Abstract
Background & Aims
The extent of HBV infection to infants of HBV/HIV-coinfected pregnant women in sub-Saharan Africa is unknown. The aim of this study was to assess prevalence of HBV infection among antiretroviral-naïve, HIV-infected pregnant women in Malawi and examine HBV transmission to their infants.
Methods
Plasma from 2048 HIV-infected, Malawian women and their infants were tested for markers of HBV infection. Study participants were provided standard-of-care health services, which included administration of pentavalent vaccine to infants at 6, 10, and 14 weeks of age.
Results
One-hundred and three women (5%) were HBsAg-positive; 70 of these HBsAg-positive women were also HBV-DNA-positive. Sixteen women (0.8%) were HBV-DNA-positive but HBsAg-negative. Five of 51 infants (9.8%) born to HBsAg-positive and/or HBV-DNA-positive women were HBV-DNA-positive by 48 weeks of age. HBV DNA concentrations of two infants of mothers who received extended lamivudine-containing anti-HIV prophylaxis were <4 log10 IU/ml compared to ≥8 log10 IU/ml in three infants of mothers who did not.
Conclusions
HBV DNA was detected in nearly 10% of infants born to HBV/HIV-coinfected women. Antenatal testing for HIV and HBV, if instituted, can facilitate implementation of prophylactic measures against infant infection by both viruses. Published by Elsevier B.V. on behalf of the European Association for the Study of the Liver.
Keywords: Hepatitis, HIV, Mother-to-child transmission, Sub-Saharan Africa, Antiviral therapy
Introduction
Hepatitis B virus (HBV) and human immunodeficiency virus type 1 (HIV) are among the leading causes of infectious disease deaths worldwide [1]. These viruses are highly endemic in sub-Saharan Africa, where infection with both viruses is frequent [2]. Pregnant women who are co-infected with HIV and HBV tend to be highly viremic for HBV [3] and may be at heightened risk of HBV transmission to their infants [4,5]. HBV infection among children in sub-Saharan Africa, in contrast to East Asia [6], has tended to be acquired during later infancy and early childhood, rather than perinatally [7,8]. HBV can be found in many body fluids including, blood, breast milk, saliva, sweat, tears, and urine [9,10]. However, several epidemiologic studies outside of Africa have shown no increased HBV transmission in breastfeeding infants compared to non-breastfeeding infants who receive appropriate anti-HBV prophylaxis [9,11,12].
Before vaccination against hepatitis B was introduced, seropositivity for hepatitis B surface antigen (HBsAg) could be detected in up to one-third of African children younger than 5 years old [7,13–15]. Acquiring hepatitis B in early childhood carries a high risk of progressing to chronic hepatitis B [16], which may progress to chronic liver disease, including hepatocellular carcinoma, in later life [17]. This risk may be even higher among those HIV-coinfected [18]. Although almost all African countries have implemented childhood vaccination against hepatitis B [19], the degree to which vaccination protects against HBV infection in infants born to HIV/HBV-coinfected women is not known.
The Breastfeeding, Antiretrovirals, and Nutrition (BAN) study, conducted in Lilongwe, Malawi, [20,21] provided an opportunity to determine the extent of HBV infection among HIV-infected pregnant women and to examine the risk of HBV transmission to their infants.
