Abstract
Cardiovascular diseases, including ventricular arrhythmias are responsible for increased mortality in patients with acromegaly. Acromegaly may cause repolarization abnormalities such as QT prolongation and impairment of repolarization reserve enhancing liability to arrhythmia. The aim of this study was to determine the short-term beat-to-beat QT variability in patients with acromegaly. Thirty acromegalic patients (23 women and 7 men, mean age±SD: 55.7±10.4 years) were compared with age- and sex-matched volunteers (mean age 51.3±7.6 years). Cardiac repolarization parameters including frequency corrected QT interval, PQ and QRS intervals, duration of terminal part of T waves (Tpeak-Tend) and short-term variability of QT interval were evaluated. All acromegalic patients and controls underwent transthoracic echocardiographic examination. Autonomic function was assessed by means of five standard cardiovascular reflex tests. Comparison of the two groups revealed no significant differences in the conventional ECG parameters of repolarization (QT: 401.1±30.6 ms vs 389.3±16.5 ms, corrected QT interval: 430.1±18.6 ms vs 425.6±17.3 ms, QT dispersion: 38.2±13.2 ms vs 36.6±10.2 ms; acromegaly vs control, respectively). However, short-term beat-to-beat QT variability was significantly increased in acromegalic patients (4.23±1.03 ms vs 3.02±0.80, P<0.0001). There were significant differences between the two groups in the echocardiographic dimensions (left ventricular end diastolic diameter: 52.6±5.4 mm vs 48.0±3.9 mm, left ventricular end systolic diameter: 32.3±5.2 mm vs 29.1±4.4 mm, interventricular septum: 11.1±2.2 mm vs 8.8±0.7 mm, posterior wall of left ventricle: 10.8±1.4 mm vs 8.9±0.7 mm, P<0.05, respectively). Short-term beat-to-beat QT variability was elevated in patients with acromegaly in spite of unchanged conventional parameters of ventricular repolarization. This enhanced temporal QT variability may be an early indicator of increased liability to arrhythmia.
Introduction
Hypertension, left ventricular hypertrophy, asymmetric septal hypertrophy, cardiomyopathy, and congestive heart failure are well-known cardiovascular complications of acromegaly caused by pituitary tumors [1]. Excessive secretion of growth hormone and IGF-1 can result in major structural and functional changes in cardiac system and arrhythmias, hypertension, and valvular heart disease are present in up to 60% of patients by the time of the diagnosis of acromegaly [1]. Clinical data suggest that a specific cardiomyopathy develops in acromegaly associated with life-threatening dysrhythmias [2]. Complex morphological and functional remodeling may be partially reversed by effective control of growth hormone and IGF-1 concentrations [2]. Moreover, acromegaly can also be associated with cardiovascular diseases contributing to increased mortality among patients [1,2]. Effective control of acromegaly with pegvisomant, a GH receptor antagonist, led to a significant improvement of Framingham risk score, and reduced the likelihood for development of coronary heart diseases, too [3].
Electrocardiographic studies also indicated cardiac rhythm abnormalities in patients with acromegaly [2,4,5]. Dysrhythmias, atrioventricular conduction delay and sick sinus syndrome were reported in sudden death in acromegalic heart disease [6]. Rodrigues et al. [7] found arrhythmias in 41% of 34 patients with acromegaly, thirteen patients had frequent ventricular extrasystoles and there were long periods of asymptomatic ventricular bigeminy in one patient. Both prevalence and severity of ventricular arrhythmia were significantly higher in acromegalic patients compared to controls, and the frequency of ventricular premature complexes increased with duration of acromegaly [8]. Higher incidence of late potential positivity, QT interval prolongation and higher QT dispersion in acromegaly patients might explain the increased susceptibility to sudden cardiac deaths from ventricular tachyarrhythmias [9]. Herrmann et al. [10] detected late potentials, a predictor of ventricular dysrhythmias in a signal-averaged electrocardiogram, in 56% of patients with active acromegaly (n = 16) and 6% of well-controlled patients (n = 32) and speculated that late potentials might indicate myocardial remodeling in acromegaly. In another study, the occurrence of late potentials were 22.9% in acromegalic vs 2.9% in control patients (P<0.001; n = 70 in both groups) and a significant association with premature ventricular complexes were seen by means of 24-h Holter ECG recording [11]. Maffei et al. [11] also described that one case of sudden cardiac death occurred during the observation period, and this acromegalic patient had late potentials, left ventricular hypertrophy, Lown 4 premature ventricular complexes, and non-sustained ventricular tachycardia.
The identification of patients with risk for serious ventricular arrhythmia and sudden cardiac death could be important during the diagnosis and treatment of acromegaly. Fatti et al. [12] described that octreotide, a somatostatin analogue, could improve abnormally prolonged QT interval in acromegalic patients. Treatment with GH receptor antagonist Pegvisomant for 6-month and 18-month (long-term) also improved rhythm abnormalities in 13 patients suffering from acromegaly [13]. However, QT interval prolongation alone cannot reliably predict the development of ventricular arrhythmias including the chaotic ventricular tachycardia, Torsades de Pointes (TdP), since cardiac repolarization reserve may be reduced even without significant changes in the duration of cardiac repolarization [14]. The short-term variability of the duration of repolarization (STVQT) [15] might be a better parameter to predict serious ventricular arrhythmias and sudden cardiac death, as it has been suggested by both animal experimental work [16–19] and recent clinical studies [20–23]. On the basis of these observations, Varkevisser et al. [24] suggested that beat-to-beat STVQT could be superior to QT interval prolongation in identifying patient populations at risk for ventricular arrhythmias and might be able to accurately predict individual risk. The aim of the present study was to determine beat-to-beat QT variability in patients with acromegaly.
Methods
Patient Population
Patients with acromegaly who are followed at the 1st Department of Internal Medicine in Szeged, Hungary, were eligible for this study. Patients were excluded if they had excessive (>5%) ectopic atrial or ventricular beats, were in a rhythm other than normal sinus, had repolarization abnormalities (i.e. early repolarization pattern, T wave inversion and complete left bundle branch block or right bundle branch block), had a permanent pacemaker or any other disorders such as serious retinopathy, symptomatic cardiac and pulmonary disease, acute metabolic disease, had excessive noise on the electrocardiographic signal that precluded analysis of the ECG waveform, were on any medication likely to affect the investigated ECG parameters or consumed significant amount of food within 3 hours or drank alcohol, coffee or smoked within 10 hours.
We studied 30 acromegalic patients, 7 males and 23 females with the age of 55.7 ± 10.4 years (all values presented are mean ± SD). A total of 30 age- and sex-matched volunteers (mean age 51.3 ± 7.6 years) without a history or evidence of heart disease were enrolled in the study as controls. All of the control individuals and acromegaly patients were of Caucasian origin. Acromegalic patient group was also divided to subgroups on the basis of medical examinations and serum diagnostic tests performed (hGH rhythm, IGF-1 level, HbA1c concentration, oral glucose tolerance test). Active acromegalic subgroup included acromegalic patients before hypophysectomy or with remnant hormonally active tumor after hypophysectomy (n = 14), as well as treated acromegalic patients with high serum IGF-1 levels in spite of long-acting somatostatin analogue octreotide or lanreotide therapy received (n = 3). Inactive acromegalic subgroup included acromegalic patients after successful hypophysectomy (n = 6) and treated acromegalic patients with an age-sex-appropriate normal IGF-1 and/or random GH < 1 ng ml-1 and/or nadir GH after OGTT < 0.4 ng ml-1 during bromocriptine, pegvisomant, or long-acting somatostatin analogue octreotide treatments (n = 7). There was no significant age difference between the active and inactive patients (56.4 ± 11.5 vs 54.8 ± 9.2 years, respectively; P = 0.69). In acromegaly group, there were 18 hypertensive patients receiving therapy (for details see S1 Table) and 12 normotensive subjects, whereas volunteers in control group did not receive antihypertensive treatment.
