Abstract
Objective
To investigate the relationship of genetic and biochemical determinants of paraoxonase 1 activity to carotid plaque as a surrogate marker of cardiovascular (CV) risk in patients with rheumatoid arthritis (RA).
Methods
The relationships between paraoxonase 1 activity, PON1 genotype (for the functional polymorphism at position 192), and carotid plaque presence were determined in 168 RA patients. After an overnight fast, blood was collected for lipoprotein analysis, and paraoxonase 1 activity was measured using paraoxon as the substrate. The PON1 Q192R genotype was determined for all patients. Lipoprotein cholesterol levels, traditional CV risk factors, medication use, and RA disease characteristics were assessed for all patients.
Results
Paraoxonase 1 activity values in the RA patients were highest for the RR genotype, intermediate for the QR genotype, and lowest for the QQ geno-type (P < 0.0001). Compared to patients with either the QQ genotype or the QR genotype, patients with the RR genotype demonstrated decreased risk of carotid plaque on multivariate analysis, controlling for traditional CV risk factors, high-sensitivity C-reactive protein levels, prednisone use, and cholesterol-lowering medication use (P < 0.05). Additional multivariate logistic regression analysis controlling for the above factors also revealed a significant association of plasma paraoxonase 1 activity with carotid plaque in RA patients. Lower plasma paraoxonase 1 activity was associated with increased risk of carotid plaque (P < 0.05).
Conclusion
The current findings suggest a relationship of the genetic determinants and activity of paraoxonase 1 to CV risk in RA patients, as assessed by the presence or absence of carotid plaque. Further CV outcome studies are warranted to validate the utility of paraoxonase 1 as a biomarker of CV risk in patients with RA.
Patients with rheumatoid arthritis (RA) have significantly increased cardiovascular (CV) morbidity and mortality that are not accounted for by traditional risk factors alone (1–3). Abnormal function of high-density lipoprotein (HDL), the carrier of so-called “good” cholesterol, has been proposed as a potential mechanism for this increased CV risk (4,5).
In addition to promoting cholesterol efflux, HDL protects low-density lipoprotein (LDL) against oxidation (6–10), and this ability has been referred to as an “antiinflammatory” function because the lipid oxidation products derived from LDL are highly proinflammatory. Paraoxonase 1 is an HDL-associated enzyme that promotes the antioxidant and antiinflammatory properties of HDL by preventing the formation of oxidized LDL and inactivating oxidized phospholipids (10–12). Lower paraoxonase 1 enzyme activity has been associated with CV events in the general population (13).
Paraoxonase 1 activity varies substantially in populations; most of this variation depends on genetic polymorphisms, particularly the Q192R polymorphism in the coding region (14), and this polymorphism has been associated with CV risk in the general population (13). In the current study, we investigated the relationship of genetic and biochemical determinants of paraoxonase 1 activity to carotid plaque as a surrogate marker of CV risk in patients with RA.
PATIENTS AND METHODS
Study design
RA patients were recruited from the rheumatology offices at the University of California, Los Angeles (UCLA) via flyers posted in the offices and in the UCLA Medical Center. All RA patients met the American College of Rheumatology 1987 revised classification criteria (15), which was verified by review of their medical records. All subjects gave written informed consent for the study under a protocol approved by the Human Research Subject Protection Committee at UCLA.
Patients provided a blood sample, underwent carotid ultrasound, and completed questionnaires as described below. Markers of inflammation, including high-sensitivity C-reactive protein (hsCRP) and erythrocyte sedimentation rate (ESR; by the Westergren method), were assessed, and lipid profiles in samples obtained after an overnight fast were measured at the UCLA clinical laboratory using standard methods. Additional blood was collected in heparinized tubes (Becton Dickinson) and stored at −80°C for paraoxonase 1 activity assays. CV risk and health information was obtained by questionnaire and chart review. Disease activity in RA patients was determined by a count of tender and swollen joints (28 joints assessed), patient's and physician's global assessments on a 0–100-mm visual analog scale (VAS), and patient's pain, fatigue, and stiffness assessments on a 0–100-mm VAS. The Disease Activity Score in 28 joints (DAS28) (16) was calculated for each patient. Disease-related disability was assessed with the Health Assessment Questionnaire disability index (HAQ DI) (17).
Carotid ultrasound imaging
A standard protocol including B (brightness)–mode gray-scale, color, and spectral Doppler techniques was used to study the carotid arteries of all participants (18). The bilateral common carotid arteries (CCAs), internal carotid arteries (ICAs), external carotid arteries, and carotid bulbs were examined. The presence of atherosclerotic plaque was defined as the presence of focal protrusion into the arterial lumen with a thickness exceeding that of the surrounding wall by at least 50%. The number, location, and sonographic appearance of all plaques were recorded. Intima-media thickness (IMT) of the far wall of the distal CCA was measured 1 cm proximal to the flow divider (the area where the column of blood in the CCA splits into the ICA and external carotid artery). Measurements were obtained at end-diastole using automated QLAB software (Philips Medical Systems), and the mean of 3 measurements was used for the CCA-IMT value. The same radiologist (NR) interpreted all studies in a blinded manner, and the same ultrasound unit (Iu22; Philips Medical Systems) was used for all participants.
