Abstract
Background
The correlation between overall survival (OS) and progression-free survival (PFS) has been evaluated in patients with metastatic or advanced gastric cancer who have received first-line and/or second-line chemotherapy. However, no corresponding analysis has been done for patients who have undergone third-line or later-line chemotherapy.
Methods
A total of 303 patients from the Phase II/III studies of apatinib were pooled (the Phase II study as a training data set, the Phase III study as a testing data set). Landmark analyses of PFS at 2 months from randomization were performed to minimize lead time bias. The Cox proportional hazard model was used to test for the significance effect of PFS rate at 2 months in predicting OS. Additionally, the PFS/OS correlations were evaluated by the normal induced copula (National Institute for Health and Care Excellence) estimation model.
Results
The median OS was 3.37 months (95% confidence interval 2.63–3.80) in patients who experienced progression at 2 months and 5.67 months in patients who did not (95% confidence interval 4.83–6.67; P<0.0001). Compared with patients who did not progress at 2 months, the adjusted hazard ratio for death was 3.39 (95% confidence interval 1.79–6.41; P<0.0001) for patients who experienced progression at 2 months. Moreover, the correlation of PFS/OS was 0.84 (95% confidence interval 0.74–0.90). Similar results were found in the testing data set.
Conclusion
These results indicate that PFS correlates strongly with OS, suggesting PFS may be a useful early endpoint for patients with advanced gastric cancer who have undergone third-line or later-line chemotherapy. These observations require prospective validation.
Keywords: gastric cancer, surrogate endpoint, progression-free survival, overall survival
Introduction
Gastric cancer is the third most common cause of cancer-related death worldwide.1 When gastric cancer is found very early, there is a better chance of recovery. However, the prognosis of patients with metastatic or advanced gastric cancer (AGC) remains poor, with a median overall survival (OS) of one year in patients treated with the commonly used first-line chemotherapy regimens (fluoropyrimidine plus a platinum agent with or without docetaxel or anthracyclines).2–6 Although there may be clinical remission or disease stabilization in many AGC patients who receive first-line chemotherapy, most will ultimately experience disease progression and be candidates for further chemotherapy. For patients who have experienced disease progression during or after first-line chemotherapy for AGC, effective second-line chemotherapy is essential. In recent years, significant achievements have been made in second-line chemotherapy for AGC.7–12 However, after failure of second-line chemotherapy, the results of further treatment are poor, yielding response rates of 0%–5% with no evidence of prolonged survival.13,14 It should be noted that further active treatments beyond second-line chemotherapy would increase with the increasing number of patients with AGC offered second-line chemotherapy. Apatinib (YN968D1), is a tyrosine kinase inhibitor that selectively inhibits vascular endothelial growth factor receptor 2, which is a new treatment option providing hope for patients with AGC who have previously failed second-line chemotherapy.15 In a Phase III study, patients with AGC who were treated with apatinib had significantly longer progression-free survival (PFS) and OS than patients given placebo (OS, 195 days versus 140 days, hazard ratio [HR] 0.71, 95% confidence interval [CI] 0.54–0.94, P<0.016; PFS, 78 days versus 53 days, HR 0.44, 95% CI 0.33–0.61, P<0.0001), but the OS benefit, weakened by the post progression survival, was weaker than the treatment effect on PFS.16 Therefore, it is likely that PFS may be a reasonable endpoint for this clinical trial.
In previous reviews, the correlation between OS and PFS has been estimated for patients with AGC in various treatment settings to evaluate surrogacy. A trial-level analysis included 36 randomized trials and demonstrated that, in patients with AGC, PFS strongly correlated with OS in clinical trial settings, and a similar conclusion was reached in patients with AGC who had undergone first-line chemotherapy based on individual-level data.17,18 Both conclusions were only applicable to patients with first-line chemotherapy, and the authors did not externally validate their findings. However, Paoletti et al reached a different conclusion, ie, that the validity of PFS as a surrogate endpoint for OS in AGC was not confirmed using individual-level patient data from the GASTRIC meta-analysis, and this study included patients on first-line treatment.19 Further, in the setting of second-line chemotherapy for patients with AGC, another trial-level analysis showed that PFS did not correlate sufficiently with OS and could not be used as an efficient surrogate endpoint.20
To the best of our knowledge, no corresponding analysis has been done for AGC patients who have had third-line or later-line chemotherapy. Therefore, the aim of this study is to examine whether PFS is a valid intermediate endpoint of OS in AGC patients who have undergone third-line or later-line treatment. We use the data from two randomized, placebo-controlled, parallel-arm clinical trials (ClinicalTrials.gov identifiers NCT00970138 and NCT01512745), which tested the molecular targeted therapy, apatinib, in similar patient populations.21 For these studies, it is reasonable that one is used as a training data set and the other is used as a testing data set.
