Abstract
Background
Prospective studies have found low bilirubin levels were an important predictive factor of cardiovascular events. However, few have yet investigated possible association between serum bilirubin level and LVH in essential hypertension. The aim of the present study was to evaluate the relationship between serum bilirubin levels with LVH in newly diagnosed hypertension patients.
Methods
The present study evaluated the relationship between serum total bilirubin level and left ventricle hypertrophy (LVH) in newly diagnosed hypertensive patients with a sample size of 344. We divided subjects into LVH group (n=138) and non-LVH group (n=206). Physical examination, laboratory tests and echocardiography were conducted. The multivariate logistic regression model was used to verify the independent association between RDW and LVH.
Results
Our results found that patients with LVH had lower bilirubin levels than non-LVH ones. Stepwise multiple linear regression analysis showed total bilirubin level (B=-0.017, P=0.008) was negatively associated with left ventricle mass index (LVMI) even adjusting for some confounders. The multiples logistic regression found total bilirubin level was independently related with of LVH, as a protective factors (OR=0.91, P=0.010).
Conclusion
As a routine and quick laboratory examination index, serum bilirubin may be treated as novel marker for evaluating LVH risk in hypertensive patients. Cohort study with larger sample size are needed.
Introduction
Hypertension is an important public health issue worldwide [1] Hypertension could damage various target organs [2] and thus raise the risks of coronary heart disease, heart failure, chronic kidney disease (CKD) and stroke [3–5]. Left ventricle hypertrophy (LVH) is a common subclinical organ damage induced by hypertension. The prevalence of LVH among hypertensive patients is about 20%–40% [6]. LVH has been suggested as a validated marker indicating the mortality of cardiovascular diseases (CVDs) [7]. Therefore, identifying specific risk factors of LVH in hypertensive patients is quite important for reducing the incidence of cardiovascular events. Many common risk factors of LVH have been confirmed through epidemiologic research, such as obesity, old age, high blood pressure, and smoking status [8].
Now, growing attention has been paid to some serological indices, such as serum bilirubin level. As the final product of heme catabolism, bilirubin is anti-inflammatory and antioxidant in vitro and in vivo [9]. Epidemiologic evidence has shown that the increase in serum bilirubin level, even within normal range [10], is a protective factor of CVDs. A clinical study suggests that people with lower serum bilirubin levels are more likely to suffer from hypertension, diabetes and obesity [11]. Moreover, prospective studies also show that low bilirubin level is a main predictive factor of cardiovascular events, such as stroke, heart failure and coronary artery disease [12–14]. However, the possible association between serum bilirubin level and the occurrence of LVH in essential hypertensive patients has been rarely investigated. Bilirubin can suppress the oxidation of blood lipids including low-density lipoprotein (LDL), and the application of bilirubin can improve the marker of anti-oxidative stress [15, 16]. Therefore, we assume that high serum bilirubin level may be a protective factor of LVH in hypertensive patients. On this basis, serum bilirubin level examination can be conducted as a cheap routine test and as a potential predictor of LVH in newly-diagnosed hypertensive patients. The aim of the present study is to evaluate the relation between serum bilirubin level and the occurrence of LVH in newly-diagnosed hypertensive patients.
Methods
Study population
This cross-sectional study preliminarily involved 408 consecutive hypertensive patients who had not received any treatment before and were enrolled in the outpatient clinic of the Third Affiliated Hospital at Southern Medical University between October 2013 and July 2014. All patients then underwent physical and Laboratory examinations. The inclusion criteria were as follows: no history of myocardial infarction, heart failure, cardiac valve disease, severe renal function impairment [defined by an estimated glomerular filtration rate (eGFR) <60 ml/min/1.73 m2], coronary bypass surgery or angioplasty, diabetes mellitus or renal insufficiency; no treatment with urate-lowering medication (allopurinol and probenecid); no secondary or malignant hypertension. Sixty-two patients who did not meet the above criteria were excluded. Therefore, 344 hypertensive patients were involved the final statistical analysis (S1 Dataset). The study protocol was approved by the Ethics Committee of Southern Medical University, and written informed consent was obtained from all participants.
