Abstract
IMPORTANCE
The US Food and Drug Administration adopted labeling for nicotine patches to allow use beyond the standard 8 weeks. This decision was based in part on data showing increased efficacy for 24 weeks of treatment. Few studies have examined whether the use of nicotine patches beyond 24 weeks provides additional therapeutic benefit.
OBJECTIVE
To compare 8 (standard), 24 (extended), and 52 (maintenance) weeks of nicotine patch treatment for promoting tobacco abstinence.
DESIGN, SETTING, AND PARTICIPANTS
We recruited 525 treatment-seeking smokers for a randomized clinical trial conducted from June 22, 2009, through April 15, 2014, through 2 universities.
INTERVENTIONS
Smokers received 12 smoking cessation behavioral counseling sessions and were randomized to 8, 24, or 52 weeks of nicotine patch treatment.
MAIN OUTCOMES AND MEASURES
The primary outcome was 7-day point prevalence abstinence, confirmed with breath levels of carbon monoxide at 6 and 12 months (intention to treat).
RESULTS
At 24 weeks, 21.7% of participants in the standard treatment arm were abstinent, compared with 27.2% of participants in the extended and maintenance treatment arms (χ 2 = 1.98; P = .17). In a multivariate model controlled for covariates, participants in the extended and maintenance treatment arms reported significantly greater abstinence rates at 24 weeks compared with participants in the standard treatment arm (odds ratio [OR], 1.70 [95% CI, 1.03-2.81]; P = .04), had a longer duration of abstinence until relapse (β = 21.30 [95% CI, 10.30-32.25]; P < .001), reported smoking fewer cigarettes per day if not abstinent (mean [SD], 5.8 [5.3] vs 6.4 [5.1] cigarettes per day; β = 0.43 [95% CI, 0.06-0.82]; P = .02), and reported more abstinent days (mean [SD], 80.5 [38.1] vs 68.2 [43.7] days; OR, 1.55 [95% CI, 1.06-2.26]; P = .02). At 52 weeks, participants in the maintenance treatment arm did not report significantly greater abstinence rates compared with participants in the standard and extended treatment arms (20.3% vs 23.8%; OR, 1.17 [95% CI, 0.69-1.98]; P = .57). Similarly, we found no difference in week 52 abstinence rates between participants in the extended and standard treatment arms (26.0% vs 21.7%; OR, 1.33 [95% CI, 0.72-2.45]; P = .36). Treatment duration was not associated with any adverse effects or adherence to the counseling regimen, but participants in the maintenance treatment arm reported lower adherence to the nicotine patch regimen compared with those in the standard and extended treatment arms (mean [SD], 3.94 [2.5], 4.61 [2.0], and 4.7 [2.4] patches/wk, respectively; F2,522 = 6.03; P = .003).
CONCLUSIONS AND RELEVANCE
The findings support the safety of long-term use of nicotine patch treatment, although they do not support efficacy beyond 24 weeks of treatment in a broad group of smokers.
TRIAL REGISTRATION
clinicaltrials.gov Identifier: NCT01047527
The transdermal nicotine patch continues to be one of the most popular medications used to treat nicotine dependence1-3 owing to its easy access, favorable adverse effect profile, and low cost. However, 6-month rates of smoking cessation rarely exceed 20%.4,5 One option that has been explored to enhance the efficacy of the nicotine patch is to extend the duration of use beyond the standard 8 weeks. Although the results of individual studies have been inconsistent6 and the findings from meta-analyses have been inconclusive,4,5 the results from a large, randomized placebo-controlled clinical trial7 showed that extending the use of the nicotine patch from 8 to 24 weeks increased 6-month smoking cessation rates to 32%. Given the favorable safety profile of the nicotine patch and some evidence supporting the benefits of extended use, the US Food and Drug Administration changed the labeling for the nicotine patch to permit extended use, and public health organizations continue to advocate for additional changes concerning long-term use.8
However, few data on the potential benefits of use of the nicotine patch beyond 24 weeks are available. Joseph and colleagues9 found that extended treatment with nicotine gum, patches, or lozenges for 52 weeks significantly increased smoking cessation rates compared with 4 weeks of treatment. However, Hall and colleagues10 reported that extended treatment with nicotine gum for 40 weeks did not increase abstinence rates, and the previously mentioned placebo-controlled randomized clinical trial7 reported that, by 52 weeks, the benefit from 24 weeks compared with 8 weeks of treatment was no longer evident. The loss of long-term benefit from the extended duration of nicotine patch treatment may be attributable to the difficulty in maintaining adherence to the treatment regimen or the diminished need for the patch with the gradual fading of intense cravings over time.6 Although treatment with the nicotine patch beyond 24 weeks—akin to treating a chronic illness, such as hypertension—may provide a therapeutic benefit, a paucity of studies in this area makes it difficult to formulate clinical and policy recommendations.
This randomized clinical trial was designed to examine whether long-term (ie, 52-week) nicotine patch treatment increases smoking cessation rates when compared with standard (ie, 8-week) or extended (ie, 24-week) treatment. The present study design also allowed for an assessment of the reproducibility of the benefit found previously from 24 weeks of treatment7 but in an independent sample of treatment-seeking smokers. In addition, we examined the differences across treatments in reported adverse effects and adherence. Overall, the study was intended to provide critical evidence for establishing clinical and policy recommendations regarding the therapeutic benefits of long-term use of nicotine patches.
Methods
Study Design
Given the widespread and easy access to nicotine patches and the goal of enhancing the study generalizability, the trial design leaned toward an effectiveness design. We selected smokers from Philadelphia and Chicago, recruited through media advertisements and considered eligible based on results of an initial telephone screening and an in-person evaluation. Participants were selected at random for 8 (standard treatment), 24 (extended treatment), or 52 (maintenance treatment) weeks of nicotine patch therapy; all participants received 12 standardized, manual-based behavioral counseling sessions for smoking cessation in accordance with established treatment guidelines.4 The primary outcome was 7-day point prevalence abstinence, confirmed with breath levels of carbon monoxide (CO) at weeks 24 and 52. The institutional review boards at the University of Pennsylvania, Philadelphia, and Northwestern University, Chicago, Illinois, approved the study. The full study protocol can be found in the trial protocol in the Supplement.
