Abstract
Analysis of motor unit discharge can provide insight into the neural control of movement in healthy and pathological states, but it is typically completed in one muscle at a time. For some research investigations, it would be advantageous to study motor unit discharge from multiple muscles simultaneously. One such example is investigation of the flexion synergy, an abnormal muscle co-activation pattern in post-stroke individuals in which activation of shoulder abductors is involuntarily coupled with that of elbow and finger flexors. However, limitations in available technology have hindered the ability to efficiently extract motor unit discharge from multiple muscles simultaneously. In this study, we propose the use of high-density surface EMG decomposition from proximal and distal flexion synergy muscles (deltoid, biceps, wrist/finger flexors) in combination with an isometric joint torque recording device in individuals with chronic stroke. This innovative approach provides the ability to efficiently analyze both motor units and joint torques that have been simultaneously recorded from the shoulder, elbow, and fingers. In preliminary experiments, 3 stroke and 5 control participants generated shoulder abduction, elbow flexion, and finger flexion torques at 10, 20, 30 and 40% of maximum torque. Motor unit spike trains could be extracted from all muscles at each torque level. Mean motor unit firing rates were significantly lower in the stroke group than in the control group for all three muscles. Within the stroke group, wrist/finger flexor motor units had the lowest coefficient of variation. Additionally, modulation of mean firing rates across torque levels was significantly impaired in all three paretic muscles. The implications of these findings and overall impact of this approach are discussed.
I. Introduction
Analysis of motor unit behavior in humans has the potential to provide a significant amount of information about the neural control of movement. This is particularly true for tasks requiring the coordinated activation of multiple muscles. For example, proximal and distal muscles in the arm serve different roles during reaching movements, with proximal muscles providing sustained postural support for the distal muscles, which provide fine motor control. Additionally, different neural strategies may be used in proximal and distal muscles to increase muscle force [1], and the muscles are presumably driven differently by cortical and brainstem pathways. Time and frequency-domain analyses of motor unit discharge can be used to clarify these neural strategies and to characterize synaptic input to proximal vs. distal motoneuron pools. However, limitations in available technology have hindered the ability to simultaneously record motor unit discharge from multiple muscles.
Acquiring and analyzing motor unit discharge from even one muscle can be more difficult and time consuming than acquisition and analysis of traditional surface EMG, and it typically requires intramuscular placement of fine-wire electrodes using a needle. Therefore, recording from multiple muscles simultaneously presents an additional logistical challenge. Advances in intramuscular decomposition algorithms [2] have improved the process by allowing semi-automatic motor unit extraction from the electromyogram (EMG), and they have enabled the acquisition of motor units at higher levels of muscle contraction. However, the method remains time consuming due to the amount of manual processing involved (up to 4 hours per 20 sec. of data) [3].
Recently developed high-density surface EMG decomposition techniques have improved upon previous methods, because they can automatically extract motor unit discharge and are non-invasive [3, 4]. The greatly improved efficiency of this approach increases the feasibility of acquiring motor unit discharge from multiple muscles simultaneously. As a result, scientific questions involving the control of motor units during multi-joint activation can now be addressed.
One application in which investigation of both proximal and distal muscles is crucial is in paretic upper extremity motor function of individuals with chronic hemiparetic stroke. Of the many biomechanical and neurological deficits that impede upper extremity movement following stroke, the emergence of abnormal muscle co-activation patterns may be the most functionally limiting, because these patterns reduce the capacity to independently control the shoulder, elbow, wrist, and finger joints.
The most prominent abnormal muscle co-activation pattern, described clinically as the flexion synergy [5], produces shoulder abduction coupled with elbow, wrist, and finger flexion, and it has been quantified extensively in static and dynamic paradigms, e.g., [6-9]. However, motor unit behavior has not yet been studied within the context of the flexion synergy. Doing so may provide significant insight into what role alternative descending neural pathways, such as those originating from the brainstem, play in flexion synergy manifestation.
In this study, we propose a method combining high-density surface EMG decomposition with an isometric joint torque recording device. This innovative approach will provide the ability to efficiently analyze motor units and joint torques collected from three muscles and joints simultaneously during the generation of volitional shoulder abduction, elbow flexion or finger flexion torques.
