Abstract
Background
Loose anagen hair syndrome (LAHS) is typically diagnosed in girls older than 2 years who present with hair that "will not grow". Hair microscopic examination shows absent inner and outer root sheaths, ruffling of the cuticle on the proximal hair shaft and deformed pigmented anagen bulbs.
Objective
The aim of the study was to assess whether there are characteristic trichoscopic features favoring the diagnosis of LAHS.
Patients and methods
Eighty nine children patients were included into the study (24 girls with LAHS, 25 with alopecia areata, 20 with telogen effluvium and 20 healthy children). In all groups trichoscopy was performed. Trichoscopy images were analyzed for abnormalities in the hairs shafts, the hair follicle openings and the interfollicular area.
Results
Dirty dots were present in all groups. A unique feature of LAHS was the presence of rectangular black granular structures which differs from dense black dots seen in patients with alopecia areata. This feature was observed in 71% of patients with LAHS. Follicular units with single hairs constituted 92,9% of hair units in these patients (65,5% in telogen effluvium and 53% in the control group). Solitary yellow dots were found in 50% of patient with LAHS and in 24% of patients with alopecia areata, but was not found in control group or in patients with telogen effluvium.
Conclusion
The trichoscopy features favoring the diagnosis of LAHS are: rectangular black granular structures, solitary yellow dots and major predominance of follicular units with single hairs.
Keywords: alopecia, dermatoscopy, dermoscopy, hair, hair loss
Background
Loose anagen hair syndrome (LAHS) is an autosomal dominant trait, with incomplete penetrance and variable and age-dependent expression. It is typically diagnosed in young girls older than 2 years who present with hair that "will not grow" or hair that is thin and sparse but adequate to cover the scalp. Usually the hair color is blonde but in the recent years LAHS in brown and black hairs was described.[1]
Clinical presentation is heterogeneous. Three primary LAHS phenotypes have been described based mainly on the clinical signs of reduced hair, hair length, increased hair shedding and altered hair texture: type A, with sparse hair that does not grow long; type B, characterized by diffuse or patchy, unruly hair; and type C, characterized by normal appearing hair with excessive shedding of loose anagen hairs and predominantly affects adults.[2] There is a tendency for the clinical presentation of patients with LAHS types A and B to evolve into LAHS type C with age.[3]
The diagnosis relies on the number and percentage of hairs in the pull test and loose anagen hairs in the trichogram. Pull test reveals more than 3 to 10 hairs which are easily and painlessly plucked.[3,4] Loose anagen hairs have absent inner and outer root sheaths, ruffling of the cuticle on the proximal hair shaft (floppy sock appearance), and deformed pigmented anagen bulbs that may appear long and tapered, twisted, or positioned at an acute angle to the long axis of the hair shaft [Fig. 1].[5,6]
Figure 1.

Microscopic hair examination in loose anagen syndrome reveals absent inner and outer root sheaths, ruffling of the cuticle on the proximal hair shaft (floppy sock appearance), and deformed pigmented anagen bulbs. (x10)
Tosti et al.[9] proposed diagnostic criteria for LAHS that include positive pull test results with painless extraction of at least 10 loose anagen hairs and the presence of more than 80% loose anagen hairs on trichogram, but these criteria may be considered too strict to identify individuals who are mildly affected to be diagnosed. Therefore revised criteria were suggested, which indicate that the diagnosis of LAHS should be established when the trichogram shows at least 70% loose anagen hairs.[1] Cantatore-Francis and Orlow proposed that LAHS should be diagnosed only when there are more than 50% loose anagen hairs in the trichogram.[10]
Trichoscopy (hair and scalp dermoscopy) is a rapid in-office technique, which has become a standard procedure in differential diagnosis of hair loss.[11,13] However, there are no specific trichoscopy features that favor the diagnosis of LAHS.[14] The differential diagnosis includes alopecia areata (AA), trichotillomania, congenital hair shafts disorders and telogen effluvium (TE). Trichotillomania and hair shafts abnormalities have characteristic trichoscopy features and can be easily recognized by trichoscopy.[14-18]
The aim of the study was to assess whether there are any specific trichoscopy features which may facilitate the the diagnosis of LAHS.