The studies described here were carried out in accordance with the Declaration of Helsinki (2000) of the World Medical Association and were approved by the Scientific and Research Ethical Committee of the Medical Research Council at the Hungarian Ministry of Health (ETT-TUKEB), under ethical approval No. 4987-0/2010-1018EKU (338/PI/010). All subjects have given written informed consent of the study.
Data Collection and Analysis
12-lead electrocardiograms were continuously recorded for 5 min at rest, in the supine position to obtain signals with the least amount of motion artefact. In all leads the ECG signals were digitized at 2000 Hz sampling rate with a multichannel data acquisition system (Cardiosys-H1 software, Experimetria Ltd, Budapest, Hungary) connected to a personal computer and stored for later off-line analysis.
Out of the repolarization parameters we analyzed the frequency corrected QT interval (QTc) using Bazett’s (QTc = QT/√RR), Fridericia (QTc = QT/[RR/1000]1/3), Framingham (QTc = QT + [0.154 * {1000-RR}]) and the Hodges formulas (QTc = QT + 1.75 * [60 000/RR-60]), the QT dispersion (QTd), the PQ and QRS intervals, the duration of terminal part of T waves (Tpeak-Tend) and the short-term variability of QT interval (STVQT).
The RR and QT intervals and duration of the T wave from the peak to the end (Tpeak-Tend) intervals were measured automatically in 30 consecutive beats (minimum number of intervals needed for variability measurements), were checked by the same expert investigator of the team for all ECGs and manually corrected if needed and were calculated as the average of 30 beats. QTc interval duration was defined as the mean duration of all QTc intervals measured. The PQ and QRS intervals were measured as the average of 15 consecutive beats. All measurements were carried out using limb lead II and in case of excessive noise in limb lead II and lead V5.
To characterize the temporal instability of beat-to-beat repolarization, Poincaré plots of the QT intervals were constructed, where each QT value is plotted against its former value. STVQT was calculated using the following formula: STVQT = ∑|QTn+1-QTn|/(30x√2), where QT represents the duration of the QT interval. This calculation defines the STV as the mean distance of points perpendicular to the line of identity in the Poincaré plot and relies on previous mathematical analysis (25).
All acromegalic patients and controls also underwent transthoracic echocardiographic examination performed by the single observer blinded to subject data for all participants. Two-dimensional echocardiographic images were obtained by Toshiba Powervision 8000 echocardiography equipment, in a number of cross-sectional planes using standard transducer positions to determine standard morphological and functional parameters.
Autonomic function was assessed by means of five standard cardiovascular reflex tests: the heart rate (HR) responses to deep breathing and to standing up (30/15 ratio), the Valsalva maneuver, the systolic blood pressure response to standing up, and the diastolic pressure change during a sustained handgrip. A score was created to express the severity of autonomic neuropathy (AN), based on the results of the five tests (normal: 0, borderline: 1, abnormal: 2). The total score was in the interval of 0 to 10.
Fasting venous blood samples were obtained from each patient and controls for the determination of serum glucose, blood urea nitrogen, creatinine, sodium and potassium levels. GH and IGF-1 were measured by chemiluminescent immunoassay (IMMULITE 1000 Immunoassay System, Siemens. GH measurement comparator: Recombinant 98/574; detection limit: 0.01 ng ml-1; intra-assay coefficients of variation: 6.0%; interassay coefficients of variation: 6.2%. IGF-1 measurement comparator: WHO IRP 87/517; detection limit: 20.0 ng ml-1; intra-assay coefficients of variation: 5.0%; interassay coefficients of variation: 9.0%).
Statistical Analysis
All data are expressed as mean±SD. Comparisons between acromegalic patients and controls for the study variables were done using the unpaired Student’s t test for normally distributed parameters, nonparametric Mann-Whitney U test for non-normal distributions, and linear regression for revealing correlations. The statistical analyses were performed using the SPSS 16.0 software package. Statistical significance was accepted at the P<0.05 level.
Results
Clinical data of acromegalic patients and control subjects
In 30 acromegalic patients studied, body weight and mean body mass index (BMI) were significantly higher (P<0.001 for both parameters) than those in age- and sex-matched volunteers (Table 1). Mean systolic blood pressure did not differ significantly between control subjects and acromegalic patients receiving standard care and treatment, however, acromegalic patients had higher diastolic blood pressure (P<0.05). The incidence of high blood pressure was 7/30 in control and 13/30 in acromegaly groups during the actual measurements. Average serum glucose and HbA1c values were also similar in both groups; incidences of diabetes were 0/30 and 1/30 in control and acromegaly groups, respectively. Incidence of impaired glucose tolerance was 0/30 in control and 4/30 in acromegalic subjects. Significant differences were seen in serum hGH (P = 0.0028) and IGF-1 (P = 0.0013) levels between acromegalic and control groups. There was no significant difference in nadir value of hGH during oral glucose tolerance test (OGTT) between active (3.40 ± 2.10 ng/ml) and inactive (1.80 ± 1.86 ng/ml) acromegalic subgroups. However, significantly higher average hGH (7.00 ± 6.73 ng/ml vs 2.03 ± 2.86 ng/ml, P = 0.0180) and IGF-1 (501.3 ± 359.6 ng/ml vs 198.5 ± 79.1 ng/ml, P = 0.0060) concentrations were measured in active acromegalic subgroup compared to inactive one.
Table 1. Clinical data of acromegalic patients and age-matched control subjects.
| Control | Acromegaly | |
|---|---|---|
| Age (years) | 51.3 ± 7.6 | 55.7 ± 10.4 |
| Weight (kg) | 68.9 ± 14.7 | 87.7 ± 19.3** |
| Height (cm) | 165.1 ± 10.5 | 168.9 ± 8.2 |
| BMI (kg m -2 ) | 25.1 ± 3.7 | 30.6 ± 5.3** |
| Systolic BP (mmHg) | 126.9 ± 13.4 | 133.2 ± 17.7 |
| Diastolic BP (mmHg) | 75.5 ± 8.5 | 82.7 ± 12.4* |
| 0 min glucose (mmol l -1 ) | 5.04 ± 0.52 | 5.40 ± 0.71 |
| 120 min glucose (mmol l -1 ) | 5.30 ± 1.30 | 6.30 ± 2.53 |
| HbA1c (%) | 5.70 ± 0.50 | 5.90 ± 0.74 |
| hGH nadir following OGTT (ng ml -1 ) | 1.02 ± 1.42 | 2.72 ± 2.13* |
| IGF-1 (ng ml -1 ) | 151.0 ± 51.4 | 370.1 ± 311.8* |
| IGF-1 x ULN | 0.50 ± 0.33 x ULN | 1.66 ± 1.59 x ULN** |
Abbreviations: BMI: body mass index; BP: blood pressure; HbA1c: glycosylated hemoglobin; hGH: human growth hormone; IGF-1: insulin-like growth factor-1; OGTT: oral glucose tolerance test; ULN: upper limit of normal value; n = 30 in each group,
*P<0.05,
**P<0.001 vs controls.
Echocardiography measurements in study subjects
There were significant differences between the two groups in the echocardiographic dimensions. Patients with acromegaly exhibited significantly higher values in left ventricular end diastolic and end systolic diameter and in interventricular septum, left ventricular posterior wall thickness compared to age-matched controls (Table 2). These results were not unexpected and were supportive of the presence of myocardial hypertrophy of in the acromegalic patients and could be related to the duration and activity of the disease. However, no significant difference was detected in the echocardiographic parameters measured between active and inactive acromegaly subgroups (EF: 66.7 ± 7.4% vs 67.9 ± 6.4%, EDD: 52.6 ± 4.9 mm vs 52.7 ± 6.1 mm, ESD: 32.1 ± 5.9 mm vs 32.5 ±4.3 mm, IVS: 10.6 ± 1.3 mm vs 11.8 ± 3.0 mm, PW: 10.5 ± 1.3 mm vs 11.2 ± 1.6 mm, respectively).