Materials and methods
Study population
A Phase II, randomized, double-blind, placebo-controlled trial served as the training data set, and was designed to assess the efficacy and safety of daily administration of apatinib as third-line or later-line treatment in patients with AGC and to determine whether a once-daily or a twice-daily regimen is better tolerated by these patients. From June 2009, 141 patients with AGC were randomized to receive 28-day cycles of placebo (n=48), apatinib 850 mg once daily (n=47), or apatinib 425 mg twice daily (n=46). Random assignment was stratified according to the number of organs with metastases (more than two sites versus up to two sites). Patients were eligible if they had progressed or been intolerant to second-line chemotherapy for AGC. Additional enrollment criteria were as follows: at least one measurable lesion as defined by Response Evaluation Criteria in Solid Tumors;22 an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; and acceptable hematologic, hepatic, and renal function. Patients with uncontrolled blood pressure on medication (>140/90 mmHg) or a bleeding tendency, and those receiving thrombolytics or anticoagulants were excluded. All participants gave their written informed consent, and approval was obtained from the relevant ethical committees.
Data from a Phase III clinical study of apatinib using the same drug doses and a similar protocol were considered as the testing data set. Study randomization was stratified according to the number of metastatic sites (more than two sites versus up to two sites). Between December 2010 and December 2012, 270 patients were randomized 2:1 to apatinib 850 mg once daily (n=180) and placebo (n=90). The inclusion and exclusion criteria for the Phase III clinical trial were similar to those for the Phase II trial.
Endpoints
PFS was considered as the primary endpoint and OS as the secondary endpoint in the Phase II clinical trial. However, in phase III clinical trial, the primary endpoint was OS and the secondary endpoint was PFS. Although the primary endpoints of the Phase II/III clinical trials were not the same, the definitions of PFS and OS in the Phase II study were the same as those in the Phase III study. PFS was defined as time from random assignment until disease progression or death, whichever occurred first. The time interval before progression or death was thus considered as PFS. OS was defined as the time from randomization to the date of death from any cause. Further, in the Phase II and III clinical trials, Response Evaluation Criteria in Solid Tumors was used to assess tumor response, and radiological assessment for disease progression was determined by five independent radiologists from different hospitals every two cycles (8 weeks). The PFS rate at 2 months was defined as a binary variable, ie, any patient experiencing any type of progression or death at or before 2 months was considered to have experienced event. Otherwise, the patient was censored.
Statistical analysis
In order to keep medication consistent in the two data sets, patients on apatinib 425 mg twice daily (n=46) were excluded from analysis, leaving 95 patients in the training data set. Landmark analyses of PFS at 2 months from randomization were performed to minimize lead time bias.23 The reason for choosing PFS rates at 2 months was that the median PFS in the Phase II clinical trial was approximately 2 months. Patients, who died before 2 months, were then excluded from the landmark analysis. 22 patients in the training data set and 40 patients in the testing data set were excluded, respectively.
The Kaplan–Meier product-limit method was used to estimate the OS distribution by the PFS rate at 2 months, and the median OS was calculated. The significance of the effect of the PFS rate at 2 months was assessed by the Cox proportional hazards regression model with adjustment for age, sex, ECOG performance status (1 versus 0), previous lines of chemotherapy (two versus three) and number of sites with metastasis (more than two sites versus up to two sites). A Bayesian semi-competing risks approach for jointly modelling PFS and OS was used to measure the correlation between PFS and OS. This new model is known as the normal induced copula estimation (NICE) model.24 Correlation between the variables can be directly derived from the joint model. This model was in a Bayesian framework so that the posterior distribution of the association parameter could be obtained, and an interval estimate of the association parameter was constructed using the posterior distribution of the association parameter. To investigate possible reasons for heterogeneity of correlation, subgroup analyses were conducted according to the vital status of patients (dead or not), ECOG performance status (1 versus 0), previous chemotherapy lines (more than three versus two chemotherapy lines), number of metastatic sites (more than two sites versus up to two sites), and treatment setting (apatinib versus placebo).