Determination of paraoxonase 1 activity
Paraoxonase 1 activity was quantified using paraoxon as the substrate and measuring the increase in the absorbance at 405 nm due to the formation of 4-p-nitrophenol over a period of 12 minutes (at 20-second intervals). Paraoxon was purchased from Sigma and was further purified using chloroform extraction. A unit of paraoxonase 1 activity was defined as the formation of 1 nmole of 4-p-nitrophenol per minute per milliliter of sample used. Enzyme activity was measured with 40-fold–diluted plasma (final concentration) in a reaction mixture containing 4 mM paraoxon working solution, 500 mM glycine/10 mM CaCl2 buffer at pH 10.5, and 155 mM NaCl/3 mM NaN3 buffer at pH 8.2.
PON1 genotyping
Genomic DNA was extracted from whole blood using the QIAamp DNA Maxi kit (Qiagen). From the 168 participants, 163 DNA samples could be analyzed. Oligonucleotide primers (5’-TATTGTTGCTGTGGGACCTGAG-3’ and 5’-CACGCTAAACCCAAATACATCTC-3’) were used to amplify a 99-bp DNA fragment covering the glutamine-to-arginine mutation at position 192. Amplification was performed using DreamTaq DNA polymerase (Fermentas) for the polymerase chain reaction. The amplified product was digested with Alw I (New England Biolabs), separated by nondenaturing acrylamide gel electrophoresis, and stained with ethidium bromide. Allele Q (glutamine) corresponded to a 99-bp fragment and allele R (arginine) corresponded to 65-bp and 34-bp fragments.
Statistical analysis
Data were analyzed using JMP IN 9.0 software (SAS Institute). Patient groups were compared using Student's t-test for continuous variables and the chi-square test of association for categorical variables, along with Fisher's exact test for small sample sizes. When needed, nonparametric Wilcoxon rank sum tests were used to analyze continuous variables. P values less than 0.05 were considered significant.
A forward stepwise logistic regression analysis was performed to evaluate correlates of carotid plaque in the RA cohort. Initial covariates included traditional CV risk factors (age, sex, ethnicity, hypertension, cholesterol levels, diabetes mellitus, family history of coronary heart disease [CHD], smoking status, and body mass index [BMI]), patient factors significantly associated with carotid plaque in bivariate analyses (Tables 1 and 2), hsCRP levels, cholesterol-lowering medication use, and PON1 genotype (19). In cases where variables were highly correlated, 1 representative variable was chosen. An additional forward stepwise multivariate logistic regression analysis was performed controlling for the above factors and using paraoxonase 1 enzyme activity in place of PON1 genotype to further assess the relationship between carotid plaque and paraoxonase 1 in patients with RA. Testing for interactions between PON1 genotype/paraoxonase 1 activity and CV risk factors (age, sex, ethnicity, hypertension, smoking, family history of CHD, and BMI) was also performed. A receiver operating characteristic curve was constructed for each model, and the sensitivity, specificity, and model accuracy are reported.
Table 1.
Demographic and clinical characteristics of the RA patients with carotid plaque compared to the RA patients without carotid plaque*
| Patients with plaque (n = 72) | Patients without plaque (n = 96) | P | |
|---|---|---|---|
| Carotid IMT, mm | 0.71 ± 0.18 | 0.58 ± 0.12 | <0.0001 |
| Age, years | 61 ± 10 | 50 ± 12 | <0.0001 |
| Female, % | 75 | 91 | 0.01 |
| Hispanic, % | 21 | 27 | 0.37 |
| ESR, mm/hour | 24 ± 21 | 25 ± 21 | 0.55 |
| hsCRP, mg/liter | 5.7 ± 13.9 | 7.6 ± 17.8 | 0.22 |
| BMI, kg/m2 | 27.7 ± 5.9 | 27.4 ± 6.8 | 0.42 |
| Hypertension, % | 59 | 17 | <0.0001 |
| Diabetes mellitus, % | 16 | 11 | 0.49 |
| Current smoker, % | 9 | 6 | 0.54 |
| Family history of CHD, % | 10 | 16 | 0.35 |
| Total cholesterol, mg/dl | 197 ± 40 | 186 ± 40 | 0.05 |
| LDL cholesterol, mg/dl | 107 ± 35 | 104 ± 33 | 0.40 |
| HDL cholesterol, mg/dl | 61 ± 19 | 59 ± 18 | 0.46 |
| Triglycerides, mg/dl | 147 ± 122 | 116 ± 83 | 0.06 |
| PON1 RR genotype, %† | 13 | 24 | 0.07 |
| Paraoxonase 1 activity, nmoles/minute/ml | 88 ± 21 | 101 ± 53 | 0.13 |
Except where indicated otherwise, values are the mean ± SD. RA = rheumatoid arthritis; IMT = intima-media thickness; ESR = erythrocyte sedimentation rate; hsCRP = high-sensitivity C-reactive protein; BMI = body mass index; CHD = coronary heart disease; LDL = low-density lipoprotein; HDL = high-density lipoprotein.
