Abstract
A 17-year-old male with diffuse axonal injury (DAI) was referred to our psychiatric clinic with a diagnosis of depression. However, further investigation indicated that he had narcolepsy without cataplexy secondary to DAI. We assessed regional volume alterations in the patient; MRI analysis showed a significant decrease in the volume of the hypothalamus, left amygdala, and brainstem. Our findings add to further understanding of the structural basis of secondary narcolepsy, and may provide basis for future neuroimaging studies on sleep disturbances in traumatic brain injury (TBI).
Citation:
Yassin W, Sugihara G, Oishi N, Kubota M, Ubukata S, Murai T, Ueda K. Hypothalamic-amygdalar-brainstem volume reduction in a patient with narcolepsy secondary to diffuse axonal injury. J Clin Sleep Med 2015;11(5):581–582.
Keywords: diffuse axonal injury, narcolepsy, hypothalamus, amygdala, MRI
Narcolepsy can occur due to several neurological conditions, in which brain trauma is most prevalent. Posttraumatic narcolepsy has not been widely investigated, and its neural underpinnings are not well understood. On the other hand, research on idiopathic narcolepsy has revealed a major role of the hypothalamus and amygdala in its pathophysiology.1–3 Here, we report a case of secondary narcolepsy showing altered volumes in these brain regions.
REPORT OF CASE
In April 2012, a 16-year-old male had an incident of traumatic brain injury (TBI) and was diagnosed as having diffuse axonal injury (DAI) (see supplemental material). One-year post injury, he was referred to our psychiatric clinic for being demotivated and fatigued with excessive daytime sleepiness, and was diagnosed with depression. Duloxetine (60 mg/day) was partially effective for treating his symptoms, of which excessive daytime sleepiness persisted throughout the 6 months of treatment. Therefore, a close investigation was conducted to examine whether or not he had posttraumatic sleep disturbance. He had excessive daytime sleepiness, with an MSLT sleep latency of 4.5 min. Sleep onset REM periods were found in 3 of 4 onsets of sleep during the test. Polysomnography ruled out both sleep apnea syndrome and periodic limb movement in sleep. HLA DQB1*0602 was negative with normal levels of orexin (hypocretin) in the cerebrospinal fluid. According to these results, the patient was diagnosed with narcolepsy type 2 (narcolepsy without cataplexy), secondary to DAI. Clinical depression was not found in this patient, as he was previously presented to us. Duloxetine was stopped then, and modafinil was started with a dose of 200 mg per day (given once in the morning), which improved his persisting symptoms.
We investigated volume alterations in the hypothalamus, amygdala, and brainstem, which have been previously associated with idiopathic narcolepsy in the literature.1–3 These regional volumes of the narcolepsy patient were compared to 12 healthy age-, handedness-, and education-matched controls. Image acquisition and volume segmentation are described in the supplemental material.
The patient had a significant decrease in the volume of the hypothalamus, left amygdala, and brainstem (Z score = 2.56 [p = 0.005], 2.70 [p = 0.003], and 2.21 [p = 0.013], respectively), but not in the right amygdala (Z score = 0.73 [p = 0.23]) (Figure 1). The significant results, except for the brainstem, survived the correction for multiple comparisons. No signifi-cant reductions were found in the total white or gray matter volumes of the patient, suggesting that he had no gross brain volume loss.
Figure 1. Regional brain volume assessment of the narcolepsy patient (1) and the healthy controls (12).