Participants
Participants were recruited from June 22, 2009, through April 15, 2014. To be eligible, participants had to be 18 years or older, to smoke at least 10 cigarettes per day, and to be interested in smoking cessation. Individuals were excluded if they had a current medical problem for which transdermal nicotine therapy is contraindicated (eg, latex allergy), had a lifetime Diagnostic and Statistical Manual of Mental Disorders (Fourth Edition) diagnosis of psychotic or bipolar disorder,11 had current suicidality identified by the Mini-International Neuropsychiatric Interview,12 or were unable to communicate in English. Women were excluded if they were pregnant, planning a pregnancy, or lactating. Written informed consent was obtained from all study participants. The statistician (E.P.W.), independently of participants, provided a computerized randomization scheme, which was stratified by site and used permuted blocks of random-sized numbers.
Procedures
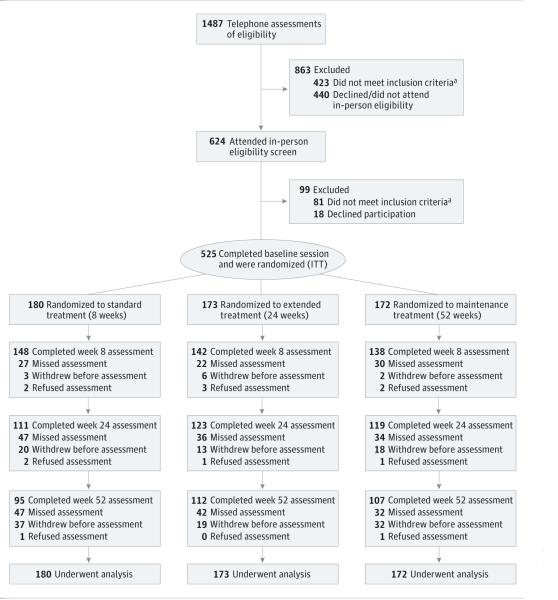
After an in-person visit to confirm eligibility, participants were randomized to 8, 24, or 52 weeks of therapy consisting of transdermal nicotine patches delivering a dose of 21 mg (Nicoderm CQ; GlaxoSmithKline) (Figure 1). All participants received 12 sessions of counseling consistent with guidelines from the US Public Health Service.4 Participants underwent in-person pre–smoking cessation counseling at baseline (2 weeks before week 0), which focused on preparing for cessation, and then set a smoking cessation date for week 0, at which time they were instructed to start using the patch. At weeks 0, 4, 8, 12, 16, 20, 24, 30, 36, 42, and 48, participants received telephone counseling that focused on managing urges and triggers to smoking and developing strategies to avoid relapse. Assessments (eg, of adverse effects) were conducted at baseline and at weeks 0, 4, 8, 12, 16, 20, 24, 30, 36, 42, 48, and 52 by telephone. Self-reports of smoking cessation (for the 7 days preceding the assessment) were biochemically confirmed using breath levels of CO at weeks 8, 24, and 52.
Figure 1. Clinical Trial FlowDiagram.
ITT indicates intention to treat.
a A list of the reasons for participant ineligibility can be found in the trial protocol in the Supplement.
Measures
At baseline, participants completed self-report measures of demographic (eg, age, race, sex) and smoking-related (eg, cigarettes per day, the Fagerström Test for Nicotine Dependence score) variables.13 A previously used checklist of nicotine patch–related adverse effects was administered.7,14 The occurrence and severity of adverse effects (eg, nausea, rash) were rated by participants (0 indicates none; 3, severe). For each point assessed, a total summary score was computed, as was the frequency of total severe adverse effects.
Daily patch use was assessed at each session using a timeline follow-back measure.15,16 Mean weekly patch use across the 3 treatment durations for each treatment arm and the number of counseling sessions completed were computed.
Self-reports of smoking were obtained using the timeline follow-back measure for smoking.15 At weeks 8, 24, and 52, participants were asked to provide a breath sample for biochemical verification of smoking status. As the primary outcome for this study, participants were considered abstinent at the assessment point if they self-reported abstinence for 7 days before the assessment and provided a breath sample with a CO level of no greater than 10 ppm.17 Participants who withdrew from the study, failed to provide a breath sample, or provided a breath sample with a CO level of greater than 10 ppm were considered smokers.
We also assessed secondary outcomes. Continuous abstinence, a stringent measure of abstinence, refers to self-reports of no smoking (even a puff) from the smoking cessation date to a follow-up assessment,16 whereas prolonged abstinence refers to sustained abstinence from the cessation date to a follow-up assessment, allowing for a grace period, which captures delayed treatment effects.18 In the present study, for prolonged abstinence, relapse was defined as 7 consecutive days of self-reported smoking from the cessation date to weeks 24 and 52 after a 2-week grace period, which may not be consistent with all past trials. Time to relapse was the duration (in days) from the cessation day until a relapse (7 consecutive days of self-reported smoking). We assessed the smoking rate (number of cigarettes per day) and number of abstinent days (24 hours).
