Abstract
Shoulder tendon injuries are frequently seen in the presence of abnormal scapular motion, termed scapular dyskinesis. The cause and effect relationship between scapular dyskinesis and shoulder injury has not been directly defined. The objective of this study was to develop and use an animal model to examine the initiation and progression of pathological changes in the rotator cuff and biceps tendon. 60 male Sprague-Dawley rats were randomized into two groups: nerve transection (to induce scapular dyskinesis, SD) or sham nerve transection (control). The animals were sacrificed 4 and 8 weeks after surgery. Shoulder function and passive joint mechanics were evaluated over time. Tendon mechanical, histological, organizational, and compositional properties were evaluated at 4 and 8 weeks. Gross observation demonstrated alterations in scapular motion, consistent with scapular “winging”. Shoulder function, passive internal range of motion, and tendon mechanical properties were significantly altered. Histology results, consistent with tendon pathology (rounded cell shape and increased cell density), were observed and protein expression of collagen III and decorin was altered. This study presents a new model of scapular dyskinesis that can rigorously evaluate cause and effect relationships in a controlled manner. These results identify scapular dyskinesis as a causative mechanical mechanism for shoulder tendon pathology.
Keywords: scapular dyskinesis, rotator cuff, animal model
Introduction
Shoulder injuries including impingement, rotator cuff disease, and biceps tendon pathology are common clinical conditions and are a significant source of joint pain, instability, and dysfunction. Injuries to the rotator cuff are particularly common and may include impingement (subacromial and internal), partial thickness tears, or complete tendon rupture. Tears may be a result of acute trauma or chronic degeneration and often begin isolated to the supraspinatus tendon with the potential to progress anteriorly to the subscapularis or posteriorly to the infraspinatus over time. In addition, long head of the biceps pathology can be found in isolation or secondary to rotator cuff tears and can be a significant source of pain.
Rotator cuff injuries are frequently seen in the presence of abnormal scapulothoracic joint kinematics.1–4 Specifically, altered scapular motion and position (termed scapular dyskinesis) has been observed in 68–100% of patients with shoulder injuries.5 The scapula provides a stable platform for rotator cuff muscle activation in order to achieve normal shoulder movements. Specifically, the rotator cuff muscles act to achieve stability through concavity compression of the humeral head on the glenoid fossa. It has been hypothesized that if a stable base does not exist, as in the case of scapular dyskinesis, the rotator cuff muscles may not be able to efficiently generate appropriate torque which may lead to muscular imbalance and instability. An unstable scapula could reduce the dynamic restraint of the glenohumeral joint provided by the rotator cuff, leading to increased joint translations, and placing the joint at increased risk for secondary injuries, including impingement and biceps pathology.6 Additionally, abnormal scapular position, such as increased protraction7, reduced upward scapular rotation, and subsequent loss of appropriate acromial elevation, may lead to compression of the rotator cuff under the acromial arch8, leading to shoulder injury. Alternatively, scapular dyskinesis may occur as a result of or in response to the shoulder injury (as a form of compensation). Therefore, the causative role scapular dyskinesis plays in injuries to the rotator cuff and biceps is still unknown.
While the prevalence of shoulder injuries and their association with scapulothoracic kinematic abnormalities is well-documented9, the cause and effect relationships between the two are not well-established, making optimal clinical management difficult. Human cadaveric studies have shown that alterations in scapular orientations and loading of the rotator cuff, deltoid, latissimus dorsi, and pectoralis major (through simulated tears and/or altering applied forces) result in abnormal joint mechanics due to disruption of the normal balance of forces and joint orientations.10–13 Additionally, in vivo human studies have identified altered scapular rhythm and position in patients with shoulder injury.3; 6; 9; 14 However, the in vivo cause and effect relationships that these mechanical alterations and disruptions have on the rotator cuff and biceps properties over time cannot be evaluated using cadaveric studies or in vivo human studies and therefore remains unknown. The underlying mechanisms and cause and effect relationships can only be addressed in an animal model where time from injury can be controlled and evaluated over time. Utilizing an animal model of abnormal scapulothoracic joint kinematics will help determine the origin of shoulder tendon injury (initial and recurring) while providing insight into the mechanical and biologic events that lead to tendon degeneration and compromised healing.
Therefore, the objective of this study was to develop and use a rat model of scapular dyskinesis to examine the initiation and progression of pathological changes in the rotator cuff and biceps tendon. We hypothesized that scapular dyskinesis would: H1) diminish shoulder function and passive joint mechanics and H2) diminish supraspinatus and biceps tendon histological, compositional, and mechanical properties.
