Abstract
Background
The recent availability of new non-invasive prenatal genetic tests for fetal aneuploidy has raised questions concerning whether and how these new tests will be integrated into prenatal medical care. Among the many factors to be considered are public understandings and preferences about prenatal testing mechanisms and the prospect of fetal aneuploidy.
Methods
To address these issues, we conducted a nation-wide mixed-method survey of 2,960 adults in the United States to explore justifications for choices among prenatal testing mechanisms. Open responses were qualitatively coded and grouped by theme.
Results
Respondents cited accuracy, followed by cost, as the most significant aspects of prenatal testing. Acceptance of testing was predicated on differing valuations of knowledge and on personal and religious beliefs. Trust in the medical establishment, attitudes towards risk, and beliefs about health and illness were also considered relevant.
Conclusions
Although a significant portion of the sample population valued the additional accuracy provided by the new non-invasive tests, they nevertheless expressed concerns over high costs. Furthermore, participants continued to express reservations about the value of prenatal genetic information per se, regardless of how it was obtained.
Keywords: Prenatal Testing, Aneuploidy, Prenatal Screening, Public Attitudes
INTRODUCTION
Recent advances in genetic analysis have made possible expanded non-invasive prenatal testing (NIPT) using massively parallel or targeted sequencing of cell-free fetal DNA (cffDNA) found in the maternal bloodstream (Bianchi et al. 2012a, 2012b; Norton et al. 2012; Palomaki et al. 2012; Sayres and Cho 2011; Zimmermann et al. 2012). Validated tests are clinically available in the United States (US) for fetal RhD blood type; fetal sex; trisomies 13, 16, 18, 21 (Down syndrome), and 22; sex chromosome aneuploidies; and selected sub-chromosomal abnormalities. NIPT has several distinguishing features, including the ability to deliver more sensitive and specific results than traditional serum screens without the procedure-related risks associated with invasive diagnostic testing (Lewis et al. 2012). In the US, cffDNA tests have entered an estimated US$1.3 billion prenatal testing market (Agarwal et al. 2013) with considerable interest from both professional and media circles (Greely 2011; Heger 2012; Norton, Rose, and Benn 2013).
The purpose of prenatal testing (including both screening tests and diagnostic tests) is to establish a risk profile for a pregnancy that may be affected by aneuploidy or certain other fetal conditions, which may allow the family to prepare for the birth both medically and socially. Patients may also use diagnostic information from invasive tests to make decisions about terminating the pregnancy. Prior to the introduction of NIPT, standards of prenatal care in the US included the offer of a first-trimester serum screening and an ultrasound for fetal trisomy, neural tube defects, and other structural anomalies. In the second trimester, patients are often offered a second serum screen, which is integrated with first trimester results to create a more accurate risk profile for the pregnancy (Cuckle et al. 2008; Farrell, Nutter, and Agatisa 2011; Malone et al. 2005; Meier et al. 2002; Wald and Bestwick 2013). Patients are usually offered amniocentesis (at fifteen to twenty-four weeks gestation) or chorionic villus sampling (CVS) (at ten to fourteen weeks gestation) to genetically test the fetus if early screens indicate high risk or there are other factors that indicate a potential fetal anomaly (Harris et al. 2004). Amniocentesis and CVS are considered diagnostic, but both are invasive and carry a slight risk of miscarriage (Caughey, Hopkins, and Norton 2006).
The International Society for Prenatal Diagnosis, the National Society of Genetic Counselors, and the American College of Obstetrics and Gynecology have released statements supporting the offer of NIPT as an aneuploidy screen for pregnancies screened as high-risk, while maintaining that NIPT is not diagnostic and results should be confirmed by invasive diagnostic methods (Devers et al. 2013; American College of Obstetrics and Gynecology 2012). However, some clinicians have begun offering NIPT along with, or in place of, first trimester serum screening (Fairbrother et al. 2013). NIPT is not diagnostic, but it is more sensitive and specific than other screening tests and allows some patients to avoid the procedure-related risk associated with diagnostic testing.
As NIPT becomes more common, it is important to understand attitudes towards this new testing modality. NIPT enters a prenatal testing landscape guided both by clinical assessments of utility and by the rhetoric of personal choice—although, as Lippman observed two decades ago, the “very existence” of these testing options “necessarily forces” women to makes choices that are “difficult, and often painful” (1994, 11). Existing studies on the views of the general US population regarding prenatal testing have not taken non-invasive prenatal genetic testing mechanisms into account (Bishop et al. 2004; Kalfoglou et al. 2005). Survey studies have been conducted on pregnant women and practitioners (Benn et al. 2014; Farrell, Agatista, and Nutter 2014; Musci et al. 2013; Sayres et al. 2011; Tischler et al. 2011), the results of which indicate support for the idea of non-invasive testing but also concerns about implementation and patient comprehension of the test’s capabilities. In the UK, Lewis et al. (2014) found enthusiasm for the use of NIPT, while Kelly and Farrimond (2011, 2012) likewise found support but reported a desire for social control of new testing mechanisms to prevent inappropriate usage.
In this study, we asked a broad sample of US adults about their attitudes towards the use of NIPT to detect aneuploidy. While pregnant women are the individuals who actually give consent for prenatal testing, we recognize that women do not make prenatal decisions in a socio-cultural vacuum. The knowledge and opinions of partners, family members, religious leaders, and other members of the social network may significantly influence patient decision making (Browner 1999; Locock and Alexander 2006; Suzumori et al. 2014). The attitudes of a broader range of stakeholders is therefore relevant to understanding how women make choices about prenatal testing.
Furthermore, prenatal technologies have historically been a target of public policy in the US, not merely as a matter of state health care provision, but at a legislative level. Some states provide prenatal screening programs, and thus control which tests are most widely available. In many states, screening may be provided through private health care but subsidized by Medicaid. The decisions of local and state non-medical actors can therefore impact the availability of prenatal testing technologies.
As these actors contemplate the potential addition of NIPT to existing testing regimes, it is important to base these decisions on broader public views on when and how prenatal testing should be conducted. We therefore conducted a nation-wide, mixed method survey of 2,960 US adults to solicit their values and opinions on prenatal testing technologies, including NIPT.
METHODS
We developed a survey instrument consisting of 25 questions, including Likert scale, multiple choice, and open response questions. Data and analysis from the quantitative portion of the study, including demographic predictors of testing choices, are published elsewhere (Allyse et al. 2014; Sayres et al. 2014). A broader team of bioethics scholars, including representatives from genetic counseling, maternal fetal medicine, and sociology, reviewed the survey’s text, including background materials, for readability and content. The introductory text and open response questions from the survey are included in Appendix 1.
The survey was distributed to a national sample through Zoomerang, an online market research firm, in February and March of 2012. Participants answered questions about their opinions on invasive versus non-invasive testing for fetal aneuploidies. All participation was voluntary, anonymous, and confidential. Participants were compensated by Zoomerang for participating with entries in sweepstakes drawings and donations to selected charities. This study was approved by the Stanford University Institutional Review Board.
The survey contained two sections. In the first, respondents received information about the typical symptoms of trisomy 13, 18, and 21 and the currently available tests available to screen for them (see Figure 1). Participants were randomized between two versions of the sample - one half received information about trisomy 13 and 18 and the other trisomy 21 - in order to avoid any cognitive bias associated with the order in which conditions were presented. This also allowed us to test whether considering higher morbidity vs. lower morbidity conditions independently would impact testing decisions (Ubel et al. 2010).
Figure 1.
