Abstract
N -methyl-D-aspartate receptors (NMDARs) are essential for several kinds of synaptic plasticity and play a critical role in learning and memory. Deficits in NMDAR functioning may be partially responsible for the learning and memory deficits associated with aging and numerous diseases. Administration of MK-801, a noncompetitive NMDAR antagonist, is commonly used as a preclinical model of NMDAR dysfunction. The objective of this study was to assess the effects of α5GABAA receptor inhibition on learning deficits in the incremental repeated acquisition (IRA) task induced by acute MK-801 administration. The IRA task, commonly used to examine factors that affect learning, begins with a single response and increments to progressively longer chains throughout a single session as behavior meets preset criteria. MK-801 (0.03–0.5 mg/kg, intraperitoneally), administered 10 min pretesting, produced a significant dose-dependent decrease in measures of IRA performance at doses greater than or equal to 0.25 mg/kg. The MK-801-induced deficit was attenuated after treatment with an α5GABAA receptor inverse agonist, L-655,708 (1 mg/kg, intraperitoneally). The present study provides the focus for, and supports the feasibility of, further in-depth definitive studies examining α5GABAA receptor inhibition as a suitable candidate for the attenuation of NMDAR-related deficits.
Keywords: γ-aminobutyric acid, α5GABAA, incremental repeated acquisition, L-655, 708, learning, mice, MK-801, N-methyl-D-aspartate
Introduction
N-methyl-D-aspartate receptors (NMDARs) play a key role in learning and memory (Bliss and Collingridge, 1993), and reductions in NMDARs are believed to contribute to learning and memory deficits (Adams et al., 2001). Application of NMDAR agonists to pharmacologically enhance the activity of NMDARs has proven difficult, however, due to risks of excitotoxicity (Fan and Raymond, 2007). An alternative method for increasing the excitatory input of glutamatergic neurons incorporates the use of pharmacological agents that decrease γ-aminobutyric acid (GABA) activity, the major inhibitory neurotransmitter of the central nervous system. Here, we attempted to create NMDAR-associated learning deficits using MK-801, a high affinity noncompetitive NMDAR antagonist (Butelman, 1989; Shapiro and Caramanos, 1990), to determine whether preferentially inhibiting α5 subunit-containing GABA A (α5GABAA) receptors would attenuate NMDA-mediated learning deficits. To test this hypothesis, we used an incremental repeated acquisition (IRA) task, which is NMDA-dependent (Cohn and Cory-Slechta, 1992), and allows for longitudinal assessment of within-subject drug effects (Bailey et al., 2010). The IRA is separated into a performance session, where the sequence of responses trained is always the same, and a learning session, where a different sequence of responses is trained each session. One session is conducted each day, and the two types of sessions alternate daily (Bailey et al., 2010; Johnson et al., 2010). Previous research suggests learning, but not performance, chains are sensitive to MK-801 administration (Bauter et al., 2003). Therefore, we first identified a dose of MK-801 that reduced accuracy in learning sessions without reducing response rates, and then tested the feasibility of using L-655,708, an inverse agonist that selectively inhibits α5GABAA receptors (Atack et al., 2006), to attenuate MK-801-induced learning deficits.
Methods
Subjects and housing
Eight FVB/NCrl male mice, obtained from Charles Rivers (Wilmington, Massachusetts, USA), were group housed with free access to water in a temperature-controlled and humidity-controlled colony room with a 12 : 12 light/dark cycle. The mice were approximately 14 weeks old at the start of the study. Body weights were maintained at between 85 and 90% of each mouse’s free feeding weight. All experimental procedures were approved by the West Virginia University Animal Care and Use Committee.
Apparatus
Testing took place in six standard model ENV-022 operant-conditioning chambers (Med Associates, St Albans, Vermont, USA) contained in sound-attenuating cubicles. An automated syringe and tubing system delivered 20 μl sweetened condensed milk, diluted 50 : 50 with water, into a constantly accessible holding cup as reinforcement. Chambers were equipped with nose-poke sensors to the left and right of the milk cup, and on the back wall across from the milk cup, which counted responses by interruption of a photobeam. One low and one high Sonalert tone (2900 and 4500 Hz, respectively) functioned as discriminative auditory stimuli. Fans ran constantly for ventilation.