Patients and methods
This retrospective study included 89 children patients, which were seen in our outpatient clinic between 2008-2015. Twenty four children (all girls) were diagnosed with LAHS (diagnosis confirmed by trichogram), 25 (11 girls and 14 boys) with alopecia areata (diagnosis confirmed by clinical examination and trichoscopy, histopathological and mycological examination only in doubtful cases), 20 (12 girls and 8 boys) with TE (diagnosis confirmed by trichogram) and 20 (11 girls and 9 boys) were healthy patients (control group). The groups were adjusted for age. The mean age of children was 5.25 (range 3-9) years in LAHS group, 6.2 (range 3-10) years in AA group, 6.5 (range 4-9) years in TE group and 6.55 (range 3-10) years in healthy control group.
Trichoscopy was performed with the use Fotofinder II (5-8 images at each, the 20- and 70-fold magnification). A total of 1126 images were analyzed by two independent blinded evaluators who evaluated abnormalities in hair shaft structure and skin surface. After the evaluation results were unblinded, the trichoscopic features were assigned to respective patients groups.
The occurrence of the scored trichoscopic criteria within each group was evaluated by Games-Howell multiple comparisons test and ANOVA. Significance level was set to p<0,001.
Results
Trichoscopy features seen in analyzed images were: dirty dots, single rectangular black granular structures, groups of black dots dense in structure, single yellow dots, clustered yellow dots, exclamation-mark hairs, percentage of pilosebaceous units with single hair, percentage of pilosebaceous units with triple hairs.
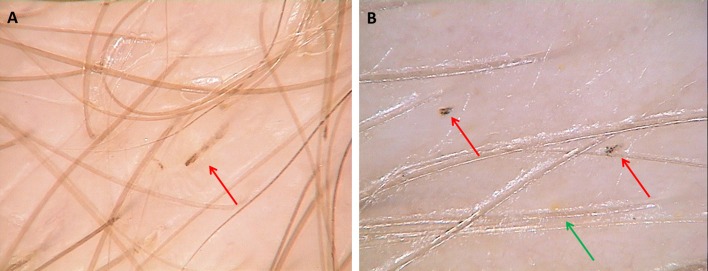
Dirty dots were a common finding in all groups: 75% in LAHS group (18/24) vs 75% in control group (15/20) vs 72% in AA group (18/25) and 72% in TE group (15/20). Statistical differences between groups were not significant. A unique feature seen only in LAHS (71%; 17/24) was the presence of rectangular black granular structures [Fig. 2] different from black dots with uniform dense structure seen in patients with AA (44%; 11/25) [Fig. 3].
Figure 2.
Trichoscopy in loose anagen hair syndrome shows black rectangular features with granular structure (A,B; red arrows) and solitary yellow dots seen by trichoscopy in loose anagen hair syndrome (B; green arrow). (x70)
Figure 3.
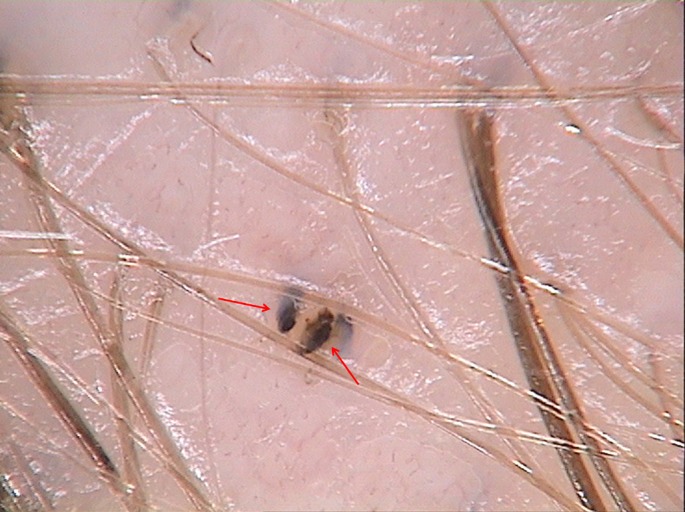
In alopecia areata trichoscopy reveals black dots which are dense in structure (red arrows). (x70)
Follicular units with single hair constituted 92,9% of hair units in LAHS group, 53,5% in control group and 65,5% in TE group (p<0,001). Follicular units with three or more hairs were seen in healthy controls (6%) and in TE (1,5%), not in LAHS. AA images were not searched for this parameter because of a different clinical presentation (focal). Solitary yellow dots [Fig. 4] were found in 50% of patient with LAHS (12/24) and this feature was not found in control group and in TE, although they was seen in 24% of patients with AA (6/25) (p<0,001). Clustered yellow dots defined as three or more yellow dots in adjacent follicular units were seen only in AA group (68%; 17/25), not in others (p<0,001). Exclamation mark hairs were seen only in children with AA (68%; 17/25, p<0,001).
