Abstract
Increased smoking and a detrimental response to tobacco smoke in the lungs of HIV/AIDS patients result in an increased risk for COPD. We aimed to determine the predictive value of a COPD screening strategy validated in the general population and to identify HIV-related factors associated with decreased lung function. Subjects at least 35 years of age at an HIV clinic in New York City completed a COPD screening questionnaire and peak flow measurement. Those with abnormal results and a random one-third of normal screens had spirometry. 235 individuals were included and 89 completed spirometry. Eleven (12%) had undiagnosed airway obstruction and 5 had COPD. A combination of a positive questionnaire and abnormal peak flow yielded a sensitivity of 20% (specificity 93%) for detection of COPD. Peak flow alone had a sensitivity of 80% (specificity 80%). Abnormal peak flow was associated with an AIDS diagnosis (p=0.04), lower nadir (p=0.001), and current CD4 counts (p=0.001). Nadir CD4 remained associated in multivariate analysis (p=0.05). Decreased FEV1 (<80% predicted) was associated with lower CD4 count nadir (p=0.04) and detectable current HIV viral load (p=0.01) in multivariate analysis. Questionnaire and peak flow together had low sensitivity, but abnormal peak flow shows potential as a screening tool for COPD in HIV/AIDS. These data suggest that lung function may be influenced by HIV-related factors.
Introduction
In 2011, the prevalence of chronic obstructive pulmonary disease (COPD) in the general population in the US was 6.8%.1 For patients infected with HIV, the prevalence of COPD is likely higher and is an increasing cause of poor respiratory health and death in this population.2–4 This increase in COPD is thought to be due to an increased prevalence of smoking in patients with HIV/AIDS5–12 in combination with a detrimental amplified inflammatory response in the lungs of those exposed to toxins in cigarette smoke.13–29 Patients with HIV/AIDS also have increased barriers to smoking cessation, which adds to the risk for COPD over time.30 Prior pulmonary infection with Pneumocystis jiroveci or other pathogens may also influence lung function.19,24 Studies show that in addition, HIV infected patients with higher HIV viral loads or lower CD4 T-cell counts have more rapid decline in pulmonary function, worse disease, and likely worse diffusing capacity than similar patients without HIV.2,4,31,32 Whether peak flow is influenced by these factors in a similar manner remains less clear.
Early identification of patients at risk for COPD could lead to earlier initiation of counseling and risk reduction strategies, and earlier treatment of moderate to severe COPD could prevent acute exacerbations and generally ease suffering.33–36 Historically, treatment of HIV itself and management of complications was the focus of care for patients infected with HIV, but as antiretroviral therapy has become more effective, more easily tolerated, and as lifespans of those infected with HIV become longer, identification and treatment of chronic conditions such as COPD is increasing in importance in HIV primary care.
Spirometry (often within the context of full pulmonary function testing) has long been the gold standard for diagnosing COPD when classic risks and symptoms are present. Advances in technology have now made office spirometry commonplace, where advanced pulmonary function data can be obtained using very small, portable machines. However, spirometry still requires significant effort by patients and coaching by providers to yield useful results. Because of these factors, along with cost, it is not feasible to institute spirometry in most HIV clinics. There is an unmet need for an easy to use, inexpensive method of screening for COPD that could be used in a primary care setting.
Peak expiratory flow is quickly and easily measured in the clinic, and evidence suggests that it correlates with FEV1 in the general population.37,38 Peak flow has been shown to be a useful predictor of COPD,39 and one study revealed that having a normal peak flow effectively excludes the possibility of stage III or IV COPD.40 Abnormal peak flow has been shown to predict COPD mortality.41 A combination of medical history/risk for COPD in combination with peak flow has successfully been used as a screening mechanism to identify those with COPD in the general population.42–44
This study aimed to explore the predictive value of a screening strategy using a brief history and peak expiratory flow measurement for the determination of pulmonary function abnormalities and the diagnosis of COPD in patients infected with HIV. We also sought to identify HIV-related factors that could influence pulmonary function.
Methods
Study design and population
The study was conducted at the HIV primary care clinics of New York Presbyterian Hospital-Weill Cornell Medical College in New York City, which provide care to approximately 2200 patients with HIV infection at two clinic sites. Study enrollment was completed from April 5, 2012 to May 25, 2013. Interested patients who met inclusion criteria (age ≥=35 years with documented HIV infection) were screened for entrance to the study. An age cutoff of 35 was used to match with the COPD screening questionnaire used and because it was presumed that age of onset of COPD could be lower in patients with HIV infection.11 Subjects were recruited via referral from clinic providers and staff, and via response to flyers posted in the waiting room. Exclusion criteria included history of asthma or COPD documented by pulmonary function testing or pulmonologist assessment and receiving chronic asthma or COPD treatment, pregnancy, end-stage renal disease on hemodialysis, decompensated liver cirrhosis, class 3 or 4 congestive heart failure, current use of systemic immunosuppressant (equivalent to ≥10 mg/day of prednisone, TNF-alpha inhibitor, or active chemotherapy), and lung resection. Patients with an acute respiratory illness (new fever, cough, or wheeze in the last 7 days) were not included, but an attempt was made to reschedule for when symptoms were resolved. The study was approved by the Weill Cornell Institutional Review Board, and all subjects provided written informed consent.
