Abstract
Knowing the context of a discourse is an essential prerequisite for comprehension. Here we used functional magnetic resonance imaging (fMRI) to disclose brain networks supporting context-dependent speech comprehension. During fMRI, 20 participants listened to 1-min spoken narratives preceded by pictures that were either contextually matching or mismatching with the narrative. Matching pictures increased narrative comprehension, decreased hemodynamic activity in Broca׳s area, and enhanced its functional connectivity with left anterior superior frontal gyrus, bilateral inferior parietal cortex, as well as anterior and posterior cingulate cortex. Further, the anterior (BA 45) and posterior (BA 44) portions of Broca׳s area differed in their functional connectivity patterns. Both BA 44 and BA 45 have shown increased connectivity with right angular gyrus and supramarginal gyrus. Whereas BA 44 showed increased connectivity with left angular gyrus, left inferior/middle temporal gyrus and left postcentral gyrus, BA 45 showed increased connectivity with right posterior cingulate cortex, right anterior inferior frontal gyrus, lateral occipital cortex and anterior cingulate cortex. Our results suggest that a fronto-parietal functional network supports context-dependent narrative comprehension, and that Broca׳s area is involved in resolving ambiguity from speech when appropriate contextual cues are lacking.
Keywords: Broca׳s area, Wernicke׳s area, fMRI, PPI, ISC, Functional connectivity, Narrative, Speech comprehension
1. Introduction
Most of us have experienced instances of joining a conversation without knowing the topic: while being able to recognise individual words and sentences, it is difficult to comprehend what is being said when lacking information about the context within which the conversation takes place. However, as soon as the topic is revealed, the subsequent as well as preceding conversation becomes clear. Previous behavioural studies have established that discourse comprehension requires knowledge of the relevant context (Bransford & Johnson, 1972; Dooling & Lachmann, 1971; Swinney, 1979). Processing of new semantic information is facilitated by predictions, based on currently available semantic representation (i.e. schema) of the narrative context. When predictions are mismatching with the incoming narrative – for example when one׳s conversation partner abruptly changes the topic – they impair understanding, as the inappropriate predictions are violated by the speech stream (Wlotko & Federmeier, 2012).
Linguistic processing during context-dependent comprehension requires a number of components: mental lexicon and associated retrieval operations, selection and integration of lexical elements into larger structures (unification), as well as attention and control/error monitoring (see, e.g., the MUC model, Hagoort, 2005). During natural speech comprehension, these operations may form a loop where incoming information is integrated with previous knowledge as early as possible (Wlotko & Federmeier, 2012). But since spoken discourse unfolds over time, critical contextual information may often be available relatively late, therefore increasing the relevance of knowing context in advance. Contextual effects in natural speech comprehension thus provide a realistic model for studying the changes in brain networks subserving semantic processing. Semantic processing on local (word or sentence) and global (narrative) levels may proceed differently. Here we study the neural mechanisms underlying use of contextual information to resolve semantic ambiguity at the narrative level.
1.1. Anatomical and functional basis of the linguistic processing network
The classic linguistic processing network comprises Broca׳s area in the left inferior frontal gyrus (IFG), and left inferior parietal cortex (IPC) encompassing angular (AG) and supramarginal gyri (SMG) that together with left middle (MTG) and superior temporal gyri (STG) form the classical Wernicke׳s area (Binder, Desai, Graves, & Conant, 2009). Broca׳s area is involved in a wide range of phonological, syntactic and semantic functions, including semantic selection (Bookheimer, 2002; Hagoort, 2005; Thompson-Schill, D'Esposito, Aguirre & Farah, 1997; Vigneau et al., 2006). Wernicke׳s area, in turn, is involved in both local and global semantic processing, e.g. initial access to and retrieval of semantic representations of words (Bookheimer, 2002; Jung-Beeman, 2005; Mesulam, 1998), as well as semantic integration during discourse processing (Binder et al., 2009; Humphries, Binder, Medler, & Liebenthal, 2006; Humphries, Binder, Medler, & Liebenthal, 2007). While the left hemisphere dominates in linguistic functions, the contribution of the right hemisphere is also significant (Bookheimer, 2002; Manenti, Cappa, Rossini, & Miniussi, 2008; Zempleni, Haverkort, Renken, & Stowe, 2007b; Zempleni, Renken, Hoeks, Hoogduin, & Stowe, 2007a). The exact boundaries of both Wernicke׳s (Binder et al., 2009; DeWitt & Rauschecker, 2012; Mesulam, 1998) and Broca׳s (Amunts et al., 1999, 2010) areas are still debated, but most researchers agree that key aspects of language processing are undertaken in this fronto-temporo-parietal language system, whose activation profile varies depending on current linguistic processing demands (Abrams et al., 2013; Tyler & Marslen-Wilson, 2008).
Several studies have confirmed the anatomical connections between Broca׳s area and superior/middle temporal gyrus as well as IPC (Catani, Jones, & Ffytche, 2005; Frey, Campbell, Pike, & Petrides, 2008; Hickok & Poeppel, 2004; Saur et al., 2008; Turken & Dronkers, 2011). The dorsal pathway, which runs from premotor cortex (including BA 44 in Broca׳s area) via arcuate fasciculus/superior longitudinal fasciculus (AF/SLF) to IPC and STG, contributes to sensory-motor mapping of sounds to articulations. The ventral pathway connects prefrontal cortex and BA 45 in Broca׳s area to middle temporal lobe via the extreme capsule fibre system (ECFS) and contributes to linguistic processing of sounds and mapping sounds to meanings (Friederici, 2012; Hickok & Poeppel, 2004; Rauschecker & Scott, 2009). It was also suggested that the dorsal pathway not only supports auditory-to-motor mapping, but also processing of syntactically complex sentences (Friederici, 2009, 2012; Friederici, Bahlmann, Heim, Schubotz, & Anwander, 2006) and can be segregated functionally into phonological and lexical-semantic processing systems (Friederici, 2012; Glasser & Rilling, 2008).
1.2. Broca׳s area׳s role in contextual understanding
Broca׳s area is a good candidate hub for the hypothesised network of brain areas involved in contextual understanding. Specifically, activation of anterior portion of Broca׳s area (BA 45) is increased when processing sentences with high rather than low ambiguity (Rodd, Davis, & Johnsrude, 2005), and participants with lesions spanning Broca׳s area (BA 44 and 45) fail to use contextually relevant information for facilitating selection of word meaning (Bedny, Hulbert, & Thompson-Schill, 2007). Broca׳s area also supports selection among semantic information held in working memory, such as decisions about task-relevant attributes of word meaning (Gabrieli, Poldrack, & Desmond, 1998; Moss et al., 2005; Thompson-Schill, D'Esposito, Aguirre & Farah, 1997). These local semantic processing functions suggest that Broca׳s area could contribute to contextual understanding by guiding selection and integration of the task-relevant text or speech features (Hagoort, 2005).
Broca׳s area can be further divided into BA 44 and BA 45 (Anwander, Tittgemeyer, von Cramon, Friederici, & Knösche, 2007; Poldrack et al., 1999). In the anatomical connectivity signatures of BA 44, the dorsal AF/SLF pathway is more dominant, and for BA 45 the ECFS tracts are more prominent (Anwander et al., 2007; Frey et al., 2008). Functional imaging studies also support this division (Amunts et al., 2004; Cooper, Hasson, & Small, 2011; Gough, Nobre, & Devlin, 2005; Hasson, Nusbaum, & Small, 2007; Poldrack et al., 1999). For example, Hagoort (2005) proposed that the posterior portions (BA 44) contribute to phonological, middle portions (BA 44/45) to syntactic and anterior portions (BA 45 and 47) to semantic unification. In line with this, during written word processing the anterior–ventral part of Broca׳s area supports semantic processing, whereas posterior–dorsal part contributes to both semantic and phonological processing (Poldrack et al., 1999). Hemodynamic activity in BA 44 is more correlated with regions involved in phonological processing, while activity in BA 45 correlates more strongly with regions attributed to syntactic and semantic processing, and it was suggested that unification components for each linguistic modality in Broca׳s area had a corresponding integration/memory component in posterior temporal cortex (Xiang, Fonteijn, Norris, & Hagoort, 2010). Another study revealed stronger functional connectivity from BA 45 to posterior occipital and temporal cortices for semantic stimuli, i.e. words than for pseudowords, letters or false fonts, while BA 44 showed similar connectivity with occipital and temporal cortices during processing of both words and phonological stimuli (pseudowords and letters). While words, pseudo-words and letters activated both subregions, the connectivity patterns indicated anterior Broca׳s area involvement in assessment of semantic representations, while posterior Broca׳s area connectivity profile corresponds to access to phonological representations associated with words (Bokde, Tagamets, Friedman, & Horwitz, 2001). Taken together, these findings suggest that in particular the anterior parts of Broca׳s area might be critical for context-dependent processing.
