Abstract
In a sample of post-menopausal premutation carrier mothers of children with the full mutation of fragile X syndrome (n = 88), this study examined the co-occurrence of the reproductive and psychiatric phenotypes associated with FMR1 premutations. Mean age at menopause was 43.1 years, and 35.2% of premutation carriers reported cessation of menses prior to age 40 (premature ovarian failure), but only 18% of carriers had been medically diagnosed by a physician as having Fragile X-associated Primary Ovarian Insufficiency. There was a significant curvilinear association between CGG repeat length and age at menopause, with women who had mid-range repeats having the earliest menopause, similar to the pattern that has been found for the psychiatric phenotype of the FMR1 premutation.
Keywords: anxiety, depression, FXPOI, FMR1 CGG repeats, menopause, premature ovarian failure, premutation
Introduction
Fragile X syndrome (FXS) is the most prevalent inherited cause of intellectual disability (Hagerman et al., 2009). This neurodevelopmental disorder is caused by an expansion to more than 200 repeats of the CGG sequence of nucleotides comprising the 5′ untranslated region of the fragile X mental retardation gene (FMR1) located on the X cromosome (Brown, 2002). Although some mothers of children with FXS themselves have the full mutation of FXS, it is more common for mothers to have the premutation of the FMR1 gene (defined as 55 to 200 CGG repeats). Recent population prevalence estimates indicate that 1 in approximately 150 females and 1 in 350 to 600 males are carriers of the premutation of FMR1 (Maenner et al., 2013; Seltzer et al., 2012a; Tassone et al., 2012).
Premutation carriers are at risk for two aging-related syndromes: fragile X-associated tremor ataxia syndrome (FXTAS) and fragile X-associated primary ovarian insufficiency (FXPOI) (Hagerman and Hagerman, 2004). FXTAS is a neurological disorder that includes progressive action tremor and gait ataxia, with associated features of parkinsonism, peripheral neuropathy, and cognitive decline. Although FXTAS is more prevalent in male premutation carriers than in females, approximately 10% of women with FMR1 CGG expansions over the age of 50 develop FXTAS. In women, FXTAS is associated with less severe disease, less cognitive decline, and some symptoms different from that of men, including higher rates of depression and obsessive thinking (Leehey, 2009). FXPOI includes absent or irregular periods, symptoms of menopause such as hot flashes, early menopause, and infertility, and affects 20-25% of FMR1 premutation carriers. Other reports have suggested increased risk of depression and anxiety in premutation carriers (Bailey et al., 2008; Roberts et al., 2009), particularly in the context of parenting stress and stressful life events (Hartley et al., 2011; Seltzer et al., 2012b). All of these symptoms associated with the premutation have incomplete penetrance, affecting only a sub-set of carriers.
Past research has examined whether these symptoms are related to the number of CGG repeats in the FMR1 gene. For some symptoms, such as FXTAS, the association of CGG repeats and the associated condition is linear, where the larger the number of repeats, the greater the risk of the syndrome (Hessl et al., 2005; Greco et al., 2006; Grigsby et al., 2006) and earlier the age of onset (Tassone et al., 2007). However, for other symptoms, the association is curvilinear. In previous research using the same sample as the current study, we found that premutation carrier mothers with mid-range CGG repeats had elevated symptoms of depression and anxiety, and had a greater vulnerability to stressful life events, than those with either a smaller or larger number of repeats (Seltzer et al., 2012b). However, not all of the carriers in our sample manifested elevated depression or anxiety; only about one-quarter of premutation carrier women were above the clinical cut-off for depression, fewer than 20% were above the clinical cut-off for anxiety, and these were most likely to have 90-110 CGG repeats. This heightened psychiatric vulnerability among those with mid-range CGG repeats was first suggested by Roberts et al. (2009) and most recently by Loesch et al. (2014), adding to the growing understanding of the non-linear effect of premutation-range CGG repeat number on psychiatric symptoms.
The literature on FXPOI suggests that it, too, manifests a curvilinear association with CGG repeat number. In the first report of this association, Sherman and colleagues provided preliminary evidence that the association between CGG repeat length and ovarian dysfunction might be non-linear, with those women with between 80 and 99 CGG repeats most vulnerable to early menopause (Sullivan et al., 2005). This non-linear association was confirmed in a later report by the same group with a larger sample including cases from the earlier study (Allen et al., 2007), and it concluded that women with mid-range numbers of CGG repeats (80 to100) had a higher risk of altered cycle traits, subfertility, and dizygotic twinning than those with a smaller or a larger number of CGG repeats within the premutation range. Further, two independent samples (Ennis et al, 2006; Tejada et al 2008) also showed a non-linear association between age at menopause and CGG repeat length such that premutations in the mid-range have increased severity of this aspect of the phenotype.