Materials and Methods
Study Design and Surgical Technique
A rat model of scapular dyskinesis was developed and used. This study was approved by the Institutional Animal Care and Use Committee (IACUC). Sixty male Sprague-Dawley rats (400–450 g) underwent unilateral surgical transection (N=30) (or sham transection (N=30)) of the spinal accessory and long thoracic nerves resulting in denervation of the trapezius and serratus anterior muscles, respectively. The rats were administered buprenorphine subcutaneously as a pre- (0.05 mg/kg) and post- (0.05 mg/kg) operative analgesia. Pre-surgical buprenorphine was administered 30 minutes prior to surgery and a local injection of lidocaine (1 mg/kg) was administered at both sites of incision. Post-surgical buprenorphine was administered 6–8 hours after surgery, then every 12 hours for the following 2 days. Briefly, animals were anesthetized and a 2 cm vertical incision was made 1 cm posterior to the left ear. The cervical nerve, clavo-trapezius, and acromio-trapezius muscles were identified. The clavo-trapezius and acromio-trapezius muscles were separated cranial to the cervical nerve using blunt dissection with Iris scissors to expose the spinal accessory nerve, located above the omotransversarius muscle. The spinal accessory nerve was transected 2 mm and 5 mm proximal to the acromio-trapezius and removed. The surgical site was irrigated with sterile saline and the overlying skin was closed with staples. Next, the animal’s forelimb was abducted and internally rotated and a 3 cm axillary incision was made in the caudal direction along the abdominal fascia, exposing the serratus anterior muscle. Next, the latissimus dorsi muscle was identified and an incision was made along the fascial interface and a blunt dissection was performed between the latissimus dorsi muscle and serratus anterior to gain proximal exposure to the serratus anterior muscle and long thoracic nerve. The long thoracic nerve was transected 2 mm and 5 mm proximal to the serratus anterior and removed. The abdominal fascia was sutured closed with 4-0 Vicryl suture (Ethicon, Inc., Blue Ash, Ohio, USA). The surgical site was irrigated with sterile saline and the overlying skin was then closed with staples. Animals were returned to un-restricted cage activity. At 4 and 8 weeks after surgery, animals were sacrificed for mechanical testing (N=10 at each time point) or for histological and immunohistochemical assays (N=5 at each time point) for the control and SD groups. For mechanical testing, the animals were frozen at −20° C until testing and for histological and immunohistochemical assays, the supraspinatus and biceps tendons were harvested immediately and fixed in formalin.
Quantitative Ambulatory Assessment
Forelimb ground reaction forces (medial/lateral, braking, propulsion, and vertical) were quantified using an instrumented walkway, as described previously.15 Briefly, the system consists of two 6 degree-of-freedom force/torque cells mounted to clear, acrylic force plates and incorporated into a walkway. Rats were acclimated to walk freely along the instrumented walkway over a period of 1 week prior to formal recording of ambulatory data. For all groups, data was collected one day prior to surgery to obtain baseline, uninjured values. Post-surgery data was collected at days 5, 7, 14, 28, 42, and 56. All data was normalized by body-weight.
Passive Joint Mechanics
Passive range of motion measurements were performed utilizing the instruments and methodology, as previously described.16 Measurements were taken under anesthesia prior to nerve transection, and at 2, 4, and 8 weeks following surgery. Under anesthesia, the forelimb was placed through a fixture and secured into the rotating clamp at 90° of elbow flexion and 90° of glenohumeral forward flexion. The scapula was manually stabilized to isolate glenohumeral motion and prevent scapulo-thoracic motion. The forelimb was then rotated through full range of internal and external rotation three times. The ROM was calculated as the difference in the average of three measures of maximal internal and external rotation. A bilinear fit utilizing least-squares optimization was applied to calculate joint stiffness in the toe and linear regions in both directions. All parameters were normalized to baseline values.
Sample Preparation for Mechanical Testing
At the time of testing, the animals were thawed and the scapula and humerus were dissected out with the biceps and supraspinatus tendons intact. For biceps testing, the tendon was isolated while still attached to the scapula at the superior aspect of the glenoid. For supraspinatus testing, the tendon was isolated while still attached to the humerus. The tendons were fine dissected under a microscope to remove muscle and excess tissue. Cross-sectional area was measured using a custom laser device3.
Tendon Mechanical Testing
Elastic and viscoelastic mechanical properties of the biceps and supraspinatus tendon were determined using uniaxial tensile testing, as previously described.17 Verhoeff stain lines were placed along the length of each tendon to divide the insertion and mid-substance regions for local optical strain measurements. The scapula and humerus were embedded in a holding fixture using polymethylmethacrylate (PMMA) and inserted into a custom testing fixture. The proximal end of the tendon was gripped with cyanocrylate annealed sand paper in custom grips. The specimen was immersed in PBS at 37°C during testing. Tensile testing of the tendon was performed as follows: preconditioning for 10 cycles from 0.1 N to 0.5 N, stress relaxation to 4% (biceps) or 5% (supraspinatus) strain at a rate of 5 %/sec for 600 sec, and ramp to failure at 0.3%/sec. Stress was calculated as force divided by initial area and 2D Lagrangian strain was determined from the stain line displacements, using custom texture tracking software. Elastic properties were calculated using a linear regression from the linear region of the stress-strain curves. For viscoelastic parameters, the stress-relaxation curve was analyzed and percent relaxation was determined using the peak and equilibrium loads.
Tendon Histology
Histologic analysis was performed to examine cellular and organizational changes in the biceps and supraspinatus tendons. Tissues were harvested immediately after sacrifice and processed using standard paraffin procedures. Sagittal sections (7 um) were collected, and stained with Hematoxylin–Eosin (H&E). Stained supraspinatus tendon sections were imaged at the insertion site and mid-substance using a microscope at 200× and 100× magnification using traditional and polarized light, respectively. Due to the unique anatomy of the biceps, it was subdivided into four regions: insertion site (INS), intra-articular space (INTRA), proximal groove (PROX), and distal groove (DIS). Cell density (number of cells/mm2) and cell shape (aspect ratio; 0–1, with 1 being a circle) were quantified in the traditional light images using a bioquantification software system (Bioquant Osteo II; BIOQUANT Image Analysis Corp, Nashville, TN, USA). Polarized light images were analyzed using custom software to evaluate tendon organization, as previously described.18 The angular deviation (AD) of the collagen orientation for each specimen, a measure of the fiber distribution spread, was calculated in each tendon location.
Tendon Immunohistochemistry
The distribution of ECM proteins was localized using immunohistochemical techniques. The same tissue specimens from histology were used and stained for collagens type II and III, the proteoglycan decorin, and the inflammatory marker, IL1-β (Table 1). The proteins were visualized using DAB, making the antibody-protein conjugate turn brown. The insertion site and mid-substance (with biceps mid-substance region subdivided into intra-articular space, proximal groove, and distal groove) of each tendon were evaluated separately. Staining results were independently graded by three blinded investigators, who were provided with previously prepared standard images, using a scale of 0–3 (0=undetectable, 1=low, 2=medium, 3=high), and the mode was used as the final score.