Testing procedure
A comprehensive medical history was obtained from each subject, and from reviewing the electronic medical record.30 Evaluation included quantification of smoking exposure, marijuana and other illicit drug exposure, and a review of both HIV and opportunistic infection history. At the time of this study, “undetectable” viral load was defined at <20 copies/mL, but the cutoff for undetectable varied when considering historical values in the chart. Each participant then underwent peak flow measurement and completed a five question respiratory questionnaire (COPD-PS).45 Peak flow was completed by standard protocol (ATS/ERS guidelines)46 using a Vitalograph asma-1 electronic peak flow meter with Vitalograph SafeTway disposable mouthpieces (Vitalograph Inc. Lenexa, KS), and the greatest value of three attempts was noted. Peak flow was deemed abnormal if the greatest value was less than 70% of predicted based on height, age, and race.44,47 The questionnaire was abnormal if the score was 5 or higher.45 Initially, those with abnormal peak flow and questionnaire went on to complete spirometry testing. After 29 enrolled subjects (July 3, 2012), no subject had both screening tests abnormal; consequently, the protocol was amended to perform spirometry for all subjects with either an abnormal peak flow or an abnormal questionnaire in an attempt to ascertain the importance of each test in the model.
Those with an abnormal questionnaire or peak flow underwent spirometry. One out of every three subjects with normal questionnaire and peak flow results was randomly selected to complete spirometry as a control. Spirometry was completed as per standard protocol outlined in ATS/ERS guidelines.46 An EasyOne (ndd Medical Technologies, Inc. Andover, MA) spirometer available at each site was used to record FEV1 and FVC. The spirometer used corrective factors internally for subject characteristics including height, weight, age, sex, and race. FEV1 and FVC values were recorded and <80% of predicted was considered abnormal as per common practice in pulmonary medicine and as is preprogrammed into the spirometer. If the pre-bronchodilator, baseline FEV1/FVC was <0.70 and the grade (reproducibility) was A–D, two puffs of albuterol (Proventil® HFA) were given, using 6” spacer tubing. After 10 min, spirometry was repeated in the same manner.
Primary HIV providers were informed of results directly if spirometry was abnormal, and a result note was completed in the electronic medical record for all subjects completing spirometry. Patients were informed if results were abnormal, and were given written materials about COPD along with advice to discuss the results further with their primary provider.
Statistical analysis
Data were managed using the Weill Cornell Medical College Research Electronic Data Capture (REDCap) database. STATA version 13 (StataCorp LP, College Station, TX) was used for statistical analysis. Unpaired Student t-tests or Wilcoxon rank-sum tests were used when comparing two groups of continuous variables, depending on data distribution. Categorical variables were compared using Pearson's χ2 or Fisher's exact tests. Univariate logistic regression analysis was done to compare demographic and HIV-related characteristics between those with abnormal versus normal peak flow, FEV1 or FVC values. Multivariate models included variables that had p values<0.1 on univariate analysis. For all analyses, p<0.05 was considered statistically significant.
Results
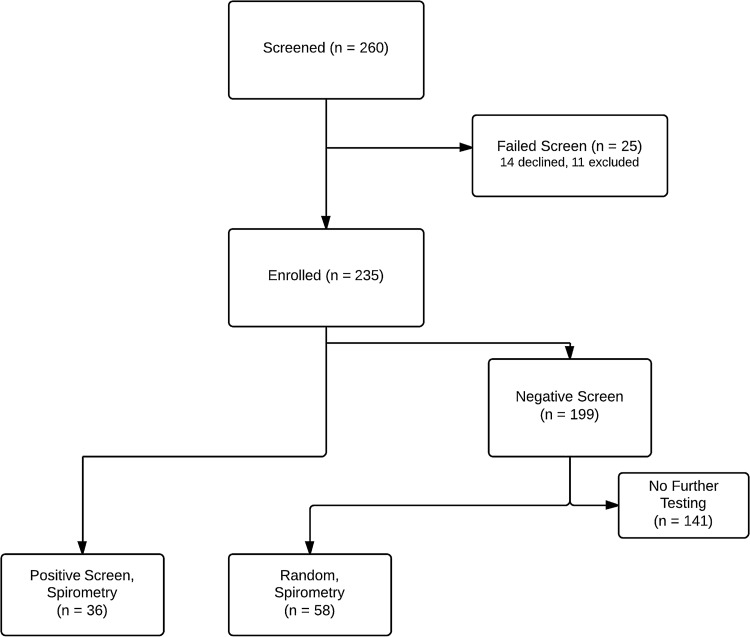
As shown in Fig. 1, 260 individuals were screened for inclusion to the study. Of these, 14 declined participation and 11 were excluded—5 had documented asthma and were receiving treatment, 2 had documented COPD and were receiving treatment, 2 had end-stage renal disease on hemodialysis, 1 had decompensated liver cirrhosis, and 1 was receiving prednisone at a dose higher than 10 mg/day. This left 235 in the final study population.
FIG. 1.
Selection of subjects.
Table 1 summarizes the demographic characteristics of the study population. Ninety-four subjects qualified for spirometry testing, either because of an abnormal screening test (n=36), or because of random selection (n=58). Five of these individuals gave only one unsatisfactory attempt at spirometry and then refused further testing—these subjects were excluded from further analysis, as no usable data was gained from the spirometry testing (3 had abnormal screening and 2 were random selections). This left 89 subjects with spirometry data: 33 qualified via abnormal screening (7 had abnormal questionnaire and peak flow, 12 had abnormal questionnaire only, and 14 had abnormal peak flow only) and 56 via random selection. See Table 1 for additional characteristics of study participants.