All analyses were based on intention-to-treat. Confidence intervals were calculated with two-sided probability coverage of 95%. All analyses were conducted using the R statistical package (R Foundation, Vienna, Austria),25 and P≤0.05 was considered to be statistically significant.
Results
Baseline characteristics
A total of 303 patients enrolled in two clinical trials with apatinib were included in present study. Baseline characteristics are outlined in Table 1. With the exception of ECOG performance status, the baseline characteristics of patients in the different data sets were similar with regard to sex ratio, age, time since initial diagnosis, prior surgery for primary tumor, disease stage, number of metastatic sites, previous lines of chemotherapy, prior radiotherapy, and laboratory variables. Further, neither trial was found to have imbalances between the experimental and control arms. Seventy-six percent of patients included in this analysis was male, the median age was 56 years, and time since initial diagnosis was 1.91 years. Seventy-three percent of patients received surgery to the primary tumor, 93% had stage IV disease, 26% had more than two metastatic sites, and 67% had already received second-line chemotherapy. Median hemoglobin, aspartate aminotransferase, blood urea nitrogen, alkaline phosphatase, lactate dehydrogenase, creatinine, albumin, and carcinoembryonic antigen levels were 11.4 g/dL, 24 IU/L, 5 mmol/L, 97 U/L, 187.5 U/L, 65 μmol/L, 40.8 g/L, and 10.46 μg/mL, respectively. Patients randomized to the testing data set had better performance status than those in the training data set.
Table 1.
Patient demographics and clinical characteristics of 303 patients randomized to two studies
| Variables | Training data set, n (%) (N=73) | Testing data set, n (%) (N=230) | Total, n (%) (N=303) |
|---|---|---|---|
| Sex | |||
| Male | 58 (79) | 172 (75) | 230 (76) |
| Female | 15 (21) | 58 (25) | 73 (24) |
| Median age, years | 54 | 58 | 56 |
| Q25–Q75 | 48–58 | 51–62 | 50–62 |
| Time since initial diagnosis, years | 2.08 | 1.86 | 1.91 |
| Q25–Q75 | 1.03–2.55 | 0.83–2.28 | 0.91–2.31 |
| ECOG PS | |||
| 0 | 4 (5) | 57 (25) | 61 (20) |
| 1 | 69 (95) | 173 (75) | 242 (80) |
| Prior surgery of primary tumor | |||
| Yes | 57 (78) | 164 (71) | 221 (73) |
| No | 16 (22) | 66 (29) | 82 (27) |
| Stage | |||
| II | 1 (1) | 2 (1) | 3 (1) |
| III | 3 (4) | 12 (5) | 15 (5) |
| IV | 69 (95) | 213 (93) | 282 (93) |
| Metastatic sites | |||
| ≤2 | 60 (82) | 164 (71) | 224 (74) |
| >2 | 13 (18) | 66 (29) | 79 (26) |
| Previous lines of chemotherapy | |||
| 2 | 52 (71) | 152 (66) | 204 (67) |
| ≥3 | 21 (29) | 78 (34) | 99 (33) |
| Prior radiotherapy | 10 (14) | 35 (15) | 45 (15) |
| Hemoglobin, g/dL | |||
| Median | 11.4 | 11.4 | 11.4 |
| Q25–Q75 | 10.7–12.6 | 10.3–12.6 | 10.4–12.6 |
| AST, IU/L | |||
| Median | 23 | 25 | 24 |
| Q25–Q75 | 19–27 | 18–37.03 | 18.1–34.2 |
| BUN, mmol/L | |||
| Median | 4.80 | 5 | 5 |
| Q25–Q75 | 3.91–5.96 | 3.91–5.88 | 3.91–5.895 |
| Alkaline phosphatase, U/L | |||
| Median | 89 | 98 | 97 |
| Q25–Q75 | 71.5–122 | 74.5–137 | 73.75–133 |
| LDH, U/L | |||
| Median | 192 | 186 | 187.5 |
| Q25–Q75 | 148–223.5 | 155.5–247.5 | 154–243.25 |
| Creatinine, μmol/L | |||
| Median | 63.5 | 65.6 | 65 |
| Q25–Q75 | 53–73 | 55.75–77 | 54.53–76.05 |
| Albumin, g/L | |||
| Median | 41.4 | 40.6 | 40.8 |
| Q25–Q75 | 38.1–45.78 | 37.1–43.65 | 37.7–44 |
| CEA, μg/mL | |||
| Median | 10.2 | 10.58 | 10.46 |
| Q25–Q75 | 2.58–65.21 | 3.45–78.83 | 3.4–76.51 |
| Treatment arm | |||
| Placebo | 31 (42) | 76 (33) | 107 (35) |
| Apatinib | 42 (58) | 154 (67) | 196 (65) |
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; AST, aspartate aminotransferase; BUN, blood urea nitrogen; LDH, lactate dehydrogenase; CEA, carcinoembryonic antigen.