Statistical Analysis
The proposed sample size was based on our expectation of at least a 10% difference in smoking cessation rates between the standard (20%) vs extended and maintenance (30%) treatment arms at week 24 and a 10% difference in smoking cessation rates between the standard and extended (16%) vs maintenance (26%) treatment arms at week 52 (power, 80%; α = .05). The sample was characterized in terms of demographic and smoking-related data (eg, race, level of nicotine dependence), and we assessed differences across treatment arms on baseline covariates using χ2 tests and analysis of variance. Following an intention-to-treat (ITT) principle, with missing abstinence data coded as smoker as in previous trials,7,9 abstinence data from all eligible randomized participants were included in the analyses. We conducted all analyses using commercially available software (SPSS, version 20.0 [IBM Corporation] and Stata, version 8 [StataCorp]). For the primary outcomes, logistic regression was used to compare 7-day point prevalence abstinence rates (confirmed by CO levels, ITT) for the standard vs extended and maintenance treatment arms at week 24 and for the maintenance vs standard and extended treatment arms at week 52. We also examined a multivariate model that compared standard vs extended treatment only on weeks 24 and 52 for 7-day point prevalence abstinence. These analyses were repeated for continuous and prolonged abstinence. Multivariate models included covariates (demographic or smoking-related variables related to abstinence or retention at P ≤ .10). We used the χ2 test to describe the treatment arms in terms of 7-day point prevalence (confirmed by CO level, ITT).
For secondary outcomes, time to relapse (in days) was examined using multivariate regression and compared the standard vs extended and maintenance treatment arms at week 24 and maintenance vs standard and extended treatment arms at week 52. All models included covariates. For smoking rate, zero-inflated negative binomial regression was used to test whether remaining in treatment improved the odds of being abstinent on a given day (reported odds ratio [OR]) and at the same time reduced the number of cigarettes smoked per day among those who were not abstinent (reported rate ratio). We used cluster correlation to adjust variances for the repeated measures. For adverse effects, we used analysis of variance to compare treatment arms in terms of the summary adverse effects measure at weeks 4, 12, and 30 and χ2 test to compare the treatment arms in terms of the frequency of severe adverse effects and individual adverse effects at these points. The analyses focused on these points because adverse effects typically peak at the start of treatment (week 4) and to assess differences after patch treatment ended for the standard (week 12) and extended (week 30) treatment arms. Adherence to the patch and counseling regimens was assessed across treatment arms.
Results
Sample Characteristics
Demographic and smoking characteristics for the ITT sample (N = 525) are shown in Table 1. Notably, African American participants constituted 48.2% of the sample (3.6% either refused or indicated another or multiple race); 17.9% of the sample had current or past major depression, and 5.9% had current substance abuse or dependence. Multivariate models included sex, sexual orientation, age, educational level, Fagerström Test for Nicotine Dependence score, and adherence to the patch regimen, which were associated with abstinence; and race and income, which were associated with retention. Study site, which was a stratification variable, was unrelated to abstinence or retention. Figure 1 describes the rate of retention across treatment arms. Although retention rates were slightly lower overall than expected, they were similar across treatment arms at all follow-ups (P > .05).
Table 1.
Participant Characteristics
| Treatment Arma |
||||
|---|---|---|---|---|
| Characteristic | Standard (n = 180) |
Extended (n = 173) |
Maintenance (n = 172) |
Total (N = 525) |
| Female sex | 50.0 | 54.9 | 47.1 | 50.7 |
|
| ||||
| Age, mean (SD), y | 45.9 (12.3) | 46.9 (12.2) | 46.4 (12.1) | 46.4 (12.1) |
|
| ||||
| White race | 48.9 | 48.0 | 48.3 | 48.4 |
|
| ||||
| Married | 68.3 | 69.4 | 69.8 | 69.1 |
|
| ||||
| Heterosexualb | 91.4 | 90.2 | 91.0 | 90.9 |
|
| ||||
| Educational level of GED or less | 28.9 | 30.6 | 33.7 | 31.0 |
|
| ||||
| Income ≤$50 000/yc | 72.6 | 70.9 | 74.5 | 72.7 |
|
| ||||
| FTND score, mean (SD)c,d | 5.3 (1.9) | 5.1 (2.1) | 5.0 (2.0) | 5.1 (2.0) |
|
| ||||
| Cigarettes smoked per day, mean (SD) | 16.9 (8.4) | 17.0 (8.8) | 17.4 (7.9) | 17.1 (8.4) |
|
| ||||
| Age at smoking initiation, mean (SD), y | 16.0 (5.2) | 16.2 (4.6) | 16.9 (5.4) | 16.4 (5.1) |
|
| ||||
| Duration of smoking, mean (SD), y | 28.6 (12.9) | 29.5 (12.7) | 28.4 (12.3) | 28.8 (12.6) |
|
| ||||
| Current/past major depression | 13.9 | 20.2 | 19.8 | 17.9 |
|
| ||||
| Current substance abuse/dependence | 4.8 | 6.8 | 6.3 | 5.9 |
Abbreviations: FTND, Fagerström Test for Nicotine Dependence; GED, General Educational Development test.
Unless otherwise indicated, data are expressed as percentage of participants. No significant differences were found in these variables across treatment arms
Indicates missing data for less than 10%.
Indicates missing data for less than 4%.
A score of 5 to 7 represents a medium to high level of nicotine dependence.
Abstinence Rates
At 24 weeks, 21.7% of participants in the standard treatment arm were abstinent compared with 27.2% of participants in the extended and maintenance treatment arms (χ 2 = 1.98; P = .17) (Figure 2). In the multivariate model of week 24, 7-day point prevalence abstinence (confirmed by CO level, ITT) controlled for covariates, participants continuing to receive treatment (extended and maintenance treatment arms) reported significantly greater abstinence rates compared with those in the standard treatment arm (OR, 1.70 [95% CI, 1.03-2.81]; P = .04) (Table 2). When multiple imputation was used instead of treating missing abstinence data as current smoking, this model did not yield significant results (P = .27). Using prolonged abstinence at week 24, we noted that participants remaining in treatment (extended and maintenance treatment arms) reported significantly greater abstinence rates compared with those in the standard treatment arm (38.3% vs 26.7%; OR, 2.15 [95% CI, 1.33-3.46]; P = .002). We found no effect of treatment arm on continuous abstinence (11.3% vs 9.4%; OR, 1.09 [95% CI, 0.59-2.00]; P = .79).
