SUMMARY
Accumulation of misfolded alpha-synuclein (α-syn) into Lewy bodies (LBs) and Lewy neurites (LNs) is a major hallmark of Parkinson’s disease (PD) and dementia with LBs (DLB). Recent studies showed that synthetic preformed fibrils (pffs) recruit endogenous α-syn and induce LB/LN pathology in vitro and in vivo, thereby implicating propagation and cell-to-cell transmission of pathological α-syn as mechanisms for the progressive spread of LBs/LNs. Here, we demonstrate that α-syn monoclonal antibodies (mAbs) reduce α-syn pff-induced LB/LN formation and rescue synapse/neuron loss in primary neuronal cultures by preventing both pff uptake and subsequent cell-to-cell transmission of pathology. Moreover, intraperitoneal (i.p.) administration of mAb specific for misfolded α-syn into nontransgenic mice injected intrastriatally with α-syn pffs reduces LB/LN pathology, ameliorates substantia nigra dopaminergic neuron loss, and improves motor impairments. We conclude that α-syn antibodies could exert therapeutic effects in PD/DLB by blocking entry of pathological α-syn and/or its propagation in neurons.
INTRODUCTION
Misfolded and aggregated disease-specific proteins such as α-syn in Parkinson’s disease (PD) and Aβ and tau in Alzheimer’s disease (AD) are common features of many neurodegenerative diseases (NDs). Self-amplification, propagation, and transmission of these misfolded proteins are hypothesized as major contributing factors to disease onset and progression. The stereotypical and topographical pattern of progressive spread of protein aggregates in the CNS of AD and PD patients supports this hypothesis (Braak and Braak, 1991; Braak et al., 2003). Moreover, the formation of α-syn-positive Lewy bodies (LBs) in fetal mesencephalic grafts in the striatum of PD patients is consistent with host-to-graft transfer of pathologic α-syn (Kordower et al., 2008; Li et al., 2008). Finally, several in vitro and in vivo studies have demonstrated entry, recruitment, and spread of misfolded α-syn and tau seeds to neighboring cells and/or anatomically connected brain regions (Costanzo and Zurzolo, 2013; Guo and Lee, 2014). Given that the cell-to-cell spread of misfolded disease protein likely involves their release followed by internalization, immunotherapy may provide a viable therapeutic approach to treat these NDs by neutralizing them in the extracellular space (Prusiner, 2012; Walker and Jucker, 2013).
Efficacy of passive immunotherapy has been demonstrated in preclinical animal models of NDs. For example, multiple Aβ antibodies were shown to reduce Aβ burden in preclinical models and several of them have progressed to clinical trials for treating AD patients (Lemere and Masliah, 2010). Immunotherapies against intracellular proteins such as tau (Boutajangout et al., 2011; d’Abramo et al., 2013; Yanamandra et al., 2013), SOD1 (Gros-Louis et al., 2010), Huntingtin (Wolfgang et al., 2005), and α-syn (Bae et al., 2012; Masliah et al., 2005, 2011) also have been explored in transgenic (Tg) mouse models and shown to decrease protein aggregation and neurodegeneration. However, only one study demonstrated a mechanism of action where antibody treatment reduced “seeding” activity of tau fibrils (Yanamandra et al., 2013). Thus, how passive immunotherapy halts cell-to-cell transmission of α-syn pathology, a process that is likely central to progression of disease and a prime therapeutic target, warrants further investigation.
Recently, we demonstrated that synthetic α-syn preformed fibrils (pffs) readily enter non-Tg neurons and recruit endogenous mouse α-syn to form LB/Lewy neurite (LN)-like pathology (Volpicelli-Daley et al., 2011). Moreover, α-syn pffs initiated temporal and spatial spread of α-syn pathology when injected into non-Tg mice, leading to motoric deficits and loss of dopaminergic neurons in the substantia nigra pars compacta (SNpc) (Luk et al., 2012a). Here, we show that α-syn monoclonal antibodies (mAbs) (Syn211 and Syn303) blocked α-syn pff entry and cell-to-cell transfer of α-syn pathology in primary neurons, thereby abrogating templated propagation and transmission of α-syn pathology to other neurons. Finally, systemic administration of Syn303 into pff-inoculated wild-type (WT) mice reduced pathologic α-syn spread, dopaminergic cell loss, and associated motor dysfunction caused by α-syn pathology. Together, these data support the therapeutic potential of α-syn immunotherapy for treatment of PD.
RESULTS
α-syn mAbs Reduced α-syn pff-Induced Insoluble Pathologic Aggregates
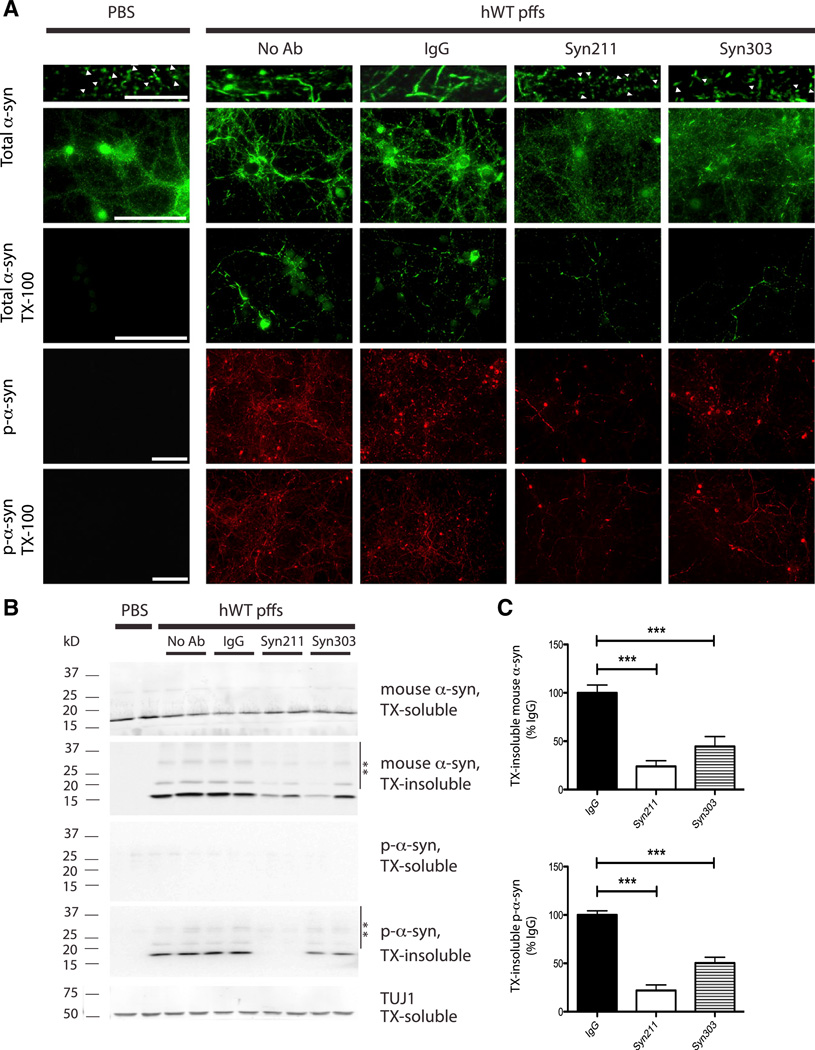
We have previously reported that synthetic α-syn pffs can seed and form LB/LN-like pathology in primary neurons (Volpicelli-Daley et al., 2011). To test whether α-syn antibodies can reduce LB/LN pathology, we treated hippocampal neurons with different α-syn mAbs 30 min before transduction with human WT (hWT) α-syn pffs and evaluated α-syn pathology 7 days posttreatment. In PBS-treated neurons, endogenous mouse α-syn proteins were localized to presynaptic compartments, as evidenced by punctate staining at axon terminals with mAb Syn202 for total α-syn (Figure 1A). However, this presynaptic pattern was clearly perturbed in hWT α-syn pff-treated neurons due to the recruitment of endogenous mouse α-syn into LB/LN-like inclusions. To determine whether these α-syn aggregates were insoluble, we extracted neurons using fixation buffer containing 1% Triton X-100 (TX-100). Under this condition, mouse α-syn within neuronal processes in PBS-treated neurons was completely extracted, whereas neurons treated with pffs showed TX-100-insoluble aggregates. Addition of Syn211 (specific for human α-syn; Giasson et al., 2000a) or Syn303 (which binds a pathological conformation of human and mouse α-syn; Duda et al., 2002) reduced the extent of fibrillar aggregates and partially restored the presynaptic localization of α-syn (Figure 1A, upper panels). In contrast, control immunoglobulin G (IgG) had no effect on α-syn aggregation.
Figure 1. α-syn mAbs Reduced α-syn pff-Induced Insoluble Pathologic Aggregates.
(A) Primary WT neurons treated with PBS or hWT α-syn pffs in the absence (no Ab) or presence of nonspecific mouse IgG (IgG) or Syn211 and Syn303 were fixed at 7 days posttreatment in 4% PFA with or without 1% TX-100 to extract soluble proteins. Neurons were stained with mAb Syn202 for endogenous mouse α-syn (total α-syn) and mAb 81A for p-α-syn. In PBS-treated neurons, α-syn localized to presynaptic compartments (white arrowheads in top panels) and was TX-100 soluble. In pff-treated neurons (no Ab), α-syn was recruited from synapses into LB/LN-like pathology that was hyperphosphorylated and TX-100 insoluble. Syn211 and Syn303, but not IgG, reduced insoluble pathologic aggregates and partially restored α-syn presynaptic localization. The scale bars represent 10 µm (top panels with highest magnification) and 100 µm (all other panels).