Blood pressure measurements
Newly diagnosed hypertension was defined as systolic blood pressure (SBP) ≥ 140 mmHg and (or) diastolic blood pressure (DBP) ≥ 90 mmHg. Blood pressures were measured using a mercury sphygmomanometer. Three measurements were taken at a 10-min interval and then averaged to define the clinic SBP or DBP.
Laboratory examinations
Serum total bilirubin, direct bilirubin, and indirect bilirubin levels were measured by the vanadate oxidation method using automatic biochemical analyzer. Hematologic test was measured using an automated hematology analyzer (Bayer Diagnostics, Newbury, and Berkshire, UK). During blood routine test [red blood cell (RBC) count, white blood cell (WBC) count, platelet count, hemoglobin, mean corpuscular volume and red cell distribution width (RDW)], fasting blood glucose, and creatinine, as well as the fasting serum lipid status including levels of total cholesterol (TC), LDL-cholesterol, high-density lipoprotein (HDL)- cholesterol, triglyceride (TG), and C-reactive protein (CRP) were recorded. Height and weight were measured, and the body mass index (BMI) was calculated as weight (kg) divided by height squared (m2). The eGFR was calculated as follows [17]: 186×SCr-1.154×age in years-0.203×1.210 (if black) ×0.742(if female) 36. Then CKD was defined as eGFR <60 ml/min/1.73 m2.
Echocardiography
All echocardiographic tests were performed on each subject with a commercially available machine (Fair Medical Company Ltd, Matsudo, Japan) using a 2.5 Hz transducer. The overall single-dimensional left ventricular measurements and the two-dimensional views were obtained according to the American Society of Echocardiography standard [18, 19]. Left ventricular mass (LVM) was calculated as follows [20]: LVM = 1.04×0.8×{(VSTd × LVIDd × PWTd) 3-(LVIDd) 3} +0.6, where IVSd is diastolic interventricular septum, LVDd is diastolic left ventricular dimension, and PWTd is diastolic posterior wall thickness. The left ventricle mass index (LVMI) was computed as = LVM/BSA (Body Surface Area, BSA). LVH was defined as: LVMI ≥ 125 g/m2 for men and ≥ 110 g/m2 for women [21].
Statistical analysis
The study patients were divided into an LVH group (n = 206) and a non-LVH group (138). Data were reported as mean ± standard deviation for quantitative variables and as percentages for qualitative variables. Whether a continuous variable was in normal distribution was assessed using the Kolmogorov—Smirnov test. Differences between groups were tested by Student t-test for continuous variables and by Chi-square test for categorical variables. Correlations between LVMI and other variables were calculated by Pearson’s or Spearman’s test as appropriate. The significant determinants for LVMI were detected with stepwise multiple linear regression, and the odds ratios (ORs) of independent variables were computed with stepwise multiple logistic regression. Collinear diagnostics within variables was applied before the regression modeling. Statistical analysis was performed on SPSS 19.0 (SPSS Inc, USA) with the significance level at P<0.05.
Results
General information of the subjects
Table 1 summarizes the demographic and clinical data of the two groups. The average ages of the two groups are 47.8±7.9 and 47.2±7.6 years old, respectively (P = 0.481). The sex ratios are not significantly different between groups. Total bilirubin and indirect bilirubin levels are both significantly lower in the LVH group than in the non-LVH group. Besides, the LVH group tends to be smokers and have higher SBP, DBP, glucose level, serum creatinine, uric acid, metabolic components, and RDW. Echocardiography shows that many indicators from the LVH group are significantly higher compared the non-LVH group. Other variables are all not different between the two groups (Table 1).