II. Methods
A. Participants
Three individuals with chronic hemiparetic stroke (mean ± SD age: 62 ± 5 years; time post-stroke: 6.5, 26.6, 17.0 years; 2 males, 1 female) and five neurologically intact individuals (mean ± SD age: 54 ± 11 years; 3 males, 2 females) completed the study. All post-stroke participants had severe upper limb impairment according to the upper limb Fugl-Meyer Motor Assessment [10] (scores of 24, 20, and 19 out of 66 possible) and the hand portion of the Chedoke-McMaster Stroke Assessment [11] (scores of 3, 3, and 2 out of 7 possible). All participants gave informed consent for participation in the study, which was approved by the Institutional Review Board of Northwestern University.
B. Experimental Apparatus
The experimental protocol was conducted in a testing device capable of measuring isometric shoulder, elbow, wrist, and finger (metacarpophalangeal) joint torques in multiple degrees of freedom simultaneously, which has been described previously [12] (Fig. 1). Participants were seated in an experimental chair with shoulder/waist restraints to prevent shoulder girdle and trunk motion. The tested forearm was placed in a fiberglass cast to rigidly interface the arm with a six degree-of-freedom load cell (JR3, Inc.). The arm was positioned in 75° shoulder abduction, 40° horizontal adduction, 90° elbow flexion, 15° pronation and 0° wrist and finger flexion/extension. Paretic fingers were positioned at 15° finger flexion to accommodate range of motion restrictions. A computer monitor displayed real-time visual feedback of joint torque data.
Fig. 1.
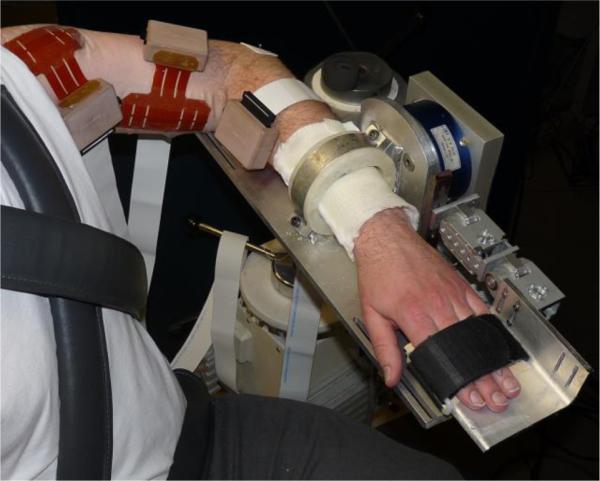
Isometric joint torque recording device with high-density surface EMG grids on the deltoid, biceps, and wrist/finger flexor muscles.
Surface EMG was recorded in bipolar fashion from grids of 64 electrodes with 8 mm inter-electrode distance (OT Bioelettronica) placed on the anterior deltoid (DELT), biceps brachii (BIC), and wrist/finger flexor (WFF) muscle bellies. The signals were amplified (x1k – 10k), band-pass filtered (10-500 Hz), and sampled at 2048 Hz (EMG-USB2, OT Bioelettronica).
C. Protocol
Each participant's maximum voluntary torques (MVTs) were measured during separate isometric torque generation in shoulder abduction (SABD), elbow flexion (EF), and finger flexion (FF) directions. Directions were randomized, and trials within a direction were repeated until three trials with peak torque within 85% of the maximum torque value were obtained, without the last trial producing the largest torque. Participants were given vigorous verbal encouragement throughout MVT trials. Visual feedback of the intended torque direction was given for all MVTs.
Participants generated submaximal torque at each of four levels (10, 20, 30, and 40% MVT) for each of the three directions (SABD, EF, and FF). The order of directions and torque levels were randomized. Visual feedback displayed real-time torque generation in the desired degree of freedom. Participants were instructed to move directly to the targeted torque level and to hold the contraction for 25-30 seconds. Up to four trials were completed for each condition. Data presented are a convenience sample from other ongoing experiments, and as such, not all trials were collected for all participants.
