Abstract
Objective:
Obstructive sleep apnea syndrome (OSA) leads to a deterioration in cognitive functions, with regard to memory and executive functions. However, few studies have investigated the impact of treatment on these cognitive functions in elderly subjects.
Methods:
The study was conducted in a large cohort of subjects aged 65 years or older (the PROOF cohort). Subjects were not diagnosed or treated for OSA. Subjects underwent a polygraphic recording. Cognitive performance was assessed in all OSA subjects at baseline and 10 years later, whether or not they were receiving continuous positive airway pressure (CPAP) therapy.
Results:
A group of 126 patients were analyzed. Only 26% of them were treated, with therapy initiated at the discretion of the primary care physician. Among treated subjects, self-reported compliance with therapy was good (> 6 h/night on average), and 66% of them reported an improvement in their quality of life. Patients receiving CPAP treatment had a higher apneahypopnea index (p = 0.006), a higher oxygen desaturation index (p < 0.001), and experienced more pronounced daytime repercussions (p = 0.004). These patients showed a statistically significant improvement in mental agility (similarities test; p < 0.0001) and memory performance (Grober and Buschke delayed free recall; p = 0.02).
Conclusion:
CPAP treatment is associated with the maintenance of memory performance over time.
Citation:
Crawford-Achour E, Dauphinot V, Saint Martin M, Tardy M, Gonthier R, Barthelemy JC, Roche F. Protective effect of long-term CPAP therapy on cognitive performance in elderly patients with severe OSA: the PROOF study. J Clin Sleep Med 2015;11(5):519–524.
Keywords: cognitive impairment, CPAP treatment, elderly, sleep apnea
Obstructive sleep apnea (OSA) is a recognized clinical entity, defined by the occurrence of complete or partial obstruction of airflow through the upper airway during sleep, leading to wakening and oxygen desaturation.1 OSA is very common among the elderly, affecting as many as 30% to 80% of people older than 65 years.2 Despite this high prevalence, OSA is largely underdiagnosed and undertreated in the elderly population.3 The hypoxia and sleep fragmentation induced by OSA lead to increased cardiovascular morbidity and mortality, as well as numerous complications, such as functional impairment, diminished quality of life, and cognitive dysfunction.4–6 Several studies have focused on the cognitive performance of younger subjects suffering from OSA. Executive functioning, vigilance, learning capacity, and coordination are impaired in subjects with OSA compared to those without.7
Somnolence and impairment in cognitive performances are two symptoms regularly reported in elderly subjects suffering from sleep apnea. More importantly, It has been clearly demonstrated that OSA is associated with a higher risk of mild cognitive impairment (MCI) or dementia, related to the percent of time spent in apnea or hypopnea.8 A meta-analysis by Engleman et al. focusing on the neuropsychological consequences of OSA, revealed impairment of attention, perception, and executive functions; these deficits tend to be more pronounced in elderly subjects.9 This was confirmed by Ju et al., who recently showed a degradation of executive functioning and episodic memory in a population of elderly subjects without dementia suffering from severe sleep apnea compared to subjects presenting mild OSA.10
BRIEF SUMMARY
Current Knowledge/Study Rationale: Sleep apnea is underdiag-nosed and undertreated in the elderly population considering the severe induced complications. This may be partly due the lack of knowledge of CPAP therapy beneficial effects and clinical tolerance.
Study Impact: This study shows that CPAP therapy may protect cognitive functions deteriorated by OSA, particularly the similarities from the Wais III test, along with good clinical self-reported compliance.
OSA is often treated with continuous positive airway pressure (CPAP) in elderly subjects. The benefits of this treatment on cardiac morbidity and mortality has been demonstrated.11 However, its benefit on cognitive function in the elderly is more difficult to prove. Aloia et al. investigated the effect of 3-month CPAP treatment on neurocognitive performance in elderly subjects without dementia. The results varied according to the tests used, but nevertheless indicated an improvement in executive functioning, even if only modest.12 In younger subjects, the effect of CPAP treatment on cognitive performance seems to be less marked.
