Abstract
Brain-derived neurotrophic factor (BDNF) plays a critical role in plasticity at glutamate synapses and the effects of repeated cocaine exposure. We recently showed that intracranial injection of BDNF into the rat nucleus accumbens (NAc), a key region for cocaine addiction, rapidly increases AMPA receptor (AMPAR) surface expression. To further characterize BDNF’s role in both rapid AMPAR trafficking and slower, homeostatic changes in AMPAR surface expression, we investigated the effects of acute (30 min) and long-term (24 h) treatment with BDNF on AMPAR distribution in NAc medium spiny neurons from postnatal rats co-cultured with mouse prefrontal cortex (PFC) neurons to restore excitatory inputs. Immunocytochemical studies showed that acute BDNF treatment increased cell surface GluA1 and GluA2 levels, as well as their co-localization, on NAc neurons. This effect of BDNF, confirmed using a protein crosslinking assay, was dependent on ERK but not AKT signaling. In contrast, long-term BDNF treatment decreased AMPAR surface expression on NAc neurons. Based on this latter result, we tested the hypothesis that BDNF plays a role in AMPAR “scaling down” in response to a prolonged increase in neuronal activity produced by bicuculline (24 h). Supporting this hypothesis, decreasing BDNF signaling with the extracellular BDNF scavenger TrkB-Fc prevented the scaling down of GluA1 and GluA2 surface levels in NAc neurons normally produced by bicuculline. In conclusion, BDNF exerts bidirectional effects on NAc AMPAR surface expression, depending on duration of exposure. Furthermore, BDNF’s involvement in synaptic scaling in the NAc differs from its previously described role in the visual cortex.
Keywords: Co-culture, mouse, rat, receptor trafficking, synaptic scaling
Introduction
Brain-derived neurotrophic factor (BDNF) belongs to the family of neurotrophins that includes neurotrophin-3, neurotrophin-4/5, and nerve growth factor. Neurotrophins promote development, modulate synaptic function, and play a vital role in synaptic plasticity (Vicario-Abejón et al., 2002; Carvalho et al., 2008). BDNF is the most abundant neurotrophin expressed in the brain (Thoenen, 1995). Synthesized as a pro-peptide, the mature form of BDNF binds to tropomyosin receptor kinase B (TrkB), which is coupled to intracellular signaling cascades such as the Ras/extracellular signal-regulated protein kinase (ERK), phosphotidylinositol-3-kinase (PI3 kinase)/Akt, and phospholipase C γ (PLCγ) pathways (Kaplan & Miller, 2000). When released in response to glutamatergic activity (Lu, 2003), BDNF activates these pathways and thereby contributes to multiple aspects of activity-dependent plasticity, including stimulation of local protein synthesis and dendritic spine formation, as well as facilitation of the induction and maintenance of long-term potentiation (LTP) (Bramham & Messaoudi, 2005; Waterhouse & Xu, 2009).
Not surprisingly, BDNF has also been implicated in behavioral and cellular plasticity produced by drugs of abuse, including cocaine (Russo et al., 2009; McGinty et al., 2010; Pickens et al., 2011). Like other forms of plasticity (Shepherd & Huganir, 2007), cocaine-induced plasticity can involve the trafficking of α-amino-3-hyroxy-5-methyl-4-isoxazole-propionic acid receptors (AMPARs) (Wolf, 2010; Pierce & Wolf, 2013). The nucleus accumbens (NAc) is a critical brain region for motivated behavior and addiction (Sesack & Grace, 2010). Studies of animal models of cocaine craving and relapse indicate that these behaviors are associated with altered BDNF levels in the NAc, as well as alterations in AMPAR surface and synaptic expression in the NAc (see Discussion). We wondered if BDNF might play a role in cocaine-induced AMPAR plasticity, based on in vitro studies showing increased cell surface and synaptic levels of AMPAR subunits following BDNF exposure (Narisawa-Saito et al., 2002; Caldeira et al., 2007; Li & Keifer, 2008; 2009) and our recent demonstration that intracranial injection of BDNF increases GluA1 surface expression in the NAc of adult drug-naïve rats (Li & Wolf, 2011).
The goal of the present study was to further characterize the interaction between BDNF and NAc AMPARs using a primary culture system amenable to direct measurements of AMPAR trafficking. In this system, characterized previously, medium spiny neurons (MSN) from postnatal rat NAc are co-cultured with prefrontal cortex (PFC) neurons from mice expressing enhanced cyan fluorescent protein (ECFP); the PFC neurons restore excitatory input onto the MSN, but can be distinguished from MSN based on fluorescence (Sun et al., 2008; Sun & Wolf, 2009). We found that acute BDNF treatment (30 min) increases AMPAR surface expression in an ERK-dependent manner, while long-term BDNF treatment (24 h) decreases AMPAR surface expression. Furthermore, BDNF is required for homeostatic reductions in AMPAR surface expression (synaptic scaling) in response to a prolonged increase in excitatory transmission. The latter finding may be relevant to BDNF’s role in addiction, as the relatively slow time-scale of synaptic scaling, at least compared to Hebbian plasticity, makes it an interesting candidate for mediating slow behavioral changes occurring during drug exposure and withdrawal.
Materials and methods
Animals
As described previously (Sun et al., 2008, Sun & Wolf, 2009), postnatal day 1 (P1) rats from pregnant Sprague-Dawley rats (Harlan, Indianapolis, IN, USA) were decapitated and used to obtain NAc neurons, while PFC cells were obtained from P1 offspring of transgenic mice expressing enhanced cyan fluorescent protein (ECFP) [strain B6.129(ICR)-Tg(ACTB-ECFP)1Nagy/J; The Jackson Laboratory, Bar Harbor, ME, USA]. The ECFP transgenic mouse line was maintained by mating homozygous ECFP male and female mice in house. All offspring express ECFP. All animal procedures were in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee of Rosalind Franklin University of Medicine and Science.
Primary neuronal cultures
NAc/PFC co-cultures were prepared as previously described (Sun et al., 2008; Sun & Wolf, 2009). Briefly, the PFC of P1 ECFP-expressing mice was dissociated with papain at 37°C and plated at a density of 30,000 cells/well onto coverslips coated with poly-D-lysine (100 μg/mL; Sigma Aldrich, St. Louis, MO, USA) in 24-well plates. Two to three days later, the NAc from P1 rats was dissociated with papain and plated at a density of 30,000 cells/well with the PFC cells. The NAc/PFC co-cultures were grown in Neurobasal medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2mM GlutaMAX, 0.5% Gentamicin and 2% B27 (Invitrogen). Half of the medium was replaced every 4 days. Cultures were used for immunocytochemical experiments between weeks 2 and 3 after plating the NAc neurons. For experiments involving Western blotting, the NAc from P1 rats was dissected and plated at a higher density (200,000 cells/well). The cultures were grown as described above. Cultures were used between 2 and 3 weeks after plating.
Drug treatments
NAc/PFC co-cultures and NAc cultures were either kept in control medium or incubated with BDNF (25 ng/mL; Sigma Aldrich), the MAPK/ERK kinase (MEK1/2) inhibitor U0126 (10 μM; Cell Signaling Technology, Danvers, MA, USA), the PI3 kinase inhibitor LY294002 (25 μM; Cell Signaling Technology) or BDNF + either inhibitor for 30 min or 24 h, as indicated, in Neurobasal media. In the synaptic scaling experiments, NAc/PFC co-cultures were kept in control medium or incubated with bicuculline (20 µM; Tocris Bioscience, Ellisville, MD, USA), TrkB-Fc (0.5 µg/mL; Sigma), or TrkB-Fc + bicuculline for 24 h.
Immunocytochemistry
For cell surface GluA1 and GluA2 double immunostaining, live neurons were incubated with polyclonal antibody to the extracellular N-terminal domain of GluA1 (1:15, PC246, aa 253–267; Calbiochem, La Jolla, CA, USA;) and monoclonal antibody to the extracellular N-terminal domain of GluA2 (1:20; MAB397, aa 175–430; Millipore, Billerica, MA, USA) in NeuroBasal media (15 min, 37°C). Cells were then fixed with 4% paraformaldehyde, blocked with 5% donkey serum in phosphate-buffered saline for 1 h and incubated for 1 h with Cy3 conjugated donkey anti-rabbit 2° antibody (1:500; Jackson ImmunoResearch, West Grove, PA, USA) and Alexa 488 conjugated donkey anti-mouse 2° antibody (1:1000; Invitrogen) without permeabilization. Incubations were performed at room temperature (RT; ~21°C).
Image analysis
Images were analyzed as described previously (Sun et al., 2005; 2008). NAc and PFC neurons were distinguished by fluorescence and morphology (Sun et al., 2008). Images of NAc MSN adjacent to PFC pyramidal neurons, selected under phase contrast imaging to avoid experimenter bias based on the intensity of fluorescence staining, were acquired with a Nikon inverted microscope using a 40 × oil objective, an ORCA-ER digital camera and MetaMorph software (Universal Imaging, Downington, PA, USA). All experimental groups compared were from the same culture preparation and were processed simultaneously. Approximately 4–6 cells from at least 5 different wells for each group were analyzed. For each image, total AMPAR surface area in a fixed length (15 µm) of process, located at least one soma diameter away from the soma, was measured using a threshold set at least 2× higher than average background fluorescence in unstained areas. We used AMPAR area rather than intensity as a measure of AMPAR density, as in our prior studies (Sun et al., 2005; Sun et al., 2008; Sun and Wolf, 2009), because we have found area to be a more sensitive measure.
Surface receptor crosslinking
After the appropriate BDNF stimulation time (30 min or 24 h), cultures were washed two times with HBSS (Invitrogen) and then incubated for 10 min with 2mM bis(sulfosuccinimidyl)suberate (BS3; Pierce, Rockford, IL, USA) in HBSS at 37°C as described previously (Gao & Wolf, 2008; Sun et al., 2008) to selectively crosslink surface-expressed receptors. This increases their apparent molecular weight, enabling them to be distinguished from unmodified intracellular AMPAR subunits by SDS-PAGE and Western blotting. Crosslinking was terminated by quenching the reaction with 100mM glycine for 10 min at 4°C.