Materials and methods
Study population and design
The BAN study screened 3572 antiretroviral-naive, HIV-infected pregnant women attending 3 antenatal clinics in Lilongwe and enrolled 2369 women who met antenatal and postnatal eligibility criteria between March 2004 and February 2009 (www.thebanstudy.org; www.ClinicalTrials.gov number NCT00164736) [20–22]. This study was a randomized, controlled, clinical trial to investigate antiretroviral prevention of mother-to-child transmission of HIV-1 during breastfeeding. Eligibility for BAN was determined using antenatal criteria (women ≥14 years of age, ≤30 weeks gestation, and with no serious complications of pregnancy, no prior antiretroviral use, CD4+ count ≥200 cells/μl, hemoglobin ≥7 g/dl and plasma alanine aminotransferase (ALT) level ≤2.5 times the upper limit of normal) and postnatal criteria (infants of birth weight ≥2000 g, no infant or maternal condition precluding study interventions, and women having accepted perinatal antiretroviral regimen and received treatment assignment within 36 h of delivery). All mothers in labor and their newborn infants received a single dose of oral nevirapine and 7 days of prophylaxis as Combivir (zidovudine, 300 mg, plus lamivudine, 150 mg) twice-daily from the onset of labor for the mothers and zidovudine (2 mg per kilogram of body weight) and lamivudine (4 mg per kilogram) twice-daily for the infants. Mother-infant pairs were randomly assigned to 3 antiretroviral intervention arms: (1) no further antiretroviral prophylaxis; (2) 28 weeks of infant nevirapine; and (3) 28 weeks of maternal antiretrovirals. Babies randomized to the infant nevirapine arm received a daily dose of nevirapine that increased according to age, ranging from 10 mg daily in the first 2 weeks to 30 mg daily for weeks 19 through 28. Mothers on the maternal antiretroviral arm received Combivir (twice daily) and either nevirapine (200 mg) once-daily for 14 days and twice-daily through week 28 (n = 39), nelfinavir (1250 mg) twice-daily through week 28 (n = 146), or Kaletra (lopinavir, 400 mg, plus ritonavir, 100 mg) twice-daily through week 28 (n = 664). These changes in the maternal antiretroviral regimen were made for reasons of safety, availability, and potency [21]. Antiretroviral adherence reports were taken at five follow-up visits, and mothers reported taking all their antiretroviral doses a mean of 89% of the time and giving all infant antiretroviral doses 94% of the time. All mother-infant pairs were escorted after their 6-week study visit to the onsite Under 5 Clinic for initiation of the Quinvaxem (Novartis Vaccines & Diagnostics) pentavalent vaccine series against diphtheria, tetanus, pertussis, hepatitis B recombinant and Haemophilus influenza type b (Hib) per standard of care. At subsequent BAN study visits, mothers were reminded to return to the Under 5 Clinic for their infants’ immunizations scheduled at 10 and 14 weeks of age. Hepatitis B immunoglobulin was not available in Malawi during the study period. Infants who tested HIV positive during the first two weeks postpartum or during BAN study follow-up were disenrolled and referred for care. The BAN study and the current viral hepatitis substudy were approved by the Malawi National Health Science Research Committee and institutional review boards at the University of North Carolina at Chapel Hill and the U.S. Centers for Disease Control and Prevention (CDC). All women provided written, informed consent for specimen storage and laboratory studies.
Laboratory methods
In March 2008, the current study began analyzing stored plasma specimens collected from women in their second and third trimester of pregnancy at pre-randomization eligibility screening through November 2007. Throughout the study, plasma specimens were shipped from −80 °C storage in Lilongwe to a repository at CDC in Atlanta. Available maternal plasma specimens (n = 2048) were transferred from the repository to CDC’s Division of Viral Hepatitis. Specimens were tested first for total antibody to hepatitis B core antigen (anti-HBc), and those anti-HBc-positive were assayed for HBsAg using the Vitros Chemiluminescence Immunoassay (Ortho Clinical Diagnostics, Rochester, NY). Specimens found HBsAg-positive were tested for hepatitis B e antigen (HBeAg) using the ETI-EBK PLUS assay (Diasorin Inc., Stillwater, MN). Specimens found HBsAg-positive underwent HBV DNA quantification using the COBAS TaqMan HBV Test (Roche Molecular, Indianapolis, IN) calibrated for quantifying 200 μl volumes by using the WHO International Standard for HBV DNA NAT assays, code 97/746. Maternal anti-HBc-positive specimens that were HBsAg-negative were tested for HBV DNA with an in-house assay based on reverse-transcription polymerase chain reaction [23]. Using serial dilutions of the WHO standard, code 97/746, it was determined that the lower limit of detection for the modified COBAS assay and the in-house assay was 1.5 log10 IU/ml of HBV DNA. Testing for hepatitis B infection in infants was limited to infants of mothers who were HBsAg-positive or HBV-DNA-positive at the screening visit and enrolled in BAN at delivery. Also, the analysis was limited to HIV-uninfected infants because the BAN study disenrolled infants who were HIV-infected in utero (as indicated by a positive HIV RNA test by 2 weeks of life) or who became HIV-infected during follow-up and therefore, specimen availability on HIV-infected infants is minimal. All available plasma samples taken at 2 weeks (n = 57) and/or 48 weeks (n = 51) from 72 HIV-uninfected infants born to HBsAg-positive or HBsAg-negative/HBV-DNA-positive mothers were assayed for HBV DNA using the COBAS TaqMan HBV Test calibrated for quantifying 200 μl volumes. For infants who were HBV-DNA-positive at 48 weeks and either HBV-DNA-negative or not tested at 2 weeks, samples from 12 and 24 weeks were also tested for HBV DNA. One infant (Infant E, see below) with a low HBV concentration first detected at 48 weeks was retested for HBV DNA on a separate aliquot to confirm that it was not falsely positive. Samples with sufficient volumes from infants found HBV-DNA-positive at 48 weeks, along with those from their mothers, were processed for nucleotide sequencing of the S gene of HBV to determine genotype and identify single-nucleotide polymorphisms associated with vaccine escape [24,25].
Statistical analysis
HBV prevalence estimates among the mothers and infants were derived with 95% confidence intervals based on the binomial exact method. Wilcoxon-Mann-Whitney tests were used to compare the maternal HBV DNA load by maternal HBeAg status, and exact Wilcoxon-Mann-Whitney tests were used to compare maternal CD4 count and HBV DNA load by infant HBV status at 48 weeks. The Fisher’s Exact test was used to evaluate an association between maternal extended lamivudine-containing prophylaxis and infant HBV status at 48 weeks. Results were considered statistically significant if the p value was <0.05. All statistical analyses were performed using SAS, version 9.2.
Results
Maternal characteristics
The median age at antenatal screening of the enrolled 2048 women was 25 (interquartile range [IQR], 22–29) years. The median CD4+ T cell count was 440 (IQR, 333–581) cells/mm3 and ALT level was 13 (IQR, 11–16) IU/L. Among the women, 989 (48.3%) tested anti-HBc-positive (Fig. 1), and of those, 103 tested HBsAg-positive, giving an overall HBsAg detection proportion of 5% (95% confidence interval [CI], 4.2–6.1%). Of the HBsAg-positive women, 70/103 (68%) were HBV-DNA-positive and 39/102 (38.2%) were HBeAg-positive (Fig. 1). Sample volume was sufficient for measuring HBV DNA concentration of 69 women, the median being 4.2 (IQR, 1.5–8.9) log10 IU/ml. The median was significantly higher in samples from 35 HBeAg-positive women (7 log10 IU/ml) compared to those from 33 HBeAg-negative women whose concentration could be measured (2.7 log10 IU/ml) (p <0.001). HBV DNA was detected in 16 samples from women who were anti-HBc-positive and HBsAg-negative. For the purpose of the current study these women were considered to have occult HBV infection, and the estimated prevalence of occult infection was therefore 0.8% (95% CI, 0.5–1.3%).
Fig. 1. Flowchart of HBV testing on 2048 HIV-infected, pregnant women screened for the Breastfeeding, Antiretrovirals, and Nutrition study.