Descriptions provided to participants of trisomy 13, 18 and 21
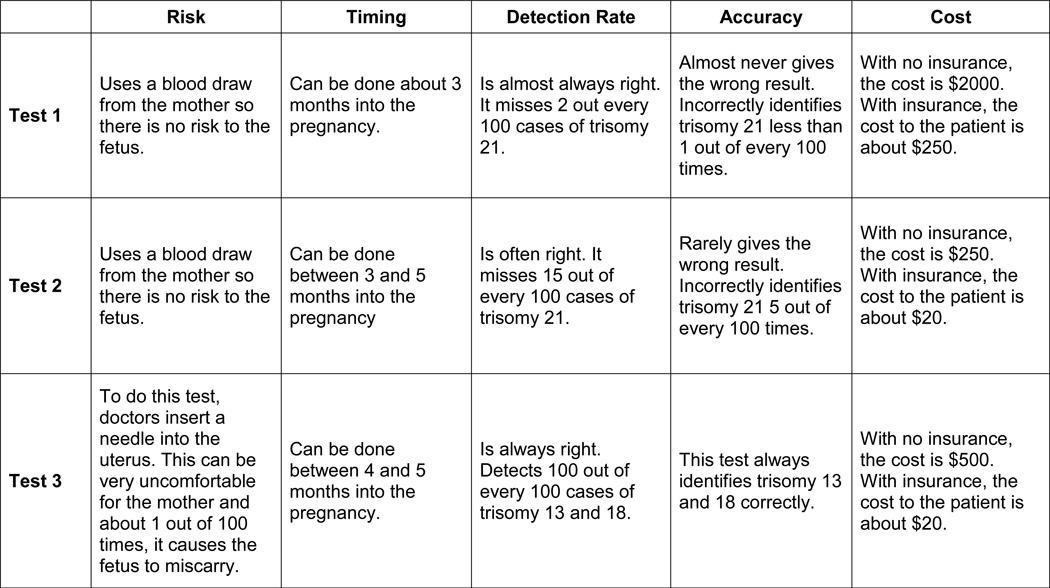
Because general knowledge of NIPT was not high in 2012, the available tests were named only “test one,” “test two,” and “test three,” but were described in terms that reflected the published performance of integrated screening (two serum screens and ultrasound), NIPT, and amniocentesis (See Figure 2). In keeping with the state of the prenatal testing market in the US at the time, NIPT was portrayed as having very high, but not diagnostic, sensitivity and specificity at a price point of $250. Participants were then invited to “think about whether or not you would want a loved one, such as a wife, sister or daughter to access these tests if she became pregnant.” After participants indicated which testing modality they would prefer, they were asked to justify their choice in an open response. The second section asked participants to self-report demographic information.
Figure 2.
Descriptions provided to participants of Tests 1, 2, and 3
Participants and Data Collection and Analysis
The survey instrument was piloted on a sample of 204 individuals to insure the clarity of the language. Opportunity for open-ended feedback on the survey instrument was provided to the pilot sample. A slightly modified survey was redistributed and a total of 2,960 surveys were completed. The survey invitation was distributed by Zoomerang, which maintains a national panel of potential respondents designed to mimic actual population demographics. Zoomerang also validates prospective participants to ensure that they are providing accurate data and ensures unique participants using digital fingerprinting (see Truesample.com for details). The only exclusion criterion was a respondent age under 18 years.
Results were coded using the qualitative software NVivo (version 10). Not all responses to the open response were usable because some respondents did not respond to all questions with legible words or coherent expressions. A total of 1,218 useable open-ended responses were returned to the trisomy 13 and 18 survey and 1,154 to the trisomy 21 survey. Qualitative data were analyzed using an iterative social science methodology, which organically extracted themes from the data. Three coders independently reviewed the data several times to identify potential codes and sub-codes. Reviewers then met over several meetings to compare codes identified and either consolidate or split potential codes. Some codes were subsumed into others.
Based on these codes, a codebook was developed that included both broad codes and a variety of sub-codes that addressed additional nuances. Three members of the research team reviewed the data independently and applied codes. The team then reviewed their coding as a group and resolved all discrepancies by discussion and collective consensus. As Bradley, Curry, and Devers (2007) described this consensus-based methodology: “The result is a single, agreed upon application of the final codes to all parts of the data” (1764). This methodology leveraged the strengths of our multidisciplinary coding team, which included coders from disciplinary backgrounds in philosophy, basic science, and social science, by elucidating and synthesizing our different but complementary perspectives on the data.
RESULTS
Demographic characteristics of all survey participants are presented in Table 1. In order to establish representativeness, we compared demographic measures to the 2010 US Census data (for gender, age, ethnicity, race, educational attainment, household income, and health insurance status) and the Pew Religious Landscape Survey of 2008 (for religion) using chi-square goodness-of-fit tests with α=0.05. The survey sample under-represented males (χ2[1, N=3164]=15.590, p<0.001); individuals who identify as Hispanic or Latino (χ2[1, N=2962]=173.432, p<0.001) or Black or African-American (χ2[1, N=3164]=74.912, p<0.001); and those who are affiliated with Evangelical (χ2[1, N=3157]=469.142, p<0.001) or other (χ2[1, N=3157]=147.939, p<0.001) denominations of Protestantism. Additionally, the distributions of respondent age (lower) (χ2[5, N=3164]=166.340, p<0.001); household income (higher) (χ2[4, N=3164]=140.058, p<0.001); and educational attainment (higher) (χ2[4, N=3164]=469.694, p<0.001) were significantly different from those of the national population. Additional demographic information, such as experience with parenting or disability, and their correlates with testing recommendations, is reported elsewhere (Sayres et al. 2014).
Table 1.
Demographics of survey respondents (n=3,133)
| Trisomy 13 & 18 n = 1642 |
Trisomy 21 n = 1491 |
National* | |
|---|---|---|---|
| AGE | |||
| 18 – 24 | 14% | 15% | 13% |
| 25 – 34 | 23% | 21% | 18% |
| 35 – 44 | 22% | 22% | 18% |
| 45 – 54 | 16% | 16% | 19% |
| 55 – 64 | 11% | 10% | 16% |
| 65 or older | 14% | 16% | 17% |
| SEX | |||
| Male | 46% | 44% | 49% |
| Female | 54% | 56% | 51% |
| HISPANIC OR LATINO | |||
| Yes | 8% | 7% | 16% |
| No | 92% | 93% | 84% |
| RACE/ETHNICITY | |||
| American Indian or Alaska Native | 2% | 2% | 2% |
| Asian | 6% | 6% | 6% |
| Black or African American | 8% | 8% | 14% |
| Native Hawaiian or Other Pacific Islander | 1% | 1% | <1% |
| White | 84% | 83% | 75% |
| Other | 2% | 3% | N/A |
| RELIGIOUS AFFLIATION | |||
| Catholic | 23% | 24% | 23% |
| Evangelical Protestant | 9% | 10% | 27% |
| Other Protestant | 18% | 16% | 27% |
| Mormon | 2% | 2% | 2% |
| Jewish | 4% | 4% | 2% |
| Buddhist | 1% | 1% | 1% |
| Muslim | 1% | 1% | <1% |
| Unaffiliated | 24% | 20% | 14% |
| Other | 20% | 24% | N/A |
| RELIGIOUS PRACTICE | |||
| Very Religious | 21% | 23% | Not Measured |
| Somewhat Religious | 41% | 42% | Not Measured |
| Not Very Religious | 22% | 20% | Not Measured |
| Not Religious | 17% | 16% | Not Measured |
| HOUSEHOLD INCOME | |||
| Under $25,000 | 20% | 20% | 25% |
| $25,000 – $49,999 | 29% | 27% | 25% |
| $50,000 – $74,999 | 23% | 23% | 18% |
| $75,000 – $99,999 | 14% | 15% | 12% |
| Over $100,000 | 15% | 15% | 20% |
| EDUCATIONAL ATTAINMENT | |||
| Some High School | 2% | 2% | 9% |
| High School Diploma | 18% | 20% | 30% |
| Some College | 31% | 30% | 23%** |
| College Diploma | 28% | 28% | |
| Some Graduate School | 7% | 5% | 27% |
| Graduate School Diploma | 14% | 15% | 11% |
| ARE YOU A PARENT? | |||
| Yes | 58% | 61% | Not Measured |
| No | 42% | 39% | Not Measured |
Based on the 2010 US Census data (age, sex, ethnicity, race, educational attainment, household income, and health insurance status) and the Pew Religious Landscape Survey of 2008 (for religion).