More recently, the data from the Sherman group’s Atlanta studies were combined with those from a cohort drawn from Nijmegen, the Netherlands (Spath et al., 2011). The Nijmegen sample, ascertained through the Radboud University Medical Center, was drawn from all families in which a proband was diagnosed with FXS between 1984 and 2008. All female relatives of these probands age 35 and older who had experienced natural menopause (i.e., not due to surgery, chemotherapy, radiation, etc.) were included in the Spath et al. study. Women with between 65 and 90 CGG repeats were found to be at heightened risk of early menopause, a lower risk range than in previous studies. There thus is a need to extend this work in additional independent samples to better define the repeat range of the high risk premutation group. This is one focus of the present research, which is based on data analyzed from a distinct US sample of post-menopausal premutation carriers.
Molecular mechanisms for the curvilinear association of the reproductive phenotype and CGG repeat length are not fully understood, but premutation carriers are known to have elevated levels of mRNA, which may be toxic (Berry-Kravis and Hall, 2011) and which have been advanced as explanations for increased risk of early menopause in those with midsize CGG repeats (Ennis et al., 2006).
Other research has examined how activation ratio (the percent of cells where the active X chromosome carries the normal repeat allele) may influence ovarian function, age at menopause, and symptoms of the psychiatric phenotype of the FMR1 premutation. Previous studies have reported that activation ratio is not associated with reproductive function (Bione et al., 2006; Murray, 2000; Rodriguez-Ravenga et al., 2009; Spath et al., 2011; Sullivan et al., 2005; Tejada et al. 2008). One additional purpose of the present study is to examine the contribution of the activation ratio to the prediction of age at menopause in premutation carrier mothers of children with the full mutation of fragile X syndrome.
The prevalence of premature ovarian failure (POF), or cessation of menses prior to age 40 years, among premutation carriers is approximately 16%, as compared to 1% in the general population (Sherman et al., 2007). Apart from POF, premutation carrier women are at increased risk of earlier menopause than women in the general population. In previous research in a different sample, we found that premutation carrier women (n = 20, most with small numbers of CGG repeats) had a significantly earlier age at menopause than controls (48.1 vs. 50.8 years, p < .05; Seltzer et al., 2012a). Another study divided its sample of carriers into three groups based on CGG repeat length, and reported that the age of menopause averaged 48.5 for those with low CGG repeats, 44.9 for those with mid-range repeats, and 47.5 for those with a high number of CGG repeats (Allen et al., 2007). In contrast, non-carriers had substantially later ages of menopause (i.e., 52.3 years). Early menopause has significance for women’s health, including an increased risk of overall mortality, cardiovascular diseases, neurological diseases, psychiatric diseases, and osteoporosis (Shuster et al., 2010).
The present study aims to extend the investigation of the association between CGG repeat length, activation ratio, and age at menopause in post-menopausal premutation carrier mothers of adolescents and adults with the full mutation of FXS.
Materials and Methods
Participants
The participants in the present study were selected from a larger sample of families of individuals with the full mutation of FXS (n = 147). This is a national sample consisting of families residing in 38 states. In the majority of these families (n = 136), the mothers themselves had the premutation of the FMR1 gene (55 to 200 CGG repeat lengths). Participants were recruited as part of a three-wave (referred to as Times 1, 2, and 3) longitudinal study of family adaptation to FXS with a focus on families of adolescents and adults with FXS (Mailick et al., in press). Each time of data collection followed the previous one by approximately 18 months. Study participation required that mothers be the biological parent of a son or daughter with the FMR1 full mutation, that the child be 12 years of age or older, and that he or she live in the parental home or have a least weekly contact with the mother either in person or by phone.
At Time 2 of the study, 121 premutation carrier mothers continued to participate in the study and they provided data on their reproductive functioning. These questions were based on the Reproductive History Questionnaire developed by Sherman and colleagues (Sullivan et al., 2005). The questions were also asked at Time 3, allowing us to capture data from additional mothers who had experienced menopause by that time.
Inclusion in the present analysis was restricted to those members of the sample who were post-menopausal. Premutation carrier mothers who did not have a menstrual period for at least the past year reported whether they ceased cycling due to factors such as a hysterectomy, chemotherapy, radiation, pregnancy or breastfeeding, severe weight loss/eating disorder, hormone medication, or natural menopause. Of the participants at Times 2 and 3, nine did not provide complete responses to the reproductive questions and were dropped from the present analysis, and another 24 were excluded either because they reported still having a menstrual period during the previous year or because they ceased cycling due to one of the factors listed above other than natural menopause. The other 88 premutation carrier mothers reported that they did not have a menstrual period during the year prior to data collection and were included in the present study.
The majority of premutation carrier mothers included in the present analysis were European American (98%), currently married (82%), had completed at least an associate’s degree (87.5%), and were currently employed (70%). Their mean age was 53.6 years (SD = 7.4, range = 39.1 to 72.3 years of age), and they had an average of 2.6 children (SD = 1.2, range = 1 to 6 children) including 1.47 children with FXS (SD = .71, range = 1 to 4 children with FXS). The target children with FXS were 23.5 years of age on average (SD = 7.2, range = 14.1 to 45.8 years of age); most were male (87%), had intellectual disability (89%), and lived in the family home (85%).