D. Data Analysis
Surface EMG channels were visually inspected and any channels with significant artifacts were discarded. Remaining channels were decomposed into motor unit spike trains with the Convolution Kernel Compensation (CKC) technique [13], which has been previously validated [3]. Each obtained motor unit spike train was analyzed and checked for quality according to the following parameters: The first and last two seconds of discharge were omitted from analysis to isolate steady firing of the motor unit while the target torque was held, removing phases of motor unit recruitment and derecruitment. Inter-spike intervals (ISI) were calculated as the difference between consecutive discharge times, and discharges with ISI less than 33 ms and greater than 250 ms were omitted [14]. The percent coefficient of variation (COV) was calculated as the standard deviation of the ISIs divided by the mean ISI multiplied by 100. Only units with COV values less than 30% were used for further analysis [3]. The instantaneous firing rate was calculated as the reciprocal of the ISI and averaged across each spike train to determine the mean firing rate. In addition, the normalized torque during the steady firing of each motor unit was isolated.
For each muscle, unpaired t-tests were used to compare group mean firing rates and COV between stroke and control groups. An ANCOVA was used to test compare linear regression slopes and intercepts between stroke and control groups. F-tests were used to determine whether individual linear regression slopes were different than zero. Two oneway ANOVAs were used to examine differences among DELT, BIC, and FF muscles within the stroke and control groups. If a significant difference among the muscles was found, the individual means were compared using an unpaired t-test and the Bonferroni correction for multiple comparisons.
III. Results
Table 1 displays the yield of decomposed motor units for DELT, BIC, and WFF during generation of 10, 20, 30, and 40% MVT. Yield is shown per participant from the best trial per condition. A total of 478 motor units were decomposed across these 71 trials. This is a substantial increase in yield compared with typical studies in the literature, which vary widely but often include 20-40 motor units in total (e.g., [15]). The WFF demonstrated consistently higher yield across participants and torque levels compared with the DELT and BIC. For many participants and muscles, motor units could be extracted during torque generation up to 40% MVT without appreciable degradation in yield.
TABLE I.
Single Trial Motor Unit Yield
| Stroke | Control | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Muscle | % MVT | S1 | S2 | S3 | C1 | C2 | C3 | C4 | C5 |
| Deltoid | 10 | 0 | 2 | 3 | 9 | 9 | - | - | 2 |
| 20 | 0 | 6 | 1 | 8 | 8 | - | - | - | |
| 30 | 0 | 2 | 2 | 6 | 10 | - | - | 1 | |
| 40 | 0 | 3 | 0 | 7 | - | - | - | - | |
| Biceps | 10 | 4 | 7 | 10 | 5 | 6 | 3 | 4 | 5 |
| 20 | 1 | - | 11 | 4 | 8 | 2 | 2 | 6 | |
| 30 | 3 | 10 | 10 | 1 | 7 | 4 | 2 | 5 | |
| 40 | 2 | - | 9 | 1 | - | - | 1 | - | |
| Wrist/Finger Flexors | 10 | 11 | - | 9 | 18 | 16 | 5 | 11 | 15 |
| 20 | 7 | - | 13 | 15 | 21 | - | 7 | - | |
| 30 | 11 | - | 13 | 19 | 14 | 6 | 10 | 9 | |
| 40 | 6 | - | 14 | 9 | - | - | 7 | - | |
A dash indicates data was not available for decomposition for the given condition. Data are from the best trial per condition for each participant.
Of the six participants for whom both DELT and BIC data were available (three stroke and three control), four participants had consistently better motor unit yield for the BIC than the DELT. The remaining two had consistently better motor unit yield for the DELT than the BIC. For one participant with stroke, no motor units of acceptable quality could be obtained from the DELT.