The objective of this study was to determine the long-term effect of CPAP treatment on cognitive function, particularly executive functioning, in a population of elderly subjects (the PROOF cohort) receiving treatment for OSA, in comparison to the spontaneous cognitive evolution of non-treated subjects of the same cohort. The subjects were homogeneous in age because of the cohort design.
METHODS
Study Population
The survey was conducted in a cohort of elderly subjects, the PROOF (PRognostic indicator OF cardiovascular and cerebrovascular events) cohort.13 The primary endpoint of the PROOF study was to investigate the effect of autonomous nervous system activity on the incidence of cardiovascular and cerebrovascular events during the aging process. The cohort was recruited between January 2001 and December 2002 from the population registered on the electoral lists for the entire district covering the city of Saint Etienne (France). To be included in the cohort, subjects had to be aged ≥ 65 years and give their informed consent to participate. Exclusion criteria comprised a history of myocardial infarction, stroke, heart failure, atrial fibrillation; type 1 (insulin-dependent) diabetes mellitus; pacemaker implantation; a pathological condition limiting life expectancy to < 5 years; a contraindication to a brain MRI; being institutionalized; or planning to move during the next 2 years.
Objective Sleep Assessment: Respiratory Polygraphy
At the beginning of the study, participants were invited to undergo ambulatory polygraphy (using a HypnoPTT device, Tyco Healthcare), giving an objective measurement of sleep parameters including apnea-hypopnea index (AHI), oxygen desaturation index (ODI), time spent at an oxygen saturation level < 90%, mean saturation level, and minimum saturation level. Apnea was defined as complete cessation of respiratory flow for ≥ 10 seconds. Hypopnea was defined as decrease in amplitude of respiratory flow ≥ 50% during ≥ 10 sec associated with 3% oxygen desaturation. OSA was considered severe if AHI was > 30/h.14 The desaturation index was defined as the number of desaturation events > 3%/h. The results were communicated to the subject's primary care physician, and treatment initiation was at their discretion. All subjects had to complete an Epworth Sleepiness Scale the night before the polygraphy. A score between 0–9 was considered normal; a score > 9 revealed excessive daytime sleepiness.
Cognitive Assessment (Neuropsychological Tests)
All subjects in the cohort underwent at the same time an assessment of cognitive performance in 2002–2003 and again in 2009–2012. The results of these evaluations were reported in a previous publication.13 The battery of tests performed included Mini-Mental State Examination (MMSE), Pichot Depression Scale evaluation, visual analogue scale (VAS) assessment of memory complaints, Grober and Buschke test, Benton Visual Retention test, Coding test (WAIS III), a Trail-Making test (TMT; parts A and B), Stroop test, Verbal and Categorical Fluency Test, and Similarities tests (WAIS III). Individuals at baseline were free of any mental disorder. All test scores show an improvement if the score is higher except the TMT test (part A and B) and the Pichot depression scale. The neuro-psychologist was blinded to whether or not participant was on CPAP therapy.
Questionnaire Concerning Prescribed Treatment and Compliance in OSA
All subjects presenting severe sleep apnea (AHI > 30) as detected by polygraphy were questioned again in 2010, initially by letter and then, if no reply or an incomplete reply was received, by a follow-up telephone call. These subjects were asked to supply several items of information: whether or not they had received CPAP therapy, and if so, whether the treatment had been stopped, and if so, for what reason; for how long they had been receiving treatment; who was monitoring their treatment; how well they tolerated the treatment; for how many nights per week and for how many hours per night the treatment was implemented. Good compliance was defined as adherence to treatment > 4 h per night and 85% of the nights of the week.12
Study Calendar
The neurocognitive evaluation was performed at the start of the study in 2002–2003, and patients were re-assessed between 2009 and 2012. The objective assessments of respiratory sleep polygraphy parameters were accomplished at the beginning of the cohort, between 2003 and 2006. The questionnaire concerning the follow-up of subjects with severe OSA was sent to these participants in 2010.
Statistical Analysis
Baseline characteristics (sociodemographic characteristics and polygraphic parameters) were compared between treated and untreated subjects. Proportions were compared between the groups using the Pearson χ2 test. Means were compared using Student t-test with equal or unequal variance hypothesis as appropriate. Results are presented as percentages for categorical variables and mean ± standard deviation (SD) for continuous variables. The population under study was divided in a dichotomous way: treated or not treated with CPAP therapy. The number of hours of use was not taken into account, as it can be overestimated in this population.
