Abstract
Purpose
Sirolimus is the eponymous inhibitor of the mammalian target of rapamycin (mTOR); however, only its analogues have been approved as cancer therapies. Nevertheless, sirolimus is readily available, has been well-studied in organ transplant patients, and demonstrates efficacy in several preclinical cancer models.
Experimental Design
Three simultaneously conducted phase I studies in advanced cancer patients utilized an adaptive escalation design to find the dose of oral, weekly sirolimus alone or in combination with either ketoconazole or grapefruit juice that achieves similar blood concentrations as its intravenously administered and approved prodrug, temsirolimus. Additionally, the effect of sirolimus on inhibition of p70S6 kinase phosphorylation in peripheral T cells was determined.
Results
Collectively, the three studies enrolled 138 subjects. The most commonly observed toxicities were hyperglycemia, hyperlipidemia, and lymphopenia in 52%, 43%, and 41% of subjects, respectively. The target sirolimus area under the concentration curve (AUC) of 3810 ng-hr/ml was achieved at sirolimus doses of 90 mg, 16 mg, and 25 mg in the sirolimus alone, sirolimus plus ketoconazole, and sirolimus plus grapefruit juice studies, respectively. Ketoconazole and grapefruit juice increased sirolimus AUC approximately 500% and 350%, respectively. Inhibition of p70 S6 kinase phosphorylation was observed at all doses of sirolimus and correlated with blood concentrations. One partial response was observed in a patient with epithelioid hemangioendothelioma.
Conclusion
Sirolimus can be feasibly administered orally, once weekly with a similar toxicity and pharmacokinetic profile compared to other mTOR inhibitors and warrants further evaluation in studies of its comparative effectiveness relative to recently approved sirolimus analogues.
Keywords: Sirolimus, mTOR, Phase 1, CYP3A modulation
Introduction
Sirolimus (rapamycin) was discovered in 1975 and initially developed as an antibiotic because of its antiproliferative properties. The realization that this effect is exerted on bacteria and human cells in an indiscriminate manner led to preclinical and clinical studies of the drug as an immunomodulator and antineoplastic agent. Sirolimus was eventually approved by regulatory authorities for prophylaxis of renal allograft rejection and is now commercially available. Moreover, it has shown activity in some cancers including Kaposi’s sarcoma and hepatocellular carcinoma in addition to tolerability in combination with other targeted agents (1–5). However, its potential in oncology was not fully explored in favor of newly patented analogues that have since been approved in the treatment of renal cell and neuroendocrine carcinoma, and have demonstrated activity in other malignancies.
The mammalian target of rapamycin (mTOR) is a serine/threonine kinase and a key regulator of protein translation via its ability to phosphorylate S6K and 4EBP1 (6–10). Sirolimus binds intracellularly to FK-506 binding protein 12 (FKBP12), inhibiting mTOR, and resulting in growth inhibition in preclinical tumor models primarily by inducing cell cycle arrest or apoptosis (11–13). Intermittent dosing schedules in animal models have demonstrated antitumor activity equivalent to continuous administration, but without prolonged immunosuppression(13).
One putative obstacle to sirolimus development has been its low bioavailability which is shared by its analogue, everolimus(14), and is not uncommon in oncologic drugs (15, 16). The pharmacokinetic profile of sirolimus has been well described in single dose experiments in healthy volunteers and multiple dose studies in renal transplant patients (17–19). Absorption after oral administration is rapid with a bioavailability of 14%, a volume of distribution of 12 L/kg (oral multiple dosing), and an elimination half-life of 63 hours (oral single dose). The agent is 92% protein bound and undergoes extensive hepatic and intestinal metabolism via CYP3A4 and CYP3A5, as well as excretion by P-glycoprotein. One potential strategy to increase bioavailability of rapamycin is co-administration with CYP3A inhibitors such as ketoconazole or grapefruit juice, the latter exerting an effect predominantly on intestinal enzymes.
Importantly, an analogue of sirolimus, temsirolimus, is a prodrug that is hepatically metabolized to the parent compound and has been approved in renal cell carcinoma at an intravenous dosage of 25 mg per week. Based on the reported temsirolimus pharmacokinetics and activity at this dose, we undertook three separate but simultaneously conducted phase 1 studies to find the dose of orally administered weekly sirolimus that would achieve exposure similar to weekly intravenous temsirolimus. We also aimed to evaluate the toxicity and pharmacokinetic profile of intermittently administered sirolimus in patients with advanced malignancies, assess the change in Cmax and AUC when sirolimus was co-administered with CYP3A inhibitors (e.g. ketoconazole and grapefruit juice), and model the relationship between sirolimus whole blood levels and inhibition of the direct mTOR target p70S6 kinase. These results build on the prior published oncology experience with sirolimus on a daily schedule (20).
Methods
Eligibility Criteria
Patients with incurable cancer and no other effective therapy, older than 18 years of age, and with Karnofsky performance status >60% were eligible to participate. Written informed consent was obtained from each subject. All subjects required laboratory parameters that included leukocytes of >3,000/ml, absolute neutrophil count >1,500/ml, platelet count >100,000/ml, total bilirubin within institutional normal limits, serum SGOT and SGPT <2.5 times institutional upper normal limits, serum triglycerides <500 mg/dl, serum creatinine within normal institutional limits, and for the sirolimus alone study, a CD4 count >500/ml. Patients with severe immunodeficient states or uncontrolled brain metastases were excluded.
Dosing Cohorts
Between October 2004 and November 2008, subjects were enrolled in three separate Institutional Review Board approved protocols at the University of Chicago (ClinicalTrials.gov Identifiers: NCT00707135, NCT00708591, NCT00375245) – sirolimus alone, sirolimus plus ketoconazole, and sirolimus plus grapefruit juice (Supplementary Table 1). Sirolimus was administered once weekly as a 1 mg/ml oral solution (sirolimus alone and sirolimus plus grapefruit juice studies) or as a 1 mg tablet (sirolimus plus ketoconazole study). All studies utilized adaptive escalation designs where sirolimus dose levels were calculated with a goal of reaching an area under the exposure curve (AUC) equivalent to that observed with the approved 25 mg dose of temsirolimus (AUC 3810 ng-hr/ml). At the completion of each cohort, the sirolimus AUC from the previous cohort was calculated as well as the relative change compared to the previous cohort AUC. An estimate of the increase required in sirolimus dose to achieve the target AUC was made assuming a linear relationship between dose and AUC. Each dose escalation was intended to reach between 75–100% of the target dose. Due to gastrointestinal side effects experienced by subjects at the 60 mg sirolimus dose, all subsequent cohorts received sirolimus in divided doses – initially doses were given 4 hours apart and then 24 hours apart in subsequent dose cohorts.
