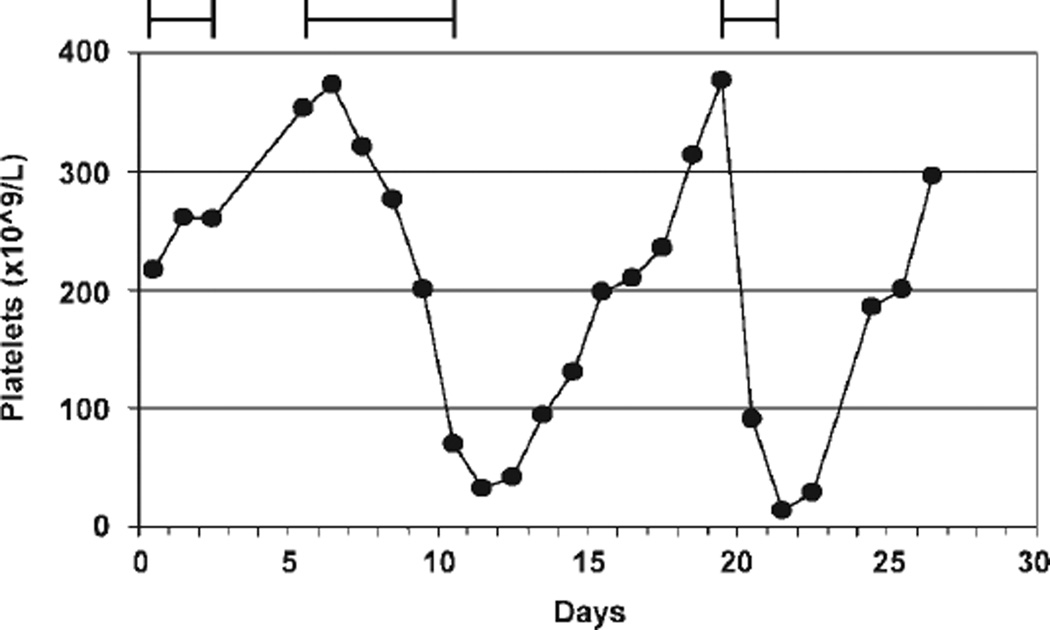
Our patient, a 30-year-old man with end-stage renal disease attributed to congenital dysplastic kidneys who had been on hemodialysis for 3.5 years, was admitted to the hospital with severe hypertension, hyperkalemia, and an infected arm arterio-venous graft after missing his regular dialysis treatment. Previously, he had been hospitalized on multiple occasions for hypertension, peptic ulcer disease, seizures, peripheral neuropathy, venous thrombosis, and failure to maintain his dialysis schedule. His blood pressure was 211/139 and his arterio-venous graft was erythematous and indurated. Serum potassium was 7.4 meq/L, hemoglobin was 9.9 gm/dL, platelet count was 217,000/µL, and white blood cell count was 9,400/µL with a normal differential. He improved with dialysis and was initially treated with piperacillin/tazobactam, gentamicin, and vancomycin for the graft infection. Blood cultures were negative. He left the hospital against medical advice on the 3rd day but returned on the 6th day, when antibiotic treatment was resumed with piperacillin/tazobactam, vancomycin, and ciprofloxacin. On Day 11, his platelet count decreased to 70,000/µL from 201,000/µL the previous day (Fig. 1). His vital signs were normal and his clinical status was stable. Hemoglobin was 8.4 gm/dL and white blood cell count was 8,000/µL, similar to previous measurements. The peripheral blood smear confirmed the decreased platelet count; red cell and white cell morphology were normal; serum lactate dehydrogenase (LDH) was 112 U/L; coagulation studies were normal.
Figure 1.
Platelet counts and piperacillin administration in the patient presented in this report. The bars at the top represent administration of piperacillin on Days 1–3, 6–10, and 19–21.
Acute, unexpected thrombocytopenia in a patient hospitalized for multiple medical problems has multiple potential etiologies. In a patient being treated for infection and at risk for additional infectious complications, sepsis must be the initial consideration because of the risk for sudden, critical deterioration. In a patient on multiple medications, drug-induced thrombocytopenia (DITP) is the other principal consideration. The patient had isolated thrombocytopenia, without evidence for microangiopathic hemolysis, consistent with both of these etiologies. The absence of evidence for microangiopathic hemolysis (no schistocytes [fragmented red blood cells] were seen on examination of the peripheral blood smear, serum LDH was normal) excluded consideration of thrombotic thrombocytopenic purpura.