Procedure
Premutation carrier mothers provided data through self-administered mail-back questionnaires and telephone interviews that typically lasted one hour. In order to participate, mothers were required to provide medical records confirming that their child had FXS. Mothers also were asked to provide a blood sample to determine their CGG repeat length and activation ratio. They were instructed to take a pre-assembled blood draw kit to a convenient location (e.g., local hospital, their doctor’s office) to have their blood drawn. The kit included instructions for the medical professional drawing the blood and a pre-paid courier container for shipping the sample to Kimball Genetics, Inc., the laboratory that assayed the samples for CGG repeat length and activation ratio. Mothers who had difficulty finding a physician or clinic in their area willing to draw the blood, or mothers who were reluctant to provide a blood sample, had the option of providing medical records indicating their CGG repeat length.
The Institutional Review Board at the University of Wisconsin-Madison approved the data collection protocol and written consent was obtained from all participants.
Measures
Age at menopause was calculated based on mother’s birth year and the self-reported year of last menstrual period, based on the Reproductive History Questionnaire (Sullivan et al., 2005). Two mothers moved in and out of menopause, as is occasionally characteristic of premutation carrier women (Wittenberger et al., 2007); for example, they experienced a year or more with no menstrual period, followed by the resumption of the menstrual cycle, followed by at least another year with no menstrual period. For our analyses, we used the age when the mother first experienced a year with no menstrual period. However, we also conducted the analyses using the age when the mother most recently experienced a year with no menstrual period and the findings were virtually identical (data available from first author). The mean age at menopause based on the earliest year when a mother did not report a menstrual period was 43.1 (s.d. = 6.1), and the age at menopause using the most recent year when the mother did not report a menstrual period was 43.5 (s.d. = 6.1). The correlation between two measures of age at menopause was 0.99.
CGG repeat length
Although all 88 mothers provided evidence from their medical record that they were premutation carriers, a quantitative count of CGG repeat length was available for only 79 of these cases. Of these 79 mothers, 10 provided medical records that specified the number of CGG repeats, whereas 69 cases sent a blood sample to Kimball Genetics, Inc. which conducted CGG repeat assays for our project. For the present sample, CGG repeat length varied from 67 to 180 CGG repeats (mean = 93.8, s.d. = 20.1).
Activation ratio
Data on activation ratio was available for 66 of the participating mothers. All activation ratio data was based on assays conducted by Kimball Genetics, Inc. Band intensities were measured by phosphorimaging, and those values were used in the following calculation: unmethylated normal/(unmethylated normal + methylated normal). In the present sample, activation ratio ranged from .11 to .92 (mean = .54, s.d. = .21), providing ample variability in this biomarker to conduct our analyses.
Other menopause symptoms
Mothers reported the frequency of selected symptoms that they ever experienced as a result of menopause, including hot flushes/flashes, depression, sleep disturbance, bone pain, night sweats, and other symptoms they associated with menopause. They reported each as 0 = not at all, 1 = a little, 2 = somewhat, and 3 = a lot. These items were taken from the Wisconsin Longitudinal Study (Hauser, Sheridan, & Warren, 1999).
Covariates
Control variables in the multivariate model included level of education and history of smoking. Maternal education was controlled as an indicator of family socioeconomic status. Maternal history of smoking was controlled because past research has shown that smoking hastens menopause by approximately one year in premutation carrier mothers and also in the general population (Allen et al., 2007; Yonker et al., 2013). Maternal education was measured on a four-point scale: 1 = less than high school, 2 = high school graduate, 3 = college graduate, and 4 = higher than college degree. Smoking was measured as a binary variable, ‘yes’; ‘no’ answer to the question of ‘have you ever smoked regularly, at least one cigarette a day?’ Smoking history was missing for one mother and she was omitted from the multivariate models.
Statistical Analysis
We first provide descriptive findings regarding the age of menopause, the percentage of participants who experienced POF, the percentage who were medically diagnosed with FXPOI, and the frequency of specific menopause symptoms.
Next, we present the results of ordinary least squares (OLS) multiple regression models to test the hypothesis that there is a curvilinear association between CGG repeat length and age at menopause. The regression equation included the following terms: 1) control variables of maternal education and smoking status; 2) linear effect of CGG repeat length; and 3) curvilinear effect of CGG repeat length (CGG repeat length squared). The sample for the regression model was based on 78 cases, as one was missing data on smoking history. We also ran a robustness test by carrying out the OLS model with the 69 cases for whom the genotyping was conducted by Kimball Genetics.
Subsequently, we added measures of activation ratio and its interaction with CGG repeats to the OLS multiple regression models to examine if inclusion of these additional terms would independently predict age at menopause. Since activation ratio data were only available on 66 cases, this regression model was restricted to this smaller number of premutation carriers.
Given the wide age range of sample members at the time of data collection (39 to 72 years of age), it is possible that recall bias regarding age at menopause may have introduced error into the data. Therefore, all of the regression models were re-estimated including age at the time of the interview as a control variable.
