The progression of liver fibrosis is a critical factor in patients with chronic liver diseases, because advanced fibrosis is a prerequisite to develop cirrhosis and its complications, and it predisposes patients to developing hepatocellular carcinoma. Currently, the only effective approach to slow down the progression of fibrosis or even induce its regression is to remove the cause of the liver disease.1 However, removal of the etiologic factor (ie, hepatitis B virus or hepatitis C virus clearance, alcohol cessation, weight loss) is not always possible and these patients would also benefit from antifibrotic therapies capable of attenuating the deposition of scar tissue in the liver. Additionally, patients with advanced fibrosis in whom the cause of the liver disease is removed (eg, a patient with alcoholic cirrhosis who stops drinking) would benefit from targeted therapies that favor fibrosis resolution and restoration of a normal liver architecture. To develop such drugs, it is essential to identify the main cellular and molecular mechanisms that mediate fibrosis resolution. Because liver tissue from patients with active fibrosis resolution is not routinely obtained for clinical practice and research purposes, experimental studies in animals with ongoing hepatic tissue repair seem appropriate to identify the molecular drivers of fibrosis resolution.
To understand its resolution, it is valuable to consider established hepatic fibrosis conceptually as having 3 components: The pathologic matrix, predominantly fibrillar collagens (collagens types I and III); the fibrogenic cell or myofibroblast (the source of both the matrix and the tissue inhibitors and metalloproteinases); and the cells that regulate matrix degradation, via secretion of matrix degrading metalloproteinase (MMPs).2,3 Each of these components potentially represents a therapeutic target. Accumulating evidence now suggests that the cells contributing to the third component, a critical source of MMPs for matrix degradation in fibrosis resolution, are monocyte-derived macrophages recruited to the liver during the inflammatory phase of injury. Furthermore, these cells populate the liver in apposition and sometimes within the hepatic matrix, so-called scar-associated macrophages (SAMs). Moreover, to degrade fibrillar matrix it is axiomatic that the MMPs derived from these SAMS must have true collagenase activity (MMP1 in the human and MMP13 in the rodent). The recruitment and function of this population are a major focus of the elegant studies presented by Yang et al in this issue of Gastroenterology.4
For architectural remodeling to occur, the balance between the factors promoting matrix accumulation (synthesis of matrix by fibrogenic factors) and remodeling (matrix breakdown mediated by MMPs) needs to alter, shifting from one that favors matrix accumulation to one of net matrix degradation. Detailed studies of rodent models have shown that cessation of injury, whether by bilioduodenal anastomosis in chronic bile duct ligation or cessation of prolonged carbon tetrachloride, results in a shift in the balance of matrix synthesis and turnover, which is characterized by apoptosis of myofibroblasts, a reduction in the hepatic tissue inhibitors and metalloproteinase levels and the production of MMPs by resident and incoming cells.3 Interestingly, studies of human liver biopsy samples, largely in the context of antiviral treatment, show parallel processes at play.5 In the longer term and associated with functional recovery architectural restoration is required with a normal organ structure and repopulation with non-pathologic cell lineages (and phenotypes).6
A crucial finding in rodent models of advanced fibrosis is that the persistent scar tissue contains not only fibrillar collagen, but is also rich in elastin (a matrix protein only susceptible to degradation by specific elastases such as MMP12). Additionally, scar tissue contains monocyte-derived macrophages, which are associated with fibrogenesis. These monocyte-derived macrophages are a potent source of a range of MMPs, including collagenases such as MMP13, able to make the first cleavage of native collagen, gelatinases (MMPs 2 and 9) able to fully degrade partially denatured collagen following the action of collagenases, and elastases including the potent macrophage metalloelastase, MMP12.4,7,8 Work by a number of groups, including the study by Yang et al in this issue of Gastroenterology, has demonstrated that macrophages are crucial to the resolution of fibrosis.4,7,9–11 Indeed, the