Table 2. Echocardiographic parameters in patients with acromegaly and age-matched controls.
| Control | Acromegaly | |
|---|---|---|
| EF (%) | 70.6 ± 5.4 | 67.2 ± 6.9* |
| EDD (mm) | 48.0 ± 3.9 | 52.6 ± 5.4* |
| ESD (mm) | 29.1 ± 4.4 | 32.3 ± 5.2* |
| IVS (mm) | 8.8 ± 0.7 | 11.1 ± 2.2** |
| PW (mm) | 8.9 ± 0.7 | 10.8 ± 1.4** |
Abbreviations: EF: ejection fraction; EDD: left ventricular end diastolic diameter; ESD: left ventricular end systolic diameter; IVS: interventricular septum; PW: posterior wall of left ventricle; n = 30 in each group
*P<0.05
**P<0.0001 vs controls
Electrocardiographic parameters in study subjects
Comparison of the two groups (acromegalic patients vs control) revealed no significant differences in heart rate, the PQ, QRS and QT intervals and the QT dispersion. In order to reliably assess the duration of ventricular repolarization and to minimize the influence of changing heart rate on the QT interval, frequency correction of the QT interval (QTc) was performed by the Bazett, Fridericia, Framingham and Hodges formulas. QTc values calculated with all the four formulas showed no significant differences between acromegalic patients and controls. However, the Tpeak-Tend interval was significantly increased in acromegalic patients compared to controls (Table 3). Electrocardiographic parameters tended to be shorter in active acromegaly subgroup compared to the data measured in inactive subgroup (RR: 859.8 ± 134.5 ms vs 901.0 ± 178.2 ms, not significant (NS), QT: 392.7 ± 28.5 ms vs 412.0 ±29.5 ms, NS; QTc Bazett: 425.0 ± 16.0 ms vs 436.8 ±20.3 ms, NS; QTc Friderica: 413.7 ± 15.6 ms vs 428.1 ±16.6 ms, P = 0.0220; QTc Framingham: 414.3 ± 14.9 ms vs 427.3 ±17.8 ms, P = 0.0376; QTc Hodges: 412.5 ± 15.4 ms vs 426.9 ±16.7 ms, P = 0.0209; Tpeak—Tend: 86.0 ± 15.7 ms vs 84.7 ±11.0 ms, NS, respectively).
Table 3. ECG parameters in patients with acromegaly and age-matched controls.
| Control | Acromegaly | |
|---|---|---|
| RR (ms) | 840.0 ± 75.0 | 877.6 ± 153.4 |
| PQ (ms) | 158.2 ± 17.7 | 158.0 ± 17.3 |
| QRS (ms) | 92.2 ± 6.5 | 95.3 ± 8.4 |
| QT (ms) | 389.3 ± 16.5 | 401.1 ± 30.0 |
| QTc (ms) Bazett | 425.6 ± 17.3 | 430.1 ± 18.6 |
| QTc (ms) Fridericia | 413.1 ± 14.5 | 419.9 ± 17.4 |
| QTc (ms) Framingham | 414.0 ± 13.7 | 419.9 ± 17.2 |
| QTc (ms) Hodges | 410.4 ± 13.8 | 418.7 ± 17.3* |
| QTd (ms) | 36.6 ± 10.2 | 38.2 ± 13.2 |
| T peak- T end (ms) | 80.0 ± 10.3 | 85.5 ± 13.6 |
| STV QT (ms) | 3.02 ± 0.80 | 4.23 ± 0.10** |
Abbreviations: QTc: frequency corrected QT interval; QTd: QT dispersion; STVQT: short-term variability of QT interval; n = 30 in each group,
*P<0.05
**P<0.001 vs controls.
Short-term beat-to-beat variability of the QT intervals
To characterize the instability of cardiac ventricular repolarization, the short-term beat-to-beat variability of the QT interval was calculated in acromegalic patients and age-matched controls. As individual representative examples (Poincaré plots, Fig 1) and grouped average data show STVQT was significantly increased by 36% in acromegalic patients compared to controls (4.23 ± 0.10 ms vs 3.12 ± 0.80, P<0.0001) (Fig 2). STVQT values did not differ significantly between active (4.16 ± 0.89 ms) and inactive (4.33 ± 1.22 ms) acromegalic patient subgroups. There was no difference between acromegalic subjects treated with antihypertensive drugs (4.33 ± 0.95 ms, n = 18) and normotensive acromegalic patients (4.10 ± 1.16 ms, n = 12). We could not find any significant correlation between the STVQT values and the left ventricular hypertrophy parameters in acromegaly patients or in the sub-groups of active and inactive patients (data not shown).
Fig 1. Representative Poincaré plots of a control individual and a patient with acromegaly.
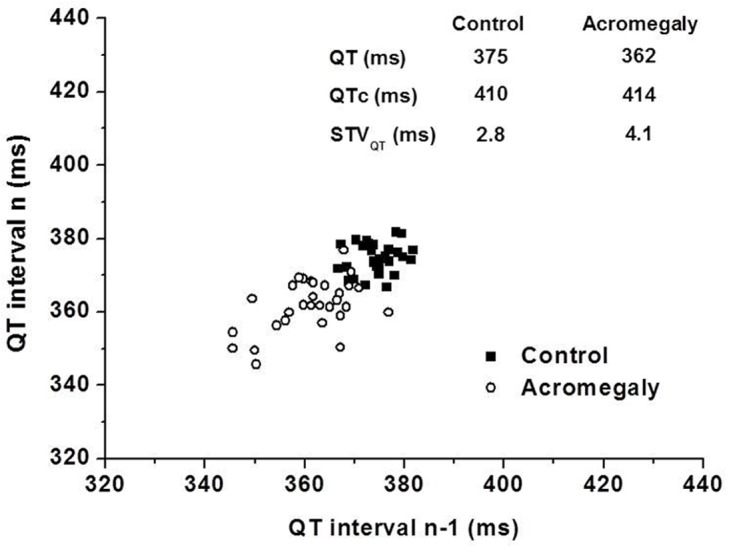
Note the larger area covered by data points obtained in the acromegalic patient illustrating increased short-term variability of the QT interval. Abbreviations: QTc, corrected QT interval by Bazett formula; STVQT, short-term variability of the QT interval.
Fig 2. Short-term beat-to-beat temporal variability of the QT interval (STVQT) in acromegalic and matched control patients.
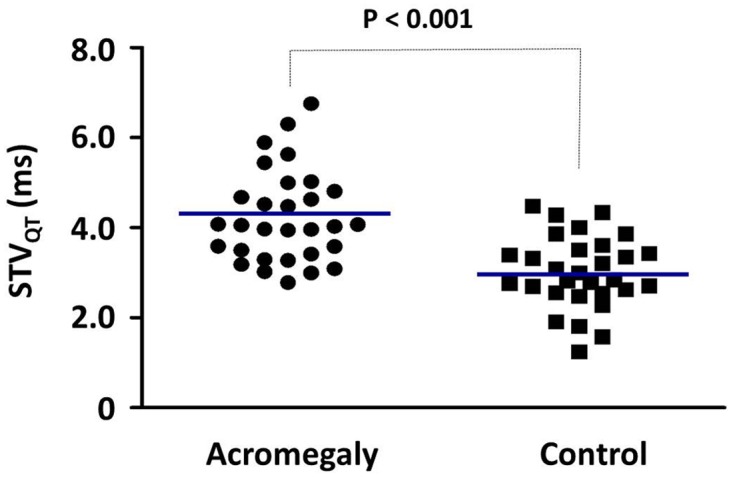
Individual values measured in n = 30 patients in each group are presented, the blue lines indicate mean values, P<0.001.
