Abstract
Inductive reasoning is an everyday process that allows us to make sense of the world by creating rules from a series of instances. Consistent with accounts of process-based fractionations of the prefrontal cortex (PFC) along the left–right axis, inductive reasoning has been reliably localized to left PFC. However, these results may be confounded by the task domain, which is typically verbal. Indeed, some studies show that right PFC activation is seen with spatial tasks. This study used fMRI to examine the effects of process and domain on the brain regions recruited during a novel pattern discovery task. Twenty healthy young adult participants were asked to discover the rule underlying the presentation of a series of letters in varied spatial locations. The rules were either verbal (pertaining to a single semantic category) or spatial (geometric figures). Bilateral ventrolateral PFC activations were seen for the spatial domain, while the verbal domain showed only left ventrolateral PFC. A conjunction analysis revealed that the two domains recruited a common region of left ventrolateral PFC. The data support a central role of left PFC in inductive reasoning. Importantly, they also suggest that both process and domain shape the localization of reasoning in the brain.
Keywords: fMRI, Frontal lobe, Executive function, Hemispheric specialization, Verbal, Spatial
Highlights
-
•
Inductive reasoning recruits left PFC in both the verbal and spatial domains.
-
•
Spatial inductive reasoning additionally recruits homologous right PFC regions.
-
•
Domain, as well as process, shapes the localization of reasoning in the brain.
1. Introduction
The prefrontal cortex (PFC) is a complex and diverse region of the brain that is critical in many higher level functions. In particular, executive functions have been broadly localized to the prefrontal cortex (e.g., Baddeley, 1986; Luria, 1966; Norman and Shallice, 1986). However, recently, various views aiming to better specify the location of individual processes have identified functional fractionations along the three directional axes. A gradient of representation has been posited along the rostro-caudal axis, with simple stimulus–response associations localized to more posterior areas and entire task-sets represented anteriorly (Badre and D'Esposito, 2009; Kim et al., 2011; Koechlin and Summerfield, 2007). Along the ventral–dorsal axis, the working memory processes of storage and manipulation have been localized to ventrolateral and dorsolateral PFC, respectively (Petrides, 2005; Rowe et al., 2000). Perhaps the first distinctions suggested were domain-based along the left–right axis. These models basically hold that the left hemisphere is the locus of verbal processing, while the right hemisphere is the seat of spatial processing (Kelley et al., 1998; Wagner et al., 1998). Evidence in support of this domain-based lateralization has come from loss of function in patients with lesions (e.g., McCarthy and Warrington, 1990; Ratcliff, 1979; Warrington and Rabin, 1970) as well as from healthy individuals (e.g., Smith et al., 1996). More recently and specific to the prefrontal cortex, the ROBBIA (Rotman-Baycrest Battery to Investigate Attention) model of executive functions suggests that distinctions along the left–right axis exist based on the process used (Stuss and Alexander, 2005). This model posits that criterion-setting processes, which allow the set up and selection of relevant task rules and are broadly defined as strategy production (Cabeza et al., 2003; Fletcher et al., 2000; Shallice, 2004), are localized to left lateral PFC, while monitoring and energization processes are found in right lateral and medial PFC, respectively (see Stuss, 2011; Vallesi, 2012, for recent reviews). How this process-based fractionation along the left–right axis of the prefrontal cortex is influenced by domain-based distinctions remains under-examined. In this study we consider the effects of domain on one criterion-setting process, inductive reasoning.
Inductive reasoning is the process of discovering a rule or pattern based on instances belonging to that rule. This complex process involves collecting and remembering instances of the rule, generating a hypothesis based on these instances, integrating new instances, and confirming the hypothesis through further observation (Crescentini et al., 2011). Supporting the ROBBIA model, left prefrontal cortex has consistently been shown to be a critical node for inductive reasoning. This has been seen in studies focused on split-brain patients (Gazzaniga and Smylie, 1984), patients with lesions (Reverberi et al., 2005a, 2005b) and healthy individuals (Crescentini et al., 2011; Goel and Dolan, 2000, 2004; Goel et al., 1997; Jia et al., 2011; Specht et al., 2009; Yang et al., 2009). Typically, though, inductive reasoning studies use verbal material. Thus these studies are less informative when addressing the effect of domain on localizations of inductive reasoning since both domain-based and process-based distinctions predict left lateralization in those cases. Rather, it is the spatial/non-verbal domain where conflicting predictions exist. Of inductive reasoning studies using non-verbal material, five have found activations in right PFC (always in conjunction with left PFC). Interestingly, none of these studies explained their results in terms of domain-based distinctions. Here we review these studies and the explanations given for the curious right PFC activations.
Specht and colleagues asked participants to complete a variant of the Wisconsin Card Sorting Test (WCST) in which the stimuli were non-verbalizable (Specht et al., 2009). Bilateral activations in dorsolateral and ventrolateral PFC were evidenced in the condition in which participants were required to induce the sorting rule when compared to either a rest condition or a condition in which the sorting rule was given. The rule given condition (when compared to rest), however, showed right-lateralized dorsolateral prefrontal cortex (DLPFC) activations and less extensive bilateral ventrolateral PFC activations. From these data the authors concluded that left DLPFC is particularly involved in inductive reasoning and hypothesis generation, while right DLPFC carries out selection and implementation of previously learned rules.
In Goel and Dolan (2000) participants had to classify drawings of novel animals based either on a given rule or through induction of a rule. A task by difficulty interaction showed an effect in right inferior PFC due to increased activation in the difficult rule induction condition and decreased activation in the rule application conditions. The authors concluded that the difficult rules required more evaluation of hypotheses than the easy rules and therefore attributed the right inferior PFC activation to hypothesis selection rather than hypothesis generation. Additionally, while both rule application and rule induction (compared to a perceptual baseline) showed bilateral PFC activations, the rule induction activations were strongly right-lateralized. The authors explained this right hemisphere dominance, in contrast to left-lateralized activations in deductive reasoning studies, by suggesting that the right hemisphere may have a special role in inference tasks, which are open-ended and often have no right or wrong answer, compatible with a role for this region in monitoring (see above). Bilateral activations in prefrontal cortex and increased activations in right-lateralized regions with increasing rule complexity were also seen in another study which examined non-verbal reasoning through a pattern finding task (Hampshire et al., 2011). Those authors did not discuss the specific role played by right PFC.
An fMRI study by Crescentini and colleagues also found bilateral PFC activations in a non-verbal reasoning task (Crescentini et al., 2011). In that study participants completed a Brixton task in which they needed to find and apply a spatial pattern to describe the movement of a colored circle among twelve positions. Comparisons of rule acquisition with rule following in this study showed bilateral mid-dorsolateral PFC activations. A closer examination of these data, however, revealed that the left and right frontal regions were differentially affected by task factors and therefore may underlie different processes. Activity in left DLPFC was modulated by rule difficulty with more activation for difficult compared to easy rules. In contrast, this area was unaffected when response time was included as a covariate, an analysis which right DLPFC did not survive. The authors did not further speculate on the specific processes performed by left and right DLPFC; however, a rule difficulty effect in left, rather than right, PFC is in discord with the results and account of the abovementioned studies.