1.3. Parietal and cingulate cortices in contextual understanding
The IPC and AG are heteromodal areas that have been implicated in a wide range of linguistic functions. Bilateral IPC is involved in initial access to semantic representations of words, giving them meaning (Jung-Beeman, 2005; Mesulam, 1998) and left AG is involved in global semantic processing during integration of linguistic material (Binder et al., 2009; Humphries et al., 2006, 2007). Left AG was reported in studies addressing semantic decisions and integration of prior experiences, for example, while solving problems that require extended periods of computation, or planning of future behaviour (Binder et al., 1999), memorisation of semantic information (Hasson et al., 2007) and combinatorial processes (Humphries et al., 2007). Activation of left parietal regions, including AG, has been reported while subjects make word versus pseudo-word decisions, but also during memory-intensive narrative comprehension, and top-down predictions of semantic content (for review see Price (2012)). Moreover, activation in bilateral IPC (including AG) increases as a function of complexity of linguistic stimulation from words to sentences to narratives (Xu, Kemeny, Park, Frattali, & Braun, 2005), suggesting that bilateral IPC may be involved in complex linguistic processing. Taken together, these studies suggest that left IPC is involved in contextual speech processing. For example, left IPC may function as a part of a hierarchical semantic process. As relevant contextual information facilitates top-down predictions of the semantic content, left IPC could be recruited when a matching context is provided during narrative listening. On earlier stages of linguistic processing left IPC might contribute to access, retrieval and maintenance of semantic representations of the verbal content. On subsequent stages of linguistic processing left IPC might support integration of linguistic information.
Finally, since contextual cues provide a cognitive filter for selection of information, constant error monitoring and control for consistency between the narrative and the contextual schema is required (Hagoort, 2005). Anterior cingulate cortex (ACC) is involved in a wide range of executive functions (Abutalebi et al., 2012; Paus, 2001), such as error monitoring (Botvinick, Cohen, & Carter, 2004; Carter, Braver, Barch, Botvinick, Noll & Cohen, 1998), selective attention (Crottaz-Herbette & Menon, 2006), and inhibition of responses (Cabeza & Nyberg, 2000). Extensive structural and functional links between ACC and prefrontal cortex suggest that the ACC could subserve general cognitive control function during narrative processing (Hagoort, 2005; Paus, 2001). Accordingly, functional connections between Broca׳s region and ACC might support monitoring of consistency between narrative and the context-providing schemas.
1.4. The current study
Here we extend the previous research on contextual effects and coherence of text and context (Maguire, Frith, & Morris, 1999; Martín-Loeches, Casado, Hernández-Tamames, & Alvarez-Linera, 2008; St George, Kutas, Martinez, & Sereno, 1999) by addressing both regional responses and functional connectivity of the brain׳s language network during context-dependent speech processing. To delineate the cortical network for context-dependent speech comprehension, participants listened to spoken narratives where contextual processing is manipulated using pictorial context, while their haemodynamic brain activity was measured with fMRI. We hypothesised that listening to ambiguous narratives with a semantically mismatching context would increase semantic selection demands due to lacking contextual cues, which would be reflected in increased activation in Broca׳s area. In addition, we hypothesised that Broca׳s area would function as the central “hub” for the fronto-temporo-parietal brain network for context-dependent speech processing. In this network, Broca׳s area is interacting with IPC to perform initial access and subsequent integration of semantic representations, and with ACC to monitor the consistency of narrative in relation to context.
2. Materials and methods
2.1. Participants
Twenty right-handed healthy adults (8 males, 12 females, ages 21–47 years, mean age 26 years) with normal or corrected to normal vision and normal hearing (self-reported) volunteered for the study. Individuals with a history of neurological or psychiatric diseases, or current medication affecting the central nervous system, were excluded. All subjects were compensated for their time, and signed informed consent forms, approved by the ethics-committee of the Helsinki and Uusimaa Hospital District.
2.2. Stimuli and experimental design
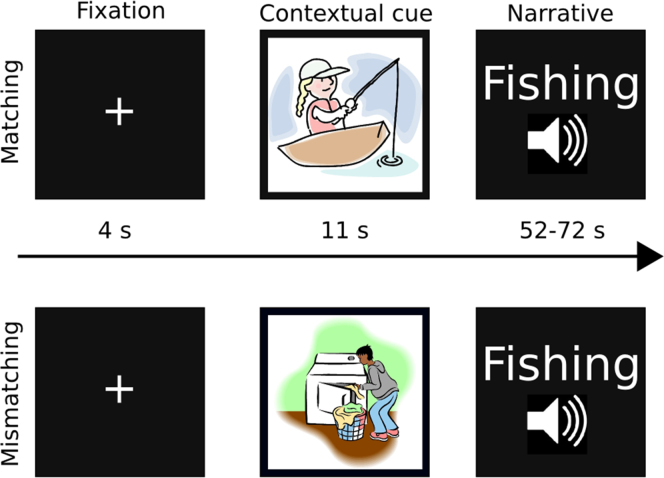
Stimuli consisted of 20 ambiguous narratives spoken in Finnish, with average duration of 62±10 s (Fig. 1). Each narrative described a complex sequence of actions such as those involved in doing laundry without providing contextual cues (e.g. without mentioning items such as washing machine, laundry soap, or actions such as washing or filling a machine) that would have revealed the action being described (c.f. Bransford and Johnson, 1972; see Appendix A for example; narratives with translations are available from corresponding author). The narratives were thus difficult to comprehend without access to relevant contextual information, but readily comprehensible when appropriate contextual cues were provided. The sentences followed the rules of Finnish grammar and contained no metaphorical vocabulary; thus their lexical meaning was accessible. In a pilot experiment, 30 independent subjects read 30 narratives preceded by either mismatching or matching picture. Subsequently they evaluated the comprehensibility of each narrative, as well as congruency of each narrative-picture pair. Twenty narratives with highest comprehension and congruency ratings in matching and lowest in mismatching context condition were selected for the fMRI experiment.
Fig. 1.
Sample trials with matching (top) and mismatching (bottom) contextual cues. Narratives described complex action sequences such as fishing in general terms that were ambiguous without the contextual cues. Before each narrative, participants saw a contextually matching or mismatching visual cue.
The selected narratives were recorded by a male voice in an acoustically shielded room. During fMRI, the stimuli were delivered using Presentation software (Neurobehavioral Systems Inc., Albany, California, USA). The narratives were played to the subjects with an UNIDES ADU2a audio system (Unides Design, Helsinki, Finland) via plastic tubes through porous EAR-tip (Etymotic Research, ER3, IL, USA) earplugs. Sound intensity was adjusted for each subject to be loud enough to be heard over the scanner noise. Contextual cue pictures were back-projected on a semitransparent screen using a 3-micromirror data projector (Christie X3, Christie Digital Systems Ltd., Mönchengladbach, Germany), and reflected via a mirror to the subject. The viewing distance was 34 cm, and the width of the projected image was 28 cm corresponding to approximately 39.5° in the visual field.
Participants listened to the narratives in a fixed, pseudo-random order while being scanned. The picture-narrative congruency was counterbalanced so that every participant received exactly the same auditory stimulation, yet the picture context for each narrative was different across the two groups. Each narrative was preceded by either matching or mismatching picture for 11 s, followed by a grey fixation cross that was shown while the narrative was played. After the narrative, the fixation cross changed to green for 4 s to mark the start of the next trial. During the cue trial the participants were instructed to look at the picture, and during the narrative trial they were instructed to focus their eyes on the fixation cross and listen to the speech while trying to comprehend its contents. The narratives were presented in two runs both consisting of 10 trials and lasting for 12.8 min with equal number of matching and mismatching trials. Each narrative was presented to each subject only once.
2.3. fMRI acquisition and preprocessing
MRI scanning was performed with General Electric Signa 3 T MRI scanner with Excite upgrade at the Advanced Magnetic Imaging Centre at the Aalto University School of Science. Whole-brain images were acquired with T2*-weighted echo-planar imaging (EPI), sensitive to blood oxygenation level-dependent (BOLD) signal contrast, using a quadrature sixteen channel head coil. The following parameters were used: 36 axial slices, 4 mm slice thickness; TR=1800 ms; TE=30 ms; Flip angle=75; FOV=240 mm; voxel size 3×3×4 mm3; ascending interleaved acquisition with no gaps between slices. A total of 450 volumes were acquired in both runs, and the first 6 volumes of each run were discarded. T1-weighted structural images were acquired at a resolution of 1×1×1 mm3.
FMRI data processing was carried out using FEAT (FMRI Expert Analysis Tool) Version 5.98, part of FSL (FMRIB׳s Software Library, www.fmrib.ox.ac.uk/fsl). First, functional images were realigned to the middle scan by rigid-body transformations with MCFLIRT to correct for subject motion. Next, non-brain matter from functional images was removed using BET (Smith, 2002). Resulting images were high-pass filtered with Gaussian-weighted least squares fitting with a 100 s cut-off. Finally, functional images were spatially smoothed using a Gaussian kernel of FWHM 8-mm. T1-weighted structural images were cleared from non-brain tissue with BET. These images were registered to the MNI152 standard space template with 2-mm resolution by first calculating transformation parameters from structural to standard space and from functional to structural space. Then, these transformations were concatenated and used to co-register functional datasets to the standard space. Both registration steps were performed using FLIRT (Jenkinson, Bannister, Brady, & Smith, 2002).