Figure 4.
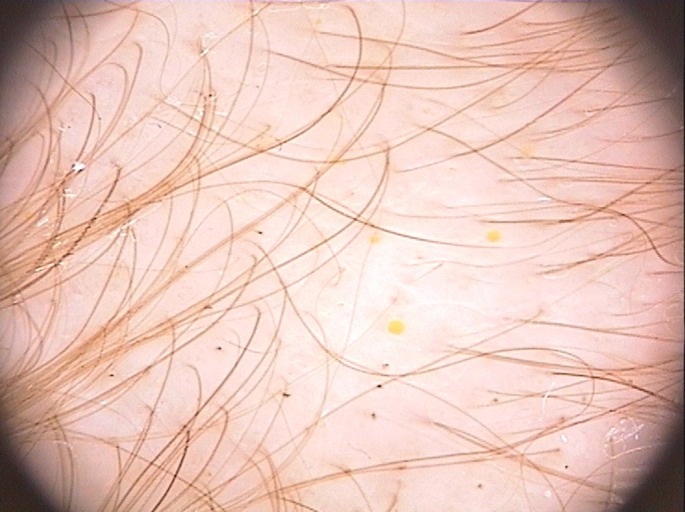
Black rectangular features and solitary yellow dots seen by trichoscopy in loose anagen hair syndrome. (x20)
The numbers and frequency of trichoscopy features in patients with LAHS, AA, TE and healthy control are presented in detail in Table 1.
Table 1. Trichoscopy features in patients with loose anagen hair syndrome, alopecia areata, telogen effluvium and healthy controls.
| LAHS number of patients (%) |
AA number of patients (%) |
TE number of patients (%) |
HC number of patients (%) |
Statistical significance | |||
|---|---|---|---|---|---|---|---|
| LAHS vs AA | LAHS vs TE | LAHS vs HC | |||||
| Dirty dots | 18/24 (75%) | 18/25 (72%) | 15/20 (72%) |
15/20 (75%) | NS | NS | NS |
| Rectangular granular black structures | 17/24 (71%) | 2/25 (8%) |
0/25 | 0/25 | p<0,001 | p<0,001 | p<0,001 |
| Groups of dense black dots | 0/24 (34%) | 11/25 (44%) | 0/25 | 0/20 | p<0,001 | P<0,001 | P<0,001 |
| Solitary yellow dots | 12/24 (50%) | 6/25 (24%) | 1/25 (4%) |
1/20 (5%) | p<0,001 | p<0,001 | p<0,001 |
| Clustered yellow dots | 0/24 (%) | 17/25 (68%) | 0/25 | 0/20 | p<0,001 | NS | NS |
| Exclamation-mark hair | 0/24 | 17/25 (68%) |
0/25 | 0/20 | p<0,001 | NS | NS |
| Percentage of follicular units with single hair | 0,929 | / | 0,535 | 0,655 | / | p<0,001 | P<0,001 |
| Percentage of follicular units with three hair | 0 | / | 0,015 | 0,06 | / | NS | p<0,001 |
| * LAHS = loose anagen hair syndrome; AA = alopecia areata; TE = telogen effluvium; HC= healthy controls; NS = not significant; | |||||||
Discussion
On scanning electron microscopy of loose anagen hair, there are distortions in the shape and a pulling back of the cuticles of the proximal hair shaft, which correspond to the ruffling of the cuticle seen with light microscopy. The main defect is presumed to be abnormal adhesion between the cuticle of the inner root sheath and the cuticle of the hair shaft. The absent inner root sheath in a pulled anagen hair in the context of LAHS supports this concept. The major pathological changes of loose anagen hair in LAHS were found in the inner root sheath of anagen follicles. These consist of vacuolization and intercellular edema in Huxley cells, and dyskeratotic changes of Henle cells and cuticle cells of both the inner root sheath and hair shaft. These structural abnormalities conceivably could interfere with the normal interdigitation of the cuticle cells of the inner root sheath and hair shaft and disturb the normal anchoring function of the inner root sheath. Ultimately this would allow hairs to be easily pulled out.[8]
Black dots seen in trichoscopy are usually assumed as a "cadaverized" hairs or hair residues in the follicular opening. These are hairs that have undergone necrosis as a result of a severe inflammatory process but are still retained within a hair follicle. Black dots are most commonly found in alopecia areata, and they may be present in groups.[19] Black structures seen in our study in patients with LAHS were solitary and differ from those seen in AA in a structure and shape. Black dots in AA usually have a dense structure, while the structures visible in LAHS are rather rectangular in shape and have a granular structure.