removal of the macrophage population at the onset of spontaneous fibrosis resolution in rodent models of liver injury prevents remodeling of fibrosis. Additionally, deletion of the macrophage population is associated with a critical drop in liver levels of key enzymes such as MMP13 and MMP12—identifying the macrophage as a crucial source of these enzymes in fibrosis resolution. Intriguingly, in the carbon tetrachloride-induced model of liver injury, the macrophages crucial for resolution are the same population that is recruited during fibrogenesis, and that contribute to fibrosis.9,10 Associated with the onset of fibrosis resolution, this same macrophage population undergoes a phenotypic switch in situ, expressing markers that define a distinct phenotype and up-regulate the expression of matrix-degrading enzymes (and survival and proliferative signals for hepatocytes and hepatic progenitor cells) after ingestion of debris.6,10
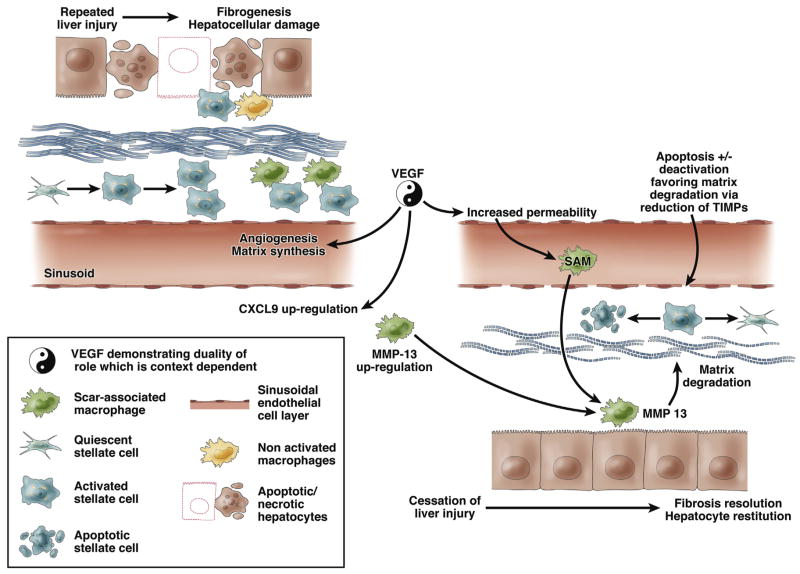
Against this background, the work by Yang et al4 provides another crucial insight to the molecular regulators of fibrosis resolution. As identified by the authors, vascular endothelial growth factor (VEGF) has previously been found to play a role in fibrogenesis via a pro-inflammatory effect acting primarily on endothelial cells. The inhibition of VEGF function in progressive fibrosis, therefore, theoretically represents an attractive therapeutic target. However, aware of the dichotomous role played by specific mediators such as macrophages in fibrosis and fibrosis resolution, Yang et al4 have undertaken detailed studies of the inhibition of VEGF in models of fibrosis resolution. Their data indicate that VEGF does indeed play a dual role in fibrosis and fibrosis resolution (Figure 1). VEGF inhibition in the resolution phase is associated not with a beneficial effect, but with a failure of matrix remodeling. Moreover, their data show that although there was no evidence of differential neutrophil migration with and without VEGF neutralization, there was a clear association with a reduced number of SAM in the absence of VEGF signaling. The authors present data indicating that VEGF promotes sinusoidal permeability, monocyte-endothelial cell adhesion, and the resulting SAM accumulation necessary for fibrosis resolution. These data also confirm a crucial role for SAM in the resolution of bile duct ligation–induced fibrosis, to complement the existing and growing literature showing the importance of these cells in parenchymal (CCl4- and dietary-induced) models of fibrosis.
Figure 1.
Dual effects of vascular endothelial growth factor (VEGF) in liver fibrogenesis and fibrosis resolution. Repeated liver injury results in hepatocellular damage (apoptosis and/or necrosis) that recruits inflammatory cells and the subsequent activation of hepatic stellate cells (HSC) mediates liver fibrosis. VEGF plays an active role in liver fibrogenesis by stimulating the synthesis of extracellular matrix proteins and favoring angiogenesis. When the cause of liver injury is removed, the liver activates mechanisms of tissue repair. Under these conditions, VEGF play an opposing role by promoting fibrosis resolution. The mechanisms include CXCL9 up-regulation and increased vascular permeability that favor the recruitment of scar tissue–associated macrophages (SAM). These events result in metalloproteinase-13 (MMP-13) up-regulation, which contributes to the degradation of extracellular matrix proteins. Regression of fibrosis is also associated by a decrease in the number of HSC owing to cell apoptosis and cell “deactivation” regaining a quiescent phenotype.
Yang et al4 go on to identify evidence for macrophage production of MMP13 as crucial for fibrosis resolution but importantly they also identify the role of the chemokine CXCL9 as critical to both the recruitment of SAM and MMP13 expression, suggesting that CXCL9 may equip SAM to undertake matrix degradation. Moreover, they show that enhanced expression of CXCL9 and MMP13 when VEGF overexpression was achieved in experimental fibrosis and evidence of enhanced matrix degradation with overexpression of either VEGF or CXCL9. Taken together, these data have further emphasized the dual role of macrophages in the development and resolution of fibrosis. Crucially, Yang et al have demonstrated a linked role for VEGF, identifying for the first time that this key mediator of fibrosis also has specific and important roles in the recruitment and phenotype of SAMs during fibrosis resolution in addition to its fibrogenic activity.