Results
Descriptive Findings – Menopause Symptoms
The mean age at menopause for the 88 post-menopausal participants was 43.1 years (s.d. = 6.1) but there was a broad range (from 30 to 56 years of age). Just over one-third (n = 31, 35.2%) of the sample reported that their menstrual period had ended prior to age 40, which signifies POF, but only half that many (n = 14; 18.2%) were medically diagnosed with FXPOI.
Mothers reported the frequency of selected menopausal symptoms they ever experienced, including hot flushes/flashes, depression, sleep disturbance, bone pain, night sweats, and other symptoms they associated with menopause (see Table 1). The great majority reported experiencing at least some of these symptoms. The most common symptom was hot flushes/flashes, with 30.7% experiencing this symptom ‘a lot’. Also common were night sweats (26.1% experienced this symptom ‘a lot’), sleep disturbance (22.7%), and depression (19.3%).
Table 1. Frequency of Menopausal Symptoms Ever Experienced by Premutation Carriers (n = 88).
| Menopausal Symptoms |
0 Not at all | 1 A little | 2 Somewhat | 3 A lot | Mean (s.d.) |
|---|---|---|---|---|---|
| Hot flushes/flashes | 11.4% | 28.4% | 29.5% | 30.7% | 1.80 (1.0) |
| Depression | 30.7% | 29.5% | 20.5% | 19.3% | 1.28 (1.1) |
| Sleep disturbance | 14.8% | 22.7% | 39.8% | 22.7% | 1.70 (1.0) |
| Bone pain | 48.9% | 22.7% | 21.6% | 6.8% | 0.86 (1.0) |
| Night sweats | 20.5% | 25.0% | 28.4% | 26.1% | 1.60 (1.1) |
| Other symptoms | 33.0% | 37.5% | 23.9% | 5.7% | 1.02 (0.9) |
Age at Menopause and CGG Repeat Length
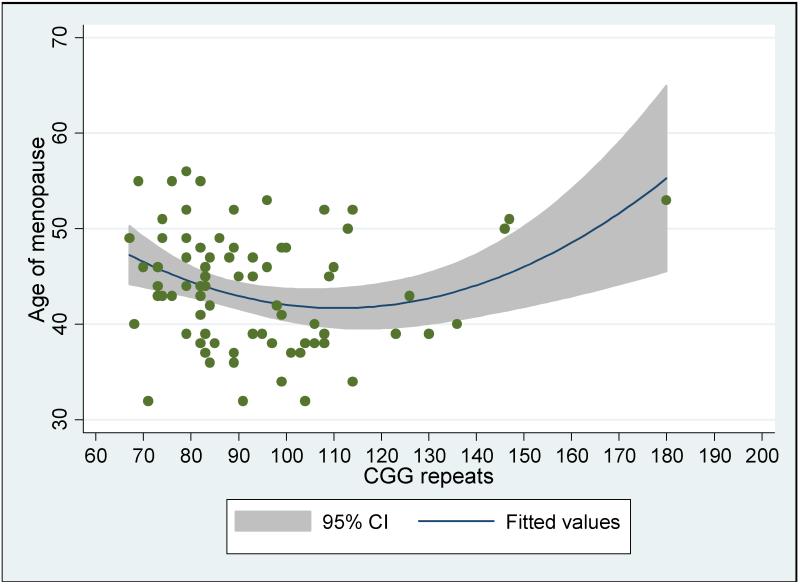
As shown in Table 2, there was a significant curvilinear association between CGG repeat length and age at menopause (b = .003, p < .01; see Figure 1). Neither maternal education nor smoking history was significantly associated with age at menopause. The effect remained significant even after the case with 180 CGG repeats was removed from the analysis (to insure that it was not just this one case that was driving the curvilinear association) and when age at the time of interview was controlled (to control for possible recall bias). The data are available from the first author.
Table 2. Unstandardized Coefficients from Multiple Regression Model of Age at Menopause on CGG Repeat Length (n = 78).
| B (S.E) | P-value | |
|---|---|---|
| Constant | 72.4 (11.7) | .000 |
| Maternal education | 1.07 (1.00) | .316 |
| Ever smoked (yes = 1) | 1.52 (1.38) | .304 |
| CGG repeat (linear) | −.62 (.21) | .005 |
| CGG repeat (curvilinear) | .003 (.001) | .005 |
| R-square | .14 |
Fig. 1. Age at menopause by CGG repeat length.
A further test of robustness was carried out by analyzing only those 69 cases with CGG repeat data from Kimball Genetics. Regression analysis with the same variables as above yielded results that were almost identical to the analysis using all cases, suggesting no confounding measurement effects of different laboratories carrying out the CGG repeat assays on the curvilinear association of CGG repeat length on age at menopause (b = .003, p < .001).
Age at Menopause and Activation Ratio
As noted, for 66 of the 78 cases included in the regression analyses reported in Table 2, we also had access to measures of activation ratio. We conducted follow up analyses on this smaller sub-sample to determine if inclusion of activation ratio in the regression models improved the prediction of age at menopause. As shown in Table 3, activation ratio was not a significant predictor of age at menopause among premutation carrier mothers, either as a main effect, as an interaction effect with CGG repeat length, or as an interaction with the curvilinear term of CGG repeat length. Nevertheless, in this analysis, the curvilinear term of CGG repeat length continued to significantly predict age at menopause (p < .05), even with activation ratio controlled.