Table I.
Primary antibodies used for immunohistochemical staining
| Protein Target | Antibody | Host | Type | Enzyme pretreatment | Dilution | Incubation period (h) | Source |
|---|---|---|---|---|---|---|---|
| Collagen II | II-116B3 | Mouse | Monoclonal | Hyaluronidase | 1:4 | 16 | DSHB, Iowa City, IA, USA |
| Collagen III | c7805 | Mouse | Monoclonal | Hyaluronidase | 1:500 | 38 | Sigma, St. Louis, MO, USA |
| Decorin | LF-113 | Rabbit | Polyclonal | Chondroitinase ABC | 1:300 | 38 | L. Fisher, Bethesda, MD, USA |
| IL1-β | AB1832 | Rabbit | Polyclonal | Pepsin | 1:250 | 16 | Millipore, Billerica, MA, USA |
Statistical Analysis
Statistical analysis was performed using SPSS version 20 (IBM, Armonk, NY). For the ambulatory assessment, multiple imputations were conducted using the Markov chain Monte Carlo method for missing data points (~10%). For both ambulatory assessment and passive joint mechanics, significance was assessed using a 2-way ANOVA with repeated measures on time with follow-up t-tests between groups at each time point. Tissue mechanics and histologic parameters between groups were assessed using a t-test. Immunohistochemistry scores were evaluated using a Mann-Whitney test. Significance was set at p<0.05, trends at p≤0.1.
Results
Ambulatory Data
Gross observational examination demonstrated clear alterations in scapular movements during forward locomotion in the rat, consistent with scapular “winging” in the human and characterized by entire medial border prominence in the closed kinetic chain activity. Additionally, shoulder function was significantly altered in the SD group (Figure 1). Specifically, the SD group had a significantly decreased vertical force and significantly increased propulsion force compared to control at all time-points (Figure 1A, 1B). No differences between groups were observed in braking force (Figure 1C). Medial-lateral force was significantly altered at early time points (5 and 7 days post-transection), with the SD group demonstrating a more medially directed force than control (Figure 1D).
Figure 1.
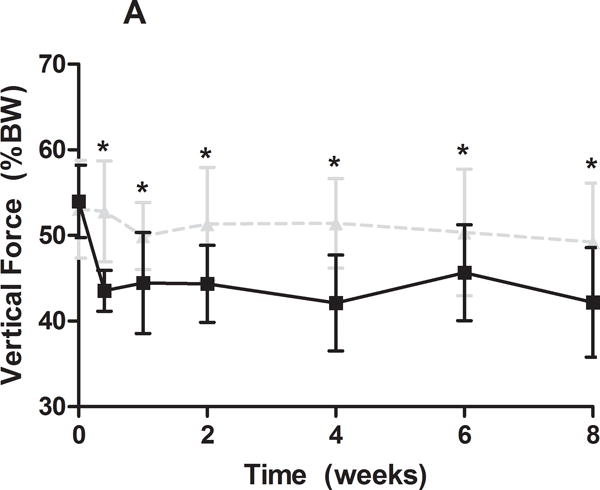
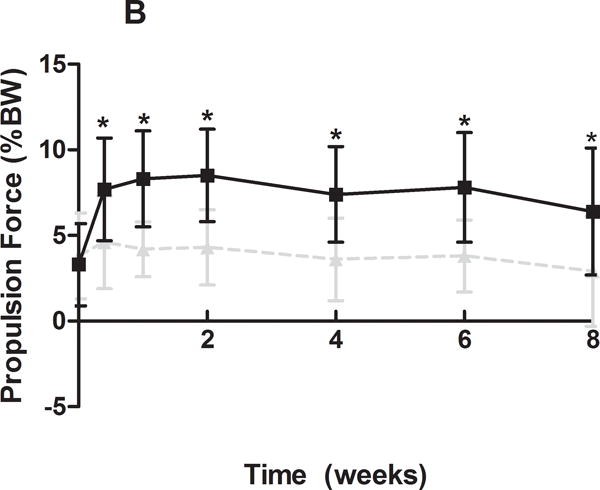
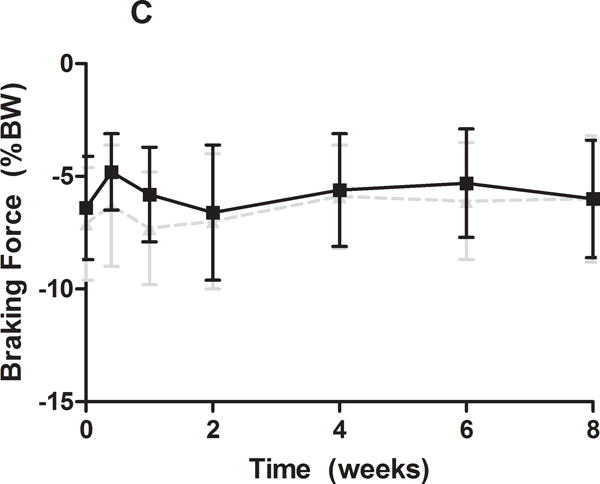
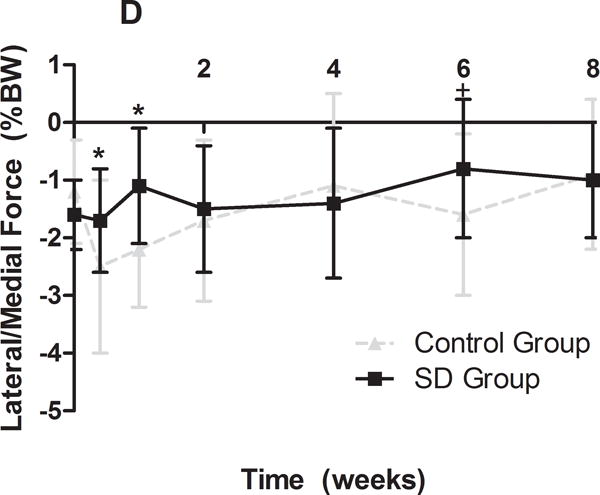
(A) The SD group had a significantly decreased vertical force compared to control at all time-points. (B) The SD group had a significantly increased propulsion force compared to control at all time-points. (C) No differences were observed in braking force. (D) The SD group had a more medially directed force at 5 and 7 days compared to control. Data is shown as mean ± standard deviation (*p<0.05) (N=15 at each time point).