(B) Western blot (WB) of mouse α-syn and p-α-syn levels in neuron lysates sequentially extracted in 1% TX-100 (TX-soluble) followed by 2% SDS (TX-insoluble) at 14 days after antibody/pff treatments. TUJ1 served as loading control. High-molecular-weight α-syn species (**) were detected in TX-insoluble fractions of pff-treated neurons. α-Syn pffs recruited endogenous mouse α-syn into TX-insoluble and hyperphosphorylated aggregates that were ameliorated by Syn211 and Syn303.
(C) Significant reduction of TX-insoluble p-α-syn pathology and decreased recruitment of endogenous mouse α-syn into aggregates in cultures treated with pffs and Syn211 or Syn303. There was no significant difference between no Ab and IgG-treated cultures, so they were grouped together. n = 3–6 independent experiments, each performed in duplicate. Values are mean ± SEM. One-way ANOVA with Bonferroni’s correction. ***p < 0.001.
Furthermore, the presence of pathological α-syn aggregates was confirmed using 81A, a mAb specific for α-syn phosphorylated at Ser129 (p-α-syn), because previously we established p-α-syn as a marker of intracellular α-syn pathology as exogenously added hWT α-syn pffs lacked this modification (Volpicelli-Daley et al., 2011; Waxman and Giasson, 2008). Notably, 81A mAb detected numerous TX-100-insoluble, p-α-syn-positive neuritic and perikaryal inclusions in pff-, but not in PBS-treated neurons. Significantly, this pathology was dramatically reduced by Syn211 and Syn303 mAb treatment (Figure 1A, lower panels).
Immunoblot analyses on soluble and insoluble α-syn fractions after sequentially extracting neurons in 1% TX-100 followed by 2% SDS further support that α-syn mAb treatment reduced LB/LN-like α-syn pathology. Unlike PBS-treated neurons, those treated with pffs showed a shift in solubility of endogenous mouse α-syn with a decrease in the TX-soluble fraction and a concomitant increase in the TX-insoluble but SDS-extractable fraction (Figure 1B). The accumulation of p-α-syn in the TX-insoluble fraction was also evident. Moreover, both mAbs to α-syn and p-α-syn detected higher-molecular-weight species of α-syn in the TX-insoluble fractions of pff-treated cultures (see ** in Figure 1B) and likely correspond to multimeric, ubiquitinated, and/or other posttranslational modified forms of α-syn (Jiang et al., 2013; Luk et al., 2009; Sampathu et al., 2003). Importantly, Syn211 or Syn303 reduced recruitment of endogenous α-syn into LB/LN-like aggregates as indicated by a 76.2% ± 5.9% and 55.4% ± 10.3% reduction in TX-insoluble α-syn, respectively (Figure 1C, top panel). The accumulation of TX-insoluble p-α-syn was also reduced by a similar degree (78.9% ± 5.7% and 49.7% ± 6.2%, respectively; Figure 1C, bottom panel). Moreover, Syn211 and Syn303 reduced α-syn pathology even in cultures where α-syn pffs were present at a 3:1 molar excess to mAbs (Figure S1). Thus, α-syn antibodies are potent in reducing pff-mediated recruitment of endogenous mouse α-syn into insoluble, hyperphosphorylated aggregates.
α-syn mAbs Reduced α-syn pff-Induced Synaptic Loss and Neuron Death
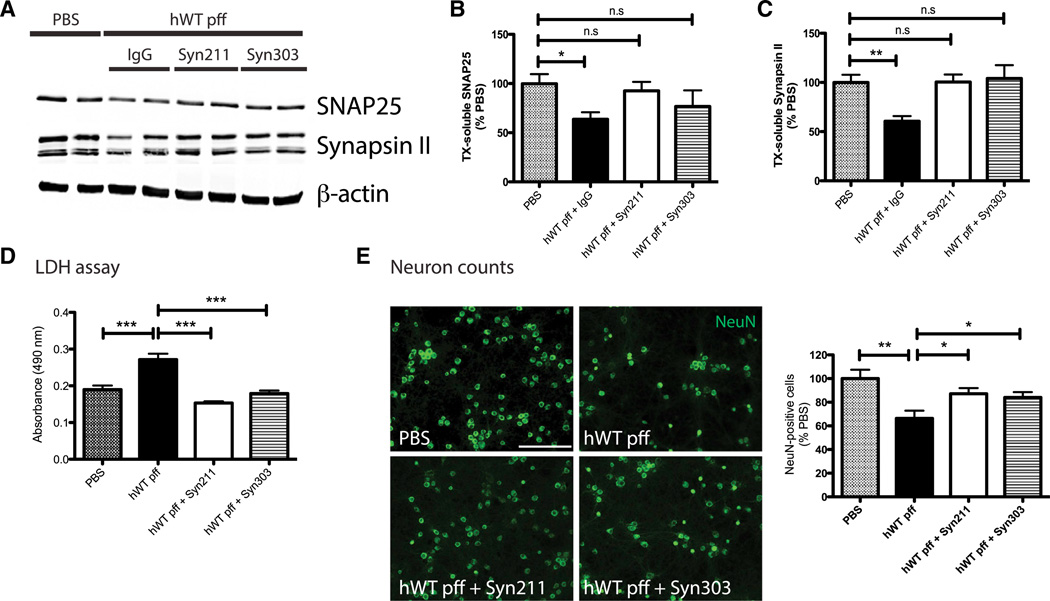
α-syn pathology induced by α-syn pffs not only depleted endogenous α-syn from presynaptic terminals but also resulted in concomitant loss of selective synaptic proteins including SNAP25 and Synapsin II (Volpicelli-Daley et al., 2011). Significantly, Syn211 or Syn303 reduced insoluble α-syn aggregates and partially restored the presynaptic localization of α-syn (see arrowheads in Figure 1A). Moreover, treatment with these mAbs also protected synaptic protein loss induced by α-syn pffs as indicated by the preservation of SNAP25 and Synapsin II at levels comparable to PBS-treated neurons (Figures 2A–2C). Further, pff addition to neurons markedly increased lactate dehydrogenase (LDH) release and neuron death as demonstrated by a ~40% reduction in the number of surviving neurons 14 days posttreatment. This pff-induced toxicity was reduced by treatment with Syn211 or Syn303 (Figures 2D and 2E).
Figure 2. α-syn mAbs Reduced α-syn pff-Induced Synaptic Loss and Neuron Death.
(A–C) α-syn pffs caused reduction in levels of SNAP25 and Synapsin II compared to PBS at 14 days posttreatment. β-actin served as loading control. Syn211 and Syn303 restored pff-induced synaptic protein loss. n = 4 independent experiments. Values are mean ± SEM. One-way ANOVA with Dunnett’s correction. *p < 0.05.
(D and E) LDH assay using culture media (D) and neuron counts (NeuN-positive cells; E) from neurons treated with PBS or hWT α-syn pffs in the absence or presence of Syn211 and Syn303 at 14 days. Syn211 and Syn303 reduced toxicity and neuron death in pff-treated cultures. n = 4 independent experiments. Values are mean ± SEM. One-way ANOVA with Dunnett’s correction. *p ;< 0.05, **p < 0.01, ***p < 0.001. The scale bar represents 100 µm.
α-syn mAbs Prevented pff Entry into Neurons
Because mAbs were added to neurons prior to introduction of hWT α-syn pffs and that Syn211 exclusively recognizes human α-syn, we hypothesized that these mAbs reduce pathology by binding directly to exogenously added hWT α-syn pffs and blocking their entry into neurons. To test this possibility, we followed the distribution and fate of both exogenously added hWT α-syn pffs and mAbs using several experimental paradigms.