2.4. Comprehension and recall measurements
After the fMRI experiment, the participants were asked to rate the comprehensibility of each narrative, and to recall as many “idea units” (see Bransford and Johnson (1972)) of each narrative as possible. Even though it is possible to memorise verbal material without understanding it, comprehension provides a significant advantage to both encoding and subsequent recall (Craik & Tulving, 1975). To obtain comprehension ratings, participants were asked to evaluate how easy it was to understand each narrative using a scale ranging from 1 (“very difficult”) to 4 (“very easy”). The idea units correspond to either individual sentences, basic semantic propositions, or phrases related to the narrative. The idea units were listed a priori, and corresponded to independent facts, for example, “You will need a sharp item for the task” (sewing) or “Sitting down is better than standing” (playing guitar). On average, the stories included 7.35 idea units (range 6–9). The idea units found in individual subjects recall reports were counted by two experts (α=0.82), and rescaled as ratio of recalled idea units to maximum amount of idea units recalled for each trial.
2.5. Analysis of regional effects
Regional responses to narratives preceded by mismatching and matching contextual cues were analysed using a FILM general linear modelling (GLM) method implemented in FSL software (FMRIB׳s Improved Linear Model; Woolrich, Ripley, Brady, & Smith, 2001). Two boxcar regressors – matching context trials and mismatching context trials – were used to model subject׳s fMRI voxel time series. The regressors included only time points when narrative was presented, i.e., onset time corresponded to the beginning of a narrative. Motion correction parameters were included in the model as effects of no interest to account for motion-related variance. After the generation of individual contrast images for the matching versus mismatching context conditions and vice versa, a second level (random effects) analysis was applied to these contrast images in a new GLM. Statistical threshold was set at Z>2.3, p<0.05, FDR-corrected (Worsley, 2002).
In addition to using GLM analysis, we quantified the temporal similarity of brain activity across subjects during mismatching and matching cue conditions using inter-subject correlation (ISC) analysis. ISC focuses on temporal similarity rather than activation amplitude of the signals in single voxels. As was shown in previous studies (Hasson, Nir, Levy, Fuhrmann, & Malach, 2004; Jääskeläinen et al., 2008; Stephens, Silbert, & Hasson, 2010; Wilson, Molnar-Szakacs, & Iacoboni, 2008), ISC can reveal consistent patterns of brain activation within a group of subjects during naturalistic stimulation, for which explicit stimulus model cannot necessarily be applied. Pearson׳s correlation coefficient was employed to derive between-subjects voxel-wise similarity measures at the group level using the toolbox developed by Kauppi, Jääskeläinen, Sams, and Tohka (2010). Data were preprocessed similarly as for GLM analyses. Motion parameters were regressed out from the data, and time series were shifted by three TRs to account for hemodynamic lag. ISC was calculated separately for each of the 20 narratives for the two counterbalancings, including all time points when the story was told (35–41 TRs), resulting in a total of 40 ISC group maps. ISC values across conditions were linearly regressed with the average behavioural comprehensibility ratings and thresholded with p<0.01, cluster-corrected with cluster size of 27 voxels.
2.6. Functional connectivity analysis
Functional connectivity was estimated using psychophysiological interactions (PPIs; Friston et al., 1997) implemented in SPM8 software (www.fil.ion.ucl.ac.uk/spm/software/spm8/). Broca׳s area was selected as a seed ROI for the PPI analyses, given its profound role in speech comprehension (Bookheimer, 2002) and its contribution to semantic selection during contextual understanding (Moss et al., 2005; Thompson-Schill, D'Esposito, Aguirre & Farah, 1997). Mean stereotactic location (MNI coordinates: −44, 23, 15) of this region in humans was derived from a previous meta-analysis on the location and functions of Broca׳s area in humans (Vigneau et al., 2006). Given the evidence suggesting differential contribution of posterior and anterior portions of Broca׳s area on various levels of linguistic processing, separate posterior and anterior ROIs were drawn on Broca׳s region: pars opercularis (MNI: −50, 14, 16) and pars triangularis (MNI: −46, 30, 6), with coordinates taken from a midpoint voxel in corresponding region in Harvard-Oxford FSL atlas1 (http://www.cma.mgh.harvard.edu/fsl_atlas.html). To avoid overlap of the seed regions, spheres with 5-mm radius were drawn around triangular and opercular subregions. The time series of each participant was computed by using the first eigenvariate from all raw voxel time series in each ROI. This BOLD time series was deconvolved using the PPI-deconvolution parameter defaults in SPM8 (Gitelman, Penny, Ashburner, & Friston, 2003) to estimate the neuronal time series for this region.
The psychophysiological interaction term (PPI regressor) was calculated as the element-by-element product of the ROI neuronal time series and a vector coding for the main effect of matching context condition. This product was re-convolved by the canonical HRF. PPI models were run separately for each participant. The model also included the main effects of task convolved by the HRF, and motion parameters as effects of no interest. These models identified regions that had greater or lesser coupling with the source region according to listening to narrative preceded by matching versus mismatching pictorial context. The resulting PPI contrast images were entered into second-level analyses to produce group-level images with statistical threshold set at T>3 and FDR-corrected at cluster level at p<0.05.
2.7. Eye movement recordings
As pupil dilation is associated with processing and memory load (Beatty, 1982), we recorded participants׳ pupil size during the narrative listening phase of the experiment. Data were recorded successfully from 17 participants with a SMI 60Hz Eye Track long-range eye tracking system (Sensomotoric Instruments GmbH, Germany). To get an absolute scale for pupil diameter, we used a reference “fake pupil”, a piece of paper with a black circle of known diameter painted on it. This fake pupil was placed in front of participants׳ eye at the beginning of the experiment, and a conversion factor was acquired. This conversion factor was subsequently used to convert the pupil diameter from pixels to millimetres. Only horizontal diameter was used for the analysis, as it is not confounded by subject׳s position or eyelid movement. Pupil size measures were cleared from blink artefacts and horizontal pupil diameters were averaged for each trial for every subject. The resulting pupil size measurements were compared between matching and mismatching context conditions.
3. Results
3.1. Behavioural results
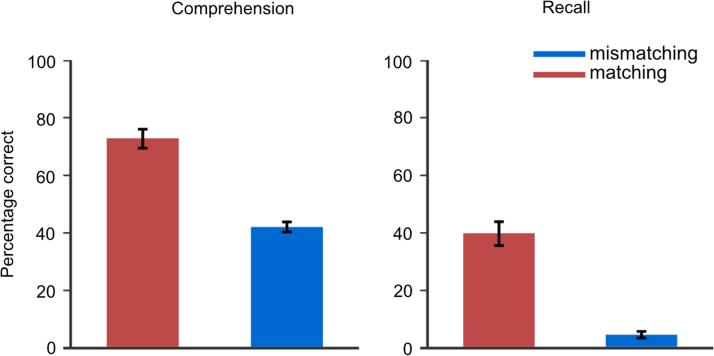
Comprehension of narratives preceded by matching context picture was significantly better than comprehension of narratives preceded by mismatching picture, t(18)=9.47, p<0.0001, d=2.74, means: 2.89 (72%) and 1.66 (42%) for matching and mismatching context conditions, respectively (Fig. 2). More idea units were also recalled for the matching context condition, t(18)=10.21, p<0.0001, d=2.94, means: 1.78 (39%) and 0.19 (4%) for matching and mismatching context conditions, respectively. Pupil diameter was not significantly influenced by context, F(16)=0.94, p=0.89 (means: 4.70 and 4.72; std: 0.75 and 0.78 for matching and mismatching context conditions, respectively).
Fig. 2.
Means and standard errors of means for comprehension (left) and recall (right) ratings.
3.2. Functional MRI
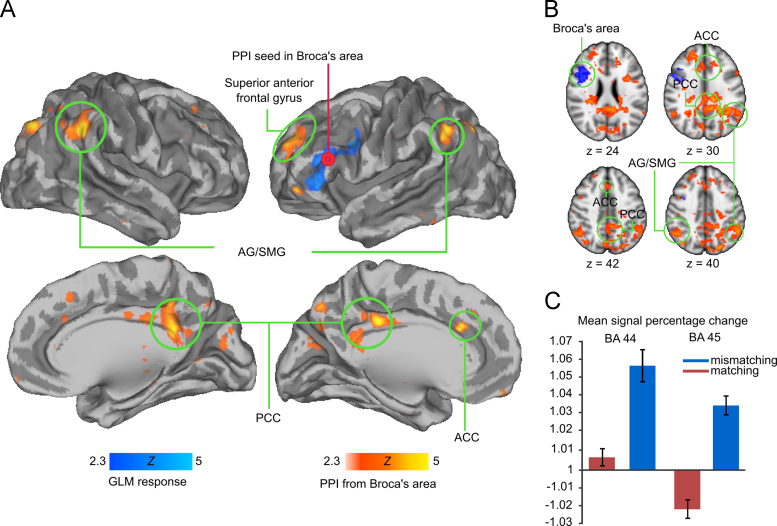
GLM analysis revealed significantly stronger (p<0.05, FDR corrected) task-evoked BOLD responses when listening to narratives preceded by contextually mismatching compared to matching pictures in the cluster that covered both left BA 44 and BA 45, as well as a portion of the left dorsal and ventral premotor cortex (see Fig. 3A and B, in blue, and Table 1). No significant differences were observed in this analysis or in the opposite contrast (matching versus mismatching).