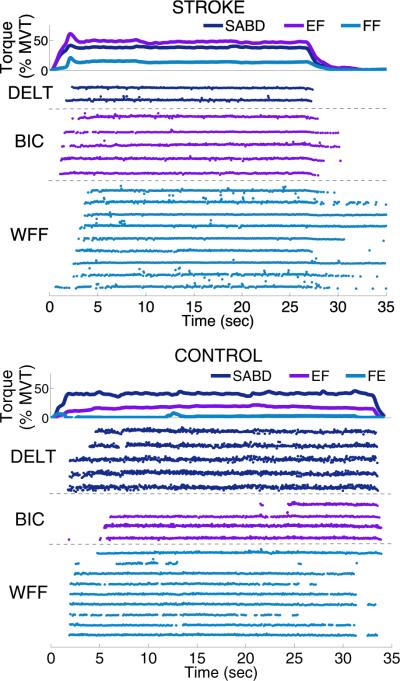
Fig. 2 displays sample data from one SABD trial at 40% MVT from one stroke participant (top panel) and one control participant (bottom panel). Motor unit instantaneous firing rates from DELT, BIC, and WFF muscles and joint torques from the shoulder, elbow, and fingers are shown. In addition to the intended 40% MVT SABD torque, the stroke participant also produced torques at the elbow and fingers, generating approximately 50% MVT EF torque and 15% MVT FF torque, consistent with previous reports quantifying flexion synergy expression at the elbow and fingers [6, 8]. Additionally, self-sustained firing of WFF motor units can be seen following the cessation of torque generation [16, 17]. The control participant produced a smaller amount of EF torque, generating approximately 20% MVT. There was only slight FE torque generated at the fingers, but many motor units could be identified in the co-contracting WFF muscles.
Fig. 2.
Motor unit instantaneous firing rates for DELT, BIC, and WFF and joint torques in SABD, EF, and FF or finger extension (FE) collected during a 40% SABD MVT trial are shown for one stroke participant (top panel) and one control participant (bottom panel).
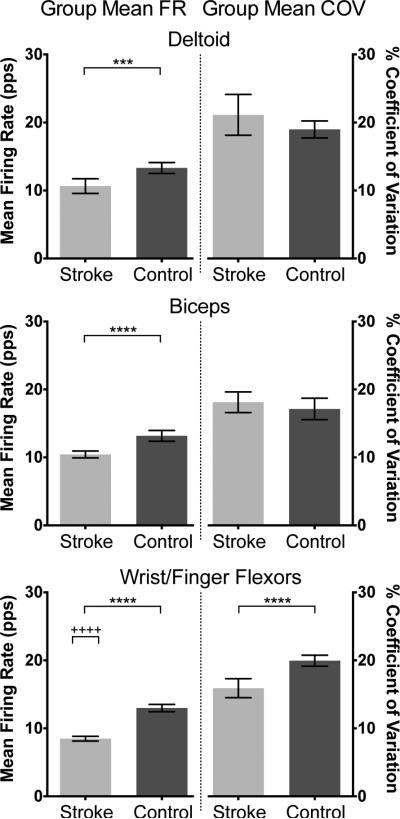
Fig. 3 shows the group mean motor unit firing rates and group mean COV for each muscle (i.e., mean firing rate and COV from each motor unit, averaged across all motor units and torque levels). Group mean firing rates in the stroke group were significantly lower than the control group in all three muscles (DELT: 10.7 vs. 13.3 pulses per second (pps), p = 0.0007; BIC: 10.4 vs. 13.2 pps, p < 0.0001; WFF: 8.5 vs. 13.0 pps, p < 0.0001). The difference in group mean rates between stroke and control groups was similar in DELT (2.6 pps) and BIC (2.8 pps) but was larger in WFF (4.5 pps).
Fig. 3.
Group mean firing rates (FRs) and coefficients of variation (COV) of each muscle for stroke and control groups across all motor units and torque levels are shown with 95% confidence intervals. Group mean FRs were lower in the stroke group for all muscles, and group mean COV was lower in the stroke group for WFF (***/**** indicate p < 0.001/p < 0.0001). In the stroke group, mean FRs were lower for WFF than for DELT or BIC (++++ indicates p < 0.0001).
The group mean COV for WFF was significantly lower in the stroke group than in the control group (15.9 vs. 19.9%, p < 0.0001). The group mean COVs for DELT and BIC were not different between stroke and control (DELT: 21.1 vs. 19.0%, p = 0.12; BIC: 18.1 vs. 17.1%, p = 0.37).
Within the stroke group, there was a significant difference in the group mean firing rates among muscles (p < 0.0001). Post-hoc comparisons revealed significant differences between DELT and WFF (p < 0.0001) and between BIC and WFF (p < 0.0001). There was no difference in group mean firing rates among muscles in the control group (p = 0.67).