Western blotting
Cultures were scraped into ice-cold lysis buffer containing phosphatase and protease inhibitors [25 mM HEPES, pH7.4, 500 mM NaCl, 2 mM EDTA, 1 mM DTT, 1 mM phenylmethyl sulfonyl fluoride, 20 mM NaF, 1 mM sodium orthovanidate, 10 mM sodium pyrophosphate, 1 μM microcystin-LF, 1 μM okadaic acid, 1X protease inhibitor cocktail set I (Calbiochem), and 0.1% Nonidet P-40 (v/v)]. Homogenates were made by sonicating lysed cells 3 times for 5 s on ice and centrifuging at 12,000 × g for 2 min. The supernatant was aliquotted and stored at −80°C. Protein concentration for each sample was determined using the Bio-Rad protein assay (Bio-Rad, Hercules, CA, USA). Samples (20 μg total protein per lane) were run on 4–15% gradient Tris-HCl gels (BioRad). For immunoblotting, proteins were transferred to PVDF membranes. Membranes were washed in dH2O and blocked with 1% goat serum with 5% non-fat dry milk in 0.05% Tween-20 in TBS, pH 7.4 for 1 hr at RT. Membranes were then incubated with one of the following primary antibodies overnight at 4°C: GluA1 (1:1000; PA137776, Pierce), GluA2 (1:1000; AB1768, Millipore), AKT (1:1000; 9272, Cell Signaling Technology), phosphorylated AKT (P-AKT; Ser473) (1:1000; 4058, Cell Signaling Technology), P-AKT (Thr308) (1:1000; 4056, Cell Signaling Technology), p-44/42 MAPK (ERK1/2) (1:1000; 9101, Cell Signaling Technology), phosphorylated p-44/42 MAPK (P-ERK1/2) (1:1000; 4377, Cell Signaling Technology), eEF2 (1:1000; 2332, Cell Signaling Technology), phosphorylated eEF2 (P-eEF2) (1:1000; 2331, Cell Signaling Technology), mTOR (1:1000; 2972, Cell Signaling Technology), or phosphorylated mTOR (P-mTOR) (1:1000; 2971, Cell Signaling Technology). Membranes were washed with TBS-Tween solution, incubated for 60 min with HRP-conjugated anti-rabbit IgG or anti-mouse IgG (1:10,000; Millipore), and washed again with TBS-Tween followed by TBS. Membranes were rinsed with dH2O, immersed in chemiluminescence (ECL) detecting substrate (GE Healthcare, Little Chalfont, UK) for 1 min and visualized using HyperFilm ECL. Phospho-antibodies were stripped from the membrane by incubating in 62.5 mM Tris, pH 6.8, 2% SDS, and 100 mM 2-mercaptoethanol for 60 min. Blots were then probed with the corresponding phosphorylation-independent antibody. Bands of interest were quantified using TotalLab (Nonlinear Dynamics Ltd., Newcastle, UK) and data were normalized to total protein in the lane determined with Ponceau S staining.
Statistical Analysis
For immunocytochemistry experiments (Figs. 1, 4 and 5), data were analyzed with a Kruskal-Wallis one-way ANOVA on ranks. When a significant group effect was found, post hoc comparisons were performed using a Dunn’s test, unless otherwise noted. For immunoblotting experiments (Figs. 2 and 3), data were analyzed with a one-way ANOVA to compare multiple groups. When a significant group effect was found, post hoc comparisons were performed using a Dunnett’s test. The criterion for significance was set at P < 0.05 (n, number of cells or lanes analyzed). All data were analyzed and all graphs were generated using SigmaStat and SigmaPlot software, respectively (Systat Software Inc., San Jose, CA, USA).
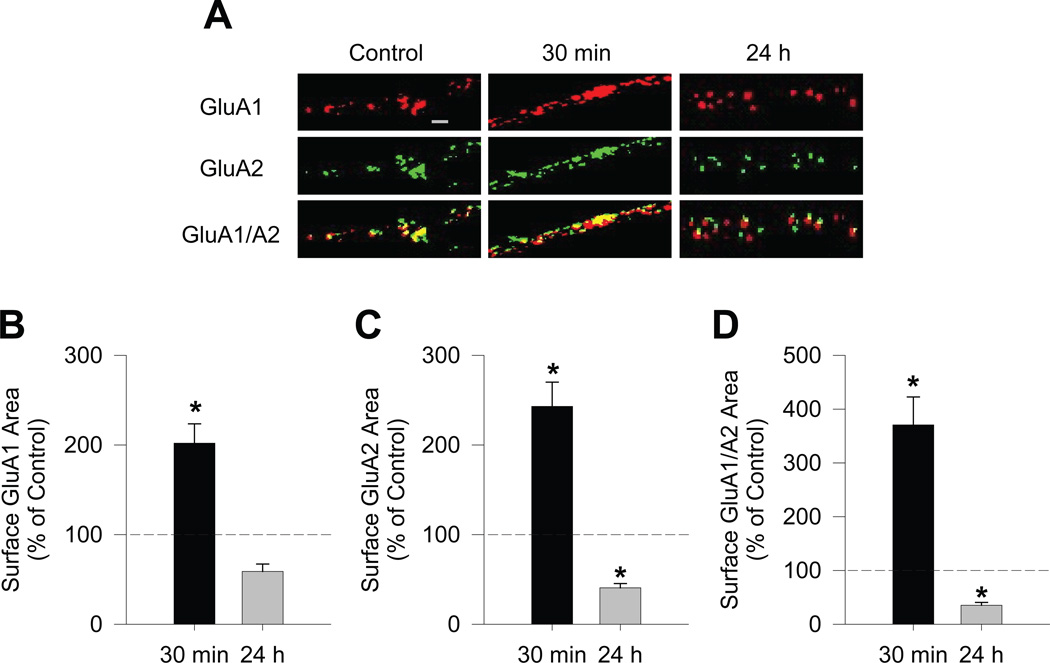
Figure 1.
Surface expression and co-localization of GluA1 and GluA2 on NAc medium spiny neurons are robustly increased after acute BDNF treatment and decreased after long-term BDNF treatment of NAc/PFC co-cultures. (A) Representative images of GluA1, GluA2 and colocalized GluA1/A2 immunostaining on medium spiny neurons in NAc/PFC co-cultures. Co-cultures were treated with BDNF (25ng/mL) for 30 min or 24 h. Surface GluA1 (red) and GluA2 (green) were detected using live cell labeling. Scale bar, 2.5μm. (B-D) Quantification of the area of cell surface GluA1 (B), GluA2 (C), and GluA1/A2 co-localization (D) (n = 18–26 cells/group). Results are shown as area of surface staining (mean + SEM), normalized to the mean of the vehicle control group, which is indicated by the dashed line (control group: GluA1, 100 ± 14.67%; GluA2, 100 ± 10.23%; GluA1/A2, 100 ± 18.12%). Data were analyzed using a one-way ANOVA on ranks followed by a Dunn’s test if group differences were found. *P < 0.05 vs. control.
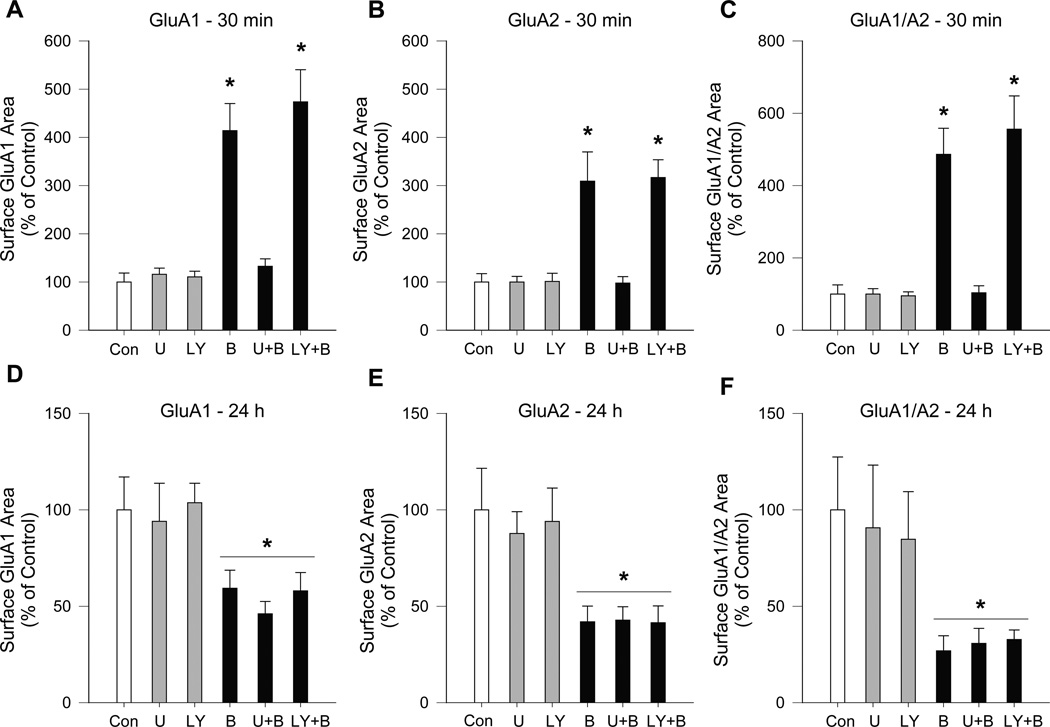
Figure 4.
The increase in surface AMPAR levels induced by acute BDNF treatment of NAc/PFC co-cultures requires MAPK activation but not the PI3 kinase pathway, whereas effects of long-term BDNF are not altered by inhibitors of either pathway. Inhibition of the MAPK pathway with U0126 (U) prevented the increase in GluA1 and GluA2 surface expression and co-localization observed after 30 min of BDNF (B) treatment, while inhibition of the PI3 kinase pathway with LY294002 (LY) had no effect (panels A-C). In contrast, the percent decrease in AMPAR subunit surface expression produced by 24 h of BDNF treatment did not differ between BDNF, U+BDNF or LY+BDNF groups (D-F). For these experiments, NAc/PFC co-cultures were treated with vehicle, BDNF (25ng/mL), U (10μM), LY (25μM), U+BDNF, or LY+BDNF for 30 min (A-C) or 24 h (D–F) (n = 17–25 cells/group). Surface GluA1 and GluA2 were measured using live cell staining. Results are presented as the area (mean + SEM) of surface GluA1 (A,D), GluA2 (B,E) or GluA1/A2 co-localization (C,F) for each time-point. Data were analyzed using a one-way ANOVA on ranks followed by a Dunn’s test. Analysis for 30 min experiment: *P < 0.05, B and LY+B groups vs. Control. Analysis for 24 h experiment: *P < 0.05, all BDNF groups (B, U+B, LY+B) vs. all non-BDNF exposed groups (Con, U, LY).
