Abstract
Study Objectives:
To clarify whether motor behaviors and/ or vocalizations during REM sleep, which do not yet fulfill diagnostic criteria for REM sleep behavior disorder (RBD) and were defined as REM sleep behavioral events (RBEs), correspond to dream enactments.
Methods:
13 subjects (10 patients with Parkinson disease [PD] and 3 healthy controls) originally identified with RBE in a prospective study (DeNoPa cohort) were reinvestigated 2 years later with 2 nights of video-supported polysomnography (vPSG). The first night was used for sleep parameter analysis. During the 2nd night, subjects were awakened and questioned for dream recall and dream content when purposeful motor behaviors and/or vocalizations became evident during REM sleep. REM sleep without atonia (RWA) was analyzed on chin EMG and the cutoff set at 18.2% as specific for RBD.
Results:
At the time of this investigation 9 of 13 subjects with previous RBE were identified with RBD based upon clinical and EMG criteria. All recalled vivid dreams, and 7 subjects were able to describe dream content in detail. Four of 13 subjects with RBE showed RWA values below cutoff values for RBD. Three of these 4 subjects recalled having non-threatening dreams, and 2 (of these 3) were able to describe these dreams in detail.
Conclusion:
RBE with RWA below the RBD defining criteria correlate to dreaming in this selected cohort. There is evidence that RBEs are a precursor to RBD.
Citation:
Muntean ML, Trenkwalder C, Walters AS, Mollenhauer B, Sixel-Döring F. REM sleep behavioral events and dreaming. J Clin Sleep Med 2015;11(5):537–541.
Keywords: REM behavioral events (RBE), REM sleep behavior disorder (RBD), REM sleep without atonia (RWA), dream recall, Parkinson disease
We recently described motor behaviors and/or vocalizations during REM sleep with a purposeful component that failed to show the substantial amount of REM sleep without atonia (RWA) required for REM sleep behavior disorder (RBD) to be classified as such according to the International Classification of Sleep Disorders 2nd edition (ICSD-2) of 2005.1 We found such phenomena in a cohort of de novo patients with Parkinson disease (PD) who had never been treated with medications for PD or RBD, as well as in age- and gender-matched healthy controls (DeNoPa cohort); we termed these phenomena REM sleep behavioral events (RBEs).2 RBEs have been proposed as a new sleep marker for PD and a possible precursor to RBD.2 Whereas RBD is understood as dream enacting behavior, the correlation of RBE to dream mentation has not yet been established. In the study presented here we sought to clarify whether or not RBE also represent dream enacting behaviors and if we can describe specific dream content during these events.
METHODS
This study was conducted as an ancillary project to the De-NoPa Kassel study, a longitudinal prospective cohort study of nonmotor symptoms and biomarkers in PD. The design of the entire DeNoPa study, recruitment process and baseline results have been reported elsewhere.2,3
BRIEF SUMMARY
Current Knowledge/Study Rationale: The study was performed to further elucidate the role of motor behaviors and/or vocalizations during REM sleep not fulfilling diagnostic criteria for REM sleep behavior disorder (RBD) and therefore defined as REM sleep behavioral events (RBE) in early Parkinson disease (PD). We sought to clarify whether RBE correspond to dream enactments.
Study Impact: As REM sleep behavioral events (RBE) have been identified as a marker for early recognition of neurodegeneration, identification of RBE as enactments of non-threatening dreams will impact the search for biomarkers in pre-motor Parkinson disease (PD). Furthermore our findings support the concept of RBE as a possible precursor to REM sleep behavior disorder (RBD).
Patients and Subjects
When this ancillary project was conceived, 13 consecutive subjects (10 patients) from the DeNoPa cohort who showed RBE at baseline were available for re-investigation at their regular 2-year follow-up study visit with 2 nights of video-supported polysomnography (vPSG) (for demographic data see Tables 1A and 1B). At the time of the follow-up study, all 10 PD patients of this cohort were receiving dopaminergic medication. None of the subjects had ever received any medication for RBD.
Table 1A.
Demographic and medication data at 2-year follow-up of 2 patients with Parkinson disease (PD) and 2 controls originally diagnosed with REM behavioral events (RBE) who presented with RBE at follow-up.