Table 1. Clinical characteristics of hypertension patients with and without LVH.
| Parameters | Non-LVH group | LVH group | P |
|---|---|---|---|
| Age, y | 47.8±7.9 | 47.2±7.6 | 0.481 |
| Sex(male) | 118(57.3%) | 89(64.5%) | 0.181 |
| Smoking, yes | 27(13.1%) | 46(33.3%) | 0.000 |
| Body mass index, kg/m2 | 26.7±3.7 | 26.4±3.2 | 0.482 |
| SBP, mmHg | 150.6±10.0 | 155.4±13.3 | 0.000 |
| DBP, mmHg | 99.0±8.1 | 101.3±10.0 | 0.022 |
| Triglyceride, mmol/dL L | 1.8±0.5 | 1.9±0.5 | 0.515 |
| HDL-cholesterol, mmol/dL | 1.1±0.2 | 1.1±0.2 | 0.289 |
| LDL- cholesterol, mmol/dL | 3.2±0.8 | 3.1±0.8 | 0.926 |
| Total cholesterol, mmol/dL | 5.1±0.9 | 5.1±0.8 | 0.995 |
| Glucose, mmol/dL | 5.6±0.6 | 5.7±0.6 | 0.040 |
| eGFR,mL/min/1.73 | 109.5±29.2 | 104.4±22.9 | 0.072 |
| Serum creatine, mmol/dL | 67.3±13.2 | 71.0±13.2 | 0.010 |
| Uric acid, μmol/L | 343.5±49.9 | 370.4±46.7 | 0.000 |
| Hs-CRP, mg/dL | 2.3±5.4 | 2.5±4.1 | 0.750 |
| Alanine aminotransferase, U/L | 32.2±27.7 | 29.5±19.5 | 0.325 |
| Aspartate aminotransferase, U/L | 25.1±10.3 | 25.1±12.0 | 0.968 |
| Blood urea nitrogen, mmol/L | 4.7±1.1 | 4.9±1.2 | 0.257 |
| Metabolic components,n | 1.6±1.0 | 1.8±1.1 | 0.145 |
| Total bilibin, μmol/L | 14.0±4.5 | 11.4±4.1 | 0.000 |
| Direct bilirubin, μmol/L | 3.2±2.4 | 3.1±2.6 | 0.999 |
| Indirect bilirubin, μmol/L | 10.8±4.5 | 8.2±4.2 | 0.000 |
| White blood cell, ×1012/L | 6.2±1.9 | 6.1±1.6 | 0.760 |
| Red blood cell, ×1012/L | 4.7±0.3 | 4.8±0.2 | 0.465 |
| Red cell distribution width (%) | 12.7±0.7 | 12.9±0.9 | 0.014 |
| Hemoglobin,g/L | 149.6±27.1 | 146.5±16.9 | 0.236 |
| Blood platelet, 103/mm3 | 242.6±54.1 | 243.3±56.0 | 0.923 |
| IVST, mm | 10.0±1.1 | 12.±1.2 | 0.000 |
| LVEDd, mm | 45.2±4.4 | 49.1±4.0 | 0.000 |
| LVESd, mm | 29.6±4.3 | 32.3±3.6 | 0.000 |
| PWT, mm | 10.1±1.7 | 11.3±1.3 | 0.000 |
| Ejection fraction, % | 65.8±5.3 | 63.5±7.3 | 0.000 |
| Left ventricular mass, g | 174.5±33.7 | 240.4±44.4 | 0.000 |
| Left ventricular mass index, g/m2 | 92.3±12.7 | 128.1±18.5 | 0.000 |
Univariate analyses
In the whole study population, total bilirubin (Fig 1) and indirect bilirubin levels (Fig 2) are negatively associated with LVMI. Sex, smoking status, SBP, glucose, serum creatinine, uric acid, blood urea nitrogen, and RDW are positively associated with LVMI. The association between LVMI and any of other variables is not significant (Table 2).
Fig 1. Scatter diagram of total bilirubin level between the LVMI.