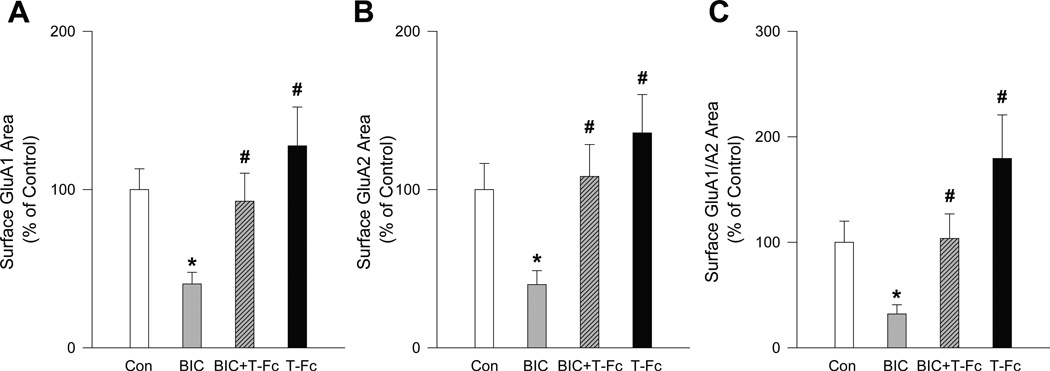
Figure 5.
BDNF mediates synaptic “scaling down” of AMPARs produced by a prolonged increase in excitatory transmission. Long-term treatment (24 h) of NAc/PFC co-cultures with the GABAA receptor bicuculline (BIC; 20 µM) produced a significant decrease in surface expression of GluA1 (A) and GluA2 (B) as well as GluA1/A2 co-localization (C). Co-incubation with the membrane-impermeable extracellular scavenger of BDNF TrkB-Fc (T-Fc, 0.5 µg/mL) prevented the decrease in GluA1 (A), GluA2 (B) and GluA1/A2 co-localization (C) normally produced by BIC, while 24 h treatment with T-Fc alone produced trends towards increased AMPAR surface expression. Results are shown as area of surface staining (mean + SEM), normalized to the mean of the respective vehicle control group. Data were analyzed using a one-way ANOVA on ranks followed by a Dunn’s test if group differences were found. * P < 0.05 vs. control, # P < 0.05 vs. BIC.
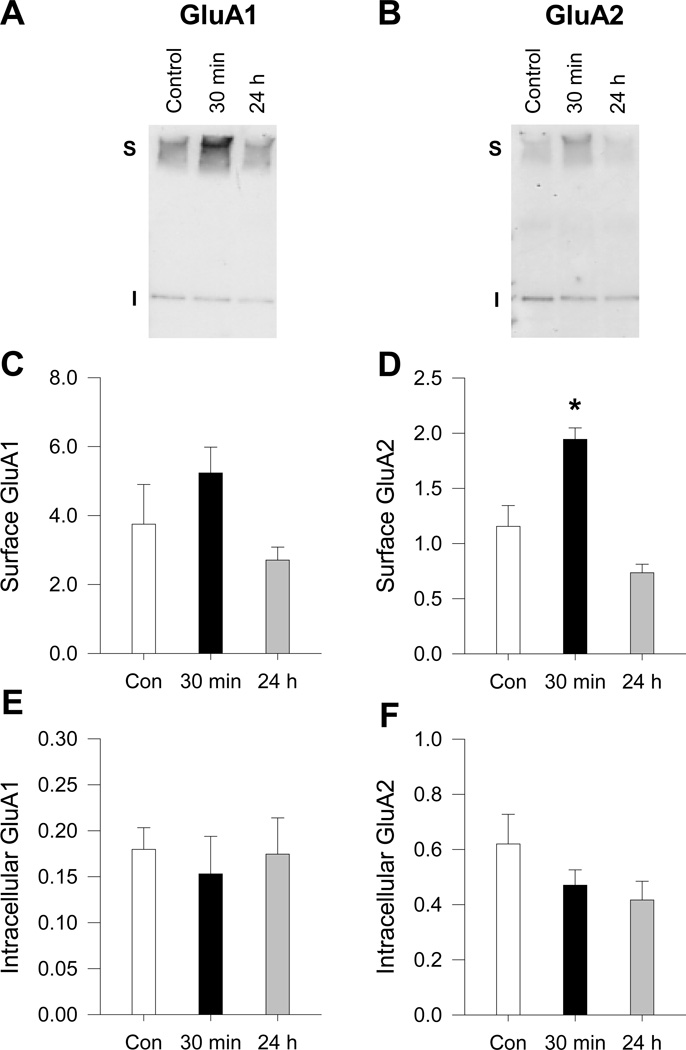
Figure 2.
BS3 crosslinking followed by Western blotting reveals that acute BDNF treatment increases AMPAR surface expression in high-density NAc cultures. NAc cultures were treated with BDNF (25ng/mL) for 30 min or 24 h. Following BDNF treatment, surface proteins were crosslinked using BS3 and Western blotting was used to quantify surface (S) and intracellular (I) levels of AMPAR subunits. (A–B) Representative blots of GluA1 (left) and GluA2 (right) show the migration pattern for the AMPAR subunits. BS3 crosslinked surface proteins migrate at a higher molecular weight than the intracellular pool, which represents unmodified receptors (~100kDa). (C-F) Effect of 30 min or 24 h incubation with BDNF on surface GluA1 (C), surface GluA2 (D), intracellular GluA1 (E), and intracellular GluA2 (F) (n = 4 wells/group). Error bars represent SEM. Data were analyzed using a one-way ANOVA followed by a Dunnett’s test if group differences were found. *P < 0.05 vs. control.
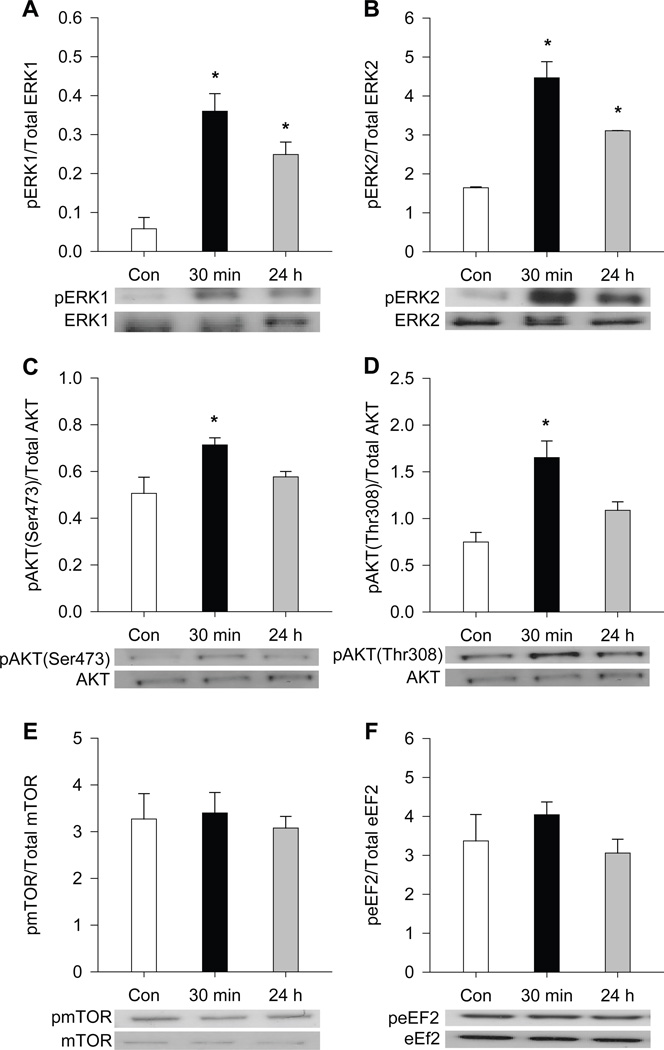
Figure 3.
Effect of BDNF treatment on phosphorylation of ERK1, ERK2, AKT, mTOR and eEF2 in high-density NAc cultures. These proteins were selected for analysis because they are downstream of BDNF-TrkB signaling. NAc cultures were stimulated with BDNF (25ng/mL) for 30 min or 24 h. Western blotting was used to determine the level of protein phosphorylation. The 30 min BDNF treatment increased phosphorylation of ERK1 (A), ERK2 (B), AKT (Ser 473) (C), and AKT (Thr 308) (D), but not mTOR (E), and eEF2 (F). The 24 h BDNF treatment only increased ERK1 and ERK2 phosphorylation. All data are shown as phospho-protein normalized to the total level of target protein determined with a phosphorylation-independent antibody. Representative blots are shown below the graphs. Error bars represent SEM (n = 4 wells/group). Data were analyzed using a one-way ANOVA followed by a Dunnett’s test if group differences were found. *P < 0.05 vs. control.
Results
Acute BDNF treatment increases while long-term BDNF treatment decreases AMPAR surface expression in NAc MSN
As in other regions (Wenthold et al., 1996; Lu et al., 2009; Reimers et al., 2011), the majority of AMPAR in the NAc are GluA1A2 receptors, along with GluA2A3 and a minority population (~10%) of GluA2-lacking receptors (Boudreau et al., 2007; Conrad et al., 2008; Reimers et al., 2011). Our prior studies of NAc MSN co-cultured with PFC neurons have implicated GluA1A2 receptors in homeostatic synaptic scaling, although these MSN also express a substantial population of homomeric GluA1 receptors on their cell surface (Sun et al., 2008; Sun & Wolf, 2009). Therefore, we focused on GluA1 and GluA2 in the present study.