For the sirolimus plus ketoconazole study, sirolimus was administered in week 1 and ketoconazole was added to all subsequent doses at a fixed dose of 200 mg bid from week 2 on day 1, followed by 200 mg daily on the next three subsequent days. Since we were trying to inhibit CYP3A, we used the standard dosage approved for fungal infections and prophylaxis. For the sirolimus plus grapefruit juice study, sirolimus was administered alone in week 1 and with grapefruit juice starting in week 2, one day prior to sirolimus. Grapefruit juice (supplied by Florida Department of Citrus), 240 cc, was administered once daily without interruption. This dosing was based on research demonstrating that the half-life of intestinal enzyme inhibition of grapefruit juice is 12 hours(21) thus providing time for modulation prior to sirolimus dosing.
A single cycle of treatment was defined as 4 consecutive weeks with no scheduled interruptions between treatment cycles. A minimum of 8 consecutive weeks (2 cycles) was administered before re-evaluation of disease status.
Dose Limiting Toxicity (DLT)
DLTs were defined as the occurrence of any of the following that were deemed at least probably drug-related in cycle 1: grade 3 or higher non-hematologic toxicity except fatigue, nausea, vomiting; grade 4 thrombocytopenia or anemia; grade 4 neutropenia or grade 3 neutropenia associated with temperature >38.3˚C; inability to complete cycle 1 due to toxicity; or delay in administration of scheduled doses of sirolimus greater than 2 weeks due to drug related toxicity of any grade. Both the sirolimus plus ketoconazole and grapefruit juice studies employed a classic '3+3' dose escalation design. In the sirolimus alone study, in order to obtain more samples for pharmacodynamic analysis, a '6+6' design was used, where dose escalation occurred if 0 or 1 out of 6 subjects at the current dose level experienced DLT. If 2 subjects experienced DLT, 6 more subjects were entered at that dose level. If a total of 2 or 3 out of 12 subjects experienced DLT, escalation proceeded to the next dose level. If 4 or more out of 12 subjects experienced DLT, then dose escalation was stopped and the previous dose was declared the maximum tolerated dose (MTD). If 3 or more of the original 6 subjects at any dose level experienced DLT, dose escalation was stopped and the next lower dose level was declared the MTD.
Sirolimus Pharmacokinetics
Sirolimus whole blood concentrations were determined as previously described at the University of Texas Medical School at Houston under the supervision of Dr. Napoli Eaton (22). The concentration data were analyzed by a noncompartmental method due to limited number of patients for each dose level. The analysis was performed using PK Solutions Software (version 2.0; Summit Research Services). In the sirolimus alone study, blood was collected on day 1 (baseline, then 2 and 4 hours post dose), day 2 (prior to dosing then 0.4 and 3 hours post dose), day 4 (48 hours post second dose), day 8 (prior to dosing), and day 29 (prior to dosing). In the sirolimus plus ketoconazole and grapefruit juice studies, blood was collected in week 1 prior to administration of sirolimus alone and then at 0.25, 0.5, 1, 2, 4, 8, 24, and 48 hours; in week 2 prior to administration of sirolimus and either ketoconazole or grapefruit juice and then at 0.25, 0.5, 1, 2, 4, 8, 24, and 48 hours; and in week 3 prior to sirolimus administration.
Sirolimus Pharmacodynamics
mTOR phosphorylates p70S6 kinase (p70S6K) at threonine 389 which most closely correlates with activity in vivo(23). p70S6K phosphorylation at Thr389 was measured in peripheral blood lymphocytes (PBL) as a potential biomarker of rapamycin activity. Only subjects in the sirolimus alone study underwent determination of phosphorylated p70S6K. Blood for collection of PBL was drawn prior to sirolimus administration at baseline and analyzed again on week 1, day 2; week 1, day 4; week 2, day 1; and week 5, day 1. PBL were separated by Lymphoprep density centrifugation followed by isolation of CD3+ cells using Human T cell Enrichment kit (StemCell Technologies Inc., Cat # 14051A) The CD3+ cells were then treated with medium or PMA and Ionomycin for 1 hour. The cells were lysed and Western blots for phospho- and total p70S6K were performed. The results were analyzed using UN-SCAN-IT software and plotted using Sigmaplot. An indirect response model (24) with rebound effect (25) was used to describe our pharmacodynamic data and estimate the inhibitory effect of sirolimus. This model was built using NONMEM (version VII, level 1, ICON, Ellicott City, MD, USA) in conjunction with a gfortran compiler. First-order conditional estimation (FOCE) with interaction and Advan 8 were applied.
Statistical Considerations
Within-patient absolute changes in pharmacokinetic parameters of sirolimus prior to and after co-administration of ketoconazole or grapefruit juice were estimated. At each dose level, the signed rank test was used to determine whether these changes were significantly different from zero. The relationship between changes in PK parameters and dose level were assessed using the nonparametric trend test. The relationship between observed (DV) and predicted p70S6 kinase phosphorylation at Thr389 was evaluated using a linear regression model among all samples. Response was assessed using RECIST 1.0.
Results
Patient Demographics
A total of 138 subjects were enrolled in the combined studies (Supplementary Table 1) and their characteristics are summarized in Supplementary Table 2.
Adverse Events
Toxicity and DLT observed on all three studies are summarized in Tables 1 and 2, respectively. The most common toxicities attributed to sirolimus observed throughout all three studies were hyperglycemia, hyperlipidemia, lymphopenia, anemia, and diarrhea. In the sirolimus alone study, the single weekly dose of 60 mg was associated with significant diarrhea and gastrointestinal upset so subsequent cohorts were administered split doses either 4 or 24 hours apart. Gastrointestinal toxicity on the split dose level consisted of diarrhea, nausea, and emesis. These were managed conservatively with antidiarrheals (loperamide or diphenoxylate/atropine as needed) and antiemetics. All subjects were able to continue therapy although one required dose interruption due to diarrhea.
Table 1.