The study by Yang et al4 may have important implications for developing future targeted therapies to favor fibrosis resolution in patients with advanced fibrosis in whom the cause of liver injury is removed. In particular, this study has identified VEGF and CXCL9 as potential molecular drivers of fibrosis resolution. Drugs inhibiting VEGF biological actions are being used to treat different types of cancer, including hepatocellular carcinoma.12 This approach has also been proposed to treat portal hypertension and attenuate liver fibrosis.13 There are no available drugs to stimulate VEGF actions in humans, which could theoretically be beneficial in promoting hepatic fibrosis resolution. Moreover, promoting VEGF actions could exacerbate portal hypertension in patients with advanced fibrosis and favor portal vein thrombosis and the growth of hepatocellular carcinoma. Therefore, this strategy does not seem feasible for patients with advanced liver diseases. Targeting CXCL9 could be an alternative strategy for promoting fibrosis resolution. However, similar to what occurs with VEGF, CXCL9 also mediates liver fibrogenesis. CXCL9, also known as monokine-induced by gamma interferon, binds CXCR3 to recruit T cells in animal models of chronic liver injury, promoting inflammation and fibrogenesis.14 The implication of the CXCL9/CXCR3 axis in promoting liver fibrosis has been recently suggested in human diseases.15–17 Therefore, both molecules seem to be “good guys” in fibrosis resolution, but “bad guys” during liver fibrogenesis. This dual biological behavior can hamper their potential use as therapeutic targets, because patients may alternate between periods of disease progression and regression. Further studies should identify new molecular drivers of fibrosis resolution that do not directly promote fibrosis progression. The study by Yang et al clearly demonstrates that the mechanisms of both processes may largely differ and that the same molecular driver could play opposing roles under different disease stages.
In summary, the work described by Yang et al highlights the importance of understanding in detail the sequence of events during fibrogenesis and spontaneous fibrosis resolution. Over the last 3 decades, the apparent intractability of fibrosis has driven researchers (appropriately) to identify antifibrotic targets, hitherto largely defined in terms of a model of fibrosis that is relentlessly progressive. This cannon of work has contributed greatly to our understanding of fibrogenesis, but recent evidence suggests that the model may have limitations in that it may not always reflect the dynamic and sometimes conflicting roles that individual mediators will play, not only during the development, but also during the resolution of fibrosis. Put another way, we need to move away from models of inflammation and fibrosis in which we identify targets that are ascribed a single function and are either considered to be bad (profibrotic) or good (antifibrotic). The ramifications for our design of antifibrotic therapies of this emerging concept are clear. In an age in which we are increasingly successful at treating the underlying cause of liver inflammation (eg, the successful deployments of antiviral therapy in chronic viral hepatitis), we must understand in detail the likely impact of blocking a specific mediator as remodeling of fibrosis commences. Perhaps we need to be more clear about whether a given intervention is antifibrotic or pro-resolution. This is not simply a semantic difference; such terminology more accurately defines both the therapeutic impact and potentially the context in which any intervention should be targeted.
At a more specific level, we likely have more to learn about the role of VEGF and SAM in liver fibrosis. Models in other organs, notably the lung, have suggested that individual VEGF isoforms may play a different role in the different stages of development, cytoprotection, cytotrophism, and inflammation, and that it is possible that the balance between specific isoforms or the expression of one or another VEGF isoform may ultimately determine the net tissue response.18 These comments notwithstanding, the study in this issue of Gastroenterology by Yang et al represents an important step forward in our understanding of the resolution of fibrosis crucially linking the function of VEGF, endothelial permeability, and the migration of monocyte-derived macrophages in the resolution of fibrosis.
Footnotes
Conflicts of interest
The authors disclose no conflicts.
Contributor Information
JOHN P. IREDALE, Centre for Inflammation Research, Queen’s Medical Research Institute, Edinburgh, United Kingdom
RAMON BATALLER, Division of Gastroenterology and Hepatology, Departments of Medicine and Nutrition, University of North Carolina at Chapel Hill, Chapel Hill, North Carolina.