Procedure
Nose-poking was autoshaped in daily 1.5-h sessions, beginning on the left nose-poke hole. In the first component of the autoshaping procedure, noncontingent milk was delivered after a 5.5-min interval, during the last 30 s of which the active nose-poke hole was illuminated and a tone sounded. Responses on the active nose-poke alternative also resulted in milk delivery on a fixed-ratio 1 schedule. After 10 correct responses or 20 noncontingent milk presentations, the mouse entered component two, in which noncontingent milk presentations were no longer administered. A fixed-ratio 1 schedule was still in effect, and the active nose-poke hole was constantly illuminated. Sessions ended either after 40 reinforced nose-pokes or when 1.5 h had passed, whichever occurred first. Training was complete when all mice acquired each of the three nose-poke responses.
The IRA procedure has been described in detail previously (Bailey et al., 2010). Briefly, the IRA consisted of daily 60-min sessions in which a maximum of a six-link chain could be reached by backward chaining. Six correct responses were required in a row to advance from a one-link chain, whereas all further chains required three consecutive correct chains to progress. The three nose-poke sensors were illuminated at the start of each session. A correct chain resulted in a low tone and delivery of milk, followed by a 5-s intertrial interval in which all lights were darkened and nose-pokes had no programmed consequences. Any incorrect response illuminated the house light for a time out period and deactivated the nose-poke devices (thus turning off the nose-poke LED lights) which lasted 5 s, and returned the current chain length to the response farthest from reinforcement. The consecutive number of correct chains completed was also reset. Responding in the last second of either the intertrial interval or time out extended the period for an additional 2 s. A unique auditory discriminative stimulus was paired with each link. IRA sessions were terminated if a mouse earned 50 reinforcers in a six-link chain or the 1-h time limit elapsed, whichever occurred first.
The first 25 sessions were performance sessions, where the chain of responses being trained was always the same. After the 25th session, days alternated between performance and learning sessions, where the response chain differed in each session. Distinct cues were associated with performance (steady nose-poke LEDs) versus learning sessions (flashing nose-poke LEDs), and a checkerboard pattern covering one wall of the experimental chamber was provided during learning sessions, but not during performance sessions. Learning sequences were presented semirandomly without replacement and were generated such that each sequence required a response on each of the three nose-poke devices; there were no consecutive responses on the same nose-poke device within a chain (e.g. BRRL), and no sequence could require more than one response in the same position as the performance chain.
Drug administration
Once baseline behavior stabilized in both the performance and learning conditions, drug administration began. An injection volume of 0.15 ml (5 ml/kg) was maintained for all injections. At least two injections were administered per dose. Drugs and vehicles were administered 10 min before each learning session in a room separate from the one containing the behavioral chambers. There was a minimum of 2 days between injections.
The dose effect curve (DEC) for intraperitoneal injections of MK-801 (0.03–0.5 mg/kg, dissolved in 0.9% saline) was completed first, followed by completion of the DEC for L-655,708 (0.1–1.0 mg/kg, dissolved in dime-thyl sulfoxide) (both from Tocris, Bristol, UK). To ensure the additional IRA training that occurred during determination of the L-655,708 DEC did not alter the ability of MK-801 to disrupt learning, the 0.25 mg/kg MK-801 dose was administered twice more. Comparison of 0.25 mg/kg MK-801 administered before, and after, determination of the L-655,708 DEC revealed no significant differences for any measure (accuracy, chain length, or total responding; repeated measures of analysis of variances P’s >0.05). Similarly, using either the more proximal 0.25 mg/kg injections, or an average of all 0.25 mg/kg injections, for comparison with the combination of both MK-801 and L-655,708, did not alter the results or interpretations of the current study. Thus, the more proximal doses were used for comparison with the combination of both MK-801 and L-655,708 and are displayed in Fig. 2.
Fig. 2.