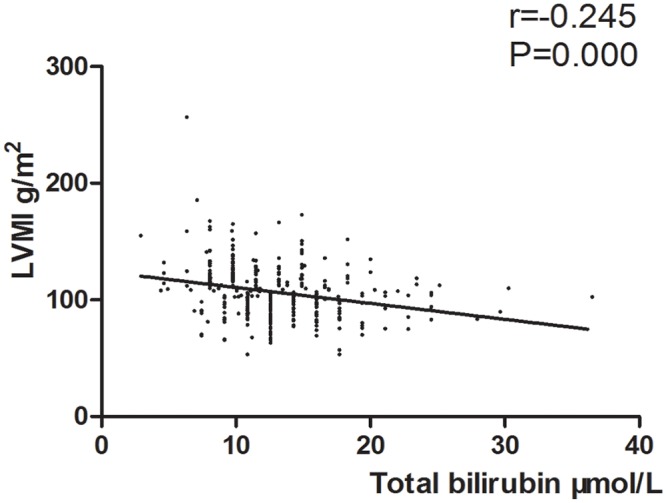
Fig 2. Scatter diagram of indirect bilirubin level between the LVMI.
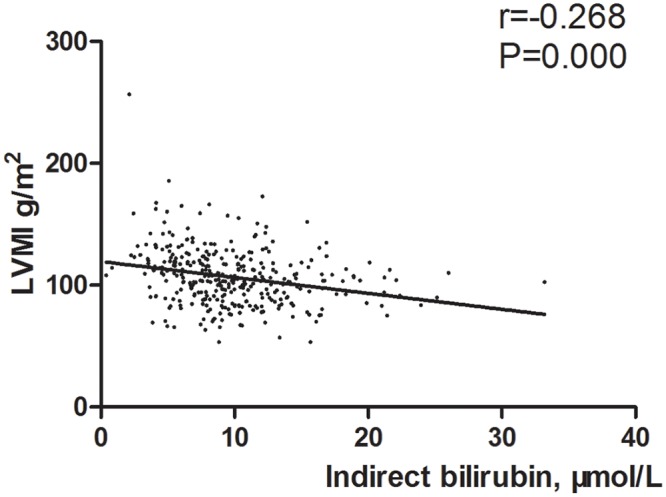
Table 2. Correlation coefficients of relation between left ventricular mass index and some parameters.
| Variables | Correlation coefficients | P |
|---|---|---|
| Age | -0.055 | 0.307 |
| Sex | 0.119* | 0.027 |
| Smoking | 0.228* | 0.000 |
| SBP, mmHg | 0.136 | 0.011 |
| DBP, mmHg | 0.089 | 0.101 |
| Triglyceride, mmol/dL L | -0.005 | 0.922 |
| HDL-cholesterol, mmol/dL | -0.057 | 0.293 |
| LDL- cholesterol, mmol/dL | 0.012 | 0.824 |
| Total cholesterol, mmol/dL | 0.004 | 0.944 |
| Glucose, mmol/dL | 0.142 | 0.008 |
| eGFR, mL/min/1.73 | -0.111 | 0.039 |
| Serum creatine, mmol/dL | 0.162 | 0.003 |
| Uric acid, μmol/L | 0.225 | 0.000 |
| Hs-CRP, mg/dL | 0.064 | 0.246 |
| Alanine aminotransferase, U/L | -0.024 | 0.656 |
| Aspartate aminotransferase, U/L | -0.022 | 0.686 |
| Blood urea nitrogen, mmol/L | 0.188 | 0.029 |
| Total bilibin, μmol/L | -0.245 | 0.000 |
| Direct bilirubin, μmol/L | -0.012 | 0.624 |
| Indirect bilirubin, μmol/L | -0.268 | 0.000 |
| White blood cell, ×1012/L | 0.032 | 0.556 |
| Red blood cell, ×1012/L | 0.060 | 0.267 |
| Red cell distribution width (%) | 0.129 | 0.017 |
| Hemoglobin,g/L | -0.053 | 0.325 |
| Blood platelet, 103/mm3 | -0.083 | 0.200 |
*Spearman correlation coefficient
Multiple linear regression analysis
To test whether the relation between total bilirubin level and LVMI was confounded by other factors, we conducted the multiple linear regression by considering a set of potential variables. The results suggest that total bilirubin (B = -0.017, P = 0.008) is negatively associated with LVMI, independent of age, sex, BMI, smoking status, creatinine, fasting glucose, eGFR, hsCRP, TG, HDL-cholesterol, TC, LDL-cholesterol, metabolic, alanine aminotranferease (ALT), aspartate aminotransferase (AST), blood urea nitrogen (BUN), WBC count, Plt count, RBC count, Hb, and Plt count. Table 3 shows the results of multiple linear regression performed to assess the independent predictors of LVMI.