The US Census only tracks completed degrees.
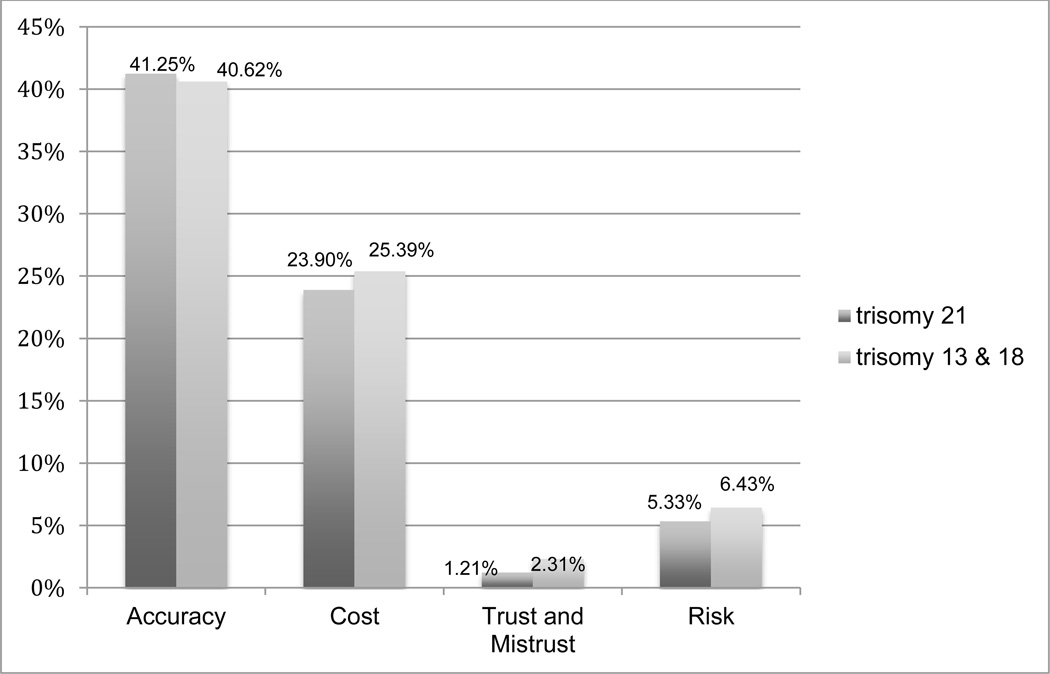
Four larger codes, developed from the data, are reported here: Accuracy, Cost, Trust or Mistrust, and Risk or Safety. Each individual’s response was coded to one or multiple relevant codes. Figure 1 shows the frequency of each code for trisomy 13/18 and trisomy 21. No statistically significant differences in the frequency of any of these codes were detected between responses to the trisomy 13/18 and trisomy 21 surveys. Because many respondents did not make a clear choice for or against NIPT (see Sayres et al. 2014), and many reasons cited for respondents’ choices referred to prenatal testing generally rather than any particular technology, we have presented this qualitative analysis by code only, rather than by test recommendation. However, where relevant, we have noted the relationship between these themes and specific recommendations for or against NIPT.
Accuracy
The stated accuracy of the NIPT was the most frequently suggested motivation for its selection. Rationales often mentioned accuracy, sometimes noting differences between the accuracy of available tests.
The accuracy [of NIPT] is more accurate than [screening]. With the accuracy from [NIPT] the mother has a better decision to make regardless the cost. [Male, 35–44 years]
Others referred more specifically to false negatives:
Would help to know for sure. My niece just had a baby with Down syndrome & was told the blood test was negative. [Female, 45–54 years]
Some respondents expressed considerable anxiety over the possibility of false positives.
I would not want to abort a normal fetus. [Male, 35–44 years]
It would be terrible to get a false positive and discontinue the pregnancy because of it. [Female, 18–24 years]
Responses like this suggest that, for these respondents, test accuracy was associated with the ability to protect “normal” pregnancies from termination.
Higher accuracy was mentioned most frequently by respondents who selected NIPT, which was presented as having a lower false positive and false negative rate than screening. However, respondents who indicated that they would elect to have invasive testing because of its diagnostic qualities also mentioned accuracy:
[NIPT] is more accurate, and when deciding whether to keep a baby or terminate the pregnancy, accuracy is paramount!! Personally, however, I've had an amniocentesis done with all of my pregnancies, and I'd recommend this route for both of my daughters if the situation arose. [Female, 45–54 years]
Respondents frequently associated amniocentesis with a feeling of certainty. Several of these respondents reported that they wanted “100% accuracy.”
Cost
The cost of testing was the second most frequent reason provided for test selection. This code included two sub-themes. In the first, cost was used to justify the selection of traditional screening over NIPT because of the higher cost of NIPT.
First of all, the blood test [NIPT] is very expensive and unnecessary. Plus…it's not even 100%. [Male, 35–44 years]
Respondents frequently weighed concerns about cost against the perceived risk of having an affected pregnancy and accuracy of results.
Well, if I was really worried I would pay for [NIPT], but [screening] is so much cheaper and won't harm the fetus, but I might freak out if I get the wrong results. [Female, 18–24 years]
Well IF insurance would cover it I would go with [NIPT]. Otherwise I would have to go with [screening]. [Female, 45 – 54 years]
The second sub-theme included a rejection of cost as a relevant factor in making testing choices. This sub-theme was more frequently used to justify the selection of NIPT over traditional screening.
If I felt so strongly that I needed to know cost would not be an issue. I think the higher accuracy rate is very important. Having the experience of an amnio to detect downs [sic] syndrome in one of my twins was very stressful. [Female, 35–44 years]
Accuracy is more important than money. A 15% failure rate [for serum screening] is unacceptable. [Female, 65 or Older]
Another aspect of this sub-theme was an assertion that cost considerations are inappropriate in pregnancy, linking the inherent value of the anticipated child to the perceived duty to do “whatever it takes” in the prenatal stage.
Babies are important no matter the cost! [NIPT] is more accurate! If I were to take the other one and it came back neg. I wouldn't feel right w/o taking [the] other one anyway! [Male, 25–34 years]
This attitude was frequently associated with an insistence that the potential child (and, by extension, any information about his/her health) is valuable beyond any financial considerations.
Finally, participants worried about the impact of the cost increase for NIPT on individuals of limited resources.
Cost. A woman of low resources not accessible to the testing. [Female, 35 – 44 years]
Don't have insurance or a lot of money. [Males, 18–24 years]
The cost to insurance which is ultimately passed on to consumer and also the personal cost upfront. If it is too expensive, many people wont do it at all. [Female, 35 – 44 years]
In these responses, the role of insurance was a consistent message, with many respondents indicating that insurance coverage was a key component of their decision making.