Passive Joint Mechanics
Passive joint mechanics were also significantly altered (Table 2). Specifically, internal range of motion was significantly greater in the SD group compared to control at all post-surgical time-points. No other differences were observed, except for an increase in toe region stiffness in external rotation in the SD group compared to control at 4 weeks post-surgery.
Table II.
Results for passive joint mechanics demonstrated increased internal range of motion (ROM) in the SD group compared to control at all time-points. Data is shown as normalized by baseline values and as mean ± standard deviation.
| Direction | Measurement | Time (wks) |
Control | SD |
|---|---|---|---|---|
| Internal | ROM | 2 | 0.93±0.21 | 1.09±0.18* |
| 4 | 0.89±0.07 | 1.01±0.17* | ||
| 8 | 0.87±0.12 | 1.02±0.16* | ||
| Toe Stiffness | 2 | 3.62±2.99 | 1.21±1.43 | |
| 4 | 3.87 ±3.83 | 1.66±1.75 | ||
| 8 | 2.99±3.64 | 1.18±1.16 | ||
| Linear Stiffness | 2 | 1.50±0.36 | 1.35±0.23 | |
| 4 | 1.47 ±0.31 | 1.30±0.28 | ||
| 8 | 1.65±0.26 | 1.51±0.22 | ||
| External | ROM | 2 | 1.20±0.13 | 1.15±0.07 |
| 4 | 1.03 ±0.10 | 0.98±0.09 | ||
| 8 | 1.26±0.16 | 1.18±0.11 | ||
| Toe Stiffness | 2 | 0.96±0.49 | 0.75±0.49 | |
| 4 | 0.82±0.29 | 1.50±0.86* | ||
| 8 | 0.87±0.42 | 1.03±0.58 | ||
| Linear Stiffness | 2 | 1.10±0.28 | 1.10±0.32 | |
| 4 | 1.16±0.34 | 0.94±0.21 | ||
| 8 | 1.25±0.43 | 1.24±0.29 |
significance p<0.05
Tendon Mechanical Properties
In the presence of scapular dyskinesis, mechanical parameters (viscoelastic and elastic) were significantly altered (Figure 2). Specifically, tendon percent relaxation was significantly greater in the SD group compared to control at 8 weeks, for both the biceps and supraspinatus tendons, indicative of inferior tissue properties (Figure 2A). No differences were observed in any tendon for cross-sectional area (S-Figure 1) or insertion elastic modulus (Figure 2B). However, tendon mid-substance elastic parameters were significantly altered. Specifically, tendon mid-substance elastic modulus was significantly decreased in the SD group compared to control, at both 4 and 8 weeks for the supraspinatus and at 8 weeks for the biceps, also indicative of inferior tissue properties (Figure 2C).
Figure 2.
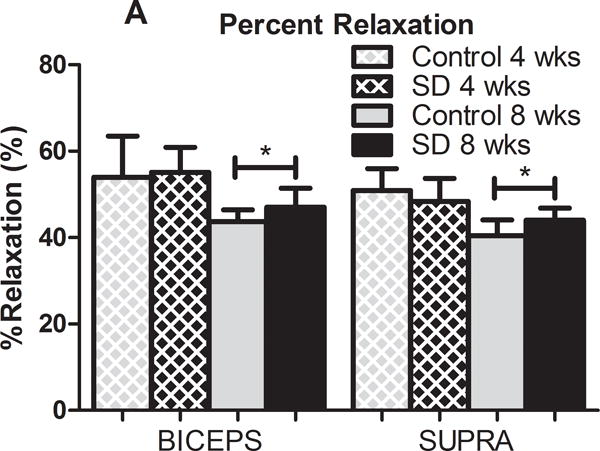
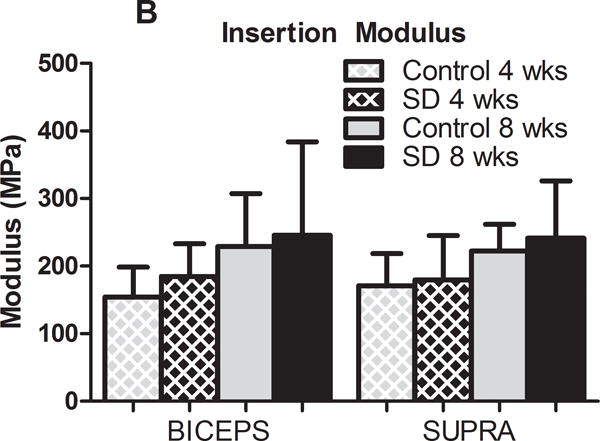
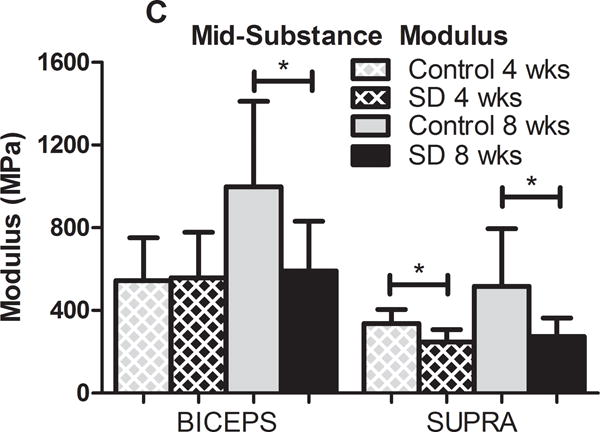
(A) The biceps and supraspinatus tendons demonstrated significantly increased percent relaxation at 8 weeks post-surgery in the SD group compared to control. (B) No differences were observed at the insertion site of any tendon at any time-point. (C) The biceps and supraspinatus tendons demonstrated significantly decreased tendon modulus at 8 weeks and both 4 and 8 weeks, respectively, in the SD group compared to control. Data is shown as mean and standard deviation (*p<0.05) (N=10 at each time point).