Autonomic function
Standard cardiovascular reflex tests indicated significant deteriorations in Valsalva ratio (P = 0.0015), 30/15 ratio (P = 0.0143), and AN score (P = 0.0023) in patients with acromegaly, however, no significant differences in systolic blood pressure response after standing up, and diastolic blood pressure response after sustained handgrip were detected between the two groups (Table 4). AN score was significantly lower in active acromegaly subgroup, than in inactive group (2.1 ± 1.7 vs 3.9 ± 2.2; P = 0.0260), whereas other autonomic functions measured did not differ significantly in our two acromegalic subgroups (heart rate variation during deep breathing: 15.5 ± 6.5 min-1 vs 11.9 ± 7.95 min-1; Valsalva ratio: 1.50 ± 0.26 vs 1.40 ± 0.29; 30/15 ratio: 1.10 ± 0.13 vs 1.10 ± 0.29; Systolic blood pressure fall after standing up: 8.6 ± 11.8 mmHg vs 9.8 ± 6.2 mmHg; Diastolic blood pressure increase after handgrip: 19.1 ± 8.2 mmHg vs 15.4 ± 8.2 mmHg; respectively). There was no significant difference between the values of autonomic parameters measured in acromegalic subjects treated with antihypertensive drugs and normotensive patients with acromegaly.
Table 4. Autonomic neuropathy parameters of acromegalic patients and age-matched control subjects.
| Control | Acromegaly | |
|---|---|---|
| Heart rate variation during deep breathing (1/min) | 17.20 ± 6.44 | 14.00 ± 7.22 |
| Valsalva ratio | 1.70 ± 0.34 | 1.40 ± 0.28* |
| 30/15 ratio | 1.20 ± 0.18 | 1.10 ± 0.21* |
| Systolic BP fall after standing up (mm Hg) | 6.40 ± 6.77 | 9.10 ± 9.66 |
| Diastolic BP increase after sustained handgrip (mm Hg) | 20.00 ± 9.22 | 17.50 ± 8.2 |
| AN score | 1.50 ± 1.28 | 2.90 ± 2.11* |
Abbreviations: AN, autonomic neuropathy; BP, blood-pressure; 30/15 ratio, immediate heart rate response to standing; n = 30 in each group,
*P<0.05
**P<0.001 vs controls.
Correlation of serum hGH and IGF-1 x ULN levels with cardiovascular data and autonomic neuropathy parameters
Pearson coefficient values indicated that neither hGH nor IGF-1 x ULN hormone level correlated with STVQT or any other ECG parameters measured (Table 5). However, serum hGH concentration negatively correlated with diastolic blood pressure (P = 0.0326), thickness of posterior wall of left ventricle (P = 0.0333), and AN score (P = 0.0131), whereas IGF-1 x ULN levels positively correlated with Valsalva ratio (P = 0.0087).
Table 5. Correlation of serum average hGH and IGF-1 x ULN level of acromegalic patients with cardiovascular data and autonomic neuropathy parameters.
| Serum average hGH level (ng ml-1) | Serum IGF-1 x ULN level | |||
|---|---|---|---|---|
| Pearson r | P value (two-tailed) | Pearson r | P value (two-tailed) | |
| Systolic BP (mmHg) | - 0.2294 | 0.2313 | 0.3206 | 0.0900 |
| Diastolic BP (mmHg) | - 0.3978 | 0.0326 * | 0.1256 | 0.5161 |
| EF (%) | 0.3170 | 0.0878 | -0.1735 | 0.3593 |
| EDD (mm) | - 0.2911 | 0.1186 | -0.1680 | 0.3749 |
| ESD (mm) | - 0.3507 | 0.0574 | -0.1076 | 0.5716 |
| IVS (mm) | - 0.2714 | 0.1469 | 0.0388 | 0.8387 |
| PW (mm) | - 0.3897 | 0.0333 * | 0.1048 | 0.5815 |
| RR (ms) | - 0.1204 | 0.5262 | - 0.1045 | 0.5826 |
| PQ (ms) | - 0.1968 | 0.2974 | - 0.0284 | 0.8817 |
| QRS (ms) | - 0.0127 | 0.9468 | - 0.3023 | 0.1044 |
| QT (ms) | - 0.2032 | 0.2815 | - 0.2360 | 0.2092 |
| QTc (ms) Bazett | - 0.1090 | 0.5663 | - 0.2084 | 0.2690 |
| QTc (ms) Fridericia | - 0.1992 | 0.2914 | - 0.2919 | 0.1175 |
| QTc (ms) Framingham | - 0.1834 | 0.3320 | - 0.2690 | 0.1506 |
| QTc (ms) Hodges | - 0.2154 | 0.2530 | - 0.2975 | 0.1103 |
| QTd (ms) | 0.1562 | 0.4099 | 0.1758 | 0.3526 |
| T peak- T end (ms) | - 0.0917 | 0.6298 | - 0.0788 | 0.6791 |
| STV QT (ms) | - 0.3401 | 0.0659 | - 0.0924 | 0.6272 |
| Heart rate variation during deep breathing (1/min) | 0.2300 | 0.2390 | - 0.1267 | 0.5206 |
| Valsalva ratio | 0.1340 | 0.4967 | 0.4864 | 0.0087 * |
| 30/15 ratio | 0.2386 | 0.2307 | 0.0167 | 0.9342 |
| Systolic BP fall after standing up (mm Hg) | - 0.3617 | 0.0586 | - 0.2206 | 0.2593 |
| Diastolic BP increase after sustained handgrip (mm Hg) | 0.2421 | 0.2146 | - 0.0762 | 0.6998 |
| AN score | - 0.4714 | 0.0131 * | - 0.2077 | 0.2987 |
Abbreviations: 30/15 ratio, immediate heart rate response to standing; AN, autonomic neuropathy; BP: blood pressure; EDD: left ventricular end diastolic diameter; EF: ejection fraction; ESD: left ventricular end systolic diameter; hGH: human growth hormone; IGF-1: insulin-like growth factor-1; ULN: upper limit of normal value; IVS: interventricular septum; PW: posterior wall of left ventricle; QTc: frequency corrected QT interval; QTd: QT dispersion; STVQT: short-term variability of QT interval; n = 30 for each number of XY pairs,
*P<0.05 for correlation.
Discussion
Although a connection between acromegaly and increased cardiovascular morbidity and mortality has been established previously, this study is the first to demonstrate increased beat-to-beat short-term variability of the QT interval in acromegalic patients. There was no significant difference between STVQT values measured in clinically and biochemically active acromegalic patients and those in inactive patients, which may suggest that elevated STVQT is related to the presence of acromegaly and not to the efficacy of the treatments applied. STVQT is a novel ECG parameter that, according to experimental [16–18] and clinical [20–23] data, more reliably predicts the development of serious ventricular arrhythmia compared to conventional ECG parameters of repolarization. STVQT values may be used to help predict individual risk for arrhythmia and sudden cardiac death in patients with acromegaly, however, the efficacy of this approach could only be confirmed during prospective clinical studies.
Cardiac rhythm abnormalities have been demonstrated by electrocardiogram and Holter studies in acromegaly [4,5]. Resting electrocardiological changes included left axis deviation, increased QT intervals, septal Q-waves, ST-T wave depression, and late potentials in acromegalic patients [7,10]. Atrial and ventricular ectopic beats, paroxysmal atrial fibrillation, paroxysmal supraventricular tachycardia, sick sinus syndrome, bundle branch block, and ventricular tachycardia were seen during physical exercise [4,5]. The severity of ventricular arrhythmias correlated with increases in left ventricular mass and the frequency of ventricular premature complexes increased with the duration of acromegaly [8]. Fatti et al. [12] detected abnormally long QTc interval before treatment in one-quarter of 30 acromegalic patients in a retrospective study. Octreotide, a somatostatin analogue, was shown to reduce QT intervals [12], and reduce the number of ventricular premature complexes in acromegalic patients [26].