Table 1.
Characteristics of Study Participants
| Characteristic | All (n=235) | Spirometry (n=89) | No Spirometry (n=146) | p Value |
|---|---|---|---|---|
| Male, n (%) | 196 (83) | 74 (83) | 122 (84) | 0.93a |
| Mean age in years, n (SD) | 49 (8.2) | 50 (6.8) | 49 (8.9) | 0.27b |
| Race | ||||
| White, n (%) | 69 (29) | 26 (29) | 43 (29) | 0.76c |
| Black, n (%) | 84 (36) | 35 (39) | 49 (34) | |
| Hispanic, n (%) | 71 (30) | 25 (28) | 46 (32) | |
| Other, n (%) | 11 (5) | 3 (3) | 11 (8) | |
| Mean BMI, kg/m2 (SD): | 26.8 (5.1) | 27.7 (5.9) | 26.3 (4.4) | 0.03b |
| Education <≤high school, n (%) | 81 (34) | 37 (42) | 44 (30) | 0.07a |
| Unemployed, n (%) | 129 (55) | 52 (58) | 77 (53) | 0.40a |
| Income <$20000/yr, n (%) | 163 (69) | 66 (74) | 97 (66) | 0.21a |
| HIV-related measures | ||||
| Risk factor MSM, n (%) | 160 (68) | 60 (67) | 100 (69) | 0.86a |
| Years since HIV diagnosis, mean (SD) | 14.9 (6.8) | 15.2 (7.0) | 14.8 (6.8) | 0.67b |
| Nadir CD4 (cells/mm3), mean (SD) | 179 (155) | 173 (139) | 183 (165) | 0.92d |
| Last CD4 (cells/mm3), mean (SD) | 601 (287) | 601 (309) | 602 (274) | 0.98b |
| AIDS diagnosis, n (%) | 151 (64) | 56 (63) | 95 (65) | 0.74a |
| OI history, n (%) | 76 (33) | 31 (35) | 45 (31) | 0.54a |
| Last viral load undetectable, n (%) | 162 (69) | 65 (73) | 97 (66) | 0.29a |
| Current HAART therapy, n (%) | 224 (95) | 87 (98) | 137 (94) | 0.17a |
| HAART years, mean (SD) | 11.0 (5.9) | 11.1 (5.9) | 11.0 (5.9) | 0.90b |
| Smoking status | ||||
| Current, n (%) | 65 (28) | 38 (43) | 27 (19) | <0.001a |
| Former, n (%) | 96 (41) | 34 (38) | 62 (43) | |
| Never, n (%) | 74 (31) | 17 (19) | 57 (39) | |
| Pack years, mean (SD) | 12.1 (15.3) | 17.6 (17.8) | 8.6 (12.3) | <0.001b |
| Past intravenous drug use, n (%) | 35 (15) | 17 (19) | 18 (12) | 0.157a |
Chi-square; bStudent t-test; cFisher's exact; dWilcoxan rank-sum.
BMI, body mass index; HAART, highly active anti-retroviral therapy; MSM, men who have sex with men; OI, opportunistic infection.
In reviewing characteristics of the 89 subjects who completed spirometry, notable differences existed between those who qualified because of an abnormal screening test and those with normal screening tests (Table 2). Subjects with abnormal screening were slightly older, less likely to have been educated beyond high school, less likely to have the HIV risk factor of men who have sex with men, had lower mean nadir CD4+ T cell counts, and less likely to have the most recent viral load be undetectable. In addition, those with abnormal screening trended towards having lower most recent CD4+ T cell counts and a higher prevalence of carrying an AIDS diagnosis.
Table 2.
Characteristics of Study Participants Who Completed Spirometry
| Characteristic | All (n=89) | Abnormal Screen (n=33) | Normal Screen (n=56) | p Value |
|---|---|---|---|---|
| Male, n (%) | 74 (83) | 25 (76) | 49 (88) | 0.15a |
| Mean age in years, n (SD) | 50 (6.8) | 52 (7.8) | 48 (5.8) | 0.01b |
| Mean BMI, kg/m2(SD) | 27.7 (5.9) | 28.3 (7.5) | 27.4 (4.7) | 0.53b |
| Education ≤=high school, n (%) | 37 (42) | 19 (58) | 18 (32) | 0.02a |
| Pack years smoking, mean (SD) | 17.7 (17.8) | 22.8 (23.1) | 14.6 (13.2) | 0.04b |
| Past Intravenous drug use, n (%) | 17 (19) | 8 (24) | 9 (16) | 0.34a |
| HIV-related measures | ||||
| Risk factor MSM, n (%) | 60 (67) | 16 (49) | 44 (79) | 0.003a |
| Nadir CD4 (cells/mm3), mean (SD) | 173 (139) | 121 (105) | 203 (148) | 0.01c |
| Last CD4 (cells/mm3), mean (SD) | 601 (309) | 527 (321) | 644 (296) | 0.08c |
| AIDS diagnosis, n (%) | 56 (63) | 25 (76) | 31 (55) | 0.05a |
| OI history, n (%) | 31 (35) | 15 (45) | 16 (29) | 0.11a |
| Last viral undetectable, n (%) | 65 (73) | 19 (58) | 46 (82) | 0.01a |
Chi-square; bStudent t-test; cWilcoxan rank-sum.
