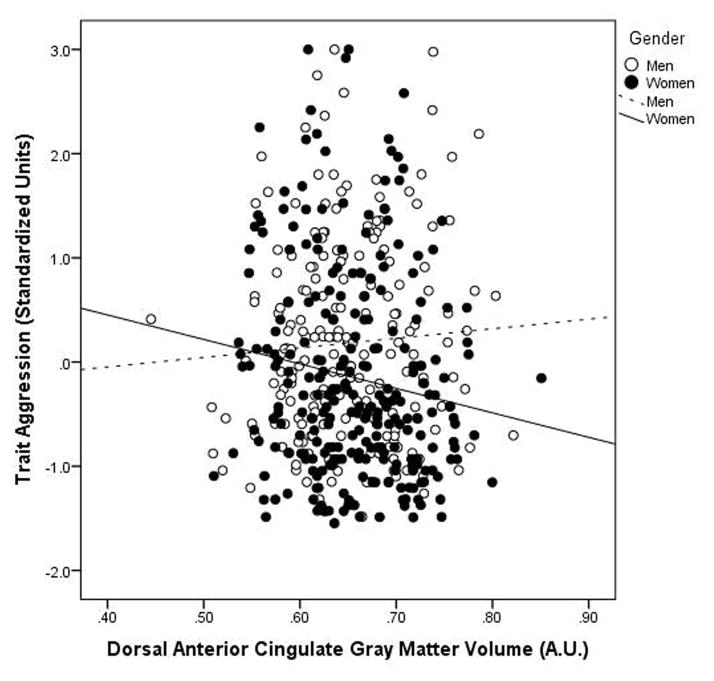
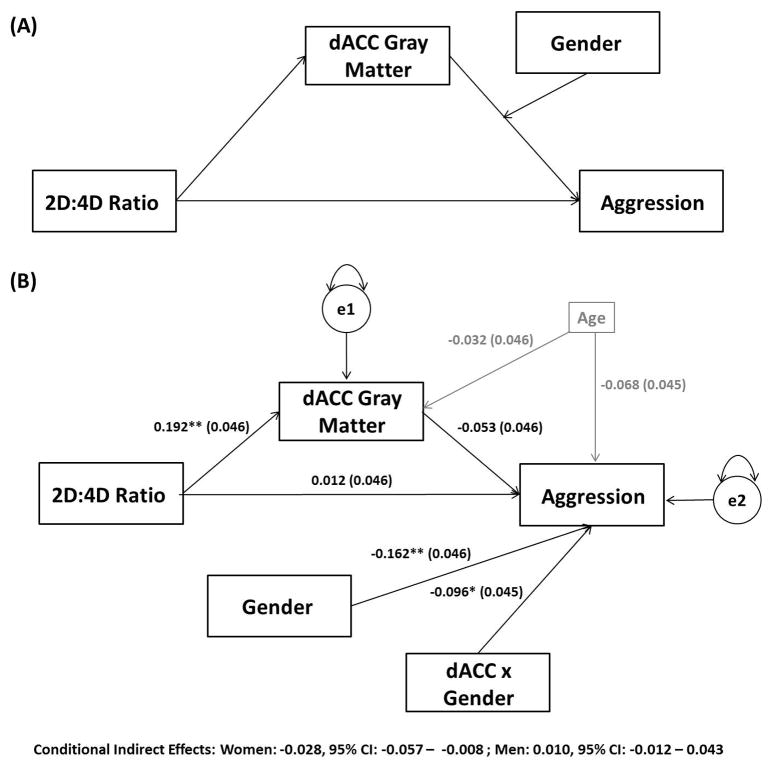
Individual differences in the gray matter volume within the dACC cluster identified within our region of interest analyses (Figure 1) significantly interacted with gender to predict BPAQ Total Scores (interaction term: β=−.096, p = .032) (Figure 2). Specifically, there was a significant negative correlation between dACC gray matter volume and BPAQ Total Scores in women (β=−.139, p = .024) but not men (β=.054, p =.411). Moderated mediation analyses (Figure 3) revealed that 2D:4D ratio from the right hand was indirectly associated with BPAQ Total scores via individual differences in dACC gray matter volume in women (β=−.028, lower limit confidence interval (LLCI)= −.057, upper limit confidence interval (ULCI)=−.008) but not men (β=.010, LLCI= −.012, ULCI=.043).