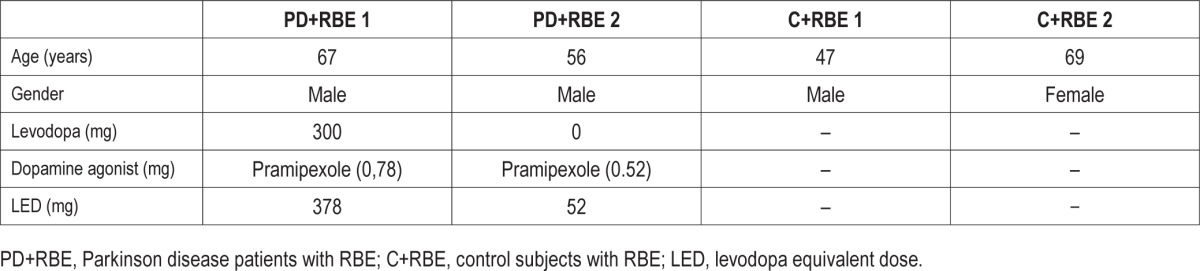
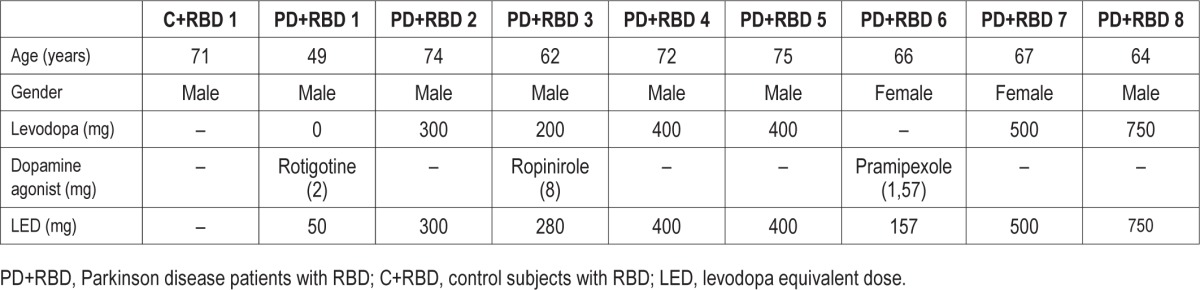
Table 1B.
Demographic and medication data at 2-year follow-up of 8 patients with Parkinson disease (PD) and 1 control originally diagnosed with REM behavioral events (RBE) who converted to RBD at follow-up.
Sleep Recordings
The first night was documented without awakening and with full polysomnographic montage, including video, to ascertain the presence of REM motor behaviors and/or vocalizations. During the second night an experienced sleep lab technician observed the subjects' sleep online on the monitor and offline during REM phases. After the first movement or vocalization with a purposeful component in any REM epoch the study participants were awakened and asked for dream recall immediately with the following questions: “Did you dream of something? Was it a dream or just a thought? Was it threatening? Can you describe your dream in detail?” The answers were noted and subjects could go back to sleep.
Standard Protocol Approvals, Registrations and Participants' Consent
All participants, as part of the Kassel DeNoPa cohort, gave consent for their data to be scientifically evaluated, and signed additional consent forms agreeing for their nighttime videos to be used for scientific and medical educational purposes. Subjects for this ancillary project also consented to being awakened during the second vPSG night. The entire DeNoPa study is registered with the German Register for Clinical trials (DRKS00000540) according to the WHO Trial Registration Dataset.