Table 3. Stepwise multiple linear regression analysis for the effect of independent variables on left ventricular mass index.
| Variable | B | S.E. | t | P |
|---|---|---|---|---|
| Constant | -1.135 | 0.443 | -2.564 | 0.011 |
| Smoking | 0.265 | 0.074 | 3.578 | 0.000 |
| Serum uric acid | 0.002 | 0.001 | 3.134 | 0.002 |
| Total bilirubin | -0.017 | 0.006 | -2.695 | 0.008 |
| SBP | 0.007 | 0.003 | 2.557 | 0.011 |
Multiple logistic regression analysis
In a stepwise multiple logistic regression model, LVH was defined as the dependent variable and other factors with LVH as covariates. The results show that total bilirubin [OR = 0.91, 95%CI (confidence interval): 0.85–0.97] is an independent protective factor of LVH. Other factors including smoking status (OR = 3.14, 95% CI: 1.66–7.00), serum acid (OR = 1.01, 95%CI: 1.00–1.02), and SBP (OR = 1.03, 95%CI: 1.01–1.06) (Table 4).
Table 4. Multivariable logistic regression analysis of prediction of LVH.
| Variable | B | S.E. | Waldχ2 | Odds ratio | 95% CI | P |
|---|---|---|---|---|---|---|
| Constant | -8.009 | 2.333 | 11.784 | - | - | 0.001 |
| Smoking | 1.227 | 0.367 | 11.194 | 3.14 | 1.66–7.00 | 0.001 |
| Serum uric acid | 0.010 | 0.003 | 9.178 | 1.01 | 1.00–1.02 | 0.002 |
| Total bilirubin | -0.090 | 0.035 | 6.625 | 0.91 | 0.85–0.97 | 0.010 |
| SBP | 0.032 | 0.013 | 6.143 | 1.03 | 1.01–1.06 | 0.013 |
Discussion
The main findings of the present study are that total serum bilirubin level is negatively associated with LVMI and serves as a protective factor of LVH in hypertensive patients without receiving any drug treatment, regardless of potential confounding factors. The same protective effect of bilirubin on LVH was observed in a hypertensive rat model [22]. In a recent report involving 114 consecutive hypertensive patients, bilirubin level is positively related with LVMI and LVH [23], which is totally different from our results. Compared with their study, our findings have several strengths that need to be addressed. On one hand, the present study involves a sample size three times larger than theirs (344 vs. 114). Moreover, their logistic regression results show quite a wide CI (1.035–12.846). The small sample size is likely to induce larger sampling error, which reduces the precision of the results. On the other hand, hypertensive patients with diabetes mellitus were excluded from our study. About 10.5% of the study population had diabetes mellitus, and most of them tended to be obesity (BMI: 31.7±5.3), which may have modest effect on our results. Thus, we extended the previous findings using a larger sample size and established that reduction in bilirubin levels may precede the development of LVH in untreated hypertensive patients.
As a major product of heme metabolism in the vessels, serum bilirubin could be poisonous for infants under excessive condition [24], but a potential antioxidant material can provide profitable protection against CVDs [25]. Actually, many epidemiologic studies demonstrate that higher serum bilirubin level is a protective factor of CVDs. As reported, serum bilirubin level is independently and negatively associated with the occurrence of carotid atherosclerosis in both males and females [26]. The causal risk reduction for type 2 diabetes (T2DM) was estimated to be 42%, which was comparable to the observational estimate [27]. This result provides strong evidence that the elevated bilirubin level is significantly associated with the reduced risk of T2DM and supports its role as a protective determinant. A prospective large-scale and community-based cohort with 8593 Korean patients shows that lower bilirubin level is an risk factor of coronary artery disease [adjusted hazard ratio:1.89 [28]. Our results support the current opinion. The exact mechanism about the association between serum bilirubin level and LVH remains unclear, but there are several possible reasons. First, bilirubin is an antioxidant, which has been confirmed in vitro and in vivo [29, 30]. Human trials also suggest that bilirubin level is positively related with the total antioxidant capacity [31]. It is generally suggested that oxidative reactions are involved in the pathophysiology of CVDs [32]. Bilirubin can suppress the oxidative modification of LDL-lipoprotein, an important part of the development of atherosclerosis. Second, serum bilirubin may be associated with LVH through the close relation with regular risk factors. It is suggested that high bilirubin levels are negatively associated with the presence of obesity, smoking status, diabetes mellitus, hypertension and metabolic syndrome. Finally, the anti-inflammatory function of bilirubin can partly explain why bilirubin level is lower in the LVH group. Animal experiments prove that the intake of bilirubin can affect the expressions of cell adhesion molecules [33]. Besides, an epidemiological study also reveals the inverse relation between bilirubin and hs-CRP [34]. Nevertheless, further studies are needed.