Fig. 3.
(A) and (B) Brain regions showing increased activity (cold colours) for narratives presented in mismatching versus matching context, and enhanced functional connectivity (hot colours) for narratives presented in matching versus mismatching context. Seed region in Broca׳s area for connectivity analysis is denoted by the red circle in the volume renders. Data are thresholded at Z>2.3, and FDR corrected (p<0.05) at the cluster level. (C) Mean signal percentage change plot shows averages for BA 44 and BA 45 seeds. Error bars show standard error of the mean.
Table 1.
MNI coordinates of peak activations obtained in the GLM and PPI analyses. The data are thresholded at p<0.05 (FDR corrected at cluster level).
| Region | Hemisphere | x | y | z | Z | Voxels |
|---|---|---|---|---|---|---|
| Regional effects in GLM (mismatching versus matching) | ||||||
| Broca׳s area (BA 44/45) | L | −43 | 18 | 18 | 5.24 | 1251 |
| Psycho-physiological interactions for mismatching versus matching (mean Broca׳s area seed) | ||||||
| Inferior parietal cortex, posterior cingulate cortex | R | 24 | −46 | 32 | 4.66 | 4864 |
| Lateral occipital cortex | R | 44 | −68 | −22 | 4.31 | 280 |
| Anterior cingulate/Paracingulate cortex | L | −12 | 22 | 28 | 4.03 | 313 |
| Angular gyrus | L | −36 | −68 | 46 | 3.92 | 732 |
| Anterior superior frontal gyrus | L | −18 | 44 | 32 | 3.91 | 455 |
| Middle frontal gyrus | R | 28 | 20 | 34 | 3.66 | 383 |
| Psycho-physiological interactions for matching versus mismatching (posterior Broca׳s area seed) | ||||||
| Postcentral gyrus | L | −42 | −26 | 36 | 4.53 | 498 |
| Angular gyrus | L | −56 | −54 | 42 | 4.17 | 538 |
| Inferior/Middle temporal gyrus | L | −50 | −18 | −26 | 4.15 | 203 |
| Supramarginal gyrus/Angular gyrus | R | 48 | −42 | 34 | 3.91 | 396 |
| Psycho-physiological interactions for matching versus mismatching (anterior Broca׳s area seed) | ||||||
| Angular gyrus, posterior cingulate cortex | R | 38 | −50 | 28 | 6.52 | 2091 |
| Anterior cingulate/Paracingulate cortex | R | 4 | 22 | 46 | 5.48 | 768 |
| Inferior frontal gyrus | R | 48 | 34 | 16 | 4.91 | 885 |
| Frontal pole | −6 | 64 | −2 | 3.97 | 264 | |
| Lateral occipital cortex | R | 36 | −66 | 30 | 4.82 | 364 |
PPI analysis revealed that in matching versus mismatching contrast connectivity increased between Broca׳s area and bilateral AG/SMG (extending to right PCC), anterior and posterior cingulate cortices, and an area in the left anterior superior frontal gyrus (Fig. 3A and B, in red, and Table 1). There were no brain regions showing increased connectivity with Broca׳s area in mismatching versus matching contrast.
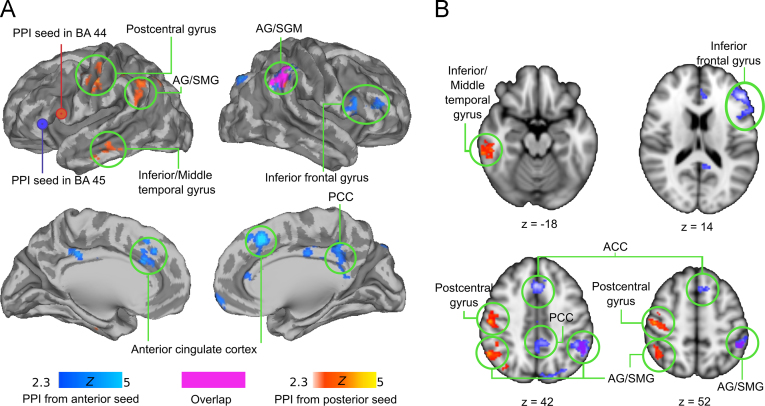
Next we studied the functional connectivity of the anterior and posterior parts of Broca׳s area separately in PPI analysis. Although the connectivity of both subregions with right AG/SMG increased while listening to matching versus mismatching narratives, the analysis revealed a clear dissociation in the regional connectivity profiles (Fig. 4 and Table 1). Left BA 44 showed additional increased connectivity with left AG, left inferior/middle temporal gyrus and left postcentral gyrus, whereas left BA 45 showed increased connectivity with right PCC, right anterior IFG, lateral occipital cortex and ACC.
Fig. 4.
(A) and (B) Brain regions showing significant functional connectivity with posterior (hot colours) and anterior (cold colours) Broca׳s area (seeds indicated by orange and blue circles respectively) during matching versus mismatching trials. Overlap is indicated in purple. Connectivity from both seeds increases only in the right inferior parietal cortex (AG/SMG, indicated by purple). Data are thresholded at Z>2.3, and FDR corrected (p<0.05) at the cluster level.
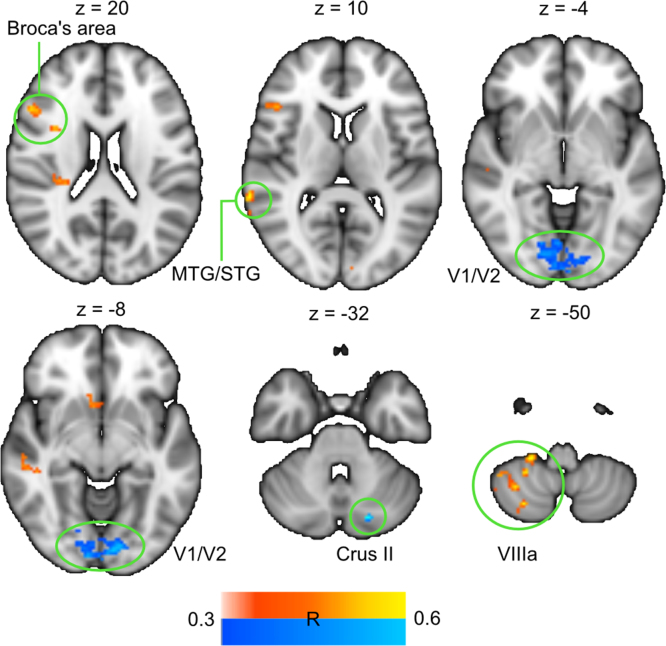
Regressing ISC against comprehension ratings revealed stronger ISC for lower comprehension ratings in anterior part of Broca׳s area, left lobule VIIIa of the cerebellum and left middle temporal gyrus (Fig. 5). This cluster in Broca׳s area overlaps with the corresponding cluster identified in the GLM analysis described above, i.e. 69% of the voxels showing significant ISC showed statistically significant activity in the GLM analysis. For higher comprehension ratings ISC was stronger in right cerebellum crus II and V1/V2 (Table 2).
Fig. 5.
Brain regions where ISC was modulated by contextual cues. Hot colours denote regions where ISC was significantly higher for contextually mismatching trials. The only area where ISC was higher in contextually matching trials was Crus II of cerebellum (in blue). Data are thresholded at cluster significance threshold of p<0.01.
Table 2.
MNI coordinates of peak activations obtained in ISC analysis. The data are thresholded at p<0.01, cluster-corrected.
| Region | Hemisphere | x | y | z | R | Voxels |
|---|---|---|---|---|---|---|
| Inter-subject correlations for mismatching versus matching | ||||||
| VIIIa | L | −30 | −62 | −58 | −0.6 | 140 |
| Crus II | R | 18 | −78 | −30 | 0.58 | 45 |
| Middle temporal gyrus | L | −46 | −32 | −8 | −0.59 | 41 |
| Broca׳s area | L | −40 | 22 | 8 | −0.56 | 109 |
| Middle temporal gyrus/Superior temporal gyrus | L | −66 | −52 | 6 | −0.58 | 80 |
| V1/V2 | LR | −12 | −86 | −18 | 0.55 | 736 |
4. Discussion
In the present study we demonstrate that a fronto-parietal functional network supports context-dependent narrative comprehension while participants listen to spoken narratives preceded by matching versus mismatching contextual cues. Matching contextual cues improved the comprehension and subsequent recall of the narratives (c.f. Bransford & Johnson, 1972; Dooling & Lachmann, 1971), and increased functional connectivity within a fronto-parietal functional network comprising Broca׳s area, bilateral AG/SMG, as well as anterior and posterior cingulate and left superior frontal cortices. Subjects׳ pupil sizes did not differ between matching and mismatching conditions, suggesting that cognitive load in the two conditions was comparable (Beatty, 1982; Kahneman & Beatty, 1966; Stanners, Coulter, Sweet, & Murphy, 1979) and that the observed differences across conditions likely cannot be explained by differences in allocation of attention or level of vigilance. Increased narrative comprehension and recall were paralleled by enhanced functional connectivity between Broca׳s area and bilateral inferior parietal cortex, anterior and posterior cingulate cortices, and left anterior superior frontal gyrus. Importantly, in spite of its enhanced connectivity in the matching condition, hemodynamic activity in Broca׳s area was stronger in conflicting condition.