Polysomnography
Standard cardiorespiratory polysomnography (PSG; Xltec: Excel Tech Ltd; Oakville, Ontario; Canada) was applied according to AASM criteria. All patients were documented with an infrared video recording synchronized to the PSG. The first night was evaluated for sleep parameters with scoring of sleep, sleep stages, periodic limb movements (PLM), and respiratory events according to standard criteria. All sleep evaluations were reviewed and supervised by board-certified sleep specialists (FSD and MLM). Sleep efficiency was defined as total sleep time (TST) / time in bed (TIB). Quantitative analysis of sleep stages was calculated as a percentage of TST. The recording of the second night was additionally monitored offline in real time mode during REM stages to identify RBE, awaken the patient, and ask for dream recall. This second night was not used for further analysis.1
REM Sleep Assessments
For this study, the same methods for measuring RWA and defining RBD were used as in the baseline evaluation two years prior.2 The videos of all first night REM sleep phases were reviewed in real time; all visible movements and noises were recorded as best as possible despite the use of blankets. Comfort moves, neck myoclonus, respiratory noises, and events related to arousals were omitted from further analysis. All motor behaviors and/or vocalizations with a purposeful component, seemingly expressive of a subject's mentation, were classified as “REM sleep behavioral events” (RBEs). These included minor movements as well as violent behaviors. At least two such events had to be present during REM sleep in order for a subject to be considered “RBE positive.”2 To measure REM without atonia (RWA), surface EMG activity of the mentalis muscle was quantified during REM sleep according to the SINBAR method4: each REM sleep 30-sec epoch was divided into ten 3-sec mini-epochs. All mini-epochs showing any muscle activity on chin EMG > 0.1 sec with an amplitude exceeding twice the background EMG activity were counted as positive for RWA. The number of RWA-positive mini-epochs was calculated as a percentage of all REM sleep mini-epochs. Increases in muscle tone associated with snoring and arousals from respiratory events were excluded. For RWA measurement, the scorer (FSD) was blinded to the video evaluation. The cutoff value for 100% specificity for RBD was set at a rate of 18.2% of any muscle activity on mentalis EMG in accordance with the results of the SINBAR Group.4 RBD was defined according to ICSD-2 only with RWA above this cutoff.
Clinical and Demographic Data