Trust and Mistrust
A small subset of responses linked their opinions about testing to a lack of trust in the medical and scientific establishment—specifically, a perception that expansions in prenatal testing is not in the best interests of patients.
There are too many tests these days that scare us all. It's best to deal with things as they come along. [Male, 25–34 years]
Because standard medicine is all too often proven as inept and corrupt and I do not blindly trust doctors unless I can fully substantiate their methods by research. [Male, 45–54 years]
Such responses often contained an implicit or explicit assumption that the medical establishment, or society more generally, views termination as the “right” decision after trisomy detection.
Pressure applied if positive for an abortion. [Male, 45–54 years]
If it indicated Downs, the doctor would most likely encourage termination of the pregnancy and I would not allow it. [Female, 55–64 years]
The theme of trust also emerged as a sense of betrayal when tests return incorrect results, damaging personal faith in the abilities of prenatal testing to provide genuine information.
Because during both my pregnancies I received false positives BOTH times…..all my kids were fine and in the end even if they were Downs positive, nothing would change. I would just be prepared as I was for children with Downs. [Female, 35–44 years, emphasis in original]
The theme of trust sometimes emerged in a sense that doctors encourage or implement testing against the wishes of the patient, even when the results may not provide genuine information.
I would not want to know before hand. I had it done during a pregnancy after asking not to and it came back highly likely. My daughter was born perfectly healthy. [Female, 45–54 years]
A lack of trust was generally applied to all testing equally, without distinction between NIPT and screening. This trust was also linked back to the issue of accuracy, with the assertion that nothing other than absolute certainty was worth obtaining.
I am currently pregnant and I would not get this test because it is not 100% accurate and I would not terminate my pregnancy based on the fact that the test could be wrong. [Female, 18–24 years]
Even this is NOT effective enough. I want 100% accuracy and would settle for nothing less. I have a sick child, and it's a living hell. I would have given anything if I could have known what he was going to develop so I could have terminated the [pregnancy]. [Female, 45 – 54 years]
These responses frequently rejected the notion of relative risk in favor of arguing for a form of guarantee of fetal health.
Risk and Safety
As anticipated, avoiding test-related risks was a significant factor for many respondents who preferred NIPT.
Don’t want no risks and don’t want no problems. [Male, 25–34 years]
I would not want to harm my unborn child. [Female, 34–44 years]
However, risk was also frequently mentioned in the context of testing as a whole, even with respect to non-invasive testing. This view appeared to regard prenatal testing in and of itself as potentially dangerous, in often unspecified ways.
Unnecessary tests that could put the mother and child at risk. [Male, 55–64]
Similarly, respondents expressed beliefs that worrying about or interpreting test results would cause emotional stresses that could negatively impact the pregnancy.
I would think this would put the mother into a stress that her body could not handle and could actually make the pregnancy worse and possible cause more damage to the unborn child. [Female, 25–34]
Thus, for many respondents, the concept of risk was multifaceted and not limited to the degree of invasiveness of testing procedures.
DISCUSSION
Commercially, NIPT has been framed as a technology designed to make the prenatal process easier and less anxiety-ridden. Companies offering NIPT portray it as a way to make prenatal testing “simpler” while providing near-diagnostic results without a risk of miscarriage. The implication of these portrayals is that the only drawbacks to prenatal information’s implicit value are uncertain accuracy (for screening tests) or the risk of miscarriage (for invasive diagnostic tests). However, the results of this study make clear that even with the option of NIPT, choosing among prenatal testing modalities remains a complex undertaking that depends on a variety of practical concerns, experiential understandings, and personal beliefs.
Accuracy and Trust and Mistrust
It is clear that, for some participants, this narrative of reassurance and reduced anxiety struck a chord. Frequent reference to the accuracy of NIPT, or the less satisfactory accuracy of serum screening, indicate that the ability of prenatal testing to provide reliable and reassuring information is valued by many participants. The remaining uncertainty, presented in the survey as a less than 1% chance that the results could be wrong, was judged negligible. This view frequently correlated with expressions of trust in technology and its ability to ensure “the mother has a better decision.” It is worth noting, however, the alternate side to this code, which contains responses where participants decried a lack of complete accuracy. In this view, respondents indicated that a core value of prenatal information is certainty. Any acknowledgement of potential inaccuracy erodes the tendency to see prenatal tests as trustworthy.
More disturbing are the explicitly stated beliefs of some respondents that the purpose of NIPT is to “encourage abortion,” suggesting that the medical establishment or broader society promotes the elimination of fetuses or children with genetic conditions (Janvier, Farlow, and Wilfond 2012; Van Riper and Choi 2011). These attitudes are counter to the expectation that increased access to genetic counseling and continued public education about disability would reduce this perceived stigma (Kaposy 2013; Parens and Asch 2003; Skotko et al. 2009). Instead, individuals continue to express views in terms of what is “supposed to happen” in a pregnancy and a feeling that, if humans interfere, then further negative consequences will ensue (Garcia et al. 2011). Even when participants expressed trust in the medical establishment, it is difficult to know whether this trust also correlates with an acceptance of pregnancy termination as an option. If so, then participants in this cohort may agree that medical providers, and society more generally, generate a prima facie assumption of termination as the natural outcome of a positive test result but do not necessarily see this as being in opposition to the interests of pregnant women.
Cost
Among survey respondents who selected serum screening over NIPT, the cost of testing was the most frequently cited reason. In part, this preference may be ascribed to the wide availability, and insurance coverage, of amniocentesis in the US. Because NIPT was not described as having diagnostic accuracy, many respondents felt that invasive confirmatory testing was inevitable and that the added cost of NIPT was unnecessary. This cost-benefit equation will be affected considerably if third-party payers, including state and private insurers, begin to provide coverage for NIPT. Currently, California is the only state to include NIPT in its universal screening program (Flessel and Goldman 2013) and, anecdotally, Medicaid coverage of NIPT is uneven across the US. If the cost of the two testing modalities were more equivalent, the emphasis of respondents on the value of “accuracy” suggests that uptake of NIPT testing would be higher. This prediction may have an impact on the payers’ cost-benefit analysis of NIPT.
The impact of the cost of testing may be particularly salient among less socioeconomically advantaged populations and minority populations in the US, who have historically had reduced access to and uptake of prenatal screening and testing (Bryant et al. 2010; Fransen et al. 2009; Kuppermann, Gates, and Washington 1996; Kuppermann et al. 2006). In certain populations, sociocultural factors may discourage the acceptance of prenatal testing, including a lingering mistrust in the medical establishments and its motivations (Corbie-Smith et al. 1999; Halbert et al. 2006; Jupka et al. 2012). In the present study, respondents often referenced personal experiences in which they or others were encouraged, in accordance with medical practice, to undergo screening and/or diagnostic tests on a fetus that proved to be “perfectly healthy,” which may be attributed to misunderstandings of risk information. However, as has been noted in previous studies (Lippman 1999; Gottfredsdottir, Björnsdóttir, and Sandall 2009), this finding also suggests that some participants trusted their own experience, personal preference, and faith more than statistical information—however accurate—offered by biomedical tests.
Risk, Informed Consent, and Declining Testing
Our results echo those of past studies showing that women may use a generic category of “risk” as an explanation for declining invasive testing, but that this category may actually serve as a proxy for other forms of prenatal anxiety; a desire to remain in ignorance of unwelcome information; or a form of parental responsibility toward a future child (Kelly 2009; Markens, Browner, and Press 1999). Certainly, many respondents in the present study favorably mentioned NIPT’s lack of test-related risk of miscarriage. However, we also see that even without this procedural risk, a significant number of respondents continued to report that they would not recommend choosing prenatal testing. This finding expands the notion of “risk” and suggests that, contrary to the message provided by commercial providers, procedural risk may not be the most relevant factor in selecting testing procedures and undergoing NIPT is not an obvious choice.