His medications on Day 11 were piperacillin/tazobactam, phenytoin, gabapentin, pantoprazole, sertraline, aliskerin, amlodipine, isosorbide mononitrate, labetalol, clonidine, hydralazine, lisinopril, kayexalate, vitamin B12 complex, calcium acetate, erythropoietin, morphine, hydromorphone, quetiapine, diphenhydramine, ondansetron, promethazine, bacitracin ointment, and heparin (given as prophylaxis, 5,000 U every 8 h and also for dialysis). Heparin-dependent platelet-reactive antibody ELISA assay was negative. Also on Day 11, blood cultures were reported as positive for vancomycin-resistant Enterococcus faecium; piperacillin/tazobactam was stopped and quinupristin/dalfopristin was started; 3 days later, quinupristin/dalfopristin was changed to daptomycin. On Day 13, the platelet count began to recover after reaching a nadir of 33,000/µL, becoming normal on Day 16 (Fig. 1). All medications other than piperacillin/tazobactam had been continued.
The list of medications was extensive, but this is similar to many patients hospitalized for multiple medical problems. Although heparin may be the most common cause of DITP, and although half of patients with heparin-induced thrombocytopenia may not have thrombotic complications recognized at the time of diagnosis, heparin does not typically cause sudden and severe thrombocytopenia [1]. The negative test for heparin-dependent antibodies was evidence against heparin as the cause of the thrombocytopenia. The recovery of his platelet count while continuing heparin confirmed that heparin was not the etiology of the thrombocytopenia. Among the other medications, there have been reports of thrombocytopenia, with varying levels of evidence, caused by piperacillin, phenytoin, pantoprazole, amlodipine, hydralazine, morphine, quetiapine, and ondansetron (www.ouhsc.edu/platelets). However, all medications except piperacillin/taxobactam were continued while the platelet count recovered to normal, excluding them from consideration of the etiology of the thrombocytopenia. At this time, Enterococcus faecium bacteremia was considered to be the etiology of the thrombocytopenia.
On Day 20, blood cultures were reported as positive for Klebsiella oxytoca and piperacillin/tazobactam was restarted. The platelet count decreased from 377,000/µL on Day 20 to 91,000/µL on Day 21 and to 18,000/µL on Day 22 at which time the patient developed hematemesis, hematochezia, and hemoptysis for which he received two units of red cells and one unit of single donor platelets. On Day 22, piperacillin/tazobactam was stopped when it was recognized that both episodes of thrombocytopenia had occurred while piperacillin/tazobactam was being administered and that all other medications had been continued during the intervening platelet count recovery. The patient’s platelet count recovered to normal 3 days after piperacillin/tazobactam was stopped (Fig. 1).
Although recurrent bacteremia remained a potential etiology of thrombocytopenia, the onset of thrombocytopenia within 1 day of resuming piperacillin/tazobactam and the recovery of the platelet count to normal within 3 days after piperacillin/tazobactam was stopped provided strong evidence that it was the cause of the thrombocytopenia. Using previously established clinical criteria [2], there appeared to be definite evidence for piperacillin/tazobactam as the etiology of the thrombocytopenia (Table I). However, some doubt persisted because of the recurrent bacteremia with multiple organisms; bacterial sepsis remained a possible though unlikely etiology of the thrombocytopenia.
TABLE I.
Clinical Criteria and Levels of Evidence for Evaluation of Patients with Suspected Drug-Induced Thrombocytopenia
Clinical criteria
|
Levels of evidence
|
Adapted from www.ouhsc.edu/platelets and Ref. [2].
Hospital records documented that our patient had received five courses of piperacillin/tazobactam of 3–10 days each between July 2006 and September 2008 with no occurrence of thrombocytopenia documented by daily platelet counts.
DITP typically occurs within 1–2 weeks after beginning daily treatment with a new drug, or after intermittent use for a longer time. The absence of thrombocytopenia during five previous treatment courses with piperacillin/tazobactam added uncertainty to the etiology of the thrombocytopenia. Also, if piperacillin/tazobactam was the etiology, it was important to determine which antibiotic of this combination was responsible. Review of the database for published reports of drug-induced thrombocytopenia (www.ouhsc.edu/platelets) documented that there have been two previous reports of thrombocytopenia caused by piperacillin. One report described thrombocytopenia related to piperacillin/tazobactam; this report documented drug-dependent, platelet-reactive antibodies with piperacillin but not with tazobactam [3]. An additional report described piperacillin-induced thrombocytopenia, providing definite evidence defined by recurrent thrombocytopenia with repeated piperacillin administration [4].