4.1. A fronto-parietal network supporting context-dependent narrative comprehension
Functional connectivity analysis revealed that Broca׳s area interacts with the linguistic processing nodes in bilateral AG and extra-linguistic ACC and PCC areas during context-dependent speech comprehension. Previously IPC regions have been associated with both local and global semantic functions, e.g. accessing word meanings and higher level semantic integration (Binder et al., 2009; Harpaz, Levkovitz, & Lavidor, 2009; Humphries et al., 2007; Price, 2000) and left AG has been shown to exhibit enhanced activity when subjects listen to titled compared to untitled narratives, possibly reflecting its involvement in processing contextual information (Martín-Loeches et al., 2008). Furthermore functional connectivity between the components of the language network increases as a function of speech intelligibility (Abrams et al., 2013). Our findings are in line with these observations, showing that functional connectivity between frontal and parietal nodes of the language network is enhanced when contextual cues are available for supporting language comprehension. We addressed here specifically the global aspect of contextual processing; therefore we can only speculate about processes that took place at the local semantic level. We propose that when a contextual cue is presented, a hierarchical representation of the context is activated. Depending on its relevance, it either facilitates or impairs processing of the following narrative. The observed functional connectivity between Broca׳s area and AG is likely related to both local and global stages of semantic processing. At the local level, the meaning of incoming word is accessed and the specific meaning is further selected based on the contextual information. At the global level, the linguistic information is integrated into a coherent narrative facilitated by the matching context.
Given that previous studies have shown that posterior cingulate cortex contributes to episodic memory retrieval (for review see Cabeza and Nyberg (2000)), the enhanced coupling between PCC and Broca׳s area during matching context conditions might reflect access to contextual knowledge in long-term memory (Binder et al., 2009). Posterior midline regions, including precuneus, medial frontal cortex and paracingulate cortex, were also shown to reliably respond only to linguistic information, which was coherent over long time scales (~30 s; Lerner, Honey, Silbert, & Hasson, 2011). Moreover, PCC was implicated in a coherence building process (Ferstl, Neumann, Bogler, & von Cramon, 2008), which may also take place during contextual understanding, when more information becomes available as the narrative develops. The relatively low recall scores may have been caused by the behavioural task taking place after the scanning session at which point details of the stories may have been forgotten; ACC, in turn, supports various executive control functions (Abutalebi et al., 2012; Paus, 2001) such as error monitoring (Botvinick et al., 2004; Carter, Braver, Barch, Botvinick, Noll & Cohen, 1998). Thus, enhanced coupling between Broca׳s area and ACC could support tracking of the consistency between the incoming narrative and the active schema provided by the context-matching picture.
We observed an increase of connectivity between left BA 45 and ACC in the matching condition. This finding might seem counterintuitive, because one would expect to see greater error monitoring activity in mismatching condition. To explain this finding, we tentatively suggest that in the matching condition consistency of the on-going narrative needs to be constantly monitored to build a coherent representation of its semantics, and this is reflected in stronger functional connectivity between left BA 45 and ACC. In mismatching case, once the narrative has been judged as inconsistent, there is no longer any need for consistency monitoring. Because of the short duration of this conflict, it probably was not reflected in the connectivity over the whole story time course. In sum, the observed functional connectivity pattern supports our hypothesis suggesting access to semantic information in AG, further selection of relevant information in Broca׳s area, re-integration of this information in AG, and consistency/error monitoring in dACC.
4.2. Functional connectivity of the anterior and posterior portions of Broca׳s area
Functional connectivity analysis of left posterior (BA 44) and anterior (BA 45) subregions of Broca׳s area revealed that these areas have differential functional connectivity profiles (see Fig. 4). While both subregions showed enhanced connectivity with right AG/SMG during matching condition – possibly reflecting interactions between sensory representation of the words and their meanings – the posterior part was also functionally coupled with left AG. This connection could reflect the initial linguistic processing, which requires both phonological and semantic components. It has been proposed that left IPC is involved in retrieval of the word meaning and subsequent integration into sentential context, without specific sensitivity to how well formed the sentence is, which is monitored by prefrontal regions (Ye, Doñamayor, & Münte, 2012). Connectivity of the anterior Broca׳s area with PCC and dACC suggests that this brain region is specifically associated with semantic processing (Hagoort, 2005) and monitors the consistency of the narrative via its connections with the dACC. However, activations in Broca׳s area (mainly BA 45, extending into 44 and 47) and left inferior parietal areas (including AG and SMG) have also been reported while processing multiple syntactic representations in ambiguous phrases (Tyler et al., 2011). Results of Humphries et al. (2007) imply that left AG is also sensitive to a combinatorial semantic structure, arising from possibility to align individual words to form a more complex meaning. These authors suggested that AG integrates semantic and syntactic information to produce the overall sentence meaning. Since activation in AG was found for cases not involving syntactic processing, they imply that AG is involved primarily in semantic integration.
We also observed increased connectivity of BA 45 with right anterior IFG and AG. These regions are activated during figurative speech processing (Bookheimer, 2002), e.g. bilateral IFG and middle temporal gyrus during processing idioms (Zempleni et al., 2007a, 2007b). It is thus possible that contextual processing involves similar global linguistic functions as comprehending less transparent figurative language.
4.3. Dissociation of response amplitudes and functional connectivity of Broca׳s area
The effects of contextual information on the response amplitudes and functional connectivity were dissociated in Broca׳s area: while the matching context markedly increased connectivity of Broca׳s area with the bilateral inferior parietal cortex, cingulate gyrus and left anterior superior frontal gyrus, we failed to see enhanced hemodynamic activity in these areas in the corresponding GLM analysis. Instead, when semantic coherence of the narrative was broken due to mismatching rather than matching contextual information, haemodynamic activity in Broca׳s area increased significantly (see Fig. 3). We propose that this reflects increased demands for semantic processing in the absence of reliable contextual cues. The role of Broca׳s area in semantic processing has been well established (Bookheimer, 2002; Hagoort, 2005; Vigneau et al., 2006) and our findings corroborate observations of previous studies where ambiguity (Rodd et al., 2005) or informativeness of sentence-level segments (Hasson et al., 2007) was manipulated. Tentatively, the stages of initial semantic access and later integration (AG) and error/consistency monitoring processes (ACC) might be similarly active during both mismatching and matching context conditions, but increase in connectivity between the areas, suggested by the correlated inter-area activity, only takes place when proper contextual information is available.
ISC was negatively rather than positively associated with comprehension ratings in Broca׳s region (BA 44 and BA 45). This finding seems contradictory, as one would expect the matching context to result in higher degree of inter-subject similarity of stimulus processing. However, we suggest that the mismatching context led participants into a shared state of increased semantic selection and integration demands, which is reflected in higher synchrony in Broca׳s area. The only regions where we observed higher ISC for higher comprehension ratings were V1/V2, possibly reflecting similarity of visually processed information, since only fixation cross was presented on the screen, and right cerebellum׳s crus II that might reflect supporting functions of cerebellum during linguistic processing (Stoodley & Schmahmann, 2009). Cerebellar activity was shown to scale with complexity of language task (Xiang et al., 2003), and greater activation during semantic processing, compared to phonological processing (McDermott, Petersen, Watson, & Ojemann, 2003), as well as involvement in semantic decision process (Noppeney & Price, 2002).
The cluster observed in our GLM analyses encompassed both BA 44 and BA 45. In the literature, BA 44 is typically reported to participate in phonological processing, and we can only speculate about its involvement in context-dependent processing. It may be possible that while listening to contextually matching narrative, the context provides a more accurate predictive model of the upcoming phonetics of the speech, thus facilitating phonological processing. Another subregion of Broca׳s area that is likely involved in semantic processing is BA 47, activation of which we would have observed with a slightly more lenient threshold.