Means of cognitive tests were compared between T0 and T1, in both treated and untreated group, using repeated MANOVA. Then the effect of CPAP treatment on cognitive functions was assessed using a model of analysis of covariance for repeated measures. In this model, the 2 repeated measures of all neuropsychological scores were considered dependent variables, and CPAP treatment was the independent variable. This model allows consideration of the expected colinearity between the neuropsychological scores with each other, as well as the expected colinearity between the 2 repeated measures of the neuropsychological scores. The neuropsychological scores were then all modelized in the same model in order to address the multiple testing problem. The results provided by the model was (1) p value for within-subject effects, assessing the difference in neuropsychological scores between repeated measures independent of CPAP treatment; (2) p value for between-subject effects, assessing the relationship between neuropsychological scores and CPAP treatment group (treated or untreated); (3) p value for the interaction between time and treatment, assessing whether changes in neuropsychological scores were similar in the CPAP treatment groups. The assumptions allowing the application of this model were verified, such as the homoscedasticity of the variances of the neuropsychological scores between CPAP treatment groups using the test of Levene.
The model was then adjusted for potential confounders using a step-wise strategy. All variables that were significantly different between treated and untreated group in the unadjusted analyses were in a first step included together in the model as independent variables. Nonsignificant variables were then removed one by one, removing the largest p value first, until all remaining variables in the model were significant.
A p value < 0.05 was considered statistically significant. All statistical tests were two-tailed. Statistical analyses were performed using SPSS (Statistical Package for the Social Sciences) version 17.0 for Windows (SPSS Inc., Chicago, IL, USA).
Ethics Committee
The PROOF study has been approved by the University Hospital of Saint-Etienne and the (CCPPRB) Rhône-Alpes Loire Ethics Committee (CCPPRB). The French Data Protection Authority (CNIL) gave its consent for the data to be entered. All the subjects who took part in the study signed a consent form in a free and enlightened manner. ClinicalTrials.gov Identifier NCT00759304.
RESULTS
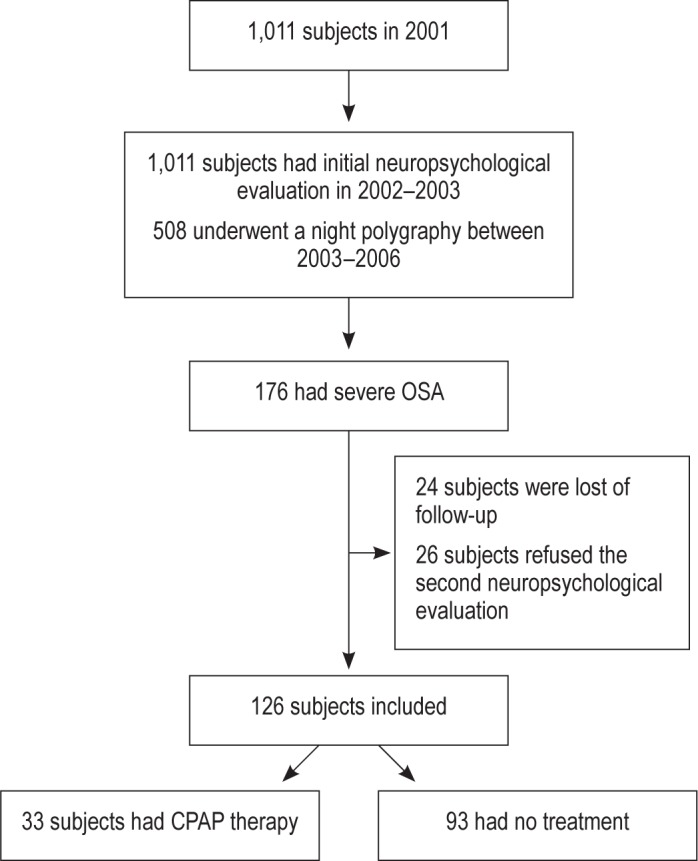
Of the entire cohort, 508 participants underwent polygraphy between 2003 and 2006, among whom 176 (35%) presented severe OSA, necessitating CPAP therapy. Among the sample, 24 subjects were lost to follow-up, and 26 subjects refused to take the second neuropsychological evaluation. A total of 126 subjects with severe OSA were assessed at the beginning of the study, then again between 2009 and 2012. Only 33 (26%) patients were treated by CPAP; the remaining 74% remained untreated due to their common decision with their primary care practitioner (Figure 1).