Percentages were determined in the 163 patients for whom DNA samples were available (69 with plaque and 94 without plaque).
Table 2.
Disease characteristics and medication use in the RA patients with carotid plaque compared to the RA patients without carotid plaque*
| Patients with plaque (n = 72) | Patients without plaque (n = 96) | P | |
|---|---|---|---|
| No. of tender joints (28-joint count) | 8±8 | 8±8 | 0.76 |
| No. of swollen joints (28-joint count) | 4 ± 5 | 4 ± 5 | 0.54 |
| DAS28 | 4.4 ± 1.8 | 4.3 ± 2.0 | 0.75 |
| Patient's assessment of pain, 0–100-mm VAS | 39 ± 29 | 41 ± 31 | 0.60 |
| Patient's global assessment of disease activity, 0–100-mm VAS | 42 ± 29 | 42 ± 31 | 0.79 |
| Physician's global assessment of disease activity, 0–100-mm VAS | 35 ± 21 | 40 ± 27 | 0.45 |
| Patient's assessment of stiffness, 0–100-mm VAS | 35 ± 29 | 38 ± 29 | 0.60 |
| Patient's assessment of fatigue, 0–100-mm VAS | 45 ± 29 | 42 ± 27 | 0.51 |
| HAQ DI score | 1.0 ± 0.79 | 0.97 ± 0.87 | 0.72 |
| Medication use, % | |||
| Cholesterol-lowering drugs | 36 | 13 | 0.0006 |
| NSAIDs | 36 | 30 | 0.50 |
| Prednisone | 36 | 19 | 0.02 |
| Methotrexate | 54 | 60 | 0.52 |
| Leflunomide | 8 | 12 | 0.61 |
| Hydroxychloroquine | 17 | 18 | 0.84 |
| TNFα inhibitors | 43 | 40 | 0.75 |
| Other biologic agents | 13 | 9 | 0.45 |
Except where indicated otherwise, values are the mean ± SD. RA = rheumatoid arthritis; DAS28 = Disease Activity Score in 28 joints; VAS = visual analog scale; HAQ DI = Health Assessment Questionnaire disability index; NSAIDs = nonsteroidal antiinflammatory drugs; TNFα = tumor necrosis factor α.
RESULTS
Demographic and clinical characteristics and laboratory findings
The clinical characteristics of the patients with carotid plaque and of those without are presented in Tables 1 and 2. Patients with plaque were older, more likely to be male, and had a greater prevalence of hypertension. Trends toward higher total cholesterol and triglyceride levels in patients with plaque compared to those without plaque were noted, although these were not statistically significant. HDL and LDL cholesterol levels were notably similar between the groups. Thirteen percent of the cohort had diabetes mellitus, with no difference in prevalence between the groups (Table 1).
Systemic inflammation measured by mean values of ESR and hsCRP was similar between patients with and those without carotid plaque. Measures of disease activity, including the patient's and physician's global disease activity scores, swollen and tender joint counts, and the DAS28, also did not differ between the groups. Disease-related disability measured by the HAQ DI was also similar (Table 2).
Use of prednisone and cholesterol-lowering medications was more common in patients with carotid plaque (Table 2). Fifty-seven percent of patients in the cohort were taking methotrexate and 42% were taking anti–tumor necrosis factor α (anti-TNFα) agents at the time of the study. No differences in the use of these agents or other disease-modifying antirheumatic drugs and nonsteroidal antiinflammatory drugs were observed between patients with and those without carotid plaque (Table 2).
One patient in the cohort had a history of CHD that included myocardial infarction. Two patients had a history of cerebrovascular disease. All 3 patients had carotid plaque on ultrasound and had the QR PON1 genotype.