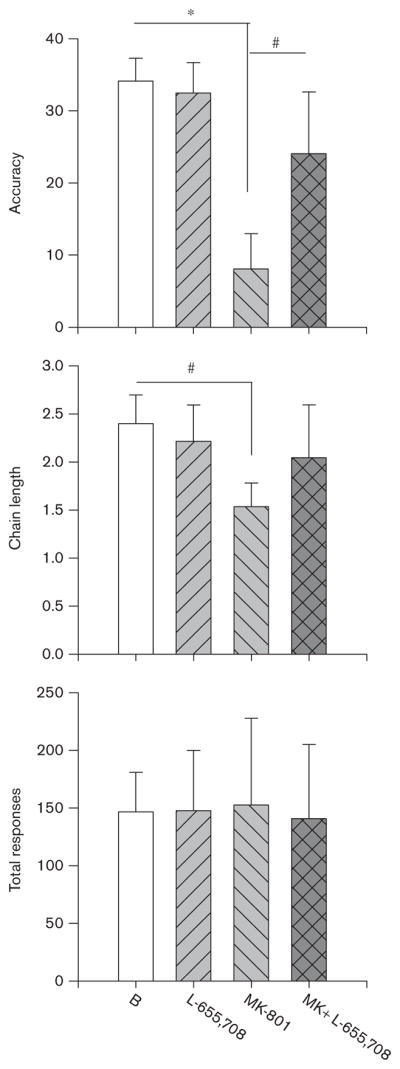
Effects of 0.25 mg/kg MK-801 in combination with 1 mg/kg L-655,708 on accuracy, chain length, and total number of responses. #P < 0.05; *P <0.01.
Next, MK-801 (0.25 mg/kg) was administered in combination with L-655,708 (1 mg/kg). The MK-801 dose (0.25 mg/kg) was selected because this dose altered accuracy without decreasing total number of responses emitted, whereas the L-655,708 dose (1 mg/kg) was selected for its potency at α5 versus α2/α3-containing or α1-containing GABAA receptors (Atack et al., 2005).
Statistical and data analysis
Dependent measures included maximum chain length reached, total nose pokes (i.e. total responses), and accuracy [(total correct/total errors +total correct) × 100]. Significant analysis of variances and repeated measures of analysis of variances were followed by Tukey’s post-hoc comparisons.
Results
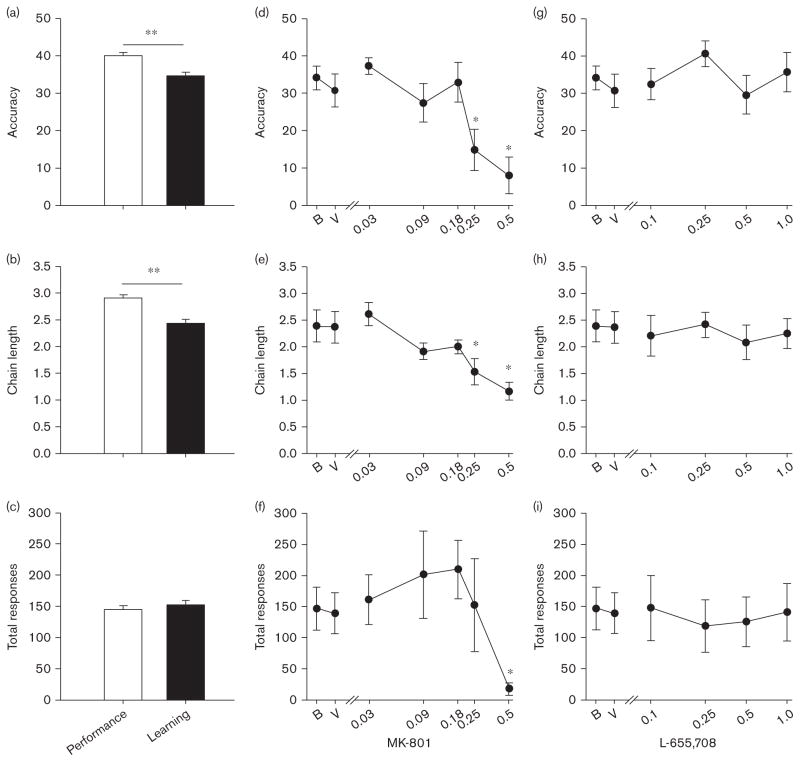
The performance chain and learning chains across all baseline sessions were first compared. Under baseline conditions, higher overall accuracy was observed during the performance chain compared with the learning chains [Fig. 1a; F(1,651) = 17.90, P < 0.001]. Likewise, the average chain length reached was greater during the performance chains [Fig. 1b; F(1,651) = 29.15, P <0.001]. To ensure these differences were not due to differences in the total number of responses, the average total responses per session were examined. There were no significant differences in total responses per session between the performance and learning chains [Fig. 1c; F(1,651) = 0.961, NS].
Fig. 1.