PFS as a surrogate endpoint of OS
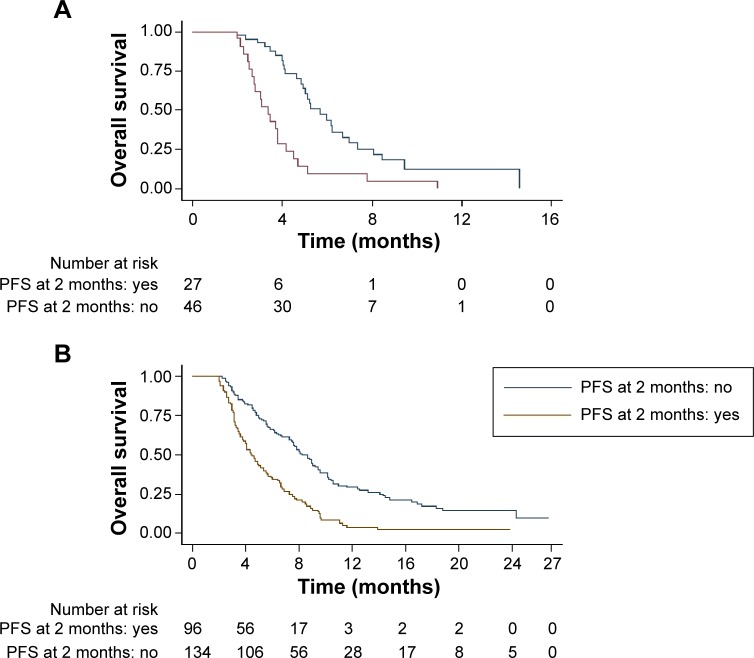
In the training data set, patients who did not experience any progression at 2 months had significantly improved OS compared with patients who did experience progression at 2 months. The median OS in patients who experienced any progression at 2 months was 3.37 months (95% CI 2.63–3.80), which was shorter than the 5.67 months in patients who did not experience any progression at 2 months (95% CI 4.83–6.67; P<0.0001). A similar result was observed in the testing data set. The median OS were 4.37 months (95% CI 3.77–5.37) and 8.23 months (95% CI 7.43–9.43; P<0.0001) in patients who experienced and did not experience progression at 2 months, respectively. The Kaplan–Meier survival curves by PFS rate at 2 months are presented in Figure 1.
Figure 1.
Kaplan–Meier survival curves by PFS at 2 months in the training data set (A) and in the testing data set (B).
Abbreviation: PFS, progression-free survival.
A multiple Cox regression model showed that disease progression at 2 months was predictive of OS in the training data set (Table 2). There were significant differences between the patients who experienced disease progression at 2 months and those who did not (adjusted HR 3.39; 95% CI 1.79–6.41; P<0.0001). Similar results were found in the testing data set, where the adjusted HR for death was 2.48 (95% CI 1.81–3.40; P<0.0001).
Table 2.