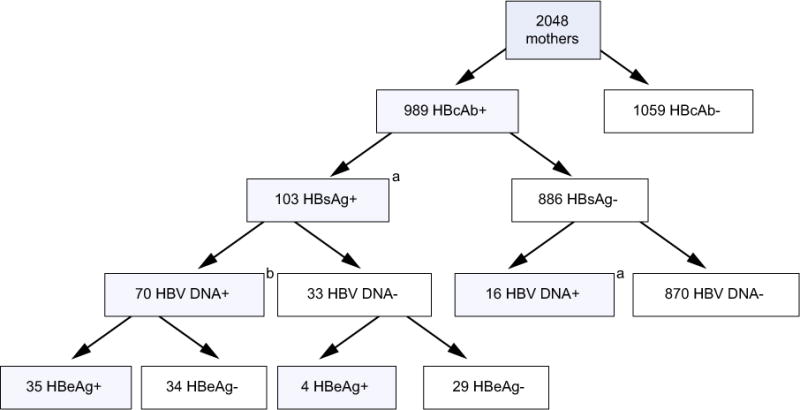
aInfant testing for hepatitis B was limited to infants born to HBsAg-positive (n = 103) or HBsAg-negative and HBV-DNA-positive (n = 16) mothers at the screening visit who enrolled in BAN at delivery (n = 95) and whose infants remained HIV-negative at 2 weeks of life (n = 79) and had plasma specimens available for HBV testing at 2 weeks (n = 57) and/or 48 weeks (n = 51) of age for a total of 72 infants tested for hepatitis B. These infants were included in the analysis of HBV infection among infants (Table 1). bVolume insufficient for HBeAg testing of one mother and for determining HBV DNA concentration in another mother.
Infant characteristics
Of the 72 infants tested at 2 weeks (n = 57) and/or 48 weeks (n = 51), five were HBV-DNA-positive (Table 1). All HBV-DNA-positive infants were born to HBeAg-positive mothers. The proportion of infants HBV-DNA-positive at 48 weeks by maternal HBV marker status was 33% (5/15) for HBeAg-positive and HBV-DNA-positive mothers, 12.8% (5/39) for HBV-DNA-positive mothers, 11.9% (5/42) for HBsAg-positive mothers, and 9.8% (5/51) for HBsAg-positive or HBV-DNA-positive mothers (Table 1). The proportion of infants HBV-DNA-positive at 48 weeks by study antiretroviral intervention arm was 2 of 20 mother-infant pairs on the maternal lamivudine-containing regimen, 1 of 13 on the infant-nevirapine regimen, and 2 of 18 on the control arm. The difference in HBV-DNA-positivity at 48 weeks of infants born to HBsAg-positive mothers who received extended prophylaxis compared to those born to HBsAg-positive mothers who did not was insignificant (2/16 [12.5%] vs. 3/26 [11.5%]). There was no statistically significant difference in the median CD4+ cell count of the 5 mothers of HBV-DNA-positive infants (651 cells/mm3) compared to 37 HBsAg-positive mothers of HBV-DNA-negative infants (441 cells/mm3). The median HBV DNA concentration was significantly higher in the 5 mothers of HBV-DNA-positive infants (8 log10 IU/ml) compared to that in 24 HBsAg-positive mothers with quantifiable HBV DNA and HBV-DNA-negative infants (3.9 log10 IU/ml) (p = 0.001). Four of the 5 mothers of HBV-DNA-positive infants had HBV DNA concentrations >7.5 log10 IU/ml (Table 2). Two infants (Infants B and C) had sufficient specimen quantity for HBV subgenomic sequencing and were determined to be infected with HBV belonging to subgenotype A1; sequencing of HBV from the corresponding mothers showed complete infant-mother identity. No nucleotide sequence polymorphisms that would confer immune-escape properties were observed.
Table 1.
Infant HBV DNA serostatus at 2 and 48 weeks of age according to maternal antenatal HBV serostatus.
| Maternal antenatal HBV serostatus | No. positive/No. tested | |
|---|---|---|
| At 2 wk | At 48 wk | |
| HBsAg-positive/HBeAg-positive/HBV DNA-positive | 1/16 (6.3%) | 5/15 (33.3%) |
| HBsAg-positive/HBeAg-negative/HBV DNA-positive | 0/18 | 0/15 |
| HBsAg-positive/HBV DNA-negative | 0/21a | 0/12a |
| HBsAg-negative/HBV DNA-positive | 0/2 | 0/9 |
| Total No. of infant samples testedb | 57 | 51 |
Includes, in denominators, 6 HBeAg-positive, 26 HBeAg-negative, and 1 HBeAg not tested.