Figure 2.
Trait aggression as a function of dorsal anterior cingulate cortex gray matter volume identified in our ROI analyses (see Results 3.2.1, Figure 1) and gender. Trait aggression plotted separately for men and women against gray matter volume from the dACC cluster exhibiting a significant positive correlation with 2D:4D ratio. Scatterplots reflect partial correlations between gray matter volumes and BPAQ trait aggression after controlling for age. BPAQ scores reflect standardized residuals. Simple slopes: (women: β=−.139, p = .024; men: β=.054, p = .411).
Figure 3.
Results from a path analysis testing the indirect effects of 2D:4D ratio on trait aggression through variability in dorsal anterior cingulate cortex gray matter identified in our ROI analyses (see Results 3.2.1, Figure 1) as a function of gender. Values represent standardized parameter estimates with standard errors presented in parentheses after controlling for age. Gray matter reductions in the dACC mediate the indirect effect of 2D:4D ratio on trait aggression in women, but not men. The circles labeled e1 and e2 denote the variance in anterior cingulate gray matter volume and trait aggression scores unaccounted for by the model. *p<0.05, **p<0.005
3.4 Typical Use of Cognitive Reappraisal and Multi-step Mediation
Based on our findings that dACC gray matter volume specifically mediated the relationship between 2D:4D ratio and BPAQ Total Scores in women, we conducted an exploratory analysis probing possible links with emotion regulation, namely cognitive reappraisal, which is associated with increased dACC activity (Ochsner and Gross, 2005). Moreover, individual differences in a person’s tendency to engage in cognitive reappraisal as measured by the ERQ is associated with increased dACC gray matter volume in women (Giuliani et al., 2011; Gross and John, 2003).
These exploratory analyses revealed that women reported more frequent use of cognitive reappraisal than men (T=2.48, p = 0.014). Further, typical use of cognitive reappraisal interacted with gender to predict BPAQ total scores (β=−.111, p = .013) such that ERQ reappraisal scores were negatively associated with BPAQ total scores in women (β=−.208, p < .001) but not men (β=.015, p = .821). Based on these results, and the moderating effect of gender in the above mediation analyses, we restricted the analyses testing whether typical use of cognitive reappraisal further mediates the indirect effect of 2D:4D ratio on BPAQ total scores via reductions in dACC gray matter to women. Multi-step mediation (Figure 4) revealed a significant indirect effect (β=−.003, LLCI= −.0115, ULCI=−.0004), suggesting that the typical use of reappraisal explained a significant proportion of the shared variance between 2D:4D ratio, dACC gray matter volume, and trait aggression.
Figure 4.
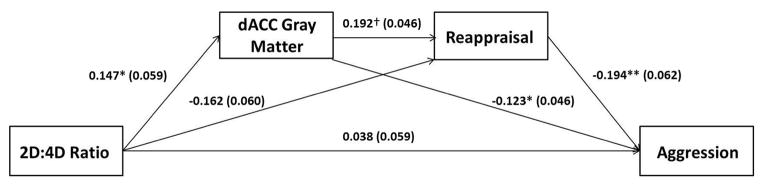
Multi-step mediation model demonstrating that dorsal anterior cingulate cortex gray matter volume identified in our ROI analyses (see Results 3.2.1, Figure 1) and typical use of cognitive reappraisal mediate the association between 2D:4D ratio and trait aggression in women. Values represent standardized parameter estimates with standard errors presented in parentheses after controlling for age. † p=.056, *p<0.05, **p<0.005
4. DISCUSSION
Our results demonstrate an association between 2D:4D ratio and gray matter volume within neural circuits that are shaped by prenatal testosterone and support threat detection and emotion regulation in trait aggression. Specifically, lower right hand 2D:4D ratio, which in part represents an index of prenatal testosterone exposure, is associated with lower gray matter volume within the dACC, which is characteristic of masculinized neural development (Chen et al., 2007; Good et al., 2001; Lombardo et al., 2012; Ruigrok et al., 2014). Additionally, our results demonstrate that reduced dACC gray matter mediates the relationship between 2D:4D ratio and increased trait aggression in women but not men. The dACC is involved with the process of cognitive reappraisal, and previous reports suggest that dACC gray matter volume is associated with a tendency to reinterpret the meaning of emotionally provocative stimuli in women (Giuliani et al., 2011; Ochsner and Gross, 2005). Our multi-step mediation model demonstrates that the indirect effect of 2D:4D ratio on trait aggression via reductions in dACC gray matter in women are further mediated by variability in tendency toward reappraisal. In addition to providing evidence that 2D:4D ratio is associated with masculinization of brain structure our results suggest specific neural and psychological mechanisms through which prenatal testosterone exposure may be expressed as individual differences in trait aggression in women.