Tendon Histology and Immunohistochemistry
A significantly more rounded cell shape was observed in the SD group in the distal groove of the biceps tendon at 8 weeks, with similar trends at the insertion, intra-articular space, and proximal groove regions at 8 weeks (S-Table 1). A significantly less rounded cell shape was observed at the insertion of the supraspinatus tendon in the SD group compared to control at 8 weeks. No other differences in cell shape were observed along the length of the supraspinatus tendon at either time point. A trend toward decreased cell density was observed at the insertion of the biceps at 8 weeks compared to control (S-Table 2). Cell density was significantly increased in the SD group at the insertion of the supraspinatus tendon at 8 weeks compared to control (Figure 3), with a similar trend at the insertion at 4 weeks and mid-substance at 8 weeks. Changes in tissue organization were observed with polarized light microscopy. Surprisingly, a significant decrease in angular deviation (indicative of more highly aligned collagen fibers) was observed in the proximal groove of the biceps at 4 weeks in the SD group compared to control, with a similar trend in the intra-articular region (S-Figure 2A). An additional trend toward increased angular deviation (indicative of greater collagen disorganization) was observed in the insertion of the biceps at 8 weeks in the SD group compared to control. No differences in tissue organization were observed in the supraspinatus tendon at either time point (S-Figure 2B).
Figure 3.
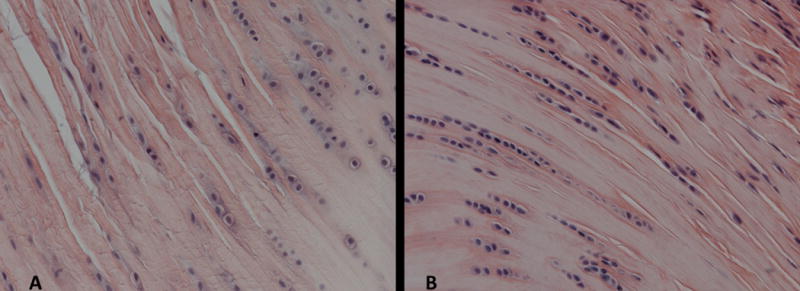
Tendon histology (supraspinatus and biceps) was quantified for cell shape and cell density at the insertion and mid-substance regions. A representative image for the supraspinatus insertion is displayed. Cell density was significantly increased (*p<0.05) in the SD group (B) compared to the control group (A) (N=5 at each time point).
Changes in protein expression were observed with immunohistochemical staining. Compared to control, collagen III was significantly increased in the SD group at the supraspinatus insertion at 4 weeks, with a similar trend at the supraspinatus insertion and mid-substance and biceps insertion at 8 weeks (Table III). However, collagen III was significantly decreased in the SD group compared to control at the distal groove of the biceps at 8 weeks. Decorin was significantly decreased in the SD group compared to control at the biceps insertion and distal groove at 4 weeks (Table III). Interestingly, however, decorin was significantly increased in the SD group compared to control at the biceps insertion at 8 weeks, with a similar trend in the supraspinatus mid-substance at 8 weeks. No differences in collagen II or IL1-β were observed (data not shown).
Table III.
At 8 weeks, collagen III staining was decreased at the biceps distal groove (DIS) in the SD group compared to control. A trend toward increased collagen III staining was also observed at the biceps insertion in the SD group compared to control. At 4 weeks, collagen III staining was increased at the supraspinatus insertion, with similar trends at the insertion and mid-substance at 8 weeks, in the SD group compared to control. At 4 weeks, decorin staining was decreased at the biceps insertion and distal groove in the SD group compared to control. At 8 weeks, decorin staining was increased at the biceps insertion in the SD group compared to control, with a similar trend at the supraspinatus mid-substance. Data is shown as median and interquartile range.