To directly assess the effect of BDNF stimulation on AMPAR trafficking in NAc neurons, NAc/PFC co-cultures were stimulated for 30 min or 24 h with BDNF (25ng/mL; concentration based on Rutherford et al., 1998) followed by live-cell labeling with antibodies directed against the extracellular N-terminal regions of GluA1 and GluA2. The 30 min time was selected to study acute effects of BDNF, while the 24 h time was selected because elevating or blocking activity for 24 h is sufficient to elicit synaptic scaling in NAc/PFC co-cultures (Sun & Wolf, 2009). Our analysis was restricted to MSN of the NAc, which were identified based on morphology and absence of the cyan fluorescence that serves as a marker for PFC neurons in the co-cultures (see Methods). Interestingly, incubation of the co-cultures with BDNF for 30 min significantly increased surface AMPAR surface expression while incubation with BDNF for 24 h significantly decreased AMPAR surface expression (Fig. 1). Analysis by Kruskal-Wallis one-way ANOVA on ranks revealed a significant difference between the three treatment groups (control, 30 min BDNF, 24 h BDNF) in levels of cell surface GluA1 (H=26.78, P<0.001), GluA2 (H=41.46, P<0.001) and GluA1/A2 co-localization (H=39.12, P<0.001). Dunn’s test post-hoc comparisons revealed that, compared to the control group, incubation with BDNF for 30 min produced a significant increase in surface expression of GluA1 (Q=2.70, P<0.05), GluA2 (Q=2.46, P<0.05) and GluA1/A2 co-localization (Q=3.08, P<0.05) (Fig. 1B–D). In contrast, Dunn’s test post-hoc comparisons revealed that, compared to the control group, incubation with BDNF for 24 h produced a significant decrease in cell surface GluA2 (Q=3.35, P<0.05) and GluA1/A2 co-localization (Q=2.57, P<0.05) (Fig. 1C,D). Although not significant (Q=1.96, P>0.05), a trend was observed towards decreased surface expression of GluA1 after 24 h of BDNF treatment (59% of control, Fig. 1B), which reached statistical significance when directly compared to the control group using a Mann-Whitney U test (U=138, P=0.023).
To confirm the results using another approach, we stimulated high-density NAc cultures with BDNF (25ng/mL) for 30 min or 24 h. Following BDNF incubation, cell surface proteins were crosslinked using the membrane impermeable, bi-functional crosslinking reagent BS3 (Boudreau & Wolf, 2005; Boudreau et al., 2012). SDS-PAGE followed by immunoblotting was used to separate the crosslinked surface proteins from the non-crosslinked intracellular proteins (Fig. 2A, B). We used “pure” NAc cultures for this experiment because PFC neurons in the co-cultures would have contributed to our protein measurements and higher density cultures in order to provide a stronger signal.
After incubation with BDNF for 30 min, the crosslinking experiments in high-density NAc cultures revealed a significant increase in GluA2 surface expression (Fig. 2D; one-way ANOVA: F(2,9) = 21.73, P = 0.006; 30 min BDNF vs. control, P < 0.05, Dunnett’s test) and a trend for GluA1 (Fig. 2C; one-way ANOVA: F(2,9) = 2.39, P = 0.14; surface GluA1 was 140% of control). These results are generally consistent with those obtained using immunocytochemistry (Fig. 1). BDNF did not significantly alter intracellular levels of GluA1 or GluA2 measured with the crosslinking assay (Fig. 2E,F; GluA1, one-way ANOVA: F(2,9) = 0.16, P = 0.86; GluA2, one-way ANOVA: F(2,9) = 1.72, P = 0.23). This could indicate that the BDNF-induced increase in AMPAR surface levels is due to synthesis of new AMPAR subunits that are targeted to the cell surface, although we cannot rule out the possible contribution of AMPAR redistribution from intracellular to surface pools. Incubation with BDNF (25ng/mL) for 24 h produced trends in the opposite direction for surface GluA1 and GluA2 (72% and 64% of control, respectively; P > 0.05 vs. control, Dunnett’s test) (Fig. 2C,D). The presence of excitatory synaptic inputs to MSN in the NAc/PFC co-cultures but not the high-density NAc cultures could explain why 24 h of BDNF incubation produced statistically significant decreases in AMPAR surface expression in the former (Fig. 1) but only trends in the latter. Taken together, immunocytochemical and protein crosslinking experiments indicate that acute BDNF incubation (30 min) increases AMPAR surface expression on MSNs of the NAc, while long-term BDNF incubation (24 h) has the opposite effect.
ERK and AKT are activated in the NAc by incubation with BDNF
In order to identify signaling pathways in NAc MSN that mediate the BDNF-induced changes in AMPAR surface expression, high-density NAc cultures were incubated with BDNF (25ng/mL) for 30 min or 24 h and then harvested in buffer containing protease and phosphatase inhibitors. Immunoblotting was used to assess phosphorylation of ERK1/2 (P-ERK1/total ERK1 and P-ERK2/total ERK2), AKT [P-AKT (Ser473)/total AKT and P-AKT (Thr308)/total AKT], mTOR (P-mTOR/total mTOR), and eEF2 (P-eEF2/total eEF2). These pathways were selected because they are downstream of the TrkB receptor (see Introduction). High-density NAc cultures are ideal for screening multiple pathways because they provide more protein for immunoblotting than the NAc/PFC co-cultures, and do not contain PFC proteins which would contribute to immunoblotting results and thus obscure selective analysis of NAc signaling pathways. Acute incubation with BDNF (30 min) significantly increased phosphorylation of ERK1, ERK2, and AKT (at both Ser473 and Thr308) (Fig. 3A–D; ERK1, one-way ANOVA: F(2,9) = 18.19, P < 0.05; ERK2, one-way ANOVA: F(2,9) = 34.93, P < 0.05; AKT Ser473, F(2,9) = 5.32, P < 0.05; AKT Thr308, one-way ANOVA: F(2,9) = 12.41, P < 0.05; Dunnett’s tests, all P < 0.05 for 30 min BDNF vs. control), while only ERK1 and ERK2 phosphorylation remained significantly elevated after long-term BDNF incubation (24 h) (Fig. 3A and B, respectively; Dunnett’s test, both P < 0.05 for 24 h BDNF vs. control). Phosphorylation of mTOR and eEF2 were not significantly altered by BDNF incubation at either time-point (Fig. 3E,F; mTOR, one-way ANOVA: F(2,9) = 0.14, P = 0.87; eEF2, one-way ANOVA: F(2,9) = 1.10, P = 0.38).
BDNF requires ERK1/2 activation to increase AMPAR surface expression in the NAc
Given that ERK1/2 phosphorylation is significantly increased by BDNF after both 30 min and 24 h of BDNF incubation and that AKT phosphorylation is significantly increased after 30 min of BDNF incubation, we wanted to determine whether either of these pathways is required for BDNF-induced changes in AMPAR surface expression. To accomplish this, NAc/PFC co-cultures were treated with the MAPK/ERK kinase (MEK1/2) inhibitor, U0126 (10μM), or the PI3 kinase inhibitor, LY294002 (25μM), alone or in combination with BDNF, for 30 min or 24 h. The inhibitors were added 20 min prior to BDNF. At the 30 min time-point, U0126 abolished the BDNF-induced increase in surface GluA1 and GluA2 expression as well as the increase in surface GluA1/A2 co-localized area (Fig. 4A–C). Analysis by Kruskal-Wallis one-way ANOVA on ranks revealed significant differences among the 6 treatment groups in cell surface levels of GluA1 (H = 61.16, P < 0.001), GluA2 (H = 44.45, P < 0.001) and GluA1/A2 co-localization (H = 59.84, P<0.001). Dunn’s test post-hoc comparisons showed that, in the BDNF + U0126 group, surface levels of GluA1 (Q=1.16), GluA2 (Q=0.23) and GluA1/A2 co-localization (Q=0.56) did not differ from the control group (P > 0.05). However, the PI3 kinase inhibitor, LY294002, was unable to block the BDNF-induced increases in surface GluA1, GluA2, or GluA1/A2 co-localization (Fig. 4A–C; Dunn’s tests, Q = 5.47, 4.75 and 5.49, respectively; P < 0.05 vs. control for all measures). Acute incubation with each inhibitor alone had no effect on surface levels of GluA1, GluA2, or GluA1/A2 co-localization (Fig. 4A–C; P>0.05 vs. control). Next, we examined the effects of long-term (24 h) incubation with U0126 (10μM) or LY294002 (25μM), alone or in combination with BDNF (25ng/mL), on AMPAR surface expression. Consistent with the results shown in Fig. 1, long-term BDNF treatment significantly decreased surface levels of GluA1, GluA2 and the area of GluA1/A2 co-localization. However, neither inhibitor affected either the magnitude of the BDNF-induced decrease in AMPAR surface expression or basal AMPAR surface expression in the absence of BDNF treatment (Fig. 4D–F). Thus, analysis by Kruskal-Wallis one-way ANOVA on ranks revealed no difference in AMPAR surface expression between all BDNF-exposed groups (BDNF, U0126+BDNF, LY294002 + BDNF) nor between the three groups that were not exposed to BDNF (Control, U0126, LY294002; P > 0.05). Subsequent ANOVAs revealed a significant decrease in all 24 h BDNF groups compared to all non-BDNF exposed groups for surface GluA1 (H = 14.92, P < 0.001), GluA2 (H = 22.06, P<0.001) and GluA1/A2 co-localization (H = 9.63, P = 0.002). Taken together, these results suggest that activation of ERK1/2 is necessary for acute BDNF incubation (30 min) to increase surface AMPAR expression, whereas this is not the case for the decrease in AMPAR surface expression produced by long-term BDNF exposure (24 h).