Adverse events observed in each protocol listed alphabetically by dose level. Numbers in parentheses represent grade 3 or 4 toxicity.
| Adverse Event | Sirolimus Alone | Sirolimus plus Ketoconazole | Sirolimus plus Grapefruit Juice | Total (n=138) |
|||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 10mg | 20mg | 30mg | 60mg | 30mg (4h) |
30mg (24h) |
45mg (24h) |
1mg | 2mg | 3mg | 4mg | 5mg | 6mg | 8mg | 16mg | 15mg | 20mg | 25mg | 30mg | 35mg | ||
| Altered taste | 1 | 1 | 1 | 1 | 1 | 5 | |||||||||||||||
| Anemia | 3 | 5 | 3 | 4(1) | 3 | 3 | 2 | 5(1) | 3 | 1 | 4(2) | 4(1) | 1 | 1 | 4 | 46(5) | |||||
| Anorexia | 1 | 1 | 1(1) | 3 | 1 | 5 | 1 | 2 | 1 | 1 | 3 | 2 | 2 | 3 | 1 | 4 | 32(1) | ||||
| Decreased platelets | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 2 | 3 | 1 | 3 | 4 | 22 | |||||||
| Diarrhea | 1 | 5 | 2 | 6(1) | 4 | 2 | 3(1) | 2 | 1 | 1 | 2 | 4 | 1(1) | 2 | 3 | 5 | 4 | 3 | 1 | 6 | 58(3) |
| Mucocutaneous dryness | 1 | 1 | 1 | 1 | 2 | 6 | |||||||||||||||
| Dyspnea | 1 | 1 | 2 | 1 | 1(1) | 6(1) | |||||||||||||||
| Edema | 1 | 3 | 1(1) | 5(1) | |||||||||||||||||
| Elevated Creatinine | 1 | 1 | |||||||||||||||||||
| Elevated Transaminases | 1 | 4 | 2 | 1 | 1 | 1(1) | 1(1) | 1 | 3 | 4 | 2 | 1 | 1 | 23(2) | |||||||
| Epistaxis | 1 | 1 | |||||||||||||||||||
| Fatigue | 2 | 4 | 1 | 5 | 1 | 4 | 1 | 4 | 1 | 2 | 1 | 3(1) | 6(1) | 2 | 2 | 2 | 5(1) | 46(3) | |||
| Fever | 2 | 1 | 1 | 4 | |||||||||||||||||
| Hand & Foot Syndrome | 1 | 1 | 2 | ||||||||||||||||||
| Hyperlipidemia | 2 | 4 | 3(1) | 6(1) | 3 | 1 | 3 | 3 | 4 | 2 | 2 | 6 | 9(1) | 1 | 1 | 2 | 7 | 59(3) | |||
| Hyperglycemia | 6 | 6 | 4 | 1 | 1 | 4(1) | 5 | 4 | 2 | 4 | 3 | 2 | 4(2) | 10(1) | 3(1) | 4(1) | 2 | 7(1) | 72(7) | ||
| Hypocalcemia | 2 | 2 | |||||||||||||||||||
| Hypertension | 1 | 1 | 2 | ||||||||||||||||||
| Hypoglycemia | 1(1) | 1(1) | |||||||||||||||||||
| Hypoxia | 2 | 2 | |||||||||||||||||||
| Infection | 1 | 1(1) | 1(1) | 1 | 1(1) | 1(1) | 1 | 1 | 3 | 1 | 1 | 1 | 14(4) | ||||||||
| Leukopenia | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 1 | 2 | 3 | 17 | ||||||||||
| Lymphopenia | 3(2) | 4(1) | 3(1) | 3 | 4 | 2 | 2(1) | 3(1) | 3(1) | 5(2) | 2 | 3(1) | 7(1) | 2(1) | 4 | 2(1) | 4(1) | 56(14) | |||
| Mood Alteration | 1 | 1 | 2 | ||||||||||||||||||
| Mucositis | 2 | 1 | 1 | 1 | 2 | 1 | 1 | 2 | 1 | 2 | 1 | 2 | 17 | ||||||||
| Myalgia | 2 | 1 | 3 | ||||||||||||||||||
| Nail Changes | 1(1) | 1(1) | |||||||||||||||||||
| Nausea | 3 | 2 | 4(1) | 6 | 2 | 1 | 2 | 2 | 1 | 2 | 1 | 4 | 2 | 2 | 6(1) | 2 | 2 | 4 | 48(2) | ||
| Neutropenia | 1 | 1 | 1 | 1 | 2 | 1 | 7 | ||||||||||||||
| Paresthesia | 1 | 1 | 2 | ||||||||||||||||||
| Pleural Effusion | 1 | 1 | |||||||||||||||||||
| Pneumonia | 2(2) | 1 | 2(2) | 5(4) | |||||||||||||||||
| Pruritis | 1 | 2 | 1 | 1 | 1 | 6 | |||||||||||||||
| Rash | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 1 | 2 | 2 | 14 | |||||||||
| Vomiting | 2 | 2 | 5 | 2 | 4 | 3 | 1 | 1(1) | 2 | 2 | 1 | 3 | 1 | 1 | 3 | 33(1) | |||||
| Weight Loss | 4 | 2 | 1 | 1 | 1 | 1 | 1 | 1 | 1 | 2 | 15 | ||||||||||
Table 2.
Dose limiting toxicities (DLT) observed in all three studies
| Study | Nature of DLT | Dose Level (mg sirolimus) |
|---|---|---|
| Sirolimus alone | Pneumonia | 20 |
| Dehydration | 30 | |
| Unable to complete cycle 1 due to toxicity (anorexia and fatigue < grade 2) |
60 | |
| Sirolimus/ketoconazole | Pneumonia | 5 |
| Hand/foot syndrome* | 6 | |
| Diarrhea* | 6 | |
| Hyperglycemia | 16 | |
| Sirolimus/grapefruit juice |
Hyperglycemia | 25 |
| Mucositis | 25 | |
| Anorexia | 30 |
These occurred concurrently in the same subject
Pharmacokinetics and Pharmacodynamics
Of the total enrolled 138 patients, 101 patients were evaluable for pharmacokinetics (Tables 3 and 4). Sirolimus concentrations were significantly increased by both ketoconazole and grapefruit juice at all dose levels (Supplementary Table 3). The highest dose levels in both studies exceeded our minimal target AUC goal of 3810 ng-hr/ml. When sirolimus was administered alone, gastrointestinal toxicity necessitated splitting the dose into two equal administrations. Although five subjects were accrued to the 45 mg-24 hours apart dose level, only two subjects were evaluable for pharmacokinetic analysis. Data from this cohort suggested that a 90 mg total weekly dose would achieve AUC close to the target, and thus accrual to the sirolimus alone study was stopped.