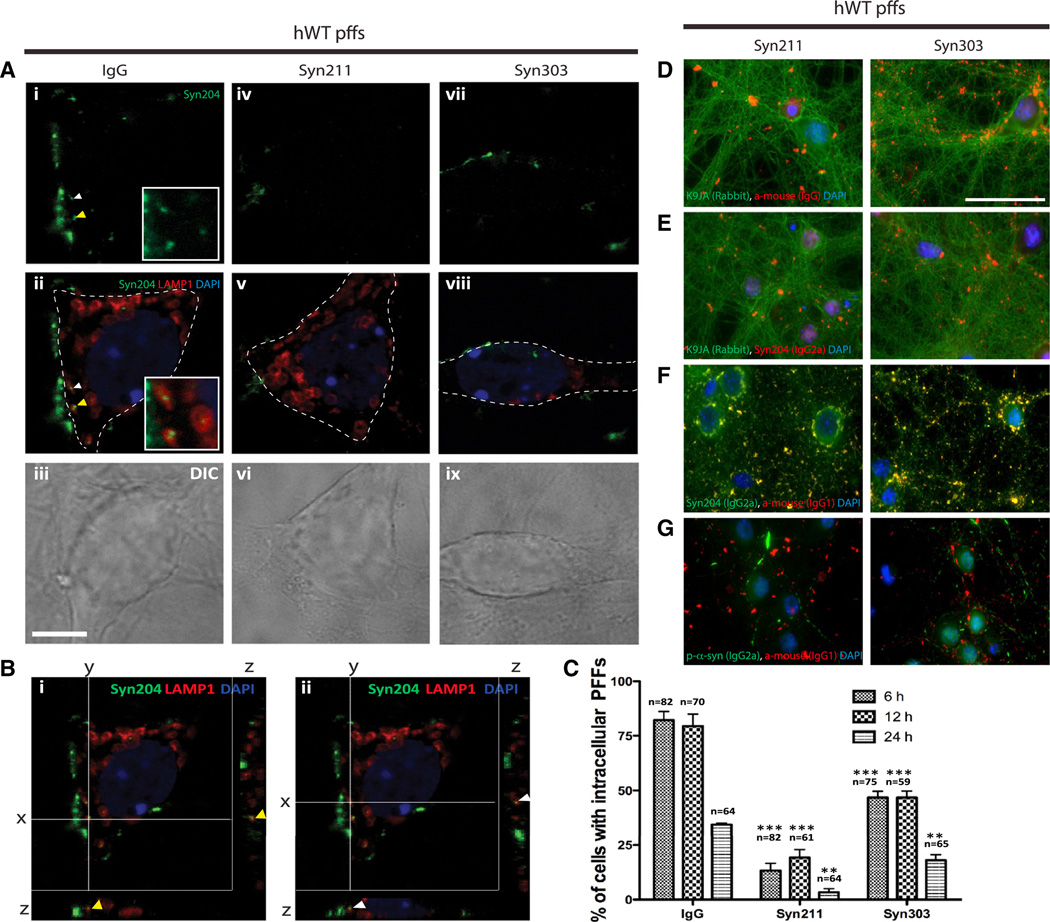
We first monitored the spatial distribution of hWT α-syn pffs in neurons treated with control IgG, Syn211, or Syn303 for 30 min followed by addition of hWT α-syn pffs and incubation for 6, 12, and 24 hr. Neurons were fixed and double labeled with Syn204 (a mAb specific to human α-syn) to detect hWT α-syn pffs and anti-LAMP1, an intracellular marker for lysosomes. Internalized pffs were defined as Syn204-positive puncta that were observed juxtaposed to LAMP1 under confocal microscopy. Within 6 hr following pff treatment, internalized pffs were readily detectable in the perikarya of neurons pretreated with control IgG (Figure 3A). Quantification showed internalized hWT α-syn pffs were present in approximately 82.2% ± 4.0% and 79.5% ± 5.4% of IgG-treated neurons at 6 and 12 hr, respectively. By 24 hr, internalized pffs were detected in only 34.4% ± 0.6% of IgG-treated neurons, suggesting the turnover of pffs. Notably, addition of either Syn211 or Syn303 significantly reduced the proportion of neurons containing internalized hWT α-syn pffs at all time points examined (Figures 3B and 3C). Furthermore, the half-life of pffs in cultures treated with either IgG, Syn211, or Syn303 were comparable (~16.3–17.0 hr). Thus, the presence of α-syn mAbs did not affect the turnover rate of internalized pffs. Together, these data suggest Syn211 or Syn303 prevented α-syn pff entry into neurons, thus reducing the amount of seeds available for templated recruitment of endogenous α-syn into pathological aggregates.
Figure 3. α-syn mAbs Remained Outside Neurons and Prevented α-syn pffs Entry into Neurons.
(A–C) Time course study characterizing the fate of internalized pffs. Neurons were treated with IgG, Syn211, and Syn303 30 min before addition of hWT α-syn pffs. Neurons were fixed at 6, 12, and 24 hr after antibody/pff treatment and double labeled with Syn204 (detect hWT α-syn pffs) and LAMP1, a lysosomal marker.
(A and B) Representative confocal images indicated intracellular pffs inside a neuron treated with IgG, but not with Syn211 or Syn303 at 6 hr. (C) Quantification of percentage of cells with intracellular pffs at 6, 12, and 24 hr posttreatment. At all time points examined, Syn211 and Syn303 prevented pffs entry into neurons, as evidenced by the reduced percentage of neurons with intracellular pffs. Furthermore, neither Syn211 nor Syn303 affected rate of pff turnover. n = 4 independent experiments. Values are mean ± SEM. One-way ANOVA with Bonferroni’s correction. **p < 0.01, ***p < 0.001.
(D and E) Double labeling of intracellular marker tau, K9JA (rabbit polyclonal antibody [pAb]) with secondary anti-mouse IgG to detect Syn211 or Syn303 (D) or with Syn204 (E). Lack of colocalization between K9JA and anti-igG secondary and K9JA and Syn204 suggested exogenously added α-syn mAbs and α-syn pffs were outside of neurons. DAPI served as nuclei marker. The scale bar represents 10 µm.
(F) Double labeling of Syn204 (IgG2a isotype) with anti-IgG1 secondary antibody to detect Syn211 and Syn303. Colocalization between Syn204 and anti-IgG1 secondary antibody suggested majority of pffs and α-syn mAbs remained outside of neurons.
(G) Double labeling of p-α-syn (81A; IgG2a isotype) with anti-IgG1 secondary antibody for Syn211 and Syn303. Lack of colocalization between 81A and anti-IgG1 secondary antibody suggested added Syn211 and Syn303 remained outside of neurons.
To further confirm that α-syn mAbs remained outside neurons, Syn211 or Syn303 was labeled with anti-mouse IgG and an intracellular marker, tau (K9JA), at 7 days after antibody/pff treatments. We found that α-syn mAbs were almost exclusively outside neurons as there was no colocalization of either mAb with K9JA (Figure 3D). Next, we found that almost all of the exogenously added hWT α-syn pffs (positive for Syn204) remained outside neurons, as evidence by the lack of Syn204 and K9JA colocalization (Figure 3E). Moreover, we used anti-mouse IgG1 as a secondary antibody to detect Syn211 or Syn303 and anti-mouse IgG2a as a secondary antibody to detect Syn204 and observed complete colocalization, indicating that most of the antibody/α-syn pffs complexes resided outside neurons (Figure 3F). In addition, anti-p-α-syn (81A; IgG2a isotype), which detects intraneuronal p-α-syn aggregates, did not colocalize with either Syn211 or Syn303, suggesting that the exogenously added α-syn antibodies remained bound to hWT α-syn pffs outside neurons (Figure 3G).
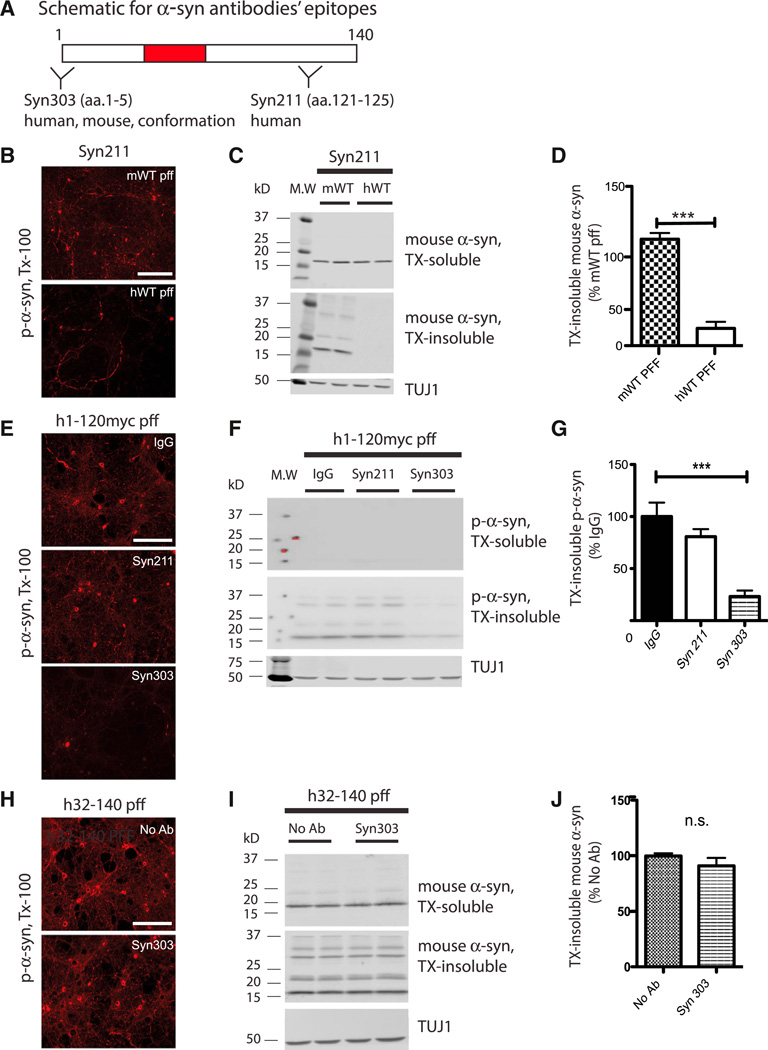
To further demonstrate blockade of α-syn pff entry as a mechanism of action of α-syn mAbs, we took advantage that Syn211 and Syn303 recognize defined epitopes in topographically distinct domains of α-syn (Figure 4A). Based on its selective recognition of amino acids 121–125 of human α-syn (Giasson et al., 2000a), we predicted that Syn211 would be ineffective in reducing α-syn pathology when neurons were treated with pffs made from recombinant mouse α-syn (mWT) or from C-terminally Myc-tagged human α-syn that was truncated at amino acid 120 (h1-120myc). Indeed, Syn211 was unable to reduce insoluble p-α-syn accumulations in mWT- (Figures 4B–4D) and h1-120myc α-syn pff-treated neurons as monitored by immunofluorescence (IF) and immunoblotting (Figures 4E–4G).