Samples were available from 72 infants for testing at 2 and/or 48 weeks: 36 infants were tested at both time points, 21 were tested only at 2 weeks, and 15 were tested only at 48 weeks.
Table 2.
HBV DNA concentration (in log10 IU/ml) of 5 infants who were HBV DNA-positive at 48 weeks and of their mothers antepartum.
| Infanta | Weeks postnatal | Mother at antepartum | |||
|---|---|---|---|---|---|
| 2 | 12 | 24 | 48 | ||
| A | 2.6 | Not testedb | Not testedb | 8.3 | 5.3 |
| B | Undetected | 3.6 | 8.9 | 8.4 | 8.0 |
| C | Undetected | 3.2 | 9.4 | 8.2 | 8.0 |
| D | Not testedb | 2.0 | Undetected | 3.2 | 7.8 |
| E | Undetected | Undetected | Undetected | 1.7c | 8.4 |
All infants received zidovudine and lamivudine for 7 d after birth, and their mothers received zidovudine/Lamivudine from onset of labor to 7 d after birth; mothers of infants in bold received 28 weeks of zidovudine/lamivudine and lopinavir-ritonavir postpartum.
Volume insufficient.
HBV DNA concentration measured in 2 separate aliquots of sample; value reflects average of the 2 measurements.
Time of infant HBV infection
Of the five HBV-DNA-positive infants, HBV DNA was detected in one (Infant A) at 2 and 48 weeks (samples at 12 and 24 weeks were not tested); in two (Infants B and C) at 12, 24, and 48 weeks, but not at 2 weeks; in one (Infant D) at 12 and 48 weeks, but not at 24 weeks (sample at 2 weeks was not tested); and in one (Infant E) at 48 weeks but not the earlier weeks (Table 2). To exclude the possibility that HBV DNA first measured in Infant E’s sample at week 48 weeks reflected false-positivity, the concentration of HBV DNA in another aliquot of that sample was measured; the value presented in Table 2 for Infant E reflects the average of values obtained from the two measurements. When examining the time of HBV DNA positivity according to the three antiretroviral intervention arms of the BAN study, Infants D and E, who were born to mothers who received 28 weeks of lamivudine-containing prophylaxis postpartum in addition to prophylaxis given during labor and one week after delivery, had HBV DNA concentrations of <4 log10 IU/ml at 48 weeks; these levels were lower compared to the ≥8 log10 IU/ml in Infants A–C whose mothers did not receive extended lamivudine-containing prophylaxis (Table 2). Infant E was included in a BAN substudy of antiretrovirals pharmacokinetics, and it was determined that the concentrations of lamivudine in breast milk (1930 ng/ml at 6 weeks, 1410 ng/ml at 12 weeks, and 898 ng/ml at 24 weeks) were about twice that in maternal plasma (1366, 765, and 327 ng/ml, respectively). Lamivudine was detectable at low levels in the infant’s plasma (53, 23, and 12 ng/ml at 6, 12, and 24 weeks, respectively.
Infants of women with occult HBV infection
None of the infants of mothers with occult HBV infection were HBV-DNA-positive. When mothers with occult HBV infection are taken into consideration, the prevalence of HBV infection in infants born to all HBV-infected mothers (5/51) is 9.8%.