MSM, men who have sex with men; OI, opportunistic infection.
Out of the 89 subjects who completed acceptable spirometry, 11 had a pre-bronchodilator FEV1/FVC ratio of less than 0.7, meeting criteria for airway obstruction. Of these 11 subjects, 2 refused bronchodilator treatment and therefore no post-bronchodilator spirometry data was available. Four of the 9 who completed post-bronchodilator spirometry showed reversibility, where FEV1/FVC was >0.7 after bronchodilator and therefore did not have COPD by definition. This left 5 individuals who qualified as having COPD by GOLD criteria. Of these, 4/5 met criteria for GOLD stage II and one was GOLD stage I. Using GOLD criteria yielded a prevalence of undiagnosed COPD in the population of 89 who underwent spirometry of 5.6%. Of these 5 subjects, 3 qualified for spirometry because of abnormal peak flow, 1 had both abnormal questionnaire and peak flow, and 1 had normal screening tests and was a random selection. Including those with post-bronchodilator reversibility beyond that of COPD criteria, the prevalence of an undiagnosed obstructive lung disease was 12.4%. A screening tool that requires a positive COPD questionnaire and an abnormal peak flow together yielded a sensitivity of 20%, specificity of 92.9%, positive predictive value (PPV) of 14.3%, and negative predictive value (NPV) of 95.1%.
Secondary outcome measures included analysis of an abnormal peak flow or a positive questionnaire alone as a predictor of COPD. The two subjects with obstruction on pre-bronchodilator spirometry who refused post-bronchodilator testing were included as false positives, resulting in abnormal peak flow alone yielding a sensitivity of 80%, specificity of 79.8%, PPV of 19.1%, and NPV of 98.5% for COPD. Abnormal peak flow in those with a smoking history showed similar results. A positive questionnaire alone yielded a sensitivity of 20%, specificity of 78.6%, PPV of 5.3%, and NPV of 94.3%.
Additional planned secondary outcome measures were to determine if demographic or HIV-related factors were associated with decreased peak flow, pre-bronchodilator FEV1 or FVC, or frank COPD. Since all 235 participants completed peak flow, HIV-related measures and demographic characteristics could be compared between those with abnormal peak flow (<70% predicted, n=27) and those with normal peak flow (n=208). Subjects with abnormal peak flow had higher pack-year smoking history (p<0.001), were less likely to have been educated beyond high school (p=0.04), more likely to have used injection drugs (p=0.004), carry an AIDS diagnosis (p=0.02), and have a history of Pneumocystis pneumonia (p=0.01); they also had a lower mean nadir CD4+ T cell count (99 [SD 96] vs. 189 [SD 159] cells/mm3; p=0.001) and a lower most recent CD4+ T cell count (455 [SD 305] vs. 620 [SD 280] cells/mm3; p=0.001). In addition, a trend was noted in those with abnormal peak flow towards having a history of bacterial pneumonia (p=0.051) or a TB infection of the lung (p=0.06).
Those subjects found to have a pre-bronchodilator FEV1 less than 80% of predicted (31/89, 35%) were more likely to have a history of AIDS (p=0.04), have a lower nadir CD4+ T cell count (p=0.004), have a lower most recent CD4+ T cell count (p=0.04), have a higher most recent viral load (p=0.047), and have the most recent viral load be detectable (p=0.005).
Subjects with pre-bronchodilator FVC less than 80% of predicted (21/89, 24%) had higher pack year smoking histories (p=0.02), were more likely to have lower nadir CD4+ T cell counts (p=0.04), higher most recent viral loads (p=0.02), and have the most recent viral load be detectable (p=0.01).
COPD diagnosis was not associated with any HIV-related measure examined, but was associated with current smoking status (p=0.03) and lower BMI (p=0.049). In multivariate analysis, having an abnormal peak flow was associated with higher pack-year smoking history (OR 1.04 per pack-year, p=0.01) with a trend towards prior injection drug use (OR 2.7, p=0.07), and having a lower CD4+ T cell count nadir (OR 0.99 per cell/mm3, p=0.052). Abnormal pre-bronchodilator FEV1 remained associated with lower mean CD4+ T cell count nadir (OR 0.99 per cell/mm3, p=0.04) and having the most recent HIV viral load be detectable (OR 5.19, p=0.005). Abnormal pre-bronchodilator FVC remained associated with having the most recent HIV viral load be detectable (OR 5.69, p=0.01), with a trend toward higher pack-year smoking history (OR 1.03 per pack-year, p=0.056) and lower income (OR 0.18 for annual income <$20,000, p=0.07).