Figure 4. Recognition of α-syn pff Epitopes by α-syn mAbs Is Required for Reduction of Pathology.
(A) Schematic representation of α-syn protein and epitopes recognized by Syn211 and Syn303. aa, amino acid.
(B–D) Syn211 was effective in reducing p-α-syn pathology when neurons were transduced with hWT, but not with mWT α-syn pffs, as evidenced by IF (B) and WB (C and D). n = 4 independent experiments. Values are mean ± SEM. Student’s t test. ***p < 0.001. The scale bar represents 100 µm. MW, molecular weight.
(E–G) Primary neurons were transduced with myc-tagged, C-terminally truncated α-syn pffs (h1-120myc) following treatment with IgG, Syn211, or Syn303. Syn303, but not Syn211, was effective in reducing insoluble pathologic aggregates as shown by IF (E) and WB (F and G). n = 6 independent experiments. Values are mean ± SEM. One-way ANOVA with Bonferroni’s correction. ***p < 0.001. The scale bar represents 100 µm.
(H–J) Primary neurons were transduced with N-terminally truncated α-syn pff (h32-140) following treatment with Syn303. As expected, Syn303 was ineffective at reducing pathology as evidenced by IF (H) and WB (I and J). n = 3 independent experiments. Values are mean ± SEM. Student’s t test. n.s., not significant. The scale bar represents 100 µm.
On the other hand, Syn303 recognizes a conformational epitope spanning across amino acids 1–5 in both human and mouse α-syn (Duda et al., 2002; Giasson et al., 2002; Figure 4A). Due to its selectivity for the α-syn N terminus, we predicted that Syn303 would reduce the extent of insoluble aggregates when neurons were treated with pffs made from h1-120myc α-syn, but not those made from N-terminally truncated human α-syn (h32-140). Indeed, Syn303 was efficacious in reducing pathologic aggregates when neurons were exposed to h1-120myc (Figures 4E–4G), but not h32-140 α-syn pffs (Figures 4H–4J). As an additional control, a Myc antibody (9E10) was able to reduce pathology when h1-120myc α-syn pffs were used, confirming blockade of exogenous pff uptake into neurons (Figure S2).
Taken together, these data support our conclusion that α-syn mAbs reduced pathology by directly interacting with the α-syn pffs added to the culture dish, rather than endogenously expressed α-syn, and that blockade of pff entry into neurons is likely the main mechanism underlying the observed reduction in pathology.
α-syn mAbs Prevented Transmission of Pathologic α-syn Aggregates
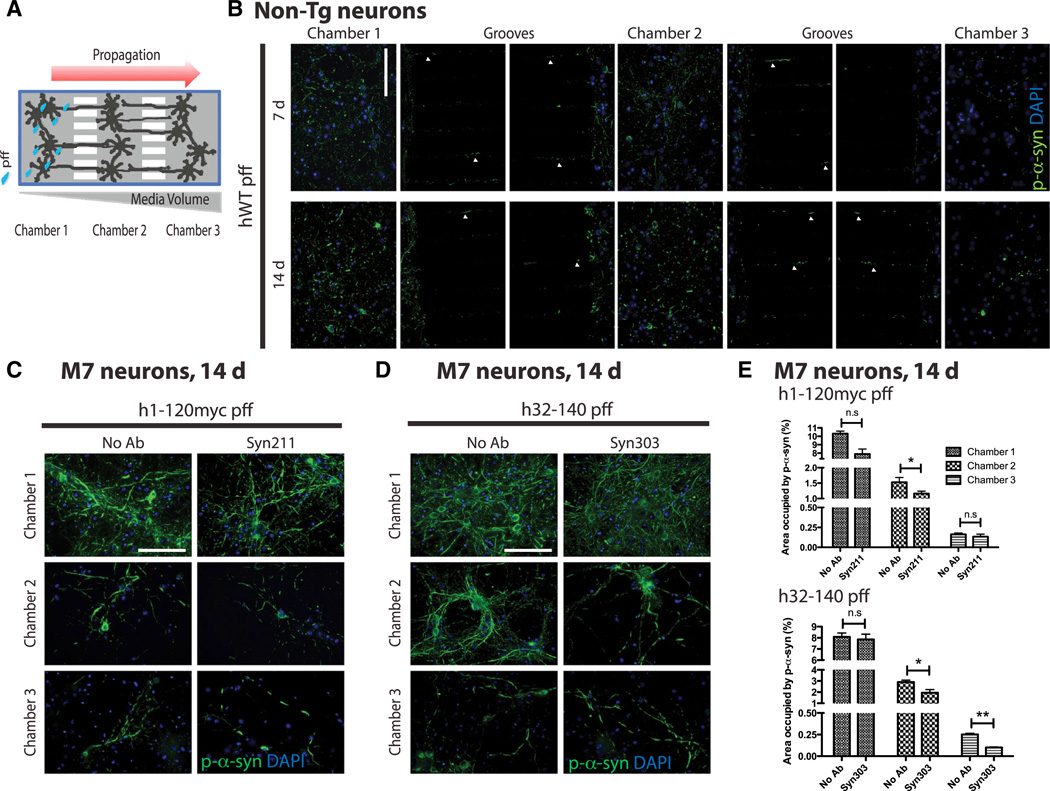
To study the effects of mAb treatment on propagation of α-syn pathology, we used microfluidic culture devices (Taylor et al., 2005), which comprised of three somal compartments connected in tandem by a series of microgrooves separating three distinct neuronal populations (Figure 5A).
Figure 5. α-syn mAbs Prevented Transmission of Pathologic α-syn Aggregates.
(A) Schematic of microfluidic neuron device with three chambers to separate neurons seeded in each chamber.
(B) Propagation of pathologic p-α-syn from C1 to C2 to C3 at 7 days and 14 days postaddition of hWT α-syn pffs into non-Tg neurons. Arrowheads indicate p-α-syn pathology formed within grooves. The scale bar represents 100 µm.
(C–E) To increase amount of transmitted pathologic α-syn aggregates in C3, neurons from Tg mice overexpressing hWT α-syn (M7 line) were used. Because passive diffusion of mAbs from C2 to C1 occurs and can potentially bind to hWT α-syn pffs in C1 to prevent pff uptake that could account for the observed reduction in transmitted pathology from C1 to C2 to C3, C- and N-terminally truncated α-syn pffs were used. Specifically, h1-120myc pffs were added to C1, whereas Syn211 was added to C2 (C) and h32-140 pffs were added to C1, whereas C2 received Syn303 treatment (D). Both Syn211 and Syn303 were ineffective in preventing C- and N-terminally truncated pff-induced pathology, respectively, in C1 when compared to systems without antibody treatment (no Ab). Syn211 partially blocked templated transmission of pathologic aggregates in C2 (C), whereas Syn303 prevented templated propagation of pathologic α-syn from C1 to C2 to C3 (D).
(E) Quantification of area occupied by mAb 81A for p-α-syn signal in C1, C2, and C3 of paired chamber devices that either received no antibody treatment (no Ab), Syn211, or Syn303. n = 3 independent experiments. Values are mean ± SEM. Student’s t test. *p < 0.05, **p < 0.01.
We first plated non-Tg neurons in all three chambers (C) and initiated α-syn pathology by adding hWT α-syn pffs to neurons in C1 at day in vitro (DIV) 7. Significantly, we observed propagation of pathologic p-α-syn aggregates to cell bodies and neurites of neurons grown in C2 (at 7 days after pff addition) followed by those in C3 (at 14 days; Figure 5B). Importantly, when h1-120myc pffs were added to C1, we were unable to detect Myc immunoreactivity in either C2 or C3, indicating that the hydrostatic gradient effectively prevented passage of these pffs from C1 (Figure S3A). Thus, our data demonstrate that pathologic α-syn induced by pffs in C1 can propagate along axons and/or dendrites contained within the microgrooves to C2 and C3. Given that neuronal processes represent the only cellular contact between individual chambers, the delayed presence of α-syn pathology in C3 supports cell-to-cell transfer of seeding material from C1 and/or C2.
To determine whether α-syn mAbs can prevent cell-to-cell transmission of pathologic α-syn aggregates in this chamber system, we added Syn211 or Syn303 to C2 30 min prior to adding pffs to C1, where the transfer of pathologic α-syn from affected neurons to naive neurons would be expected to occur. Compared with no α-syn mAb treatment, we observed a highly significant reduction of α-syn pathology in C1 and C2 at 7 and 14 days following treatment with Syn211 and Syn303 (Figures S3B–S3D). Passive diffusion of mAbs from C2 to C1 could in part account for this reduction in α-syn aggregates due to the hydrostatic gradient used to confine pffs to C1 (Figure 5A) and hence preventing pff uptake. Moreover, because only a limited number of the axons from each chamber can pass through the microgrooves into the next, we were unable to accurately quantify the amount of α-syn pathology in C3 using primary neurons from non-Tg mice.