Acromegalic cardiomyopathy is frequently present at diagnosis and the majority of patients with acromegaly meet echocardiographic criteria for left ventricular hypertrophy [5]. A possible reason is that acromegalic patients are sometimes diagnosed only after longer duration (7–10 years) of the disease. No significant difference in left ventricle hypertrophy was observed between active and inactive acromegaly patients in our study, which may indicate that adequate treatment of acromegaly could not turn back the process. Cardiac performance of acromegalic patients during physical exercise depends on left ventricular diastolic function under resting condition [27]. Ciulla et al. [28] found elevated myocardial echoreflectivity and increased QTd in acromegalic patients and explained these changes by long-term, blood pressure-independent cardiac hypertrophy and prolonged exposure to high serum concentrations of hGH and IGF-1. Baykan et al. [29] analyzed echocardiographic parameters by tissue and two-dimensional Doppler imaging in acromegalic patients and found that GH level positively correlated with interventricular septum thickness. Our observations regarding these parameters were unexpectedly different. Myocardial hypertrophy in relevant animal models has been shown to result in electrophysiological remodeling where the expression of potassium channels critical for repolarization and repolarization reserve (such as IKs), is significantly reduced, creating an arrhythmia substrate of increased spatial heterogeneity and temporal instability of repolarization and leading to increased arrhythmia susceptibility in the heart [14,30–32]. Patients with acromegaly may also develop congestive heart failure, the ratio was less than 3% (10 of 330 consecutive patients) in a study performed in 2 centers [33]. Recent studies indicated that IKs, IKr, IK1, and Ito potassium channels were down-regulated [34–36] and the persistent or slowly-inactivating sodium current was also increased in chronic heart failure [37]. Additionally, acromegalic patients could also develop coronary heart disease and most patients have systemic complications affecting the Framingham risk score [38]. GH receptor antagonist therapy improved the score and reduced the risk for coronary heart diseases [3]. In acromegalic patients, increased stiffness of ascending aorta was described [39] and ambulatory arterial stiffness indexes might have an important role in predicting cardiovascular risk [40]. Several mechanisms have been implicated in the development of ventricular arrhythmias in the settings of myocardial ischemia and myocardial infarction [41]. The surviving ventricular myocytes in the border zone next to the infarcted area play a particularly important role in the development of arrhythmias [42,43]. In these cells, a consistent downregulation of different potassium channels has been found, including Ito [42], IK1 [44], IKr and IKs [45]. The QT variability index, among the first ECG parameters used to characterize temporal variability of repolarization, has been shown to more reliably predict myocardial ischemia and myocardial infarction associated serious ventricular arrhythmia development compared to more conventional ECG parameters [15,46,47]. It should be noted that myocardial fibrosis occurring in acromegaly [48] can also contribute to the underlying arrhythmia substrate in the heart due to disturbances in conduction.
Animal studies support the cardiovascular findings of clinical observations on acromegalic patients. Overexpression of bovine GH gene increased cardiac mass, induced hypertrophy of left ventricle, and deteriorated cardiac systolic function in adult female transgenic mice [49]. The long-term exposure to high serum GH concentration also resulted in impaired high-energy phosphate metabolism and mitochondrial ultrastructural changes in the heart muscle of mice [49]. Bovine GH transgenic mice also developed a salt-resistant form of hypertension and structural narrowing of the resistance vasculature [50].
Our observations indicate deterioration in autonomic function assessed by standard cardiovascular reflex tests in acromegalic patients. AN score was significantly worse in inactive acromegalic patients and there was no apparent difference between acromegalic subgroups in other autonomic parameters measured, which may suggest that these neuropathy parameters are long-term consequences of acromegaly and cannot be reverted by the control of the disease. Among the tests primarily reflecting parasympathetic functions, the Valsalva ratio and 30/15 ratio were significantly decreased in acromegaly, whereas heart rate variation during deep breathing was not changed significantly. The tests demonstrating sympathetic activity, such as systolic blood pressure fall after standing up and diastolic blood pressure increase after sustained handgrip, did not change significantly in acromegalic patients. These reflex tests indicate a moderate parasympathetic dysfunction in our study, which could represent a predisposition to proarrhythmic activity in acromegalic patients. Increased risk of sudden cardiac death and ventricular arrhythmia has been associated with decreased parasympathetic and increased sympathetic activity [51]. Parasympathetic activation has been considered as antiarrhythmic regarding the development of ventricular fibrillation in pathological settings; for a recent review see [52]. There are conflicting data published about the cardiac autonomic functions in patients with acromegaly [53–57]. Dural et al. [53] provided evidence of sympathovagal imbalance due to sympathetic hypertone in acromegalic patients. Acromegaly was significantly associated with cardiac autonomic dysfunction independent from the presence of hypertension or diabetes mellitus [53]. Comunello et al. [54] analyzed 24 h frequency domain heart rate variability and found a correlation between reduced sympathovagal balance and pathological conditions, such as diabetes or hypertension in acromegalic patients. Chemla et al. [55] found that 10±6 months successful treatment of acromegaly could increase parasympathetic modulation and decrease sympathetic modulation of the night time heart variability and this effect was unrelated to changes in sleep apnea status. In contrast to our observations, sympathovagal imbalance due to increased vagal tone was demonstrated as a new risk factor for arrhythmias and syncope in acromegalic patients with left ventricle hypertrophy, although with normal heart rate, normal QT interval, and normal ejection function [56]. High frequency bands in orthostatism, but not in clinostatism, were higher in acromegalic patients than in normal subjects [56]. However, Seravalle et al. [57] have recently detected significantly decreased adrenergic tone through direct recording of muscle sympathetic nerve activity in newly diagnosed acromegalic patients with insulin resistance, but without cardiac hypertrophy.
Determination of beat-to-beat STVQT is an intensively investigated new and non-invasive method for assessment of proarrhythmic risk [14,24]. QT interval measurements provide physiological information regarding the duration of cardiac repolarization. However, simple QT interval measurements are not always reliable in arrhythmic risk prediction. Ventricular repolarization is governed by a fine balance of inward and outward ionic currents. Under normal conditions impairment of one type of outward potassium channels is not likely to cause excessive QT prolongation, since other types of potassium channels provide sufficient repolarizing capacity. This was termed as repolarisation reserve [58,59]. Temporal STVQT proved to be a more sensitive predictor of Torsades de Pointes ventricular tachycardia development than conventional QT parameters, such as QT and rate corrected QT intervals or the spatial QT interval dispersion, in case of experimentally impaired repolarization reserve [17,18,60]. There are numerous examples for the association between different pathophysiological conditions and attenuated repolarization reserve caused by electrophysiological remodelling. In addition, in experimental studies ventricular hypertrophy and chronic heart failure (CHF) were associated with decreased repolarisation reserve and/or high incidence of proarrhythmic events [17,61–63]. The significance and sensitivity of STVQT as a predictor for electrical remodelling and proarrhythmia has recently been confirmed in clinical conditions in connection with CHF [22]. Increased STVQT in the context of moderate CHF may reflect a latent repolarization disorder and increased susceptibility to sudden death in patients with dilated cardiomyopathy, which is not identified by a prolonged QT interval. In this study, increased STVQT was the strongest indicator with an odds ratio of 1.52 (95% confidence interval 1.20 to 2.07, P = 0.007) for a history of documented ventricular tachycardia [22].
Varkevisser et al. [24] has recently reviewed the studies in which beat-to-beat STVQT was a better indicator than QT interval prolongation for identification of healthy subjects or patients at risk for ventricular arrhythmias. In this regard, we have recently demonstrated that professional soccer players with hypertrophied hearts had increased STVQT both in resting conditions and after exercise [64]. Significantly increased baseline STVQT was able to identify patients with diminished repolarization reserve exhibiting drug-induced Torsades de Pointes [20] and those with inherited long QT syndrome [21]. Increase in STVQT and prolongation of QT interval were observed in patients receiving cardiotoxic doxorubicin therapy [65]. A prospective clinical trial, the EUTrigTreat clinical study, was completed to investigate arrhythmogenic risk factors, including beat-to-beat variability of repolarization in sudden cardiac death risk stratification in patients with implantable cardioverter defibrillator [66]. Similar, prospective trials may elucidate the benefit of the use of STVQT in other patient populations including acromegaly.
Increased temporal instability of cardiac repolarization characterized by elevated STVQT in pathological situations, including acromegaly, can refer to impairment of repolarization reserve and increased propensity for arrhythmias [14]. In this setting, even relatively weak inhibition of potassium channels by seemingly harmless medications and/or dietary constituents may lead to sudden and unexpected excessive QT prolongation and development of Torsades de Pointes ventricular tachycardia [14].