The results reported here underscore the value of intermediate neural phenotypes in facilitating the search for likely small effects of lower 2D:4D ratio on increased self-reported aggression (Hönekopp and Watson, 2011) by indirectly modeling shared variance between underlying biology and distal emergent processes (Hyde et al., 2011). Additionally, the employment of intermediate neural phenotypes allows for the identification of plausible psychological mechanisms linking prenatal testosterone exposure and trait aggression thereby suggesting avenues for future research. The effect of 2D:4D ratio on dACC gray matter could potentially impact multiple processes. Our findings that dACC gray matter volume and cognitive reappraisal mediate the relationship between 2D:4D ratio and self-reported aggression suggest that prenatal testosterone exposure shapes trait aggression in women by affecting the capacity to regulate aggressive responses. Consistent with this interpretation, previous research has demonstrated that gray matter volume within the dACC is negatively correlated with self-reported aggression, but positively correlated with the likelihood to engage in explicit emotion regulation (Boes et al., 2008; Giuliani et al., 2011).
Alternatively, the dACC has been implicated in social and moral decision making (Forbes and Grafman, 2010; Greene et al., 2001). Previous research has demonstrated that testosterone administration results in impaired cognitive empathy and more utilitarian moral decision making, effects which are predicted by 2D:4D ratio (Montoya et al., 2013; Van Honk et al., 2011). Future research should explore the role of 2D:4D ratio and dACC gray matter in emotion regulation, response inhibition, and social and moral decision making to more broadly assess which processes may be influenced by prenatal testosterone exposure.
In addition to informing future research strategies, the observation that the indirect effect of 2D:4D ratio on trait aggression via reductions in dACC gray matter is further mediated by variability in cognitive reappraisal may help to explain the gender differences observed in our results. Previous studies have identified associations between 2D:4D ratio and aggression in men, but not women (Hönekopp and Watson, 2011). It is important to note that no direct effect between 2D:4D ratio and trait aggression is observed in our sample, and the indirect effect of 2D:4D ratio on trait aggression is mediated by dACC volume and cognitive reappraisal. Previous reports demonstrate that women have greater dACC gray matter volume than men (Chen et al., 2007; Good et al., 2001; Ruigrok et al., 2014), and our data demonstrates that women report engaging in cognitive reappraisal with greater frequency than men. Further, we only observed a negative association between typical use of reappraisal and trait aggression in women. The finding that cognitive reappraisal further mediates the effect of dACC gray matter on trait aggression, and is only associated with self-reported aggression in women, may explain why the indirect effects of 2D:4D ratio were not significant in men. Modeling indirect effects of 2D:4D on aggression through other neural phenotypes and processes may yield different results.
It is interesting to note that within a priori regions of interest we observed an association between gray matter volume and right but not left hand 2D:4D ratio. Prior work suggests that right hand 2D:4D ratio may be a more sensitive metric of prenatal exposure to sex steroids than left hand 2D:4D (Manning et al., 2014). Research in animal models demonstrates that the sexual dimorphism of the right paw 2D:4D ratio emerges earlier than that of the left paw (Zheng and Cohn, 2011), and research in humans has reported stronger associations between right hand 2D:4D ratio and variables of interest such as sperm numbers and concentrations of testosterone, estrogen, and prolactin (Manning et al., 1998). Our results are broadly in line with these observations; however, future research will be required to determine if gray matter volume is consistently more sensitive to the 2D:4D ratio of the right hand.