References
- 1.Schuppan D, Kim YO. Evolving therapies for liver fibrosis. J Clin Invest. 2013;123:1887–1901. doi: 10.1172/JCI66028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gao B, Bataller R. Alcoholic liver disease: pathogenesis and new therapeutic targets. Gastroenterology. 2011;141:1572–1585. doi: 10.1053/j.gastro.2011.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Iredale JP. Models of liver fibrosis: exploring the dynamic nature of inflammation and repair in a solid organ. J Clin Invest. 2007;117:539–548. doi: 10.1172/JCI30542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Yang L, Kwon J, Popov Y, et al. Vascular endothelial growth factor promotes fibrosis resolution and repair in mice. Gastroenterology. 2014;146:1339–1350. doi: 10.1053/j.gastro.2014.01.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kweon YO, Goodman ZD, Dienstag JL, et al. Decreasing fibrogenesis: an immunohistochemical study of paired liver biopsies following lamivudine therapy for chronic hepatitis B. J Hepatol. 2001;35:749–755. doi: 10.1016/s0168-8278(01)00218-5. [DOI] [PubMed] [Google Scholar]
- 6.Boulter L, Govaere O, Bird TG, et al. Macrophage-derived Wnt opposes Notch signaling to specify hepatic progenitor cell fate in chronic liver disease. Nat Med. 2012;18:572–579. doi: 10.1038/nm.2667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fallowfield JA, Mizuno M, Kendall TJ, et al. Scar-associated macrophages are a major source of hepatic matrix metalloproteinase-13 and facilitate the resolution of murine hepatic fibrosis. J Immunol. 2007;178:5288–5295. doi: 10.4049/jimmunol.178.8.5288. [DOI] [PubMed] [Google Scholar]
- 8.Pellicoro A, Aucott RL, Ramachandran P, et al. Elastin accumulation is regulated at the level of degradation by macrophage metalloelastase (MMP-12) during experimental liver fibrosis. Hepatology. 2012;55:1965–1975. doi: 10.1002/hep.25567. [DOI] [PubMed] [Google Scholar]
- 9.Baeck C, Wei X, Bartneck M, et al. Pharmacological inhibition of the chemokine C-C motif chemokine ligand 2 (monocyte chemoattractant protein 1) accelerates liver fibrosis regression by suppressing Ly-6C(+) macrophage infiltration in mice. Hepatology. 2014;59:1060–1072. doi: 10.1002/hep.26783. [DOI] [PubMed] [Google Scholar]
- 10.Ramachandran P, Pellicoro A, Vernon MA, et al. Differential Ly-6C expression identifies the recruited macrophage phenotype, which orchestrates the regression of murine liver fibrosis. Proc Natl Acad Sci U S A. 2012;109:E3186–E3195. doi: 10.1073/pnas.1119964109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Duffield JS, Forbes SJ, Constandinou CM, et al. Selective depletion of macrophages reveals distinct, opposing roles during liver injury and repair. J Clin Invest. 2005;115:56–65. doi: 10.1172/JCI22675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sampat KR, O’Neil B. Antiangiogenic therapies for advanced hepatocellular carcinoma. Oncologist. 2013;18:430–438. doi: 10.1634/theoncologist.2012-0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rosmorduc O. Antiangiogenic therapies in portal hypertension: a breakthrough in hepatology. Gastroenterol Clin Biol. 2010;34:446–469. doi: 10.1016/j.gcb.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 14.Sahin H, Borkham-Kamphorst E, Kuppe C, et al. Chemokine Cxcl9 attenuates liver fibrosis-associated angiogenesis in mice. Hepatology. 2012;55:1610–1619. doi: 10.1002/hep.25545. [DOI] [PubMed] [Google Scholar]
- 15.Tacke F, Zimmermann HW, Berres ML, et al. Serum chemokine receptor CXCR3 ligands are associated with progression, organ dysfunction and complications of chronic liver diseases. Liver Int. 2011;31:840–849. doi: 10.1111/j.1478-3231.2011.02504.x. [DOI] [PubMed] [Google Scholar]
- 16.Wasmuth HE, Lammert F, Zaldivar MM, et al. Antifibrotic effects of CXCL9 and its receptor CXCR3 in livers of mice and humans. Gastroenterology. 2009;137:309–319. doi: 10.1053/j.gastro.2009.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zeremski M, Petrovic LM, Chiriboga L, et al. Intrahepatic levels of CXCR3-associated chemokines correlate with liver inflammation and fibrosis in chronic hepatitis C. Hepatology. 2008;48:1440–1450. doi: 10.1002/hep.22500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Medford AR, Millar AB. Vascular endothelial growth factor (VEGF) in acute lung injury (ALI) and acute respiratory distress syndrome (ARDS): paradox or paradigm? Thorax. 2006;61:621–626. doi: 10.1136/thx.2005.040204. [DOI] [PMC free article] [PubMed] [Google Scholar]