This finding calls into question the logic of standard clinical informed consent procedures for prenatal testing: women undergo considerable counseling and make clear decisions about invasive procedures whereas non-invasive serum screening is frequently held to lower standards of consent (Constantine et al. 2013; Favre et al. 2007; Seror et al. 2009). This disparity is significant because NIPT, relying as it does on a blood draw, combines the physical characteristics of serum screening with an information load that approaches that of diagnostic invasive testing. Indeed, an erosion of informed consent process has been one of the most persistent concerns associated with the introduction of NIPT (de Jong et al.2010; Deans and Newson 2011; van den Heuvel et al. 2010). In light of the views expressed here, the complexity of decision making about NIPT suggests that provisions should be made to expand the informed consent process for NIPT. Many observers have noted that the non-invasive nature of NIPT may encourage providers to view this testing as having “no downside” (Schmitz, Henn, and Netzer 2009; Schmitz, Netzer, and Henn 2009), and there is evidence that practitioners may feel that less stringent informed consent procedures are appropriate when offering non-invasive testing (van den Heuvel et al. 2010). Some commentators within the clinical arena even framed replacing the offer of invasive with non-invasive prenatal testing as an “ethical imperative” (Ravitsky 2009).
These concerns are relevant here because, as our results show, there are populations who, when considering the options outside of the potentially overwhelming context of prenatal care visits, would continue to advocate declining testing even when done non-invasively. This supports arguments that more careful attention must be paid to the informed consent process for non-invasive testing, so that individuals are not surprised by information that they would not have elected to receive. This is particularly true, given studies that have shown that, due to its routinization, and the large number of blood draws pregnant women endure, traditional serum screening is already frequently folded into routine prenatal care and many women are not aware that they are being tested or for what (Constantine et al. 2014). This denies women the chance to make an informed decision about whether screening provides information they would value. When asked, a sample of pregnant women expressed dismay that this might also occur in NIPT, arguing that being tested without one’s knowledge would be “not ethical” (Farrell, Agatista, and Nutter 2014, 625).
The second inference we may draw from the responses of those who said they would recommend declining testing is that a subset of the population perceives the information generated by these tests as inherently damaging or undesirable, even when it is acquired non-invasively and with high sensitivity and specificity. Clinical practice guidelines recommend the offer of prenatal testing to all women, and professional society recommendations collectively form a standard of practice to which physicians generally adhere. This practice shapes a discourse in which prenatal testing, and the information it provides, become a routinized part of the medicalized pregnancy in the US (Browner 1995; Press 1997; Rothenberg and Thompson1994).
This paradigm has recently been challenged by studies that find individuals tend to overestimate the benefit of procedures such as early and frequent screening for conditions such as certain cancers (Gigerenzer, Mata, and Frank 2009), and that such procedures may have little added utility in detecting preventable conditions while causing emotional and psychiatric stress with negative health implications (Miller 2012). Indeed, even when additional information is not harmful, evidence suggests that it is not always beneficial (Bernhardt et al., 2012; Biesecker, Schwartz, and Marteau 2013; Bloss, Schork, and Topol 2011; Case et al. 2005; Leithner et al., 2004; Tercyak et al. 2001).
Some survey respondents who did not consider termination of a pregnancy an option nevertheless considered information about the potential phenotype of the fetus valuable, because it would allow them to come to terms with a diagnosis and prepare mentally and physically. However, others saw this information as providing no benefit—or worse, as anxiety-producing, with potentially negative effects on the mother’s health.
Limitations
As described in the results, the demographic profile of the study population does not mirror the US population perfectly. In particular, income, education, and gender are distributed differently than in the national population. Minority populations, especially ethnic Hispanics and African Americans, are under-represented in this sample. On the one hand, this disparity is problematic; because higher income and education frequently predict acceptance of prenatal testing, the results of the current study may over-estimate the approval of the availability of prenatal testing (Kuppermann et al. 2006). On the other hand, given the high price point and limited availability of NIPT in the current climate, it is likely that individuals of higher socioeconomic status will be the first to encounter NIPT. As such, the study population, while not perfectly representative of the national population, may more accurately reflect the current market for new modalities in prenatal testing.
The second limitation in studies that ask participants to anticipate their health care decisions is their hypothetical nature, which may reflect discrepancies between individuals’ reported actions and real-world decision making. This is especially true of behavior that may be stigmatized in some way, such as pregnancy termination. Additionally, positive social pressures towards the value of unconditional parental love and the social acceptance of individuals with disabilities may exert subtle pressures upon participant responses. It is quite possible that individuals who report that they would decline testing because they would not consider termination and “love the child no matter what” might make very different decisions when faced with a high-risk pregnancy. By the same token, individuals who report a willingness to consider termination may find, after learning more about life with trisomy, that they wish to continue the pregnancy. Certainly, significant differences have been shown between those who report that they would not consider termination and those who ultimately elect to undergo elective termination (Henshaw 2010; Jones et al. 2008; Joyce et al. 2009).
CONCLUSIONS
It is as yet unclear how much of an impact the availability of NIPT will have on prenatal care in the US. However, uptake of available tests suggests a positive trend towards the widespread availability of NIPT (Allyse et al. 2014; Larion et al. 2014; Musci et al. 2013; Taylor, Chock, and Hudgins 2013). Additionally, recent studies have begun to validate the use of NIPT to test for an expanded range of genetic conditions, including subchromosomal abnormalities. The expansion of NIPT beyond the capabilities of current serum screening methods provides further evidence that NIPT is likely to become a part of the prenatal care process. Its increasingly common use suggests that third-party payers, practitioners, regulators, and professional societies should undertake proactive consideration of how to amend current models of prenatal standards of care to ensure the ethical clinical integration of these technologies.
The results presented here argue for the management of cost and insurance coverage to encourage more equitable access, the restructuring of the informed consent process to address the very real desire of some patients not to receive prenatal information, and the need for careful attention to demonstrating clinical and analytical utility in order to maintain patients’ trust. Importantly, clinicians should address the perception that a woman who receives positive prenatal test results should be encouraged to consider termination.
Supplementary Material
Figure 3.
Frequency of codes from open-ended response questions
Acknowledgments
FUNDING: This project was supported by NIH grant P50-HG003389 [Center for Integrating Ethics and Genetic Research]. Dr. Michie is additionally supported by NIH grant K99-HG006452. Dr. Cho is additionally supported by NIH grant 1-U54-RR024374-01A1 [Stanford Center for Clinical and Translational Education and Research]. Select findings from this manuscript were presented at the 16th International Conference on Prenatal Diagnosis and Therapy in Miami, Florida, in June 2012.
Footnotes
AUTHOR CONTRIBUTIONS: MA, LCS, and MKC designed and distributed the survey instrument as well as contributed to the analysis of the results and the preparation of the manuscript. TG contributed to analysis of results. MM contributed significantly to the revision of the manuscript.
CONFLICTS OF INTEREST: The authors report that they have no conflicts of interest to declare.
ETHICAL APPROVAL: This study was approved by the institutional review board at Stanford University.
Contributor Information
Megan Allyse, Institute for Health and Aging, University of California San Francisco.
Lauren Carter Sayres, Duke University Medical School.
Taylor Goodspeed, University of Pennsylvania Law School.
Marsha Michie, Institute for Health and Aging, University of California San Francisco.