Discussion
The BAN study, conducted in Malawi, provided the setting for one of the largest descriptions of HBV infection among HIV-infected, pregnant women in sub-Saharan Africa and allowed for the extent of HBV infection in their infants to be determined. Of the 2048 women tested, almost half (48.3%) were anti-HBc-positive, indicating that a substantial proportion had prior HBV infection. The proportion of HBsAg-positivity, signifying active HBV infection, was 5%. It is of the same magnitude as the 4% determined from a study in Uganda and Rwanda of 247 HIV-infected, pregnant women [26], but lower than the 8%–36% estimated from smaller studies of HIV-infected, pregnant women in other African countries [27–31]. Of the HBsAg-positive women in our study, 68% were HBV-DNA-positive, which is similar to the 71% observed in Uganda and Rwanda [26], but higher than the 27% observed in Cote d’Ivoire [31]. The 38% HBeAg positivity among HBsAg-positive women in our study is similar to the 33% found in HIV-infected, pregnant women in Uganda and Rwanda [26], but higher than the 0% to 22% reported from smaller studies of HIV-infected, pregnant women from Cameroon and Cote d’Ivoire [30,31]. The prevalence of occult HBV infection in this study was 0.8%, which contrasts with the 10%–20% observed in HIV-infected patients in South Africa [32–34] and Cote D’Ivoire [35]. Although variations across studies in the detection of these various HBV serological markers may reflect differences in the size, demography and stage of HIV disease of the study populations and the genotypic distribution of endemic HBV strains, the usage of assays with disparate performance characteristics likely contributed. As expected and consistent with the findings from elsewhere [26,28], women positive for HBeAg had significantly higher HBV DNA concentrations compared to those who were negative.
We found that 11.9% of infants born to HBsAg-positive women were positive for HBV DNA at 48 weeks of age. Maternal HBeAg status was the most significant predictor of infant HBV infection, which is expected since HBeAg-positive women tend to be highly viremic and therefore highly infectious. Non-immunized infants born to HBeAg-positive, HBsAg-positive mothers have a higher probability of HBV transmission of 70–90% compared to <10% probability of transmission for non-immunized infants of HBeAg-negative, HBsAg-positive mothers [36]. All 5 HBV-DNA-positive infants in this study were born to HBeAg-positive mothers, four of whom had HBV DNA concentrations >7.5 log10 IU/ml. The routes along which HBV may be transmitted from mother to infant are transplacental, perinatal and post-natal [37–39]. Body fluids from which HBV could be transmitted postnatally include breast milk, blood, saliva, sweat, tears and urine [9,10]. Postnatal transmission from other members of the household cannot be excluded [40,41]; however, the fact that all infants in our study were born to HBeAg-positive women with high HBV DNA concentration argues in favour of transmission from their mothers. For Infants B and C, the particularly high viremia of their mothers and the complete mother-infant identity in the HBV S gene further suggest that transmission was maternal.
Of the four infants (A, B, C, and E) tested at two and 48 weeks, one infant (A) first tested HBV-DNA-positive at 2 weeks, 2 infants (B and C) at 12 weeks, and the remaining infant (E) at 48 weeks. Whereas positivity at 2 weeks in Infant A may reflect transplacental, perinatal, or early post-natal HBV infection, positivity from 12 weeks in infants B and C reflects perinatal or post-natal infection. The infections in infants D and E despite maternal prophylaxis with lamivudine-containing antiretrovirals could be due to resistance mutations, but there was insufficient specimen volume to sequence the viral genomes. Alternatively, the relatively low HBV DNA concentrations in infants D and E whose mothers received 28 weeks of lamivudine-containing antiretroviral prophylaxis suggest that lamivudine prophylaxis given to mothers during breastfeeding could have suppressed HBV replication in their infants. One of these infants (Infant E) was tested for lamivudine in plasma and was determined to have detectable lamivudine at 6, 12, and 24 weeks when the infant was HBV-negative. These observations are based on a relatively small sample set, however. Further investigations are needed to confirm that lamivudine in breast milk plays a role against HBV in the infant, particularly in view of reports stating that lamivudine can attain a higher concentration in breast milk compared to plasma, and breastfeeding infants exposed to it may carry low but detectable levels of lamivudine in their plasma [42].