Figure 1. Flowchart of the population under study.
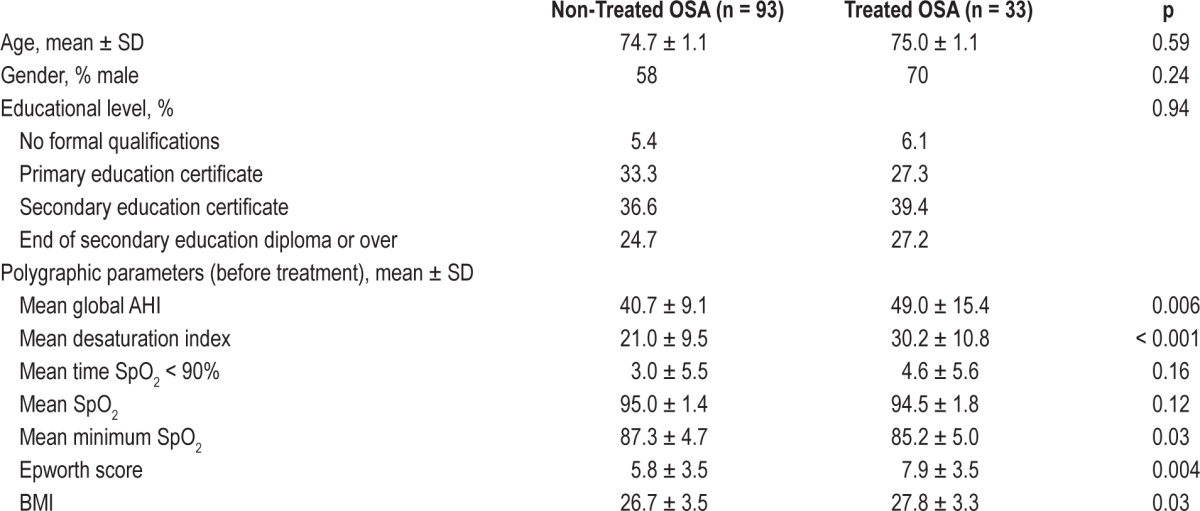
Sociodemographic and Polygraphic Characteristics of the Study Population
The mean age of the subjects in 2010 was 74.8 ± 1.1. Men represented the majority (> 60%) of the population. The 2 groups, treated and non-treated, did not differ to a statistically significant extent with regard to age, sex, or socioeducational level. With respect to polygraphic parameters, treated subjects presented more severe OSA, had a higher BMI, and experienced more pronounced daytime repercussions of sleep apnea reflected by higher scores on the Epworth Sleepiness Scale (Table 1).
Table 1.
Sociodemographic and polygraphic parameters of the treated and non-treated population (n = 126).
Among the subjects receiving CPAP treatment for OSA, the mean duration of treatment was 44.5 ± 26.3 months. Only 2 subjects stopped treatment because of intolerance. The self-reported mean compliance with treatment was 7.0 ± 0.2 nights/ week and 6.6 ± 1.1 h/night. All participants of the treated group estimated their compliance > 4 h/night. The treatment was well tolerated; only 30% of subjects reported adverse effects, none of which led to treatment cessation. All subjects were regularly monitored by a pulmonologist.
Changes in Neurocognitive Parameters in Treated and Non-Treated Subjects
The changes in the different parameters over time (within-subject - time) for each group (treated and not treated) and for all subjects, as a function of treatment (between-subjects - treatment), and as a function of both effect of time and treatment with respect to changes in cognitive performance (time-treatment) are summarized in Table 2.
Table 2.
Comparison of neuropsychological parameters between treated and non-treated OSA subjects (n = 126 patients with T0 and T1 analysis).