The present study has several limitations. First and most importantly, the nature of cross-sectional design does not allow us to confirm the cause-and-effect relation. Actually, the serum bilirubin level is very valuable as a reference in clinic, no matter the lower serum bilirubin is a cause or effect of LVH. After all, the serum bilirubin level could be thought as a relative index to LVH. Second, some inflammation markers (interleukin-6 and -10) were not reported. Bilirubin without other clinical index may not provide enough information for clinicians. Third, it should be noted that our findings are based on the selected Asian population with moderate primary hypertension. Patients with CKD or cardiovascular diseases were excluded from this study. Therefore, the extrapolation to other populations will be limited. Nevertheless, this point makes our results independent from some confounders. Finally, although our results prove an inverse association different from previous research, the specific mechanism cannot be illustrated clearly. Therefore, further studies regarding the role of serum bilirubin on the development of LVH are needed.
In conclusion, total serum bilirubin level is inversely associated with the development of LVH in the untreated hypertensive patients after adjustment of some potential confounders. Thus, as a routine quick laboratory examination index, serum bilirubin level may be treated as a novel marker for evaluation of target organ damage risk (especially LVH) in hypertensive patients. Subsequent studies should focus on the exact biological mechanism.
Supporting Information
(XLS)
Acknowledgments
We thank all our colleagues working in the Department of Epidemiology and Health Statistics, School of public health of Southern Medical University.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
The authors have no support or funding to report.
References
- 1. Muntner P, Krousel-Wood M, Hyre AD, Stanley E, Cushman WC, Cutler JA, et al. (2009) Antihypertensive prescriptions for newly treated patients before and after the main antihypertensive and lipid-lowering treatment to prevent heart attack trial results and seventh report of the joint national committee on prevention, detection, evaluation, and treatment of high blood pressure guidelines. Hypertension 53: 617–623. 10.1161/HYPERTENSIONAHA.108.120154 [DOI] [PubMed] [Google Scholar]
- 2. ESH/ESC Task Force for the Management of Arterial Hypertension (2013) 2013 Practice guidelines for the management of arterial hypertension of the European Society of Hypertension (ESH) and the European Society of Cardiology (ESC): ESH/ESC Task Force for the Management of Arterial Hypertension. J Hypertens 31: 1925–1938. 10.1097/HJH.0b013e328364ca4c [DOI] [PubMed] [Google Scholar]
- 3. van den Hoogen PCW, Feskens EJM, Nagelkerke NJD, Menotti A, Kromhout D. (2000) The relation between blood pressure and mortality due to coronary heart disease among men in different parts of the world. Seven Countries Study Research Group. N. Engl. J. Med 342, 1–8. [DOI] [PubMed] [Google Scholar]
- 4. Verdecchia P, Reboldi G, Angeli F, Trimarco B, Mancia G, Pogue J, et al. (2015) Systolic and diastolic blood pressure changes in relation with myocardial infarction and stroke in patients with coronary artery disease. Hypertension 65: 108–114. 10.1161/HYPERTENSIONAHA.114.04310 [DOI] [PubMed] [Google Scholar]