Figure 2. Seven-Day Point Prevalence Abstinence Rates by Treatment Arm and Assessment Point.
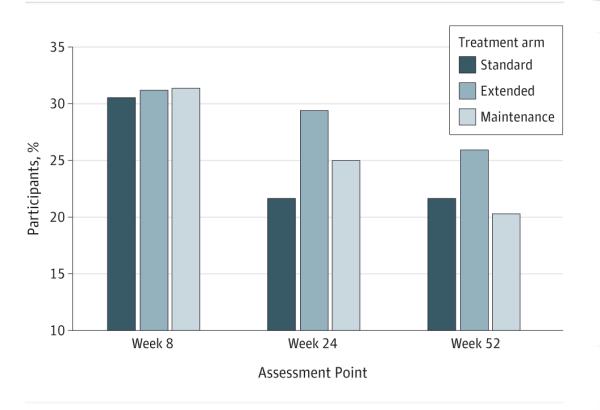
Abstinence was confirmed by breath levels of carbon monoxide (intention to treat). Standard treatment indicates 8 weeks of patch treatment (n = 180); extended treatment, 24 weeks of patch treatment (n = 173); and maintenance treatment, 52 weeks of patch treatment (n = 172). According to a multivariate model for standard and extended vs maintenance treatment arms at week 24, the odds ratio (OR) was 1.70 (95%CI, 1.03-2.81; P = .04). According to a multivariate model for standard and extended treatment vs maintenance treatment at week 52, the OR was 1.17 (95%CI, 0.69-1.98; P = .57).
Table 2.
Multivariate Logistic Regression Analysis of Smoking Abstinence at Weeks 24 and 52 by Treatment Arm, Controlling for Covariates
| Covariate | OR (95% CI) | P Value |
|---|---|---|
| Model 1 a | ||
|
| ||
| Sex (reference, female) | 0.71 (0.44-1.15) | .16 |
|
| ||
| Race (reference, African American) | 0.89 (0.53-1.47) | .64 |
|
| ||
| Adherence to patch regimen | 1.48 (1.29-1.71) | <.001 |
|
| ||
| Age | 1.02 (1.00-1.04) | .07 |
|
| ||
| Educational level (reference, ≥some college) |
0.71 (0.40-1.24) | .23 |
|
| ||
| Income (reference, ≥$50 000/y) | 0.85 (0.49-1.48) | .56 |
|
| ||
| Sexual orientation (reference, heterosexual) | 2.66 (1.26-5.61) | .01 |
|
| ||
| FTND score | 0.88 (0.78-0.99) | .04 |
|
| ||
| Treatment arm (reference, standard treatment) |
1.70 (1.03-2.81) | .04 |
|
| ||
| Model 2 b | ||
|
| ||
| Sex (reference, female) | 0.85 (0.52-1.39) | .53 |
|
| ||
| Race (reference, white) | 1.48 (0.87-2.53) | .15 |
|
| ||
| Adherence to patch regimen | 1.68 (1.41-2.01) | <.001 |
|
| ||
| Age | 1.02 (0.99-1.04) | .18 |
|
| ||
| Educational level (reference, ≥some college) |
0.49 (0.27-0.89) | .19 |
|
| ||
| Income (reference, ≥$50 000/y) | 0.67 (0.38-1.20) | .18 |
|
| ||
| Sexual orientation (reference, heterosexual) | 2.18 (1.01-4.73) | .05 |
|
| ||
| FTND score | 0.86 (0.76-0.98) | .02 |
|
| ||
| Treatment arm (reference, standard and extended treatment) |
1.17 (0.69-1.98) | .57 |
Abbreviations: FTND, Fagerström Test for Nicotine Dependence; OR, odds ratio.
Indicates week 24 abstinence by standard (8-week) treatment arm compared with extended (24-week) and maintenance (52-week) treatment arms.
Indicates week 52 abstinence by standard (8-week) and extended (24-week) treatment arms compared with maintenance (52-week) treatment arm.
Abstinence was confirmed by breath levels of carbon monoxide (intention to treat). Standard treatment indicates 8 weeks of patch treatment (n = 180); extended treatment, 24 weeks of patch treatment (n = 173); and maintenance treatment, 52 weeks of patch treatment (n = 172). According to a multivariate model for standard and extended vs maintenance treatment arms at week 24, the odds ratio (OR) was 1.70 (95% CI, 1.03-2.81; P = .04). According to a multivariate model for standard and extended treatment vs maintenance treatment at week 52, the OR was 1.17 (95% CI, 0.69-1.98; P = .57).
At 52 weeks, 23.8% of participants in the standard and extended treatment arms were abstinent compared with 20.3% of those in the maintenance treatment arm (χ 2 = 0.8; P = .44) (Figure 2). In a multivariate model of 7-day point prevalence abstinence at week 52 (confirmed by CO levels, ITT) controlled for covariates, participants in the maintenance treatment arm did not report significantly greater abstinence rates compared with those in the standard and extended treatment arms (23.8% vs 20.3%; OR, 1.17 [95% CI, 0.69-1.98]; P = .57) (Table 2). The results were similar for week 52 for prolonged (23.5% vs 24.4%; OR, 1.39 [95% CI, 0.83-2.36]; P = .21) and continuous (9.1% vs 8.1%; OR, 1.34 [95% CI, 0.65-2.78]; P = .43) abstinence.