Finally, Yang and associates asked older participants to perform a numerical inductive reasoning task (Yang et al., 2009). These authors found bilateral DLPFC activations and explained the bilaterality as a possible effect of aging.
The interpretations suggested by the authors of these studies may adequately explain the data found in their own study, however extending them to data from other studies is problematic. Yang and colleagues' role for aging cannot explain the data from the other four studies, all of which were conducted using younger adults. In contrast, a hypothesis selection account could explain the data from the five discussed studies; however it would suggest that all studies of inductive reasoning should show activity in right PFC, a fact that is challenged by the collection of studies that found only left-lateralized activity. However, a domain-based account which suggests that the domain of the task impacts the hemisphere(s) used during inductive reasoning can explain the results of all five studies as well as the absence of right PFC activation in verbal inductive reasoning studies. The critical test for this explanation is to examine verbal and non-verbal tasks using the same inductive reasoning paradigm with the same type of stimuli and a common set of participants. To the authors’ knowledge, no such study has been completed.
The present study addresses whether the domain of the to-be-induced pattern affects the hemisphere(s) used during inductive reasoning. We completed an fMRI study using a novel pattern finding task which crucially included both spatial and verbal patterns composed of the same elements. The patterns were created from letters presented in varied spatial locations that formed either shapes/designs constituting a category (with random letters) or words belonging to a semantic category (with random locations). Participants were asked to infer the category (in the experimental condition) or apply a known rule which required working memory (in the control condition). The common stimuli allow us to make strong observations on the effects of domain on the lateralization of the inductive reasoning process. A prediction based solely on the process-based distinction would suggest that left-lateralized PFC activations will be present for both the verbal and spatial domains. A purely domain-based lateralization account would predict left-lateralized activations for the verbal domain and right-lateralized activations for the spatial domain. However, given the studies presented above, combined activations could also be expected, that is, left PFC activations for the verbal domain and bilateral activations for the spatial domain.
2. Material and methods
2.1. Participants
Twenty-one healthy university students (16 females; mean age=22.8, SD=.6, range 22–24) participated in the study. All were right-handed native Italian speakers with no known neurological or psychiatric problems. Additionally, all participants reported having normal color vision, which was confirmed with the Ishihara Color Vision Test (Ishihara, 1972). The study was approved by the ethical committee of “Istituto IRCCS E. Medea – La Nostra Famiglia.” All participants gave written informed consent and were compensated for their time. Participants were naïve with respect to the specific aims and comparisons of the study. One female participant was subsequently excluded from all analyses due to a low rate of pattern discovery and difficulties synchronizing her performance and neuroimaging data.
2.2. Task procedure
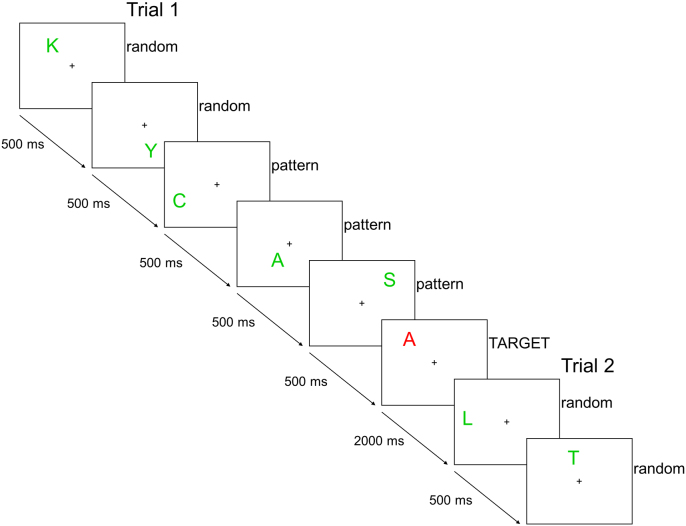
Participants viewed a series of capital letters (the 21 letters of the Italian alphabet) in varied spatial locations within a rectangle which had a fixation cross at the center. The items (i.e., letters) were presented one at a time and were grouped into trials consisting of six to twenty items. The final item of each trial was a target, indicated by its red color; the remaining items were non-targets and colored green. Participants were asked to make a choice response as quickly as possible via button press when a target item appeared. To aid in the choice response, the red target items were presented on screen for 2000 ms, while the non-target items were presented for 500 ms each. After presentation of a target item, the task continued with another trial (see Fig. 1). The task consisted of multiple blocks each with three phases; the phases differed in the composition of the trials and the choice response to the target items.
Fig. 1.
Example of items during pattern discovery and confirmation phases where the pattern exemplar is CASA (house). (For interpretation of the references to color in this figure, the reader is referred to the web version of this article.)
The first phase constituted the experimental condition, focusing on pattern discovery. In this phase the trials consisted of two parts: first, a series of two to twelve random (filler) items, then, a sequence of four to eight pattern items. The spatial location and letter identity was randomly determined for each of the random items, as was the number of random items in each trial. As these items were all non-targets, they were colored green. Among the pattern items, the final (target) item was red, while the other items were green. These items followed a pattern which belonged to one of two possible domains, spatial and verbal. Patterns in the spatial domain represented geometric figures or designs (e.g., a horizontal line, a square, an N shape; see Appendix A for a full list of the patterns used). In the verbal domain, the patterns represented semantic categories (e.g., fruits, parts of the body, weather phenomena; see Appendix A for a full list of the categories used). For both domains, “pattern” refers to a general category, not a specific sequence of positions or letters. Instead, each pattern category consisted of ten such specific sequences, or exemplars, two of each length (4–8 items). For example, the verbal pattern category “fruits” included the exemplars pera (pear), pesca (peach), and fragola (strawberry), among others. In the spatial domain, the general category “horizontal line” indicated that the items were presented at a single y-value starting at one edge of the rectangle and proceeding in equally spaced steps to the opposite edge. In both domains, the exemplars were designed so that the appearance of the final item of each exemplar (the target item) could be fully predicted once the pattern was acquired. When a spatial pattern was presented, the spatial location of each pattern item was determined by the specific exemplar, while the letter identity was randomly selected. Similarly, for verbal patterns, the letter identity was determined by the specific exemplar, and the spatial location was randomly selected. The participants were instructed that they would see random items followed by a sequence belonging to a general category which culminated with a red item. They were asked to discover the pattern and, when each red item appeared, to make a choice response between having discovered the pattern category and not having discovered it (specific response buttons were counterbalanced across participants). In half of the blocks the domain of the pattern category was indicated, limiting the participants' search to only one domain (separate search). In the other half of the blocks the domain was not indicated, and participants needed to search both domains in parallel (parallel search). The phase ended when the participant indicated that she/he had discovered the category or after five trials without pattern discovery. In the first case, the participant advanced to the second phase. In the latter, the participant was told the category, and then proceeded to another pattern discovery phase with a new pattern category.