To overcome these two limitations, we used primary neurons generated from homozygous Tg mice overexpressing hWT α-syn (M7 line; Giasson et al., 2002) to increase the amount of α-syn pathology and enhance our ability to detect pathological transmission to neurons in C3. Moreover, to unequivocally identify cell-to-cell transmitted species, we used pffs assembled from α-syn lacking the complementary extreme N- or C-terminal regions recognized by the mAbs. If cell-to-cell transfer of α-syn pathology indeed occurs in C2 and C3, α-syn mAbs should block this process because the transmissible α-syn species is likely comprised of full-length α-syn (i.e., endogenous human or mouse α-syn in the M7 neurons) rather than the truncated α-syn in pffs. Indeed, when h1-120myc α-syn pffs was added to C1, Syn211 added to C2 was ineffective in blocking h-120myc pff-induced pathology in C1 but partially effective in blocking α-syn pathology in C2, supporting the interpretation that some of the transmissible species in C2 are human α-syn (Figures 5C and 5E). When h32-140 α-syn pffs were added to C1 and Syn303 to C2, Syn303 was ineffective in blocking α-syn pathology elicited by h32-140 pffs in C1. However, Syn303 reduced propagation of α-syn pathology in C2 and C3 (Figures 5D and 5E). Because the numbers of DAPI nuclei were similar in chamber pairs with and without mAb treatment (data not shown), the reduction in p-α-syn by α-syn mAbs indeed reflects the blockade of cell-to-cell transmission of α-syn pathology. Furthermore, because Syn303 is a species-independent mAb specific for a misfolded α-syn conformer and does not immunostain endogenous and synaptically localized α-syn, the transmissible species are likely misfolded α-syn conformers that are subsequently released and internalized by nearby neurites and cell bodies to template the recruitment of endogenous α-syn in neurons in C2 and C3. Thus, our results are consistent with Syn303 reducing pathologic α-syn propagation in C2 and C3, whereas Syn211 was only able to ameliorate pathology in C2 (Figure 5E).
Taken together, these data demonstrate that α-syn mAbs could prevent cell-to-cell transmission of pathologic α-syn aggregates.
Syn303 Reduced Pathological α-syn Spread In Vivo
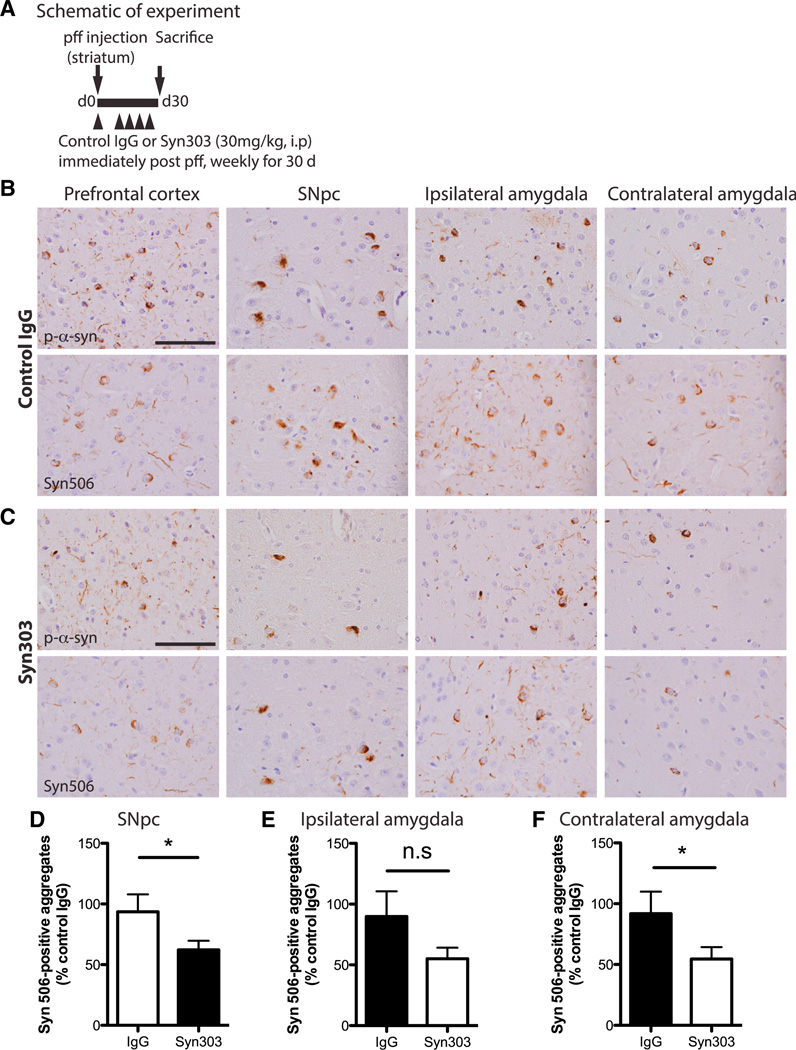
Because Syn303 can reduce pathologic α-syn aggregates in primary neurons and recognizes misfolded α-syn in LBs/LNs in PD brains and various PD mouse models (Giasson et al., 2000b; Irwin et al., 2012; Lim et al., 2011; Luk et al., 2012b), we asked whether Syn303 could reduce α-syn pathology in vivo. We used a previously established mouse model of sporadic PD where we showed that a single intrastriatal injection of mWT α-syn pffs into young WT mice resulted in progressive accumulation of misfolded and hyperphosphorylated α-syn aggregates at neuroanatomically connected areas including the SNpc and amygdala (Luk et al., 2012a). To this end, WT mice were inoculated with mWT α-syn pffs and immediately treated with Syn303 or control IgG (30 mg/kg, intraperitoneally [i.p.]) followed by weekly treatment for 30 days (Figure 6A). Mice were sacrificed posttreatment, and brains were processed for immunohistochemistry (IHC) to detect α-syn pathology using 81A mAb for p-α-syn and Syn506 mAb for misfolded α-syn in LB/LN-like aggregates (Luk et al., 2012a).
Figure 6. Systemic Treatment of Syn303 Reduced Spread of pff-Induced α-syn Pathology In Vivo.
(A) Schematic representation of experimental design.
(B and C) IHC with mAb 81A for p-α-syn and mAb Syn506 for misfolded α-syn. Pff inoculation resulted in widespread α-syn pathology at sites with connection to the striatum such as the ipsilateral (ipsi) prefrontal cortex, ipsi SNpc, and ipsi and contralateral (contra) amygdala (B). Syn303 treatment significantly reduced transmission of α-syn pathology to these regions (C). The scale bar represents 100 µm.
(D–F) Quantitative analyses of Syn506-positive aggregates from rostral to caudal SNpc (D), ipsi (E), and contra amygdala (F) confirmed the qualitative observations. n = 8 mice per treatment group. Values are mean ± SEM. Student’s t test. *p < 0.05.
In pff-inoculated, control-IgG-treated mice, pathologic α-syn aggregates were detected in multiple brain regions including frontal cortex, SNpc, and the amygdala, primarily ipsilateral to the injection site (Figure 6B). Importantly, Syn303 treatment reduced α-syn pathology in these same areas (Figure 6C). Quantitative analysis of Syn506-positive aggregates in control-IgG-and Syn303-treated mice showed significant reduction of α-syn pathology in the ipsilateral SNpc and contralateral amygdala (Figures 6D and 6F) and a trend toward reduction in the ipsilateral amygdala (Figure 6E). Thus, the simultaneous administration of Syn303 mAb and α-syn pffs led to reduction in LB/LN pathology in our PD mouse model.
To determine whether i.p. administration of Syn303 led to an appreciable mAb concentration within the brain that demonstrates effective target engagement (i.e., binding of extracellular pathologic α-syn), we measured Syn303 levels in the cerebral spinal fluid (CSF) at 1, 3, and 7 days following a single i.p. injection (10 mg/kg). This dose was chosen based on the use of mAbs in prior preclinical models, which ranges from 1 to 10 mg/kg (d’Abramo et al., 2013; Masliah et al., 2011). Within 1 day following injection, CSF levels of Syn303 reached 0.1% ± 0.002% of the plasma levels (Figure S4), comparable to CSF levels achieved by other antibodies administered via this route (Demattos et al., 2012; Wang et al., 2008). Whereas the half-life of Syn303 mAb in plasma was approximately 60 hr, CSF levels of Syn303 persisted for the duration of the experiment. These data further suggest that the observed reduction in pathology was indeed due to presence of Syn303 mAb in the brain of mWT pff-injected mice.
To test whether Syn303 immunotherapy could retard disease progression, we initiated treatment with either control IgG or Syn303 (10 mg/kg) in separate cohorts at 7 days post pffs injection. At this time point, some α-syn pathology already is detectable (Figure S5), thus permitting us to determine whether this mAb could reduce misfolded α-syn propagation. Following this treatment protocol (Figure S6A), we observed a significant reduction in numbers of Syn506-positive aggregates in the SNpc and contralateral amygdala of Syn303-treated mice by 60 days after pff injection (Figures S6B, S6C, S6F, and S6G) and a trend toward reduction in the ipsilateral amygdala (Figures S6D and S6E). These data demonstrate that Syn303 treatment can retard the spread of α-syn pathology in vivo.