As secondary analysis, 7-day point prevalence abstinence among participants in the extended treatment arm only was greater at week 24 (29.5%) compared with those in the standard treatment arm controlled for covariates (21.7%; OR, 1.90 [95% CI, 1.06-3.40]; P = .03). Prolonged abstinence was also greater for the extended (40.5%) vs standard (26.7%; OR, 1.85 [95% CI, 1.1-3.1]; P = .02) treatment arms controlled for covariates, but rates for continuous abstinence were similar (11.6% vs 11.7%). Comparisons at week 52 were not significant for 7-day point prevalence (26.0% vs 21.7%; OR, 1.33 [95% CI, 0.72-2.45]; P = .36), continuous abstinence (9.8% vs 8.3%; P = .71), or prolonged abstinence controlled for covariates (27.2% vs 20.0%; OR, 1.41 [95% CI, 0.79-2.51]; P = .25).
Secondary Outcomes
Participants in the standard treatment arm showed significantly fewer days until relapse (mean [SD], 72 [62.6] days) compared with those in the extended and maintenance treatment arms (mean [SD], 89 [66.5] days) (β = 21.30 [95% CI, 10.30-32.25]; P < .001). In contrast, assessment of the number of days until relapse to week 52 showed that participants in the standard or extended treatment arm (mean [SD], 134.1 [141.6] days) reported similar time until relapse compared with those in the maintenance treatment arm (mean [SD], 146.7 [145.1] days). Last, participants in the extended and maintenance treatment arms reported abstinence on more days (weeks 9-24) (mean [SD], 80.5 [38.1] days) than those in the standard treatment arm (mean [SD], 68.2 [43.7] days) (OR, 1.55 [95% CI, 1.06-2.26]; P = .02) and reported smoking fewer cigarettes per day on smoking days (mean [SD], 5.8 [5.3] vs 6.4 [5.1] cigarettes per day; β = 0.43 [95% CI, 0.06-0.82]; P = .02). During weeks 25 through 52, participants in the maintenance treatment arm reported smoking fewer cigarettes per day on smoking days (mean [SD], 5.4 [4.6] cigarettes per day) compared with those in the standard and extended treatment arms (mean [SD], 7.5 [6.3] cigarettes per day) (incidence rate ratio, 0.71 [95% CI, 0.54-0.93]; P = .01), but no significant difference in abstinent days between groups was found.
Adherence and Adverse Effects
Participants in the maintenance treatment arm used significantly fewer patches per week (mean [SD], 3.94 [2.5]) than did participants in the standard (mean [SD], 4.61 [2.0]) and extended (mean [ SD], 4. 7 [2.4 ]) treatment arms (F2,522 = 6.03; P = .003). When we defined adherence as wearing the patch for at least 6 days per week,7 participants in the maintenance treatment arm showed the lowest rate of adherence (32.0%) compared with those in the standard (38.3%) and extended (47.4%) treatment arms (χ 2 = 8.71; P = .01). For participants in the maintenance treatment arm, the mean number of days per week of nicotine patch use significantly diminished from weeks 24 to 52 (mean [SD], 4.0 [3.4] d/wk) compared with weeks 1 through 24 (mean [SD], 4.5 [2.4] d/wk) (F1,171 = 8.19; P = .005), and we found no significant difference in weekly patch use from weeks 1 through 24 between participants in the extended and maintenance treatment arms (P = .41). Adherence to nicotine patch regimens increased the likelihood for cessation at weeks 24 and 52 overall (P < .05) but did not interact with or mediate treatment arm effects on outcomes at week 24 or week 52 (P > .05). Participants in the extended treatment arm completed more counseling sessions (mean [SD], 9.3 [3.6] sessions) than those in the standard (mean [SD], 8.5 [3.6] sessions) and maintenance (mean [SD], 8.7 [3.6] sessions) treatment arms, but the comparison was not statistically significant (P = .12).
We found no significant differences among the treatment arms in terms of the summary of adverse effects or the frequency of severe adverse effects at weeks 4, 12, and 30 (Table 3; P > .05). For individual adverse effects, nausea increased in the extended treatment arm (χ2 = 7.12; P = .03) and diarrhea increased in the maintenance treatment arm (χ 2 = 6.92; P = .03) at week 4. Serious adverse events (ie, self-reported events resulting in disability/incapacity or death) were determined by site physicians (Table 3). We found 4 (2.2%), 2 (1.2%), and 8 (4.7%) serious adverse events in the standard, extended, and maintenance treatment arms, respectively (P > .05).
Table 3.
Adverse Effects and Serious Adverse Events by Treatment Arm
| Treatment Arma |
|||
|---|---|---|---|
| Variable | Standard (8 wk) |