The second phase was intended to confirm that the participant had discovered a category. The stimuli in this phase followed the same design as in the pattern discovery phase, that is, random items followed by pattern items, the last of which was the red target. The pattern category in this phase was the same as in the preceding pattern discovery phase, though the specific exemplars were not repeated from the previous phase. Participants were asked to predict, based on their knowledge of the category, when the red target would appear and to respond as quickly as possible to its appearance. The choice response in this phase was between being confident in their original determination of the category and feeling that they had originally mistaken the category (specific response buttons were counterbalanced across participants). This phase contained the same number of trials as the preceding pattern discovery phase (e.g., if a participant signaled that s/he had discovered the pattern category after four trials in the discovery phase, the second phase would also include four trials). The confirmation phase was followed in all cases by a third phase.
The third and final phase was a control condition. Participants completed a modified 1-back task that controlled primarily for the working memory demands of the pattern discovery phase. The items in this phase did not include any pattern items; instead each trial consisted of six to twenty random items, the last of which was a red target item. The participants were asked to make a response based on the red target and the item preceding it. Since the appearance of the target item could not be predicted, participants had to continuously retain and update the previous item, a process which resembled the working memory requirements of the pattern discovery phase. The specific instructions for this phase were based on the domain of the category in the preceding phases. For spatial categories, participants were asked to press the left button if the target and preceding item were both to the left of the fixation cross and the right button if at least one of these two items was to the right (instructions with the opposite sides were given to half of the participants). For verbal categories, participants were asked to press the left button if the target and the preceding item were both consonants and the right button if at least one of these two items was a vowel (instructions with the opposite sides were given to half of the participants). This phase contained the same number of trials as the preceding pattern discovery phase.
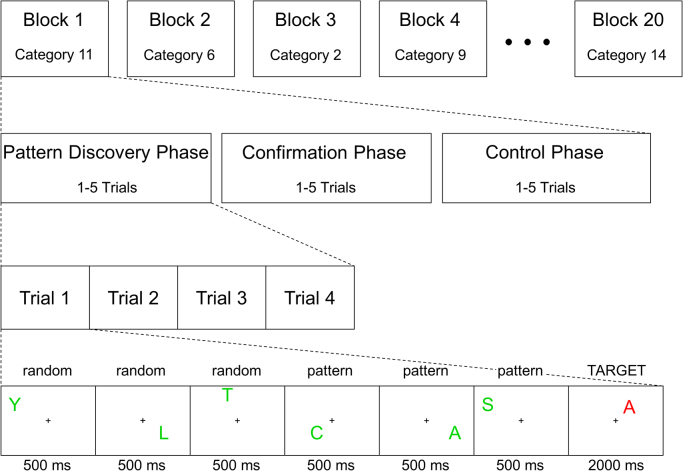
Thus, a complete block consisted of a pattern discovery phase, a confirmation phase using the same pattern category, and a working memory control phase using the same domain (see Fig. 2 for an overview of the entire task structure). Upon completion of a block, a new block with a new pattern category began. Reminders of the specific task and how to respond to the target items were given prior to each phase in every block after a blank screen of jittered length (2000–8000 ms in 250 ms intervals). Participants were trained on the structure of the task at least one day (mean=2.7 days, SD=1.7 days) prior to scanning. The training session consisted of four guided practice blocks with the experimenter present to clarify any questions and eight training blocks (divided evenly across domain and search type). In the training session, participants were asked to provide a written or drawn description of each category after the confirmation phase to ensure that they had learned to actively search for and discover a category. This check was not feasible during the experimental session. However, it was unnecessary (beyond the training session) given that we were interested in the brain processes employed during the full time span of inductive reasoning, not in the final outcome, which is by definition open-ended (Thagard, 2001). During scanning each participant viewed 20 novel categories (half spatial, half verbal) which were different from the categories used during the training session. These 20 categories were split into four runs, each containing five blocks. A behavioral pilot study of 60 categories was completed to select the categories for the experimental session, as well as those used in the training session. Twelve healthy native Italian speakers participated in the pilot study (one was later excluded due to color-blindness), none of whom participated in the fMRI study. These participants completed a modified version of the above-described task (the control phase instructions differed) on 30 categories each. The final selections were made to ensure that sufficient data would be obtained in the fMRI study. Thus, the selected categories had a high discovery rate (M=92.4%, range: 80%–100%) and required more than one presentation for discovery on average (M=2.03, range: 1.4–2.8). Forty categories were selected for the experimental session which were split into two matched sets of 20 categories each (see Appendix A for a list of the 40 categories used). Half of the participants received each set. Within each set of categories the order of presentation was randomized for each participant. Additionally, the search type (that is, separate or parallel) was randomized for each participant, though always distributed equally across the domains. Finally, the ten exemplars of each category were presented in a random order for each participant.
Fig. 2.
Overview of the task structure. Note that the order of the categories and the number of random items changed for each participant. Further note that the number of trials and presence of any given confirmation and control phases were dependent on the participant's performance.
2.3. Image acquisition and preprocessing
Images were acquired at the Santa Maria della Misericordia Hospital on a 3-Tesla Philips Achieva whole-body scanner with an 8-channel head coil. Head movement was minimized through cushioning within the coil. Functional volumes were obtained using a whole-head T2⁎-weighted echoplanar image (EPI) sequence (repetition time [TR]=2 s, echo time=35 ms, flip angle=90, 34 transverse axial slices with interleaved acquisition, 3.5×3.59×4 mm3 voxel resolution, field of view [FOV]=23 cm, acquisition matrix=64×64, SENSE factors: 2 in the anterior–posterior direction). The number of volumes acquired varied for each participant and run given the performance-based nature of the task (mean volumes per run=241, SD=39, range 180–341). The first four volumes of each run were discarded to allow the magnetization to reach steady-state. Anatomical images were acquired between the 2nd and 3rd runs of functional images. Stimuli were presented using Presentation software (www.neurobs.com) and viewed through MR-compatible goggles mounted to the head coil, which were adjusted so that each participant's vision was corrected-to-normal. Responses were made and recorded through two MR-compatible response pads using the left and right index fingers.
The fMRI data were pre-processed and statistically analyzed using SPM8 (Statistical Parameter Mapping; Wellcome Department of Cognitive Neurology, UCL, London, UK). Functional images were spatially realigned and unwarped to account for head movement during the experiment using a 4th degree B-Spline interpolation. To normalize the images, a transformation matrix between the mean functional volume and a standard functional Montreal Neurological Institute (MNI) template (EPI.nii) was generated using a 6th degree B-Spline algorithm. The realigned images were normalized based on this matrix with a 2 mm3 voxel size. Lastly, the resulting images were spatially smoothed with an 8 mm full-width-at-half-maximum Gaussian filter.