Limitations of the study: It is important to note that in the present study the duration of acromegaly from the diagnosis can be defined (10–30 years), however the exact onset of the disease is not determinable and furthermore the duration since the remission in the inactive acromegalics is also not known. Therefore the real exposure time of increased hGH level before the diagnosis and effective treatment of the disease is not known and our active and inactive patients groups can be heterogenous in this regard. Moreover, the actual hormone levels used for correlation calculations with echocardiography and other cardiovascular parameters do not necessarily correspond to the duration of the disease. Because of our unexpected negative correlation between the GH level and the posterior wall thickness, further echocardiographical studies are warranted to examine the relationship between GH and IGF-1 levels and echocardiographic parameters in a larger series of acromegalic patients. A prospective study on newly diagnosed acromegaly patients could answer the question whether effective treatment would have any time-related effects on the changes in STVQT variability and autonomic cardiovascular functions.
In conclusion, STVQT is increased in patients with acromegaly while more conventional parameters of ventricular repolarization were unchanged. STVQT values did not differ between active and inactive acromegalic patients and did not correlate with actual serum concentrations of hGH and IGF-1. These observations may suggest that elevated short-term beat-to-beat variability is a consequence of the disease and not related directly to current treatment or condition of the patient. The elevated STVQT suggests instability of ventricular repolarization and may be an early indicator of increased liability to arrhythmia in patients with acromegaly. Further prospective clinical studies are needed to identify individual risk for ventricular arrhythmias in acromegalic patients.
Supporting Information
Abbreviations: BMI: body mass index; hGH: human growth hormone; IGF-1: insulin-like growth factor-1; OGTT: oral glucose tolerance test; ULN: upper limit of normal value.
(DOC)
Data Availability
All relevant data are within the paper.
Funding Statement
This work was supported by grants from the Hungarian Research Fund [OTKA NK-104331, CNK-77855], the National Development Agency and co-financed by the European Social Fund [TÁMOP-4.2.2A-11/1/KONV-2012-0073, TÁMOP-4.2.2./B-10/1-2010-0012; GOP-1.1.1-11-2011-00812-0035], the National Research, Development and Innovation Office [PIAC_13-1-2013-0201], Hungarian National Office for Research and Technology (TECH_08_A1_CARDIO 08), the Hungarian Academy of Sciences (János Bolyai Research Scholarship to AN, IB, CsL), and by the HU-RO Cross-Border Cooperation Programmes (HURO/0901/137-HU-RO_TRANSMED). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Melmed S. Agromegaly. N Engl J Med. 2006;355: 2558–2573. 10.1056/NEJMra062453 [DOI] [PubMed] [Google Scholar]
- 2. Clayton RN. Cardiovascular function in acromegaly. Endocr Rev. 2003;24: 272–277. 10.1210/er.2003-0009 [DOI] [PubMed] [Google Scholar]
- 3. Berg C, Petersenn S, Lahner H, Herrmann BL, Buchfelder M, Droste M, et al. Cardiovascular risk factors in patients with uncontrolled and long-term acromegaly: Comparison with matched data from the general population and the effect of disease control. J Clin Endocrinol Metab. 2010;95: 3648–3656. 10.1210/jc.2009-2570 [DOI] [PubMed] [Google Scholar]
- 4. Colao A, Vitale G, Pivonello R, Ciccarelli A, Di Somma C, Lombardi G. The heart: an end-organ of GH action. Eur J Endocrinol. 2004;151: S93–S101. 10.1530/eje.0.151S093 [DOI] [PubMed] [Google Scholar]
- 5. Rhee SS, Pearce EN. The endocrine system and the heart: a review. Rev Esp Cardiol. 2009;64: 220–231. 10.1016/j.rec.2010.10.016 [DOI] [PubMed] [Google Scholar]
- 6. Rossi L, Thiene G, Caregaro L, Giordano R, Lauro S. Dysrhythmias and sudden death in acromegalic heart disease. A clinicopathologic study. Chest. 1977;72: 495–498. 10.1378/chest.72.4.495 [DOI] [PubMed] [Google Scholar]
- 7. Rodrigues EA, Caruana MP, Lahiri A, Nabarro JDN, Jacobs HS, Raftery EB. Subclinical cardiac dysfunction in acromegaly: evidence for a specific disease of heart muscle. Br Heart J. 1989;62: 185–194. 10.1136/hrt.62.3.185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kahaly G, Olshausen KV, Mohr-Kahaly S, Erbel R, Boor S, Beyer J, et al. Arrhythmia profile in acromegaly. Eur Heart J. 1992;13: 51–56. [DOI] [PubMed] [Google Scholar]
- 9. Mohamed AL, Yusoff K, Muttalif AR, Khalid BA. Markers of ventricular tachyarrythmias in patients with acromegaly. Med J Malaysia. 1999;54: 338–345. [PubMed] [Google Scholar]
- 10. Herrmann BL, Bruch C, Saller B, Ferdin S, Dagres N, Ose C, et al. Occurrence of ventricular late potentials in patients with acromegaly. Clin Endocrinol (Oxf.). 2001;55: 201–207. 10.1046/j.1365-2265.2001.01319.x [DOI] [PubMed] [Google Scholar]
- 11. Maffei P, Martini C, Milanesi A, Corfini A, Mioni R, de Carlo E, et al. Late potentials and ventricular arrhythmias in acromegaly. Int J Cardiol. 2005;104: 197–203. 10.1016/j.ijcard.2004.12.010 [DOI] [PubMed] [Google Scholar]
- 12. Fatti LM, Scacchi M, Lavezzi E, Pecori Giraldi F, De Martin M, Toja P, et al. Effects of treatment with somatostatin analogues on QT interval duration in acromegalic patients. Clin Endocrinol (Oxf.). 2006;65: 626–630. 10.1111/j.1365-2265.2006.0639.x [DOI] [PubMed] [Google Scholar]
- 13. Auriemma RS, Pivonello R, De Martino MC, Cudemo G, Grasso LFS, Galdiero M, et al. Treatment with GH receptor antagonist in acromegaly: effect on cardiac arrhythmias. Eur J Endocrinol. 2013;168: 15–22. 10.1530/EJE-12-0596 [DOI] [PubMed] [Google Scholar]
- 14. Varró A, Baczkó I. Cardiac ventricular repolarization reserve: a principle for understanding drug-related proarrhythmic risk. Br J Pharmacol. 2011;164: 14–36. 10.1111/j.1476-5381.2011.01367.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Berger RD, Kasper EK, Baughman KL, Marban E, Calkins H, Tomaselli GF. Beat-to-beat QT interval variability: novel evidence for repolarization lability in ischemic and nonischemic dilated cardiomyopathy. Circulation. 1997;96: 1557–1565. 10.1161/01.CIR.96.5.1557 [DOI] [PubMed] [Google Scholar]
- 16. Thomsen MB, Verduyn SC, Stengl M, Beekman JD, de Pater G, van Opstal J, et al. Increased short-term variability of repolarization predicts d-sotalol-induced torsades de pointes in dogs. Circulation. 2004;110: 2453–2459. 10.1161/01.CIR.0000145162.64183.C8 [DOI] [PubMed] [Google Scholar]
- 17. Thomsen MB, Truin M, van Opstal JM, Beekman JD, Volders PG, Stengl M, et al. Sudden cardiac death in dogs with remodeled hearts is associated with larger beat-to-beat variability of repolarization. Basic Res Cardiol. 2005;100: 279–287 10.1007/s00395-005-0519-6 [DOI] [PubMed] [Google Scholar]