Extended (24 wk) |
Maintenance (52 wk) |
| No. of serious adverse eventsb | |||
|
| |||
| Weeks 1-8 | 1 | 0 | 3 |
|
| |||
| Weeks 9-24 | 0 | 1 | 3 |
|
| |||
| Weeks 25-52 | 3 | 1 | 2 |
|
| |||
| Week 4 Adverse Effects | |||
|
| |||
| No. of participants | 165 | 148 | 146 |
|
| |||
| Summary score, mean (SD)c | 5.3 (6.0) | 4.5 (5.1) | 5.0 (4.9) |
|
| |||
| Frequency of severe adverse effects | 21 (12.7) | 22 (14.9) | 16 (11.0) |
|
| |||
| Headached | 2 (1.2) | 5 (3.4) | 0 |
|
| |||
| Nausea | 2 (1.2) | 1 (0.7) | 3 (2.1) |
|
| |||
| Coughing | 4 (2.4) | 2 (1.4) | 4 (2.7) |
|
| |||
| Pounding heart | 0 | 0 | 2 (1.4) |
|
| |||
| Diarrhead | 0 | 0 | 3 (2.1) |
|
| |||
| Insomnia | 7 (4.2) | 7 (4.7) | 4 (2.7) |
|
| |||
| Skin redness | 5 (3.0) | 5 (3.4) | 3 (2.1) |
|
| |||
| Dizziness | 0 | 1 (0.7) | 2 (1.4) |
|
| |||
| Light-headedness | 2 (1.2) | 1 (0.7) | 2 (1.4) |
|
| |||
| Sweating | 9 (5.5) | 4 (2.7) | 2 (1.4) |
|
| |||
| Watery eyes | 0 | 2 (1.4) | 0 |
|
| |||
| Coldness in hands or feet | 2 (1.2) | 0 | 0 |
|
| |||
| Disturbing dreams | 5 (3.0) | 5 (3.4) | 3 (2.1) |
|
| |||
| Vomiting | 1 (0.6) | 0 | 1 (0.7) |
|
| |||
| Shortness of breath | 3 (1.8) | 1 (0.7) | 1 (0.7) |
|
| |||
| Rapid heart beat | 1 (0.6) | 0 | 2 (1.4) |
|
| |||
| Week 12 Adverse Effects | |||
|
| |||
| No. of participants | 128 | 137 | 121 |
|
| |||
| Summary score, mean (SD)c | 4.1 (5.1) | 3.6 (4.7) | 3.9 (4.4) |
|
| |||
| Frequency of severe adverse effects | 13 (10.2) | 9 (6.6) | 11 (9.1) |
|
| |||
| Headache | 1 (0.8) | 0 | 3 (2.5) |
|
| |||
| Nausea | 1 (0.8) | 0 | 1 (0.8) |
|
| |||
| Coughing | 4 (3.1) | 2 (1.5) | 2 (1.7) |
|
| |||
| Pounding heart | 0 | 1 (0.7) | 1 (0.8) |
|
| |||
| Diarrhea | 2 (1.6) | 0 | 0 |
|
| |||
| Insomnia | 6 (4.7) | 4 (2.9) | 4 (3.3) |
|
| |||
| Skin redness | 1 (0.8) | 1 (0.7) | 0 |
|
| |||
| Dizziness | 0 | 0 | 1 (0.8) |
|
| |||
| Light-headedness | 0 | 0 | 0 |
|
| |||
| Sweating | 1 (0.8) | 0 | 0 |
|
| |||
| Watery eyes | 2 (1.6) | 2 (1.5) | 1 (0.8) |
|
| |||
| Coldness in hands or feet | 1 (0.8) | 0 | 1 (0.8) |
|
| |||
| Disturbing dreams | 1 (0.8) | 1 (0.7) | 4 (3.3) |
|
| |||
| Vomiting | 1 (0.8) | 0 | 0 |
|
| |||
| Shortness of breath | 0 | 1 (0.7) | 1 (0.7) |
|
| |||
| Rapid heart beat | 0 | 0 | 2 (1.7) |
|
| |||
| Week 30 Adverse Effects | |||
|
| |||
| No. of participants | 103 | 116 | 103 |
|
| |||
| Summary score, mean (SD)c | 3.7 (5.5) | 2.7 (4.1) | 3.3 (3.8) |
|
| |||
| Frequency of severe adverse effects | 5 (4.9) | 3 (2.6) | 7 (6.8) |
|
| |||
| Headache | 1 (1.0) | 0 | 0 |
|
| |||
| Nausea | 0 | 0 | 1 (1.0) |
|
| |||
| Coughing | 1 (1.0) | 1 (0.9) | 0 |
|
| |||
| Pounding heart | 2 (1.9) | 1 (0.9) | 1 (1.0) |
|
| |||
| Diarrhea | 0 | 0 | 1 (1.0) |
|
| |||
| Insomnia | 2 (1.9) | 2 (1.7) | 0 |
|
| |||
| Skin redness | 0 | 0 | 2 (1.9) |
|
| |||
| Dizziness | 0 | 0 | 0 |
|
| |||
| Light-headedness | 0 | 0 | 0 |
|
| |||
| Sweating | 1 (1.0) | 1 (0.9) | 0 |
|
| |||
| Watery eyes | 2 (1.9) | 1 (0.9) | 2 (1.9) |
|
| |||
| Coldness in hands or feet | 1 (1.0) | 0 | 0 |
|
| |||
| Disturbing dreams | 0 | 1 (0.9) | 1 (0.9) |
|
| |||
| Vomiting | 0 | 0 | 0 |
|
| |||
| Shortness of breath | 1 (1.0) | 0 | 0 |
|
| |||
| Rapid heart beat | 1 (1.0) | 1 (0.9) | 0 |
Unless otherwise indicated, data are expressed as number (percentage) of participants.
Self-reported by participants during the trial (standard treatment arm: cancer, kidney stones, pneumonia, and death [withdrawn]; extended treatment arm: cysts and cancer; maintenance treatment arm: infection, kidney disease, pericarditis, hypertension [withdrawn], chronic obstructive pulmonary disease, dehydration, abdominal pain, and death [withdrawn]).
Indicates the mean total score on all adverse effect checklist items; individual adverse effects are noted only if considered severe.
Comparison across treatment arm is significant (P < .05).
Discussion
To help determine the therapeutic benefit of long-term treatment with nicotine patches, this study evaluated whether treating smokers with nicotine patches beyond 24 weeks increases the likelihood of abstinence. Overall, the results replicate a previous finding7—that providing treatment-seeking smokers with 24 weeks of nicotine patches compared with 8 weeks increases the likelihood that they will be abstinent at 24 weeks—and show that treatment to 52 weeks, although safe, yields no additional therapeutic benefit. These findings and their clinical and policy implications are discussed below.