Multivariable proportional hazards model of progression-free survival at 2 months predicting overall survival stratified on study
| Variables | Training data set (II)
|
Testing data set (III)
|
|---|---|---|
| Adjusted HR (95% CI; P-value)* | Adjusted HR (95% CI; P-value)* | |
| Any progression at 2 months | ||
| Yes versus no | 3.39 (1.79–6.41; <0.0001) | 2.48 (1.81–3.40; <0.0001) |
| Age | 0.98 (0.94–1.03; 0.469) | 1.01 (0.99–1.02; 0.414) |
| Sex | ||
| Female versus male | 1.54 (0.64–3.72; 0.337) | 0.84 (0.59–1.19; 0.326) |
| ECOG PS | ||
| 1 versus 0 | 3.81 (0.50–28.93; 0.196) | 2.02 (1.40–2.93; <0.0001) |
| Previous lines of chemotherapy | ||
| ≥3 versus 2 | 1.47 (0.78–2.78; 0.235) | 0.79 (0.56–1.07; 0.126) |
| Metastatic sites (n) | ||
| >2 versus ≤2 | 1.54 (0.73–3.23; 0.258) | 1.46 (1.05–2.03; 0.023) |
Note:
Adjusted for age, sex, ECOG PS, previous lines of chemotherapy and number of metastatic sites in Cox model.
Abbreviations: HR, hazard ratio; CI, confidence interval; ECOG, Eastern Cooperative Oncology Group; PS, performance status.
Correlation between PFS and OS
In the training data set, the correlation between PFS and OS estimated by the NICE model was 0.84 (95% CI 0.74–0.90, Table 3). There was a strong correlation between these two endpoints, indicating that PFS was a good surrogate endpoint for OS in this setting. Using the testing data set, the correlation between PFS and OS was 0.81 (95% CI 0.76–0.86), which also indicated a strong correlation between these two endpoints (Table 3).
Table 3.
Correlation between progression-free survival and overall survival
| Subgroup | Training data set (II)
|
Testing data set (III)
|
||
|---|---|---|---|---|
| n | Correlation (95% CI)* | n | Correlation (95% CI)* | |
| Total | 73 | 0.84 (0.74–0.90) | 230 | 0.81 (0.76–0.86) |
| ECOG PS 0 | 4 | – | 57 | 0.83 (0.71–0.91) |
| ECOG PS 1 | 69 | 0.83 (0.72–0.90) | 173 | 0.80 (0.73–0.85) |
| Two previous lines of chemotherapy lines | 52 | 0.85 (0.71–0.92) | 152 | 0.81 (0.74–0.87) |
| Three or more previous lines of chemotherapy | 21 | 0.75 (0.34–0.91) | 78 | 0.79 (0.66–0.86) |
| Up to two metastatic sites | 60 | 0.81 (0.68–0.97) | 164 | 0.79 (0.71–0.85) |
| More than two metastatic sites | 13 | 0.88 (0.64–0.97) | 66 | 0.84 (0.75–0.90) |
| Confirmed death | 49 | 0.80 (0.66–0.89) | 187 | 0.83 (0.78–0.87) |
| Placebo | 31 | 0.79 (0.60–0.90) | 76 | 0.82 (0.73–0.88) |
| Apatinib | 42 | 0.86 (0.73–0.93) | 154 | 0.82 (0.75–0.87) |
Note:
Evaluated by the normal induced copula estimation model.
Abbreviations: ECOG, Eastern Cooperative Oncology Group; PS, performance status; CI, confidence interval.
In an additional subgroup analysis (Table 3), based on the training data set, the correlation between PFS and OS tended to be higher in patients with previous second-line chemotherapy (0.85; 95% CI 0.71–0.92) than that in patients with third-line or later-line chemotherapy (0.75; 95% CI 0.34–0.91), and to be higher in patients with more than two metastatic sites (0.88; 95% CI 0.64–0.97) than that in patients with up to two metastatic sites (0.81; 95% CI 0.68–0.97). These correlations were also confirmed in the testing data set. The PFS/OS correlation in 42 patients who received apatinib (0.86; 95% CI 0.73–0.93) was stronger than that in 31 patients who received placebo (0.79; 95% CI 0.60–0.90). However, a similar result was not found in the testing data set. Moreover, in a subgroup analysis of patients experiencing death after 2 months, the correlation between these endpoints was 0.80 (95% CI 0.66–0.89) in the training data set and 0.83 (95% CI 0.78–0.87) in the testing data set. Since there were only four patients with an ECOG performance status of 0, the PFS/OS correlation could not be estimated by the NICE model. However, in a subgroup analysis of patients with ECOG performance status of 1, the correlation between these endpoints was 0.83 (95% CI 0.72–0.90). This correlation, verified in the testing data set, also indicated a strong correlation between PFS and OS in patients with ECOG performance status of 1 (0.80; 95% CI 0.73–0.85). In summary, the PFS/OS correlations were strong in all of the subset analyses, ie, higher than 0.75.