- 18. Cs Lengyel, Varró A, Tábori K, Papp JGy, Baczkó I. Combined pharmacological block of IKr and IKs increases short-term QT interval variability and provokes torsades de pointes. Br J Pharmacol. 2007;151: 941–951. 10.1038/sj.bjp.0707297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanton G, Yvon A, Racaud A. Temporal variability of QT interval and changes in T wave morphology in dogs as markers of the clinical risk of drug-induced proarrhythmia. J Pharmacol Toxicol Meth. 2008;57: 194–201. 10.1016/j.vascn.2008.03.001 [DOI] [PubMed] [Google Scholar]
- 20. Hinterseer M, Thomsen MB, Beckmann BM, Pfeufer A, Schimpf R, Wichmann HE, et al. Beat-to-beat variability of QT intervals is increased in patients with drug-induced long-QT syndrome: a case control pilot study. Eur Heart J. 2008;29: 185–190. 10.1093/eurheart/ehm586 [DOI] [PubMed] [Google Scholar]
- 21. Hinterseer M, Beckmann BM, Thomsen MB, Pfeufer A, Dalla Pozza R, Loeff M, et al. Relation of increased short-term variability of QT interval to congenital long-QT syndrome. Am J Cardiol. 2009;103: 1244–1248. 10.1016/j.amjcard.2009.01.011 [DOI] [PubMed] [Google Scholar]
- 22. Hinterseer M, Beckmann BM, Thomsen MB, Pfeufer A, Ulbrich M, Sinner MF, et al. Usefulness of short-term variability of QT intervals as a predictor for electrical remodeling and proarrhythmia in patients with nonischemic heart failure. Am J Cardiol. 2010;106: 216–220. 10.1016/j.amjcard.2010.02.033 [DOI] [PubMed] [Google Scholar]
- 23. Oosterhof P, Tereshchenko LG, van der Heyden MAG, Ghanem RN, Fetics BJ, Berger RD, et al. Short-term variability of repolarization predicts ventricular tachycardia and sudden cardiac death in patients with structural heart disease: A comparison with QT variability index. Heart Rhythm. 2011;8: 1584–1590. 10.1016/j.hrthm.2011.04.033 [DOI] [PubMed] [Google Scholar]
- 24. Varkevisser R, Wijers SC, van der Heyden MAG, Beekman JDM, Meine M, Vos MA. Beat-to-beat variability of repolarization as a new biomarker for proarrhythmia in vivo. Heart Rhythm. 2012;9: 1718–1726. 10.1016/j.hrthm.2012.05.016 [DOI] [PubMed] [Google Scholar]
- 25. Brennan M, Palaniswami M, Kamen P. Do existing measures of Poincaré plot geometry reflect nonlinear features of heart rate variability? IEEE Trans Biomed Eng. 2001;48: 1342–1347. 10.1109/10.959330 [DOI] [PubMed] [Google Scholar]
- 26. Suyama K, Uchida D, Tanaka T, Saito J, Noguchi Y, Nakamura S, et al. Octreotide improved ventricular arrhythmia in an acromegalic patient. Endocr J. 2000;47 Suppl: S73–S75. doi: 10.1507endocrj.47.SupplMarch_S73 [DOI] [PubMed] [Google Scholar]
- 27. Spinelli L, Petretta M, Verderame G, Carbone G, Venetucci AA, Petretta A, et al. Left ventricular diastolic function and cardiac performance during exercise in patients with acromegaly. J Clin Endocrinol Metab. 2003;88: 4105–4109. 10.1210/jc.2003-030462 [DOI] [PubMed] [Google Scholar]
- 28. Ciulla M, Arosio M, Barelli MV, Paliotti R, Poretti S, Valentini P, et al. Blood pressure-independent cardiac hypertrophy in acromegalic patients. J Hypertens. 1999;17: 1965–1969. 10.1097/00004872199917121-00028 [DOI] [PubMed] [Google Scholar]
- 29. Baykan M, Erem C, Gedikli O, Hacihasanoğlu A, Erdoğan T, Koçak M, et al. Assessment of the Tei index by tissue Doppler imaging in patients with acromegaly: serum growth hormone level is associated with the Tei index. Echocardiography. 2008;25: 374–380. 10.1111/j.1540-8175.2007.00615 [DOI] [PubMed] [Google Scholar]
- 30. Vos MA, de Groot SHM, Verduyn SC, van der Zande J, Leunissen HDM, Cleutjens JP, et al. Enhanced susceptibility for acquired torsade de pointes arrhythmias in the dog with chronic, complete AV block is related to cardiac hypertrophy and electrical remodelling. Circulation. 1998;98: 1125–1135. 10.1161/01.CIR.98.11.1125 [DOI] [PubMed] [Google Scholar]
- 31. Volders PGA, Sipido KR, Vos MA, Spätjens RLHMG, Leunissen JDM, Carmeliet E, et al. Downregulation of delayed rectifier K+ currents in dogs with chronic complete atrioventricular block and acquired torsades de pointes. Circulation. 1999;100: 2455–2461. 10.1161/01.CIR.100.24.2455 [DOI] [PubMed] [Google Scholar]
- 32. Varró A, Baczkó I. Possible mechanisms of sudden cardiac death in top athletes: a basic cardiac electrophysiological point of view. Pflügers Arch: Eur J Physiol. 2010;460: 31–41. 10.1007/s00424-010-0798-0 [DOI] [PubMed] [Google Scholar]
- 33. Bihan H, Espinosa C, Valdes-Socin H, Salenave S, Young J, Levasseur S, et al. Long-term outcome of patients with acromegaly and congestive heart failure. J Clin Endocrinol Metab. 2004;89: 5308–5313. 10.1210/jc.2004-0821 [DOI] [PubMed] [Google Scholar]
- 34. Kääb S, Dixon J, Duc J, Ashen D, Näbauer M, Beuckelmann DJ, et al. Molecular basis of transient outward potassium current downregulation in human heart failure: a decrease in Kv4.3 mRNA correlates with a reduction in current density. Circulation. 1998;98: 1383–1393. 10.1161/01.CIR.98.14.1383 [DOI] [PubMed] [Google Scholar]
- 35. Näbauer M, Kääb S. Potassium channel down-regulation in heart failure. Cardiovasc Res. 1999;37: 324–334. 10.1016/S0008-6363(97)00274-5 [DOI] [PubMed] [Google Scholar]
- 36. Li GR, Lau CP, Ducharme A, Tardif JC, Nattel S. Transmural action potential and ionic current remodeling in ventricles of failing canine hearts. Am J Physiol Heart Circ Physiol. 2002;283: H1031–H1041. 10.1152/ajpheart.00105.2002 [DOI] [PubMed] [Google Scholar]
- 37. Valdivia CR, Chu WW, Pu J, Foell JD, Haworth RA, Wolff MR, et al. Increased late sodium current in myocytes from a canine heart failure model and from failing human heart. J Mol Cell Cardiol. Ha2005;38: 475–483. 10.1016/j.yjmcc.2004.12.012 [DOI] [PubMed] [Google Scholar]
- 38. Bogazzi F, Battolla L, Spinelli C, Rossi G, Gavioli S, Di Bello V, et al. Risk factors for development of coronary heart disease in patients with acromegaly: a five year prospective study. J Clin Endocrinol Metab. 2007;92: 4271–4277. 10.1210/jc.2007-1213 [DOI] [PubMed] [Google Scholar]
- 39. Nemes A, Gavallér H, Csajbók É, Julesz J, Forster T, Csanády M. Aortic stiffness is increased in acromegaly—A transthoracic echocardographic study. Int J Cardiol. 2008;124: 121–123. 10.1016/j.ijcard.2006.11.196 [DOI] [PubMed] [Google Scholar]
- 40. Dassie F, Grillo A, Carretta R, Fabris B, Macaluso L, Bardelli M, et al. Ambulatory arterial stiffness indexes in acromegaly. Eur J Endocrinol. 2012;166: 199–205. 10.1530/EJE-11-0835 [DOI] [PubMed] [Google Scholar]
- 41. Janse MJ, Wit AL. Electrophysiological mechanisms of ventricular arrhythmias resulting from myocardial ischemia and infarction. Physiol Rev. 1989;69: 1049–1069. [DOI] [PubMed] [Google Scholar]
- 42. Lue WM, Boyden PA. Abnormal electrical properties of myocytes from chronically infarcted canine heart. Alterations in Vmax and the transient outward current. Circulation. 1992;85: 1175–1188. 10.1161/01.CIR.85.3.1175 [DOI] [PubMed] [Google Scholar]