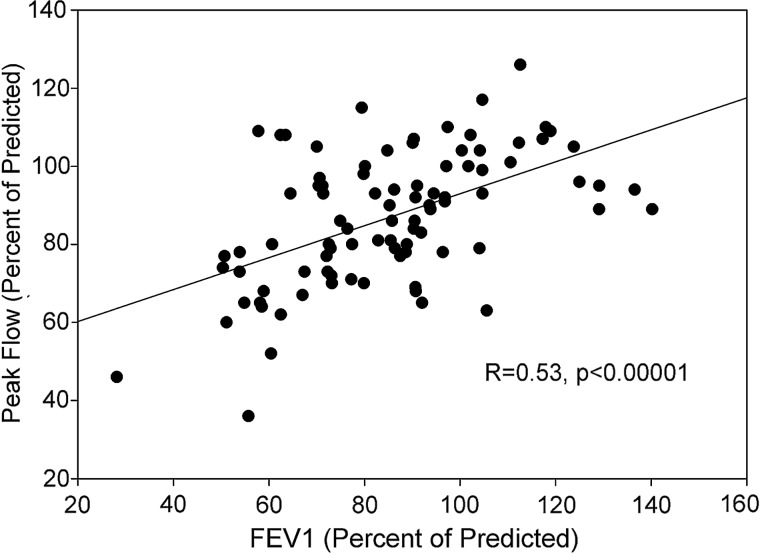
As shown in Fig. 2, percent of predicted peak flow correlated with percent of predicted FEV1 (R=0.53, p<0.00001).
FIG. 2.
Correlation of peak flow and FEV1 in subjects completing spirometry.
Discussion
The combination of higher smoking rates, amplified inflammatory response to tobacco smoke, and increased barriers to cessation has led to an increased prevalence of many smoking-related conditions in patients infected with HIV.7,9,48,49 Studies show an increase in COPD in this population, and as in the general population, this number is likely under-reported due to lack of screening and in HIV infected patients, a historical emphasis of HIV treatment as a priority over testing and management of chronic diseases.2,5,50 In our pilot study of a COPD screening strategy in an HIV clinic, we found that 12% of those completing spirometry had evidence of previously undiagnosed obstructive lung disease, revealing clear deficits in the recognition of pulmonary disease in this population. Six percent of subjects completing spirometry met GOLD criteria for COPD, whereas the others had undiagnosed asthma, undifferentiated obstructive lung disease, or refused further testing to be able to make a diagnosis. These results are similar to estimates of COPD prevalence in the general population,1 but given that we excluded those with known obstructive lung disease, the true COPD prevalence was likely substantially higher.
The population in this study was similar in demographic characteristics to the overall HIV population at the two clinic sites, where the majority of subjects were MSM, most were prescribed anti-retroviral therapy with a majority having undetectable viral loads, and where most subjects had some exposure to tobacco smoke (data not shown). Subjects who underwent spirometry were representative of the study population but did have a significantly higher rate of smoking and pack year tobacco exposure. This is due to the fact that a positive smoking history made it much more likely to have a positive screening questionnaire or abnormal peak flow, thereby qualifying a subject for spirometry. Patients with an abnormal screening test were also older, less educated, had poorer control of HIV (lower nadir CD4+ T cell counts and more likely to have the most recent viral load be detectable), and were less likely to be MSM, suggesting potential risk factors for a positive screen, or potential confounding variables.
Office spirometry can be used to help diagnose COPD in the clinic setting, but is often not feasible due to cost and implementation issues. The quality of spirometry results is heavily dependent on coaching and subject investment in participation, which was a notable barrier in this study. As shown in our study, the combination of a COPD screening questionnaire and peak flow was very poor in predicting COPD in patients with HIV infection. Peak flow alone showed more predictive potential and might be useful as a screen to rule out COPD in HIV infected patients. The COPD screening questionnaire was not predictive of COPD either alone or in combination with peak flow in this population. It is unclear why this test performed so poorly in this study compared to previous studies of the general population. Potential differences in patient populations including age, education, socioeconomic status, and possible confounding past or co-existing medical conditions could have affected test performance, and further studies are needed to assess the validity of the COPD-PS in the HIV-infected population.
In addition, further studies are needed to determine how peak flow predicts COPD in a larger population of patients infected with HIV and how it might be combined with other screening tools or historical factors to be useful in a clinic setting. In our study, the sensitivity of peak flow in predicting COPD might be underestimated because those patients with known COPD were excluded and the smoking rate of our population was lower than that seen in other studies of HIV infected patients. Peak flow did show a correlation with FEV1 in the study population, suggesting that peak flow might be useful in predicting FEV1 in patients with HIV infection. Benefits of peak flow testing include low cost, ease of administration, speed of testing, and based on this study, high negative predictive value. The disadvantages are that peak flow has a low positive predictive value (more false positives), and there is less consensus on what constitutes an abnormal test relative to spirometry.
In addition to smoking-related factors, evidence suggests more advanced immunosuppression and poor control of HIV (i.e., lower CD4+ T cell counts and higher HIV viral loads) have an influence on lung function as well.2,31,51,52,53 This study corroborated previous results. After controlling for smoking and other potential confounders in multivariate analysis, both abnormal FEV1 and abnormal FVC remained closely associated with having a detectable most recent viral load, suggesting some effect of poor HIV control on lung function. In addition, abnormal FEV1 correlated with lower historical CD4+ T cell count nadir, suggesting that the degree of past immune suppression could contribute to later lung disease. Previous studies noted a correlation between current CD4+ T cell count and decreased lung function, but the association seen here with nadir CD4+ count and decreased lung function could be an additional useful marker in determining COPD risk that is available for most HIV infected patients in care. Peak flow appeared in univariate analysis to be influenced by historical CD4 values and history of Pneumocystis pneumonia along with prior injection drug use, but was most influenced by prior pack year smoking based on multivariate analysis.