2.4. Behavioral statistical analyses
Three behavioral measures were analyzed to ensure that general alertness and difficulty did not differ across the phases and domains. Hit rate was used to assess alertness. We chose this measure as opposed to accuracy because accuracy is intrinsically different across the phases. Both the pattern discovery and confirmation phases have no correct or incorrect response and therefore no true measure of accuracy, while the control phase does have correct responses, which would be reflected in the accuracy measure. The hit rate, which measures percentage of responses on target items without reference to the specific response, is, on the other hand, equivalent across the phases. Response times (RT) allowed us to compare the general difficulty of the phases, as well as check if participants had acquired a pattern prior to the confirmation phase as suggested by reduced RTs in this phase. Finally, the number of presentations needed for pattern discovery was used to compare the difficulty across the domains and search types. Two repeated measures two-way ANOVAs with phase (pattern discovery, confirmation, control) and domain (spatial, verbal) were conducted on the hit rate and RT data. Reported results reflect a Greenhouse–Geisser correction. Further, three repeated measures two-way ANOVAs with domain (spatial, verbal) and search type (separate, parallel) were conducted on the hit rate, RT, and ‘trials to discovery’ data from the pattern discovery phase.
2.5. fMRI statistical analyses
First-level analyses were performed for each participant using a General Linear Model. The data were modeled using nine conditions as regressors:
-
-
four experimental pattern discovery conditions: spatial pattern-separate search, verbal pattern-separate search, spatial pattern-parallel search, and verbal pattern-parallel search;
-
-
two confirmation conditions: spatial and verbal;
-
-
two working memory control conditions: spatial and verbal; and
-
-
a no pattern discovery/incorrect pattern discovery condition.
The final condition included discovery phases in which the pattern was not discovered in five trials as well as the confirmation and corresponding discovery phases in cases where the participant indicated during the confirmation phase that s/he had misjudged the category during the initial discovery phase. Each condition consisted of a series of epochs, modeled as boxcar functions, convolved with a canonical hemodynamic response function. An epoch was defined as a given phase within a given block, the duration of which was determined by the onset of the first item and offset of the final item in that particular phase. The average epoch duration was sixteen seconds, with no difference between the phases. We chose to use epochs as opposed to single events since we were interested in the continuous processing throughout the condition and not solely at the point of stimulus onset or participant response. Estimates of head movement given by realignment were included in the matrix as six additional regressors of no interest. The four runs were modeled within a single GLM as separate sessions and therefore four regressors of no interest were also included to model sustained and unspecific differences between the runs. Slow signal drifts were removed using a 128 s high-pass filter. For each participant, a t-contrast averaged across the runs was extracted for each of the eight conditions of interest (that is, those listed above excluding ‘no pattern discovery/incorrect pattern discovery’). The SPM group maps were generated with a random-effects model within SPM8 using the individual contrast maps. A “full factorial” ANOVA model was used consisting of one factor with eight levels (conditions). Three primary t-contrasts of interest were extracted. The first was a simple contrast between the four pattern discovery conditions (spatial pattern-separate search, verbal pattern-separate search, spatial pattern-parallel search, and verbal pattern-parallel search; each +1) and the two control conditions (spatial and verbal; each −2). The second was a simple contrast between the two confirmation conditions (spatial and verbal; each +1) and the two control conditions (each −1). The third was a simple contrast between the four pattern discovery conditions (each +1) and the two confirmation conditions (each −2). Six additional t-contrasts were extracted which mirrored the first three contrasts, but within each domain (e.g., spatial pattern-separate search vs. spatial control). These contrasts used only the separate search conditions (among the pattern discovery conditions) since the aim was to determine the pattern of activity specific to each domain and during parallel search conditions both domains may be searched. The effects of searching multiple domains was examined with a final t-contrast between pattern discovery parallel search conditions (each +1) and pattern discovery separate search conditions (each −1). The statistical significance was set at peak-wise p<.05, corrected for multiple comparisons using a Family-wise Error correction. The brodmann template in MRIcroN (http://www.mccauslandcenter.sc.edu/mricro/mricron/) was used to find the likely Brodmann area (BA) for each cluster. Similarly, the Hammers-mith n30r83 atlas (© Copyright Imperial College of Science, Technology and Medicine 2007, All rights reserved; www.brain-development.org; Hammers et al., 2003; Gousias et al., 2008), which is a probabilistic atlas of neuroanatomy, was used to find the likely anatomical region for each cluster.
3. Results
3.1. Behavioral results
We first report analyses on the behavioral measures. Data from phases in which the pattern was not discovered within five trials (3.3%) and phases for which the participant reported having misjudged the pattern (6%) were not considered. A two-way (phase by domain) repeated measures ANOVA on the percentage of hits on target items revealed no significant main effects and no interaction (ps>.253, see Table 1 for values). A two-way (domain by search type) repeated measures ANOVA within the pattern discovery phase also showed no main effects or interaction (ps>.291).
Table 1.
Behavioral results.
| Hits on targets | RT (ms) | # Trials to discovery | |
|---|---|---|---|
| Pattern discovery phase | 96.6% (1.6%) | 853 (44) | 1.99 (0.14) |
| Spatial | 96.2% (1.8%) | 836 (44) | 2.08 (0.16) |
| Verbal | 97.2% (1.6%) | 871 (52) | 1.90 (0.15) |
| Separate | 97.3% (1.3%) | 811 (44) | 1.84 (0.14) |
| Parallel | 96.6% (2.2%) | 879 (50) | 2.15 (0.15) |
| Confirmation phase | 97.5% (0.6%) | 390 (36) | |
| Spatial | 97.3% (0.9%) | 361 (39) | |
| Verbal | 97.5% (0.9%) | 422 (33) | |
| Control phase | 99.0% (0.4%) | 812 (36) | |
| Spatial | 99.0% (0.6%) | 765 (35) | |
| Verbal | 98.9% (0.6%) | 870 (45) | |
Note: Values shown are means with standard errors in parentheses.
A two-way (phase by domain) repeated measures ANOVA on response times (RTs) revealed main effects of phase (F(1.735,32.970)=83.802, p<.001) and domain (F(1,19)=11.221, p=.003), but no interaction between them (p=.206). Follow-up t-tests (evaluated at α=.017 to correct for multiple comparisons) revealed that RTs were shorter in the confirmation phase compared to both the pattern discovery and control phases (t(19)=11.013, p<.001 and t(19)=9.623, p<.001, respectively), which did not differ from one another (p=.225). Shorter RTs during the confirmation phase were expected, as the arrival of the target item was predictable only during this phase. This may be taken as verification that the participants had formed a hypothesis during the pattern discovery phase. Though an alternative explanation that the shorter RTs were due to the choice decision in this phase being easier than in the other phases cannot be ruled out. Responses to spatial trials were faster than to verbal trials in the confirmation and control phases (t(19)=3.650, p=.002 and t(19)=3.401, p=.003, respectively), but not in the pattern discovery phase (p=.365). A two-way (domain by search type) repeated measures ANOVA on RTs within the pattern discovery phase showed no main effects or interaction (ps>.132).