Mildred K Cho, Stanford Center for Biomedical Ethics and Department of Pediatrics, Stanford Medical School.
REFERENCES
- American College of Obstetricians and Gynecologists (ACOG) Noninvasive prenatal testing for fetal aneuploidy. Committee Opinion No. 545. Obstetrics & Gynecology. 2012;120(6):1532–1534. doi: 10.1097/01.AOG.0000423819.85283.f4. [DOI] [PubMed] [Google Scholar]
- Agarwal A, Sayres L, Cho LMK, Cook-Deegan R, Chandrasekharan S. Commercial landscape of noninvasive prenatal testing in the United States. Prenatal Diagnosis. 2013;33(6):521–531. doi: 10.1002/pd.4101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allyse M, Sayres LC, Goodspeed TA, Cho MK. Attitudes towards non-invasive prenatal testing for aneuploidy among US adults of reproductive age. Journal of Perinatology. 2014;34(6):429–434. doi: 10.1038/jp.2014.30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asch A. Disability equality and prenatal testing: contradictory or compatible. Florida State University Law Review. 2002;30(2):315–342. [PubMed] [Google Scholar]
- Benn P, Chapman AR, Erickson K, et al. Obstetricians' and gynecologists' practice and opinions of expanded carrier testing and non-invasive prenatal testing. Prenatal Diagnosis. 2014;34(2):145–152. doi: 10.1002/pd.4272. [DOI] [PubMed] [Google Scholar]
- Bernhardt BA, Soucier D, Hanson K, Savage MS, Jackson L, Wapner RJ. Women's experiences receiving abnormal prenatal chromosomal microarray testing results. Genetics in Medicine. 2012;15:139–145. doi: 10.1038/gim.2012.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi D, Platt L, Golberg J, Abuhamad A, Sehnert A, Rava R. Whole genome maternal plasma DNA sequencing detects autosomal and sex chromosome aneuploidies. Prenatal Diagnosis. 2012a;32(Suppl. 1):3–4. [Google Scholar]
- Bianchi D, Platt L, Golberg J, Abuhamad A, Sehnert A, Rava R. Genome-wide fetal aneuploidy detection by maternal plasma DNA sequencing. Obstetrics & Gynecology. 2012b;119(5):890–891. doi: 10.1097/AOG.0b013e31824fb482. [DOI] [PubMed] [Google Scholar]
- Biesecker BB, Schwartz MD, Marteau TM. Enhancing informed choice to undergo health screening: a systematic review. American Journal of Health Behavior. 2013;37(3):351–359. doi: 10.5993/AJHB.37.3.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop AJ, Marteau TM, Armstrong D, et al. Women and health care professionals' preferences for down's syndrome screening tests: a cojoint analysis study. British Journal of Obstetrics & Gynaecology. 2004;111(8):775–779. doi: 10.1111/j.1471-0528.2004.00197.x. [DOI] [PubMed] [Google Scholar]
- Bloss CS, Schork NJ, Topol EJ. Effect of direct-to-consumer genomewide profiling to assess disease risk. New England Journal of Medicine. 2011;364(6):524–534. doi: 10.1056/NEJMoa1011893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley EH, Curry LA, Devers KJ. Qualitative data analysis for health services research: developing taxonomy, themes, and theory. Health Services Research. 2007;42(4):1758–1772. doi: 10.1111/j.1475-6773.2006.00684.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Browner CH. Lost in translation: lessons from California on the implementation of state-mandated fetal diagnosis in the context of globalization. In: Browner CH, Sargent CF, editors. Reproduction, globalization, and the state: new theoretical and ethnographic perspectives. Durham, NC: Duke University Press; 2011. pp. 204–223. [Google Scholar]
- Browner CH, Press NA. The normalization of prenatal diagnostic screening. In: Ginsburg F, Rap F, editors. Conceiving the new world order: the global politics of reproduction. Berkeley, CA: University of California Press; 1995. pp. 292–306. [Google Scholar]
- Browner CH, Preloran HM. Male partners' role in Latinas' amniocentesis decisions. Journal of Genetic Counseling. 1999;8(2):85–108. doi: 10.1023/a:1022890714866. [DOI] [PubMed] [Google Scholar]
- Bryant AS, Worjoloh A, Caughey AB, Washington AE. Racial/ethnic disparities in obstetric outcomes and care: prevalence and determinants. American Journal of Obstetrics and Gynecology. 2010;202(4):335–343. doi: 10.1016/j.ajog.2009.10.864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case DO, Andrews JE, Johnson JD, Allard SL. Avoiding versus seeking: the relationship of information seeking to avoidance, blunting, coping, dissonance, and related concepts. Journal of the Medical Library Association. 2005;93(3):353–362. [PMC free article] [PubMed] [Google Scholar]
- Caughey AB, Hopkins LM, Norton ME. Chorionic villus sampling compared with amniocentesis and the difference in the rate of pregnancy loss. Obstetrics & Gynecology. 2006;108(3 Part 1):612–616. doi: 10.1097/01.AOG.0000232512.46869.fc. [DOI] [PubMed] [Google Scholar]
- Cole-Turner R. Faith meets the human genome project: Religious factors in the public response to genetics. Public Understanding of Science. 1999;8(3):207–214. [Google Scholar]
- Constantine M, Allyse M, Rockwood T, Wall M, De Vries R. Imperfect informed consent for prenatal screening: lessons from the Quad screen. Clinical Ethics. 2014;9:17–27. [Google Scholar]
- Corbie-Smith G, Thomas SB, Williams MV, Moody-Ayers S S. Attitudes and beliefs of African Americans toward participation in medical research. Journal of General Internal Medicine. 1999;14(9):537–546. doi: 10.1046/j.1525-1497.1999.07048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuckle HS, Malone FD, Wright D, et al. Contingent screening for down syndrome--results from the FaSTER trial. Prenatal Diagnosis. 2008;28(2):89–94. doi: 10.1002/pd.1913. [DOI] [PubMed] [Google Scholar]
- Deans Z, Newson AJ. Should non-invasiveness change informed consent procedures for prenatal diagnosis? Health Care Analysis. 2011;19(2):122–132. doi: 10.1007/s10728-010-0146-8. [DOI] [PubMed] [Google Scholar]
- de Jong A, Dondorp WJ, de Die-Smulders CE, Frints SG, de Wert GM. Non-invasive prenatal testing: ethical issues explored. European Journal of Human Genetics. 2010;18(3):272–277. doi: 10.1038/ejhg.2009.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devers P, Cronister S, Ormond K, Facio F, Brasington C, Flodman P. Noninvasive prenatal testing/noninvasive prenatal diagnosis: the position of the National Society of Genetic Counselors. Journal of Genetic Counseling. 2013;22(3):291–295. doi: 10.1007/s10897-012-9564-0. [DOI] [PubMed] [Google Scholar]
- Fairbrother G, Johnson S, Musci TJ, Song K. Clinical experience of noninvasive prenatal testing with cell-free DNA for fetal trisomies 21, 18, and 13 in a general screening population. Prenatal Diagnosis. 2013;33(6):580–583. doi: 10.1002/pd.4092. [DOI] [PubMed] [Google Scholar]
- Farrell R, Nutter B, Agatisa PK. Meeting patients' education and decision-making needs for first trimester prenatal aneuploidy screening. Prenatal Diagnosis. 2011;31(13):1222–1228. doi: 10.1002/pd.2867. [DOI] [PubMed] [Google Scholar]