Fig. 4 shows the mean firing rates of individual motor units (for all motor units represented in Table 1), plotted as a function of the corresponding mean torque level. Linear regression lines and equations are shown to demonstrate how motor unit firing rates are modulated across torque level. The slopes of all linear regression lines were significantly different from zero for the control group but none were significantly different for the stroke group. Comparing slopes between stroke and control groups for each muscle, there were significant differences in BIC and FF muscles but not DELT (DELT: p = 0.177; BIC: p = 0.010; FF: p = 0.017). However, the intercept was significantly different between groups for DELT (p = 0.0001).
Fig. 4.
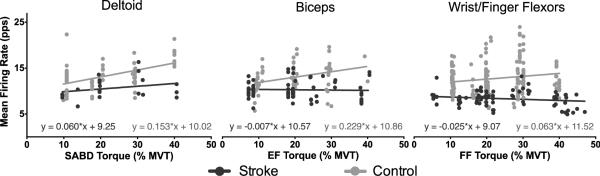
Mean firing rates of individual motor units during steady torque generation are shown as function of torque level. Linear regression slopes for control data only demonstrated motor unit rate modulation (significantly different than zero for all muscles). Rate modulation was different between stroke and control groups for BIC and WFF (linear regression slopes were significantly different) but not DELT
IV. Discussion
This study proposes the use of high-density surface EMG decomposition in combination with an isometric joint torque recording device to enable the simultaneous measurement of motor unit discharges from the three primary flexion synergy muscles (DELT, BIC, WFF) along with joint torques generated at the shoulder, elbow, and fingers in the paretic upper limb of individuals post-stroke.
Preliminary results from three individuals with stroke and five healthy control individuals demonstrate the feasibility, efficiency, and increased yield of this approach in both populations. The increased efficiency will be particularly useful for analyses requiring large amounts of data (e.g., 60 to 90 sec), such as estimation of coherence. Increased motor unit yield allows for construction of a cumulative motor unit spike train (CST) which, if containing a sufficient number of motor units, provides a faithful estimate of the behavior of the entire motor unit population in a given motor pool [18]. Analysis of a CST is advantageous compared to analysis of single or paired motor units, because the CST more accurately reflect the common synaptic input to the motor pool [18]. To further increase motor unit yield, this approach can be combined with intramuscular recordings to isolate additional motor units from deeper portions of the muscle [3]. Additionally, the number of muscles that can be recorded from simultaneously with this approach is bounded by the number of data acquisition channels available, which is currently 256. Although the preliminary results presented here demonstrate simultaneous recording from three muscles of the flexion synergy, it is currently possible to record from a total of four muscles (with 64 channels per muscle).
The approach presented in this study has the ability to provide rich data for many types of motor unit analyses in both healthy and pathological populations. For example, cross-correlation and coherence analyses, which can provide insight into the structure and function of synaptic inputs to a motoneuron pool, provide information with which to speculate about the descending pathways that may be involved [19]. These analyses are typically performed within a given muscle, but with the availability of simultaneous motor units recordings from multiple muscles, we plan to extend these techniques to investigate whether cross-correlation and coherence between muscles of the flexion synergy may explain their manifestation.
Results from this study demonstrate decreased firing rates and lack of rate modulation in the stroke group that are consistent with previous reports from biceps brachii [20, 21] and tibialis anterior [22, 23]. Interestingly, the current findings also demonstrate differences in motor unit behavior in proximal (DELT, BIC) vs. distal muscles (WFF), underscoring the need to examine motor unit behavior in a variety of muscles. The group mean firing rates in the stroke group were lower for distal motor units than for proximal motor units. Accordingly, the difference in group mean firing rates between stroke and control groups was largest for distal motor units, as was the difference in group mean COV.
The physiological mechanisms underlying the impaired rate modulation in the stroke group are not fully clear. Changes in descending drive to the motor pool occur following stroke, likely including both synaptic and neuromodulatory inputs. A change in these inputs could alter intrinsic motoneuron properties, potentially leading to dramatic increases in motoneuron excitability and/or changes in firing behavior [24]. Additionally, rate modulation could be impaired due to a combination of excitation and co-active inhibition to the motoneuron pool [20, 25]. The decrease in COV in distal motor units may suggest decreased synaptic noise due to stroke-induced loss of descending drive from corticospinal pathways. This would preferentially impact distal muscles, because they have a much higher proportion of corticospinal innervation than proximal muscles.