BDNF signaling is required for “scaling down” of AMPAR surface expression in NAc MSN in response to a prolonged increase in neuronal activity
To determine if the decrease in AMPAR surface expression observed following long-term BDNF exposure was related to synaptic scaling, we assessed whether interfering with BDNF activity would prevent the decrease in GluA1 and GluA2 surface expression (“scaling down”) normally observed in NAc neurons following a prolonged increase in excitatory transmission (Sun & Wolf, 2009). NAc/PFC co-cultures were treated for 24 h with the GABAA receptor antagonist bicuculline (20 µM) alone or in the presence of a membrane-impermeable scavenger of extracellular BDNF, TrkB-Fc (0.5 µg/mL; concentration based on Jourdi et al., 2009). A separate group was treated for 24 h with TrkB-Fc alone (0.5 µg/mL). As reported previously (Sun & Wolf, 2009), enhancing excitatory synaptic activity by blocking GABAA receptor transmission with bicuculline produced a significant decrease in AMPAR surface expression (Fig. 5A–C). Analysis by Kruskal-Wallis one-way ANOVA on ranks revealed a significant difference between the four treatment groups (control, bicuculline, bicuculline +TrkB-Fc, TrkB-Fc) in surface levels of GluA1 (H = 17.25, P<0.001), GluA2 (H = 15.81, P = 0.001) and GluA1/A2 co-localization (H = 17.73, P < 0.001). Dunn’s test post-hoc comparisons revealed a significant decrease in surface GluA1, GluA2 and GluA1/A2 co-localization in the bicuculline-treated group compared to the control group (Q = 3.39, 2.77 and 2.89, respectively, P < 0.05). Interestingly, decreasing BDNF signaling in the presence of bicuculline prevented the bicuculline-induced decrease in surface GluA1, GluA2 and GluA1/A2 co-localization (Q = 2.68, 2.99 and 2.92, respectively; P < 0.05, bicuculline vs. bicuculline + TrkB-Fc). Incubation for 24 h with TrkB-Fc alone did not significantly alter AMPAR surface expression (Fig. 5A–C; P > 0.05 TrkB-Fc compared to control), which could simply be due to low endogenous BDNF levels under basal conditions since TrkB-Fc competes with native TrkB receptors for BDNF (Binder et al., 1999). However, incubation with TrkB-Fc alone produced trends for AMPAR surface expression (GluA1, 128% of control; GluA2, 136% of control; GluA1A2 co-localization, 179% of control; Fig 5A–C), potentially suggesting that low levels of endogenous BDNF may be exerting small effects that are opposed by TrkB-Fc. Together, these results indicate that BDNF release mediates the “scaling down” of AMPAR surface expression in NAc MSN produced by a long-term enhancement in synaptic activity.
Discussion
MSN, which comprise approximately 90% of neurons in the NAc, are GABAergic projection neurons that play a critical role in motivated behaviors including addiction (Meredith et al., 2008). We have previously developed and characterized a NAc/PFC co-culture system in which PFC pyramidal neurons make excitatory synapses onto the MSN; AMPAR trafficking at these synapses can be studied using immunocytochemical methods (Sun et al., 2008; Sun & Wolf, 2009). Our present results show that acute BDNF treatment (30 min) increases AMPAR surface expression in MSN in NAc/PFC co-cultures, whereas long-term BDNF (24 h) decreases AMPAR surface expression. Furthermore, BDNF appears to mediate the decrease in AMPAR surface expression associated with synaptic scaling elicited by chronic elevation of neuronal activity with bicuculline (24 h). Although there are caveats associated with extrapolating from in vitro to in vivo systems (see below), these results help define basic synaptic mechanisms operating in MSN of the NAc, which facilitates the development of hypotheses to account for in vivo findings on cocaine-induced changes in BDNF and AMPAR levels in this brain region.
Acute BDNF treatment produces a rapid increase in AMPAR surface expression
In order to determine the effects of BDNF treatment on AMPAR surface expression and the signaling pathways involved, we used two separate in vitro approaches, immunocytochemical analysis of NAc/PFC co-cultures and surface receptor crosslinking in high-density “pure” NAc cultures. The former is preferable for localizing receptors, whereas the latter offers advantages for screening signaling pathways via immunoblotting since more protein is available and immunoblotting results are not “contaminated” by the presence of PFC proteins. We found that acute (30 min) treatment of cultures with BDNF (25ng/mL) increases surface expression of the AMPAR subunits GluA1 and GluA2, along with their co-localization. Our results are in general agreement with results from other in vitro systems. Caldeira et al. (2007) showed that treatment of hippocampal cultures with BDNF for 30 min increases levels of surface GluA1, an effect that is dependent on translation. Furthermore, in an organotypic slice preparation, they showed that when GluA1 is over-expressed, leading to formation of homomeric GluA1 receptors, BDNF can induce synaptic insertion of these receptors (Caldeira et al., 2007). Narisawa-Saito et al. (2002), using cultured cortical neurons, showed that BDNF induced a translocation of GluA2-containing AMPARs to the surface that is evident within 30 min. Nakata & Nakamura (2007) showed that BDNF rapidly increases AMPAR trafficking to the postsynaptic density in cultured cortical neurons. Finally, BDNF treatment of turtle brain stem preparations enhances synaptic delivery of GluA1- and GluA4-containing AMPARs (Li & Keifer, 2008; 2009; Keifer & Zheng, 2010). In two of the studies mentioned above, the rapid BDNF-induced increase in AMPAR surface expression was followed by a slower increase in total protein levels of AMPAR subunits (Caldeira et al., 2007; Narisawa-Saito et al., 2002; see also Narisawa-Saito et al., 1999a,b) which was paralleled by an increase in AMPAR-interacting proteins (Jourdi et al., 2003). Our immunocytochemical studies, which analyzed AMPAR levels on the surface of neuronal processes, do not provide information about total cellular AMPAR protein levels, whereas our protein crosslinking results may suggest an increase in total GluA1 and GluA2 protein levels after 30 min of BDNF incubation.
An interesting aspect of BDNF’s effects on AMPAR trafficking in different in vitro preparations (summarized above) is that they indicate the potential for BDNF to regulate both GluA2-containing AMPARs and GluA2-lacking Ca2+-permeable AMPARs (CP-AMPARs). Whether the type of AMPAR regulated reflects differences in cell types, brain regions, or experimental conditions is unclear. Our present results indicate that BDNF regulates GluA2-containing AMPARs (GluA1A2) in MSN in NAc/PFC co-cultures. This is consistent with our prior work in the same culture system demonstrating that synaptic scaling in MSN involves GluA1A2 receptors, despite the existence of a substantial population of homomeric GluA1 receptors in these cultured MSN (Sun & Wolf, 2009). However, we cannot rule out a role for homomeric GluA1 receptors in rapid AMPAR trafficking between intracellular, extrasynaptic and synaptic pools elicited by D1 dopamine receptor stimulation or glycine-induced synaptic activation of MSN, as only GluA1 was measured in these studies (Sun et al., 2008). Returning to BDNF, while our current in vitro results indicate a selective effect on GluA1A2 receptors, we obtained a different result when we examined BDNF’s effect on AMPAR trafficking in the NAc of adult rats (Li & Wolf, 2011). In this prior study, we observed that micro-injection of BDNF into the core subregion increased cell surface levels of GluA1 but not GluA2 or GluA3 30 min after BDNF injection, suggesting increased CP-AMPAR levels; this effect was transient, returning to baseline 3 h post-injection (Li & Wolf, 2011). Potential explanations for the different findings include the age of the neurons and the effects of many neurotransmitters and modulators in the intact NAc that are absent in cultures.
ERK activation is necessary for the acute BDNF-induced increase in surface AMPAR expression
We next wanted to determine which pathways downstream of BDNF signaling are activated by acute and long-term treatment of NAc MSNs with BDNF. We chose to investigate ERK1/2, AKT, mTOR, and eEF2. ERK is activated through the Ras-MAPK/ERK signaling cascade, downstream of BDNF binding to the TrkB receptor, via a series of phosphorylation events (Frank et al., 1996). ERK activation subsequently leads to activation of transcription factors such as cAMP-response element binding protein (CREB) (Fields et al., 1997) and thus influences transcriptional events (Whitmarsh, 2007), potentially including BDNF-induced changes in the synthesis of new AMPAR subunits (Caldeira et al., 2007; Narisawa-Saito et al., 2002; see also Narisawa-Saito et al., 1999a,b). However, ERK also has non-transcriptional actions (Sweatt, 2004), including an important role in AMPAR trafficking (see below). AKT phosphorylation is indicative of activation of the PI3 kinase signaling pathway, another downstream target of BDNF (Sarbassov et al., 2005). BDNF can trigger local protein synthesis through activation of mTOR (Takei et al., 2004) and recent evidence has shown that the activation of mTOR by BDNF increases GluA1 transcription (Slipczuk et al., 2009). The protein eEF2, which is required for the elongation step of translation (Ryazanov et al., 1988), is also involved in local protein synthesis stimulated by BDNF (Inamura et al., 2005).
We observed that acute (30 min) stimulation of NAc cultures with BDNF leads to the phosphorylation of ERK and AKT, but not mTOR or eEF2. This discovery led us to the next experiment in which we inhibited MEK1/2 (upstream from ERK1/2) with the inhibitor U0126 or PI3 kinase (upstream of AKT) with the inhibitor LY294002 to determine if activation of either pathway is required for BDNF-induced changes in AMPAR surface expression. We found that MEK1/2 inhibition and not PI3 kinase inhibition prevented the acute BDNF-induced increase in AMPAR surface expression. This is in agreement with work showing that ERK activation is necessary for BDNF-induced synaptic delivery of GluA1 and GluA4 in turtle brain stem (Li & Keifer, 2008; 2009). Similarly, work in other systems has demonstrated that ERK activation is part of the cascade leading to activity-dependent increases in surface AMPAR expression (Zhu et al., 2002; Qin et al., 2005; Keifer et al., 2007). Our lab has also shown a positive relationship between ERK activation and AMPAR surface expression in the adult rat NAc during withdrawal from repeated cocaine injections (Boudreau et al., 2007, 2009). Of course, many additional signaling pathways also contribute to AMPAR synaptic insertion (Derkach et al., 2007; Shepherd & Huganir, 2007; Anggono & Huganir, 2012).