- Farrell RM, Agatisa PK, Nutter B. What women want: lead considerations for current and future applications of noninvasive prenatal testing in prenatal care. Birth. 2014;41(3):276–282. doi: 10.1111/birt.12113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrimond R, Kelly S. Public viewpoints on new non-invasive prenatal genetic tests. Public Understanding of Science. 2011;22(6):730–744. doi: 10.1177/0963662511424359. [DOI] [PubMed] [Google Scholar]
- Favre R, Duchange N, Vayssière C, et al. How important is consent in maternal serum screening for Down syndrome in France? Information and consent evaluation in maternal serum screening for Down syndrome: a French study. Prenatal Diagnosis. 2007;27(3):197–205. doi: 10.1002/pd.1656. [DOI] [PubMed] [Google Scholar]
- Flessel M, Goldman S S. California prenatal screening program to include noninvasive testing. 2013 Available at: http://www.acog.org/About_ACOG/ACOG_Departments/District_Newsletters/District_IX/September_2013/California_Prenatal_Screening_Program. [Google Scholar]
- Fransen MP, Wildschut HIJ, Vogel I, Mackenbach JP, Steegers EAP, Essink-Bot ML. Ethnic differences in considerations whether or not to participate in prenatal screening for Down syndrome. Prenatal Diagnosis. 2009;29(13):1262–1269. doi: 10.1002/pd.2391. [DOI] [PubMed] [Google Scholar]
- Gigerenzer G, Mata J, Frank R. Public knowledge of benefits of breast and prostate cancer screening in Europe. Journal of the National Cancer Institute. 2009;101(17):1216–1220. doi: 10.1093/jnci/djp237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfresdóttir H, Björnsdóttir K, Sandall J. How do prospective parents who decline prenatal screening account for their decision? A qualitative study. Social Science & Medicine. 2009;69(2):274–277. doi: 10.1016/j.socscimed.2009.05.004. [DOI] [PubMed] [Google Scholar]
- Greely HT. Get ready for the flood of fetal gene screening. Nature. 2011;469(7330):289–291. doi: 10.1038/469289a. [DOI] [PubMed] [Google Scholar]
- Halbert CH, Armstrong K, Gandy OH, Jr, Shaker L. Racial differences in trust in health care providers. Archives of Internal Medicine. 2006;166(8):896–901. doi: 10.1001/archinte.166.8.896. [DOI] [PubMed] [Google Scholar]
- Harris RA, Washington AE, Nease RF, Kuppermann M. Cost utility of prenatal diagnosis and the risk-based threshold. Lancet. 2004;363(9405):276–282. doi: 10.1016/S0140-6736(03)15385-8. [DOI] [PubMed] [Google Scholar]
- Heger M. As rivals vie for share of noninvasive trisomy testing market, physicians see value for screening. [accessed February 2014];Genome Web. 2012 Jul 3; Available at: http://www.genomeweb.com/sequencing/rivals-vie-share-noninvasive-trisomy-testing-market-physicians-see-value-screeni. [Google Scholar]
- Henshaw SK. Issues in contraception and abortion: the debate rages. In: Finkel M, editor. Public health in the 21st century. Santa Barbara, CA: Praeger; 2010. pp. 347–370. [Google Scholar]
- Janvier A, Farlow B, Wilfond BS. The experience of families with children with trisomy 13 and 18 in social networks. Pediatrics. 2012;130(2):293–298. doi: 10.1542/peds.2012-0151. [DOI] [PubMed] [Google Scholar]
- Jones RK, Zolna MR, Henshaw SK, Finer LB. Abortion in the United States: incidence and access to services, 2005. Perspectives on Sexual and Reproductive Health. 2008;40(1):6–16. doi: 10.1363/4000608. [DOI] [PubMed] [Google Scholar]
- Joyce TJ, Henshaw SK, Dennis A, Finer LB, Blanchard K. The impact of state mandatory counseling and waiting period laws on abortion: a literature review. New York: Guttmacher Institute; 2009. [Google Scholar]
- Jupka KA, Weaver NL, Sanders-Thompson VL, Caito NM, Kreuter MW. African American adults’ experiences with the health care system: in their own words. Journal of Health Disparities Research and Practice. 2012;2(3):17–32. [Google Scholar]
- Kalfoglou AL, Doksum B T, et al. Opinions about new reproductive genetic technologies: Hopes and fears for our genetic future. Fertility and Sterility. 2005;83(6):1612–1621. doi: 10.1016/j.fertnstert.2005.01.090. [DOI] [PubMed] [Google Scholar]
- Kaposy C. A disability critique of the new prenatal test for Down syndrome. Kennedy Institute of Ethics Journal. 2013;23(4):299–324. doi: 10.1353/ken.2013.0017. [DOI] [PubMed] [Google Scholar]
- Kelly SE. Choosing not to choose: reproductive responses of parents of children with genetic conditions or impairments. Sociology of Health and Illness. 2009;31(1):81–97. doi: 10.1111/j.1467-9566.2008.01110.x. [DOI] [PubMed] [Google Scholar]
- Kelly SE, Farrimond HR. Non-invasive prenatal genetic testing: a study of public attitudes. Public Health Genomics. 2012;15(2):73–81. doi: 10.1159/000331254. [DOI] [PubMed] [Google Scholar]
- Kuppermann M, Gates E E, Washington AE. Racial-ethnic differences in prenatal diagnostic test use and outcomes: preferences, socioeconomics, or patient knowledge? Obstetrics & Gynecology. 1996;87(5 Part 1):675–682. doi: 10.1016/0029-7844(96)00017-8. [DOI] [PubMed] [Google Scholar]
- Kuppermann M, Learman LA, Gates E, et al. Beyond race or ethnicity and socioeconomic status: predictors of prenatal testing for Down syndrome. Obstetrics & Gynecology. 2006;107(5):1087–1097. doi: 10.1097/01.AOG.0000214953.90248.db. [DOI] [PubMed] [Google Scholar]
- Larion MS, Warsof SL, Romary L, Mlynarczyk M, Peleg D, Abuhamad AZ. Uptake of non-invasive prenatal testing at a large academic referral center. American Journal of Obstetrics and Gynecology. 2014 doi: 10.1016/j.ajog.2014.06.038. doi: http://dx.doi.org/10.1016/j.ajog.2014.06.038 [Epub ahead of print]/ [DOI] [PubMed] [Google Scholar]
- Leithner K, Maar A, Fischer-Kern M, Hilger E, Löffler-Stastka H, Ponocny-Seliger, E E. Affective state of women following a prenatal diagnosis: predictors of a negative psychological outcome. Ultrasound in Obstetrics & Gynecology. 2004;23(3):240–246. doi: 10.1002/uog.978. [DOI] [PubMed] [Google Scholar]
- Lewis C, Hill M, Skirton H, Chitty LS. Non-invasive prenatal diagnosis for fetal sex determination: Benefits and disadvantages from the service users' perspective. European Journal of Human Genetics. 2012;20(11):1127–1133. doi: 10.1038/ejhg.2012.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lippman A. The genetic construction of prenatal testing: choice, consent, or conformity for women? In: Rothenberg KH, Thomson EJ, editors. Women & prenatal testing: facing the challenges of genetic technology. Columbus, OH: Ohio State University Press; 1994. pp. 9–34. [Google Scholar]
- Lippman A. Embodied knowledge and making sense of prenatal diagnosis. Journal of Genetic Counseling. 1999;8(5):255–274. doi: 10.1023/A:1022901131305. [DOI] [PubMed] [Google Scholar]
- Locock L, Alexander J. Just a bystander? Men's place in the process of fetal screening and diagnosis. Social Science & Medicine. 2006;62(6):1349–1359. doi: 10.1016/j.socscimed.2005.08.011. [DOI] [PubMed] [Google Scholar]