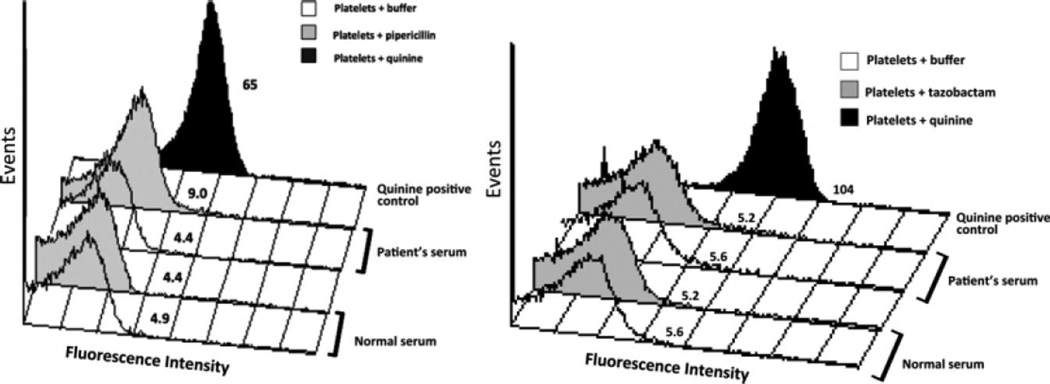
Piperacillin as the etiology of the thrombocytopenia in our patient was confirmed by documentation of piperacillin-dependent platelet-reactive antibodies and by the absence of tazobactam-dependent platelet-reactive antibodies (Fig. 2) [5]. Our patient’s platelet count has been normal for the following 6 months.
Figure 2.
Demonstration of a piperacillin-dependent, platelet-reactive antibody in the patient presented in this report (left) and absence of a detectable tazobactam-dependent, platelet-reactive antibody (right). Results shown are representative of three independent studies that gave essentially identical results. Platelet median fluorescence intensity (MFI values shown) obtained with our patient’s serum plus piperacillin (9.0) differed significantly from those obtained with our patient’ serum plus buffer (4.4) and with normal serum plus piperacillin (4.4) (P < 0.01, Student’s t-test). The much stronger drug-dependent reaction of serum from a patient with quinine-induced thrombocytopenia plus quinine (65) is also shown.
Commentary
Acute thrombocytopenia is an uncommon adverse effect of many common medications, typically caused by drug-dependent platelet-reactive antibodies [6,7]. The risk of thrombocytopenia with any single drug is rare, but bleeding complications in affected patients can be severe, even fatal [2,7]. A case–control study estimated the risk of acute thrombocytopenia with trimethoprim/sulfamethoxazole and quinine, the two drugs that are the most commonly reported causes of thrombocytopenia, to be 38 and 26 cases/106 users/week of exposure, respectively [8]. Quinine-induced thrombocytopenia may be even more frequent than this estimate because it is commonly overlooked when it is taken in a beverage [9] or as an occasional remedy for leg cramps [10,11]. The diagnosis of DITP can be particularly difficult in elderly patients who take many drugs [12] and in hospitalized patients who have complex illnesses [13], such as the patient described in this report. Patients with drug-induced thrombocytopenia are often initially diagnosed as having immune thrombocytopenic purpura, which may result in inappropriate management [14].