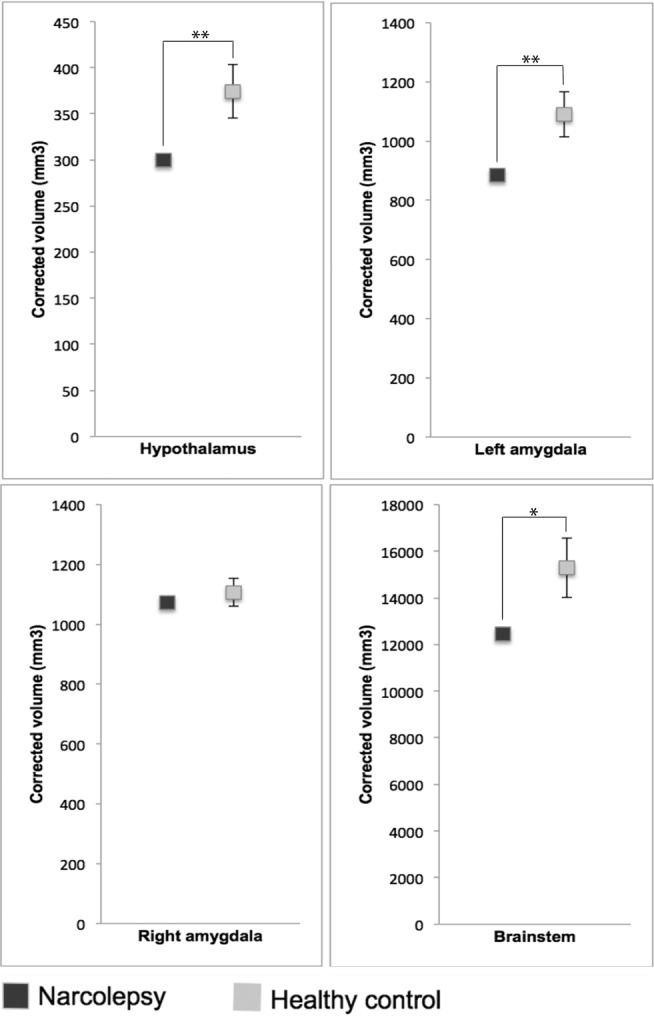
In the controls, the mean volume is presented. Error bars represent standard deviations. **p < 0.01, *p < 0.05.
DISCUSSION
DAI is a type of TBI that involves damage to the white matter. However, gray matter regions can also be affected, leading to diverse secondary outcomes, such as our case of narcolepsy. In general, secondary narcolepsy occurs due to several conditions: tumor, stroke, trauma as well as others neurological disorders. Although the precise mechanism underlying secondary narcolepsy remains unknown, it has been suggested that this condition is linked to hypothalamic lesions that usually result in orexin deficiency. The neuronal loss represented by the reduction in the volume of the hypothalamus, as in our case, is likely to be the primary cause of narcolepsy, as TBI was previously shown to cause chronic sleepiness by affecting the hypothalamus.4 Recent evidence from mice suggests that the amygdala is responsible for triggering cataplexy,5 which is typically brought on by positive emotions. In humans, positive emotions are induced by electrical stimulations in the left, but not right, amygdala.6 These findings suggest that the intact function in the left amygdala is important for cataplexy to occur, which further supports the notion that the volumetric reduction in the left amygdala might have led to the absence of cataplexy in our patient. To our knowledge, this is the first report to show regional volume alterations in a patient with narcolepsy secondary to DAI. The reduction in the volume of the hypothalamus, amygdala and to a lesser extent the brainstem, models a pathway volume reduction associated with the hypothalamic projections related to narcolepsy.
Our findings add to the understanding of the underlying structural basis of secondary narcolepsy, and may provide basis for future neuroimaging studies on sleep disturbances in TBI.
DISCLOSURE STATEMENT
This study was supported by The General Insurance Association of Japan, and JSPS KAKENHI Grant Number 23791328 and 26461766. This study was performed at Kyoto University and was approved by the Committee on Medical Ethics of Kyoto University and carried out in accordance with The Code of Ethics of the World Medical Association. After a complete description of the study to the participants, written informed consent was obtained. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank Drs. Hitoshi Sasaki, Hironobu Fujiwara, Shiraku Son, and Kimito Hirose, as well as a special thanks to Dr. Masaki Nakamura for their contribution to this manuscript.
SUPPLEMENTAL MATERIAL
The patient had loss of consciousness that continued for 3 days before he showed nonverbal reactions to stimuli. He spoke spontaneously 1 week after the accident, and showed retrograde amnesia for few hours and posttraumatic amnesia for about 2 weeks. Computed tomography after the accident showed spotty hemorrhages in the brain parenchyma with absence of large focal lesions. Thus, he was diagnosed with diffuse axonal injury. The patient had one generalized seizure few months before undergoing PSG and MSLT. His Epworth Sleepiness Scale score was 18/24, indicating that he had excessive daytime sleepiness; MSLT sleep latency was as short as 4.5 min. The sleep onset REM period was found in 3 of 4 onsets of sleep during the test. Polysomnography ruled out both sleep apnea syndrome and periodic limb movement in sleep.