- Malone FD, Canick JA, Ball RH, et al. First-trimester or second-trimester screening, or both, for down's syndrome. New England Journal of Medicine. 2005;353(19):2001–2011. doi: 10.1056/NEJMoa043693. [DOI] [PubMed] [Google Scholar]
- Markens S, Browner C, Press N. 'Because of the risks': How US pregnant women account for refusing prenatal screening. Social Science & Medicine. 1999;49(3):359–369. doi: 10.1016/s0277-9536(99)00097-0. [DOI] [PubMed] [Google Scholar]
- Meier C, Huang T, Wyatt PR, Summers AM. Accuracy of expected risk of Down syndrome using the second-trimester triple test. Clinical Chemistry. 2002;48(4):653–655. [PubMed] [Google Scholar]
- Miller AB. New data on prostate-cancer mortality after PSA screening. New England Journal of Medicine. 2012;366(11):1047–1048. doi: 10.1056/NEJMe1200185. [DOI] [PubMed] [Google Scholar]
- Musci TJ, Fairbrother G, Batey A, Bruursema J, Struble C, Song K. Non-invasive prenatal testing with cell-free DNA: US physician attitudes toward implementation in clinical practice. Prenatal Diagnosis. 2013;33(5):424–428. doi: 10.1002/pd.4091. [DOI] [PubMed] [Google Scholar]
- Norton ME, Brar H, Weiss J, et al. Non-Invasive chromosomal evaluation (NICE) study: results of a multicenter prospective cohort study for detection of fetal trisomy 21 and trisomy 18. American Journal of Obstetrics and Gynecology. 2012;207(2):137.e1–137e8. doi: 10.1016/j.ajog.2012.05.021. [DOI] [PubMed] [Google Scholar]
- Norton ME, Rose NC, Benn P. Noninvasive prenatal testing for fetal aneuploidy: clinical assessment and a plea for restraint. Obstetrics & Gynecology. 2013;121(4):847–850. doi: 10.1097/AOG.0b013e31828642c6. [DOI] [PubMed] [Google Scholar]
- Palomaki GE, Deciu C, Kloza EM, et al. DNA sequencing of maternal plasma reliably identifies trisomy 18 and trisomy 13 as well as Down syndrome: an international collaborative study. Genetics in Medicine. 2012;13(3):296–305. doi: 10.1038/gim.2011.73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parens E, Asch A. Disability rights critique of prenatal genetic testing: Reflections and recommendations. Mental Retardation and Developmental Disabilities Research Reviews. 2003;9(1):40–47. doi: 10.1002/mrdd.10056. [DOI] [PubMed] [Google Scholar]
- Press N, Browner CH. Why women say yes to prenatal diagnosis. Social Science & Medicine. 1997;45(7):979–989. doi: 10.1016/s0277-9536(97)00011-7. [DOI] [PubMed] [Google Scholar]
- Ravitsky V. Non-invasive prenatal diagnosis: an ethical imperative. Nature Reviews Genetics. 2009;10(10):733. doi: 10.1038/nrg2631-c1. [DOI] [PubMed] [Google Scholar]
- Rothenberg KH, Thomson EJ. Women and prenatal testing: facing the challenges of genetic technology. Columbus, OH: Ohio State University Press; 1994. [Google Scholar]
- Sayres LC, Cho MK. Cell-Free fetal nucleic acid testing: a review of the technology and its applications. Obstetrical & Gynecological Survey. 2011;66(7):431–442. doi: 10.1097/OGX.0b013e31822dfbe2. [DOI] [PubMed] [Google Scholar]
- Sayres LC, Allyse M, Goodspeed TA, Cho MK. Demographic and Experiential Correlates of Public Attitudes Towards Cell-Free Fetal DNA Screening. Journal of Genetic Counseling. 2014 doi: 10.1007/s10897-014-9704-9. [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sayres LC, Allyse M, Norton ME, Cho MK. Cell-free fetal DNA testing: a pilot study of obstetric healthcare provider attitudes toward clinical implementation. Prenatal Diagnosis. 2011;31(11):1070–1076. doi: 10.1002/pd.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz D, Henn W W, Netzer C. Commentary: No risk, no objections? Ethical pitfalls of cell-free fetal DNA and RNA testing. British Medical Journal. 2009;339:b2690. doi: 10.1136/bmj.b2690. [DOI] [PubMed] [Google Scholar]
- Schmitz D, Netzer C, Henn W. An offer you can't refuse? Ethical implications of non-invasive prenatal diagnosis. Nature Reviews Genetics. 2009;10:515. doi: 10.1038/nrg2631. [DOI] [PubMed] [Google Scholar]
- Seror V, Ville Y Y. Prenatal screening for Down syndrome: women's involvement in decision-making and their attitudes to screening. Prenatal Diagnosis. 2009;29(2):120–128. doi: 10.1002/pd.2183. [DOI] [PubMed] [Google Scholar]
- Skotko BG, Kishnani PS, Capone GT and the Down Syndrome Diagnosis Study Group. Prenatal diagnosis of Down syndrome: how best to deliver the news. American Journal of Medical Genetics Part A. 2009;149A(11):2361–2367. doi: 10.1002/ajmg.a.33082. [DOI] [PubMed] [Google Scholar]
- Suzumori N, Ebara T, Kumagai K, et al. Non-specific psychological distress in women undergoing noninvasive prenatal testing because of advanced maternal age. Prenatal Diagnosis. 2014;34(11):1055–1060. doi: 10.1002/pd.4427. [DOI] [PubMed] [Google Scholar]
- Taylor JB, Chock VY, Hudgins L. NIPT in a clinical setting: an analysis of uptake in the first months of clinical availability. Journal of Genetic Counseling. 2013;23(1):72–78. doi: 10.1007/s10897-013-9609-z. [DOI] [PubMed] [Google Scholar]
- Tercyak KP, Johnson SB, Roberts SF, Cruz AC. Psychological response to prenatal genetic counseling and amniocentesis. Patient Education and Counseling. 2001;43(1):73–84. doi: 10.1016/s0738-3991(00)00146-4. [DOI] [PubMed] [Google Scholar]
- Tischler R, Hudgins L, Blumenfeld YJ, Greely HT, Ormond KE. Noninvasive prenatal diagnosis: pregnant women's interest and expected uptake. Prenatal Diagnosis. 2011;31(13):1292–1299. doi: 10.1002/pd.2888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ubel PA, Smith DM, Zikmund-Fisher BJ, et al. Testing whether decision aids introduce cognitive biases: results of a randomized trial. Patient Education and Counseling. 2010;80(2):158–163. doi: 10.1016/j.pec.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Heuvel A, Chitty L, Dormandy E, et al. Will the introduction of non-invasive prenatal diagnostic testing erode informed choices? An experimental study of health care professionals. Patient Education and Counseling. 2010;78(1):24–28. doi: 10.1016/j.pec.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Van Riper M, Choi H. Family-provider interactions surrounding the diagnosis of Down syndrome. Genetics in Medicine. 2011;13(8):714–716. doi: 10.1097/GIM.0b013e3182209f21. [DOI] [PubMed] [Google Scholar]
- Wald NJ, Bestwick JP. Incorporating DNA sequencing into current prenatal screening practice for Down's syndrome. PLoS One. 2013;8(3):e58732. doi: 10.1371/journal.pone.0058732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmermann B, Hill M, Gemelos G, et al. Noninvasive prenatal aneuploidy testing of chromosomes 13, 18, 21, X, and Y, using targeted sequencing of polymorphic loci. Prenatal Diagnosis. 2012;32(13):1233–1241. doi: 10.1002/pd.3993. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.