Table 3. Unstandardized Coefficients from Multiple Regression Models of Age at Menopause on CGG Repeat Length and Activation Ratio (n = 66).
| Model 1 | Model 2 | Model 3 | ||||
|---|---|---|---|---|---|---|
| B (S.E) | P-value | B (S.E) | P-value | B (S.E) | P-value | |
| Constant | 73.9 (13.1) | .000 | 74.0 (13.5) | .000 | 124.0 (35.4) | .001 |
| Maternal education | 1.11 (1.09) | .316 | 1.11 (1.11) | .320 | 1.35 (1.09) | .221 |
| Ever smoked (yes = 1) | 1.62 (1.56) | .304 | 1.62 (1.57) | .308 | 1.54 (1.60) | .340 |
| CGG repeat (linear) | −.65 (.22) | .005 | −.65 (.23) | .006 | −1.45 (0.63) | .024 |
| CGG repeat (curvilinear) |
.003 (.001) | .005 | .003 (.001) | .005 | .006 (.003) | .035 |
| Activation ratio | −.081 (3.55) | .982 | −84.4 (70.7) | .238 | ||
| Activation ratio × CGG repeat (linear) |
1.29 (1.31) | .329 | ||||
| Activation ratio × CGG repeat (curvilinear) |
−.004 (.006) | .474 | ||||
| R-square | .15 | .15 | .22 | |||
Discussion
The risk of early menopause is elevated in premutation carrier women. The average age at menopause in the present cohort was 43.1 years, which is nearly 10 years earlier than reported in the US general population (Gold, 2011). In our sample, just under two-fifths (18.2%) were medically diagnosed with FXPOI, in line with past reports (Rodriguez-Revenga et al., 2009). However, twice that percentage (35.2%) had POF, as their menses ceased prior to age 40. This disparity reflects a significant under-diagnosis of FXPOI.
We also examined the association between two biomarkers of the FMR1 premutation and age at menopause – CGG repeat length and activation ratio. We found a significant curvilinear association between CGG repeat length and age at menopause, similar to past studies (Allen et al., 2007; Ennis et al., 2006; Spath et al., 2011; Sullivan et al., 2005; Tejada et al., 2008), with the earliest age at menopause observed in the present sample among those who had between 90 and 110 CGG repeats. This is a slightly higher zone of greatest risk of early menopause than reported in previous research, which was found to be 65-90 repeats. However, this difference may be due to the repeat length frequency distribution in the various studies. Future research is needed to provide a more precise estimate of the zone of greatest vulnerability to early menopause, as there is variability from study to study.
Activation ratio did not significantly predict age at menopause, again similar to past studies that reported no association between this biomarker and menopausal age among carrier women (Bione et al., 2006; Murray, 2000; Rodriguez-Revenga et al., 2009; Spath et al., 2011; Sullivan et al., 2005; Tejada et al., 2008). However, in a previous study that implicated activation ratio in the clinical manifestation of POF (Bodega et al., 2006), the pedigrees of three families were reported in which each had two sisters with FMR1 CGG expansions who were discordant for activation ratio. In all three instances, the sister with the lower percentage of active mutated alleles did not have POF whereas the sister with the higher percentage had POF. Other clinical reports suggest that activation ratio may be related to FMR1-related symptom severity (Berry-Kravis et al., 2005; Heine-Suner et al., 2003). Thus, future research with larger samples should continue to probe the association between activation ratio, CGG repeat length, and age at menopause.
Some premutation carrier women who have mid-range CGG repeats may also be at increased risk of depression, anxiety, and a blunted cortisol response, especially in the context of environmental stress (Roberts et al., 2009; Seltzer et al., 2012b). However, this increased mental health vulnerability is manifested by only a sub-set of premutation carrier women. In our prior research drawn from the same sample as the present research, we found that 25.6% of premutation carrier women were above the clinical cut-off for depression, 17.1% were above the clinical cut-off for anxiety, and 13.4% were above the clinical cutoffs for both depression and anxiety (Seltzer et al., 2012b). Similarly, early menopause is characteristic of only a sub-set of premutation carriers; in the present sample, 35.2.8% had menopause at age 40 or earlier. Future research should examine whether the same premutation carriers are at increased risk of both mental health symptoms and early menopause, and whether these two domains of vulnerability are independent or interactive. It is possible that depression and anxiety are exacerbated by early menopause and heightened menopausal symptoms, as well as by exposure to environmental stress. Alternatively, a different sub-set of carrier women may be vulnerable to early menopause than to elevated mental health symptoms.