Table 3.
Pharmacokinetic analysis (non-compartmental analysis) of sirolimus with or without ketoconazole or grapefruit juice
| Sirolimus Dose (mg) [n] |
Keto 0=non 1=Keto |
GFJ 0=non 1=GFJ |
Cmax (ng/ml)* |
Cmax CV%* |
AUC∞ (ng-hr/ml)* |
AUC∞ CV%* |
T1/2 (hr) |
T1/2 CV% |
CL/F (l/hr)* |
CL/F CV%* |
|---|---|---|---|---|---|---|---|---|---|---|
| 1 [6] | 0 | - | 5.4 | 31.4% | 186 | 102% | 82 | 156% | 10.3 | 87.2% |
| 1 | - | 25.4 | 36.8% | 935 | 30% | 50 | 47% | 1.2 | 43.7% | |
| 2 [6] | 0 | - | 9.9 | 59.6% | 271 | 74% | 45 | 41% | 9.5 | 37.8% |
| 1 | - | 33.2 | 47.8% | 1347 | 31% | 52 | 28% | 1.8 | 58.5% | |
| 3 [6] | 0 | - | 17.4 | 54.9% | 400 | 26% | 35 | 36% | 8.0 | 27.3% |
| 1 | - | 49.2 | 62.2% | 2,448 | 73% | 84 | 101% | 1.6 | 43.8% | |
| 4 [6] | 0 | - | 21.3 | 35.7% | 456 | 42% | 36 | 35% | 10.0 | 37.4% |
| 1 | - | 66.8 | 71.9% | 2,897 | 79% | 61 | 50% | 2.1 | 53.5% | |
| 5 [6] | 0 | - | 25.8 | 43.2% | 676 | 46% | 35 | 27% | 9.0 | 49.9% |
| 1 | - | 71.2 | 70.1% | 3,144 | 72% | 57 | 35% | 2.8 | 81.5% | |
| 6 [3] | 0 | - | 15.0 | 55.5% | 510 | 63% | 78 | 76% | 15.6 | 62.0% |
| 1 | - | 74.6 | 92.7% | 2,620 | 38% | 62 | 60% | 2.6 | 45.3% | |
| 8 [1] | 0 | - | 25.2 | - | 420 | - | 18 | - | 19.1 | - |
| 1 | - | 85.2 | - | 1,624 | - | 34 | - | 4.9 | - | |
| 16 [2] | 0 | - | 47.4 | 15.8% | 1,456 | 17% | 25 | 79% | 11.2 | 17.1% |
| 1 | - | 123.0 | 7.6% | 5,007 | 3% | 32 | 6% | 3.2 | 3.2% | |
| 15 [5] | - | 0 | 50.3 | 28.1% | 858 | 37% | 36 | 71% | 20.5 | 52.4% |
| - | 1 | 127.9 | 52.0% | 4,361 | 52% | 46 | 41% | 5.9 | 109.4% | |
| 20 [5] | - | 0 | 45.6 | 73.0% | 863 | 82% | 26 | 44% | 36.9 | 71.6% |
| - | 1 | 105.3 | 65.8% | 2,797 | 62% | 53 | 52% | 11.5 | 79.1% | |
| 25 [4] | - | 0 | 84.7 | 68.0% | 1,449 | 77% | 30 | 47% | 32.6 | 102.4% |
| - | 1 | 173.9 | 55.3% | 4,538 | 80% | 40 | 29% | 9.2 | 79.5% | |
| 30 [5] | - | 0 | 68.5 | 36.1% | 1,814 | 48% | 30 | 37% | 18.7 | 39.9% |
| - | 1 | 82.1 | 37.5% | 2,419 | 17% | 35 | 21% | 12.6 | 14.7% | |
| 35 [8] | - | 0 | 68.1 | 62.3% | 1,799 | 72% | 43 | 58% | 29.4 | 64.0% |
| - | 1 | 129.7 | 65.3% | 6,655 | 83% | 56 | 103% | 12.8 | 135.3% | |
p<0.05 for combined dose levels
Table 4.
Calculated sirolimus pharmacokinetics. Mean values are shown for each parameter.
| Dose Cohort (mg) |
N | CL/F (l/h) [SD] | Tmax (hours) [SD] |
Cmax (ng/ml) [SD] | AUC (ng*hr/ml) [SD] |
|---|---|---|---|---|---|
| 10 | 6 | 16.922 [6.08] | 2.3 [0.01] | 9.67 [2.48] | 615 [130] |
| 20 | 7 | 18.736 [11.0] | 2.3 [0.09] | 19.27 [5.69] | 1084 [581] |
| 30 | 6 | 16.148 [10.4] | 2.3 [0.16] | 43.47 [8.43] | 2713 [1346] |
| 60 | 8 | 19.588 [13.6] | 2.2 [0.16] | 57.72 [22.6] | 3142 [1680] |
| 30, 4 hours apart (total 60 mg) |
5 | 19.786 [15.2] | 2.2 [0.17] | 32.93 [10.7] | 2054 [1123] |
| 30, 24 hours apart (total 60 mg) |
3 | 17.087 [2.90] | 2.3 [0.17] | 83.87 [18.8] | 3677 [1460] |
| 45, 24 hours apart (total 90 mg) |
2 | 26.842 [1.1] | 2.1[0.02] | 42.75 [0.08] | 3356 [138] |
We chose to examine a phosphorylation site on the direct mTOR substrate, Thr389 on p70S6K, as a pharmacodynamic marker of sirolimus activity in the sirolimus alone study. We observed inhibition of phospho-p70S6K 48 hours after administration of sirolimus at all dose levels (Figure 1, p = 0.006). Interestingly, even at the 10 mg weekly dose, phospho-p70S6K activity was suppressed 1 week after the initial dose (data not shown).
Figure 1.