For this study, age, gender, and data on dopaminergic medication were analyzed. Levodopa equivalent doses were calculated as a daily dose according to Tomlinson et al.5 History of possible RBD was assessed by evaluation of questions 6.1 to 6.4 of the RBD screening questionnaire (RBD-SQ),6 asking for previous nocturnal occurrence of vocalizations (question 6.1), motor behaviors (question 6.2 and 6.3), or any indication of nocturnal disturbances around the bed (question 6.4) occurring during the last 2 years since baseline evaluation.
Statistical Analysis
The age and levodopa equivalent dosages were compared between subjects with persisting RBE and subjects who converted to RBD using the Mann-Whitney U-test. The significance level was set at p < 0.05.
RESULTS
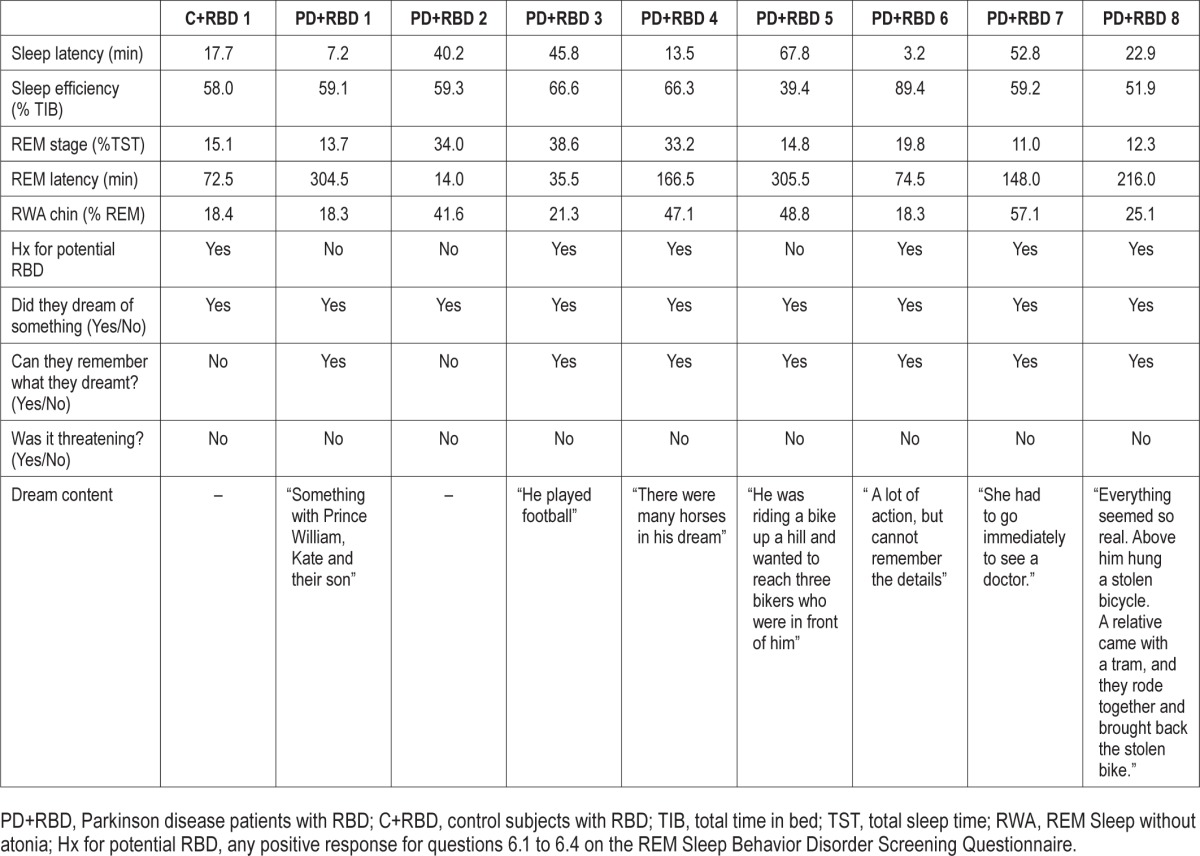
Based on the diagnostic criteria for RBD (according to the ICSD-2, which was in effect at the time of the baseline evaluation and follow-up), 9 (8 PD patients and 1 control) of the 13 subjects enrolled in this study originally identified with RBE at baseline presented with RBD (RWA values > 18.2%). When awakened during their REM behavioral episodes, they all reported vivid but non-threatening dreams, and 7 of them could more or less accurately describe their dreams. Three of 4 subjects who continued to show RBE but not excessive RWA at follow-up reported having dreamt something upon being awakened at the time of a behavioral event. Two of 3 subjects were able to remember more detailed dream content that was described as non-threatening.
Of the 9 subjects who converted to RBD, 6 (67%) answered affirmatively to at least one of the RBD-SQ questions (6.1 to 6.4), denoting a positive history of dream enacting vocalizations with screaming, talking, or laughing (n = 6); behaviors suggestive of RBD such as additional violent movements in sleep (n = 5); and bed falls (n = 1) during the 2 years since their baseline evaluation. In the RBE group, only 1 of 4 subjects (25%) gave a positive history suggestive of RBD with vocalizations and violent movements in sleep during 2-year follow-up. Paradoxically, this was the one subject who could not recall dreaming on being awakened at the time of follow-up, although he repetitively showed seemingly purposeful, gesticulating movements of the arms as well as vocalizations during REM sleep and was classified as RBE-positive accordingly. For details, see Tables 2A and 2B.
Table 2A.
Polysomnographic data, dream recall, and dream content at 2-year follow-up of 2 patients with Parkinson disease (PD) and 2 controls originally diagnosed with REM behavioral events (RBE) who presented with RBE at follow-up.
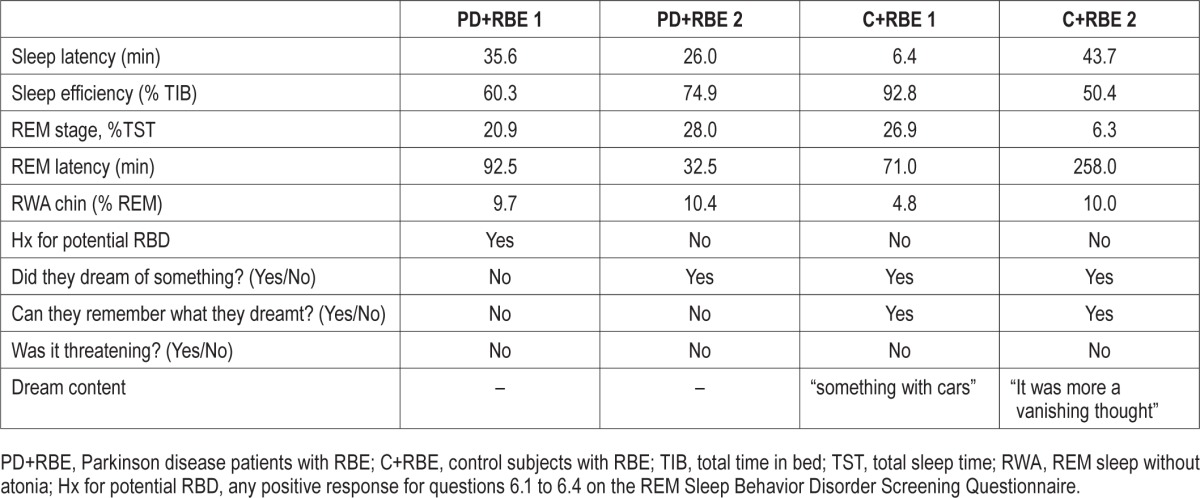
Table 2B.