(a) Accuracy, (b) chain length, and (c) total number of responses of incremental repeated acquisition performance and learning chains at baseline. Effects of MK-801 on (d) accuracy, (e) chain length, and (f) total number of responses emitted. Effects of L-655,708 on (g) accuracy, (h) chain length, and (i) total number of responses emitted. **Performance versus learning, P <0.001; *P <0.05 versus baseline.
MK-801 dose dependently decreased accuracy [Fig. 1d; F(6,30) = 6.61, P <0.001], chain length [Fig. 1e; F(6,30) = 10.96, P <0.001], and total responding [Fig. 1f; F(6,30) = 4.24, P < 0.005]. Though accuracy and chain length were decreased at both the 0.25 and 0.5 mg/kg doses of MK-801 (Fig. 1d and e), the total number of responses were not significantly decreased by 0.25 mg/kg MK-801 (Fig. 1f). Thus, the 0.25 mg/kg dose of MK-801 was identified as a dose that altered accuracy without decreasing total number of responses emitted. Despite testing a range of doses of L-655,708, no differences in accuracy [Fig. 1g; F(5,25) =2.43, P = 0.063], chain length [Fig. 1h; F(5,25) = 1.70, P = NS], or total responding [Fig. 1i; F(5,25) = 0.50, NS] were observed.
MK-801 (0.25 mg/kg) was administered in combination with 1 mg/kg of L-655,708. L-655,708 attenuated the MK-801-induced decreases in accuracy [Fig. 2a; F(3,15) =9.03, P <0.001]; accuracy in the MK +L-655,708 condition was significantly higher than when MK-801 was administered alone (P <0.05). For chain length [Fig. 2b; F(3,15) =5.82, P <0.01], there was a trend towards increasing chain length; in the presence of L-655,708, MK-801 no longer significantly decreased chain length, but there was also no significant difference between MK-801 and MK +L-655,708 (P >0.05). The total number of responses was similar across all conditions [Fig. 2c; F(3,15) =0.04, NS], suggesting that any differences among treatments were not due to alterations in the total number of responses emitted.
Discussion
NMDARs are centrally involved in cognitive processes, and deregulation of NMDARs is thought to underlie the synaptic dysfunction observed in many diseases. GABA receptors limit the synaptic activity of NMDARs (Davies et al., 1990), and blocking the activity of GABA receptors can enhance the synaptic activation of NMDARs (Herron et al., 1985). The doses of L-665,708 used in the present study were selected because previous work in mice has shown these doses to be effective in preventing memory blockade by etomidate (Martin et al., 2009) and isoflurane (Saab et al., 2010) and because in-vivo potency of L-655,708 at α5 relative to α2/α3-containing or α1-containing GABAA receptor is about five-fold and 20-fold, respectively (Atack et al., 2005). To date, the effects of α5GABAA receptor inhibition have largely been studied within the context of general cognitive enhancement (e.g. Chambers et al., 2003, 2004). Interestingly, no improvements in IRA measures were noted following L-665,708 administration in the current study.
Despite a lack of general cognitive enhancement by L-655,708, concurrent administration of L-655,708 attenuated MK-801-induced decreases in accuracy. Changes in learning could not be attributed to changes in general activity because exposure to MK-801 (0.25 mg/kg), L-655,708, or MK-801 plus L-655,708 did not alter the total number of responses emitted. Although L-655,708 is selective for α5GABAA receptors (Atack et al., 2005), it is possible that the current results attributed to reduced α5GABAA receptor activity could be due to effects on other GABAA receptor subtypes.
In summary, the present investigation indicates that L-655,708, an α5GABAA inverse agonist, can attenuate learning deficits caused by decreased NMDAR activity. Future studies are needed to examine other dose combinations of MK-801 and L-655,708 and determine whether the effects of higher doses of MK-801, or chronic MK-801, could be attenuated.
Acknowledgments
The project described was supported by the National Institute of General Medical Sciences, U54GM104942, and the Alzheimer’s Association, NIRG-12–242187 (M.N.R.).
Footnotes
Conflicts of interest
There are no conflicts of interest.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or Alzheimer’s Association.