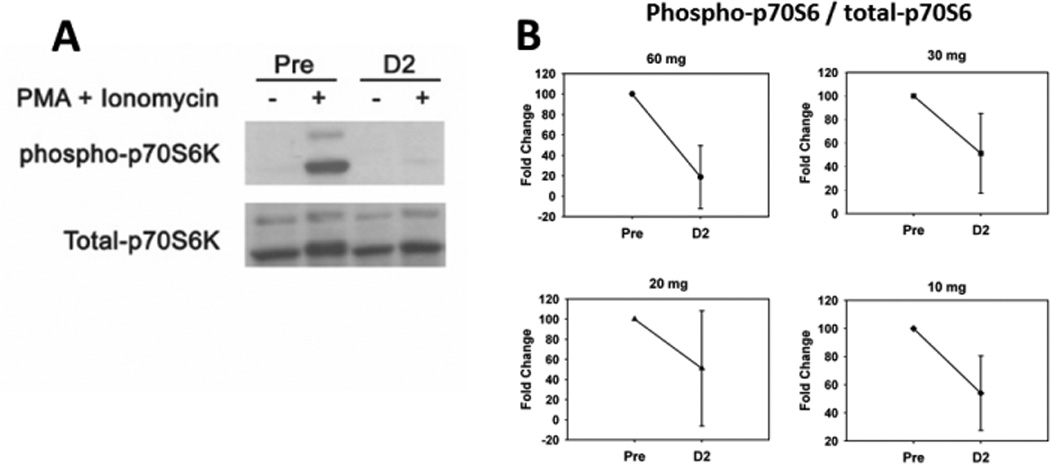
(A) Representative Western blot from a subject sample probed for phosphorylated and total p70S6 kinase. Cryo-preserved human CD3+ cells were treated with or without PMA (0.5 µg/ml) plus Ionomycin (0.025 µg/ml) for 1 hr at 37° C. Cells were lysed and proteins were resolved by SDS-PAGE and transferred onto PVDF membranes. The blots were probed with anti-phosphorylated p70S6 kinase (Thr389) (108D2, Cell Signaling Technology) and developed according to manufacture’s procedure. The blots were then stripped and reprobed for total p70S6 kinase (Cell Signaling Technology). (B) The results were scanned and the densities of phospho- and total p70S6 kinase were analyzed using UNSCANIT software. Results were averaged by dose cohort and plotted using Simgaplot software. Pre = samples prior to administration of sirolimus. D2 = samples collected on day 2 after administration of sirolimus. Error bars represent standard deviation. P value (p = 0.006) was calculated using paired t-test.
In addition, we modeled the relationship between suppression of phospho-p70S6K and sirolimus concentration. In our model, the change of phospho-p70S6K was described by kin (zero-order constant rates for gain) and kout (first-order constant rate for loss) where baseline phosphorylation is 100%. Sirolimus has an inhibitory effect on the kin decreasing the level of phospho-p70S6K. The inhibition exhibits linear correlation with the concentration of sirolimus (Csiro) with a coefficient of keffect. The observed and predicted phosphorylation levels were well correlated (Figure 2A). The production rate of phospho-p70S6K decreases to kin × (1–keffect × Csiro), while the patients are taking sirolimus. Paradoxically, in six out of twelve subjects we observed a rebound effect on phospho-p70S6K where phosphorylated protein levels exceeded baseline at later time points (Figure 2B). We determined that the rebound was driven by the concentration of sirolimus, expressed as ( is the concentration of sirolimus in the rebound compartment). The estimated phospho-p70S6K in addition to the rebound is our prediction for the patients with rebound effect. The typical value for kin, kout, keffect and krebound are 5.5, 0.05, 0.08 ml/ng, and 11.3 ml/ng, respectively.
Figure 2.
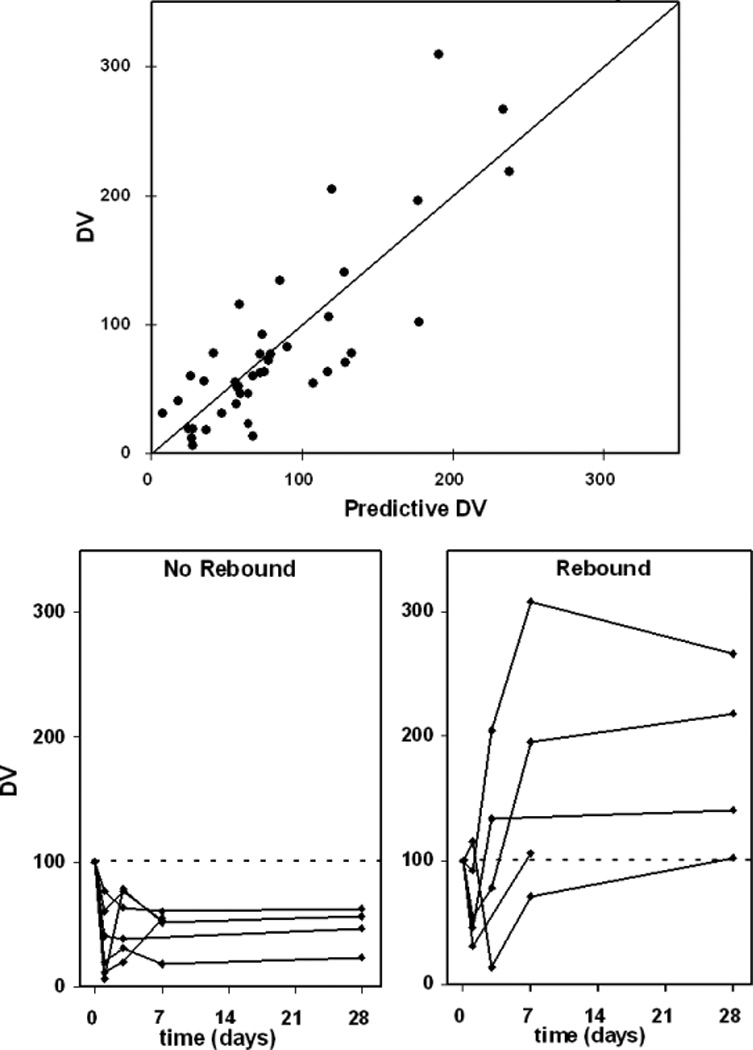
(A) Correlation between observations (DV) and predictions of p70S6 kinase phosphorylation at Thr389 among all samples (R2=0.69, linear regression). An outlier with DV=427 was excluded. (B) Time course of observed phosphorylated p70S6 kinase, by individual subject rebound status.
Efficacy
In the collective studies, one partial response was observed. This subject, diagnosed with epithelioid hemangioendothelioma with hepatic metastases, was treated with sirolimus and grapefruit juice. She had been previously treated with sorafenib and developed progressive disease. She remains on sirolimus (with grapefruit juice) more than 3 years after enrollment. Stable disease was observed in 16 (40%), 16 (28%), and 11 (27%) subjects in the sirolimus alone, sirolimus plus ketoconazole, and sirolimus plus grapefruit juice studies, respectively.