Future directions include developing the ability to track individual motor units over different contractions and implementing a recently introduced metric to assess accuracy of motor units identified using the CKC technique [26].
Acknowledgment
The authors wish to thank Jessica Wilson, B.S. for assistance with participant recruitment and data collection.
This work was supported by NIH grant R01-HD039343 and T32 EB009406 (JPAD), European Research Council Advanced Grant DEMOVE No. 267888 (DF), and a Promotion of Doctoral Studies scholarship from the Foundation for Physical Therapy, Inc. (LCM).
Contributor Information
Laura C. Miller, Department of Biomedical Engineering and the Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, USA..
Christopher K. Thompson, Department of Physiology, Northwestern University, Chicago, IL, USA.
Francesco Negro, Department of Neurorehabilitation Engineering, University Medical Center Göttingen, Georg-August University, Göttingen, Germany..
C. J. Heckman, Department of Physiology, Northwestern University, Chicago, IL, USA.
Dario Farina, Department of Neurorehabilitation Engineering, University Medical Center Göttingen, Georg-August University, Göttingen, Germany..
Julius P.A. Dewald, Department of Biomedical Engineering and the Department of Physical Therapy and Human Movement Sciences, Northwestern University, Chicago, IL, USA..
REFERENCES
- 1.Kukulka CG, Clamann HP. Comparison of the recruitment and discharge properties of motor units in human brachial biceps and adductor pollicis during isometric contractions. Brain Res. 1981 Aug 24;219:45–55. doi: 10.1016/0006-8993(81)90266-3. [DOI] [PubMed] [Google Scholar]
- 2.McGill KC, Lateva ZC, Marateb HR. EMGLAB: an interactive EMG decomposition program. J Neurosci Methods. 2005 Dec 15;149:121–33. doi: 10.1016/j.jneumeth.2005.05.015. [DOI] [PubMed] [Google Scholar]
- 3.Holobar A, Minetto MA, Botter A, Negro F, Farina D. Experimental analysis of accuracy in the identification of motor unit spike trains from high-density surface EMG. IEEE Trans Neural Syst Rehabil Eng. 2010 Jun;18:221–9. doi: 10.1109/TNSRE.2010.2041593. [DOI] [PubMed] [Google Scholar]
- 4.De Luca CJ, Adam A, Wotiz R, Gilmore LD, Nawab SH. Decomposition of surface EMG signals. J Neurophysiol. 2006 Sep;96:1646–57. doi: 10.1152/jn.00009.2006. [DOI] [PubMed] [Google Scholar]
- 5.Brunnstrom S. Movement therapy in hemiplegia: a neurophysiological approach. Harper and Row; New York: 1970. [Google Scholar]
- 6.Dewald J, Beer R. Abnormal joint torque patterns in the paretic upper limb of subjects with hemiparesis. Muscle Nerve. 2001;24:273–283. doi: 10.1002/1097-4598(200102)24:2<273::aid-mus130>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 7.Sukal T, Ellis M, Dewald J. Shoulder abduction-induced reductions in reaching work area following hemiparetic stroke: neuroscientific implications. Exp Brain Res. 2007;176:594–602. doi: 10.1007/s00221-007-1029-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller LC, Dewald JP. Involuntary paretic wrist/finger flexion forces and EMG increase with shoulder abduction load in individuals with chronic stroke. Clin Neurophysiol. 2012 Jun;123:1216–25. doi: 10.1016/j.clinph.2012.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ellis MD, Drogos J, Carmona C, Keller T, Dewald JP. Neck rotation modulates flexion synergy torques indicating an ipsilateral reticulospinal source for impairment in stroke. J Neurophysiol. 2012 Sep 5; doi: 10.1152/jn.01030.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Fugl-Meyer A, Jaasko L, Leyman I, Olsson S, Steglind S. The post-stroke hemiplegic patient. 1. A method for evaluation of physical performance. Scand J Rehabil Med. 1975;7:13–31. [PubMed] [Google Scholar]