| Tendon | Location | Col III | Decorin | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 4 weeks | 8 weeks | 4 weeks | 8 weeks | ||||||||||
| Control | SD | p-value | Control | SD | p-value | Control | SD | p-value | Control | SD | p-value | ||
| Biceps | INS | 2 (1-2) | 1 (1-1) | 0.16 | 1 (1-2) | 2 (2-2)+ | 0.10+ | 2.5 (2-3) | 1 (1-1.25)* | 0.02* | 1 (1-2) | 3 (2-3)* | 0.02* |
| INTRA | 1 (1-1) | 1 (0-1) | 0.13 | 2 (1-2) | 2 (1-2) | 0.50 | 2 (1.5-2.5) | 1.5 (1-2) | 0.28 | 2 (1.5-2.5) | 2 (2-3) | 0.32 | |
| PROX | 0 (0-1) | 0 (0-1) | 0.45 | 1 (1-1) | 1 (1-1) | 0.35 | 2 (1-2) | 1 (1-1.25) | 0.17 | 2 (1-2) | 1 (1-2) | 0.24 | |
| DIS | 0 (0-1) | 0 (0-1) | 0.45 | 1 (1-1) | 0 (0-1)* | 0.03* | 2 (2-2.25) | 1 (1-1)* | 0.01* | 1 (1-2) | 2 (1-2) | 0.29 | |
| Supra | INS | 0.5 (0-1) | 1 (1-2)* | 0.04* | 1.5 (0.75-2) | 2 (2-2)+ | 0.14+ | 2 (1.75-2) | 3 (2.5-3) | 0.18 | 2 (2-2.5) | 2.5 (1.75-3) | 0.43 |
| MID | 0.5 (0-1) | 0 (0-1) | 0.45 | 0.5 (0-1) | 2 (1-1)+ | 0.08+ | 2 (1.5-2.25) | 2.5 (1.5-3) | 0.38 | 2 (1-2) | 2 (2-2.25)+ | 0.07+ | |
p<0.05
p≤0.1
Discussion
While the prevalence of shoulder impingement and its association with scapulothoracic kinematic abnormalities is well-documented, the cause and effect relationships remain unknown, making optimal clinical management difficult. In this animal model, we were able to prescribe scapular dyskinesis and evaluate the effect on the supraspinatus and the long head of the biceps in a controlled manner.
Results of this study demonstrate that scapular dyskinesis alters shoulder function and passive joint mechanics. Specifically, vertical force decreased and propulsion force increased in the SD group compared to control. Additionally, gross observational examination demonstrated clear alterations in scapular movements during forward locomotion, consistent with scapular “winging” in the human and characterized by entire medial border prominence in the closed kinetic chain activity. These changes indicate an alteration in the loading environment of this model and may place the glenohumeral joint at increased risk for degenerative injury. Specifically, altered scapular orientation infers altered acromial position which may lead to mechanical impingement of the rotator cuff and biceps and subsequent deficits in mechanical properties.7 Additionally, passive joint mechanics were significantly altered in the SD group, with the SD group having increased internal range of motion. This may be due to a capsular inefficiency as a result of the reduced dynamic restraint. Previous studies have demonstrated that abnormal scapular orientation, specifically excessive protraction, may place increased strains on the joint capsule, increasing risk of injury.19 Additionally, the unstable scapula may diminish the efficiency of the rotator cuff to effectively compress the humeral head into the glenoid fossa thereby requiring additional support from the static restraints, such as the joint capsule for stability. The resultant increased stress on the capsule could progress to the observed increased joint laxity.
Scapular dyskinesis also led to compromised tendon properties. Specifically, in the SD group, tendon elastic modulus was diminished in the mid-substance of both tendons. Additionally, percent relaxation, a viscoelastic parameter, was significantly increased. Previous studies have demonstrated that diminished elastic modulus and increased percent relaxation are indicative of inferior tissue properties as observed in injured and tendinopathic tendons.20; 21 The changes observed in the presence of scapular dyskinesis may be a result of altered acromial position and subsequent reduced subacromial space, leading to tendon mechanical abrasion and wear. Alternatively, these changes could be a result of the increased demand (overload and overuse) placed on the rotator cuff and biceps in an attempt to restore dynamic stability to the glenohumeral joint, despite the presence of an unstable scapula. The location specific tendon changes (mid-substance region) may be due to its anatomic location under the acromial arch during forward flexion, resulting in impingement.
Histologic changes, consistent with tendon pathology, were also observed in each tendon. Specifically, the SD group had more rounded cell morphology in the biceps tendon at 8 weeks. These changes have been observed previously in injured tendon and may be indicative of increased cellular activity and/or a compressive loading environment. Surprisingly, the SD group had a less rounded cell shape at the supraspinatus tendon insertion. The cellular changes observed in the supraspinatus tendon of the SD group are less than 10% different compared to controls and therefore may not be scientifically relevant. The changes observed in the biceps tendon were greater (~30% change) and therefore may be indicative of changes in cell behavior. Additionally, the SD group had a greater cell density at the supraspinatus tendon insertion, which may be indicative of increased cellular metabolic activity in response to the altered loading environment. Tendon collagen organization was also altered in the biceps, with greater organization observed in the SD group at 4 weeks in the proximal groove and intra-articular space. However, at 8 weeks there was greater disorganization in the SD group compared to control at the insertion. The tendon’s differential organization at 4 and 8 weeks may be associated with a varied adaptation in response to changes in loading over time.
Structural changes were accompanied by changes in protein expression. Specifically, collagen III was significantly altered in both tendons. Collagen III was increased in the supraspinatus tendon and at the insertion of the biceps tendon in the SD group. Accumulation of collagen III in tendon has been associated with microtrauma, scar formation, and a decrease in mechanical strength.22; 23 Additionally, collagen III was decreased in the distal groove of the biceps tendon in the SD group. The location of this change is consistent with the location of differences in cell morphology in the biceps tendon and may be indicative of alterations in cellular function and subsequent extracellular-matrix deposition. Decorin was also significantly altered in both tendons. Specifically, decorin was decreased at the biceps insertion and distal groove at 4 weeks and interestingly, increased at the biceps insertion and supraspinatus mid-substance at 8 weeks, in the SD group. Decorin is the primary proteoglycan found in tendon and is involved in regulation of fibrillogenesis and may play a role in the tendon response to injury.24 The initial decrease in decorin observed at 4 weeks is consistent with previous studies that have demonstrated that decorin is initially downregulated in injured tendon.22 Interestingly, the increase in decorin observed at 8 weeks may be a result of the tendon’s adaptive response to the change in loading and is consistent with the increased proteoglycan content observed with tendinopathy.25 No differences in the cartilage marker, collagen II, or the pro-inflammatory cytokine, IL1-β, were observed. Previous studies have identified IL1-β as a possible initiator of tendinopathy and therefore IL1-β was selected for this study as an important inflammatory marker to be examined.2; 26 The results observed for IL1-β indicate that chronic inflammation may not play a role in the mechanical changes observed in this study; however, future studies are warranted to further elucidate the acute and chronic inflammatory responses following injury.