- 5. Faraco G, Iadecola C (2013) Hypertension: a harbinger of stroke and dementia. Hypertension 62: 810–817. 10.1161/HYPERTENSIONAHA.113.01063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Subramaniam A, Shanmugam MK, Perumal E, Li F, Nachiyappan A, Dai X, et al. (2013) Potential role of signal transducer and activator of transcription (STAT)3 signaling pathway in inflammation, survival, proliferation and invasion of hepatocellular carcinoma. Biochim Biophys Acta 1835: 46–60. 10.1016/j.bbcan.2012.10.002 [DOI] [PubMed] [Google Scholar]
- 7. Sahіn DY, Gür M, Elbasan Z, Kalkan GY, Ozdoğru I, Kivrak A, et al. (2013)Myocardial performance index and aortic dispensability in patients with different left ventricle geometry in newly diagnosed essential hypertension. Blood Press. 22: 329–335. 10.3109/08037051.2013.778006 [DOI] [PubMed] [Google Scholar]
- 8. Levy D, Murabito JM, Anderson KM, Christiansen JC, Castelli WP (1992) Echocardiographic left ventricular hypertrophy: clinical characteristics. The Framingham Heart Study. Clin Exp Hypertens 14: 85–97. [DOI] [PubMed] [Google Scholar]
- 9. Stocker R, Yamamoto Y, McDonagh AF, Glazer AN, Ames BN (1987) Bilirubin is an antioxidant of possible physiological importance. Science. 235: 1043–1046. [DOI] [PubMed] [Google Scholar]
- 10. Vitek L (2012) The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol 3: 55 10.3389/fphar.2012.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kimm H, Yun J, Jo J, Jee S (2009) Low serum bilirubin level as an independent predictor of stroke incidence: a prospective study in Korean men and women. Stroke. 40: 3422–3427. 10.1161/STROKEAHA.109.560649 [DOI] [PubMed] [Google Scholar]
- 12. Perlstein T, Pande R, Beckman J, Creager M (2008) Serum total bilirubin level and prevalent lower-extremity peripheral arterial disease: National Health and Nutrition Examination Survey (NHANES) 1999 to 2004. Arteriosclerosis, thrombosis, and vascular biology. 28: 166–172. [DOI] [PubMed] [Google Scholar]
- 13. Hopkins PN, Wu LL, Hunt SC, James BC, Vincent GM, Williams RR. (1996) Higher Serum Bilirubin Is Associated With Decreased Risk for Early Familial Coronary Artery Disease. Arteriosclerosis, Thrombosis, and Vascular Biology. 16: 250–255. [DOI] [PubMed] [Google Scholar]
- 14. Horsfall L, Nazareth I, Petersen I (2012) Cardiovascular events as a function of serum bilirubin levels in a large, statin-treated cohort. Circulation. 126: 2556–2564. 10.1161/CIRCULATIONAHA.112.114066 [DOI] [PubMed] [Google Scholar]
- 15. Regression Modeling Strategies: With Applications to Linear Models, Logistic Regression, And Survival Analysis. New York, NY: Springer; 2001. [Google Scholar]
- 16. Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, et al. (2005) Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol 25: 155–160. [DOI] [PubMed] [Google Scholar]
- 17.(2001) IV. NKF-K/DOQI Clinical Practice Guidelines for Anemia of Chronic Kidney Disease: update 2000. Am J Kidney Dis 37: S182–S238. [DOI] [PubMed] [Google Scholar]
- 18. Lang RM, Badano LP, Mor-Avi V, Afilalo J, Armstrong A, Erannde L, et al. (2015) Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American society of echocardiography and the European association of cardiovascular imaging. J Am Soc Echocardiogr 28: 1–39. 10.1016/j.echo.2014.10.003 [DOI] [PubMed] [Google Scholar]
- 19. Porter TR, Abdelmoneim S, Belcik JT, McCulloch ML, Mulvagh SL, Olson JJ, et al. (2014) Guidelines for the cardiac sonographer in the performance of contrast echocardiography: a focused update from the American Society of Echocardiography. J Am Soc Echocardiogr 27: 797–810. 10.1016/j.echo.2014.05.011 [DOI] [PubMed] [Google Scholar]