Polysomnographic data, dream recall, and dream content at 2-year follow-up of 8 patients with Parkinson disease (PD) and 1 control originally diagnosed with REM behavioral events (RBE) who converted to RBD at follow-up.
Mean age in the group remaining RBE positive was determined at 60 ± 10 years (n = 4), compared to 67 ± 8 years in the group that converted to RBD (n = 9; p = 0.28). Levodopa equivalent dose of the 2 PD patients in the RBE group was calculated at 215 ± 231 mg, and for the 8 PD patients in the converter group at 355 ± 214 mg (p = 0.51). Gender was predominantly male in both groups (RBE: 3/4 male; converters: 7/9 male). For details see Tables 1A and 1B.
DISCUSSION
The main finding of this study is that REM-associated behaviors without a substantial amount of RWA, defined as RBE, are indeed associated with dreaming. Although subjects with RBE do not show the amount of RWA required for a positive RBD diagnosis, their mostly mild motor events and/or vocalizations correspond to reports of nonviolent, non-threatening dreams. This implies a relationship between the benign content of the dreams and the mildness of motor and/or vocal manifestation during REM in patients with RBE. Thus, the REM-associated phenomena may be less frequently noticed and reported by patients and/or bed-partners and would not lead to consultation of a sleep specialist. Accordingly, the rate of a history suggestive of possible RBD was higher in the converter group, with subjects reporting the occurrence of violent movements, talking, screaming, or laughing in their sleep and bed falls during the last two years. The fact that one of the four RBE-positive subjects could not remember dreaming when awakened may be attributed to the practical conduction of the study whereby participants were awakened at the first visible manifestation of motor behaviors and/or vocalizations during REM sleep, although later episodes might have been more elaborate.
The second important finding is that 9 of 13 RBE positive subjects at baseline converted to RBD after two years. They continued to show purposeful motor behaviors and/or vocalizations during REM sleep and developed RWA exceeding the validated cutoff value for diagnosis of RBD.3 They also reported more vivid and elaborate dreams. This supports the hypothesis that RBE is a precursor to RBD.
In our original and previously published observations,2 we refrained from labelling REM behavioral events that did not meet the necessary RWA cutoff for defining RBD as subclinical or preclinical RBD as suggested by other authors,7,8 because this implies a transition to “clinical” RBD. At that time it was unclear whether RBE would convert to RBD. Moreover, no definition of subclinical RBD related to the video assessments is known.
Aging, disease progression, and dopaminergic medication have to be considered influential factors for the transition of RBE to RBD. At baseline, patients did not receive any medication for PD. At two years follow-up—at the time of this investigation—all PD patients were treated with dopaminergic medication. An association between higher doses of levodopa, older age, and the presence of RBD in PD patients has been reported in the literature,9 but could not be detected in the small group of patients investigated here. Control subjects did not receive dopaminergic treatment, and the levodopa equivalent doses of PD patients did not differ between those with RBE and those who converted to RBD. Neither was there a signifi-cant difference in age between RBE subjects and converters. Further monitoring of subjects with RBE may shed more light on factors determining conversion to RBD.
REM sleep is considered a state of reactivation of neo-cortical and subcortical areas that are also active during wakefulness, with the difference that the sensations and movements during dreaming in REM stage are fictitious,10 and physiologic atonia during REM sleep prevents physical enactment. The induction of seemingly real, wake-state sensations, and movements during REM sleep when atonia is lost is impressive and can even lead to lucid dreaming.8 This did not occur in any of our 13 subjects. Activated areas include the amygdala, the parahippocampal cortex, and the deep frontal white matter, which could explain the increased emotional content of the dreams in REM sleep.11 Whether or not activation levels of these areas are lower in patients with RBE compared to those in RBD, needs to be elucidated in further studies.