Our study has several limitations. The sample size was relatively small, and several subjects were not able to complete full spirometry to make a diagnosis of COPD. Subjects were enrolled via convenience sampling, but efforts were made to recruit using a variety of means, including provider/staff referral, approach by study staff, and posting of flyers. The study population was representative of the overall population at our HIV clinic, but because the clinic has only two sites, both in Manhattan in New York City, it may not represent the broader population of individuals infected with HIV. In addition, the majority of subjects were male and MSM, possibly limiting generalizability. While the rate of smoking in our study population was higher than that of the general population, it is lower than that seen in previous studies of smoking in the HIV-infected population.7,8,10,12 This is a testament to the anti-smoking interventions widely implemented in the city of New York in general and cessation programs targeted at the HIV-infected population7,54–57 but does decrease the generalizability. Participants were at least 35 years old, since COPD risk would not be expected to be high before this age. Because the study population was generally younger, there is the potential for COPD under-diagnosis using GOLD criteria of fixed FEV1/FVC ratio of <0.7 as abnormal. Patients with a prior history of obstructive lung disease were excluded, limiting the population further.
The accuracy of several medical history variables such as smoking or drug exposure history, CD4+ nadir, or most recent viral load were dependent on appropriate charting in the electronic medical record or recall by subjects. Spirometry is a standardized test, but the quality of results is effort dependent. Despite full review of study protocol with each subject, informed consent, and coaching by study staff, several subjects refused spirometry attempts beyond the initial trial, leading to the inability to assess reproducibility in those individuals. Peak flow is less studied with regards to COPD screening, but a standardized method was used to determine which results were abnormal based on validated methods from previous studies.44,47 No CT imaging or diffusing capacity measurement was completed as part of this study due to cost constraints, and therefore it remains unclear whether abnormal spirometry results could represent emphysematous or other structural changes in the lung for which patient infected with HIV are at higher risk based on previous studies.11,58
As shown in this study, COPD and other obstructive lung diseases remain underdiagnosed in HIV infected individuals. HIV-related factors, including poor viral control and magnitude of CD4 T-cell suppression can influence lung function, but further studies are needed to elucidate the exact mechanisms. Peak flow measurement is a simple, low-cost test that shows some potential as a screening tool to help diagnose COPD. Further studies are needed to test peak flow in combination with other clinical or historical factors in predicting COPD.
Acknowledgments
We would like to thank Dr. Byron Thomashow and Steven Nelson for their input on the initial design of this project, Ravi Kesari for his assistance in testing, and Valery Hughes, FNP for her help in recruitment and study organization. We would also like to acknowledge and thank the COPD Alliance for the use of the COPD Population Screener (Appendix).
This work was supported by the National Institutes of Health, the National Institute of Allergy and Infectious Diseases [K24 AI078884], [T32 AI007613], the Agency for Healthcare Research and Quality [T32 HS000066] and the Weill Cornell Medical College Clinical and Translational Science Center [UL1-RR024996]. The contents of this publication are solely the responsibility of the authors and do not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
Author Disclosure Statement
MJG has served as consultant to Pfizer and has received research grants from Pfizer. These are unrelated to the present study. DKS and RJK have no potential conflicts to report.
References
- 1.Ford ES, Croft JB, Mannino DM, Wheaton AG, Zhang X, Giles WH. COPD surveillance–United States, 1999–2011. Chest 2013;144:284–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Gingo MR, Morris A, Crothers K. Human immunodeficiency virus-associated obstructive lung diseases. Clin Chest Med 2013;34:273–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schwarcz SK, Vu A, Hsu LC, Hessol NA. Changes in causes of death among persons with AIDS: San Francisco, California, 1996–2011. AIDS Patient Care STDS 2014;28:517–523 [DOI] [PubMed] [Google Scholar]
- 4.Leung JM, Liu JC, Mtambo A, et al. . The determinants of poor respiratory health status in adults living with human immunodeficiency virus infection. AIDS Patient Care STDS 2014;28:240–247 [DOI] [PubMed] [Google Scholar]
- 5.Crothers K. Chronic obstructive pulmonary disease in patients who have HIV infection. Clin Chest Med 2007;28:575–587, vi [DOI] [PubMed] [Google Scholar]
- 6.Humfleet GL, Delucchi K, Kelley K, Hall SM, Dilley J, Harrison G. Characteristics of HIV-positive cigarette smokers: A sample of smokers facing multiple challenges. AIDS Educ Prev 2009;21:54–64 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lifson AR, Lando HA. Smoking and HIV: Prevalence, health risks, and cessation strategies. Curr HIV/AIDS Rep 2012;9:223–230 [DOI] [PubMed] [Google Scholar]