Our concept is that the rectangular shape of black structures corresponds to rectangular shape of an anagen follicle, which has become "rapidly empty" in the course of LAHS.
Yellow dots are follicular openings lacking hair shafts but filled with sebum or keratotic material. In cases of LAHS the solitary yellow dots are seen next to rectangular black structures. The chronology of events is unknown. We hypothesize that most probably the black structure is visible short after the anagen hair is lost and over time a yellow dot develops and is increasingly visible before a new upright regrowing hair or pig-tail hair [Fig. 5] appears.
Figure 5.
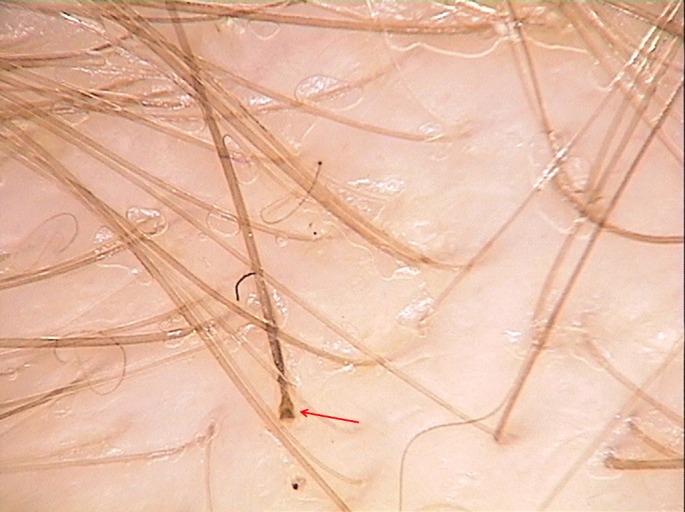
Easily pull out hair with rectangular anagen bulb in loose anagen syndrome seen by trichoscopy (red arrow). Pig-tail hairs are sometimes seen as a result of hair regrowth.
Follicular units with single hair constituted 92,9% of hair units in LAHS group and 65,5% in control group and the difference was statistically significant (p<0,001). Units with 3 hairs were found in healthy patients and in the TE group (AA group was not examined for this parameter because of focal hair loss). Triple hair units can be assumed as good hair health condition. A pulled hair with an anagen bulb is rarely seen in LAHS by trichoscopy [Fig. 5].
Conclusion
Trichoscopy may be helpful in differential diagnosis of LAHS. The characteristic trichoscopy features of the disease are: solitary rectangular black granular structures (which differ from densely structured black dots seen in alopecia areata), solitary yellow dots, and major predominance of follicular units with single hairs.