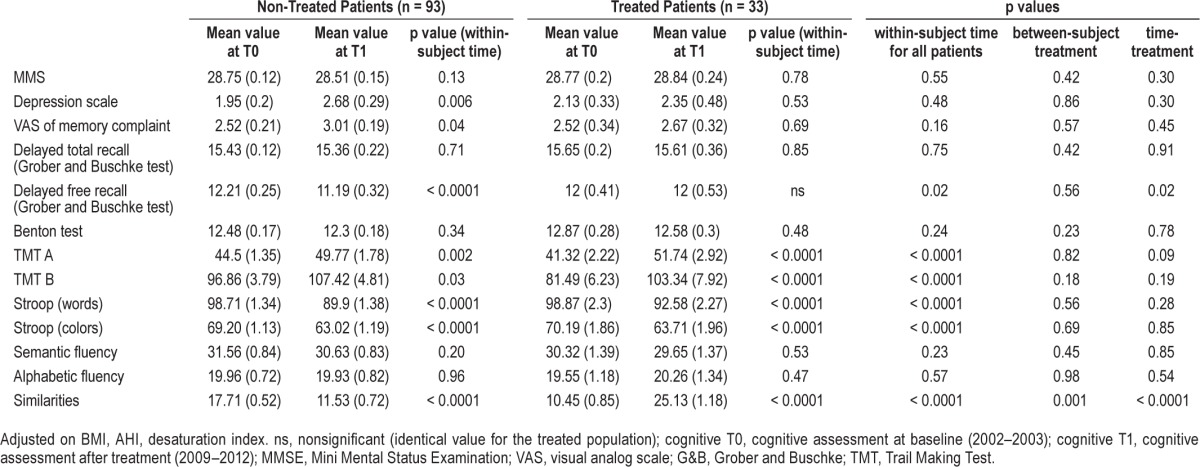
Deterioration of performance was observed with time in the Trail-Making test (parts A and B), the number of delayed free recalls, the Stroop test, and the WAIS III Similarities test. Comparison of the means obtained in cognitive scores in the 2 groups, without taking into account the effect of time, revealed a statistically significant difference with respect to the Similarities test—the scores achieved at the time of the baseline evaluation indicating a greater impairment in the group of subjects who would receive treatment. No statistically significant differences were seen in other cognitive parameters. When both the effect of time and the effect of treatment were taken into account, an improvement in scores on the Similarities test (Figure 2) as well as maintenance of performance in terms of delayed free recall was observed in subjects with sleep apnea receiving CPAP treatment.
Figure 2.
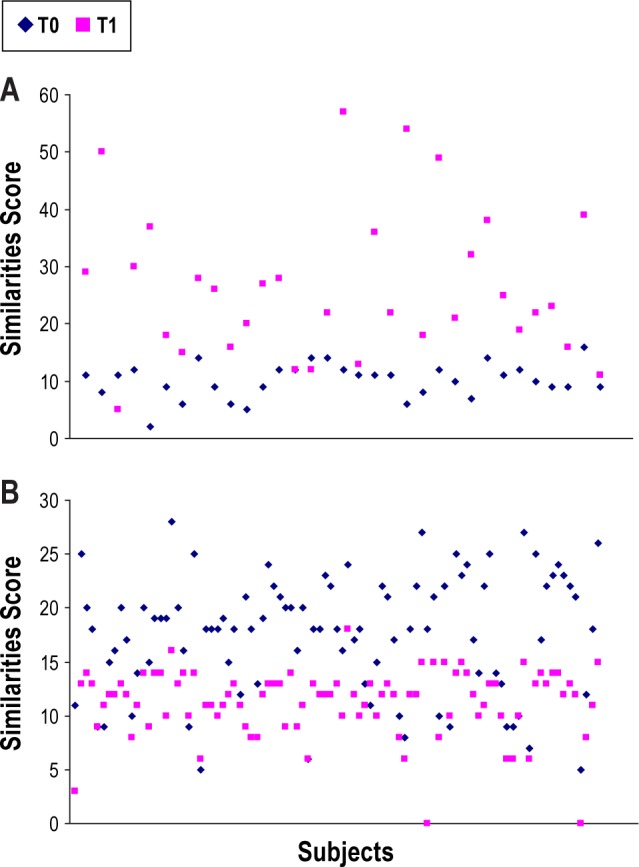
(A) Similarities test at T0 and T1 for each subjects under CPAP therapy (n = 33). (B) Similarities test at T0 and T1 for each subject without CPAP therapy treatment (n = 93).