Discussion
This is the first study based on individual patient data to evaluate whether PFS is a reasonable surrogate endpoint for OS in patients with third-line or later-line chemotherapy for AGC. Our results show that disease progression at 2 months was strongly predictive of OS in 303 patients with third-line or later-line chemotherapy for AGC. In the training data set, the median OS was 5.67 months in patients who experienced any type of progression at 2 months and was significantly longer than in patients who did not experience progression (3.37 months; P<0.0001). Further, the adjusted HR for death was 3.39 in patients who did and did not progress at 2 months. Similarly, in the testing data set, these findings were initially validated using 230 patients with AGC.
Other results suggest that there was a reasonable correlation between OS and PFS, with 0.84 (95% CI 0.74–0.90) and 0.81 (95% CI 0.76–0.86), respectively. A similar result has been reported in patients with AGC treated by first-line chemotherapy.18 In the current analysis, the results indicate that PFS may be used as a surrogate endpoint in trials of patients with third-line or later-line chemotherapy for AGC. In addition, the results from these Phase II and III studies of apatinib indicate that median OS and median PFS were significantly longer in the apatinib group than in the placebo group, which satisfies the Prentice criteria.26 These observations could be explained by a strong association between PFS and OS. A further explanation could be that the results of multiple sequential salvage therapies after failure of third-line or later-line chemotherapy were poor and the post progression survival was short (2.3 months in the training data set, 3.7 months in the testing data set). According to the results of Broglio et al the HR for OS is largely influenced by median post progression survival.27 When the P-value for improvement in PFS was 0.001, more than 90% probability for statistical significance in OS could be achieved if median post progression survival was 2 months but less than 20% if median post progression survival was 24 months.
Our results suggest that PFS may serve as an intermediate endpoint for OS. However, this analysis had some unavoidable limitations. It is worth noting that patients enrolled in these studies were required to meet the inclusion criteria and be deemed appropriate for participation in clinical trials, so our findings cannot be generalized to the entire population of patients with AGC. Further, the allocation proportions of the enrolled patients were different between the two data sets (1:1 in the training data set and 1:2 in the testing data set). If the relationship between PFS and OS in patients randomized to the experimental group was different from that in patients randomized to the control group, the overall PFS/OS association would be influenced by the imbalance in enrolment of patients between the treatment group and the control group in the clinical trial. At present, relatively few randomized studies involving third-line or later-line treatment in patients with AGC are available, so our analysis was based on only two clinical studies that used the same drug and included a very limited number of patients. Therefore, more research is needed to evaluate the surrogacy of PFS for OS in future randomized clinical trials of third-line or later-line chemotherapy in patients with AGC.
Nevertheless, our results are noteworthy, and for three main reasons. First, individual patient data were obtained from two randomized controlled trials that used uniform inclusion criteria, monitoring, and definitions of progression. Second, both trials used the same experimental regimen (apatinib 850 mg once daily) and the same control arm (placebo). Third, an independent data set was use to validate the results of the current analysis.
Conclusion
Our present study results indicate that PFS at 2 months predicts OS in patients having third-line or later-line chemotherapy for AGC. PFS correlates strongly with OS, suggesting that PFS may be used as a surrogate endpoint for OS. Although PFS and progression at 2 months seem to be strongly associated with OS, the clinical relevance of the association between PFS and OS has been questioned by regulatory bodies and agencies and needs to be validated by ongoing randomized trials. A critical task remains, namely to develop and validate intermediate endpoints of OS to accelerate drug approval and improve survival in patients who are going to die of AGC.