4.4. Context-dependent language processing in the brain
Our findings significantly extend the results of earlier studies addressing contextual effects and coherence of text and context (Maguire et al., 1999; Martín-Loeches et al., 2008; St George et al., 1999). St George et al. (1999) contrasted reading titled and untitled paragraphs word by word and found right middle temporal regions being particularly important for integrative processes during discourse processing. In PET study of Maguire et al. (1999) the subjects were presented with auditory passages that were either readily comprehensible or unusual stories that required prior knowledge to comprehend. Prior knowledge was manipulated by presenting relevant, irrelevant or no visual cue before the story. The authors found involvement of anterior and ventral parts of the medial parietal and posterior cingulate cortices when unusual story was given with relevant prior knowledge. Finally, a more recent study used fMRI to address effects of story coherence on brain activation, employing textual long narratives with or without a title that made paragraphs globally coherent (Martín-Loeches et al., 2008). This study found left AG activation while processing titled paragraphs and linked it to global coherence processing. While our results are in line with this interpretation, some other studies did not find the same regions involved during contextual understanding process. Methodological differences are a possible source of discrepancy, e.g., selection of a priori regions of interest (St George et al., 1999) or use of PET (Maguire et al., 1999) that has lower temporal resolution than fMRI, or the fact that two of these earlier studies presented discourses as text and context as a title (Martín-Loeches et al., 2008; St George et al., 1999), in contrast to our study that involved spoken narratives and pictorial context. Indeed, several studies have compared neural mechanisms of written and spoken language comprehension and have shown activation of different cortical areas depending on modality of stimulation (Buchweitz, Mason, Tomitch, & Just, 2009; Constable et al., 2004; Michael, Keller, Carpenter, & Just, 2001). Listening to versus reading sentences results in activation of more anterior sites of Broca׳s area, possibly reflecting more intensive semantic processing (Hagoort, 2005) during listening comprehension: it was specifically suggested that spoken language provides listener with prosodic cues, while written language offers punctuation as a means of information parsing (Michael et al., 2001).
Finally, it has been suggested that language comprehension can be treated as language-guided mental simulation of the described situation (Martín-Loeches et al., 2008; Zwaan & Rapp, 2006). From this point of view, different situational models could be created as a function of narrative content, and consequently, result in different patterns of brain activity. Even the same content can lead to different situational models being constructed due to level of global coherence achieved by reader and listener and lead to variable brain activation patterns (Martín-Loeches et al., 2008). Given the profound role of Broca׳s area in particularly spoken language processing our study, manipulating the contextual information of spoken instead of textual narratives, is possibly more sensitive for detecting contextual effects in Broca׳s area.
Narrative comprehension requires active prediction, selection, and integration of task-relevant information (Desimone & Duncan, 1995; Gibson, 1979). If the context is unknown, several alternative explanations of the current discourse segment can be prepared in parallel when listening to the unfolding story (Cisek & Kalaska, 2010; Wlotko & Federmeier, 2012). Given that Broca׳s area is engaged by tasks where response is selected among several alternatives (Thompson-Schill, D'Esposito, Aguirre & Farah, 1997), we suggest that the increased activation of Broca׳s area (as well as increased ISC) during mismatching context condition reflects this region׳s role in selecting among semantic information held in working memory (Gabrieli et al., 1998; Moss et al., 2005; Thompson-Schill, D'Esposito, Aguirre & Farah, 1997). Conversely, absence of stronger Broca׳s area activation during matching context condition could be explained by automatic retrieval processes (Moss et al., 2005; Poldrack et al., 1999) since context provides clear prediction for the subsequent narrative. While a matching contextual cue clearly defines the narrative topic and thus provides a stable schema for prediction of the narrative, the conflicting context renders meaning of the narrative inaccessible, and as the narrative unfolds interpretations become obsolete in quick succession (Wlotko & Federmeier, 2012). As a result, more extensive selection process is engaged in the contextually conflicting condition. While building representation of a word or a coherent sentence may start as soon as they are heard, narrative evolves over time and requires re-integration whenever new information becomes accessible (Wlotko & Federmeier, 2012; Zempleni et al., 2007a, 2007b). It is possible that semantic processing of complex contextual speech is hierarchical, where inputs are exchanged between the nodes such as AG (initial semantic access and later semantic integration) and Broca׳s area (selection and unification) with involvement of other extra-linguistic regions such as ACC or PCC that contribute to specific aspects of semantic processing such as error monitoring or episodic memory retrieval respectively.
5. Conclusions
We conclude that Broca׳s area and its connections with the inferior parietal and cingulate cortices play a critical role in context-dependent narrative comprehension. Listening to narrative disambiguated with appropriate context facilitates access to and selection of relevant semantic information and further integration of words and sentences into a coherent narrative. We propose that at the neural level, such contextual understanding is supported by enhanced connectivity of posterior and anterior portions of Broca׳s area with fronto-parietal network of brain regions. Speculatively, we suggest that within this network the semantic processing involves AG, which participates in access to semantic representation of incoming information. This semantic representation is further selected and unified into larger units in Broca׳s area on the basis of available context. In the next stage these larger units are matched and updated to a new representation in AG. The whole process might be monitored by dACC for consistency of narrative in relation to context.
Funding
This work was supported by the aivoAALTO grant from the Aalto University, Academy of Finland (Grant #251125 to L.N. and Grant #138145 to I.P.J.), Kordelin Foundation scholarship to D.S. and the European Research Council (Starting Grant #313000 to L.N.).
Author contributions
DS: Designed the experiments, acquired and analysed fMRI and behavioural data, wrote the manuscript
EG: Analysed fMRI data.
JL: Analysed fMRI data.
JS: Designed the experiment, wrote the manuscript.
IJ: Designed the experiment, wrote the manuscript.
MS: Designed the experiment, wrote the manuscript.
LN: Designed the experiment, supervised data analysis, and wrote the manuscript.
Acknowledgements
We thank Marita Kattelus for her help with the data acquisition. The calculations presented above were performed using computer resources within the Aalto University School of Science “Science-IT” project.
Footnotes
These seeds overlap with regions observed in GLM analysis (100% for BA 45, 14% for BA 44). Coordinates were taken from an atlas instead of functional results to avoid selection bias (i.e. ‘double-dipping’). Nevertheless, we confirmed in ROI analysis that each of these seeds in Broca׳s area was significantly activated in the GLM analysis of contextual narrative processing (p<0.05 SVC), see Section 3.
Appendix A. Sample narrative translated to English.
If the environment were polluted, the whole business would be useless. If our spot is very popular, the results may not be as good as expected. Of course, it is nice to have companion but others may also compete for the same limited shares. Accidents are also possible, so one should be prepared to provide first aid for the common injuries. Of course, one can use a phone to call for help, but as we are far out from the hospital the cell phone might not work or it may run out of battery. Operating the equipment takes a good deal of practice and skill in the labile environment. Performing the actions in the right sequence is critical; otherwise one simply ends up tangling everything. Even when everything is done right, a great deal of patience and attention are required to achieve the best results, even though on many occasions one must return to home without having any success at all.
Appropriate contextual cue=Fishing,
Inappropriate contextual cue=Doing laundry.