Changes in Neurocognitive Parameters in Treated and Untreated Subjects in a Multivariate Model
Adjustment of the initial model was proposed on the basis of sociodemographic characteristics (sex, BMI, and socio-educational level), and polygraphic parameters (AHI and de-saturation index). The trends described above remained after this adjustment. Neither depth of desaturation nor severity of OSAS affected performance in the neurocognitive tests conducted before and after treatment.
DISCUSSION
The main result of this study suggests that long-term CPAP therapy is associated with a protective effect on cognitive performance in elderly OSA patients. Almost 15% of the PROOF cohort had unknown OSA at the age of 66 years old (age of diagnosis), with a mean Epworth score less than 9. This ascertainment might explain why subjects were not previously diagnosed.
This study highlighted several important points concerning OSA in the elderly. First, the population with a diagnosis of severe OSA was predominantly male, slightly overweight, and with a clinical profile approaching that of young subjects with OSA15,16; while previous reports have described a considerably more heterogeneous profile in elderly subjects.17 The prevalence of severe OSA was comparable to that reported in the literature for elderly subjects.15 Treated and untreated patients did not differ with respect to age, sex, or level of education. In contrast, treated subjects presented more severe apnea and deeper hypoxia, as well as more pronounced daytime repercussions of OSA in terms of somnolence, which may underline that a significant clinical pattern is needed for the general practitioner to go for CPAP therapy.
From the neurocognitive standpoint, this study emphasized several points. Firstly, the subjects with sleep apnea showed a more severe deterioration of executive functioning and episodic memory over time. Secondly, CPAP treatment enabled maintenance of certain cognitive functions, notably with regard to memory (delayed free recall), attention, and executive functioning (WAIS III Similarities test). Thirdly, when CPAP therapy was prescribed, self-reported compliance was excellent, with treatment used for at least 6 hours per night. The level of treatment tolerance and acceptability was fully satisfactory. Only two patients stopped their CPAP treatment. Similarly satisfactory compliance has been shown in a population of subjects older than 65 years, particularly in those with severe OSA.18
It is worth noting that few subjects were treated. Treatment initiation was at the discretion of the primary care physician. Unfortunately, a small proportion of the subjects received CPAP therapy, and only the more symptomatic subjects may have being considered. These results demonstrate lack of awareness of the potential impact of OSA in the elderly, even though OSA was recently shown to be associated with a higher risk of cardiovascular mortality among elderly subjects.19
One of the main results of this study is the association of CPAP therapy on a cognitive test: the similarities test (WAIS III). These results are of considerable importance as the PAQUID French epidemiological study has shown that the similarities test from the WAIS III was one of the more specific tests correlated with later occurrence of dementia.20 Thus, treating the sleep apnea population could be a way to delay the occurrence of cognitive impairment.
Ju et al. also showed the effect of severe OSA on episodic memory and executive functioning.10 In their study, cognitive performance was related to the severity of the disorder in terms of AHI and hypoxia. Such a relationship was not evident in our study, probably because subjects already had severe OSA in the inclusion period; in this case, the AHI may have had a lower impact because all individuals included had a score over 30. On the basis of smaller groups, similar results were obtained, with treated subjects showing less impairment of delayed free recall and better preservation of executive functioning.12 Cooke et al. also reported similar results, with treated subjects showing less deterioration of verbal episodic memory and improvement in more specific tests of executive functioning.21 Neuroimaging studies reinforce these findings, as improvement has been showed in specific regions such as the hippocampus or frontal structures affecting memory, attention, and executive functioning.22,23
This study has some limitations. While the long period between the two cognitive evaluations constitute the original intent of this study to show the long-term effect of CPAP therapy, as the mean time treatment was 44.5 months, this long period could impair the cognitive function of patients, and we cannot be absolutely sure that neuropsychological modifications are not due to another pathophysiological process. Nevertheless, in our study, patients under treatment tended to maintain their cognitive function even when taking into account this time period. This bias would tend to reinforce our results. Also, a large number of subjects were lost to follow-up or contributed to incomplete data. However, as the subjects lost to follow-up were probably those with poorer health, this factor should not modify the nature of the results. One may also regret the absence of more ecological tests to evaluate executive performance, which might have highlighted executive dysfunction in subjects with sleep apnea and the effect of treatment on these. Also, treatment compliance was only self-reported and subjects might have overestimated their adherence with CPAP therapy. However, the comparison of the two groups did not take into account nightly duration of CPAP therapy, but only if individuals were using CPAP therapy. Finally, allocation to treatment was not random, as this study was a cohort study, but several confounding factors have been included in the analysis. The confirmation of these results will require further randomized clinical studies to confirm the CPAP effect on cognitive performance.