First, this study confirmed the results from the previous trial,7 providing support for the therapeutic benefit of 24 weeks of transdermal nicotine therapy compared with 8 weeks (including a model comparing extended with standard therapy only). This result was found for point prevalence and prolonged abstinence, but not for continuous abstinence, which is a more conservative indicator of cessation because it does not capture delayed effects of treatment. However, as in the first trial, smoking cessation rates at week 52 were not significantly higher for the extended vs standard treatment arms. These results are based on a tightly controlled efficacy trial and now a more effectiveness-leaning clinical trial. In fact, the 24-week ITT abstinence rates for 8 vs 24 weeks of treatment across the 2 trials are very similar, indicating that 6-month smoking cessation rates can be increased significantly if smokers continue to use nicotine patches for 24 weeks vs 8 weeks. Furthermore, as was seen in the initial trial,7 24 weeks of treatment does not increase adverse effects compared with 8 weeks of treatment. Last, several other indicators of treatment response suggest that 24 weeks of treatment enhances outcomes compared with 8 weeks, including increased prolonged abstinence (although prolonged abstinence may be defined differently in this trial), a shorter time to relapse, a reduced smoking rate (although the clinical benefits of reduced smoking remain equivocal), and more abstinent days. Together these findings further support the policy change of the US Food and Drug Administration and individual clinical recommendations allowing smokers to use nicotine patches beyond the standard 8 weeks to increase the potential for smoking cessation.8
We did not see an additional therapeutic advantage from continuing nicotine patch therapy beyond 24 weeks. Across all measures of abstinence, even including secondary outcome measures, we found no indication that nicotine patch use for 52 weeks increases therapeutic benefits beyond 24 weeks. This result suggests that treating nicotine dependence among the general population of treatment-seeking smokers with long-term use of nicotine patches—akin to how hypertension is treated—requires additional study. This result is consistent with the findings of a study of extended nicotine gum treatment.10 One possible explanation for this result is that smokers have difficulty adhering to a patch treatment regimen for such a long period. We found that participants receiving maintenance therapy reported significantly lower adherence compared with those receiving standard and extended therapy, which worsened after week 24. Although we did not detect any relationship between adherence and adverse effects, factors not assessed herein (eg, psychological effects of access to nicotine patches for 52 weeks) may be important to examine as drivers of low adherence to a patch therapy regimen. Alternatively, only subgroups of smokers may benefit from long-term treatment (eg, those with high levels of dependence), and future studies should explore smoker characteristics that may predict better therapeutic response to long-term patch treatment. These findings are in contrast to the therapeutic benefit of 48 weeks of nicotine replacement therapy reported previously,9 although differences between the studies include the treatment provided (nicotine patches vs any nicotine replacement therapy), design (8, 24, and 52 weeks of treatment vs 4 and 52 weeks of treatment), and sample demographics (eg, 48.2% African American vs 3.6%). Thus, although long-term patch use (even to 52 weeks) appears safe, clinical or policy recommendations for use of nicotine patches beyond 24 weeks to increase abstinence rates may require additional study.
Study limitations should be considered. First, the lack of a placebo and the limited inclusion and exclusion criteria may have reduced internal validity. Participants were aware of their treatment arm, and past studies19,20 have found that expectations about treatment arm assignment can affect outcomes. Future studies should consider alternative designs, including differential randomization ratios at the assessment points or adaptive/sequential multiple assignment randomized trial designs. Likewise, the inclusion of smokers with comorbid psychiatric conditions may reduce the comparability of the present findings to past studies. Second, adherence to the nicotine patch therapy regimen was low, particularly among the participants in the maintenance treatment arm, among whom adherence worsened over time. This finding is not unique to this study,7 but it highlights the need for the inclusion of treatment to improve adherence.21 Third, nicotine patches are not the most effective pharmacotherapy for smoking cessation. Future studies should examine the possibility that long-term treatment with varenicline tartrate or combination nicotine replacement therapy may yield divergent results. Finally, as in many trials, attrition was an issue, although the retention rates were similar across treatment arms. Although we used an ITT approach, this method is conservative, which can reduce the potential detection of treatment effects.
Conclusions
Despite these limitations, the present study provides additional support for the benefits and safety of 24 weeks of nicotine patch therapy for promoting smoking cessation. From a randomized placebo-controlled clinical trial7 and an effectiveness-leaning trial (present study), we have evidence that 6-month smoking cessation rates can be increased significantly with 24 weeks compared with 8 weeks of nicotine patch use. The cost-effectiveness of 24 weeks of treatment, compared with 8 weeks, has been demonstrated previously as well.7 However, perhaps because of difficulty with long-term adherence to the patch regimen, a maintenance approach to treating nicotine dependence (defined as 52 weeks) provides no additional therapeutic benefit compared with 24 weeks of treatment. Future studies should evaluate additional interventions to address treatment adherence or explore long-term use of more effective medications. Individual clinical and population-based policy recommendations should continue to advise that long-term use of nicotine patches (even to 52 weeks) is safe. However, additional studies are needed to assess the therapeutic benefits of the nicotine patch beyond 24 weeks to facilitate additional clinical and policy recommendations.
Supplementary Material
Acknowledgments
Funding/Support: This study was supported by grants R01 DA025078 and R01 DA033681 from the National Institute on Drug Abuse and grants R01 CA165001 and P50 CA143187 from the National Cancer Institute.
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Schnoll had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schnoll, Leone, Hitsman. Acquisition, analysis, or interpretation of data: All authors.
Drafting of the manuscript: Schnoll, Goelz, Gariti, Wileyto, Hitsman.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: Schnoll, Wileyto. Obtained funding: Schnoll, Hitsman. Administrative, technical, or material support: Goelz, Veluz-Wilkins, Blazekovic, Powers, Leone, Gariti.
Study supervision: Goelz, Veluz-Wilkins, Gariti, Hitsman.
Conflict of Interest Disclosures: Drs Schnoll and Hitsman report receiving varenicline (Chantix) and placebo free of charge from Pfizer for use in ongoing National Institutes of Health–supported clinical trials. Dr Schnoll also reports having provided consultation to Pfizer and GlaxoSmithKline. No other disclosures were reported.
Supplemental content at jamainternalmedicine.com
Contributor Information
Robert A. Schnoll, Department of Psychiatry, University of Pennsylvania, Philadelphia.
Patricia M. Goelz, National Comprehensive Cancer Network, Ft Washington, Pennsylvania.
Anna Veluz-Wilkins, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Sonja Blazekovic, Department of Psychiatry, University of Pennsylvania, Philadelphia.