References
- Abrams D.A., Ryali S., Chen T., Balaban E., Levitin D.J., Menon V. Multivariate activation and connectivity patterns discriminate speech intelligibility in Wernicke׳s, Broca׳s, and Geschwind׳s areas. Cerebral Cortex. 2013;23(7):1703–1714. doi: 10.1093/cercor/bhs165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abutalebi J., Della Rosa P.A., Green D.W., Hernandez M., Scifo P., Keim R. Bilingualism tunes the anterior cingulate cortex for conflict monitoring. Cerebral Cortex. 2012;22(9):2076–2086. doi: 10.1093/cercor/bhr287. [DOI] [PubMed] [Google Scholar]
- Amunts K., Lenzen M., Friederici A.D., Schleicher A., Morosan P., Palomero-Gallagher N. Broca׳s region: novel organizational principles and multiple receptor mapping. PLoS Biology. 2010;8(9):16. doi: 10.1371/journal.pbio.1000489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amunts K., Schleicher A., Bürgel U., Mohlberg H., Uylings H.B., Zilles K. Broca׳s region revisited: cytoarchitecture and intersubject variability. Journal of Comparative Neurology. 1999;412(2):319–341. doi: 10.1002/(sici)1096-9861(19990920)412:2<319::aid-cne10>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- Amunts K., Weiss P.H., Mohlberg H., Pieperhoff P., Eickhoff S., Gurd J.M. Analysis of neural mechanisms underlying verbal fluency in cytoarchitectonically defined stereotaxic space—the roles of Brodmann areas 44 and 45. NeuroImage. 2004;22(1):42–56. doi: 10.1016/j.neuroimage.2003.12.031. [DOI] [PubMed] [Google Scholar]
- Anwander A., Tittgemeyer M., von Cramon D.Y., Friederici A.D., Knösche T.R. Connectivity-based parcellation of Broca׳s area. Cerebral Cortex. 2007;17(4):816–825. doi: 10.1093/cercor/bhk034. [DOI] [PubMed] [Google Scholar]
- Beatty J. Task-evoked pupillary responses, processing load, and the structure of processing resources. Psychological Bulletin. 1982;91(2):276–292. [PubMed] [Google Scholar]
- Bedny M., Hulbert J.C., Thompson-Schill S.L. Understanding words in context: the role of Broca׳s area in word comprehension. Brain Research. 2007;1146(18):101–114. doi: 10.1016/j.brainres.2006.10.012. [DOI] [PubMed] [Google Scholar]
- Binder J.R., Desai R.H., Graves W.W., Conant L.L. Where is the semantic system? A critical review and meta-analysis of 120 functional neuroimaging studies. Cerebral Cortex. 2009;19(12):2767–2796. doi: 10.1093/cercor/bhp055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder J.R., Frost J.A., Hammeke T.A., Bellgowan P.S. F., Rao S.M., Cox R.W. Conceptual processing during the conscious resting state: a functional MRI study. Journal of Cognitive Neuroscience. 1999;11(1):80–93. doi: 10.1162/089892999563265. [DOI] [PubMed] [Google Scholar]
- Bokde A.L. W., Tagamets M.-A., Friedman R.B., Horwitz B. Functional interactions of the inferior frontal cortex during the processing of words and word-like stimuli. Neuron. 2001;30(2):609–617. doi: 10.1016/s0896-6273(01)00288-4. [DOI] [PubMed] [Google Scholar]
- Bookheimer S. Functional MRI of language: new approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Botvinick M.M., Cohen J.D., Carter C.S. Conflict monitoring and anterior cingulate cortex: an update. Trends in Cognitive Sciences. 2004;8(12):539–546. doi: 10.1016/j.tics.2004.10.003. [DOI] [PubMed] [Google Scholar]
- Bransford J.D., Johnson M.K. Contextual prerequisites for understanding: some investigations of comprehension and recall. Journal of Verbal Learning and Verbal Behavior. 1972;11(6):717–726. [Google Scholar]
- Buchweitz A., Mason R., Tomitch L., Just M. Brain activation for reading and listening comprehension: an fMRI study of modality effects and individual differences in language comprehension. Psychology & Neuroscience. 2009;2(2):111–123. doi: 10.3922/j.psns.2009.2.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Nyberg L. Imaging cognition II: an empirical review of 275 PET and fMRI studies. Journal of Cognitive Neuroscience. 2000;12(1):1–47. doi: 10.1162/08989290051137585. [DOI] [PubMed] [Google Scholar]
- Carter C.S., Braver T.S., Barch D.M., Botvinick M.M., Noll D., Cohen J.D. Anterior cingulate cortex, error detection, and the online monitoring of performance. Science. 1998;280(5364):747–749. doi: 10.1126/science.280.5364.747. [DOI] [PubMed] [Google Scholar]
- Catani M., Jones D.K., Ffytche D.H. Perisylvian language networks of the human brain. Annals of Neurology. 2005;57(1):8–16. doi: 10.1002/ana.20319. [DOI] [PubMed] [Google Scholar]
- Cisek P., Kalaska J.F. Neural mechanisms for interacting with a world full of action choices. Annual Review of Neuroscience. 2010;33:269–298. doi: 10.1146/annurev.neuro.051508.135409. [DOI] [PubMed] [Google Scholar]
- Constable R.T., Pugh K.R., Berroya E., Mencl W.E., Westerveld M., Ni W. Sentence complexity and input modality effects in sentence comprehension: an fMRI study. NeuroImage. 2004;22(1):11–21. doi: 10.1016/j.neuroimage.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Cooper E.A., Hasson U., Small S.L. Interpretation-mediated changes in neural activity during language comprehension. NeuroImage. 2011;55(3):1314–1323. doi: 10.1016/j.neuroimage.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craik F.I. M., Tulving E. Depth of processing and the retention of words in episodic memory. Journal of Experimental Psychology: General. 1975;104:168–294. [Google Scholar]
- Crottaz-Herbette S., Menon V. Where and when the anterior cingulate cortex modulates attentional response: combined fMRI and ERP evidence. Journal of Cognitive Neuroscience. 2006;18(5):766–780. doi: 10.1162/jocn.2006.18.5.766. [DOI] [PubMed] [Google Scholar]
- Desimone R., Duncan J. Neural mechanisms of selective visual attention. Annual Review of Neuroscience. 1995;18:193–222. doi: 10.1146/annurev.ne.18.030195.001205. [DOI] [PubMed] [Google Scholar]
- DeWitt I., Rauschecker J.P. Phoneme and word recognition in the auditory ventral stream. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(8):505–514. doi: 10.1073/pnas.1113427109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dooling D.J., Lachmann R. Effects of comprehension on retention of prose. Journal of Experimental Psychology. 1971;88(2):216–222. [Google Scholar]
- Ferstl E.C., Neumann J., Bogler C., von Cramon D.Y. The extended language network: a meta-analysis of neuroimaging studies on text comprehension. Human Brain Mapping. 2008;29(5):581–593. doi: 10.1002/hbm.20422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey S., Campbell J.S. W., Pike G.B., Petrides M. Dissociating the human language pathways with high angular resolution diffusion fiber tractography. Journal of Neuroscience. 2008;28(45):11435–11444. doi: 10.1523/JNEUROSCI.2388-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friederici A.D. Pathways to language: fiber tracts in the human brain. Trends in Cognitive Sciences. 2009;13(4):175–181. doi: 10.1016/j.tics.2009.01.001. [DOI] [PubMed] [Google Scholar]
- Friederici A.D. The cortical language circuit: from auditory perception to sentence comprehension. Trends in Cognitive Sciences. 2012;16(5):262–268. doi: 10.1016/j.tics.2012.04.001. [DOI] [PubMed] [Google Scholar]
- Friederici A.D., Bahlmann J., Heim S., Schubotz R.I., Anwander A. The brain differentiates human and non-human grammars: functional localization and structural connectivity. Proceedings of the National Academy of Sciences of the United States of America. 2006;103(7):2458–2463. doi: 10.1073/pnas.0509389103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Friston K.J., Buechel C., Fink G.R., Morris J., Rolls E., Dolan R.J. Psychophysiological and modulatory interactions in neuroimaging. NeuroImage. 1997;6(3):218–229. doi: 10.1006/nimg.1997.0291. [DOI] [PubMed] [Google Scholar]
- Gabrieli J.D. E., Poldrack R.A., Desmond J.E. The role of left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(3):906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson J.J. Houghton Mafflin; Boston: 1979. The ecological approach to visual perception. [Google Scholar]
- Gitelman D.R., Penny W.D., Ashburner J., Friston K.J. Modeling regional and psychophysiologic interactions in fMRI: the importance of hemodynamic deconvolution. NeuroImage. 2003;19(1):200–207. doi: 10.1016/s1053-8119(03)00058-2. [DOI] [PubMed] [Google Scholar]
- Glasser M.F., Rilling J.K. DTI tractography of the human brain׳s language pathways. Cerebral Cortex. 2008;18(11):2471–2482. doi: 10.1093/cercor/bhn011. [DOI] [PubMed] [Google Scholar]
- Gough P.M., Nobre A.C., Devlin J.T. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. Journal of Neuroscience. 2005;25(35):8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in Cognitive Sciences. 2005;9(9):416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Harpaz Y., Levkovitz Y., Lavidor M. Lexical ambiguity resolution in Wernicke׳s area and its right homologue. Cortex. 2009;45(9):1097–1103. doi: 10.1016/j.cortex.2009.01.002. [DOI] [PubMed] [Google Scholar]
- Hasson U., Nir Y., Levy I., Fuhrmann G., Malach R. Intersubject synchronization of cortical activity during natural vision. Science. 2004;303(5664):1634–1640. doi: 10.1126/science.1089506. [DOI] [PubMed] [Google Scholar]
- Hasson U., Nusbaum H.C., Small S.L. Brain networks subserving the extraction of sentence information and its encoding to memory. Cerebral Cortex. 2007;17(12):2899–2913. doi: 10.1093/cercor/bhm016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G., Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92(1–2):67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Humphries C., Binder J.R., Medler D.A., Liebenthal E. Syntactic and semantic modulation of neural activity during auditory sentence comprehension. Journal of Cognitive Neuroscience. 2006;18(4):665–679. doi: 10.1162/jocn.2006.18.4.665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries C., Binder J.R., Medler D.A., Liebenthal E. Time course of semantic processes during sentence comprehension: an fMRI study. NeuroImage. 2007;36(3):924–932. doi: 10.1016/j.neuroimage.2007.03.059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jääskeläinen I.P., Koskentalo K., Autti T., Balk M., Kauramäki J., Pomren C. Inter-subject synchronization of prefrontal cortex hemodynamic activity during natural viewing. Open Neuroimaging Journal. 2008;2:14–19. doi: 10.2174/1874440000802010014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jenkinson M., Bannister P., Brady M., Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. NeuroImage. 2002;17(2):825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jung-Beeman M. Bilateral brain processes for comprehending natural language. Trends in Cognitive Sciences. 2005;9(11):512–518. doi: 10.1016/j.tics.2005.09.009. [DOI] [PubMed] [Google Scholar]