Discussion
These three studies confirm that oral administration of sirolimus is feasible in oncology patients, extending the work of Jimeno and colleagues (20). Our studies demonstrate that weekly oral sirolimus can achieve drug exposure (AUC) similar to that observed with its parenteral prodrug, temsirolimus, and the recommended phase 2 doses from this study are 90 mg, 16 mg, and 35 mg when administered alone, with ketoconazole, and with grapefruit juice, respectively. Notably, the target AUC was attainable at significantly lower sirolimus doses when combined with CYP3A inhibitors, either ketoconazole or grapefruit juice. In fact, when siroliums was combined with grapefruit juice, target AUC was observed at 15, 25, and 35 mg. Toxicity observed with weekly sirolimus was typical of other mTOR inhibitors with glucose, lipid, and hemotologic alterations being most common. In general, weekly sirolimus was well tolerated with relatively few serious adverse events or dose-limiting toxicities as defined in these studies.
These findings carry important implications for development of mTOR inhibitors and oral antineoplastic agents. Sirolimus is commercially available with a safety record much longer than any of its analogues. Moreover, the patents for sirolimus for most oncology uses have expired, and thus the potential exists for substantial cost savings over its analogues. Therefore, sirolimus represents a viable cancer drug whose development would offer several advantages with further cost savings realized by combining the drug with agents that inhibit its metabolism. We hope that our phase I studies provide a basis for initiation of comparative effectiveness studies relative to the more expensive sirolimus analogues.
Many drugs contain warnings to avoid grapefruit juice or other members of the Rutaceae family, including Seville oranges and pummelo, but this is the first cancer study to harness this drug-food interaction. In 1989, grapefruit juice was serendipitously found to inhibit the metabolism of the calcium channel blocker, felodipine (26, 27). Further research discovered that grapefruit juice is a potent inhibitor of intestinal CYP3A4, CYP1A2, and CYP2A6 (27). In fact, small bowel enterocyte CYP3A4 protein levels begin to decrease within hours of grapefruit juice administration and the effect is maximal if grapefruit juice is ingested simultaneously or within the previous four hours of drug administration. Furthermore, the half-life of the effect is approximately 12 hours and enzyme levels are reduced by a mean of 62% even after 5 days of grapefruit juice consumption (21). The reduction in protein level is likely post-transcriptional requiring de novo CYP3A4 synthesis (27). Although the magnitude of the interaction is highly variable, it is reproducible within individuals and seems to be dependent on small bowel CYP3A4 content, i.e. persons with the highest intestinal CYP3A4 content have the largest reduction in enzyme levels and subsequently the greatest effect on drug metabolism (27–30).
Interestingly, different grapefruit juice formulations appear to vary in inhibitory potency and therefore, in consultation with the Florida Department of Citrus, we employed a frozen concentrate product that was tested for furanocoumarin levels prior to delivery of each batch. This ensured consistency across cohorts and is something that must be kept in mind for future studies or applications. Of course, one advantage of grapefruit juice is that it is non-toxic without risk of overdose. Therefore, we have at our disposal an agent that can markedly increase drug bioavailability (in this study by approximately 350%) and, critically in the current environment, decrease prescription drug spending on many agents metabolized by P450 enzymes.
The optimal dose and schedule of sirolimus and its analogues are still being debated with current administration schedules varying from weekly (temsirolimus), every other week (ridaforolimus), or daily (everolimus). Daily dosing of sirolimus, the universal and approved schedule employed in the organ allograft setting, has been explored in cancer patients and appears feasible (20). One could, therefore, reasonably expect success administering sirolimus in the same indications as its analogues. Based on the relatively long half-life of sirolimus, our studies hypothesized that sirolimus could be administered on a weekly schedule which would, in turn, simplify administration and provide a period of immune cell recovery and reduce immunosuppressive effects. Weekly administration appears feasible and acceptable to patients.
In all subjects, a decrease in PBL phospho-p70S6K expression in response to a dose of sirolimus was observed at 48 hours as expected. However, at later time points in some subjects, PBL phospho-p70S6K expression increased above baseline, which was labeled a rebound effect. The mechanisms underlying this rebound are unclear since sirolimus dosing was continued as planned. Feedback loops clearly exist that can stimulate protein activation in response to mTOR inhibition and such a look has been described linking p70S6K inhibition with acivation of AKT through IRS1(31). Whether this was operational in subjects enrolled in this study and why this was not observed in all subjects needs further investigation. At this time, the impact of the rebound in phospho-p70S6K on efficacy and immune function is unclear.
This work and that of others (32–34) would suggest that the optimal biologic dose of mTOR inhibitors may be lower than those currently being administered. This study demonstrated target inhibition, albeit in peripheral blood, at an appreciably lower dose than the highest dose administered. In fact, lower doses of all the available mTOR inhibitors have not been fully explored for comparative effectiveness. Dose finding studies are not necessarily profitable for manufacturers, especially if the drug has been approved, priced, and lower doses prove to be equally active. However, the societal benefits with respect to quality of life and health care costs could be substantial. Zidovudine in the treatment of human immunodeficiency virus provides a poignant example of the benefits associated with examination of lower doses (35, 36).
There are challenges to incorporating grapefruit juice, ketoconazole, or any inhibitor of drug metabolism into regular practice. For a non-drug product like grapefruit juice one has to assure consistent potency. Moreover, interactions with other concomitantly administered medications must be examined cautiously as well as adherence to regimens that would presumably incorporate at least two agents. In a clinical trial setting we were able to monitor and control these factors. However, there is now considerable evidence that sirolimus can be tolerably and feasibly administered to cancer patients(1–5, 20). There are ostensible and indubitable advantages to developing sirolimus over other mTOR inhibitors while this work describes strategies to improve bioavailability by combining sirolimus with CYP3A inhibitors. Funding for this development should be critically contemplated by governmental and public agencies, as well as potentially nonprofit biomedical companies (37).
Supplementary Material
Statement of Translational Relevance.