BDNF mediates “scaling down” of AMPAR surface expression in NAc neurons
Synaptic scaling is a form of homeostatic plasticity in which prolonged increases in neuronal activity lead to a compensatory decrease in excitatory transmission that is often mediated by a decrease in synaptic AMPAR levels (scaling down). Prolonged activity blockade produces the opposite effect, that is, an increase in excitatory transmission that is often mediated by increased synaptic AMPAR levels (scaling up) (Turrigiano, 2008). As BDNF is synthesized and released in an activity dependent manner (Lu, 2003), BDNF levels could serve as signals for changes in neuronal activity and thereby mediate synaptic scaling. Indeed, extensive studies in visual cortex have shown that decreasing neuronal activity (e.g., with TTX) leads to a decrease in endogenous BDNF release that is responsible for scaling up of mEPSC amplitude in pyramidal neurons. Thus, the increase in mEPSC amplitude produced by prolonged activity blockade with TTX was prevented by co-application of BDNF, whereas scavenging BDNF with a TrkB-IgG fusion protein reproduced the effect of activity blockade (Rutherford et al., 1998). On the other hand, 48 h incubation with BDNF alone did not decrease mEPSC amplitude (Rutherford et al., 1998), and scavenging BDNF did not affect scaling down produced by chronic depolarization with KCl (Leslie et al., 2001). Thus, BDNF is not a bidirectional mediator of synaptic scaling in pyramidal neurons of the visual cortex. While it mediates scaling up, other cellular messengers must be responsible for scaling down of synaptic strength in visual cortical pyramidal neurons in response to prolonged increases in activity (Rutherford et al., 1998; Turrigiano, 2008).
These prior findings were important in motivating us to test the effects of longer-term (24 h) BDNF exposure on AMPAR trafficking in NAc neurons. Whereas acute (30 min) BDNF treatment markedly increased AMPAR surface expression, the longer incubation produced the opposite effect, namely a decrease in AMPAR surface expression. Although this is not what would have been predicted based on results in visual cortex (above), we proceeded to test the possibility that, in NAc neurons, BDNF plays a role in scaling down of AMPAR surface expression in response to prolonged increases in activity. As expected from our past results (Sun & Wolf, 2009), 24 h treatment with the GABAA receptor antagonist bicuculline decreased GluA1 and GluA2 surface expression, indicative of scaling down. This effect was blocked when we co-applied the neurotrophin scavenger TrkB-Fc and thereby decreased BDNF signaling. Thus, increasing exogenous BDNF levels decreased AMPAR surface expression and blocking endogenous BDNF transmission with TrkB-Fc prevented bicuculline-induced scaling down of AMPAR surface expression. Together, these results suggest that increasing neuronal activity with bicuculline leads to increased BDNF release, which in turn scales down AMPAR surface expression. Although we did not distinguish synaptic from extrasynaptic AMPAR pools in the present study, we showed previously, in an identical co-culture system, that both synaptic and extrasynaptic AMPARs “scale up” in response to bicuculline; furthermore, scaling of surface AMPARs in response to activity blockade or enhancement is accompanied by parallel changes in total AMPAR subunit levels measured in permeabilized neurons (Sun & Wolf, 2009).
It is surprising that BDNF mediates scaling down of AMPAR surface expression in cultured NAc MSN (present results), but scaling up in pyramidal neurons in visual cortical cultures (Rutherford et al., 1998). One potentially relevant difference is that principal neurons of the NAc (MSN) are inhibitory GABAergic neurons, while those of the visual cortex are excitatory pyramidal neurons. Interestingly, whereas addition of exogenous BDNF does not alter mEPSC amplitude in pyramidal neurons in visual cortical cultures, it increases mEPSC amplitude in GABAergic interneurons in the same cultures (Rutherford et al., 1998). This is intriguing, as it shows that the nature of BDNF’s effect can vary with cell phenotype. However, this effect in the cortical GABAergic interneurons is still opposite to what we observe in GABAergic MSN of the NAc, so variables other than GABAergic phenotype must be important. Further supporting this, whereas long-term activity blockade (TTX or AMPAR antagonists) produces scaling up of AMPARs in cultured NAc MSN (Sun & Wolf, 2009), the same activity blockade fails to scale up mEPSC amplitude in GABAergic interneurons in visual cortical cultures (Turrigiano et al., 1998). Another consideration is that activity levels in NAc-PFC co-cultures are likely to be lower than in visual cortical cultures, due to a population of GABAergic principal neurons in the former but not the latter. Therefore, BDNF production in cortical cultures may be near-saturating under control conditions (see Rutherford et al., 1998 for discussion), whereas lower basal BDNF production in NAc-PFC co-cultures may render BDNF a more useful signal for communicating increases in neuronal activity. Finally, the source of BDNF in the two culture systems is different. In the case of visual cortical cultures, TTX inhibits neuronal activity by acting on the same cells in which scaling is measured (pyramidal neurons), whereas in the case of the NAc-PFC co-cultures, the TTX is acting on the excitatory PFC neurons to reduce activity, whereas we are measuring scaling in the NAc MSN that are postsynaptic to these PFC neurons.
It remains unclear how elevation of BDNF levels for 24 h leads to scaling down of AMPAR levels. In parallel experiments, we showed that this BDNF treatment activates ERK but not AKT, mTOR or EF2; however, MEK1/2 inhibition did not prevent BDNF-induced decreases in AMPAR surface expression. Future studies should examine the possible role of BDNF signaling through PLCγ and whether BDNF regulates signaling molecules that have previously been implicated in bicuculline-induced scaling down of AMPAR transmission, such as Homer1a (Hu et al., 2010) and MeCP2 (Qiu et al., 2012; Zhong et al., 2012).
BDNF and cocaine-induced plasticity
BDNF has been shown to play an important role in animal models of cocaine addiction (McGinty et al., 2010; Pickens et al., 2011; Loweth et al., 2013) as has its downstream target, ERK (Lu et al., 2006; Girault et al., 2007; Whitfield et al., 2011). BDNF is highly expressed in many addiction-related brain regions (Meredith et al., 2002). Although BDNF mRNA is present in the NAc (e.g., Graham et al., 2007; Bahi et al., 2008), brain regions that project to the NAc, such as the PFC, provide a major source of BDNF through axonal transport (Altar et al., 1997). Many different types of cocaine exposure increase BDNF levels in the NAc (e.g., Grimm et al., 2003; Graham et al., 2007; Bahi et al., 2008), although the consequences for cocaine seeking are complex and depend on the regimen, the withdrawal time, and the subregion of NAc studied (Graham et al., 2007; 2009; Bahi et al., 2008; Berglind et al., 2007, 2009; Whitfield et al., 2011; Li et al., 2013). In some cases, BDNF appears to modify addiction-related behaviors through its effects on glutamate transmission in the NAc (e.g., Berglind et al., 2009). However, even though both BDNF (above) and AMPARs (Wolf, 2010) are clearly important for addiction-related plasticity in the NAc, and prior studies in other cell types demonstrate that BDNF regulates AMPAR trafficking (Narisawa-Saito et al., 2002; Caldeira et al., 2007; Li & Keifer, 2008; 2009; Keifer & Zheng, 2010), it has been difficult to understand the relationship between cocaine’s effects on BDNF and AMPAR trafficking. By using PFC-NAc co-cultures to directly explore this relationship, we hope to define “rules” for BDNF-AMPAR relationships in NAc MSN that will help us interpret in vivo findings. For example, our 30 min results suggest that acute increases in BDNF levels may acutely increase AMPAR levels in NAc neurons. This could help explain a report that transient elevation of BDNF in the NAc shell after cocaine self-administration is important for promoting cocaine use and relapse in cocaine-experienced animals (Graham et al., 2007), since elevation of NAc AMPAR levels is associated with increased motivation for cocaine (Wolf, 2010). Our 24 h results may be relevant to understanding consequences of the long-term elevation of BDNF observed in the NAc and other regions during withdrawal from extended access cocaine self-administration (Grimm et al., 2003). This type of cocaine exposure leads to a progressive intensification (“incubation”) of cocaine craving (Pickens et al., 2011) that ultimately depends upon increased CP-AMPAR levels in the NAc (Conrad et al., 2008). The finding that 24 h elevation of BDNF decreases AMPAR levels in cultured NAc neurons suggests that withdrawal-dependent in vivo increases in BDNF and CP-AMPAR levels are not causally related, a prediction that has recently received experimental support (Li et al., 2013).
It is important to emphasize that there are many caveats associated with extrapolating from the present in vitro results to the role of BDNF in the intact NAc of cocaine-exposed rodents. A major limitation of our in vitro paradigm is that adding BDNF to the media obviously does not mimic physiological patterns of release. Indeed, acute and gradual increases in BDNF concentration elicit different effects on synaptic transmission (Ji et al., 2010). There are also concerns about TrkB desensitization (Almeida et al., 2005). Furthermore, our co-culture model utilizes immature neurons and does not reproduce the full circuitry that regulates MSN activity. Despite these limitations, our results demonstrate that BDNF can regulate AMPAR trafficking in NAc MSN on different time-scales. This is relevant not only to addiction-related plasticity, but also to another pathological situation – social defeat and resultant social avoidance – that is mediated by a persistent elevation of BDNF signaling in the NAc (Berton et al., 2006; Krishnan et al., 2007).
Acknowledgements
This work was supported by US Public Health Service grants DA015835 and DA029099 (M.E.W.) and postdoctoral National Research Service Award DA030844 (J.A.L.).
Footnotes
The authors declare no conflict of interest.