CONCLUSIONS
OSA is currently recognized as a risk factor for hippocampal atrophy,24 and its implication in the onset or exacerbation of cognitive disorders should be seriously taken into account. As symptoms are often absent and medical awareness of this field is still emerging, particular attention should be paid to screening and treatment of OSA in the elderly population. CPAP therapy may help maintain cognitive performance, but further study is needed to confirm this finding.
DISCLOSURE STATEMENT
This was not an industry supported study. The authors have indicated no financial conflicts of interest. The work was performed in Saint-Etienne University Hospital.
ABBREVIATIONS
- AHI
apnea-hypopnea index
- CPAP
continuous positive airway pressure
- MCI
mild cognitive impairment
- OSA
obstructive sleep apnea
- SD
standard deviation
- VAS
visual analogue scale
REFERENCES
- 1.Guilleminault C, Tilkian A, Dement WC. The sleep apnea syndromes. Annu Rev Med. 1976;27:465–84. doi: 10.1146/annurev.me.27.020176.002341. [DOI] [PubMed] [Google Scholar]
- 2.Young T, Peppard PE, Gottlieb DJ. Epidemiology of obstructive sleep apnea: a population health perspective. Am J Respir Crit Care Med. 2002;165:1217–39. doi: 10.1164/rccm.2109080. [DOI] [PubMed] [Google Scholar]
- 3.Onen F, Onen H. [Obstructive sleep apnea and cognitive impairment in the elderly] Psychol Neuropsychiatr Vieil. 2010;8:163–9. doi: 10.1684/pnv.2010.0219. [DOI] [PubMed] [Google Scholar]
- 4.Launois SH, Pepin JL, Levy P. Sleep apnea in the elderly: a specific entity? Sleep Med Rev. 2007;11:87–97. doi: 10.1016/j.smrv.2006.08.005. [DOI] [PubMed] [Google Scholar]
- 5.Dam TT, Ewing S, Ancoli-Israel S, Ensrud K, Redline S, Stone K. Association between sleep and physical function in older men: the osteoporotic fractures in men sleep study. J Am Geriatr Soc. 2008;56:1665–73. doi: 10.1111/j.1532-5415.2008.01846.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Greenberg GD, Watson RK, Deptula D. Neuropsychological dysfunction in sleep apnea. Sleep. 1987;10:254–62. doi: 10.1093/sleep/10.3.254. [DOI] [PubMed] [Google Scholar]
- 7.Beebe DW, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 8.Yaffe K, Laffan AM, Harrison SL, et al. Sleep-disordered breathing, hypoxia, and risk of mild cognitive impairment and dementia in older women. JAMA. 2011;306:613–9. doi: 10.1001/jama.2011.1115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Engleman H, Joffe D. Neuropsychological function in obstructive sleep apnoea. Sleep Med Rev. 1999;3:59–78. doi: 10.1016/s1087-0792(99)90014-x. [DOI] [PubMed] [Google Scholar]
- 10.Ju G, Yoon IY, Lee SD, Kim TH, Choe JY, Kim KW. Effects of sleep apnea syndrome on delayed memory and executive function in elderly adults. J Am Geriatr Soc. 2012;60:1099–103. doi: 10.1111/j.1532-5415.2012.03961.x. [DOI] [PubMed] [Google Scholar]
- 11.Yaggi HK, Concato J, Kernan WN, Lichtman JH, Brass LM, Mohsenin V. Obstructive sleep apnea as a risk factor for stroke and death. N Engl J Med. 2005;353:2034–41. doi: 10.1056/NEJMoa043104. [DOI] [PubMed] [Google Scholar]