PON1 genotype and paraoxonase 1 activity
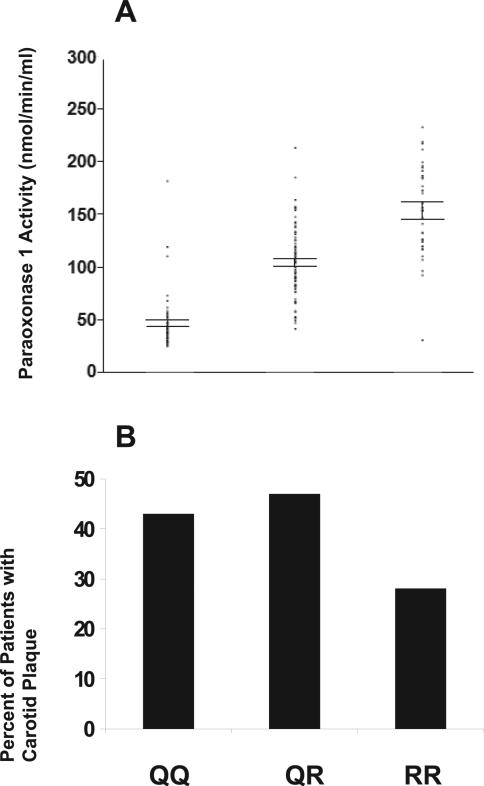
The relationship between the PON1 Q192R genotype and paraoxonase 1 activity in patients with RA is shown in Figure 1A. Significant differences in paraoxonase 1 activity were noted between genotypes. Patients with the RR genotype had the highest plasma paraoxonase 1 activity (mean ± SD 154.0 ± 44.9 nmoles/minute/ml), followed by patients with the QR genotype (mean ± SD 104.7 ± 32.6 nmoles/minute/ml), followed by patients with the QQ genotype (mean ± SD 47.2 ± 25.8 nmoles/ minute/ml) (P < 0.0001 for all comparisons) (Figure 1A). The frequencies of patients with the 3 genotypes were as follows: 32% with QQ, 48% with QR, and 20% with RR.
Figure 1.
A, Association of paraoxonase 1 activity with genotype in rheumatoid arthritis (RA) patients. The PON1 Q192R genotype demonstrated a significant dose-dependent association with paraoxonase 1 activity. Paraoxonase 1 activity values in the RA patients were highest for the RR genotype (n = 32), intermediate for the QR genotype (n = 78), and lowest for the QQ genotype (n = 53) (P < 0.0001). Horizontal bars represent ± 1 SEM; dots represent individual patients. B, Association of carotid plaque with PON1 genotype in RA patients. There was a trend toward a smaller percentage of RA patients with the RR genotype to have carotid plaque (28%) as compared to patients with the QR genotype (47%) or the QQ genotype (43%) (P = 0.07).
Association of PON1 genotype with carotid plaque in RA patients
The percentage of patients with carotid plaque was examined within each of the PON1 genotypes (Figure 1B), and multivariate logistic regression analysis was performed to evaluate an association of the PON1 genotype with carotid plaque in RA patients, while controlling for important CV risk factors and other significant correlates of carotid plaque noted in bivariate analyses (Tables 1 and 2). Compared to patients with either the QQ or the QR genotype, patients with the RR genotype demonstrated a significantly decreased risk of carotid plaque after controlling for traditional CV risk factors, hsCRP levels, prednisone use, and cholesterol-lowering medication use (P < 0.05) (model 1 in Table 3). No significant interactions were observed between the PON1 genotype and traditional CV risk factors. The accuracy of the model was 81%, with a sensitivity of 81% and a specificity of 80%. The unadjusted sensitivity and specificity values for the PON1 genotype were 87% and 25%, respectively. A trend was noted toward a lower mean ± SD carotid IMT (0.60 ± 0.14 mm) in patients with the RR genotype compared to patients with the QR genotype (0.63 ± 0.17 mm) or patients with the QQ genotype (0.65 ± 0.16 mm) (P = 0.18 for RR versus QQ).
Table 3.
Stepwise logistic regression analyses of variables associated with carotid plaque in the patients with RA*
| Explanatory variable | Model 1, OR (95% CI) | Model 2, OR (95% CI) |
|---|---|---|
| Age, years | 1.09 (1.04-1.14)† | 1.09 (1.04-1.14)† |
| BMI, kg/m2 | 0.94 (0.87-1.01) | 0.95 (0.88-1.01) |
| Female sex | 0.26 (0.08-0.76)† | 0.29 (0.09-0.85)† |
| Hypertension | 4.69 (1.77-13.43)† | 4.97 (1.95-13.62)† |
| Prednisone use | 2.00 (0.78-5.21) | 2.10 (0.84-5.40) |
| PON1 RR genotype | 0.26 (0.08-0.78)† | - |
| Paraoxonase 1 activity, nmoles/minute/ml | - | 0.99 (0.98-0.99)† |
Model 1 evaluated the association of the PON1 genotype with carotid plaque. Model 2 evaluated the association of paraoxonase 1 activity with carotid plaque. Odds ratios (ORs) for the continuous variables age, body mass index (BMI), and paraoxonase 1 activity reflect 1 unit change in the predictor (i.e., per 1 year in age, per 1.0 unit change in BMI, per 1.0 unit change in paraoxonase 1 activity). RA = rheumatoid arthritis; 95% CI = 95% confidence interval.