- 20. Varis JP, Puukka PJ, Karanko HM, Jula AM (2014) Risk assessment of echocardiographic left ventricular hypertrophy with electrocardiography, body mass index and blood pressure. Blood Press 23: 39–46. 10.3109/08037051.2013.803313 [DOI] [PubMed] [Google Scholar]
- 21. Leoncini G, Viazzi F, Parodi D, Ratto E, Vettoretti S, Vaccaro V, et al. (2004) Creatinine clearance and signs of end-organ damage in primary hypertension. J Hum Hypertens 18: 511–516. [DOI] [PubMed] [Google Scholar]
- 22. Ndisang JF, Jadhav A (2009) Upregulating the heme oxygenase system suppresses left ventricular hypertrophy in adult spontaneously hypertensive rats for 3 months. J Card Fail. 15:616–28. 10.1016/j.cardfail.2009.02.003 [DOI] [PubMed] [Google Scholar]
- 23. Ayaz T, Durakoglugil ME, Kocaman SA, Durakoglugil T, Erdogan T, Sahin SB, et al. (2014) Bilirubin Level is Associated with Left Ventricular Hypertrophy Independent of Blood Pressure in Previously Untreated Hypertensive Patients. Korean Circ J 44: 336–343. 10.4070/kcj.2014.44.5.336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Tiribelli C, Ostrow JD (2005) The molecular basis of bilirubin encephalopathy and toxicity: report of an EASL Single Topic Conference. J Hepatol. 43:156–156. [DOI] [PubMed] [Google Scholar]
- 25. Mayer M (2000) Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 46:1723–1727. [PubMed] [Google Scholar]
- 26. Kawamoto R, Ninomiya D, Hasegawa Y, Kasai Y, Kusunoki T, Ohtsuka N, et al. (2014) Mildly Elevated Serum Bilirubin Levels Are Negatively Associated with Carotid Atherosclerosis among Elderly Persons. PLoS One. 9: e114281 10.1371/journal.pone.0114281 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Abbasi A, Deetman PE, Corpeleijn E, Gansevoort RT, Gans RO, Hillege HL, et al. (2014) Bilirubin as a potential causal factor in type 2 diabetes risk: a Mendelian randomization study. Diabetes. 64:1459–1469. 10.2337/db14-0228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Song YS, Koo BK, Cho NH, Moon MK (2014) Effect of low serum total bilirubin levels (</ = 0.32 mg/dl) on risk of coronary artery disease in patients with metabolic syndrome. Am J Cardiol 114: 1695–1700. 10.1016/j.amjcard.2014.08.043 [DOI] [PubMed] [Google Scholar]
- 29. Parfenova H, Leffler CW, Basuroy S, Liu J, Fedinec AL (2012) Antioxidant roles of heme oxygenase, carbon monoxide, and bilirubin in cerebral circulation during seizures. J Cereb Blood Flow Metab 32: 1024–1034. 10.1038/jcbfm.2012.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kotani K, Tsuzaki K, Sakane N (2014) The relationship between gamma-glutamyltransferase (GGT), bilirubin (Bil) and small dense low-density lipoprotein (sdLDL) in asymptomatic subjects attending a clinic for screening dyslipidaemias. Ann Acad Med Singapore 43: 216–219. [PubMed] [Google Scholar]
- 31. Vitek L, Jirsa M, Brodanova M, Kalab M, Marecek Z, Danzig V, et al. (2002) Gilbert syndrome and ischemic heart disease: a protective effect of elevated bilirubin levels. Atherosclerosis 160: 449–456. [DOI] [PubMed] [Google Scholar]
- 32. Mayer M (2000) Association of serum bilirubin concentration with risk of coronary artery disease. Clin Chem. 46:1723–1727. [PubMed] [Google Scholar]
- 33. Vitek L (2012) The role of bilirubin in diabetes, metabolic syndrome, and cardiovascular diseases. Front Pharmacol 3:55 10.3389/fphar.2012.00055 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lippi G, Targher G (2012)Further insights on the relationship between bilirubin and C-reactive protein. Clin Chem Lab Med 50:2229–2230. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(XLS)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.