- 12.Aloia MS, Ilniczky N, Di Dio P, Perlis ML, Greenblatt DW, Giles DE. Neuropsychological changes and treatment compliance in older adults with sleep apnea. J Psychosom Res. 2003;54:71–6. doi: 10.1016/s0022-3999(02)00548-2. [DOI] [PubMed] [Google Scholar]
- 13.Barthelemy JC, Pichot V, Dauphinot V, et al. Autonomic nervous system activity and decline as prognostic indicators of cardiovascular and cerebrovascular events: the ‘PROOF’ Study. Study design and population sample. Associations with sleep-related breathing disorders: the ‘SYNAPSE’ Study. Neuroepidemiology. 2007;29:18–28. doi: 10.1159/000108914. [DOI] [PubMed] [Google Scholar]
- 14.Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement techniques in clinical research. The Report of an American Academy of Sleep Medicine Task Force. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 15.Young T, Palta M, Dempsey J, Skatrud J, Weber S, Badr S. The occurrence of sleep-disordered breathing among middle-aged adults. N Engl J Med. 1993;328:1230–5. doi: 10.1056/NEJM199304293281704. [DOI] [PubMed] [Google Scholar]
- 16.Strohl KP, Redline S. Recognition of obstructive sleep apnea. Am J Respir Crit Care Med. 1996;154:279–89. doi: 10.1164/ajrccm.154.2.8756795. [DOI] [PubMed] [Google Scholar]
- 17.Teramoto S, Inoue Y, Ouchi Y. Clinical significance of geriatric sleep apnea syndrome. Geriatr Gerontol Int. 2002;2:163–71. [Google Scholar]
- 18.Harada M, Taniguchi M, Ohi M, et al. Acceptance and short-term tolerance of nasal continous positive airway pressure therapy in elderly patients with obstructive sleep apnea. Sleep Biol Rhythms. 2004;2:53–6. [Google Scholar]
- 19.Martinez-Garcia MA, Campos-Rodriguez F, Catalan-Serra P, et al. Cardiovascular mortality in obstructive sleep apnea in the elderly. role of long-term CPAP Treatment: a prospective observational trial. Am J Respir Crit Care Med. 2012;186:909–16. doi: 10.1164/rccm.201203-0448OC. [DOI] [PubMed] [Google Scholar]
- 20.Fabrigoule C, Rouch I, Taberly A, et al. Cognitive process in preclinical phase of dementia. Brain. 1998;121:135–41. doi: 10.1093/brain/121.1.135. [DOI] [PubMed] [Google Scholar]
- 21.Cooke JR, Ayalon L, Palmer BW, et al. Sustained use of CPAP slows deterioration of cognition, sleep, and mood in patients with Alzheimer's disease and obstructive sleep apnea: a preliminary study. J Clin Sleep Med. 2009;5:305–9. [PMC free article] [PubMed] [Google Scholar]
- 22.Lee SD, Ju G, Kim JW, Yoon IY. Improvement of EEG slowing in OSAS after CPAP treatment. J Psychosom Res. 2012;73:126–31. doi: 10.1016/j.jpsychores.2012.04.007. [DOI] [PubMed] [Google Scholar]
- 23.Canessa N, Castronovo V, Cappa SF, et al. Obstructive sleep apnea: brain structural changes and neurocognitive function before and after treatment. Am J Respir Crit Care Med. 2011;183:1419–26. doi: 10.1164/rccm.201005-0693OC. [DOI] [PubMed] [Google Scholar]
- 24.Fotuhi M, Do D, Jack C. Modifiable factors that alter the size of the hippocampus with ageing. Nat Rev Neurol. 2012;8:189–202. doi: 10.1038/nrneurol.2012.27. [DOI] [PubMed] [Google Scholar]