References
- Tosti A, Piraccini BM. Loose anagen hair syndrome and loose anagen hair. Arch Dermatol. 2002;138:521–522. doi: 10.1001/archderm.138.4.521. [DOI] [PubMed] [Google Scholar]
- Chapman DM, Miller RA. An objective measurement of the anchoring strength of anagen hair in an adult with the loose anagen hair syndrome. J Cutan Pathol. 1996;23:288–292. doi: 10.1111/j.1600-0560.1996.tb01481.x. [DOI] [PubMed] [Google Scholar]
- Olsen EA, Bettencourt MS, Coté NL. The presence of loose anagen hairs obtained by hair pull in the normal population. J Investig Dermatol Symp Proc. 1999;4:258–260. doi: 10.1038/sj.jidsp.5640225. [DOI] [PubMed] [Google Scholar]
- Chong AH, Sinclair R. Loose anagen syndrome: a prospective study of three families. Australas J Dermatol. 2002;43:120–124. doi: 10.1046/j.1440-0960.2002.00572.x. [DOI] [PubMed] [Google Scholar]
- Price VH, Gummer CL. Loose anagen syndrome. J Am Acad Dermatol. 1989;20(2 Pt 1):249–256. doi: 10.1016/s0190-9622(89)70030-x. [DOI] [PubMed] [Google Scholar]
- Abdel-Raouf H, El-Din WH, Awad SS, Esmat A, Al-Khiat M, Abdel-Wahab H, Fakahani H, Al-Domyati M, El-Din Anber T, El-Tonsy MH. Loose anagen hair syndrome in children of Upper Egypt. J Cosmet Dermatol. 2009;8:103–107. doi: 10.1111/j.1473-2165.2009.00434.x. [DOI] [PubMed] [Google Scholar]
- Dey V, Thawani M. Loose anagen hair syndrome in black-haired Indian children. Pediatr Dermatol. 2013;30:579–583. doi: 10.1111/pde.12208. [DOI] [PubMed] [Google Scholar]
- Mirmirani P, Uno H, Price VH. Abnormal inner root sheath of the hair follicle in the loose anagen hair syndrome: an ultrastructural study. J Am Acad Dermatol. 2011;64:129–134. doi: 10.1016/j.jaad.2010.01.026. [DOI] [PubMed] [Google Scholar]
- Tosti A, Peluso AM, Misciali C, Venturo N, Patrizi A, Fanti PA. Loose anagen hair. Arch Dermatol. 1997;133:1089–1093. [PubMed] [Google Scholar]
- Cantatore-Francis JL, Orlow SJ. Practical guidelines for evaluation of loose anagen hair syndrome. Arch Dermatol. 2009;145:1123–1128. doi: 10.1001/archdermatol.2009.220. [DOI] [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy: a new method for diagnosing hair loss. J Drugs Dermatol. 2008;7:651–654. [PubMed] [Google Scholar]
- Rudnicka L, Olszewska M, Rakowska A, Slowinska M. Trichoscopy update 2011. J Dermatol Case Rep. 2011;5:82–88. doi: 10.3315/jdcr.2011.1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olszewska M, Rudnicka L, Rakowska A, Kowalska-Oledzka E, Slowinska M. Trichoscopy. Arch Dermatol. 2008;144:1007. doi: 10.1001/archderm.144.8.1007. [DOI] [PubMed] [Google Scholar]
- Lencastre A, Tosti A. Role of trichoscopy in children's scalp and hair disorders. Pediatr Dermatol. 2013;30:674–682. doi: 10.1111/pde.12173. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Kowalska-Oledzka E, Slowinska M, Rosinska D, Rudnicka L. Hair shaft videodermoscopy in netherton syndrome. Pediatr Dermatol. 2009;26:320–322. doi: 10.1111/j.1525-1470.2008.00778.x. [DOI] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Czuwara J, Olszewska M, Rudnicka L. Dermoscopy as a tool for rapid diagnosis of monilethrix. J Drugs Dermatol. 2007;6:222–224. [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Kowalska-Oledzka E, Rudnicka L. Trichoscopy in genetic hair shaft abnormalities. J Dermatol Case Rep. 2008;2:14–20. doi: 10.3315/jdcr.2008.1009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowska A, Slowinska M, Olszewska M, Rudnicka L. New trichoscopy findings in trichotillomania: flame hairs, V-sign, hook hairs, hair powder, tulip hairs. Acta Derm Venereol. 2014;94:303–306. doi: 10.2340/00015555-1674. [DOI] [PubMed] [Google Scholar]
- Kowalska-Oledzka E, Slowinska M, Rakowska A, Czuwara J, Sicinska J, Olszewska M, Rudnicka L. 'Black dots' seen under trichoscopy are not specific for alopecia areata. Clin Exp Dermatol. 2012;37:615–619. doi: 10.1111/j.1365-2230.2012.04401.x. [DOI] [PubMed] [Google Scholar]