This study has several limitations. First, the use of a quadruped animal does not exactly replicate the human condition. However, it has been well-established that the rat shoulder has similar bony architecture and soft tissue anatomy as the human.27 The presence of the acromial arch in the rat is particularly important because during forward locomotion, the supraspinatus passes repetitively under it, leading to supraspinatus tendinopathy. This is similar to what occurs in humans during repetitive overhead activity21. Secondly, acute transection of both the spinal accessory and long thoracic nerves to induce scapular dyskinesis does not exactly mimic the clinical scenario. Specifically, several factors may contribute to scapular dysfunction clinically including muscle imbalance, nerve injury, postural abnormality, anatomical disruption, capsular contracture, or proprioceptive dysfunction28; 29. For this model, we utilized a nerve injury mechanism and were able to successfully and repeatably create scapular “winging”, which is consistent with observations found clinically in patients with scapular dyskinesis. Additionally, by utilizing an animal model, we were able to rigorously evaluate its effect in a controlled manner. Despite these limitations, results clearly demonstrate that scapular dyskinesis caused mechanical and structural consequences in the supraspinatus and biceps tendons.
This is the first study to directly identify scapular dyskinesis as a causative mechanical mechanism for the development of pathological changes in the supraspinatus and biceps tendons. Specifically, scapular dyskinesis permanently diminished shoulder function and tendon mechanical, structural, and compositional properties. Identification of scapular dyskinesis as a mechanism of pathological changes will help inform and guide clinicians in developing optimal long-term rehabilitation strategies. Alternatively, early interventions, such as preventative neuromuscular training, can also be considered, as has been successfully demonstrated in prevention of anterior-cruciate ligament (ACL) injuries30. Future studies should be performed to quantify and/or classify the position of the scapula in this model in order to allow for a closer comparison to the clinical situation. Additionally, longer time points could be examined in order to further assess the progression of tendon damage. Next, this model will be utilized to examine the effect of scapular dyskinesis in the presence of overuse and following supraspinatus tendon repair in order to help define the in vivo mechanical processes which lead to rotator cuff and biceps tendon degeneration and compromise tendon healing potential following repair.
Supplementary Material
Acknowledgments
This study was funded by NIH/NIAMS (R01AR056658), NIH (T32AR556680), and the Penn Center for Musculoskeletal Disorders (P30AR050950).
References
- 1.Timmons MK, Thigpen CA, Seitz AL, et al. Scapular kinematics and subacromial-impingement syndrome: a meta-analysis. J Sport Rehabil. 2012;21:354–370. doi: 10.1123/jsr.21.4.354. [DOI] [PubMed] [Google Scholar]
- 2.Green RA, Taylor NF, Watson L, et al. Altered scapula position in elite young cricketers with shoulder problems. J Sci Med Sport. 2013;16:22–27. doi: 10.1016/j.jsams.2012.05.017. [DOI] [PubMed] [Google Scholar]
- 3.Struyf F, Nijs J, Baeyens JP, et al. Scapular positioning and movement in unimpaired shoulders, shoulder impingement syndrome, and glenohumeral instability. Scand J Med Sci Sports. 2011;21:352–358. doi: 10.1111/j.1600-0838.2010.01274.x. [DOI] [PubMed] [Google Scholar]
- 4.Laudner KG, Myers JB, Pasquale MR, et al. Scapular dysfunction in throwers with pathologic internal impingement. J Orthop Sports Phys Ther. 2006;36:485–494. doi: 10.2519/jospt.2006.2146. [DOI] [PubMed] [Google Scholar]
- 5.Warner JJ, Micheli LJ, Arslanian LE, et al. Scapulothoracic motion in normal shoulders and shoulders with glenohumeral instability and impingement syndrome. A study using Moire topographic analysis. Clin Orthop Relat Res. 1992:191–199. [PubMed] [Google Scholar]
- 6.Kibler WB, Sciascia A, Wilkes T. Scapular dyskinesis and its relation to shoulder injury. J Am Acad Orthop Surg. 20:364–372. doi: 10.5435/JAAOS-20-06-364. [DOI] [PubMed] [Google Scholar]
- 7.Solem-Bertoft E, Thuomas KA, Westerberg CE. The influence of scapular retraction and protraction on the width of the subacromial space. An MRI study. Clin Orthop Relat Res. 1993:99–103. [PubMed] [Google Scholar]
- 8.Seitz AL, McClure PW, Finucane S, et al. Mechanisms of rotator cuff tendinopathy: intrinsic, extrinsic, or both? Clin Biomech (Bristol, Avon) 2011;26:1–12. doi: 10.1016/j.clinbiomech.2010.08.001. [DOI] [PubMed] [Google Scholar]
- 9.Ludewig PM, Reynolds JF. The association of scapular kinematics and glenohumeral joint pathologies. J Orthop Sports Phys Ther. 2009;39:90–104. doi: 10.2519/jospt.2009.2808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mihata T, Jun BJ, Bui CN, et al. Effect of scapular orientation on shoulder internal impingement in a cadaveric model of the cocking phase of throwing. J Bone Joint Surg Am. 94:1576–1583. doi: 10.2106/JBJS.J.01972. [DOI] [PubMed] [Google Scholar]