- 43. Cabo C, Boyden PA. Electrical remodeling of the epicardial border zone in the canine infarcted heart: a computational analysis. Am J Physiol Heart Circ Physiol. 2003;284: H372–H384. 10.1152/ajpheart.00512.2002 [DOI] [PubMed] [Google Scholar]
- 44. Pinto JM, Boyden PA. Reduced inward rectifying and increased E-4031-sensitive K+ current density in arrhythmogenic subendocardial Purkinje myocytes from the infarcted heart. J Cardiovasc Electrophysiol. 1998;9: 299–311. 10.1111/j.1540-8167.1998.tb00915.x [DOI] [PubMed] [Google Scholar]
- 45. Jiang M, Cabo C, Yao J, Boyden PA, Tseng G. Delayed rectifier K currents have reduced amplitudes and altered kinetics in myocytes from infarcted canine ventricle. Cardiovasc Res. 2000;48: 34–43. 10.1016/S0008-6363(00)00159-0 [DOI] [PubMed] [Google Scholar]
- 46. Murabayashi T, Fetics B, Kass D, Nevo E, Gramatikov B, Berger RD. Beat-to-beat QT interval variability associated with acute myocardial ischemia. J Electrocardiol. 2002;35: 19–25. 10.1054/jelc.2002.30250 [DOI] [PubMed] [Google Scholar]
- 47. Haigney MC, Zareba W, Gentlesk PJ, Goldstein RE, Illovsky M, McNitt S, et al. QT interval variability and spontaneous ventricular tachycardia or fibrillation in the Multicenter Automatic Defibrillator Implantation Trial (MADIT) II patients. J Am Coll Cardiol. 2004;44: 1481–1487. 10.1016/j.jacc.2004.06.063 [DOI] [PubMed] [Google Scholar]
- 48. Lie JT, Grossman SJ. Pathology of the heart in acromegaly: anatomic findings in 27 autopsied patients. Am Heart J. 1980;100: 41–52. 10.1016/0002-8703(80)90277-X [DOI] [PubMed] [Google Scholar]
- 49. Bollano E, Omerovic E, Bohlooly-Y M, Kujacic V, Madhi B, Törnell J, et al. Impairment of cardiac function and bioenergetics in adult transgenic mice overexpressing the bovine growth hormone gene. Endocrinology. 2000;141: 2229–2235. 10.1210/endo.141.6.7486 [DOI] [PubMed] [Google Scholar]
- 50. Bohlooly-Y M, Carlson L, Olsson B, Gustafsson H, Andersson IJL, Törnell J, et al. Vascular function and blood pressure in GH transgenic mice. Endocrinology. 2001;142: 3317–3323. 10.1210/endo.142.8.8296 [DOI] [PubMed] [Google Scholar]
- 51. Lahiri MK, Kannankeril PJ, Goldberger JJ. Assessment of autonomic function in cardiovascular disease: physiological basis and prognostic implications. J Am Coll Cardiol. 2008;51: 1725–1733. 10.1016/j.jacc.2008.01.038 [DOI] [PubMed] [Google Scholar]
- 52. Shen MJ, Zipes DP. Role of the autonomic nervous system in modulating cardiac arrhythmias. Circ Res. 2014;114: 1004–1021. 10.1161/CIRCRESAHA.113.302549 [DOI] [PubMed] [Google Scholar]
- 53. Dural M, Kabakcı C, Çınar N, Erbaş T, Canpolat U, Gürses KM, et al. Assessment of cardiac autonomic functions by heart rate recovery, heart rate variability and QT dynamicity parameters in patients with acromegaly. Pituitary. 2014;17: 163–170. 10.1007/s11102-013-0482-4 [DOI] [PubMed] [Google Scholar]
- 54. Comunello A, Dassie F, Martini C, De Carlo E, Mioni R, Battocchio M, et al. Heart rate variability is reduced in acromegaly patients and improved by treatment with somatostatin analogues. Pituitary. 2014. September 27; In Press. 10.1007/s11102-014-0605-6 [DOI] [PubMed] [Google Scholar]
- 55. Chemla D, Attal P, Maione L, Veyer A-S, Mroue G, Baud D, et al. Impact of successful treatment of acromegaly on overnight heart rate variability and sleep apnea. J Clin Endocrinol Metab. 2014;99: 2925–2931. 10.1210/jc.2013-4288 [DOI] [PubMed] [Google Scholar]
- 56. Resmini E, Casu M, Patrone V, Murialdo G, Bianchi F, Giusti M, et al. Sympathovagal imbalance in acromegalic patients. J Clin Endocrinol Metab. 2006;91: 115–120. 10.1210/jc.2005-1506 [DOI] [PubMed] [Google Scholar]
- 57. Seravalle G, Carzaniga C, Attanasio R, Grassi G, Lonati L, Facchini C, et al. Decreased adrenergic tone in acromegaly: evidence from direct recording of muscle sympathetic nerve activity. Clin Endocrinol (Oxf.). 2012;77: 262–267. 10.1111/j.1365-2265.2012.04335.x [DOI] [PubMed] [Google Scholar]
- 58. Roden DM. Taking the “idio” out of “idiosyncratic”—predicting torsades de pointes. Pacing Clin Electrophysiol. 1998;21: 1029–1034. 10.1111/j.1540-8159.1998.tb00148.x [DOI] [PubMed] [Google Scholar]
- 59. Varró A, Baláti B, Iost N, Takács J, Virág L, Lathrop DA, et al. The role of the delayed rectifier component Iks in dog ventricular muscle and Purkinje fibre repolarization. J Physiol (London). 2000;523: 67–81. 10.1111/j.1469-7793.2000.00067.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Thomsen MB. Double pharmacological challenge on repolarization opens new avenues for drug safety research. Br J Pharmacol. 2007;151: 909–911. 10.1038/sj.bjp.0707299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Volders PG, Sipido KR, Vos MA, Kulcsár A, Verduyn SC, Wellens HJ. Cellular basis of biventricular hypertrophy and arrhythmogenesis in dogs with chronic complete atrioventricular block and acquired torsade de pointes. Circulation. 1998;98: 1136–1147. 10.1161/01.CIR.98.11.1136 [DOI] [PubMed] [Google Scholar]
- 62. Zicha S, Xiao L, Stafford S, Cha TJ, Han W, Varró A, et al. Transmural expression of transient outward potassium current subunits in normal and failing canine and human hearts. J Physiol (London). 2004;561: 735–748. 10.1113/jphysiol.2004.075861 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Stengl M, Ramakers C, Donker DW, Nabar A, Rybin AV, Spätjens RL, et al. Temporal patterns of electrical remodeling in canine ventricular hypertrophy: Focus on IKs downregulation and blunted β-adrenergic activation. Cardiovasc Res. 2006;72: 90–100. 10.1016/j.cardiores.2006.07.015 [DOI] [PubMed] [Google Scholar]
- 64. Cs Lengyel, Orosz A, Hegyi P, Komka Z, Udvardy A, Bosnyák E, et al. Increased short-term variability of the QT interval in professional soccer players: possible implications for arrhythmia prediction. PLoS ONE. 2011;6: e18751 10.1371/journal.pone.0018751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Ritsema van Eck HJ, Broeyer FJ, van Herpen G, Burggraaf J, Kors JA. Short-term QT variability: a marker for reduced repolarization reserve in anthracyclin therapy. Computers in Cardiology. 2009;36: 585–588. [Google Scholar]
- 66. Seegers J, Vos MA, Flevari P, Willems R, Sohns C, Vollmann D, et al. Rationale, objectives, and design of the EUTrigTreat clinical study: a prospective observational study for arrhythmia risk stratification and assessment of interrelationships among repolarization markers and genotype. Europace. 2012;14: 416–422. 10.1093/europace/eur352 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Abbreviations: BMI: body mass index; hGH: human growth hormone; IGF-1: insulin-like growth factor-1; OGTT: oral glucose tolerance test; ULN: upper limit of normal value.
(DOC)
Data Availability Statement
All relevant data are within the paper.