Limitations of this study include the small number of participants, and for reasons of comfort and acceptance, participants were only awakened once and not after every behavioral episode during REM sleep. Therefore, we might have missed some more intense dream content. However, we could show that RBE do indeed correlate to nonthreatening dreams, and thus also represent dream-enacting behaviors. Furthermore, we present preliminary evidence that RBE are a precursor to RBD as the majority of subjects identified with RBE at baseline had developed RBD after 2 years.
DISCLOSURE STATEMENT
The study was supported by unrestricted research grants from the Paracelsus-Elena-Klinik, Kassel, Germany and unrestricted research grants from TEVA Pharma/Lundbeck and GE Healthcare. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Petra Schwarz, Norbert Drescher and Timo Zindel for their excellent practical support in the conduction of the vPSGs. Our thanks also go to Dr. Johannes Zimmermann, Dept. of Psychology, University Kassel, Germany, for his help with the statistics.
ABBREVIATIONS
- PD
Parkinson disease
- PSG
polysomnography
- RBD
REM sleep behavior disorder
- RBD-SQ
RBD screening questionnaire
- RBEs
REM sleep behavioral events
- REM
rapid eye movement
- RWA
REM sleep without atonia
- vPSG
video-supported polysomnography
REFERENCES
- 1.American Academy of Sleep Medicine. International classification of sleep disorders, 2nd ed.; diagnostic and coding manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Rapid eye movement sleep behavioral events: a new marker for neurodegeneration in early Parkinson disease? Sleep. 2014;37:431–8. doi: 10.5665/sleep.3468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mollenhauer B, Trautmann E, Sixel-Doring F, et al. Nonmotor and diagnostic findings in subjects with de novo Parkinson disease of the DeNoPa cohort. Neurology. 2013;81:1226–34. doi: 10.1212/WNL.0b013e3182a6cbd5. [DOI] [PubMed] [Google Scholar]
- 4.Frauscher B, Iranzo A, Gaig C, et al. Normative EMG values during REM sleep for the diagnosis of REM sleep behavior disorder. Sleep. 2012;35:835–47. doi: 10.5665/sleep.1886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tomlinson CL, Stowe R, Patel S, Rick C, Gray R, Clarke CE. Systematic review of levodopa dose equivalency reporting in Parkinson's disease. Mov Disord. 2010;25:2649–53. doi: 10.1002/mds.23429. [DOI] [PubMed] [Google Scholar]
- 6.Stiasny-Kolster K, Mayer G, Schafer S, Moller JC, Heinzel-Gutenbrunner M, Oertel WH. The REM sleep behavior disorder screening questionnaire--a new diagnostic instrument. Mov Disord. 2007;22:2386–93. doi: 10.1002/mds.21740. [DOI] [PubMed] [Google Scholar]
- 7.Schenck CH, Mahowald MW. Subclinical REM sleep behavior disorder and its clinical and research implications. Sleep. 2008;31:1627. doi: 10.1093/sleep/31.12.1627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ferri R, Fantini ML, Schenck CH. The role of REM sleep without atonia in the diagnosis of REM sleep behavior disorder: past errors and new challenges. Sleep Med. 2014;15:1007–8. doi: 10.1016/j.sleep.2014.05.006. [DOI] [PubMed] [Google Scholar]
- 9.Sixel-Doring F, Trautmann E, Mollenhauer B, Trenkwalder C. Age, drugs, or disease: what alters the macrostructure of sleep in Parkinson's disease? Sleep Med. 2012;13:1178–83. doi: 10.1016/j.sleep.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 10.Hobson JA. REM sleep and dreaming: towards a theory of protoconsciousness. Nat Rev Neurosci. 2009;10:803–13. doi: 10.1038/nrn2716. [DOI] [PubMed] [Google Scholar]
- 11.Nofzinger EA, Mintun MA, Wiseman M, Kupfer DJ, Moore RY. Forebrain activation in REM sleep: an FDG PET study. Brain Res. 1997;770:192–201. doi: 10.1016/s0006-8993(97)00807-x. [DOI] [PubMed] [Google Scholar]