Little is known about the disease pathology underlying FXPOI. It does not appear to be related to an FMRP deficit since women with the full mutation, or those with reduced FMRP, do not have ovarian dysfunction. Like FXTAS, the increased FMR1 mRNA containing the long repeat tract is most likely involved in the etiology. This has recently been supported by two premutation mouse models for FXPOI (Hoffman et al., 2012; Lu et al., 2012). However, there is little speculation for the cause of the non-linear association of repeat length and FXPOI. Future biological research is needed to determine how the FMR1 premutation is toxic to the ovarian system, and how the number of CGG repeats in the premutation range dictates risk.
This study was not without limitations. Since all participants in this study were mothers, premutation carriers who had very early menopause that interfered with their ability to have children were not included. Additionally, since all participants had a child with full mutation FXS, then premutation carriers with low numbers of CGG repeats might have been undersampled (as such carriers are at lower risk of having a child with the full mutation) relative to the population. Thus, the frequency of repeat length alleles may differ from other samples. Population-based studies of female premutation carriers, not selected based on the criterion of having a child with FXS, are needed to ascertain the age at menopause in an unselected sample.
In other research conducted by our group (Seltzer et al., 2012a), we reported the results of a pilot study in which we screened 6747 DNA samples from a representative population. We found that the average age at menopause among the 20 female premutation carriers in that cohort (who were not ‘reverse-ascertained’ from a child with FXS) was 48.1, whereas the controls who were selected from the same population reached menopause at age 50.8 (p < .05). Nearly half of the premutation carriers in the population-based pilot study had low numbers of CGG repeats (55-60). Thus, early menopause is a characteristic of premutation carriers, even on the low end of the premutation CGG repeat range. In that sample, we were not able to test whether the association between CGG repeat length and age at menopause was curvilinear, as the sample included few women with mid-range numbers of CGG repeats. However, the mean age at natural menopause of the women in that sample who had between 55 and 60 CGG repeats was 50.8 years, whereas the mean age for those with between 60 and 110 CGGs was 45.1 years, again suggesting that those with mid-range repeats were at greater vulnerability of early menopause.
To conclude, the latest estimates put the prevalence of premutation carriers at over 1 million Americans (Maenner et al., 2013), and thus study of the phenotype of the premutation has substantial public health significance. Most past research on the premutation phenotype has been carried out with men. However, a substantial sub-group of women who carry the premutation are at risk of early menopause, and this risk is greatest among those with mid-range CGG repeats. The extent to which early menopause contributes to other aspects of the premutation phenotype in women, such as osteoporosis or mental health symptoms, warrants study in future research.
Acknowledgements
This study was supported by a grant from the Eunice Kennedy Shriver National Institute of Child Health and Human Development to the University of North Carolina (P30 HD003100-S1) to support a Fragile X Research Center at three additional sites. The present analyses were based on data collected at the UW-Madison Waisman Center site (M. Mailick, PI). We are extremely appreciative of the families who participated in this study, without whom our research would not have been possible. We would like to thank the National Fragile X Foundation for providing informational materials to share with families. We are also grateful for the support we received from the Waisman Center Core Grant (P30 HD03352).
Contributor Information
Marsha R. Mailick, Waisman Center, University of Wisconsin-Madison
Jinkuk Hong, Waisman Center, University of Wisconsin-Madison.
Jan Greenberg, Waisman Center, University of Wisconsin-Madison.
Leann Smith, Waisman Center, University of Wisconsin-Madison.
Stephanie Sherman, Department of Human Genetics, Emory University, Atlanta, GA.
References
- Allen EG, Sullivan AK, Marcus M, Small C, Dominguez C, Epstein MP, Sherman SL. Examination of reproductive aging milestones among women who carry the FMR1 premutation. Hum Reprod. 2007;22:2142–2152. doi: 10.1093/humrep/dem148. [DOI] [PubMed] [Google Scholar]