References
- Almeida RD, Manadas BJ, Melo CV, Gomes JR, Mendes CS, Grãos MM, Carvalho RF, Carvalho AP, Duarte CB. Neuroprotection by BDNF against glutamate-induced apoptotic cell death is mediated by ERK and PI3-kinase pathways. Cell Death Differ. 2005;12:1329–1343. doi: 10.1038/sj.cdd.4401662. [DOI] [PubMed] [Google Scholar]
- Altar CA, Cai N, Bliven T, Juhasz M, Conner JM, Acheson AL, Lindsey RM, Wiegland SJ. Anterograde transport of brain-derived neurotrophic factor and its role in the brain. Nature. 1997;389:856–860. doi: 10.1038/39885. [DOI] [PubMed] [Google Scholar]
- Anggono V, Huganir RL. Regulation of AMPA receptor trafficking and synaptic plasticity. Curr. Opin. Neurobiol. 2012;22:461–469. doi: 10.1016/j.conb.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bahi A, Boyer F, Dreyer J-L. Role of accumbens BDNF and TrkB in cocaine-induced psychomotor sensitization, conditioned place preference and reinstatement in rats. Psychopharmacology. 2008;199:169–182. doi: 10.1007/s00213-008-1164-1. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, See RE, Fuchs RA, Ghee SM, Whitfield TW, Jr, Miller SW, McGinty JF. A BDNF infusion into the medial prefrontal cortex suppresses cocaine seeking in rats. Eur. J. Neurosci. 2007;26:757–766. doi: 10.1111/j.1460-9568.2007.05692.x. [DOI] [PubMed] [Google Scholar]
- Berglind WJ, Whitfield TW, Jr, LaLumiere RT, Kalivas PW, McGinty JF. A single intra-PFC infusion of BDNF prevents cocaine-induced alterations in extracellular glutamate within the nucleus accumbens. J. Neurosci. 2009;29:3715–3719. doi: 10.1523/JNEUROSCI.5457-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berton O, McClung CA, Dileone RJ, Krishnan V, Renthal W, Russo SJ, Graham D, Tsankova NM, Bolanos CA, Rios M, Monteggia LM, Self DW, Nestler EJ. Essential role of BDNF in the mesolimbic dopamine pathway in social defeat stress. Science. 2006;311:864–868. doi: 10.1126/science.1120972. [DOI] [PubMed] [Google Scholar]
- Binder DK, Routbort MJ, Ryan RE, Yancopoulos GD, McNamara JO. Selective inhibition of kindling development by intraventricular administration of TrkB receptor body. J. Neurosci. 1999;19:1424–1436. doi: 10.1523/JNEUROSCI.19-04-01424.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Wolf ME. Behavioral sensitization to cocaine is associated with increased AMPA receptor surface expression in the nucleus accumbens. J. Neurosci. 2005;25:9144–9151. doi: 10.1523/JNEUROSCI.2252-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Reimers JM, Milovanovic M, Wolf ME. Surface AMPA receptors in the rat nucleus accumbens increase during cocaine withdrawal but internalize after cocaine challenge in association with altered activity of mitogen-activated protein kinases. J. Neurosci. 2007;27:10621–10635. doi: 10.1523/JNEUROSCI.2163-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Ferrario CR, Glucksman MJ, Wolf ME. Signaling pathway adaptations and novel protein kinase A substrates related t o behavioral sensitization to cocaine. J. Neurochem. 2009;110:363–377. doi: 10.1111/j.1471-4159.2009.06140.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boudreau AC, Milovanovic M, Conrad KL, Nelson C, Ferrario CR, Wolf ME. A protein cross-linking assay for measuring cell surface expression of glutamate receptor subunits in the rodent brain after in vivo treatments. Chapter 5, Units 5.30, 1–19. Curr. Protocols Neurosci. 2012 doi: 10.1002/0471142301.ns0530s59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog. Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Caldeira MV, Melo CV, Pereira DB, Carvalho R, Correia SS, Backos DS, Carvalho AL, Esteban JA, Duarte CB. Brain-derived neurotrophic factor regulates the expression and synaptic delivery of α-amino-3-hydroxy-5-methyl-4-isoxazole propionic acid receptor subunits in hippocampal neurons. J. Biol. Chem. 2007;282:12619–12628. doi: 10.1074/jbc.M700607200. [DOI] [PubMed] [Google Scholar]
- Carvalho AL, Caldeira MV, Santos SD, Duarte CB. Role of the brain-derived neurotrophic factor at glutamatergic synapses. Brit. J. Pharmacol. 2008;153(S1):310–324. doi: 10.1038/sj.bjp.0707509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad KL, Tseng KY, Uejima JL, Reimers JM, Heng L, Shaham Y, Marinelli M, Wolf ME. Formation of accumbens GluR2-lacking AMPA receptors mediates incubation of cocaine craving. Nature. 2008;454:118–121. doi: 10.1038/nature06995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Derkach VA, Oh MC, Guire ES, Soderling TR. Regulatory mechanisms of AMPA receptors in synaptic plasticity. Nat. Rev. Neurosci. 2007;8:101–113. doi: 10.1038/nrn2055. [DOI] [PubMed] [Google Scholar]
- Fields RD, Eshete F, Stevens B, Itoh K. Action potential-dependent regulation of gene expression: temporal specificity in Ca2+, cAMP-responsive element binding proteins, and mitogen-activated protein kinase signaling. J. Neurosci. 1997;17:7252–7266. doi: 10.1523/JNEUROSCI.17-19-07252.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frank L, Ventimiglia R, Anderson K, Lindsay RM, Rudge JS. BDNF down-regulates neurotrophin responsiveness, TrkB Protein and TrkB mRNA levels in cultured rat hippocampal neurons. Eur. J. Neurosci. 1996;8:1220–1230. doi: 10.1111/j.1460-9568.1996.tb01290.x. [DOI] [PubMed] [Google Scholar]
- Gao C, Wolf ME. Dopamine receptors regulate NMDA receptor surface expression in prefrontal cortex neurons. J. Neurochem. 2008;106:2489–2501. doi: 10.1111/j.1471-4159.2008.05597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girault JA, Valjent E, Caboche J, Herve D. ERK2: a logical AND gate critical for drug-induced plasticity. Curr Opin Pharmacol. 2007;7:77–85. doi: 10.1016/j.coph.2006.08.012. [DOI] [PubMed] [Google Scholar]
- Graham DL, Edwards S, Bachtell RK, DiLeone RJ, Rios M, Self DW. Dynamic BDNF activity in nucleus accumbens with cocaine use increases self-administration and relapse. Nat. Neurosci. 2007;10:1029–1037. doi: 10.1038/nn1929. [DOI] [PubMed] [Google Scholar]
- Graham DL, Krishnan V, Larson EB, Graham A, Edwards S, Bachtell RK, Simmons D, Gent LM, Berton O, Bolanos CA, DiLeone RJ, Parada LS, Nestler EJ, Self DW. Tropomyosin-Related Kinase B in the mesolimbic dopamine system: region-specific effects on cocaine reward. Biol. Psychiat. 2009;65:696–701. doi: 10.1016/j.biopsych.2008.09.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm JW, Lu L, Hayashi T, Hope BT, Su TP, Shaham Y. Time-dependent increases in brain-derived neurotrophic factor protein levels within the mesolimbic dopamine system after withdrawal from cocaine: implications for incubation of cocaine craving. J. Neurosci. 2003;23:742–747. doi: 10.1523/JNEUROSCI.23-03-00742.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu J-H, Park JM, Park S, Xiao B, Dehoff MH, Kim S, Hayashi T, Schwarz MK, Huganir RL, Seeburg PH, Linden DJ, Worley PF. Homeostatic scaling requires Group I mGluR activation mediated by Homer1a. Neuron. 2010;68:1128–1142. doi: 10.1016/j.neuron.2010.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inamura N, Nawa H, Takei N. Enhancement of translation elongation in neurons by brain-derived neurotrophic factor: implications for mammalian target of rapamycin signaling. J. Neurochem. 2005;95:1438–1445. doi: 10.1111/j.1471-4159.2005.03466.x. [DOI] [PubMed] [Google Scholar]
- Ji Y, Lu Y, Yang F, Shen W, Tang TT, Feng L, Duan S, Lu B. Acute and gradual increases in BDNF concentration elicit distinct signaling and functions in neurons. Nat. Neurosci. 2010;13:302–309. doi: 10.1038/nn.2505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Iwakura Y, Narisawa-Saito M, Ibaraki K, Xiong H, Watanabe M, Hayashi Y, Takei N, Nawa H. Brain-derived neurotrophic factor signal enhances and maintains the expression of AMPA receptor-associated PDZ proteins in developing cortical neurons. Dev. Biol. 2003;263:216–230. doi: 10.1016/j.ydbio.2003.07.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jourdi H, Hsu Y-T, Zhou M, Qin Q, Bi X, Baudry M. Positive AMPA receptor modulation rapidly stimulates BDNF release and increases dendritic mRNA translation. J. Neurosci. 2009;29:8688–8697. doi: 10.1523/JNEUROSCI.6078-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaplan DR, Miller FD. Neurotrophin signal transduction in the nervous system. Curr. Opin. Neurobiol. 2000;10:381–391. doi: 10.1016/s0959-4388(00)00092-1. [DOI] [PubMed] [Google Scholar]
- Keifer J, Zheng ZQ, Zhu D. MAPK signaling pathways mediate AMPA receptor trafficking in an in vitro model of classical conditioning. J. Neurophysiol. 2007;97:2067–2074. doi: 10.1152/jn.01154.2006. [DOI] [PubMed] [Google Scholar]
- Keifer J, Zheng Z. AMPA receptor trafficking and learning. Eur. J. Neurosci. 2010;32:269–277. doi: 10.1111/j.1460-9568.2010.07339.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishnan V, Han MH, Graham DL, Berton O, Renthal W, Russo SJ, Laplant Q, Graham A, Lutter M, Lagace DC, Ghose S, Reister R, Tannous P, Green TA, Neve RL, Chakravarty S, Kumar A, Eisch AJ, Self DW, Lee FS, Tamminga CA, Cooper DC, Gershenfeld HK, Nestler EJ. Molecular adaptations underlying susceptibility and resistance to social defeat in brain reward regions. Cell. 2007;131:391–404. doi: 10.1016/j.cell.2007.09.018. [DOI] [PubMed] [Google Scholar]