Our whole-brain exploratory analyses 2D:4D ratio failed to identify gray matter correlates of 2D:4D ratio and it is unclear why we did not observe any associations between 2D:4D ratio and gray matter variability within other hypothesized regions including the amygdala, hippocampus, hypothalamus, and OFC. Lombardo et al. (2012) report that prenatal testosterone during the second trimester is positively associated with reduced gray matter within the subgenual ACC and posterior lateral OFC, areas which did not exhibit significant associations with 2D:4D ratio in our analyses. Prenatal testosterone is additionally associated with other gray matter variation not observed here (Lombardo et al., 2012). Animal models have demonstrated that 2D:4D ratio can be influenced by the actions of the androgen or the estrogen receptor (Zheng and Cohn, 2011), which may explain discrepancies between our results and analyses that solely assess prenatal testosterone exposure. Future research will be required to replicate our results, and determine which mechanisms (androgen vs estrogen receptor signaling) contribute to these relationships.
There are, of course, limitations to our current study. First, it is difficult to fully reconcile findings in animal models to the analyses reported here. Animal models have demonstrated the prenatal testosterone can affect dendritic arborization and the number of neurons within sexually dimorphic brain regions (Cooke and Woolley, 2005; Morris et al., 2004). Assessment of gray matter morphometry with high resolution structural MRI cannot determine if volumetric reductions reflect reduced dendrites, neuronal count, or some other property of neural architecture. Second, while the Buss-Perry Aggression Questionnaire is a well-validated measure and frequently used in psychological research, it nonetheless retains the limitations inherent to self-report. Further, individual differences in aggressive behavior are likely influenced by initial reactions to perceived social challenge, as well as the ability to regulate those reactions. Our dependent measure cannot differentiate between these two sources of variance, and it is likely that identifying the structural correlates of aggressive behavior would benefit from more refined metrics.
HIGHLIGHTS.
We tested links between brain structure, aggression and prenatal androgen exposure
Prenatal androgen exposure was indirectly assessed using the hand 2D:4D ratio
Lower 2D:4D ratio (i.e., masculinized) predicts decreased dACC gray matter
Decreased dACC gray matter mediates association between 2D:4D ratio and aggression
This pathway is only present in women and reflects typical use of reappraisal
Footnotes
AUTHORS’ CONTRIBUTIONS
AXG performed statistical analyses, participated in study design and data collection, and helped draft the manuscript. REM participated in data collection. SRR participated in data collection. JMC helped draft the manuscript, participated in study design, and aided with statistical analyses. ARH conceived of the study, participated in study design and helped draft the manuscript. All authors read and approved the final manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Archer J. Testosterone and human aggression: an evaluation of the challenge hypothesis. Neurosci Biobehav Rev, Relationship between the Brain and Aggression. 2006;30:319–345. doi: 10.1016/j.neubiorev.2004.12.007. [DOI] [PubMed] [Google Scholar]