Piperacillin/tazobactam (Zosyn®) is a broad-spectrum antibiotic often used for managing severe infections in hospitalized patients [15,16]. Although piperacillin-induced thrombocytopenia has only been previously reported twice [3,4], the Platelet and Neutrophil Immunology Laboratory of the BloodCenter of Wisconsin (BCW) has identified pipericillin-dependent, platelet-reactive antibodies [5] in 13 patients (in addition to our patient) referred for testing over 10 years, 2000–2009. In seven cases, the antibodies had relatively weak, but reproducible, drug-dependent reactions with normal platelets similar to our patient (Fig. 1). In the other six patients, the antibodies were much stronger. However, there was no correlation between antibody strength measured by flow cytometry and the severity of thrombocytopenia (data not shown). Clinical data were obtained in 11 of the 13 patients. Their clinical presentations were similar to our patient and typical of immune-mediated DITP, in which patients present with thrombocytopenia after exposure to the sensitizing drug for about 1 week (Table II) [2,6,7]. In the 12 patients summarized in Table I, the median exposure time before onset of thrombocytopenia was 6.5 days, however, there was considerable variability. Patients 1 and 11 developed thrombocytopenia after only 1 day of piperacillin; their physicians were confident that they had not been previously exposed to piperacillin. In contrast, our patient had received piperacillin five times during the previous 28 months without developing thrombocytopenia. Table I also demonstrates that piperacillin-induced thrombocytopenia is typically severe (median nadir platelet count, 15,500/µL) but that recovery to a normal platelet count occurred promptly (median, 5 days).
TABLE II.
Clinical Course of Piperacillin-Induced Thrombocytopenia in 12 Patients
| Patient | Baseline platelet count (×10−3/µL) |
Days receiving piperacillin before thrombocytopenia |
Nadir platelet count (×10−3/µL) |
Days to normal platelet count after piperacillin discontinued |
|---|---|---|---|---|
| 1 | 250 | 1 | 2 | 4 |
| 2 | 212 | 6 | 14 | 5 |
| 3 | 237 | 7 | 18 | 5 |
| 4 | 310 | 6 | 10 | 14 |
| 5 | 250 | 7 | 5 | >6a |
| 6 | 332 | 9 | 25 | 3 |
| 7 | Normal | 7 | 3 | 5 |
| 8 | 820 | 10 | 20 | 4 |
| 9 | 238 | 6 | 17 | 5 |
| 10 | 255 | 5 | 10 | 7 |
| 11 | 215 | 1 | 40 | >7a |
| 12 (1st) | 353 | 5 | 33 | 5 |
| 12 (2nd) | 377 | 1 | 14 | 3 |
| Median valuesb | 250 | 6 | 15.5 | 5 |
Clinical data are available on 11 of 13 patients in whom piperacillin-dependent platelet-reactive antibodies were documented at the BloodCenter of Wisconsin. Patient 12 is the patient presented in this report.
Daily platelet counts were not recorded until platelet ≥150,000/µL.
Median values for the 11 patients from the BloodCenter of Wisconsin and the first episode of thrombocytopenia for our patient.
The occurrence of thrombocytopenia after only 1 day of piperacillin treatment in Patients 1 and 11 suggests that the patients may have had pre-existing piperacillin-specific, platelet-reactive antibodies. “Naturally occurring” antibodies are known to be capable of causing acute thrombocytopenia in patients treated for the first time with the RGD-mimetic platelet function inhibitors, tirofiban, and eptifibatide [6]. In addition, three patients who experienced acute thrombocytopenia within 1 day of their first known exposure to vancomycin were found to have vancomycin-dependent, platelet-reactive antibodies, possibly naturally occurring [13]. It seems possible that Patients 1 and 11 had naturally occurring, piperacillin-dependent antibodies, perhaps related environmental exposure to β-lactam antibiotics or closely related chemicals. However, such antibodies are not common in the general population. In screening 499 normal blood donors, we did not find a single individual with a piperacillin-dependent platelet-reactive antibody (Bougie DW, Curtis BR, Aster RH, unpublished observations).
Pipericillin is a six-substituted aminopenicillanic acid that has the broadest antibacterial spectrum of the penicillins [16] and has been implicated as a cause of immune hemolytic anemia [17] as well as thrombocytopenia. Like other penicillins, at pH 9.8, piperacillin becomes covalently linked to free amino groups on membrane glycoproteins [18]. Leger et al. found that about 90% of normal persons possess naturally occurring IgM antibodies that agglutinate piperacillin-coated red cells [18]. Preincubation of these antibodies with soluble piperacillin abolished their ability to react with piperacillin-coated cells, consistent with the possibility that they are typical hapten-specific immunoglobulins [19]. In contrast, antibodies from patients with piperacillin-induced hemolytic anemia were of the IgG isotype and, although they reacted with piperacillin-coated red cells, these reactions were not inhibited by preincubation with soluble drug. This indicated that the specificity of piperacillin-dependent antibodies capable of causing hemolytic anemia is distinctly different from that of the naturally occurring antibodies that recognize piperacillin-coated cells. The 14 antibodies associated with pipericillin-induced thrombocytopenia in this study behaved like those implicated in hemolytic anemia [17] in that they recognized platelets in the presence of soluble piperacillin and did not require the target cells to be precoated with the drug for binding to occur. How soluble drug at pharmacologic concentrations promotes binding of a drug-dependent antibody to a membrane protein and causes cell destruction is an unresolved problem that has been investigated for more than 50 years [20]. Bougie et al. have proposed a model that calls for structural elements of the drug to react with both antibody and the target protein, increasing the affinity of the two macromolecules for each other and causing drug to be trapped at the antigen–antibody interface [5]. This model provides a potential explanation for why excess soluble drug does not inhibit antibody binding. The behavior of piperacillin-dependent, platelet-reactive antibodies identified in our cases is consistent with this model.