- 8.Mamary EM, Bahrs D, Martinez S. Cigarette smoking and the desire to quit among individuals living with HIV. AIDS Patient Care STDS 2002;16:39–42 [DOI] [PubMed] [Google Scholar]
- 9.Reynolds NR. Cigarette smoking and HIV: More evidence for action. AIDS Educ Prev 2009;21:106–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tesoriero JM, Gieryic SM, Carrascal A, Lavigne HE. Smoking among HIV positive New Yorkers: Prevalence, frequency, and opportunities for cessation. AIDS Behav 2010;14:824–835 [DOI] [PubMed] [Google Scholar]
- 11.Diaz PT, King MA, Pacht ER, et al. . Increased susceptibility to pulmonary emphysema among HIV-seropositive smokers. Ann Intern Med 2000;132:369–372 [DOI] [PubMed] [Google Scholar]
- 12.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc 2011;8:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brusselle GG, Joos GF, Bracke KR. New insights into the immunology of chronic obstructive pulmonary disease. Lancet 2011;378:1015–1026 [DOI] [PubMed] [Google Scholar]
- 14.Cota-Gomez A, Flores AC, Ling X-F, Varella-Garcia M, Flores SC. HIV-1 Tat increases oxidant burden in the lungs of transgenic mice. Free Radic Biol Med 2011;51:1697–1707 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gil L, Martínez G, González I, et al. . Contribution to characterization of oxidative stress in HIV/AIDS patients. Pharmacol Res 2003;47:217–224 [DOI] [PubMed] [Google Scholar]
- 16.Kaner RJ, Santiago F, Crystal RG. Up-regulation of alveolar macrophage matrix metalloproteinases in HIV1(+) smokers with early emphysema. J Leukoc Biol 2009;86:913–922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lassiter C, Fan X, Joshi PC, et al. . HIV-1 transgene expression in rats causes oxidant stress and alveolar epithelial barrier dysfunction. AIDS Res Ther 2009;6:1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sethi S, Murphy TF. Infection in the pathogenesis and course of chronic obstructive pulmonary disease. N Engl J Med 2008;359:2355–2365 [DOI] [PubMed] [Google Scholar]
- 19.Shipley TW, Kling HM, Morris A, et al. . Persistent pneumocystis colonization leads to the development of chronic obstructive pulmonary disease in a nonhuman primate model of AIDS. J Infect Dis 2010;202:302–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Petrache I, Diab K, Knox KS, et al. . HIV associated pulmonary emphysema: A review of the literature and inquiry into its mechanism. Thorax 2008;63:463–469 [DOI] [PubMed] [Google Scholar]
- 21.Buhl R, Meyer A, Vogelmeier C. Oxidant-protease interaction in the lung. Prospects for antioxidant therapy. Chest 1996;110:267S–272S [DOI] [PubMed] [Google Scholar]
- 22.Micoli KJ, Mamaeva O, Piller SC, et al. . Point mutations in the C-terminus of HIV-1 gp160 reduce apoptosis and calmodulin binding without affecting viral replication. Virology 2006;344:468–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mocchegiani E, Giacconi R, Costarelli L. Metalloproteases/anti-metalloproteases imbalance in chronic obstructive pulmonary disease: Genetic factors and treatment implications. Curr Opin Pulm Med 2011;17:S11–S19 [DOI] [PubMed] [Google Scholar]
- 24.Morris A, Alexander T, Radhi S, et al. . Airway obstruction is increased in pneumocystis-colonized human immunodeficiency virus-infected outpatients. J Clin Microbiol 2009;47:3773–3776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Sundaram M, Saghayam S, Priya B, et al. . Changes in antioxidant profile among HIV-infected individuals on generic highly active antiretroviral therapy in southern India. Int J Infect Dis 2008;12:e61–e66 [DOI] [PubMed] [Google Scholar]
- 26.Pacht ER, Diaz P, Clanton T, Hart J, Gadek JE. Alveolar fluid glutathione decreases in asymptomatic HIV-seropositive subjects over time. Chest 1997;112:785–788 [DOI] [PubMed] [Google Scholar]
- 27.Treitinger A, Spada C, Verdi JC, et al. . Decreased antioxidant defence in individuals infected by the human immunodeficiency virus. Eur J Clin Invest 2000;30:454–459 [DOI] [PubMed] [Google Scholar]
- 28.Wood KL, Knox KS, Wang Y, Day RB, Schnizlein-Bick C, Twigg HL. Apoptosis of CD57+ and CD57− lymphocytes in the lung and blood of HIV-infected subjects. Clin Immunol 2005;117:294–301 [DOI] [PubMed] [Google Scholar]
- 29.Yearsley MM, Diaz PT, Knoell D, Nuovo GJ. Correlation of HIV-1 detection and histology in AIDS-associated emphysema. Diagn Mol Pathol 2005;14:48–52 [DOI] [PubMed] [Google Scholar]
- 30.Shirley DK, Kesari RK, Glesby MJ. Factors associated with smoking in HIV-infected patients and potential barriers to cessation. AIDS Patient Care STDS 2013;27:604–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Drummond MB, Merlo CA, Astemborski J, et al. . The effect of HIV infection on longitudinal lung function decline among IDUs: A prospective cohort. AIDS 2013;27:1303–1311 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kristoffersen US, Lebech A-M, Mortensen J, Gerstoft J, Gutte H, Kjaer A. Changes in lung function of HIV-infected patients: A 4.5-year follow-up study. Clin Physiol Funct Imaging 2012;32:288–295 [DOI] [PubMed] [Google Scholar]
- 33.Decramer M, Celli B, Kesten S, Lystig T, Mehra S, Tashkin DP. Effect of tiotropium on outcomes in patients with moderate chronic obstructive pulmonary disease (UPLIFT): A prespecified subgroup analysis of a randomised controlled trial. Lancet 2009;374:1171–1178 [DOI] [PubMed] [Google Scholar]