Participants required on average two presentations to discover the patterns. This did not differ between spatial and verbal patterns (p=.156), though there was a significant effect of search type, with separate search leading to quicker discovery (F(1,19)=21.539, p<.001). There was no interaction between domain and search type (p=.873).
3.2. fMRI results
The planned comparison of all pattern discovery conditions to all working memory control conditions showed activations in several areas including bilateral ventrolateral prefrontal cortex (BA 45), right orbitofrontal cortex (BA 47), medial prefrontal cortex (BA 8), anterior cingulate cortex (BA 32), left posterior temporal cortex (BA 37), and bilateral cerebellum (see Table 2). A separate planned comparison of all confirmation conditions to all control conditions showed activations in similar areas, though less extensive (see Table 2), including bilateral ventrolateral prefrontal cortex (BA 45) and medial prefrontal cortex (BA 8). A comparison of all pattern discovery conditions to all confirmation conditions yielded no activations at the specified threshold.
Table 2.
Activated regions.
| Anatomical localization | BA | MNI coordinates |
Cluster p-corr. | Peak p-corr. | Peak Z-value | Voxels per cluster | ||
|---|---|---|---|---|---|---|---|---|
| x | y | z | ||||||
| Pattern discovery vs. control activations | ||||||||
| L. inferior frontal gyrus | 45 | −52 | 36 | 6 | <.0001 | <.0001 | >10 | 2226 |
| −56 | 24 | 18 | <.0001 | 7.24 | ||||
| −56 | 18 | 8 | <.0001 | 7.17 | ||||
| L. superior frontal gyrus | 8 | −2 | 22 | 64 | <.0001 | <.0001 | 7.19 | 1016 |
| −6 | 22 | 54 | <.0001 | 7.09 | ||||
| 8 | 30 | 46 | <.0001 | 6.55 | ||||
| L. cerebellum | −22 | −88 | −26 | <.0001 | <.0001 | 7.04 | 881 | |
| −30 | −82 | −24 | <.0001 | 6.47 | ||||
| −12 | −84 | −36 | <.0001 | 6.16 | ||||
| L. posterior temporal lobe | 37 | −56 | −56 | −20 | <.0001 | <.0001 | 6.89 | 455 |
| −48 | −62 | −24 | <.0001 | 6.58 | ||||
| −48 | −52 | −26 | <.0001 | 6.24 | ||||
| R. inferior frontal gyrus | 45 | 56 | 36 | 8 | <.0001 | <.0001 | 6.68 | 485 |
| 58 | 30 | 14 | <.0001 | 5.63 | ||||
| 42 | 46 | 8 | 0.006 | 5.11 | ||||
| R. lateral orbital gyrus | 47 | 38 | 36 | −18 | <.0001 | <.0001 | 6.47 | 262 |
| 34 | 22 | −16 | <.0001 | 6.15 | ||||
| R. cerebellum | 20 | −82 | −46 | <.0001 | <.0001 | 6.26 | 783 | |
| 26 | −70 | −30 | <.0001 | 6.07 | ||||
| 44 | −70 | −30 | <.0001 | 5.97 | ||||
| L. anterior cingulate gyrus | 32 | −8 | 38 | 18 | <.0001 | <.0001 | 5.96 | 91 |
| R. posterior cingulate gyrus | 23 | 4 | −36 | 30 | <.0001 | <.0001 | 5.74 | 144 |
| L. lingual gyrus | 17 | −2 | −66 | 4 | <.0001 | <.0001 | 5.73 | 75 |
| R. anterior cingulate gyrus | 32 | 12 | 40 | 8 | 0.002 | 0.002 | 5.3 | 33 |
| 14 | 36 | 16 | 0.03 | 4.74 | ||||
| L. lingual gyrus | 17 | −2 | −94 | −10 | 0.003 | 0.004 | 5.2 | 28 |
| R. middle frontal gyrus | 10 | 34 | 58 | 14 | 0.004 | 0.013 | 4.94 | 25 |
| R. inferior frontal gyrus | 48 | 46 | 18 | 16 | 0.002 | 0.014 | 4.92 | 36 |
| 44 | 10 | 20 | 0.018 | 4.86 | ||||
| L. caudate nucleus | −14 | 10 | 0 | 0.007 | 0.02 | 4.84 | 16 | |
| R. caudate nucleus | 14 | 10 | 6 | 0.027 | 0.028 | 4.75 | 3 | |
| L. middle frontal gyrus | 6 | −44 | 8 | 40 | 0.027 | 0.035 | 4.7 | 3 |
| R. posterior temporal lobe | 20 | 68 | −42 | −12 | 0.037 | 0.042 | 4.66 | 1 |
| R. lateral orbital gyrus | 50 | 36 | −16 | 0.037 | 0.043 | 4.65 | 1 | |
| R. cerebellum | 34 | −74 | −50 | 0.037 | 0.048 | 4.63 | 1 | |
| R. posterior orbital gyrus | 38a | 28 | 18 | −24 | 0.037 | 0.049 | 4.62 | 1 |
| Confirmation vs. control activations | ||||||||
| L. inferior frontal gyrus | 45 | −52 | 36 | 4 | <.0001 | <.0001 | 6.37 | 839 |
| −30 | 22 | −14 | <.0001 | 5.76 | ||||
| −56 | 14 | 2 | <.0001 | 5.75 | ||||
| R. posterior orbital gyrus | 38a | 34 | 22 | −16 | <.0001 | <.0001 | 5.75 | 87 |
| R. superior frontal gyrus | 32 | 10 | 32 | 46 | <.0001 | 0.001 | 5.49 | 66 |
| L. cerebellum | −22 | −78 | −28 | 0.001 | 0.006 | 5.11 | 44 | |
| L. superior frontal gyrus | 8 | −2 | 26 | 54 | 0.004 | 0.008 | 5.04 | 25 |
| L. posterior temporal lobe | 37 | −52 | −58 | −20 | 0.001 | 0.013 | 4.94 | 45 |
| R. inferior frontal gyrus | 45 | 54 | 36 | 4 | 0.007 | 0.016 | 4.89 | 17 |
| 48 | 30 | 2 | 0.032 | 4.73 | ||||
| Spatial pattern discovery vs. control | ||||||||
| L. inferior frontal gyrus | 45 | −52 | 36 | 4 | 0.002 | 0.003 | 5.27 | 32 |
| L. cerebellum | −22 | −86 | −26 | 0.001 | 0.005 | 5.15 | 40 | |
| R. inferior frontal gyrus | 45 | 54 | 38 | 4 | 0.002 | 0.005 | 5.14 | 36 |
| Verbal pattern discovery vs. control | ||||||||
| L. lateral orbital gyrus | 38a | −40 | 26 | −16 | <.0001 | <.0001 | 5.89 | 128 |
| L. inferior frontal gyrus | 45 | −56 | 24 | 18 | <.0001 | <.0001 | 5.97 | 274 |
| −52 | 36 | 4 | 0.002 | 5.61 | ||||
| −48 | 22 | 4 | 0.005 | 5.41 | ||||
| L. superior frontal gyrus | 8 | −6 | 22 | 54 | 0.011 | 0.01 | 5.20 | 11 |
| Spatial and verbal conjunction | ||||||||
| L. inferior frontal gyrus | 45 | −52 | 36 | 4 | 0.003 | 0.003 | 5.27 | 29 |
These clusters were on the border between the frontal orbital gyrus and the temporal pole.