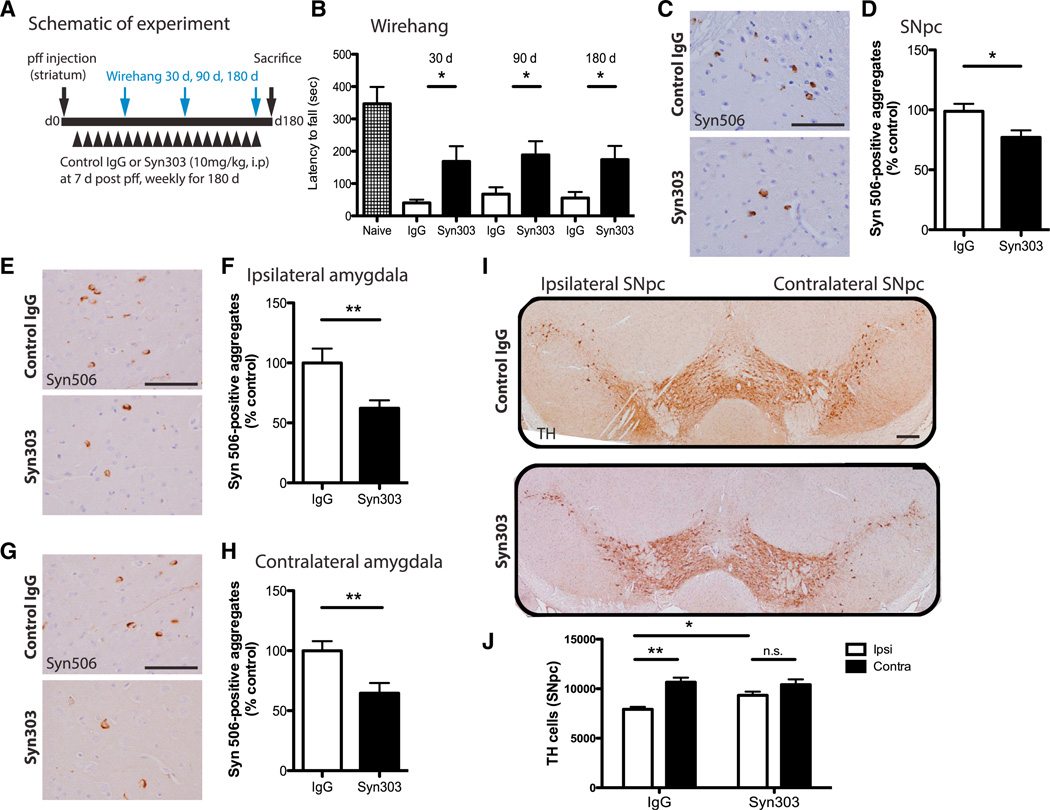
Because the accumulation of α-syn aggregates in the SNpc compromises the survival of dopaminergic neurons over time, resulting in motor impairments in pff-injected mice, we asked whether long-term anti-α-syn immunotherapy could ameliorate these deficits. To this end, another cohort of mice was treated with Syn303 or control IgG (10 mg/kg) at 7 days post pff inoculation and then continued weekly for 180 days (Figure 7A). The animals tolerated long-term antibody treatment well, as they gained weight normally and appeared healthy until the predefined termination of the experiment. To determine efficacy of immunotherapy, mice were evaluated on the wire hang test for grip strength at 30, 90, and 180 days, where we had observed impairment in this task (Luk et al., 2012a). At all time points tested, Syn303 significantly improved grip strength and motor coordination (Figure 7B). These mice also exhibited loss of motor learning and coordination at 90 and 180 days post pff inoculation, as assessed by the rotarod task. However, rotarod performance was not significantly improved with Syn303 compared to IgG treatment at these time points (Figure S7A). IHC analyses showed a significant reduction in Syn506-positive aggregates in the SNpc (30% reduction) as well as both ipsilateral (40% reduction) and contralateral amygdala (40% reduction) of Syn303- compared to IgG-treated mice (Figures 7C–7H). To determine whether treatment with Syn303 could prevent loss of tyrosine hydroxylase (TH)-expressing SNpc neurons at 180 days post pff inoculation, we conducted IHC using TH antibody in IgG- and Syn303-treated mice (Figure 7I). Quantification of TH-positive neurons showed significant loss in the ipsilateral SNpc compared to contralateral SNpc of IgG-treated mice (Figures 7I and 7J). Syn303 treatment not only ameliorated TH cell loss in the ipsilateral SNpc compared to the contralateral SNpc (Figure 7J) but also rescued TH-positive cells in the ipsilateral SNpc of Syn303- compared to IgG-treated mice (Figure 7J).
Figure 7. Syn303 Reduced pff-Induced Motor Dysfunction and Dopaminergic Cell Loss.
(A) Schematic representation of experimental design.
(B) Wire hang performance of WT mice at 30 days, 90 days, and 180 days post pff inoculation with control IgG or Syn303 treatment. Longer latency to fall equated to better performance. n = 15 mice per treatment group. Student’s t test. *p < 0.05.
(C–H) Syn303 treatment significantly reduced pathologic spread of Syn506-positive aggregates in the SNpc (C and D) and ipsi (E and F) and contra amygdala (G and H). n = 12 mice per treatment group. Values are mean ± SEM. Student’s t test. *p < 0.05, **p < 0.01. The scale bars represent 100 µm.
(I) TH cells loss was evident in the ipsi, but not the contra, SNpc of control IgG-treated mice. Syn303 treatment ameliorated TH cell loss. The scale bar represents 100 µm.
(J) Quantification of TH-positive cells showed ~35% TH cell loss in the ipsi SNpc compared to the contra SNpc in control IgG-treated mice at 180 days post pff inoculation. Syn303 treatment ameliorated this TH cell loss. n = 12 mice per treatment group. Values are mean ± SEM. One-way ANOVA with Bonferroni’s correction. *p < 0.05, **p < 0.01.
We also assessed changes in astrocyte and microglia activation via IHC with GFAP and Iba1 antibodies, respectively, but did not observe marked differences in the extent of astrogliosis and microgliosis in IgG- versus Syn303-treated mice (Figures S7B–S7E). We surmise this reflects the modest SNpc neurodegeneration at this time.
Taken together, α-syn immunotherapy using Syn303 reduced pathologic spread of α-syn aggregates in vivo, ameliorated motor dysfunction mediated by LB pathology, and rescued TH cell loss.
DISCUSSION
Our study demonstrates efficacy of anti-α-syn immunotherapy in reducing LB/LN-like pathology formation and spread in physiologically relevant in vitro and in vivo models of PD-like synucleinopathy. Indeed, in primary neurons treated with α-syn pffs, α-syn mAbs were capable of impeding recruitment of endogenous α-syn into pathologic aggregates as well as preserving normal synaptic localization of α-syn, mitigating synaptic and neuron loss caused by pathologic α-syn. Likewise, in WT mice inoculated with α-syn pffs into the striatum, anti-α-syn immunotherapy reduced the spread of pathologic α-syn, rescuing dopaminergic SNpc neuron loss and the associated motor deficits induced by transmission of α-syn pathology. Thus, α-syn mAbs interrupt a reiterative chain of detrimental changes following the templated conversion of normal endogenous mouse α-syn by α-syn pffs in neurons.
Several lines of evidence support the self-templating, amplification, and cell-to-cell transmission of misfolded protein seeds as the underlying mechanism for the progression and manifestation of several major NDs (Costanzo and Zurzolo, 2013; Guo and Lee, 2014; Walker and Jucker, 2013). Here, we extend these previous observations by demonstrating that LB/LN-like pathology induced by α-syn pffs propagates both intracellularly within neurons and intercellularly between neurons through the use of a triple microfluidic chamber system that allows for physical isolation of distinct neuronal populations. Using this system, we demonstrate that α-syn pathology induced by α-syn pffs within one chamber is progressively amplified and propagated to downstream neuron populations. Moreover, the efficacy of α-syn mAbs in reducing pathological α-syn and spread advocates targeting extracellular protein seeds as viable therapeutic options for the treatment of NDs, and our triple chamber neuronal system will be useful for future testing of compounds that may retard or halt cell-to-cell transmission of disease proteins like α-syn.
Our study suggests two possible mechanisms by which α-syn mAbs reduced pathologic α-syn. First, α-syn mAbs could block pff uptake into neurons, thereby limiting the amount of seeds available for recruitment of endogenous α-syn into pathologic aggregates. Indeed, the majority of added pffs remained outside neurons, where they colocalized with the α-syn mAbs, but not with intracellular neuronal markers. Furthermore, dramatically lower number of neurons with internalized α-syn pffs was observed in the presence of α-syn mAbs, and the turnover rate of intracellular pffs was not affected by mAb treatment. Moreover, in h1-120myc α-syn pff-treated culture, a Myc antibody dramatically reduced α-syn pathology, thereby providing unequivocal evidence that eliminating the exogenous source is driving pathology reduction. This concept of inhibiting fibril entry as a mechanism of immunotherapy was also demonstrated recently for tau antibodies in tau-overexpressing nonneuronal cell models (Kfoury et al., 2012; Yanamandra et al., 2013) as well as in a Tg mouse model of tauopathy (Yanamandra et al., 2013).