- 34.Jenkins CR, Jones PW, Calverley PMA, et al. . Efficacy of salmeterol/fluticasone propionate by GOLD stage of chronic obstructive pulmonary disease: Analysis from the randomised, placebo-controlled TORCH study. Respir Res 2009;10:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Spencer S, Calverley PM, Sherwood Burge P, Jones PW. Health status deterioration in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:122–128 [DOI] [PubMed] [Google Scholar]
- 36.Bridevaux P-O, Gerbase MW, Probst-Hensch NM, Schindler C, Gaspoz J-M, Rochat T. Long-term decline in lung function, utilisation of care and quality of life in modified GOLD stage 1 COPD. Thorax 2008;63:768–774 [DOI] [PubMed] [Google Scholar]
- 37.Kelly CA, Gibson GJ. Relation between FEV1 and peak expiratory flow in patients with chronic airflow obstruction. Thorax 1988;43:335–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Llewellin P, Sawyer G, Lewis S, et al. . The relationship between FEV1 and PEF in the assessment of the severity of airways obstruction. Respirology 2002;7:333–337 [DOI] [PubMed] [Google Scholar]
- 39.Jackson H, Hubbard R. Detecting chronic obstructive pulmonary disease using peak flow rate: Cross sectional survey. BMJ 2003;327:653–654 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Perez-Padilla R, Vollmer WM, Vázquez-García JC, Enright PL, Menezes AMB, Buist AS. Can a normal peak expiratory flow exclude severe chronic obstructive pulmonary disease? Int J Tuberc Lung Dis 2009;13:387–393 [PMC free article] [PubMed] [Google Scholar]
- 41.Hansen EF, Vestbo J, Phanareth K, Kok-Jensen A, Dirksen A. Peak flow as predictor of overall mortality in asthma and chronic obstructive pulmonary disease. Am J Respir Crit Care Med 2001;163:690–693 [DOI] [PubMed] [Google Scholar]
- 42.Badgett RG, Tanaka DJ, Hunt DK, et al. . Can moderate chronic obstructive pulmonary disease be diagnosed by historical and physical findings alone? Am J Med 1993;94:188–196 [DOI] [PubMed] [Google Scholar]
- 43.Badgett RG, Tanaka DJ, Hunt DK, et al. . The clinical evaluation for diagnosing obstructive airways disease in high-risk patients. Chest 1994;106:1427–1431 [DOI] [PubMed] [Google Scholar]
- 44.Nelson SB, LaVange LM, Nie Y, et al. . Questionnaires and pocket spirometers provide an alternative approach for COPD screening in the general population. Chest 2012;142:358–366 [DOI] [PubMed] [Google Scholar]
- 45.Martinez FJ, Raczek AE, Seifer FD, et al. . Development and initial validation of a self-scored COPD Population Screener Questionnaire (COPD-PS). COPD 2008;5:85–95 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Miller MR, Hankinson J, Brusasco V, et al. . Standardisation of spirometry. Eur Respir J 2005;26:319–338 [DOI] [PubMed] [Google Scholar]
- 47.Hankinson JL, Odencrantz JR, Fedan KB. Spirometric reference values from a sample of the general U.S. population. Am J Respir Crit Care Med 1999;159:179–187 [DOI] [PubMed] [Google Scholar]
- 48.Rahmanian S, Wewers ME, Koletar S, Reynolds N, Ferketich A, Diaz P. Cigarette smoking in the HIV-infected population. Proc Am Thorac Soc 2011;8:313–319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Shirley DK, Kaner RJ, Glesby MJ. Effects of smoking on non-AIDS-related morbidity in HIV-infected patients. Clin Infect Dis 2013;57:275–282 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Morris A, George MP, Crothers K, et al. . HIV and chronic obstructive pulmonary disease: Is it worse and why? Proc Am Thorac Soc 2011;8:320–325 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Drummond MB, Kirk GD, Astemborski J, et al. . Association between obstructive lung disease and markers of HIV infection in a high-risk cohort. Thorax 2012;67:309–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Crothers K, McGinnis K, Kleerup E, et al. . HIV infection is associated with reduced pulmonary diffusing capacity. J Acquir Immune Defic Syndr 2013;64:271–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Crothers K, Huang L, Goulet JL, et al. . HIV infection and risk for incident pulmonary diseases in the combination antiretroviral therapy era. Am J Respir Crit Care Med 2011;183:388–395 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Matthews AK, Conrad M, Kuhns L, Vargas M, King AC. Project Exhale: Preliminary evaluation of a tailored smoking cessation treatment for HIV-positive African American smokers. AIDS Patient Care STDS 2013;27:22–32 [DOI] [PubMed] [Google Scholar]
- 55.Moadel AB, Bernstein SL, Mermelstein RJ, Arnsten JH, Dolce EH, Shuter J. A randomized controlled trial of a tailored group smoking cessation intervention for HIV-infected smokers. J Acquir Immune Defic Syndr 2012;61:208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wewers ME, Neidig JL, Kihm KE. The feasibility of a nurse-managed, peer-led tobacco cessation intervention among HIV-positive smokers. J Assoc Nurses AIDS Care 2000;11:37–44 [DOI] [PubMed] [Google Scholar]
- 57.Zwiebel MA, Hughes V. Smoking cessation efforts in one New York City HIV clinic. J Assoc Nurses AIDS Care 2010;21:11–15 [DOI] [PubMed] [Google Scholar]
- 58.Gelman M, King MA, Neal DE, Pacht ER, Clanton TL, Diaz PT. Focal air trapping in patients with HIV infection: CT evaluation and correlation with pulmonary function test results. Am J Roentgenol 1999;172:1033–1038 [DOI] [PubMed] [Google Scholar]