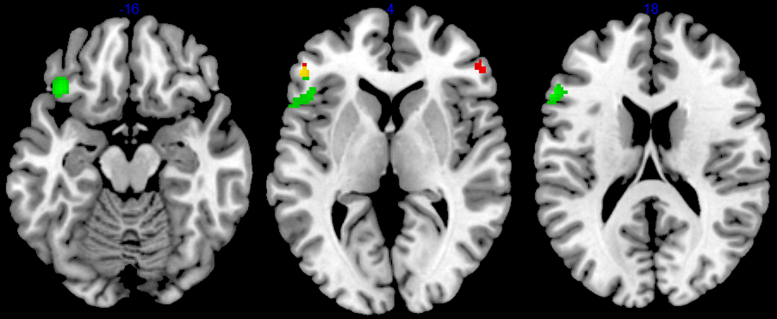
Planned comparisons within each domain addressed the central issue of the role of domain in localization of brain activity. The comparison of the spatial separate search condition to the spatial control condition revealed activations in bilateral ventrolateral prefrontal cortex (BA 45) and left cerebellum (see Table 2 and Fig. 3). The comparison of the verbal separate search condition to the verbal control condition (see Table 2 and Fig. 3) showed activations in left ventrolateral prefrontal cortex (BA 45) and medial prefrontal cortex (BA 8). The comparison of the confirmation condition to the control condition within the spatial domain yielded a single activation close to the right posterior orbital gyrus (BA 38). The same comparison in the verbal domain resulted in activations in the left inferior frontal gyrus (BA 45/47). Comparisons between pattern discovery and confirmation conditions within domains showed no activations. The final planned comparison between parallel search conditions and separate search conditions yielded no areas of activation.
Fig. 3.
Activations for the separate search pattern discovery vs. control phases within the spatial and verbal domains. Red—spatial domain, green—verbal domain, yellow—overlapping spatial and verbal activations. Slices are at z=−16, z=4, and z=18 in MNI coordinates. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.)
Three post-hoc analyses further explored the results of the single domain pattern discovery vs. control comparisons. First, a statistical conjunction analysis of these two within domain contrasts showed an overlap of activation in left ventrolateral prefrontal cortex (BA 45). The second and third analyses examined differences in the pattern discovery vs. control comparisons between the domains; neither contrast (spatial vs. verbal, verbal vs. spatial) yielded areas of activation.
4. Discussion
The present study used a novel pattern finding paradigm to localize the brain regions implicated in inductive reasoning. The use of stimuli which afforded both spatial and verbal patterns allowed us to compare the areas activated by each domain. Inductive reasoning, above and beyond working memory requirements (which were controlled for with the baseline tasks), activated areas in bilateral ventrolateral prefrontal cortex (BA 45), right orbitofrontal cortex (BA 47), medial prefrontal cortex (BA 8), anterior cingulate cortex (BA 32), and more posterior regions. When focused on the individual domains a subset of these regions was activated. The spatial domain showed activations in bilateral ventrolateral prefrontal cortex. The verbal domain, on the other hand, showed activations in medial PFC and left ventrolateral PFC, the location of which overlapped with the left hemisphere activation found for the spatial domain. These data highlight two points. First, the left prefrontal cortex has a central role in inductive reasoning, independent of the domain. Second, both process and domain influence the brain regions employed during reasoning.
Though inductive reasoning activated a network of frontal and temporal brain regions, the results of the conjunction analysis demonstrated that left ventrolateral prefrontal cortex is uniquely important for this process. Both the spatial and verbal domains showed activation in the left PFC, which rules out the possibility that the left-lateralization seen in the verbal task was due solely to the task domain employed. Additionally, left PFC activation is consistent with results from previous studies on inductive reasoning (Crescentini et al., 2011; Goel and Dolan, 2000, 2004; Goel et al., 1997; Jia et al., 2011; Reverberi et al., 2005a, 2005b; Yang et al., 2009). The centrality of left PFC in inductive reasoning also adds further support to the ROBBIA model of executive functions (Stuss and Alexander, 2005).
The right PFC activation seen in the spatial domain is consistent with a subset of inductive reasoning studies that made use of non-verbal stimuli (Crescentini et al., 2011; Goel and Dolan, 2000; Hampshire et al., 2011; Specht et al., 2009; Yang et al., 2009). Previous explanations of this right PFC activation (see Introduction) do not adequately explain the present results. Accounts suggesting that right PFC is used for hypothesis selection, such as those by Specht and colleagues and Goel and colleagues, are insufficient. Activation in right PFC was not seen for the verbal domain in this study. Thus, a hypothesis selection account of the present data would imply that selection is needed in the spatial domain, but not in the verbal domain. This possibility is unlikely given the similarities between the verbal and spatial tasks. The paradigm was designed to utilize identical stimuli and general structure across the domains, with the only difference between them residing in the specific factor of interest, the domain of the pattern. Additionally, the difficulty of the spatial and verbal patterns was matched (no differences in the pattern discovery phase on hit rate, RTs, or trials to discovery). A related account, also by Goel and colleagues, posits that right ventrolateral PFC is used for set-shifts (or lateral transformations) (Goel and Vartanian, 2004). This explanation can be excluded by the same logic as above, that is, there is no evidence that the spatial patterns necessitated more set-shifts than the verbal patterns did. A final interpretation, suggested by Yang and colleagues, that right PFC activation is related to aging does not directly apply to this study given that our participants were all young adults. However, Yang et al.'s results could be viewed as evidence for compensatory activity, in which case bilateral activity could be expected in particularly demanding tasks. This understanding would then present the same difficulty as the previously discussed accounts, that is, it would be unclear why right PFC activation was seen in the spatial, but not the verbal domain. We, instead, posit that the presence of right PFC activation is related to the use of a spatial task.
While the results of the direct spatial vs. verbal comparison may slightly contest this assertion, it is important to note that most divisions drawn in the prefrontal cortex are actually gradients. Thus a gradient nature may be responsible for the absence of a statistically detectable difference. However, a dominance of the right hemisphere in the spatial domain has been suggested by numerous studies concerned with diverse paradigms and processes. Studies of working memory have shown activations in the right hemisphere when spatial information must be retained (e.g., Smith et al., 1996). Patients with lesions in the right hemisphere have shown deficits in perceptual matching (Warrington and Rabin, 1970) and mental rotation (Ratcliff, 1979). Right prefrontal activations were noted in a spatial monitoring task (Vallesi and Crescentini, 2011). Indeed, spatial attention is thought to rely more heavily on the right hemisphere than the left (Corbetta and Shulman, 2002; Vallar, 1998); hemi-spatial neglect occurs more frequently with right-sided than left-sided lesions. This reliance may be due to the right hemisphere's ability to direct attention to both hemifields, while the left hemisphere can only attend to the right hemifield (Mesulam, 1981). Thus, it is apparent that the right hemisphere is utilized for a range of functions in the spatial domain.