A second potential mechanism for α-syn mAbs is the prevention of cell-to-cell transmission of pathologic α-syn. In this regard, the utilization of the triple microfluidic system allowed us to demonstrate that α-syn mAbs impeded progressive propagation and spread of pathologic α-syn from neurons grown in one chamber to another. Presumably, this occurred within neurons that had internalized α-syn pffs, but more studies are needed to confirm this issue. Importantly, the two mechanisms we propose to account for how α-syn mAbs reduce pathologic α-syn are not mutually exclusive, and it is plausible that they could operate simultaneously.
Consistent with our in vitro findings, the α-syn mAb Syn303 was effective in arresting initiation and halting progression of α-syn pathology in vivo. By treating WT mice with Syn303 immediately after intrastriatal inoculation of mWT α-syn pffs, which presumably inhibits pff uptake, we demonstrated a reduction in pathologic α-syn aggregates in brain regions with direct connection to the striatum, including prefrontal cortex, SNpc, and amygdala. By treating the mice at 7 days post α-syn pff inoculation, a time at which some α-syn pathology was already observed, Syn303 was able to inhibit transmission of α-syn pathology and retard disease progression, as evidenced by reduced pathologic spread of α-syn throughout multiple CNS regions. Currently, it is unclear whether α-syn mAbs mediate the clearance of Lewy pathology in our sporadic PD model of WT mice. Based on previous reports of active and passive α-syn immunization in Tg mice overexpressing human α-syn, α-syn antibodies might facilitate degradation of α-syn aggregates by promoting lysosomal and/or autophagic clearance (Masliah et al., 2005, 2011) whereas another passive α-syn immunotherapy study supported the role of microglia in antibody-mediated clearance of α-syn aggregates (Bae et al., 2012). Future in vivo studies should aim at determining the metabolic fate of the antibody/α-syn complexes as well as sorting out the exact mechanism(s) of immune therapy in vivo.
Despite a lack of understanding of disease mechanisms underlying transmissible α-syn release and uptake into neurons in synucleinopathies brains and factors responsible for initiating α-syn misfolding and fibrillization, our study supports that the transmissible species likely comprised of endogenous misfolded α-syn for the following reasons. First, using triple chamber system cultured with M7 neurons that overexpress human α-syn, we showed that both Syn211, a C-terminal, human-specific α-syn mAb, and Syn303, a species-independent, N-terminal mAb against misfolded α-syn, retarded the spread of pathologic α-syn from neurons grown in one chamber to the other two downstream chambers, even when C- and N-terminally truncated α-syn pffs were added to seed recruitment of endogenous α-syn. This suggests that the transmissible species are most likely endogenous α-syn protein (human or mouse α-syn), because Syn211 and Syn303 continued to inhibit propagation, even though neither of the mAbs recognize exogenously added C- and N-terminally truncated α-syn pffs, respectively. Second, we postulated that the transmitted species are likely misfolded α-syn because mAb Syn303 only recognizes misfolded, but not normal, α-syn, and this mAb is effective in preventing α-syn pathology propagation from C1 to C2 and then to C3. We also found that 81A, a mAb against p-α-syn, was less effective at reducing pathologic α-syn formation and spread in both in vitro and in vivo systems (data not shown). Together, these data suggest that the transmissible α-syn species originate from templated recruitment of endogenously expressed α-syn protein that is misfolded, but not necessarily phosphorylated.
Previous reports of anti-α-syn immunotherapy showed efficacy of C-terminal α-syn mAbs recognizing linear α-syn epitopes in clearing intracellular aggregates (Bae et al., 2012; Masliah et al., 2011). Similarly, Syn211, which binds to amino acids 121–125 of human α-syn, was very potent in preventing fibrils entry and reducing α-syn pathology in our primary neuron systems. Higher affinity of Syn211 for α-syn compared to Syn303 (data not shown) likely accounted for the observed increase in efficacy of Syn211 compared with Syn303 in our in vitro experiments. However, because our sporadic PD-like model uses non-Tg mice, whether our human-specific Syn211 is effective in vivo remains to be determined. Nonetheless, the efficacy of Syn303, an N-terminal mAb to misfolded α-syn, demonstrates that both conformation and affinity are important determinants for successful α-syn immunotherapy.
Timing of intervention by α-syn immunotherapy is another important factor to be considered. In primary neurons, α-syn mAbs were effective when added 30 min prior to and up to 1 day post pff addition (data not shown). In our in vivo model, α-syn immunotherapy reduced pathological α-syn spread if given immediately or up to 7 days post pff inoculation in mice. Thus, immunotherapy against disease proteins may only be efficacious in treatment of NDs early during the course of disease.
Finally, our recent studies suggest that there may be α-syn “strains,” which, when transmitted from cell to cell, can give rise to morphologically distinct Lewy pathology that could account for different types of synucleinpathies with diverse clinical phenotypes (Guo et al., 2013). We speculate that the transmissible species of pathological α-syn may reflect adaptation of the specific conformations templated by the initial seeds such that it may be possible to design therapeutic antibodies and/or compounds that are specific to these conformers. Perhaps strain-independent mAbs that bind a wide variety of misfolded forms of α-syn will be required to robustly block propagation in vivo, as strains may evolve during the disease process. Future studies will be required to address this question.
In conclusion, the observation that antibodies targeting aberrant species of α-syn markedly reduce uptake and spread of PD-like LB/LN-like pathology provides evidence to support the hypothesis that transmission of pathological α-syn is a central event in the progression of PD and related synucleinopathies. Thus, targeting transmissible misfolded species of α-syn is a potential strategy to halt progression of PD and related synucleinopathies.
EXPERIMENTAL PROCEDURES
Animals
WT mice (CD1 or C57BL6/C3H background) were purchased from Charles River Laboratories. All studies were performed according to the National Institutes of Health (NIH) Guide for the Care and Use of Experimental Animals and approved by the University of Pennsylvania Institutional Animal Care and Use Committee.
Primary Neuronal Cultures, Fibril Transduction, and Antibody Treatment in Primary Neurons
Primary hippocampal cultures were prepared from E16 to E18 CD1 or M7 mouse brains and maintained as described (Volpicelli-Daley et al., 2011). Fibril transduction was performed at 7–10 DIV as described (Volpicelli-Daley et al., 2011). For antibody treatment, neurons were treated with α-syn mAbs 30 min prior to α-syn pff addition. Unless otherwise noted, 6 µg/ml and 15 µg/ml of antibody were used in most IHC and biochemical experiments, respectively. Each experiment was performed in duplicates and repeated 3–10 times. Transduced neurons were harvested for indirect IF and sequential extraction (see Supplemental Information for details).
Microfluidic Chambers
Triple compartments microfluidic devices were obtained from Xona Microfluidic (TCND500). Glass coverslips were prepared and coated as described before being affixed to microfluidic devices (Volpicelli-Daley et al., 2011). Approximately 120,000 neurons were plated per chamber. At DIV7, 3 µg of α-syn mAbs were added to C2 at 30 min before addition of 0.5 µg α-syn pff to C1. To control for direction of flow, a 75 µl difference in media volume was maintained between C1 and C2 and C2 and C3 according to the manufacturers’ instructions. Neurons were fixed 7 or 14 days after mAb/pff treatments using 4% paraformaldehyde and 4% sucrose in PBS. Devices were then removed from cover glass for IF (see Supplemental Information for details).
LDH Toxicity Assay
LDH release assay (Promega; CytoTox 96 NonRadioactive Cytotoxicity Assay) was performed according to manufacturer’s instruction (see Supplemental Information for details).
Stereotaxic Injections of α-syn pff, Antibody Treatment, and IHC
All surgical procedures were performed as previously described (Luk et al., 2012a). An equal proportion of male and female mice were used for each experiment. Either immediately or at 7 days post pff injection, mice were treated with Syn 303 or control IgG1 antibody at 30 mg/kg or 10 mg/kg via i.p. injections, respectively. They received weekly i.p. injections of antibodies until sacrifice at predetermined periods: 30 days, 60 days, and 180 days. IHC was done as previously described (Luk et al., 2012a, 2012b; see Supplemental Information for details).
Direct ELISA
A direct α-syn ELISA using mouse α-syn pff as coating material was used to determine the levels of α-syn antibody in plasma and CSF (see Supplemental Information for details).
Behavioral Assessments
Behavioral tests were performed as described (Luk et al., 2012a), except that the wire hang test was stopped if mice remained suspended for over 10 min (see Supplemental Information for details).
Statistics
For pairwise comparisons, Student’s t tests were used. For comparisons of more than two treatment groups, one-way ANOVA with Bonferroni or Dunnett’s post hoc tests were used. Statistical significance was set to p < 0.05. Values are shown as mean ± SEM unless otherwise indicated.
Supplementary Material
ACKNOWLEDGMENTS
We thanked Drs. Laura Volpicelli-Daley, Kurt Brunden, Jing Guo, Laurel Vana, and Selcuk Tanik for insightful discussions and Dawn Riddle, Victoria Kehm, and Martine White for technical assistance. We thanked the University of Pennsylvania Veterinary Imaging Core for the use of the confocal imaging system. This work was supported by NIH/NIA training grant T32-AG000255, the NIH/NINDS Morris K. Udall Parkinson’s Disease Center of Excellence, P50 NS053488, the Michael J. Fox Foundation, the Keefer family, and the Parkinson Council.