- Bailey DB, Sideris J, Roberts J, Hatton D. Child and genetic variables associated with maternal adaptation to fragile X syndrome: A multidimensional analysis. Am J Med Genet Part A. 2008;146A:720–729. doi: 10.1002/ajmg.a.32240. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Hall DA. Executive dysfunction in young FMR1 premutation carriers: forme fruste of FXTAS or new phenotype? Neurology. 2011;77(7):612–321. doi: 10.1212/WNL.0b013e3182299f98. [DOI] [PubMed] [Google Scholar]
- Berry-Kravis E, Potanos K, Weinberg D, Zhou L, Goetz CG. Fragile X-associated tremor/ataxia syndrome in sisters related to X-inactivation. Ann Neurol. 2005;57:144–147. doi: 10.1002/ana.20360. [DOI] [PubMed] [Google Scholar]
- Bione S, Benedetti S, Goegan M, Menditto I, Marozzi A, Ferrari M, Toniolo D. Skewed X-chromosome inactivation is not associated with premature ovarian failure in a large cohort of Italian patients. Am J Med Genet Part A. 2006;140A:1349–1351. doi: 10.1002/ajmg.a.31312. [DOI] [PubMed] [Google Scholar]
- Bodega B, Bione S, Dalpra L, Toniolo D, Ornaghi F, Vegetti W, Ginelli E, Marozzi A. Influence of intermediate and uninterrupted FMR1 CGG expansions in premature ovarian failure manifestation. Hum Reprod. 2006;21:952–957. doi: 10.1093/humrep/dei432. [DOI] [PubMed] [Google Scholar]
- Brown WT. The molecular biology of fragile X mutation. In: Hagerman R, Hagerman PJ, editors. Fragile X syndrome: Diagnosis, treatment, and research. 3rd ed John Hopkins University Press; Baltimore, MD: 2002. pp. 110–135. [Google Scholar]
- Ennis S, Ward D, Murray A. Nonlinear association between CGG repeat number and age of menopause in FMR1 premutation carriers. Eur J Hum Genet. 2006;14:253–255. doi: 10.1038/sj.ejhg.5201510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold EB. The timing of the age at which natural menopause occurs. Obstet Gynecol Clin North Am. 2011;38:425–440. doi: 10.1016/j.ogc.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greco CM, Berman RF, Martin RM, Tassone F, Schwartz PH, Chang A, Trapp BD, Iwahashi C, Brunberg J, Grigsby J, Hessl D, Becker EJ, Papazian J, Leehey MA, Hagerman RJ, Hagerman PJ. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- Grigsby J, Leehey MA, Jacquemont S, Brunberg JA, Hagerman RJ, Wilson R, Epstein JH, Greco CM, Tassone F, Hagerman PJ. Cognitive impairment in a 65-year old male with the Fragile X-Associated Tremor/Ataxia Syndrome (FXTAS) Cognitive and Behavioral Neurology. 2006;3:165–167. doi: 10.1097/01.wnn.0000213906.57148.01. [DOI] [PubMed] [Google Scholar]
- Hagerman PJ, Berry-Kravis E, Kaufmann WE, Ono MY, Tartaglia N, Laschiewicz A, Tranfaglia M. Advances in the treatment of fragile X syndrome. Pediatrics. 2009;123:378–390. doi: 10.1542/peds.2008-0317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hagerman PJ, Hagerman RJ. The fragile-X premutation: A maturing perspective. Am J Hum Genet. 2004;74:805–816. doi: 10.1086/386296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hartley S, Seltzer MM, Hong J, Greenberg J, Smith L, Almeida D, Coe C, Abbeduto L. Cortisol response to behavior problems in FMR1 premutation mothers of adolescents and adults with fragile X syndrome: A diathesis-stress model. International Journal of Behavior and Development. 2011;36:53–61. doi: 10.1177/0165025411406857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauser RM, Sheridan JT, Warren JR. Socioeconomic achievements of siblings in the life course: New findings from the Wisconsin Longitudinal Study. Research on Aging. 1999;21:338–378. [Google Scholar]
- Heine-Suner D, Torres-Juan L, Morla M, Busquets X, Barcelo F, Pico G, Bonilla L, Govea N, Bernues M, Rosell J. Fragile-X syndrome and skewed X-chromosome inactivation within a family: A female member with complete inactivation of the functional X chromosome. Am J Med Genet. 2003;122:108–114. doi: 10.1002/ajmg.a.20160. [DOI] [PubMed] [Google Scholar]
- Hessl D, Tassone F, Loesch DZ, Berry-Kravis E, Leehey MA, Gane LW, Barbato I, Rice C, Gould E, Hall DA, Grigsby J, Wegelin JA, Harris S, Lewin F, Weinberg D, Hagerman PJ, Hagerman RJ. Abnormal elevation of FMR1 mRNA is associated with psychological symptoms in individuals with the fragile X premutation. Am J Med Genet Part B. 2005;139B:115–121. doi: 10.1002/ajmg.b.30241. [DOI] [PubMed] [Google Scholar]