- Leslie KR, Nelson SB, Turrigiano GG. Postsynaptic depolarization scales quantal amplitude in cortical pyramidal neurons. J. Neurosci. 2001;21:RC170, 1–6. doi: 10.1523/JNEUROSCI.21-19-j0005.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keifer J. Coordinate action of pre- and postsynaptic brain-derived neurotrophic factor is required for AMPAR trafficking and acquisition in vitro classical conditioning. Neuroscience. 2008;155:686–697. doi: 10.1016/j.neuroscience.2008.06.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Keifer J. BDNF-induced delivery of AMPAR subunits is differentially dependent on NMDA receptors and requires ERK. Neurobiol. Learn. Mem. 2009;91:243–249. doi: 10.1016/j.nlm.2008.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Wolf ME. Brain-derived neurotrophic factor rapidly increases AMPA receptor surface expression in rat nucleus accumbens. Eur. J. Neurosci. 2011;34:190–198. doi: 10.1111/j.1460-9568.2011.07754.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, DeJoseph MR, Urban JH, Bahi A, Dreyer JL, Meredith GE, Ford KA, Ferrario CR, Loweth JA, Wolf ME. Different roles of BDNF in nucleus accumbens core versus shell during the incubation of cue-Induced cocaine craving and its long-term maintenance. J. Neurosci. 2013;33:1130–1142. doi: 10.1523/JNEUROSCI.3082-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loweth JA, Tseng KY, Wolf ME. Adaptations in AMPA receptor transmission in the nucleus accumbens contributing to incubation of cocaine craving. Neuropharmacol. 2013 doi: 10.1016/j.neuropharm.2013.04.061. [Epub May 30 2013] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu B. BDNF and activity-dependent synaptic modulation. Learning & Memory. 2003;10:86–98. doi: 10.1101/lm.54603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu L, Koya E, Zhai H, Hope BT, Shaham Y. Role of ERK in cocaine addiction. Trends Neurosci. 2006;29:695–703. doi: 10.1016/j.tins.2006.10.005. [DOI] [PubMed] [Google Scholar]
- Lu W, Shi Y, Jackson AC, Bjorgan K, During MJ, Sprengel R, Seeburg PH, Nicoll RA. Subunit composition of AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGinty JF, Whitfield TW, Jr, Berglind WJ. Brain-derived neurotrophic factor and cocaine addiction. Brain Res. 2010;1314:183–193. doi: 10.1016/j.brainres.2009.08.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meredith GE, Callen S, Scheuer DA. Brain-derived neurotrophic factor expression is increased in the rat amygdala, piriform cortex and hypothalamus following repeated amphetamine administration. Brain Res. 2002;949:218–227. doi: 10.1016/s0006-8993(02)03160-8. [DOI] [PubMed] [Google Scholar]
- Meredith GE, Baldo BA, Andrezjewski ME, Kelley AE. The structural basis for mapping behavior onto the ventral striatum and its subdivisions. Brain Struct. Funct. 2008;213:17–27. doi: 10.1007/s00429-008-0175-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakata H, Nakamura S. Brain-derived neurotrophic factor regulates AMPA receptor trafficking to post-synaptic densities via IP3R and TRPC calcium signaling. FEBS Lett. 2007;581:2047–2054. doi: 10.1016/j.febslet.2007.04.041. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Silva AJ, Yamaguchi T, Hayashi T, Yamamoto T, Nawa H. Growth factor-mediated Fyn signaling regulates alpha-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid (AMPA) receptor expression in rodent neocortical neurons. Proc. Natl. Acad. Sci. USA. 1999a;96:2461–2466. doi: 10.1073/pnas.96.5.2461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narisawa-Saito M, Carnahan J, Araki K, Yamaguchi T, Nawa H. Brain-derived neurotrophic factor regulates the expression of AMPA receptor proteins in neocortical neurons. Neuroscience. 1999b;88:1009–1014. doi: 10.1016/s0306-4522(98)00496-5. [DOI] [PubMed] [Google Scholar]
- Narisawa-Saito M, Iwakura Y, Kawamura M, Araki K, Kozaki S, Takei N, Nawa H. Brain-derived neurotrophic factor regulates surface expression of alpha-amino-3-hydroxy-5-methyl-4-isoxazoleproprionic acid receptors by enhancing the N-ethylmaleimide sensitive factor/GluR2 interaction in developing neocortical neurons. J. Biol. Chem. 2002;277:40901–40910. doi: 10.1074/jbc.M202158200. [DOI] [PubMed] [Google Scholar]
- Pickens CL, Airavaara M, Theberge F, Fanous S, Hope BT, Shaham Y. Neurobiology of the incubation of drug craving. Trends Neurosci. 2011;34:411–420. doi: 10.1016/j.tins.2011.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierce RC, Wolf ME. Psychostimulant-induced neuroadaptations in nucleus accumbens AMPA receptor transmission. In: AddictionPierce RC, Kenny PJ, editors. Cold Spring Harbor Perspectives in Medicine. Cold Spring Harbor, New York: Cold Spring Harbor Laboratory Press; 2013. pp. 121–134. Epub Dec 10, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin Y, Zhu Y, Baumgart JP, Stornetta RL, Seidenman K, Mack V, van Aelst L, Zhu JJ. State-dependent Ras signalling and AMPA receptor trafficking. Genes Dev. 2005;19:2000–2015. doi: 10.1101/gad.342205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu Z, Sylwestrak EL, Lieberman DN, Zhang Y, Liu X-Y, Ghosh A. The Rett syndrome protein MeCP2 regulates synaptic scaling. J. Neurosci. 2012;32:989–994. doi: 10.1523/JNEUROSCI.0175-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reimers JM, Milovanovic M, Wolf ME. Quantitative analysis of AMPA receptor subunit composition in addiction-related brain regions. Brain Res. 2011;1367:223–233. doi: 10.1016/j.brainres.2010.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Russo SJ, Mazei-Robison MS, Ables JL, Nestler EJ. Neurotrophic factors and structural plasticity in addiction. Neuropharmacology. 2009;56:73–82. doi: 10.1016/j.neuropharm.2008.06.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rutherford LC, Nelson SB, Turrigiano GG. BDNF has opposite effects on the quantal amplitude of pyramidal neuron and interneuron excitatory synapses. Neuron. 1998;21:521–530. doi: 10.1016/s0896-6273(00)80563-2. [DOI] [PubMed] [Google Scholar]
- Ryazanov AG, Shestakova EA, Natapov PG. Phosphorylation of elongation factor 2 by EF-2 kinase affects rate of translation. Nature. 1988;334:170–173. doi: 10.1038/334170a0. [DOI] [PubMed] [Google Scholar]
- Sarbassov DD, Guertin DA, Ali SM, Sabatini DM. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science. 2005;307:1098–1101. doi: 10.1126/science.1106148. [DOI] [PubMed] [Google Scholar]
- Sesack SR, Grace AA. Cortico-basal ganglia reward network: microcircuitry. Neuropsychopharmacology. 2010;35:27–47. doi: 10.1038/npp.2009.93. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu. Rev. Cell Dev. Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- Slipczuk L, Beckinschtein P, Katche C, Cammarota M, Izquierdo I, Medina JH. BDNF activates mTOR to regulate GluR1 expression required for memory formation. PLoS One. 2009;4:e6007. doi: 10.1371/journal.pone.0006007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Zhao Y, Wolf ME. Dopamine receptor stimulation modulates AMPA receptor synaptic insertion in prefrontal cortex neurons. J. Neurosci. 2005;25:7342–7351. doi: 10.1523/JNEUROSCI.4603-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Milovanovic M, Zhao Y, Wolf ME. Acute and chronic dopamine receptor stimulation modulates AMPA receptor trafficking in nucleus accumbens neurons cocultured with prefrontal cortex neurons. J. Neurosci. 2008;28:4216–4230. doi: 10.1523/JNEUROSCI.0258-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun X, Wolf ME. Nucleus accumbens neurons exhibit synaptic scaling that is occluded by repeated dopamine pre-exposure. Eur. J. Neurosci. 2009;30:539–550. doi: 10.1111/j.1460-9568.2009.06852.x. [DOI] [PubMed] [Google Scholar]
- Sweatt JD. Mitogen-activated protein kinases in synaptic plasticity and memory. Curr. Opin. Neurobiol. 2004;14:311–317. doi: 10.1016/j.conb.2004.04.001. [DOI] [PubMed] [Google Scholar]
- Takei N, Inamura N, Kawamura M, Namba H, Hara K, Yonezawa K, Nawa H. Brain-derived neurotrophic factor induces mammalian target of rapamycin-dependent local activation of translation machinery and protein synthesis in neuronal dendrites. J. Neurosci. 2004;24:9760–9769. doi: 10.1523/JNEUROSCI.1427-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thoenen H. Neurotrophins and neuronal plasticity. Science. 1995;270:593–598. doi: 10.1126/science.270.5236.593. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG, Leslie KR, Desai NS, Rutherford LC, Nelson SB. Activity-dependent scaling of quantal amplitude in neocortical neurons. Nature. 1998;391:892–895. doi: 10.1038/36103. [DOI] [PubMed] [Google Scholar]
- Turrigiano GG. The self-tuning neuron: synaptic scaling of excitatory synapses. Cell. 2008;135:422–435. doi: 10.1016/j.cell.2008.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vicario-Abejón C, Owens D, McKay R, Segal M. Role of neurotrophins in central synapse formation and stabilization. Nat. Rev. Neurosci. 2002;3:965–974. doi: 10.1038/nrn988. [DOI] [PubMed] [Google Scholar]
- Waterhouse EG, Xu B. New insights into the role of brain-derived neurotrophic factor in synaptic plasticity. Mol. Cell. Neurosci. 2009;42:81–89. doi: 10.1016/j.mcn.2009.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenthold RJ, Petralia RS, Blahos J, Niedzielski AS. Evidence for multiple AMPA receptor complexes in hippocampal CA1/CA2 neurons. J. Neurosci. 1996;16:1982–1989. doi: 10.1523/JNEUROSCI.16-06-01982.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitfield TW, Jr, Shi X, Sun WL, McGinty JF. The suppressive effect of an intra-prefrontal cortical infusion of BDNF on cocaine-seeking is Trk receptor and extracellular signal-regulated protein kinase mitogen-activated protein kinase dependent. J. Neurosci. 2011;31:834–842. doi: 10.1523/JNEUROSCI.4986-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitmarsh AJ. Regulation of gene transcription by mitogen-activated protein kinase signaling pathways. Biochim Biophys Acta. 2007;1773:1285–1298. doi: 10.1016/j.bbamcr.2006.11.011. [DOI] [PubMed] [Google Scholar]
- Wolf ME. The Bermuda triangle of cocaine-induced neuroadaptations. Trends Neurosci. 2010;33:391–398. doi: 10.1016/j.tins.2010.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhong X, Li H, Chang Q. MeCP2 phosphorylation is required for modulating synaptic scaling through mGluR5. J. Neurosci. 2012;32:12841–12847. doi: 10.1523/JNEUROSCI.2784-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JJ, Qin Y, Zhao M, van Aiest L, Malinow R. Ras and Rap control AMPA receptor trafficking during synaptic plasticity. Cell. 2002;110:443–455. doi: 10.1016/s0092-8674(02)00897-8. [DOI] [PubMed] [Google Scholar]