Sirolimus is the first mTOR inhibitor to be discovered and utilized pre-clinically in neoplastic disease. It is a commercially available agent with approval as prophylaxis against solid organ allograft rejection. These three simultaneously conducted phase I studies in advanced cancer patients utilized an adaptive escalation design to find the dose of oral, weekly sirolimus alone or in combination with either ketoconazole or grapefruit juice that achieves whole blood concentrations associated with activity of other mTOR inhibitors and inhibition of p70 S6 kinase. Weekly sirolimus was feasibly administered with a similar toxicity and pharmacokinetic profile compared to other mTOR inhibitors. Moreover, sirolimus whole blood concentrations are significantly modulated by ketoconazole and grapefruit juice. These studies provide dosing for future phase 2 and 3 studies of sirolimus in mTOR-responsive malignancies with the potential for significantly lower costs to society compared to sirolimus analogues.
Acknowledgements
Dan King (Florida Department of Citrus), Dominick’s Supermarkets, Human Immunologic Monitoring Facility, Pharmacology Core of the University of Chicago Comprehensive Cancer Center
Support: This work was supported by National Institutes of Health (R21CA112951 to E.E.W.C and P30 CA14599 to the University of Chicago Comprehensive Cancer Center); Department of Medicine, University of Chicago Pilot Project Grant; and the William F. O’Connor Foundation.
Footnotes
Parts of this manuscript have been presented in abstract form at the following scientific meetings: American Society of Clinical Oncology annual meeting 2006, 2007, 2008; and American Association of Cancer Research annual meeting 2009.
References
- 1.Stallone G, Schena A, Infante B, Di Paolo S, Loverre A, Maggio G, et al. Sirolimus for Kaposi's sarcoma in renal-transplant recipients. N Engl J Med. 2005;352:1317–1323. doi: 10.1056/NEJMoa042831. [DOI] [PubMed] [Google Scholar]
- 2.Reardon DA, Quinn JA, Vredenburgh JJ, Gururangan S, Friedman AH, Desjardins A, et al. Phase 1 trial of gefitinib plus sirolimus in adults with recurrent malignant glioma. Clin Cancer Res. 2006;12:860–868. doi: 10.1158/1078-0432.CCR-05-2215. [DOI] [PubMed] [Google Scholar]
- 3.Rizell M, Andersson M, Cahlin C, Hafstrom L, Olausson M, Lindner P. Effects of the mTOR inhibitor sirolimus in patients with hepatocellular and cholangiocellular cancer. Int J Clin Oncol. 2008;13:66–70. doi: 10.1007/s10147-007-0733-3. [DOI] [PubMed] [Google Scholar]
- 4.Desar IM, Timmer-Bonte JN, Burger DM, van der Graaf WT, van Herpen CM. A phase I dose-escalation study to evaluate safety and tolerability of sorafenib combined with sirolimus in patients with advanced solid cancer. Br J Cancer. 2010;103:1637–1643. doi: 10.1038/sj.bjc.6605777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gangadhar TC, Cohen EE, Wu K, Janisch L, Geary D, Kocherginsky M, et al. Two drug interaction studies of sirolimus in combination with sorafenib or sunitinib in patients with advanced malignancies. Clin Cancer Res. 2011;17:1956–1963. doi: 10.1158/1078-0432.CCR-10-2061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Maki N, Sekiguchi F, Nishimaki J, Miwa K, Hayano T, Takahashi N, et al. Complementary DNA encoding the human T-cell FK506-binding protein, a peptidylprolyl cis-trans isomerase distinct from cyclophilin. Proc Natl Acad Sci U S A. 1990;87:5440–5443. doi: 10.1073/pnas.87.14.5440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Standaert RF, Galat A, Verdine GL, Schreiber SL. Molecular cloning and overexpression of the human FK506-binding protein FKBP. Nature. 1990;346:671–674. doi: 10.1038/346671a0. [DOI] [PubMed] [Google Scholar]
- 8.Hara K, Maruki Y, Long X, Yoshino K, Oshiro N, Hidayat S, et al. Raptor, a binding partner of target of rapamycin (TOR), mediates TOR action. Cell. 2002;110:177–189. doi: 10.1016/s0092-8674(02)00833-4. [DOI] [PubMed] [Google Scholar]
- 9.Brunn GJ, Hudson CC, Sekulic A, Williams JM, Hosoi H, Houghton PJ, et al. Phosphorylation of the translational repressor PHAS-I by the mammalian target of rapamycin. Science. 1997;277:99–101. doi: 10.1126/science.277.5322.99. [DOI] [PubMed] [Google Scholar]
- 10.Gingras AC, Raught B, Sonenberg N. Regulation of translation initiation by FRAP/mTOR. Genes Dev. 2001;15:807–826. doi: 10.1101/gad.887201. [DOI] [PubMed] [Google Scholar]
- 11.Houchens DP, Ovejera AA, Riblet SM, Slagel DE. Human brain tumor xenografts in nude mice as a chemotherapy model. Eur J Cancer Clin Oncol. 1983;19:799–805. doi: 10.1016/0277-5379(83)90012-3. [DOI] [PubMed] [Google Scholar]
- 12.Eng CP, Sehgal SN, Vezina C. Activity of rapamycin (AY-22,989) against transplanted tumors. J Antibiot (Tokyo) 1984;37:1231–1237. doi: 10.7164/antibiotics.37.1231. [DOI] [PubMed] [Google Scholar]
- 13.Houghton PJ, Huang S. mTOR as a target for cancer therapy. Curr Top Microbiol Immunol. 2004;279:339–359. doi: 10.1007/978-3-642-18930-2_20. [DOI] [PubMed] [Google Scholar]
- 14.Crowe A, Bruelisauer A, Duerr L, Guntz P, Lemaire M. Absorption and intestinal metabolism of SDZ-RAD and rapamycin in rats. Drug Metab Dispos. 1999;27:627–632. [PubMed] [Google Scholar]
- 15.Ryan CJ, Smith MR, Fong L, Rosenberg JE, Kantoff P, Raynaud F, et al. Phase I clinical trial of the CYP17 inhibitor abiraterone acetate demonstrating clinical activity in patients with castration-resistant prostate cancer who received prior ketoconazole therapy. J Clin Oncol. 28:1481–1488. doi: 10.1200/JCO.2009.24.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kang SP, Ratain MJ. Inconsistent labeling of food effect for oral agents across therapeutic areas: differences between oncology and non-oncology products. Clin Cancer Res. 16:4446–4451. doi: 10.1158/1078-0432.CCR-10-0663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.MacDonald A, Scarola J, Burke JT, Zimmerman JJ. Clinical pharmacokinetics and therapeutic drug monitoring of sirolimus. Clin Ther. 2000;22(Suppl B):B101–B121. doi: 10.1016/s0149-2918(00)89027-x. [DOI] [PubMed] [Google Scholar]