When DITP is suspected, the following six steps are appropriate. (1) The essential first step is to take a complete history of all drugs that the patient is taking, including not only prescribed drugs that are used regularly but also occasionally used over-the-counter medicines, herbal remedies [21,22], and quinine-containing beverages [9]. (2) The second step in the evaluation is to determine which among the multiple medications that the patient is taking may have triggered DITP. This decision may be helped by a database that regularly evaluates all case reports of DITP (www.ouhsc.edu/platelets). (3) The next step is to determine if the patient fulfills the clinical criteria for a causal relation of the drug to thrombocytopenia [2]. In our case, we provide a definite level of clinical evidence: therapy with piperacillin/tazobactam preceded thrombocytopenia, recovery was complete and sustained after the drug was discontinued, other drugs were continued or reintroduced with a sustained normal platelet count, other causes for thrombocytopenia were excluded, and re-exposure to the drug resulted in recurrent thrombocytopenia [2]. (4) Next, it is important to confirm the etiology of the DITP by testing for the presence of a drug-dependent platelet-reactive antibody. (5) When the drug-induced etiology of the thrombocytopenia is established, the case should be reported to the FDA Adverse Event Reporting System, using the MedWatch website (https://www.accessdata.fda.gov/scripts/medwatch/). (6) Finally, if there are few reports describing definite clinical evidence of a causal relation of the drug to thrombocytopenia, it is important to publish a case report.
When these steps are completed, the information about the adverse event of DITP will be appropriately documented and disseminated. This will strengthen the ability of physicians to assess future patients with unexpected thrombocytopenia.
Footnotes
Conflict of interest: Nothing to report.
References
- 1.Warkentin TE. Think if HIT. In: Berliner N, Linker C, Schiffer CA, editors. Hematology. Washington, D.C.: American Society of Hematology; 2006. pp. 408–414. 2006. [Google Scholar]
- 2.George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, Vondracek T. Drug-induced thrombocytopenia: A systematic review of published case reports. Ann Int Med. 1998;129:886–990. doi: 10.7326/0003-4819-129-11_part_1-199812010-00009. [DOI] [PubMed] [Google Scholar]
- 3.Pérez-Vázquez A, Pastor JM, Riancho JA. Immune thrombocytopenia caused by piperacillin/tazobactam. Clin Infect Dis. 1998;27:650–651. doi: 10.1086/517143. [DOI] [PubMed] [Google Scholar]
- 4.Olivera E, Lakhani P, Watanakunakorn C. Isolated severe thrombocytopenia and bleeding caused by piperacillin. Scand J Infect Dis. 1992;24:815–817. doi: 10.3109/00365549209062471. [DOI] [PubMed] [Google Scholar]
- 5.Bougie DW, Wilker PR, Aster RH. Patients with quinine-induced immune thrombocytopenia have both “drug-dependent” and “drug-specific“ antibodies. Blood. 2006;108:922–927. doi: 10.1182/blood-2006-01-009803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Aster RH, Bougie DW. Drug-induced immune thrombocytopenia. N Engl J Med. 2007;357:580–587. doi: 10.1056/NEJMra066469. [DOI] [PubMed] [Google Scholar]