P < 0.05.
Association of paraoxonase 1 activity with carotid plaque in RA patients
To further examine a potential relationship of paraoxonase 1 to CV risk in RA patients, the paraoxonase assay was used to assess the activity of the paraoxonase 1 enzyme in plasma from RA patients. In bivariate analysis (Table 1), mean ± SD plasma paraoxonase 1 activity tended to be lower in patients with carotid plaque (88 ± 21 nmoles/minute/ml) than in patients without carotid plaque (101 ± 53 nmoles/minute/ml) (P = 0.13). Controlling for traditional CV risk factors and significant correlates of carotid plaque identified in bivariate analyses, multivariate logistic regression analysis showed a significant association of plasma paraoxonase 1 activity with carotid plaque in RA patients. Lower plasma paraoxonase 1 activity was associated with increased risk of carotid plaque, while higher plasma paraoxonase 1 activity was associated with decreased risk of carotid plaque (P < 0.05) (model 2 in Table 3). No significant interactions were observed between paraoxonase 1 activity and traditional CV risk factors. The accuracy of the model was 82%, with a sensitivity of 81% and a specificity of 83%. The unadjusted sensitivity and specificity values for paraoxonase 1 activity were 63% and 56%, respectively.
Association of paraoxonase 1 activity with systemic inflammation in RA patients
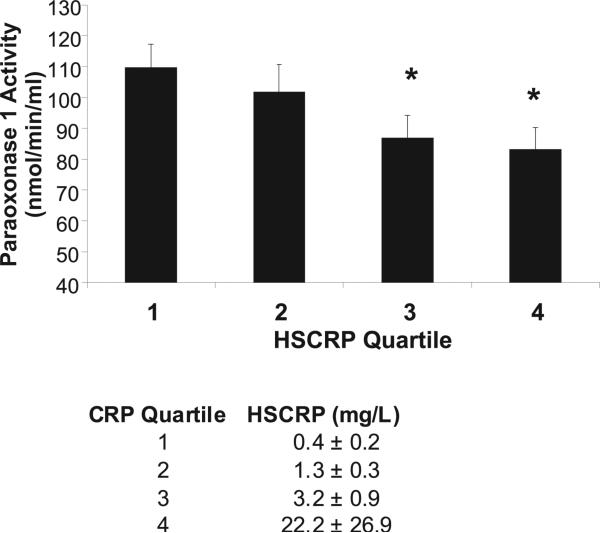
A modest but significant correlation between plasma paraoxonase 1 activity and systemic inflammation as measured by the hsCRP level was noted in this RA cohort (r = −0.21, P = 0.006). Higher levels of hsCRP were associated with decreased paraoxonase 1 activity. Paraoxonase 1 activity was also examined within quartiles of hsCRP values, and decreasing paraoxonase 1 activity was noted with increasing hsCRP quartile (Figure 2). No correlation was observed between paraoxonase 1 activity and inflammation as measured by the ESR (r = 0.009, P = 0.90).
Figure 2.
Mean ± SEM paraoxonase 1 activity within quartiles of high-sensitivity C-reactive protein (hsCRP) in the rheumatoid arthritis patient cohort. Higher quartiles of hsCRP were associated with lower paraoxonase 1 activity. * = P < 0.05 versus quartile 1. The mean ± SD hsCRP value is presented for each quartile.
DISCUSSION
Epidemiologic studies have consistently shown that HDL cholesterol levels are inversely associated with CV events in the general population (20). However, increasing amounts of data, including the failure of 2 drugs that increased HDL cholesterol levels to reduce CV risk in large clinical trials (21,22), suggest that the function, rather than the level, of HDL cholesterol may be a better determinant of the protective capacity of HDL.
The antiatherogenic function of HDL involves its ability to promote cholesterol efflux directly from peripheral tissues, as well as its ability to protect LDL against oxidation and neutralize already-formed oxidized phospholipids that produce inflammation in the arterial wall (6–10). The latter function has been shown to be abnormal in RA patients compared to controls and may be particularly relevant to RA patients, who may have a lesser histologic extent of atherosclerosis but a greater degree of arterial inflammation in comparison to matched controls (23).
Paraoxonase 1 is an HDL-associated enzyme that promotes the antioxidant function of HDL by protecting phospholipids in LDL from oxidation and by inactivating already-formed oxidized phospholipids (10,11). Paraoxonase 1 also protects HDL itself from oxidation, thereby directly promoting the antiinflamma-tory capacity of HDL (12). Previous studies have suggested that paraoxonase 1 activity is decreased in RA patients as compared to healthy controls (24,25) and may be increased following RA treatment (26). The PON1 Q192R genotype distribution in RA patients compared to controls has also been examined, with varying results in different populations (25,27). In the current study, we first observed that the PON1 Q192R polymorphism is functional in RA patients, resulting in marked differences in plasma paraoxonase 1 activity depending on the PON1 Q192R genotype. Patients with the RR genotype had significantly higher paraoxonase 1 activity compared to patients with either the QR geno-type or the QQ genotype, and patients with the QR genotype had significantly higher paraoxonase 1 activity compared to patients with the QQ genotype.