The specific function of the right PFC in the current pattern finding task has two possible interpretations, which cannot be distinguished using the data presented here. First, the right PFC could perform inductive reasoning processes specific to non-verbal domains and thus would be additionally recruited when the task falls outside the verbal domain. This interpretation is supported by the fact that the left and right activations in the spatial domain are homologs, suggesting that they may be performing the same function. However, such an inference may be premature in light of Crescentini and colleagues' findings showing differential effects of task factors on homologous regions of PFC (Crescentini et al., 2011). The second interpretation is that inductive reasoning occurs in left PFC but recruits content information from domain specific regions, which adhere to domain-based hemispheric lateralization. This account may be supported by the larger area of activation for the verbal domain in the left hemisphere. Additional support comes from the bilateral inductive reasoning and right-lateralized rule following activations seen in Specht et al. (2009).
Regardless of the specific interpretation of the right PFC activation, the results appear to corroborate previous findings which suggest that where an operation is carried out in the brain is dependent not only on the process being used, but also on the domain in which that process is occurring. Langdon and Warrington (2000) noted impairments on spatial reasoning in patients with left hemisphere lesions as well as in patients with right hemisphere lesions; however only patients with left hemisphere lesions were impaired on verbal reasoning tasks. In the memory field, Johnson and colleagues found right PFC activation for recognizing previously presented objects and bilateral PFC activation for recognizing previously presented words (Johnson et al., 2003). These authors concluded that the activation in PFC “depend[ed] on the specific combination of information and process.” The localizations evidenced in the present study during inductive reasoning in the verbal and spatial domains add support to this understanding of the roles of both domain and process.
The majority of the sample in the current study was female, whereas previous neuroimaging literature on reasoning has often tested males. Given this gap, future studies should combine domain and sex differences factorially to disentangle their roles in driving brain activations. Additionally, future studies could benefit from ensuring the completion of induction prior to subsequent conditions. In the present study participants may have used the confirmation phase to complete the integrating new instances of the rule and confirming the hypothesis steps of the inductive reasoning process. This supposition is based on the absence of a difference between the pattern discovery and confirmation phases, the similarity of the pattern discovery vs. control and confirmation vs. control comparisons, and the seemingly fast average discovery rate of two presentations. This potential bleeding of induction into the confirmation phase, however, does not negate the induction processes occurring during the pattern discovery phase.
The present findings confirm the role of the left prefrontal cortex as the primary seat of inductive reasoning, and criterion-setting processes more broadly. However, they also demonstrate that right prefrontal cortex is additionally recruited when spatial material must be inferred. This outcome extends our knowledge of the roles that process and domain play on the distribution of brain activity. Specifically, regions related to both the process used and the domain used appear to be activated in a given task. Thus it would benefit future studies aimed at localizing specific processes to take into consideration the potential effects of domain. Furthermore, it will be important to understand for each process whether areas of domain-related activation are performing the same process but specific to the domain or rather are performing content-gathering functions.
Acknowledgments
AV and LB are funded by the European Research Council Starting Grant LEX-MEA (GA #313692) to AV. We thank Olga Puccioni and Laura Riontino for their help in data collection. We also thank Marta Maieron and the Neuroradiology Unit of the Azienda Ospedaliero-Universitaria S. Maria della Misericordia, Udine, for their kind support in MRI sequence preparation and acquisition. We additionally thank the anonymous reviewers who provided useful comments on previous versions of this manuscript.
Appendix A. Supplementary material
Supplementary material
References
- Baddeley A.D. Clarendon Press; Oxford: 1986. Working Memory. [Google Scholar]
- Badre D., D’Esposito M. Is the rostro-caudal axis of the frontal lobe hierarchical? Nat. Rev. Neurosci. 2009;10:659–669. doi: 10.1038/nrn2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R., Locantore J.K., Anderson N.D. Lateralization of prefrontal activity during episodic memory retrieval: evidence for the production-monitoring hypothesis. J. Cognit. Neurosci. 2003;15:249–259. doi: 10.1162/089892903321208187. [DOI] [PubMed] [Google Scholar]
- Corbetta M., Shulman G.L. Control of goal-directed and stimulus-driven attention in the brain. Nat. Rev. Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Crescentini C., Seyed-Allaei S., De Pisapia N., Jovicich J., Amati D., Shallice T. Mechanisms of rule acquisition and rule following in inductive reasoning. J. Neurosci. 2011;31:7763–7774. doi: 10.1523/JNEUROSCI.4579-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P.C., Shallice T., Dolan R.J. Sculpting the response space – an account of left prefrontal activation at encoding. NeuroImage. 2000;12:404–417. doi: 10.1006/nimg.2000.0633. [DOI] [PubMed] [Google Scholar]
- Gazzaniga M.S., Smylie C.S. Dissociation of language and cognition: a psychological profile of two disconnected right hemispheres. Brain. 1984;107:145–153. doi: 10.1093/brain/107.1.145. [DOI] [PubMed] [Google Scholar]
- Goel V., Dolan R.J. Anatomical segregation of component processes in an inductive inference task. J. Cognit. Neurosci. 2000;12:110–119. doi: 10.1162/08989290051137639. [DOI] [PubMed] [Google Scholar]
- Goel V., Dolan R.J. Differential involvement of left prefrontal cortex in inductive and deductive reasoning. Cognition. 2004;93:B109–B121. doi: 10.1016/j.cognition.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Goel V., Gold B., Kapur S., Houle S. The seats of reason? An imaging study of deductive and inductive reasoning. NeuroReport. 1997;8:1305–1310. doi: 10.1097/00001756-199703240-00049. [DOI] [PubMed] [Google Scholar]