METHODS
Imaging Techniques: MRI Acquisition
Diffusion-weighted data were acquired using single-shot spin- echo echo-planar sequences and structural MRI data using 3-dimensional magnetization-prepared rapid gradient echo (3D MPRAGE) sequences, on a 3.0-T MRI unit (Trio; Siemens) with a 40-mT/m gradient and a receiver-only 8-channel phased array head coil. Diffusion-weighted data parameters were: echo time, 96 millisecond; repetition time, 10 500 milliseconds; 96 × 96 matrix; field of view, 192 × 192 mm; 70 contiguous axial slices of 2.0 mm thickness; 81 non-collinear motion-probing gradients; and b = 1,500 s/mm2. The b = 0 images were scanned before every 9 diffusion-weighted images, consisting of 90 volumes in total. The 3D MPRAGE imaging parameters were: echo time, 4.38 milliseconds; repetition time, 2,000 milliseconds; inversion time, 990 milliseconds; field of view, 225 × 240 mm; 240 × 256 matrix; resolution, 0.9375 × 0.9375 × 1.0 mm3; and 208 total axial sections without intersection gaps.
Structural MRI Data Preprocessing and Measurement
The 3D-MPRAGE images as well as the volumes of the selected brain regions were obtained using the automated segmentation function of FreeSurfer software package version 5.0.0 (http://surfer.nmr.mgh.harvard.edu/), except for the hypothalamus, which was manually drawn based on an atlas,6 checked by 2 experts in neuroanatomy (K.U., W.Y.) and used when consensus was achieved. All volumes were corrected for total intracranial volume. In brief, the processing stream included a Talairach transformation of each subject's native brain, removal of non-brain tissue, volumetric subcortical labeling, and surface based segmentation of GM/WM tissue.
Healthy Controls
The healthy control group was gender-, age-, and handedness- matched to the patient. They had no history of brain trauma, neurological, or psychiatric diseases.
RESULTS
PSG was conducted at night, while MSLT was conducted the next day (daytime) after the patient had 6 h of sleep.
On overnight PSG, his total sleep time was 6 h with the lowest recorded SpO2 of 95%. REM sleep was observed 7.5 min after he fell asleep (during PSG) with a sleep latency of 3 h.
On MSLT, conducted on the day following PSG, sleep latencies were 7 min 30 sec; 1 min; 3 min 30 sec; and 6 min; so the average time of sleep latency was 4 min 30 sec. REM sleep was observed in the naps 1, 3, and 4 of MSLT with a REM latency of 7 min 30 sec; 4 min; and 4 min, respectively. Total sleep time of MSLT was 14 min 30 sec; 16 min; 15 min; and 14 min 30 sec, for the sessions 1, 2, 3, and 4, respectively. He was not on duloxetine at the time of MSLT.
The above results are consistent with the diagnosis of narcolepsy. We have added the above information to the supplemental material under Results, as shown in Table S1.
Results of PSG and MSLT
REFERENCES
- 1.Draganski B, Geisler P, Hajak G, et al. Hypothalamic gray matter changes in narcoleptic patients. Nat Med. 2002;8:1186–88. doi: 10.1038/nm1102-1186. [DOI] [PubMed] [Google Scholar]
- 2.Menzler K, Belke M, Unger M, et al. DTI reveals hypothalamic and brainstem white matter lesions in patients with idiopathic narcolepsy. Sleep Med. 2012;13:736–42. doi: 10.1016/j.sleep.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 3.Poryazova R, Schnepf B, Werth E, et al. Evidence for metabolic hypothalamoamygdala dysfunction in narcolepsy. Sleep. 2009;32:607–13. doi: 10.1093/sleep/32.5.607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Baumann CR, Bassetti CL, Valko PO, et al. Loss of hypocretin (orexin) neurons with traumatic brain injury. Ann Neurol. 2009;66:555–9. doi: 10.1002/ana.21836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Burgess CR, Oishi Y, Mochizuki T, Peever JH, Scammell TE. Amygdala lesions reduce cataplexy in orexin KO mice. J Neurosci. 2013;33:9734–42. doi: 10.1523/JNEUROSCI.5632-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lanteaume L, Khalfa S, Régis J, Marquis P, Chauvel P, Bartolomei F. Emotion induction after direct intracerebral stimulations of human amygdala. Cereb Cortex. 2007;17:1307–13. doi: 10.1093/cercor/bhl041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Results of PSG and MSLT