We next observed a significant association of the PON1 Q192R polymorphism with carotid plaque in RA patients after controlling for traditional CV risk factors and other significant correlates of plaque noted on bivariate analysis. Patients with the RR genotype had less risk of carotid plaque compared to patients with the QR or QQ genotype.
The PON1 Q192R polymorphism and the functional activity of paraoxonase 1 have previously been associated with both prevalent coronary artery disease and incident CV events in the general population (13). In a prospective study of 1,399 patients undergoing diagnostic coronary angiography, patients with the QQ genotype had an increased risk of major cardiac events, and patients with paraoxonase 1 activity in the highest quartile had the lowest risk of events (13).
We also examined plasma paraoxonase 1 activity in our cohort to further evaluate the association of paraoxonase 1 with CV risk in patients with RA. These data demonstrated a significant association of paraoxonase 1 activity with carotid plaque on multivariate analysis. Higher plasma paraoxonase 1 activity was associated with lower risk of plaque.
The presence of carotid plaque on ultrasound has previously been assessed and validated as a surrogate marker of CV risk in RA patients (28). Evans et al described a 636-patient RA cohort in which 66 incident acute coronary events occurred during 3,403 person-years of followup (28). In this cohort, the presence of unilateral carotid plaque at baseline was associated with a 2.5-fold increased risk of acute coronary syndrome (28). The current cohort consisted primarily of RA patients without known CV disease. Three patients did have a known history of CV events, and all 3 of these patients had carotid plaque on ultrasound.
Kerekes et al previously evaluated a potential association of CCA-IMT and flow-mediated dilation and nitroglycerine-mediated vasodilation with serum paraoxonase 1 activity in 52 RA patients and 40 matched healthy controls (29). While paraoxonase 1 activity was not associated with any of the CV risk measures in this analysis, paraoxonase 1 activity was significantly correlated with serum levels of TNFα and interleukin-6 in the RA patients, suggesting a possible relationship to RA disease activity (29).
A modest but significant correlation between plasma paraoxonase 1 activity and systemic inflammation as measured by the hsCRP level was noted in our RA cohort. Higher hsCRP levels were associated with lower paraoxonase 1 activity. A previous proteomics study of HDL by our group also demonstrated a trend toward decreased levels of paraoxonase 1 protein in association with abnormal HDL with poor antioxidant capacity in patients with active inflammatory RA (30). In addition, Popa et al have reported increased paraoxonase 1 activity following therapy with infliximab in a cohort of 45 RA patients (26).
To our knowledge, this is the first study to evaluate both the genetic and biochemical determinants of paraoxonase 1 activity in relation to CV risk in RA patients. Certainly, however, the study has several limitations. Given the relatively small sample size, the data must be treated as preliminary and must be validated in larger cohort studies. In addition, studies of hard CV end points, including death from CV causes and myocardial infarction, remain the “gold standard” in assessing potential CV biomarkers and mechanisms of increased CV risk. The feasibility of these studies is often limited by the numbers of patients required, as well as by the duration of followup needed in a relatively rare disease. In addition, while multivariate analysis was performed to account for other patient factors associated with the presence of carotid plaque in this cohort, it is possible that a role was played by other disease factors that were not assessed. Finally, most of our RA patient population was Caucasian; further investigation is warranted in other ethnic groups.
In summary, our findings suggest a significant association of the PON1 Q192R polymorphism and plasma paraoxonase 1 activity with CV risk in RA patients, as measured by the presence of carotid plaque. Our data support previous work implicating abnormal HDL function as a potential mechanism and biomarker of CV risk in RA patients. Further large-scale studies, including studies of both biochemical and genetic determinants of paraoxonase 1, are warranted to confirm these findings. Identification of alternative pathways that account for the increased CV risk in RA patients remains important so that appropriate primary prevention strategies and targeted CV therapeutics can be developed.
Acknowledgments
Supported by the NIH (National Heart, Lung, and Blood Institute grants 5K23-HL-094834 to Dr. Charles-Schoeman and 5R01-HL-082823 to Dr. Reddy) and the Arthritis Foundation, Southern California Chapter.
Footnotes
Dr. Charles-Schoeman has received consulting fees and speaking fees from Pfizer (more than $10,000) and has received research grants from Bristol-Myers Squibb and Pfizer. Dr. Ranganath has received consulting fees from UCB (less than $10,000) and has received research grants from UCB and Bristol-Myers Squibb.