- Arnold AP, Breedlove SM. Organizational and activational effects of sex steroids on brain and behavior: A reanalysis. Horm Behav. 1985;19:469–498. doi: 10.1016/0018-506X(85)90042-X. [DOI] [PubMed] [Google Scholar]
- Auger J, Le Denmat D, Berges R, Doridot L, Salmon B, Canivenc-Lavier MC, Eustache F. Environmental levels of oestrogenic and antiandrogenic compounds feminize digit ratios in male rats and their unexposed male progeny. Proc R Soc B Biol Sci. 2013;280:20131532. doi: 10.1098/rspb.2013.1532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey AA, Hurd PL. Finger length ratio (2D:4D) correlates with physical aggression in men but not in women. Biol Psychol. 2005;68:215–222. doi: 10.1016/j.biopsycho.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Benderlioglu Z, Nelson RJ. Digit length ratios predict reactive aggression in women, but not in men. Horm Behav. 2004;46:558–564. doi: 10.1016/j.yhbeh.2004.06.004. [DOI] [PubMed] [Google Scholar]
- Boes AD, Tranel D, Anderson SW, Nopoulos P. Right anterior cingulate: A neuroanatomical correlate of aggression and defiance in boys. Behav Neurosci. 2008;122:677–684. doi: 10.1037/0735-7044.122.3.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown WM, Hines M, Fane BA, Breedlove SM. Masculinized Finger Length Patterns in Human Males and Females with Congenital Adrenal Hyperplasia. Horm Behav. 2002;42:380–386. doi: 10.1006/hbeh.2002.1830. [DOI] [PubMed] [Google Scholar]
- Buck JJ, Williams RM, Hughes IA, Acerini CL. In-utero androgen exposure and 2nd to 4th digit length ratio—comparisons between healthy controls and females with classical congenital adrenal hyperplasia. Hum Reprod. 2003;18:976–979. doi: 10.1093/humrep/deg198. [DOI] [PubMed] [Google Scholar]
- Buss AH, Perry M. The Aggression Questionnaire. J Pers Soc Psychol. 1992;63:452–459. doi: 10.1037/0022-3514.63.3.452. [DOI] [PubMed] [Google Scholar]
- Chen X, Sachdev PS, Wen W, Anstey KJ. Sex differences in regional gray matter in healthy individuals aged 44–48 years: A voxel-based morphometric study. NeuroImage. 2007;36:691–699. doi: 10.1016/j.neuroimage.2007.03.063. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Woolley CS. Gonadal hormone modulation of dendrites in the mammalian CNS. J Neurobiol. 2005;64:34–46. doi: 10.1002/neu.20143. [DOI] [PubMed] [Google Scholar]
- Efron B. Better Bootstrap Confidence Intervals. J Am Stat Assoc. 1987;82:171–185. doi: 10.1080/01621459.1987.10478410. [DOI] [Google Scholar]
- Emerton BC, Jerram M, Deckersbach T, Dougherty DD, Fulwiler C, Gansler DA. A Comparison of Voxel-Based Morphometry and Volumetry Methods in the Context of the Neural Basis of Aggression. Brain Imaging Behav. 2009;3:332–341. doi: 10.1007/s11682-009-9075-2. [DOI] [Google Scholar]
- Forbes CE, Grafman J. The Role of the Human Prefrontal Cortex in Social Cognition and Moral Judgment*. Annu Rev Neurosci. 2010;33:299–324. doi: 10.1146/annurev-neuro-060909-153230. [DOI] [PubMed] [Google Scholar]
- Giuliani NR, Drabant EM, Gross JJ. ANTERIOR CINGULATE CORTEX VOLUME AND EMOTION REGULATION: IS BIGGER BETTER? Biol Psychol. 2011;86:379–382. doi: 10.1016/j.biopsycho.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Good CD, Johnsrude I, Ashburner J, Henson RNA, Friston KJ, Frackowiak RSJ. Cerebral Asymmetry and the Effects of Sex and Handedness on Brain Structure: A Voxel-Based Morphometric Analysis of 465 Normal Adult Human Brains. NeuroImage. 2001;14:685–700. doi: 10.1006/nimg.2001.0857. [DOI] [PubMed] [Google Scholar]
- Greene JD, Sommerville RB, Nystrom LE, Darley JM, Cohen JD. An fMRI investigation of emotional engagement in moral judgment. Science. 2001;293:2105–2108. doi: 10.1126/science.1062872. [DOI] [PubMed] [Google Scholar]