- 18.Mahalati K, Kahan BD. Clinical pharmacokinetics of sirolimus. Clin Pharmacokinet. 2001;40:573–585. doi: 10.2165/00003088-200140080-00002. [DOI] [PubMed] [Google Scholar]
- 19.Napoli KL, Taylor PJ. From beach to bedside: history of the development of sirolimus. Ther Drug Monit. 2001;23:559–586. doi: 10.1097/00007691-200110000-00012. [DOI] [PubMed] [Google Scholar]
- 20.Jimeno A, Rudek MA, Kulesza P, Ma WW, Wheelhouse J, Howard A, et al. Pharmacodynamic-guided modified continuous reassessment method-based, dose-finding study of rapamycin in adult patients with solid tumors. J Clin Oncol. 2008;26:4172–4179. doi: 10.1200/JCO.2008.16.2347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lundahl J, Regardh CG, Edgar B, Johnsson G. Relationship between time of intake of grapefruit juice and its effect on pharmacokinetics and pharmacodynamics of felodipine in healthy subjects. Eur J Clin Pharmacol. 1995;49:61–67. doi: 10.1007/BF00192360. [DOI] [PubMed] [Google Scholar]
- 22.Napoli KL. practical guide to the analysis of sirolimus using high-performance liquid chromatography with ultraviolet detection. Clin Ther. 2000;22(Suppl B):B14–B24. doi: 10.1016/s0149-2918(00)89019-0. [DOI] [PubMed] [Google Scholar]
- 23.Harris TE, Lawrence JC., Jr TOR signaling. Sci STKE. 2003;2003:re15. doi: 10.1126/stke.2122003re15. [DOI] [PubMed] [Google Scholar]
- 24.Abou Hammoud H, Simon N, Urien S, Riou B, Lechat P, Aubrun F. Intravenous morphine titration in immediate postoperative pain management: population kinetic-pharmacodynamic and logistic regression analysis. Pain. 2009;144:139–146. doi: 10.1016/j.pain.2009.03.029. [DOI] [PubMed] [Google Scholar]
- 25.Gardmark M, Brynne L, Hammarlund-Udenaes M, Karlsson MO. Interchangeability and predictive performance of empirical tolerance models. Clin Pharmacokinet. 1999;36:145–167. doi: 10.2165/00003088-199936020-00005. [DOI] [PubMed] [Google Scholar]
- 26.Bailey DG, Malcolm J, Arnold O, Spence JD. Grapefruit juice-drug interactions. Br J Clin Pharmacol. 1998;46:101–110. doi: 10.1046/j.1365-2125.1998.00764.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lown KS, Bailey DG, Fontana RJ, Janardan SK, Adair CH, Fortlage LA, et al. Grapefruit juice increases felodipine oral availability in humans by decreasing intestinal CYP3A protein expression. J Clin Invest. 1997;99:2545–2553. doi: 10.1172/JCI119439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bailey DG, Arnold JM, Bend JR, Tran LT, Spence JD. Grapefruit juice-felodipine interaction: reproducibility and characterization with the extended release drug formulation. Br J Clin Pharmacol. 1995;40:135–140. [PMC free article] [PubMed] [Google Scholar]
- 29.Bailey DG, Arnold JM, Spence JD. Grapefruit juice and drugs. How significant is the interaction? Clin Pharmacokinet. 1994;26:91–98. doi: 10.2165/00003088-199426020-00002. [DOI] [PubMed] [Google Scholar]
- 30.Bailey DG, Bend JR, Arnold JM, Tran LT, Spence JD. Erythromycin-felodipine interaction: magnitude, mechanism, and comparison with grapefruit juice. Clin Pharmacol Ther. 1996;60:25–33. doi: 10.1016/S0009-9236(96)90163-0. [DOI] [PubMed] [Google Scholar]
- 31.Zheng Y, Zhang W, Pendleton E, Leng S, Wu J, Chen R, et al. Improved insulin sensitivity by calorie restriction is associated with reduction of ERK and p70S6K activities in the liver of obese Zucker rats. J Endocrinol. 2009;203:337–347. doi: 10.1677/JOE-09-0181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.O'Donnell A, Faivre S, Burris HA, 3rd, Rea D, Papadimitrakopoulou V, Shand N, et al. Phase I pharmacokinetic and pharmacodynamic study of the oral mammalian target of rapamycin inhibitor everolimus in patients with advanced solid tumors. J Clin Oncol. 2008;26:1588–1595. doi: 10.1200/JCO.2007.14.0988. [DOI] [PubMed] [Google Scholar]
- 33.Tanaka C, O'Reilly T, Kovarik JM, Shand N, Hazell K, Judson I, et al. Identifying optimal biologic doses of everolimus (RAD001) in patients with cancer based on the modeling of preclinical and clinical pharmacokinetic and pharmacodynamic data. J Clin Oncol. 2008;26:1596–1602. doi: 10.1200/JCO.2007.14.1127. [DOI] [PubMed] [Google Scholar]
- 34.Tabernero J, Rojo F, Calvo E, Burris H, Judson I, Hazell K, et al. Dose- and schedule-dependent inhibition of the mammalian target of rapamycin pathway with everolimus: a phase I tumor pharmacodynamic study in patients with advanced solid tumors. J Clin Oncol. 2008;26:1603–1610. doi: 10.1200/JCO.2007.14.5482. [DOI] [PubMed] [Google Scholar]
- 35.Collier AC, Bozzette S, Coombs RW, Causey DM, Schoenfeld DA, Spector SA, et al. A pilot study of low-dose zidovudine in human immunodeficiency virus infection. N Engl J Med. 1990;323:1015–1021. doi: 10.1056/NEJM199010113231502. [DOI] [PubMed] [Google Scholar]
- 36.Fischl MA, Parker CB, Pettinelli C, Wulfsohn M, Hirsch MS, Collier AC, et al. A randomized controlled trial of a reduced daily dose of zidovudine in patients with the acquired immunodeficiency syndrome. The AIDS Clinical Trials Group. N Engl J Med. 1990;323:1009–1014. doi: 10.1056/NEJM199010113231501. [DOI] [PubMed] [Google Scholar]
- 37.Conti RM, Meltzer DO, Ratain MJ. Nonprofit biomedical companies. Clin Pharmacol Ther. 2008;84:194–197. doi: 10.1038/clpt.2008.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