Lindsay Powers, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
Frank T. Leone, Pulmonary, Allergy, and Critical Care Division, University of Pennsylvania Presbyterian Medical Center, Philadelphia.
Peter Gariti, Department of Psychiatry, University of Pennsylvania, Philadelphia.
E. Paul Wileyto, Department of Biostatistics and Epidemiology, University of Pennsylvania School of Medicine, Philadelphia.
Brian Hitsman, Department of Preventive Medicine, Feinberg School of Medicine, Northwestern University, Chicago, Illinois.
REFERENCES
- 1.Fix BV, Hyland A, Rivard C, et al. Usage patterns of stop smoking medications in Australia, Canada, the United Kingdom, and the United States: findings from the 2006-2008 International Tobacco Control (ITC) Four Country Survey. Int J Environ Res Public Health. 2011;8(1):222–233. doi: 10.3390/ijerph8010222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Kasza KA, Hyland AJ, Borland R, et al. Effectiveness of stop-smoking medications: findings from the International Tobacco Control (ITC) Four Country Survey. Addiction. 2013;108(1):193–202. doi: 10.1111/j.1360-0443.2012.04009.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Shiffman S, Brockwell SE, Pillitteri JL, Gitchell JG. Use of smoking-cessation treatments in the United States. Am J Prev Med. 2008;34(2):102–111. doi: 10.1016/j.amepre.2007.09.033. [DOI] [PubMed] [Google Scholar]
- 4.Fiore MC, Jaen CR, Baker TB, et al. Treating Tobacco Use and Dependence: 2008 Update. US Public Health Service; Rockville, MD: 2008. US Public Health Service Clinical Practice Guideline. [Google Scholar]
- 5.Stead LF, Perera R, Bullen C, et al. Nicotine replacement therapy for smoking cessation. Cochrane Database Syst Rev. 2012;11:CD000146. doi: 10.1002/14651858.CD000146.pub4. [DOI] [PubMed] [Google Scholar]
- 6.Carpenter MJ, Jardin BF, Burris JL, et al. Clinical strategies to enhance the efficacy of nicotine replacement therapy for smoking cessation: a review of the literature. Drugs. 2013;73(5):407–426. doi: 10.1007/s40265-013-0038-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schnoll RA, Patterson F, Wileyto EP, et al. Effectiveness of extended-duration transdermal nicotine therapy: a randomized trial. Ann Intern Med. 2010;152(3):144–151. doi: 10.7326/0003-4819-152-3-201002020-00005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Fucito LM, Bars MP, Forray A, et al. Addressing the evidence for FDA nicotine replacement therapy label changes: a policy statement of the Association for the Treatment of Tobacco Use and Dependence and the Society for Research on Nicotine and Tobacco. Nicotine Tob Res. 2014;16(7):909–914. doi: 10.1093/ntr/ntu087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Joseph AM, Fu SS, Lindgren B, et al. Chronic disease management for tobacco dependence: a randomized, controlled trial. Arch Intern Med. 2011;171(21):1894–1900. doi: 10.1001/archinternmed.2011.500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hall SM, Humfleet GL, Muñoz RF, Reus VI, Robbins JA, Prochaska JJ. Extended treatment of older cigarette smokers. Addiction. 2009;104(6):1043–1052. doi: 10.1111/j.1360-0443.2009.02548.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.American Psychiatric Association . Diagnostic and Statistical Manual of Mental Disorders. 4 American Psychiatric Association; Washington, DC: 1994. [Google Scholar]
- 12.Sheehan DV, Lecrubier Y, Sheehan KH, et al. The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry. 1998;59(suppl 20):22–33. [PubMed] [Google Scholar]
- 13.Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86(9):1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 14.Schnoll RA, Patterson F, Wileyto EP, Tyndale RF, Benowitz N, Lerman C. Nicotine metabolic rate predicts successful smoking cessation with transdermal nicotine: a validation study. Pharmacol Biochem Behav. 2009;92(1):6–11. doi: 10.1016/j.pbb.2008.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown R, Burgess E, Sales S, Whiteley J. Reliability and validity of a smoking timeline follow-back interview. Psychol Addict Behav. 1998;12(2):101–112. [Google Scholar]
- 16.Goelz PM, Audrain-McGovern JE, Hitsman B, et al. The association between changes in alternative reinforcers and short-term smoking cessation. Drug Alcohol Depend. 2014;138:67–74. doi: 10.1016/j.drugalcdep.2014.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.SRNT Subcommittee on Biochemical Verification Biochemical verification of tobacco use and cessation. Nicotine Tob Res. 2002;4(2):149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- 18.Hughes JR, Keely JP, Niaura RS, Ossip-Klein DJ, Richmond RL, Swan GE. Measures of abstinence in clinical trials: issues and recommendations. Nicotine Tob Res. 2003;5(1):13–25. [PubMed] [Google Scholar]
- 19.Mooney M, White T, Hatsukami D. The blind spot in the nicotine replacement therapy literature: assessment of the double-blind in clinical trials. Addict Behav. 2004;29(4):673–684. doi: 10.1016/j.addbeh.2004.02.010. [DOI] [PubMed] [Google Scholar]
- 20.Schnoll RA, Epstein L, Audrain J, et al. Can the blind see? participant guess about treatment arm assignment may influence outcome in a clinical trial of bupropion for smoking cessation. J Subst Abuse Treat. 2008;34(2):234–241. doi: 10.1016/j.jsat.2007.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shiffman S, Sweeney CT, Ferguson SG, Sembower MA, Gitchell JG. Relationship between adherence to daily nicotine patch use and treatment efficacy: secondary analysis of a 10-week randomized, double-blind, placebo-controlled clinical trial simulating over-the-counter use in adult smokers. Clin Ther. 2008;30(10):1852–1858. doi: 10.1016/j.clinthera.2008.09.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.