- 11.Gowland C, Stratford P, Ward M, Moreland J, Torresin W, Van Hullenaar S, et al. Measuring physical impairment and disability with the Chedoke-McMaster Stroke Assessment. Stroke. 1993;24:58–63. doi: 10.1161/01.str.24.1.58. [DOI] [PubMed] [Google Scholar]
- 12.Sukal-Moulton T, Krosschell KJ, Gaebler-Spira DJ, Dewald JP. Motor impairment factors related to brain injury timing in early hemiparesis. Part I: expression of upper-extremity weakness. Neurorehabil Neural Repair. 2014 Jan;28:13–23. doi: 10.1177/1545968313500564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Holobar A, Zazula D. Multichannel Blind Source Separation Using Convolution Kernel Compensation. Signal Processing, IEEE Transactions on. 2007;55:4487–4496. [Google Scholar]
- 14.Watanabe K, Gazzoni M, Holobar A, Miyamoto T, Fukuda K, Merletti R, et al. Motor unit firing pattern of vastus lateralis muscle in type 2 diabetes mellitus patients. Muscle Nerve. 2013 Feb 28; doi: 10.1002/mus.23828. [DOI] [PubMed] [Google Scholar]
- 15.Enoka RM, Fuglevand AJ. Motor unit physiology: some unresolved issues. Muscle Nerve. 2001 Jan;24:4–17. doi: 10.1002/1097-4598(200101)24:1<4::aid-mus13>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 16.McPherson J, Ellis M, Heckman C, Dewald J. Evidence for increased activation of persistent inward currents in individuals with chronic hemiparetic stroke. J Neurophysiol. 2008;100:3236–3243. doi: 10.1152/jn.90563.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Mottram CJ, Wallace CL, Chikando CN, Rymer WZ. Origins of Spontaneous Firing of Motor Units in the Spastic-Paretic Biceps Brachii Muscle of Stroke Survivors. J Neurophysiol. 2010 Sep 22; doi: 10.1152/jn.00463.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Negro F, Farina D. Factors Influencing the Estimates of Correlation between Motor Unit Activities in Humans. PLoS One. 2012;7:e44894. doi: 10.1371/journal.pone.0044894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Farmer SF, Halliday DM, Conway BA, Stephens JA, Rosenberg JR. A review of recent applications of cross-correlation methodologies to human motor unit recording. J Neurosci Methods. 1997 Jun 27;74:175–87. doi: 10.1016/s0165-0270(97)02248-6. [DOI] [PubMed] [Google Scholar]
- 20.Mottram CJ, Heckman CJ, Powers RK, Rymer WZ, Suresh NL. Disturbances of Motor Unit Rate Modulation are Prevalent in Muscles of Spastic-Paretic Stroke Survivors. J Neurophysiol. 2014 Feb 26; doi: 10.1152/jn.00389.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gemperline JJ, Allen S, Walk D, Rymer WZ. Characteristics of motor unit discharge in subjects with hemiparesis. Muscle Nerve. 1995 Oct;18:1101–14. doi: 10.1002/mus.880181006. [DOI] [PubMed] [Google Scholar]
- 22.Chou LW, Palmer JA, Binder-Macleod S, Knight CA. Motor unit rate coding is severely impaired during forceful and fast muscular contractions in individuals post stroke. J Neurophysiol. 2013 Jun;109:2947–54. doi: 10.1152/jn.00615.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Jakobsson F, Grimby L, Edstrom L. Motoneuron activity and muscle fibre type composition in hemiparesis. Scand J Rehabil Med. 1992 Sep;24:115–9. [PubMed] [Google Scholar]
- 24.Heckman CJ, Johnson M, Mottram C, Schuster J. Persistent inward currents in spinal motoneurons and their influence on human motoneuron firing patterns. Neuroscientist. 2008 Jun;14:264–75. doi: 10.1177/1073858408314986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Powers RK, Elbasiouny SM, Rymer WZ, Heckman CJ. Contribution of intrinsic properties and synaptic inputs to motoneuron discharge patterns: a simulation study. J Neurophysiol. 2012 Feb;107:808–23. doi: 10.1152/jn.00510.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holobar A, Minetto MA, Farina D. Accurate identification of motor unit discharge patterns from high-density surface EMG and validation with a novel signal-based performance metric. J Neural Eng. 2014 Feb;11:016008. doi: 10.1088/1741-2560/11/1/016008. [DOI] [PubMed] [Google Scholar]