- Gross JJ, John OP. Individual differences in two emotion regulation processes: Implications for affect, relationships, and well-being. J Pers Soc Psychol. 2003;85:348–362. doi: 10.1037/0022-3514.85.2.348. [DOI] [PubMed] [Google Scholar]
- Hayes AF. PROCESS: A versatile computational tool for observed variable mediation, moderation, and conditional process modeling. 2012 Manuscr. Submitt. Publ. [Google Scholar]
- Hiraishi K, Sasaki S, Shikishima C, Ando J. The Second to Fourth Digit Ratio (2D:4D) in a Japanese Twin Sample: Heritability, Prenatal Hormone Transfer, and Association with Sexual Orientation. Arch Sex Behav. 2012;41:711–724. doi: 10.1007/s10508-011-9889-z. [DOI] [PubMed] [Google Scholar]
- Hönekopp J, Watson S. Meta-analysis of the relationship between digit-ratio 2D:4D and aggression. Personal Individ Differ, Digit Ratio (2D:4D) and Individual Differences Research. 2011;51:381–386. doi: 10.1016/j.paid.2010.05.003. [DOI] [Google Scholar]
- Hyde LW, Bogdan R, Hariri AR. Understanding risk for psychopathology through imaging gene–environment interactions. Trends Cogn Sci, Special Issue: The Genetics of Cognition. 2011;15:417–427. doi: 10.1016/j.tics.2011.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuepper Y, Hennig J. Behavioral Aggression Is Associated with the 2D:4D Ratio in Men but Not in Women. J Individ Differ. 2007;28:64–72. doi: 10.1027/1614-0001.28.2.64. [DOI] [Google Scholar]
- Lephart ED. A review of brain aromatase cytochrome P450. Brain Res Rev. 1996;22:1–26. doi: 10.1016/0165-0173(96)00002-1. [DOI] [PubMed] [Google Scholar]
- Lombardo MV, Ashwin E, Auyeung B, Chakrabarti B, Taylor K, Hackett G, Bullmore ET, Baron-Cohen S. Fetal Testosterone Influences Sexually Dimorphic Gray Matter in the Human Brain. J Neurosci. 2012;32:674–680. doi: 10.1523/JNEUROSCI.4389-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lutchmaya S, Baron-Cohen S, Raggatt P, Knickmeyer R, Manning JT. 2nd to 4th digit ratios, fetal testosterone and estradiol. Early Hum Dev. 2004;77:23–28. doi: 10.1016/j.earlhumdev.2003.12.002. [DOI] [PubMed] [Google Scholar]
- MacLusky NJ, Walters MJ, Clark AS, Toran-Allerand CD. Aromatase in the Cerebral Cortex, Hippocampus, and Mid-Brain: Ontogeny and Developmental Implications. Mol Cell Neurosci. 1994;5:691–698. doi: 10.1006/mcne.1994.1083. [DOI] [PubMed] [Google Scholar]
- Malas MA, Dogan S, Hilal Evcil E, Desdicioglu K. Fetal development of the hand, digits and digit ratio (2D: 4D) Early Hum Dev. 2006;82:469–475. doi: 10.1016/j.earlhumdev.2005.12.002. [DOI] [PubMed] [Google Scholar]
- Manning J, Kilduff L, Cook C, Crewther B, Fink B. Digit Ratio (2D:4D): A Biomarker for Prenatal Sex Steroids and Adult Sex Steroids in Challenge Situations. Front Endocrinol. 2014;5:9. doi: 10.3389/fendo.2014.00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manning JT, Kilduff LP, Trivers R. Digit ratio (2D:4D) in Klinefelter’s syndrome. Andrology. 2013;1:94–99. doi: 10.1111/j.2047-2927.2012.00013.x. [DOI] [PubMed] [Google Scholar]
- Manning JT, Scutt D, Wilson J, Lewis-Jones DI. The ratio of 2nd to 4th digit length: a predictor of sperm numbers and concentrations of testosterone, luteinizing hormone and oestrogen. Hum Reprod. 1998;13:3000–3004. doi: 10.1093/humrep/13.11.3000. [DOI] [PubMed] [Google Scholar]
- Mann MA, Svare B. Prenatal testosterone exposure elevates maternal aggression in mice. Physiol Behav. 1983;30:503–507. doi: 10.1016/0031-9384(83)90212-3. [DOI] [PubMed] [Google Scholar]