- Hoffman GE, Le WW, Entezam A, Otsuka N, Tong ZB, Nelson L, Flaws JA, McDonald JH, Jafar S, Usdin K. Ovarian abnormalities in a mouse model of fragile X primary ovarian insufficiency. J Histochem Cytochem. 2012;60(6):439–456. doi: 10.1369/0022155412441002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leehey MA. Fragile X-associated Tremor/Ataxia Syndrome (FXTAS): Clinical phenotype, diagnosis and treatment. J Investig Med. 2009;57(8):830–836. doi: 10.231/JIM.0b013e3181af59c4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loesch DZ, Bui MQ, Hammersley E, Schneider A, Storey E, Stimpson P, Burgess T, Francis D, Slater H, Tassone F, Hagerman RJ, Hessl D. Psychological status in female carriers of premtuation FMR1 allele showing a complex relationship with size of CGG expansion. Clin Genet. 2014 doi: 10.1111/cge.12347. Doi:10.111/cage 12347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu C, Lin L, Tan H, Wu H, Sherman SL, Gao F, Jin P, Chen D. Fragile X premutation RNA is sufficient to cause primary ovarian insufficiency in mice. Hum Mol Genet. 2012;21(23):5039–5047. doi: 10.1093/hmg/dds348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maenner M, Baker M, Broman K, Tian J, Barnes J, Atkins A, McPherson E, Hong J, Brilliant M, Mailick M. FMR1 CGG expansions: Prevalence and sex ratios. Am J Med Genet Part B. 2013;162B:466–473. doi: 10.1002/ajmg.b.32176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mailick MR, Greenberg JS, Smith L, Sterling A, Brady N, Warren SF, Hong J. Fragile X-associated disorders: How the family environment and genotype interact. In: Burack J, Schmidt L, editors. Cultural and contextual perspectives on development at risk. Cambridge University Press; Cambridge: In press. [Google Scholar]
- Murray A. Premature ovarian failure and the FMR1 gene. Seminars in Reproductive Medicine. 2000;18:59–66. doi: 10.1055/s-2000-13476. [DOI] [PubMed] [Google Scholar]
- Roberts JE, Bailey DB, Mankowski J, Ford A, Sideris J, Weisenfeld LA, Golden RN. Mood and anxiety disorders in females with the FMR1 premutation. Am J Med Genet Part B. 2009;150B:130–139. doi: 10.1002/ajmg.b.30786. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Revenga L, Madrigal I, Badenas C, Xuncla M, Jimenez L, Mila M. Premature ovarian failure and fragile X female premutation carriers: no evidence for a skewed X- chromosome inactivation pattern. Menopause (New York, NY) 2009;16(5):944–949. doi: 10.1097/gme.0b013e3181a06a37. [DOI] [PubMed] [Google Scholar]
- Seltzer MM, Baker MW, Hong J, Maenner M, Greenberg JS, Mandel D. Prevalence of CGG expansions of the FMR1 gene in a US population-based sample. Am J Med Genet Part B. 2012a;2159B:589–97. doi: 10.1002/ajmg.b.32065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seltzer MM, Barker ET, Greenberg JS, Hong J, Coe C, Almeida D. Differential sensitivity to life stress in FMR1 premutation carrier mothers of children with fragile X syndrome. Health Psychol. 2012b;31:612–622. doi: 10.1037/a0026528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SL, Taylor K, Allen EG. FMR1 premutation: A leading cause of inherited ovarian dysfunction. In: Arrieta I, Penagarikano O, Telez M, editors. Fragile Sites: New Directions and Changing Perspectives. Nova Science Publishers; Hauppauge, NY: 2007. pp. 299–320. [Google Scholar]
- Spath MA, Feuth TB, Smits AP, Yntema HG, Braat DD, Thomas CM, van Kessel AG, Sherman SL, Allen EG. Predictors and risk model development for menopausal age in fragile X premutation carriers. Genetics in Medicine: Official journal of the American College of Medical Genetics. 2011;13(7):643–650. doi: 10.1097/GIM.0b013e31821705e5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sullivan AK, Marcus M, Epstein MP, Allen EG, Anido AE, Paquin JJ, Yadav-Shah M, Sherman SL. Association of FMR1 repeat size with ovarian dysfunction. Hum Reprod. 2005;20:402–412. doi: 10.1093/humrep/deh635. [DOI] [PubMed] [Google Scholar]
- Tassone F, Iong KP, Tong TH, Lo J, Gan LW, Berry-Kravis E, Nguyen D, Mu LY, Laffin J, Bailey DB, Hagerman RJ. FMR1 CGG allele size and prevalence ascertained through newborn screening in the United States. Genome Medicine. 2012;4:100. doi: 10.1186/gm401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tassone F, Adams J, Berry-Kravis EM, Cohen SS, Brusco A, Leehey MA, Li L, Hagerman RJ, Hagerman PJ. CGG repeat length correlates with age of onset of motor signs of the fragile X-associated tremor/ataxia syndrome (FXTAS) Am J Med Genet Part B. 2007;144B:566–569. doi: 10.1002/ajmg.b.30482. [DOI] [PubMed] [Google Scholar]
- Tejada MI, Garcia-Alegria E, Bilbao A, Martinez-Bouzas C, Beristain E, Poch M, Ramos-Arroyo MA, Lopez B, Fernandez Carvajal I, Ribate MP, Ramos F. Analysis of the molecular parameters that could predict the risk of manifesting premature ovarian failure in female premutation carriers of fragile X syndrome. Menopause (New York, NY) 2008;15(5):945–9. doi: 10.1097/gme.0b013e3181647762. [DOI] [PubMed] [Google Scholar]
- Wittenberger MD, Hagerman RJ, Sherman SL, McConkie-Rosell A, Welt CK, Rebar RW, Corrigan EC, Simpson JL, Nelson LM. The FMR1 premutation and reproduction. Fertil Steril. 2007;87:456–465. doi: 10.1016/j.fertnstert.2006.09.004. [DOI] [PubMed] [Google Scholar]
- Yonker JA, Chang V, Roetker NS, Hauser TS, Hauser RM, Atwood CA. Hypothalamic-pituitary-gonadal axis homeostasis predicts longevity. AGE. 2013;35(1):129–138. doi: 10.1007/s11357-011-9342-1. [DOI] [PMC free article] [PubMed] [Google Scholar]