AUTHOR CONTRIBUTIONS
All authors were involved in drafting the article or revising it critically for important intellectual content, and all authors approved the final version to be published. Dr. Charles-Schoeman had full access to all of the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Study conception and design. Charles-Schoeman, Reddy.
Acquisition of data. Charles-Schoeman, Lee, Shahbazian, Gorn, FitzGerald, Ranganath, Taylor, Ragavendra, McMahon.
Analysis and interpretation of data. Charles-Schoeman, Lee, Shahbazian, Elashoff, Reddy.
REFERENCES
- 1.Fischer LM, Schlienger RG, Matter C, Jick H, Meier CR. Effect of rheumatoid arthritis or systemic lupus erythematosus on the risk of first-time acute myocardial infarction. Am J Cardiol. 2004;93:198–200. doi: 10.1016/j.amjcard.2003.09.037. [DOI] [PubMed] [Google Scholar]
- 2.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, et al. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–7. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 3.Del Rincon I, Williams K, Stern MP, Freeman GL, Escalante A. High incidence of cardiovascular events in a rheumatoid arthritis cohort not explained by traditional cardiac risk factors. Arthritis Rheum. 2001;44:2737–45. doi: 10.1002/1529-0131(200112)44:12<2737::AID-ART460>3.0.CO;2-%23. [DOI] [PubMed] [Google Scholar]
- 4.Charles-Schoeman C, Watanabe J, Lee YY, Furst DE, Amjadi S, Elashoff D, et al. Abnormal function of high-density lipoprotein is associated with poor disease control and an altered protein cargo in rheumatoid arthritis. Arthritis Rheum. 2009;60:2870–9. doi: 10.1002/art.24802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Charles-Schoeman C, Lee YY, Grijalva V, Amjadi S, FitzGerald J, Ranganath VK, et al. Cholesterol efflux by high density lipoproteins is impaired in patients with active rheumatoid arthritis. Ann Rheum Dis. 2012;71:1157–62. doi: 10.1136/annrheumdis-2011-200493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Navab M, Hama SY, Anantharamaiah GM, Hassan K, Hough GP, Watson AD, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: steps 2 and 3. J Lipid Res. 2000;41:1495–508. [PubMed] [Google Scholar]
- 7.Navab M, Hama SY, Cooke CJ, Anantharamaiah GM, Chaddha M, Jin L, et al. Normal high density lipoprotein inhibits three steps in the formation of mildly oxidized low density lipoprotein: step 1. J Lipid Res. 2000;41:1481–94. [PubMed] [Google Scholar]
- 8.Navab M, Hama-Levy S, Van Lenten BJ, Fonarow GC, Cardinez CJ, Castellani LW, et al. Mildly oxidized LDL induces an increased apolipoprotein J/paraoxonase ratio. J Clin Invest. 1997;99:2005–19. doi: 10.1172/JCI119369. published erratum appears in J Clin Invest 1997;99:3043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Watson AD, Navab M, Hama SY, Sevanian A, Prescott SM, Stafforini DM, et al. Effect of platelet activating factor-acetylhydrolase on the formation and action of minimally oxidized low density lipoprotein. J Clin Invest. 1995;95:774–82. doi: 10.1172/JCI117726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackness MI, Arrol S, Durrington PN. Paraoxonase prevents accumulation of lipoperoxides in low-density lipoprotein. FEBS Lett. 1991;286:152–4. doi: 10.1016/0014-5793(91)80962-3. [DOI] [PubMed] [Google Scholar]
- 11.Durrington PN, Mackness B, Mackness MI. Paraoxonase and atherosclerosis. Arterioscler Thromb Vasc Biol. 2001;21:473–80. doi: 10.1161/01.atv.21.4.473. [DOI] [PubMed] [Google Scholar]
- 12.Costa LG, Cole TB, Jarvik GP, Furlong CE. Functional genomic of the paraoxonase (PON1) polymorphisms: effects on pesticide sensitivity, cardiovascular disease, and drug metabolism. Annu Rev Med. 2003;54:371–92. doi: 10.1146/annurev.med.54.101601.152421. [DOI] [PubMed] [Google Scholar]
- 13.Bhattacharyya T, Nicholls SJ, Topol EJ, Zhang R, Yang X, Schmitt D, et al. Relationship of paraoxonase 1 (PON1) gene polymorphisms and functional activity with systemic oxidative stress and cardiovascular risk. JAMA. 2008;299:1265–76. doi: 10.1001/jama.299.11.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Costa LG, Vitalone A, Cole TB, Furlong CE. Modulation of paraoxonase (PON1) activity. Biochem Pharmacol. 2005;69:541–50. doi: 10.1016/j.bcp.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 15.Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS, et al. The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum. 1988;31:315–24. doi: 10.1002/art.1780310302. [DOI] [PubMed] [Google Scholar]