- 11.Thompson WO, Debski RE, Boardman ND, 3rd, et al. A biomechanical analysis of rotator cuff deficiency in a cadaveric model. Am J Sports Med. 1996;24:286–292. doi: 10.1177/036354659602400307. [DOI] [PubMed] [Google Scholar]
- 12.Labriola JE, Lee TQ, Debski RE, et al. Stability and instability of the glenohumeral joint: the role of shoulder muscles. J Shoulder Elbow Surg. 2005;14:32S–38S. doi: 10.1016/j.jse.2004.09.014. [DOI] [PubMed] [Google Scholar]
- 13.Konrad GG, Jolly JT, Labriola JE, et al. Thoracohumeral muscle activity alters glenohumeral joint biomechanics during active abduction. J Orthop Res. 2006;24:748–756. doi: 10.1002/jor.20062. [DOI] [PubMed] [Google Scholar]
- 14.Timmons MK, Thigpen CA, Seitz AL, et al. Scapular kinematics and subacromial-impingement syndrome: a meta-analysis. J Sport Rehabil. 21:354–370. doi: 10.1123/jsr.21.4.354. [DOI] [PubMed] [Google Scholar]
- 15.Sarver JJ, Dishowitz MI, Kim SY, et al. Transient decreases in forelimb gait and ground reaction forces following rotator cuff injury and repair in a rat model. J Biomech. 43:778–782. doi: 10.1016/j.jbiomech.2009.10.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sarver JJ, Peltz CD, Dourte L, et al. After rotator cuff repair, stiffness–but not the loss in range of motion–increased transiently for immobilized shoulders in a rat model. J Shoulder Elbow Surg. 2008;17:108S–113S. doi: 10.1016/j.jse.2007.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Peltz CD, Perry SM, Getz CL, et al. Mechanical properties of the long-head of the biceps tendon are altered in the presence of rotator cuff tears in a rat model. J Orthop Res. 2009;27:416–420. doi: 10.1002/jor.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Gimbel JA, Van Kleunen JP, Mehta S, et al. Supraspinatus tendon organizational and mechanical properties in a chronic rotator cuff tear animal model. J Biomech. 2004;37:739–749. doi: 10.1016/j.jbiomech.2003.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Weiser WM, Lee TQ, McMaster WC, et al. Effects of simulated scapular protraction on anterior glenohumeral stability. Am J Sports Med. 1999;27:801–805. doi: 10.1177/03635465990270061901. [DOI] [PubMed] [Google Scholar]
- 20.Dourte LM, Perry SM, Getz CL, et al. Tendon properties remain altered in a chronic rat rotator cuff model. Clin Orthop Relat Res. 2010;468:1485–1492. doi: 10.1007/s11999-009-1206-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Soslowsky LJ, Thomopoulos S, Tun S, et al. Neer Award 1999. Overuse activity injures the supraspinatus tendon in an animal model: a histologic and biomechanical study. J Shoulder Elbow Surg. 2000;9:79–84. [PubMed] [Google Scholar]
- 22.Berglund M, Reno C, Hart DA, et al. Patterns of mRNA Expression for Matrix Molecules and Growth Factors in Flexor Tendon Injury: Differences in the Regulation Between Tendon and Tendon Sheath. The Journal of Hand Surgery. 2006;31:1279–1287. doi: 10.1016/j.jhsa.2006.06.011. [DOI] [PubMed] [Google Scholar]
- 23.Eriksen HA, Pajala A, Leppilahti J, et al. Increased content of type III collagen at the rupture site of human Achilles tendon. Journal of Orthopaedic Research. 2002;20:1352–1357. doi: 10.1016/S0736-0266(02)00064-5. [DOI] [PubMed] [Google Scholar]
- 24.Dunkman A, Buckley M, Mienaltowski M, et al. The Tendon Injury Response is Influenced by Decorin and Biglycan. Annals of Biomedical Engineering. 2013;42:619–630. doi: 10.1007/s10439-013-0915-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Joseph M, Maresh CM, McCarthy MB, et al. Histological and molecular analysis of the biceps tendon long head post-tenotomy. Journal of Orthopaedic Research. 2009;27:1379–1385. doi: 10.1002/jor.20868. [DOI] [PubMed] [Google Scholar]
- 26.Gotoh M, Hamada K, Yamakawa H, et al. Significance of granulation tissue in torn supraspinatus insertions: an immunohistochemical study with antibodies against interleukin-1 beta, cathepsin D, and matrix metalloprotease-1. J Orthop Res. 1997;15:33–39. doi: 10.1002/jor.1100150106. [DOI] [PubMed] [Google Scholar]
- 27.Soslowsky LJ, Carpenter JE, DeBano CM, et al. Development and use of an animal model for investigations on rotator cuff disease. J Shoulder Elbow Surg. 1996;5:383–392. doi: 10.1016/s1058-2746(96)80070-x. [DOI] [PubMed] [Google Scholar]
- 28.Kibler WB. The role of the scapula in athletic shoulder function. Am J Sports Med. 1998;26:325–337. doi: 10.1177/03635465980260022801. [DOI] [PubMed] [Google Scholar]
- 29.Kibler WB, McMullen J. Scapular dyskinesis and its relation to shoulder pain. J Am Acad Orthop Surg. 2003;11:142–151. doi: 10.5435/00124635-200303000-00008. [DOI] [PubMed] [Google Scholar]
- 30.Myklebust G, Engebretsen L, Braekken IH, et al. Prevention of anterior cruciate ligament injuries in female team handball players: a prospective intervention study over three seasons. Clin J Sport Med. 2003;13:71–78. doi: 10.1097/00042752-200303000-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