Acknowledgments
Data was provided by Jiangsu Hengrui Medicine Co., Ltd, Lianyungang, People’s Republic of China. The work was supported by the National Natural Science Foundation of China (81273184, 81473070), the National Natural Science Foundation of China Grant for Young Scientists (81302512), and the Priority Academic Program Development of Jiangsu Higher Education Institutions.
Footnotes
Disclosure
None of the authors have any financial or personal conflicts of interest to disclose in relation to this work.
References
- 1.Stewart BW, Wild CP. World Cancer Report 2014. International Agency for Research on Cancer; 2014. [Accessed March 25, 2015]. Available from: http://www.iarc.fr/en/publications/pdfs-online/wcr/ [Google Scholar]
- 2.Kang YK, Kang WK, Shin DB, et al. Capecitabine/cisplatin versus 5-fluorouracil/cisplatin as first-line therapy in patients with advanced gastric cancer: a randomised phase III noninferiority trial. Ann Oncol. 2009;20:666–673. doi: 10.1093/annonc/mdn717. [DOI] [PubMed] [Google Scholar]
- 3.Koizumi W, Narahara H, Hara T, et al. S-1 plus cisplatin versus S-1 alone for first-line treatment of advanced gastric cancer (SPIRITS trial): a phase III trial. Lancet Oncol. 2008;9:215–221. doi: 10.1016/S1470-2045(08)70035-4. [DOI] [PubMed] [Google Scholar]
- 4.Ajani JA, Buyse M, Lichinitser M, et al. Combination of cisplatin/S-1 in the treatment of patients with advanced gastric or gastroesophageal adenocarcinoma: results of noninferiority and safety analyses compared with cisplatin/5-fluorouracil in the First-Line Advanced Gastric Cancer Study. Eur J Cancer. 2013;49:3616–3624. doi: 10.1016/j.ejca.2013.07.003. [DOI] [PubMed] [Google Scholar]
- 5.Bang YJ, Van Cutsem E, Feyereislova A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): a phase 3, open-label, randomised controlled trial. Lancet. 2010;376:687–697. doi: 10.1016/S0140-6736(10)61121-X. [DOI] [PubMed] [Google Scholar]
- 6.Cunningham D, Starling N, Rao S, et al. Capecitabine and oxaliplatin for advanced esophagogastric cancer. N Engl J Med. 2008;358:36–46. doi: 10.1056/NEJMoa073149. [DOI] [PubMed] [Google Scholar]
- 7.Thuss-Patience PC, Hofheinz RD, Arnold D, et al. Perioperative chemotherapy with docetaxel, cisplatin and capecitabine (DCX) in gastro-oesophageal adenocarcinoma: a phase II study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Ann Oncol. 2012;23:2827–2834. doi: 10.1093/annonc/mds129. [DOI] [PubMed] [Google Scholar]
- 8.Kang JH, Lee SI, Lim do H, et al. Salvage chemotherapy for pretreated gastric cancer: a randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol. 2012;30:1513–1518. doi: 10.1200/JCO.2011.39.4585. [DOI] [PubMed] [Google Scholar]
- 9.Thuss-Patience PC, Kretzschmar A, Bichev D, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer – a randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO) Eur J Cancer. 2011;47:2306–2314. doi: 10.1016/j.ejca.2011.06.002. [DOI] [PubMed] [Google Scholar]
- 10.Ford HE, Marshall A, Bridgewater JA, et al. Docetaxel versus active symptom control for refractory oesophagogastric adenocarcinoma (COUGAR-02): an open-label, phase 3 randomised controlled trial. Lancet Oncol. 2014;15:78–86. doi: 10.1016/S1470-2045(13)70549-7. [DOI] [PubMed] [Google Scholar]
- 11.Fuchs CS, Tomasek J, Yong CJ, et al. Ramucirumab monotherapy for previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (REGARD): an international, randomised, multicentre, placebo-controlled, phase 3 trial. Lancet. 2014;383:31–39. doi: 10.1016/S0140-6736(13)61719-5. [DOI] [PubMed] [Google Scholar]