- Kahneman D., Beatty J. Pupil diameter and load on memory. Science. 1966;154(3756):1583–1585. doi: 10.1126/science.154.3756.1583. [DOI] [PubMed] [Google Scholar]
- Kauppi J.-P., Jääskeläinen I.P., Sams M., Tohka J. Inter-subject correlation of brain hemodynamic responses during watching a movie: localization in space and frequency. Front Neuroinformatics. 2010;4:5. doi: 10.3389/fninf.2010.00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner Y., Honey C.J., Silbert L.J., Hasson U. Topographic mapping of a hierarchy of temporal receptive windows using a narrated story. Journal of Neuroscience. 2011;31(8):2906–2915. doi: 10.1523/JNEUROSCI.3684-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire E.A., Frith C.D., Morris R.G. M. The functional neuroanatomy of comprehension and memory: the importance of prior knowledge. Brain. 1999;122(10):1839–1850. doi: 10.1093/brain/122.10.1839. [DOI] [PubMed] [Google Scholar]
- Manenti R., Cappa S.F., Rossini P.M., Miniussi C. The role of the prefrontal cortex in sentence comprehension: an rTMS study. Cortex. 2008;44:337–344. doi: 10.1016/j.cortex.2006.06.006. [DOI] [PubMed] [Google Scholar]
- Martín-Loeches M., Casado P., Hernández-Tamames J.A., Alvarez-Linera J. Brain activation in discourse comprehension: a 3t fMRI study. NeuroImage. 2008;41(2):614–622. doi: 10.1016/j.neuroimage.2008.02.047. [DOI] [PubMed] [Google Scholar]
- McDermott K.B., Petersen S.E., Watson J.M., Ojemann J.G. A procedure for identifying regions preferentially activated by attention to semantic and phonological relations using functional magnetic resonance imaging. Neuropsychologia. 2003;41(3):293–303. doi: 10.1016/s0028-3932(02)00162-8. [DOI] [PubMed] [Google Scholar]
- Mesulam M. From sensation to cognition. Brain. 1998;121(6):1013–1052. doi: 10.1093/brain/121.6.1013. [DOI] [PubMed] [Google Scholar]
- Michael E.B., Keller T.A., Carpenter P.A., Just M.A. fMRI investigation of sentence comprehension by eye and by ear: modality fingerprints on cognitive processes. Human Brain Mapping. 2001;13(4):239–252. doi: 10.1002/hbm.1036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moss H.E., Abdallah S., Fletcher P., Bright P., Pilgrim L., Acres K. Selecting among competing alternatives: selection and retrieval in the left inferior frontal gyrus. Cerebral Cortex. 2005;15(11):1723–1735. doi: 10.1093/cercor/bhi049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noppeney U., Price C.J. A PET study of stimulus- and task-induced semantic processing. NeuroImage. 2002;15(4):927–935. doi: 10.1006/nimg.2001.1015. [DOI] [PubMed] [Google Scholar]
- Paus T. Primate anterior cingulate cortex: where motor control, drive and cognition interface. Nature Reviews Neuroscience. 2001;2(6):417–424. doi: 10.1038/35077500. [DOI] [PubMed] [Google Scholar]
- Poldrack R.A., Wagner A.D., Prull M.W., Desmond J.E., Glover G.H., Gabrieli J.D. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. NeuroImage. 1999;10(1):15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Price C.J. The anatomy of language: contributions from functional neuroimaging. Journal of Anatomy. 2000;197(3):335–359. doi: 10.1046/j.1469-7580.2000.19730335.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price C.J. A review and synthesis of the first 20 years of PET and fMRI studies of heard speech, spoken language and reading. NeuroImage. 2012;62(2):816–847. doi: 10.1016/j.neuroimage.2012.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rauschecker J.P., Scott S.K. Maps and streams in the auditory cortex: nonhuman primates illuminate human speech processing. Nature Neuroscience. 2009;12(6):718–724. doi: 10.1038/nn.2331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodd J.M., Davis M.H., Johnsrude I.S. The neural mechanisms of speech comprehension: fMRI studies of semantic ambiguity. Cerebral Cortex. 2005;15(8):1261–1269. doi: 10.1093/cercor/bhi009. [DOI] [PubMed] [Google Scholar]
- Saur D., Kreher B.W., Schnell S., Kümmerer D., Kellmeyer P., Vry M.S. Ventral and dorsal pathways for language. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(46):18035–18040. doi: 10.1073/pnas.0805234105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith S.M. Fast robust automated brain extraction. Human Brain Mapping. 2002;17(3):143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stanners R.F., Coulter M., Sweet A.W., Murphy P. The pupillary response as an indicator of arousal and cognition. Motivation and Emotion. 1979;3(4):319–340. [Google Scholar]
- St George M., Kutas M., Martinez A., Sereno M.I. Semantic integration in reading: engagement of the right hemisphere during discourse processing. Brain. 1999;122(7):1317–1325. doi: 10.1093/brain/122.7.1317. [DOI] [PubMed] [Google Scholar]
- Stephens G.J., Silbert L.J., Hasson U. Speaker-listener neural coupling underlies successful communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107(32):14425–14430. doi: 10.1073/pnas.1008662107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoodley C.J., Schmahmann J.D. Functional topography in the human cerebellum: a meta-analysis of neuroimaging studies. NeuroImage. 2009;44(2):489–501. doi: 10.1016/j.neuroimage.2008.08.039. [DOI] [PubMed] [Google Scholar]
- Swinney D. Lexical access during sentence comprehension: (re)consideration of context effects. Journal of Verbal Learning and Verbal Behavior. 1979;18(6):645–659. [Google Scholar]
- Thompson-Schill S.L., D´Esposito M., Aguirre G.K., Farah M.J. Role of left inferior prefrontal cortex in retrieval of semantic knowledge:a re-evaluation. Proc. Nat. Acad. Sci. USA. 1997;94(26):14792–14797. doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turken A.U., Dronkers N.F. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in Systems Neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L.K., Marslen-Wilson W. Fronto-temporal brain systems supporting spoken language comprehension. Philosophical Transactions of the Royal Society of London B: Biological Sciences. 2008;363(1493):1037–1054. doi: 10.1098/rstb.2007.2158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler L.K., Marslen-Wilson W.D., Randall B., Wright P., Devereux B.J., Zhuang J. Left inferior frontal cortex and syntax: function, structure and behaviour in patients with left hemisphere damage. Brain. 2011;134:415–431. doi: 10.1093/brain/awq369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vigneau M., Beaucousin V., Hervé P.Y., Duffau H., Crivello F., Houdé O. Meta-analyzing left hemisphere language areas: phonology, semantics, and sentence processing. NeuroImage. 2006;30(4):1414–1432. doi: 10.1016/j.neuroimage.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Wilson S.M., Molnar-Szakacs I., Iacoboni M. Beyond superior temporal cortex: intersubject correlations in narrative speech comprehension. Cerebral Cortex. 2008;18(1):230–242. doi: 10.1093/cercor/bhm049. [DOI] [PubMed] [Google Scholar]
- Wlotko E.W., Federmeier K.D. So that׳s what you meant! Event-related potentials reveal multiple aspects of context use during construction of message-level meaning. NeuroImage. 2012;62(1):356–366. doi: 10.1016/j.neuroimage.2012.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woolrich M.W., Ripley B.D., Brady M., Smith S.M. Temporal autocorrelation in univariate linear modeling of FMRI data. NeuroImage. 2001;14(6):1370–1386. doi: 10.1006/nimg.2001.0931. [DOI] [PubMed] [Google Scholar]
- Worsley K.J. Statistical analysis of activation images. In: Jezzard P.M., Matthews P.M., Smith S.M., editors. Functional MRI: an introduction to methods. Oxford University Press; New York: 2002. [Google Scholar]
- Xiang H.D., Lin C., Ma X., Zhang Z., Bower J.M., Weng X. Involvement of the cerebellum in semantic discrimination: an fMRI study. Human Brain Mapping. 2003;18(3):208–214. doi: 10.1002/hbm.10095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiang H.D., Fonteijn H.M., Norris D.G., Hagoort P. Topographical functional connectivity pattern in the perisylvian language networks. Cerebral Cortex. 2010;20(3):549–560. doi: 10.1093/cercor/bhp119. [DOI] [PubMed] [Google Scholar]
- Xu J., Kemeny S., Park G., Frattali C., Braun A. Language in context: emergent features of word, sentence, and narrative comprehension. NeuroImage. 2005;25(3):1002–1015. doi: 10.1016/j.neuroimage.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Ye Z., Doñamayor N., Münte T.F. Brain network of semantic integration in sentence reading: insights from independent component analysis and graph theoretical analysis. Human Brain Mapping. 2012;35(2):367–376. doi: 10.1002/hbm.22182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zempleni M.Z., Haverkort M., Renken R., Stowe L.A. Evidence for bilateral involvement in idiom comprehension: an fMRI study. NeuroImage. 2007;34(3):1280–1291. doi: 10.1016/j.neuroimage.2006.09.049. [DOI] [PubMed] [Google Scholar]
- Zempleni M.Z., Renken R., Hoeks J.C., Hoogduin J.M., Stowe L.A. Semantic ambiguity processing in sentence context: evidence from event-related fMRI. NeuroImage. 2007;34(3):1270–1279. doi: 10.1016/j.neuroimage.2006.09.048. [DOI] [PubMed] [Google Scholar]
- Zwaan R.A., Rapp D.N. Discourse comprehension. In: Traxler M.J., Gernsbacher A.M., editors. Handbook of psycholinguistics. 2nd ed. Academic Press; San Diego: 2006. [Google Scholar]