References
- Adams MM, Smith TD, Moga D, Gallagher M, Wang Y, Wolfe BB, et al. Hippocampal dependent learning ability correlates with N-methyl-D-aspartate (NMDA) receptor levels in CA3 neurons of young and aged rats. J Comp Neurol. 2001;432:230–243. doi: 10.1002/cne.1099. [DOI] [PubMed] [Google Scholar]
- Atack JR, Alder L, Cook SM, Smith AJ, McKernan RM. In vivo labelling of α5 subunit-containing GABA (A) receptors using the selective radioligand [3 H]L-655,708. Neuropharmacology. 2005;49:220–229. doi: 10.1016/j.neuropharm.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Atack JR, Bayley PJ, Seabrook GR, Wafford KA, McKernan RM, Dawson GR. L-655,708 enhances cognition in rats but is not proconvulsant at a dose selective for alpha5-containing GABA (A) receptors. Neuropharmacology. 2006;51:1023–1029. doi: 10.1016/j.neuropharm.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Bailey JM, Johnson JE, Newland MC. Mechanisms and performance measures in mastery-based incremental repeated acquisition: behavioral and pharmacological analyses. Psychopharmacology (Berl) 2010;209:331–341. doi: 10.1007/s00213-010-1801-3. [DOI] [PubMed] [Google Scholar]
- Bauter MR, Brockel BJ, Pankevich DE, Virgolini MB, Cory-Slechta DA. Glutamate and dopamine in nucleus accumbens core and shell: sequence learning versus performance. Neurotoxicology. 2003;24:227–243. doi: 10.1016/S0161-813X(02)00167-5. [DOI] [PubMed] [Google Scholar]
- Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- Butelman ER. A novel NMDA antagonist, MK-801, impairs performance in a hippocampal-dependent spatial learning task. Pharmacol Biochem Behav. 1989;34:13–16. doi: 10.1016/0091-3057(89)90345-6. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Broughton HB, Collinson N, Cook S, Dawson GR, et al. Identification of a novel, selective GABA (A) α5 receptor inverse agonist which enhances cognition. J Med Chem. 2003;46:2227–2240. doi: 10.1021/jm020582q. [DOI] [PubMed] [Google Scholar]
- Chambers MS, Atack JR, Carling RW, Collinson N, Cook SM, Dawson GR, et al. An orally bioavailable, functionally selective inverse agonist at the benzodiazepine site of GABA (A) alpha5 receptors with cognition enhancing properties. J Med Chem. 2004;47:5829–5832. doi: 10.1021/jm040863t. [DOI] [PubMed] [Google Scholar]
- Cohn J, Cory-Slechta DA. Differential effects of MK-801, NMDA and scopolamine on rats learning a four-member repeated acquisition paradigm. Behav Pharmacol. 1992;3:403–413. [PubMed] [Google Scholar]
- Davies CH, Davies SN, Collingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fan MM, Raymond LA. N-methyl-D-aspartate (NMDA) receptor function and excitotoxicity in huntington’s disease. Prog Neurobiol. 2007;81:272–293. doi: 10.1016/j.pneurobio.2006.11.003. [DOI] [PubMed] [Google Scholar]
- Herron CE, Williamson R, Collingridge GL. A selective N-methyl-D-aspartate antagonist depresses epileptiform activity in rat hippocampal slices. Neurosci Lett. 1985;61:255–260. doi: 10.1016/0304-3940(85)90473-2. [DOI] [PubMed] [Google Scholar]
- Johnson JM, Bailey JM, Johnson JE, Newland MC. Performance of BALB/c and C57BL/6 mice under an incremental repeated acquisition of behavioral chains procedure. Behav Processes. 2010;84:705–714. doi: 10.1016/j.beproc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin LJ, Oh GH, Orser BA. Etomidate targets alpha5 gamma-aminobutyric acid subtype a receptors to regulate synaptic plasticity and memory blockade. Anesthesiology. 2009;111:1025–1035. doi: 10.1097/ALN.0b013e3181bbc961. [DOI] [PubMed] [Google Scholar]
- Saab BJ, Maclean AJ, Kanisek M, Zurek AA, Martin LJ, Roder JC, Orser BA. Short-term memory impairment after isoflurane in mice is prevented by the alpha5 gamma-aminobutyric acid type a receptor inverse agonist L-655,708. Anesthesiology. 2010;113:1061–1071. doi: 10.1097/ALN.0b013e3181f56228. [DOI] [PubMed] [Google Scholar]
- Shapiro M, Caramanos Z. NMDA antagonist MK-801 impairs acquisition but not performance of spatial working and reference memory. Psychobiology. 1990;18:231–243. [Google Scholar]