- 7.Aster RH, Curtis BR, Mcfarland JG, Bougie DW. Durg-induced immune thrombocytopenia: Pathogenesis, diagnosis and management. J Thromb Haemost. 2009;7:911–918. doi: 10.1111/j.1538-7836.2009.03360.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kaufman DW, Kelly JP, Johannes CB, Sandler A, Harmon D, Stolley PD, Shapiro S. Acute thrombocytopenic purpura in relation to the use of drugs. Blood. 1993;82:2714–2718. [PubMed] [Google Scholar]
- 9.Belkin GA. Cocktail purpura: An unusual case of quinine sensitivity. Ann Intern Med. 1967;66:583. doi: 10.7326/0003-4819-66-3-583. [DOI] [PubMed] [Google Scholar]
- 10.Kojouri K, Perdue JJ, Medina PJ, George JN. Occult quinine-induced thrombocytopenia. J Okla State Med Assoc. 2000;93:519–521. [PubMed] [Google Scholar]
- 11.Reddy JC, Shuman MA, Aster RH. Quinine/quinidine-induced thrombocytopenia: A great imitator. Arch Intern Med. 2004;164:218–220. doi: 10.1001/archinte.164.2.218. [DOI] [PubMed] [Google Scholar]
- 12.Kaufman DW, Kelly JP, Rosenberg L, Anderson TE, Mitchell AA. Recent patterns of medication use in the ambulatory adult population of the United States. The Slone survey. JAMA. 2002;287:337–344. doi: 10.1001/jama.287.3.337. [DOI] [PubMed] [Google Scholar]
- 13.Von Drygalski A, Curtis BR, Bougie DW, Mcfarland JG, Ahl S, Limbu I, Baker KR, Aster RH. Vancomycin-induced immune thrombocytopenia. N Engl J Med. 2007;356:904–910. doi: 10.1056/NEJMoa065066. [DOI] [PubMed] [Google Scholar]
- 14.Neylon AJ, Saunders PWG, Howard MR, Proctor SJ, Taylor PRA. Clinically significant newly presenting autoimmune thrombocytopenic purpura in adults: A prospective study of a population-based cohort of 245 patients. Br J Haematol. 2003;122:966–974. doi: 10.1046/j.1365-2141.2003.04547.x. [DOI] [PubMed] [Google Scholar]
- 15.Young M, Plosker GL. Piperacillin/Tazobactam. A pharmacoeconomic review of its use in moderate to severe bacterial infections. Pharmacoeconomics. 2001;19:1135–1175. doi: 10.2165/00019053-200119110-00006. [DOI] [PubMed] [Google Scholar]
- 16.Petri WA. Penicillins, cephalosporines and other lactam antibiotics. In: Brunton LL, Lazo JS, Parker KR, editors. The Pharmacologic Basis of Therapeutics. 11th ed. New York: McGraw-Hill; 2006. pp. 1127–1154. [Google Scholar]
- 17.Arndt PA, Garratty G, Hill J, Kasper M, Chandrasekaran V. Two cases of immune haemolytic anaemia, associated with anti-piperacillin, detected by the ‘immune complex’ method. Vox Sang. 2002;83:273–278. doi: 10.1046/j.1423-0410.2002.00188.x. [DOI] [PubMed] [Google Scholar]
- 18.Leger RM, Arndt PA, Garratty G. Serologic studies of piperacillin antibodies. Transfusion. 2008;48:2429–2434. doi: 10.1111/j.1537-2995.2008.01852.x. [DOI] [PubMed] [Google Scholar]
- 19.Park BK, Kitteringham NR, Powell H, Pirmohamed M. Advances in molecular toxicology—Towards understanding idiosyncratic drug toxicity. Toxicology. 2000;153:39–60. doi: 10.1016/s0300-483x(00)00303-6. [DOI] [PubMed] [Google Scholar]
- 20.Shulman NR. A mechanism of cell destruction in individuals sensitized to foreign antigens and its implications in autoimmunity. Combined clinical staff conference at the National Institutes of Health. Ann Intern Med. 1964;60:506–521. doi: 10.7326/0003-4819-60-3-506. [DOI] [PubMed] [Google Scholar]
- 21.Azuno Y, Yaga K, Sasayama T, Kimoto K. Thrombocytopenia induced byJui, a traditional Chinese herbal medicine. Lancet. 1999;354:304–305. doi: 10.1016/S0140-6736(99)01336-7. [DOI] [PubMed] [Google Scholar]
- 22.Ohmori T, Nishii K, Hagihara A, Takeda M, Sekido K. Acute thrombocytopenia induced byJui, a traditional herbal medicine. J Thromb Haemost. 2004;2:1479–1480. doi: 10.1111/j.1538-7836.2004.00786.x. [DOI] [PubMed] [Google Scholar]