- McCarthy MM, Arnold AP. Reframing sexual differentiation of the brain. Nat Neurosci. 2011;14:677–683. doi: 10.1038/nn.2834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, van Honk J. Testosterone, cortisol, and serotonin as key regulators of social aggression: A review and theoretical perspective. Motiv Emot. 2012;36:65–73. doi: 10.1007/s11031-011-9264-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Montoya ER, Terburg D, Bos PA, Will GJ, Buskens V, Raub W, van Honk J. Testosterone administration modulates moral judgments depending on second-to-fourth digit ratio. Psychoneuroendocrinology. 2013;38:1362–1369. doi: 10.1016/j.psyneuen.2012.12.001. [DOI] [PubMed] [Google Scholar]
- Morris JA, Jordan CL, Breedlove SM. Sexual differentiation of the vertebrate nervous system. Nat Neurosci. 2004;7:1034–1039. doi: 10.1038/nn1325. [DOI] [PubMed] [Google Scholar]
- Nelson RJ, Trainor BC. Neural mechanisms of aggression. Nat Rev Neurosci. 2007;8:536–546. doi: 10.1038/nrn2174. [DOI] [PubMed] [Google Scholar]
- Ochsner KN, Gross JJ. The cognitive control of emotion. Trends Cogn Sci. 2005;9:242–249. doi: 10.1016/j.tics.2005.03.010. [DOI] [PubMed] [Google Scholar]
- Ökten A, Kalyoncu M, Yariş N. The ratio of second- and fourth-digit lengths and congenital adrenal hyperplasia due to 21-hydroxylase deficiency. Early Hum Dev. 2002;70:47–54. doi: 10.1016/S0378-3782(02)00073-7. [DOI] [PubMed] [Google Scholar]
- Preacher KJ, Rucker DD, Hayes AF. Addressing Moderated Mediation Hypotheses: Theory, Methods, and Prescriptions. Multivar Behav Res. 2007;42:185–227. doi: 10.1080/00273170701341316. [DOI] [PubMed] [Google Scholar]
- Rivas Mp, Moreira Lma, Santo Lde, Marques Acss, El-Hani Cn, Toralles Mbp. New studies of second and fourth digit ratio as a morphogenetic trait in subjects with congenital adrenal hyperplasia. Am J Hum Biol. 2014;26:559–561. doi: 10.1002/ajhb.22545. [DOI] [PubMed] [Google Scholar]
- Ruigrok ANV, Salimi-Khorshidi G, Lai MC, Baron-Cohen S, Lombardo MV, Tait RJ, Suckling J. A meta-analysis of sex differences in human brain structure. Neurosci Biobehav Rev. 2014;39:34–50. doi: 10.1016/j.neubiorev.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- vom Saal FS. Models of Early Hormonal Effects on Intrasex Aggression in Mice. In: Svare BB, editor. Hormones and Aggressive Behavior. Springer; US: 1983. pp. 197–222. [Google Scholar]
- Sheehan DV, Lecrubier Y, Sheehan KH, Amorim P, Janavs J, Weiller E, Hergueta T, Baker R, Dunbar GC. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59:22–33. [PubMed] [Google Scholar]
- Van Anders SM, Vernon PA, Wilbur CJ. Finger-length ratios show evidence of prenatal hormone-transfer between opposite-sex twins. Horm Behav. 2006;49:315–319. doi: 10.1016/j.yhbeh.2005.08.003. [DOI] [PubMed] [Google Scholar]
- Van Honk J, Schutter DJ, Bos PA, Kruijt AW, Lentjes EG, Baron-Cohen S. Testosterone administration impairs cognitive empathy in women depending on second-to-fourth digit ratio. Proc Natl Acad Sci. 2011;108:3448–3452. doi: 10.1073/pnas.1011891108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura T, Gomes MC, Pita A, Neto MT, Taylor A. Digit ratio (2D:4D) in newborns: Influences of prenatal testosterone and maternal environment. Early Hum Dev. 2013;89:107–112. doi: 10.1016/j.earlhumdev.2012.08.009. [DOI] [PubMed] [Google Scholar]
- Zheng Z, Cohn MJ. Developmental basis of sexually dimorphic digit ratios. Proc Natl Acad Sci. 2011;108:16289–16294. doi: 10.1073/pnas.1108312108. [DOI] [PMC free article] [PubMed] [Google Scholar]