- Goel V., Vartanian O. Dissociating the roles of right ventral lateral and dorsal lateral prefrontal cortex in generation and maintenance of hypotheses in set-shift problems. Cereb. Cortex. 2004;15:1170–1177. doi: 10.1093/cercor/bhh217. [DOI] [PubMed] [Google Scholar]
- Gousias I.S., Rueckert D., Heckemann R.A., Dyet L.E., Boardman J.P., Edwards A.D., Hammers A. Automatic segmentation of brain MRIs of 2-year-olds in 83 regions of interest. NeuroImage. 2008;40:672–684. doi: 10.1016/j.neuroimage.2007.11.034. [DOI] [PubMed] [Google Scholar]
- Hammers A., Allom R., Koepp M.J., Free S.L., Myers R., Lemieux L., Duncan J.S. Three-dimensional maximum probability atlas of the human brain, with particular reference to the temporal lobe. Hum. Brain Mapp. 2003;19:224–247. doi: 10.1002/hbm.10123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hampshire A., Thompson R., Duncan J., Owen A.M. Lateral prefrontal cortex subregions make dissociable contributions during fluid reasoning. Cereb. Cortex. 2011;21:1–10. doi: 10.1093/cercor/bhq085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishihara S. Kanehara Shuppan; Tokyo: 1972. Tests for Colour-Blindness. [Google Scholar]
- Jia X., Liang P., Lu J., Yang Y., Zhong N., Li K. Common and dissociable neural correlates associated with component processes of inductive reasoning. NeuroImage. 2011;56:2292–2299. doi: 10.1016/j.neuroimage.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Johnson M.K., Raye C.L., Mitchell K.J., Greene E.J., Anderson A.W. fMRI evidence of an organization of prefrontal cortex by both type of process and type of information. Cereb. Cortex. 2003;13:265–273. doi: 10.1093/cercor/13.3.265. [DOI] [PubMed] [Google Scholar]
- Kelley W.M., Miezin F.M., McDermott K.B., Buckner R.L., Raichle M.E., Cohen N.J., Petersen S.E. Hemispheric specialization in human dorsal frontal cortex and medial temporal lobe for verbal and nonverbal memory encoding. Neuron. 1998;20:927–936. doi: 10.1016/s0896-6273(00)80474-2. [DOI] [PubMed] [Google Scholar]
- Kim C., Johnson N.F., Cilles S.E., Gold B.T. Common and distinct mechanisms of cognitive flexibility in prefrontal cortex. J. Neurosci. 2011;31:4771–4779. doi: 10.1523/JNEUROSCI.5923-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koechlin E., Summerfield C. An information theoretical approach to prefrontal executive function. Trends Cognit. Sci. 2007;11:229–235. doi: 10.1016/j.tics.2007.04.005. [DOI] [PubMed] [Google Scholar]
- Langdon D., Warrington E.K. The role of the left hemisphere in verbal and spatial reasoning tasks. Cortex. 2000;36:691–702. doi: 10.1016/s0010-9452(08)70546-x. [DOI] [PubMed] [Google Scholar]
- Luria A.R. Harper & Row Publishers; New York: 1966. Human Brain and Psychological Processes. [Google Scholar]
- McCarthy R.A., Warrington E.K. Auditory verbal span of apprehension: a phenomenon in search of a function? In: Vallar G., Shallice T., editors. 167–186. CUP; Cambridge: 1990. (Short Term Memory). [Google Scholar]
- Mesulam M.M. A cortical network for directed attention and unilateral neglect. Ann. Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- Norman D.A., Shallice T. Attention to action: willed and automatic control of behavior. In: Davidson R.J., Schwartz G.E., Shapiro D., editors. 1–18. Plenum Press; New York: 1986. (Consciousness Self Regulation: Advances in Research). [Google Scholar]
- Petrides M. Lateral prefrontal cortex: architectonic and functional organization. Philos. Trans. R. Soc. B Biol. Sci. 2005;360:781–795. doi: 10.1098/rstb.2005.1631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ratcliff G. Spatial thought, mental rotation and the right cerebral hemisphere. Neuropsychologia. 1979;17:49–54. doi: 10.1016/0028-3932(79)90021-6. [DOI] [PubMed] [Google Scholar]
- Reverberi C., D'Agostini S., Skrap M., Shallice T. Generation and recognition of abstract rules in different frontal lobe subgroups. Neuropsychologia. 2005;43:1924–1937. doi: 10.1016/j.neuropsychologia.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Reverberi C., Lavaroni A., Gigli G.L., Skrap M., Shallice T. Specific impairments of rule induction in different frontal lobe subgroups. Neuropsychologia. 2005;43:460–472. doi: 10.1016/j.neuropsychologia.2004.06.008. [DOI] [PubMed] [Google Scholar]
- Rowe J.B., Toni I., Josephs O., Frackowiak R.S., Passingham R.E. The prefrontal cortex: response selection or maintenance within working memory? Science. 2000;288:1656–1660. doi: 10.1126/science.288.5471.1656. [DOI] [PubMed] [Google Scholar]
- Shallice T. The fractionation of supervisory control. In: Gazzaniga M.S., editor. edition. 943–956. MIT Press; Cambridge (MA): 2004. (The Cognitive Neurosciences III). [Google Scholar]
- Smith E.E., Jonides J., Koeppe R.A. Dissociating verbal and spatial working memory using PET. Cereb. Cortex. 1996;6:11–20. doi: 10.1093/cercor/6.1.11. [DOI] [PubMed] [Google Scholar]
- Specht K., Lie C., Shah N.J., Fink G.R. Disentangling the prefrontal network for rulse selection by means of a non-verbal variant of the Wisconsin card sorting task. Hum. Brain Mapp. 2009;30:1734–1743. doi: 10.1002/hbm.20637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuss D.T. Functions of the frontal lobes: Relation to executive functions. J. Int. Neuropsychol. Soc. 2011;17:759–765. doi: 10.1017/S1355617711000695. [DOI] [PubMed] [Google Scholar]
- Stuss D.T., Alexander M.P. Is there a dysexecutive syndrome? Philos. Trans. R. Soc. B Biol. Sci. 2005;362:901–915. doi: 10.1098/rstb.2007.2096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thagard P. Induction. In: Wilson R.A., Keil F.C., editors. 399–400. MIT Press; Cambridge (MA): 2001. (The MIT Encyclopedia of the Cognitive Sciences). [Google Scholar]
- Vallar G. Spatial hemineglect in humans. Trends Cognit. Sci. 1998;2:87–97. doi: 10.1016/s1364-6613(98)01145-0. [DOI] [PubMed] [Google Scholar]
- Vallesi A. Organization of executive functions: hemispheric asymmetries. J. Cognit. Psychol. 2012;24:367–386. [Google Scholar]
- Vallesi A., Crescentini C. Right fronto-parietal involvement in monitoring spatial trajectories. NeuroImage. 2011;57:558–564. doi: 10.1016/j.neuroimage.2011.04.061. [DOI] [PubMed] [Google Scholar]
- Wagner A.D., Poldrack R.A., Eldridge L.L., Desmond J.E., Glover G.H., Gabrieli J.D.E. Material-specific lateralization of prefrontal activation during episodic encoding and retrieval. NeuroReport. 1998;9:3711–3717. doi: 10.1097/00001756-199811160-00026. [DOI] [PubMed] [Google Scholar]
- Warrington E.K., Rabin P. Perceptual matching in patients with cerebral lesions. Neuropsychologia. 1970;8:475–487. doi: 10.1016/0028-3932(70)90043-6. [DOI] [PubMed] [Google Scholar]
- Yang Y.H., Liang P.P., Lu S.F., Li K.C., Zhong N. The role of the DLPFC in inductive reasoning of MCI patients and normal agings: an fMRI study. Sci. China Ser. C: Life Sci. 2009;52:789–795. doi: 10.1007/s11427-009-0089-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material