Footnotes
SUPPLEMENTAL INFORMATION
Supplemental Information includes Supplemental Experimental Procedures, seven figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2014.05.033.
REFERENCES
- Bae EJ, Lee HJ, Rockenstein E, Ho DH, Park EB, Yang NY, Desplats P, Masliah E, Lee SJ. Antibody-aided clearance of extracellular α-synuclein prevents cell-to-cell aggregate transmission. J. Neurosci. 2012;32:13454–13469. doi: 10.1523/JNEUROSCI.1292-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutajangout A, Ingadottir J, Davies P, Sigurdsson EM. Passive immunization targeting pathological phospho-tau protein in a mouse model reduces functional decline and clears tau aggregates from the brain. J. Neurochem. 2011;118:658–667. doi: 10.1111/j.1471-4159.2011.07337.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braak H, Braak E. Neuropathological stageing of Alzheimer-related changes. Acta Neuropathol. 1991;82:239–259. doi: 10.1007/BF00308809. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rüb U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson’s disease. Neurobiol. Aging. 2003;24:197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Costanzo M, Zurzolo C. The cell biology of prion-like spread of protein aggregates: mechanisms and implication in neurodegeneration. Biochem. J. 2013;452:1–17. doi: 10.1042/BJ20121898. [DOI] [PubMed] [Google Scholar]
- d’Abramo C, Acker CM, Jimenez HT, Davies P. Tau passive immunotherapy in mutant P301L mice: antibody affinity versus specificity. PLoS ONE. 2013;8:e62402. doi: 10.1371/journal.pone.0062402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demattos RB, Lu J, Tang Y, Racke MM, Delong CA, Tzaferis JA, Hole JT, Forster BM, McDonnell PC, Liu F, et al. A plaque-specific antibody clears existing β-amyloid plaques in Alzheimer’s disease mice. Neuron. 2012;76:908–920. doi: 10.1016/j.neuron.2012.10.029. [DOI] [PubMed] [Google Scholar]
- Duda JE, Giasson BI, Mabon ME, Lee VM, Trojanowski JQ. Novel antibodies to synuclein show abundant striatal pathology in Lewy body diseases. Ann. Neurol. 2002;52:205–210. doi: 10.1002/ana.10279. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Jakes R, Goedert M, Duda JE, Leight S, Trojanowski JQ, Lee VM. A panel of epitope-specific antibodies detects protein domains distributed throughout human α-synuclein in Lewy bodies of Parkinson’s disease. J. Neurosci. Res. 2000a;59:528–533. doi: 10.1002/(SICI)1097-4547(20000215)59:4<528::AID-JNR8>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Murray IV, Chen Q, Souza JM, Hurtig HI, Ischiropoulos H, Trojanowski JQ, Lee VM. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000b;290:985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- Giasson BI, Duda JE, Quinn SM, Zhang B, Trojanowski JQ, Lee VM. Neuronal alpha-synucleinopathy with severe movement disorder in mice expressing A53T human alpha-synuclein. Neuron. 2002;34:521–533. doi: 10.1016/s0896-6273(02)00682-7. [DOI] [PubMed] [Google Scholar]
- Gros-Louis F, Soucy G, Larivière R, Julien JP. Intracerebro-ventricular infusion of monoclonal antibody or its derived Fab fragment against misfolded forms of SOD1 mutant delays mortality in a mouse model of ALS. J. Neurochem. 2010;113:1188–1199. doi: 10.1111/j.1471-4159.2010.06683.x. [DOI] [PubMed] [Google Scholar]
- Guo JL, Lee VM. Cell-to-cell transmission of pathogenic proteins in neurodegenerative diseases. Nat. Med. 2014;20:130–138. doi: 10.1038/nm.3457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo JL, Covell DJ, Daniels JP, Iba M, Stieber A, Zhang B, Riddle DM, Kwong LK, Xu Y, Trojanowski JQ, Lee VM. Distinct α-synuclein strains differentially promote tau inclusions in neurons. Cell. 2013;154:103–117. doi: 10.1016/j.cell.2013.05.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Irwin DJ, White MT, Toledo JB, Xie SX, Robinson JL, Van Deerlin V, Lee VM, Leverenz JB, Montine TJ, Duda JE, et al. Neuropathologic substrates of Parkinson disease dementia. Ann. Neurol. 2012;72:587–598. doi: 10.1002/ana.23659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang P, Gan M, Yen SH. Dopamine prevents lipid peroxidation-induced accumulation of toxic α-synuclein oligomers by preserving autophagy-lysosomal function. Front. Cell. Neurosci. 2013;7:81. doi: 10.3389/fncel.2013.00081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kfoury N, Holmes BB, Jiang H, Holtzman DM, Diamond MI. Trans-cellular propagation of Tau aggregation by fibrillar species. J. Biol. Chem. 2012;287:19440–19451. doi: 10.1074/jbc.M112.346072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14:504–506. doi: 10.1038/nm1747. [DOI] [PubMed] [Google Scholar]
- Lemere CA, Masliah E. Can Alzheimer disease be prevented by amyloid-β immunotherapy? Nat. Rev. Neurol. 2010;6:108–119. doi: 10.1038/nrneurol.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, Lashley T, Quinn NP, Rehncrona S, Björklund A, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat. Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- Lim Y, Kehm VM, Lee EB, Soper JH, Li C, Trojanowski JQ, Lee VM. α-Syn suppression reverses synaptic and memory defects in a mouse model of dementia with Lewy bodies. J. Neurosci. 2011;31:10076–10087. doi: 10.1523/JNEUROSCI.0618-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Song C, O’Brien P, Stieber A, Branch JR, Brunden KR, Trojanowski JQ, Lee VM. Exogenous α-synuclein fibrils seed the formation of Lewy body-like intracellular inclusions in cultured cells. Proc. Natl. Acad. Sci. USA. 2009;106:20051–20056. doi: 10.1073/pnas.0908005106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm V, Carroll J, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Pathological α-synuclein transmission initiates Parkinson-like neurodegeneration in nontransgenic mice. Science. 2012a;338:949–953. doi: 10.1126/science.1227157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luk KC, Kehm VM, Zhang B, O’Brien P, Trojanowski JQ, Lee VM. Intracerebral inoculation of pathological α-synuclein initiates a rapidly progressive neurodegenerative α-synucleinopathy in mice. J. Exp. Med. 2012b;209:975–986. doi: 10.1084/jem.20112457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Adame A, Alford M, Crews L, Hashimoto M, Seubert P, Lee M, Goldstein J, Chilcote T, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46:857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Masliah E, Rockenstein E, Mante M, Crews L, Spencer B, Adame A, Patrick C, Trejo M, Ubhi K, Rohn TT, et al. Passive immunization reduces behavioral and neuropathological deficits in an α-synuclein transgenic model of Lewy body disease. PLoS ONE. 2011;6:e19338. doi: 10.1371/journal.pone.0019338. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Prusiner SB. Cell biology. A unifying role for prions in neurodegenerative diseases. Science. 2012;336:1511–1513. doi: 10.1126/science.1222951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sampathu DM, Giasson BI, Pawlyk AC, Trojanowski JQ, Lee VM. Ubiquitination of alpha-synuclein is not required for formation of pathological inclusions in alpha-synucleinopathies. Am. J. Pathol. 2003;163:91–100. doi: 10.1016/s0002-9440(10)63633-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor AM, Blurton-Jones M, Rhee SW, Cribbs DH, Cotman CW, Jeon NL. A microfluidic culture platform for CNS axonal injury, regeneration and transport. Nat. Methods. 2005;2:599–605. doi: 10.1038/nmeth777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volpicelli-Daley LA, Luk KC, Patel TP, Tanik SA, Riddle DM, Stieber A, Meaney DF, Trojanowski JQ, Lee VM. Exogenous α-synuclein fibrils induce Lewy body pathology leading to synaptic dysfunction and neuron death. Neuron. 2011;72:57–71. doi: 10.1016/j.neuron.2011.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker LC, Jucker M. Seeds of dementia. Sci. Am. 2013;308:52–57. doi: 10.1038/scientificamerican0513-52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin. Pharmacol. Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- Waxman EA, Giasson BI. Specificity and regulation of casein kinase-mediated phosphorylation of α-synuclein. J. Neuropathol. Exp. Neurol. 2008;67:402–416. doi: 10.1097/NEN.0b013e3186fc995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolfgang WJ, Miller TW, Webster JM, Huston JS, Thompson LM, Marsh JL, Messer A. Suppression of Huntington’s disease pathology in Drosophila by human single-chain Fv antibodies. Proc. Natl. Acad. Sci. USA. 2005;102:11563–11568. doi: 10.1073/pnas.0505321102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanamandra K, Kfoury N, Jiang H, Mahan TE, Ma S, Maloney SE, Wozniak DF, Diamond MI, Holtzman DM. Anti-tau antibodies that block tau aggregate seeding in vitro markedly decrease pathology and improve cognition in vivo. Neuron. 2013;80:402–414. doi: 10.1016/j.neuron.2013.07.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.