- 16.Prevoo ML, van 't Hof MA, Kuper HH, van Leeuwen MA, van de Putte LB, van Riel PL. Modified disease activity scores that include twenty-eight–joint counts: development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38:44–8. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 17.Fries JF, Spitz P, Kraines RG, Holman HR. Measurement of patient outcome in arthritis. Arthritis Rheum. 1980;23:137–45. doi: 10.1002/art.1780230202. [DOI] [PubMed] [Google Scholar]
- 18.Bild DE, Detrano R, Peterson D, Guerci A, Liu K, Shahar E, et al. Ethnic differences in coronary calcification: the Multi-Ethnic Study of Atherosclerosis (MESA). Circulation. 2005;111:1313–20. doi: 10.1161/01.CIR.0000157730.94423.4B. [DOI] [PubMed] [Google Scholar]
- 19.Charles-Schoeman C, Khanna D, Furst DE, McMahon M, Reddy ST, Fogelman AM, et al. Effects of high-dose atorvastatin on antiinflammatory properties of high density lipoprotein in patients with rheumatoid arthritis: a pilot study. J Rheumatol. 2007;34:1459–64. [PubMed] [Google Scholar]
- 20.Rader DJ. Molecular regulation of HDL metabolism and function: implications for novel therapies. J Clin Invest. 2006;116:3090–100. doi: 10.1172/JCI30163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barter PJ, Caulfield M, Eriksson M, Grundy SM, Kastelein JJ, Komajda M, et al. the ILLUMINATE Investigators Effects of torcetrapib in patients at high risk for coronary events. N Engl J Med. 2007;357:2109–22. doi: 10.1056/NEJMoa0706628. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz GG, Olsson AG, Abt M, Ballantyne CM, Barter PJ, Brumm J, et al. the dal-OUTCOMES Investigators Effects of dalcetrapib in patients with a recent acute coronary syndrome. N Engl J Med. 2012;367:2089–99. doi: 10.1056/NEJMoa1206797. [DOI] [PubMed] [Google Scholar]
- 23.Aubry MC, Maradit-Kremers H, Reinalda MS, Crowson CS, Edwards WD, Gabriel SE. Differences in atherosclerotic coronary heart disease between subjects with and without rheumatoid arthritis. J Rheumatol. 2007;34:937–42. [PubMed] [Google Scholar]
- 24.Isik A, Koca SS, Ustundag B, Celik H, Yildirim A. Paraoxonase and arylesterase levels in rheumatoid arthritis. Clin Rheumatol. 2007;26:342–8. doi: 10.1007/s10067-006-0300-8. [DOI] [PubMed] [Google Scholar]
- 25.Tanimoto N, Kumon Y, Suehiro T, Ohkubo S, Ikeda Y, Nishiya K, et al. Serum paraoxonase activity decreases in rheumatoid arthritis. Life Sci. 2003;72:2877–85. doi: 10.1016/s0024-3205(03)00195-4. [DOI] [PubMed] [Google Scholar]
- 26.Popa C, van Tits LJ, Barrera P, Lemmers HL, van den Hoogen FH, van Riel PL, et al. Anti-inflammatory therapy with tumour necrosis factor α inhibitors improves high-density lipoprotein cholesterol antioxidative capacity in rheumatoid arthritis patients. Ann Rheum Dis. 2009;68:868–72. doi: 10.1136/ard.2008.092171. [DOI] [PubMed] [Google Scholar]
- 27.Elfasakhany F, Mona M, Hussein M, Beyari M, Abou-Elnoeman S. Paraoxonase 1 (PON1) Glu 192 Arg gene polymorphism and serum paraoxonase activity in rheumatoid arthritis among Egyptians. Tanta Med Sc J. 2010;5:103–11. [Google Scholar]
- 28.Evans MR, Escalante A, Battafarano DF, Freeman GL, O'Leary DH, del Rincon I. Carotid atherosclerosis predicts incident acute coronary syndromes in rheumatoid arthritis. Arthritis Rheum. 2011;63:1211–20. doi: 10.1002/art.30265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kerekes G, Szekanecz Z, Der H, Sandor Z, Lakos G, Muszbek L, et al. Endothelial dysfunction and atherosclerosis in rheumatoid arthritis: a multiparametric analysis using imaging techniques and laboratory markers of inflammation and autoimmunity. J Rheumatol. 2008;35:398–406. [PubMed] [Google Scholar]
- 30.Watanabe J, Charles-Schoeman C, Miao Y, Elashoff D, Lee YY, Katselis G, et al. Proteomic profiling following immunoaffinity capture of high-density lipoprotein: association of acute-phase proteins and complement factors with proinflammatory high-density lipoprotein in rheumatoid arthritis. Arthritis Rheum. 2012;64:1828–37. doi: 10.1002/art.34363. [DOI] [PMC free article] [PubMed] [Google Scholar]