- 12.Ohtsu A, Ajani JA, Bai Y-X, et al. Everolimus for previously treated advanced gastric cancer: results of the randomized, double-blind, Phase III GRANITE-1 study. J Clin Oncol. 2013;31:3935–3943. doi: 10.1200/JCO.2012.48.3552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hawkes E, Okines AF, Papamichael D, et al. Docetaxel and irinotecan as second-line therapy for advanced oesophagogastric cancer. Eur J Cancer. 2011;47:1146–1151. doi: 10.1016/j.ejca.2010.12.021. [DOI] [PubMed] [Google Scholar]
- 14.Park SH, Kim YS, Hong J, et al. Mitomycin C plus S-1 as second-line therapy in patients with advanced gastric cancer: a noncomparative phase II study. Anticancer Drugs. 2008;19:303–307. doi: 10.1097/cad.0b013e3282f46ad8. [DOI] [PubMed] [Google Scholar]
- 15.Tian S, Quan H, Xie C, et al. YN968D1 is a novel and selective inhibitor of vascular endothelial growth factor receptor-2 tyrosine kinase with potent activity in vitro and in vivo. Cancer Sci. 2011;102:1374–1380. doi: 10.1111/j.1349-7006.2011.01939.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Qin S. Phase III study of apatinib in advanced gastric cancer: a randomized, double-blind, placebo-controlled trial. J Clin Oncol. 2014;32:4003. [Google Scholar]
- 17.Shitara K, Ikeda J, Yokota T, et al. Progression-free survival and time to progression as surrogate markers of overall survival in patients with advanced gastric cancer: analysis of 36 randomized trials. Invest New Drugs. 2012;30:1224–1231. doi: 10.1007/s10637-011-9648-y. [DOI] [PubMed] [Google Scholar]
- 18.Shitara K, Matsuo K, Muro K, et al. Progression-free survival and post-progression survival in patients with advanced gastric cancer treated with first-line chemotherapy. J Cancer Res Clin Oncol. 2013;139:1383–1389. doi: 10.1007/s00432-013-1452-y. [DOI] [PubMed] [Google Scholar]
- 19.Paoletti X, Oba K, Bang YJ, et al. Progression-free survival as a surrogate for overall survival in advanced/recurrent gastric cancer trials: a meta-analysis. J Natl Cancer Inst. 2013;105:1667–1670. doi: 10.1093/jnci/djt269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shitara K, Matsuo K, Muro K, et al. Correlation between overall survival and other endpoints in clinical trials of second-line chemotherapy for patients with advanced gastric cancer. Gastric Cancer. 2014;17:362–370. doi: 10.1007/s10120-013-0274-6. [DOI] [PubMed] [Google Scholar]
- 21.Li J, Qin S, Xu J, et al. Apatinib for chemotherapy-refractory advanced metastatic gastric cancer: results from a randomized, placebo-controlled, parallel-arm, phase II trial. J Clin Oncol. 2013;31:3219–3225. doi: 10.1200/JCO.2013.48.8585. [DOI] [PubMed] [Google Scholar]
- 22.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. J Natl Cancer Inst. 2000;92:205–216. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 23.Anderson JR, Cain KC, Gelber RD. Analysis of survival by tumor response. J Clin Oncol. 1983;1:710–719. doi: 10.1200/JCO.1983.1.11.710. [DOI] [PubMed] [Google Scholar]
- 24.Fu H, Wang Y, Liu J, et al. Joint modeling of progression-free survival and overall survival by a Bayesian normal induced copula estimation model. Stat Med. 2013;32:240–254. doi: 10.1002/sim.5487. [DOI] [PubMed] [Google Scholar]
- 25.R Core Team R: A language and environment for statistical computing. 2012. [Accessed March 25, 2015]. Available from: http://web.mit.edu/r/r_v3.0.1/fullrefman.pdf.
- 26.Prentice RL. Surrogate endpoints in clinical trials: definition and operational criteria. Stat Med. 1989;8:431–440. doi: 10.1002/sim.4780080407. [DOI] [PubMed] [Google Scholar]
- 27.Broglio KR, Berry DA. Detecting an overall survival benefit that is derived from progression-free survival. J Natl Cancer Inst. 2009;101:1642–1649. doi: 10.1093/jnci/djp369. [DOI] [PMC free article] [PubMed] [Google Scholar]