The current study had some limitations. First, our testing algorithm did not include testing for HBsAg and HBV DNA in anti-HBc-negative women; nonetheless, the likelihood of losing anti-HBc antibody is small and more typically encountered during severe immunosuppression [43]. Second, the blood volume from study infants was not sufficient for HBsAg testing, and infant blood was tested only for HBV DNA as marker of active HBV infection. Third, the infants in this study were born to mothers who met the eligibility criteria for randomization to a BAN postnatal intervention, which included serum ALT level ≤2.5 times the upper limit of normal and a CD4+ cell count ≥200 cells/ll. However, only two pregnant women with abnormally high ALT activities were excluded from the study. The BAN study also excluded infants with documented HIV infection at 2 weeks (implying in utero infection). Therefore, the infants in this study may not fully represent the general population of infants born to HIV-infected pregnant women and could present an underestimate of the true HBV transmission in this population. Another limitation is associated with the administration of the pentavalent vaccine as per standard of care in an onsite immunization clinic and not as part of the BAN study, in the course of which vaccination records were not linked to the BAN study. Nonetheless, the 5 infants who became HBV positive could be considered to have been vaccinated, particularly as all 5 infants were recorded to have been brought by their mothers to attend the 6-, 12-, and 48-week visits. Finally, based on the BAN study design, all women and infants were given lamivudine (along with zidovudine) for 7 days after delivery. Although the exposure of the infants to lamivudine was short, the possibility that this anti-HBV drug [1] may have interrupted perinatal transmission or suppressed HBV replication during the first week of life, thereby mitigating infection or delaying detection of HBV infection in the infants, cannot be ruled out.
In summary, among 2048 HIV-infected, pregnant women studied in Malawi, 5% and 4.2% were seropositive for HBsAg and HBV DNA, respectively. Among the HBsAg-seropositive and HBV-DNA-seropositive mothers, HBV infection in infants was observed in 11.9% and 12.8%, respectively. Given the high endemicity of HIV and HBV infection in sub-Saharan Africa, and the fact that HBV infection early in life carries a high risk for developing chronic hepatitis B later in life, it is recommended that efficient strategies are implemented to prevent HBV infection during infancy. Currently, the WHO recommends that all infants should receive their first dose of hepatitis B vaccine as soon as possible after birth, preferably within 24 h [44]. The logistical challenges of delivering the birth dose in certain resource-limited settings need consideration, however [45]. Similar considerations pertain to passive immunoprophylaxis, which is recommended, along with vaccination, for infants of HBsAg-positive mothers, particularly those who also are HBeAg-seropositive [44]. Administration of antivirals during pregnancy is another promising approach to prophylaxis against infant HBV infection [1,42], particularly for highly viremic women. Nonetheless, the cost and feasibility of viral load testing before and after antiviral therapy require consideration. Since the BAN Study, the Malawi Ministry of Health has implemented Option B+, in which all HIV-infected pregnant and breastfeeding women are eligible for lifelong antiretroviral therapy regardless of CD4 count. The regimen of choice is tenofovir, lamivudine, and efavirenz, two of which (tenofovir and lamivudine) are active against HBV. Implementation of Option B+ would be expected to further reduce HBV transmission but, in light of our study’s results, further research should be performed to assess its true impact. Currently, there is no routine antenatal screening for HBV infection in sub-Saharan Africa. Ideally, HBV testing should be integrated with the antenatal HIV testing so that pregnant women may be given care appropriate to their coinfected state and their infants receive timely and complete prophylaxis against infection by both viruses.
Acknowledgments
We are grateful to the following: BAN Study Team at University of North Carolina Chapel Hill, Centers for Disease Control and Prevention, Atlanta, and UNC Project team in Lilongwe including: L Adair, Y Ahmed, M Ait-Khaled, S Albrecht, S Bangdiwala, R Bayer, M Bentley, B Bramson, E Bobrow, N Boyle, S Butera, C Chasela, C Chavula, J Chimerang’ambe, M Chigwenembe, M Chikasema, N Chikhungu, D Chilongozi, G Chiudzu, L Chome, A Cole, A Corbett, A Corneli, A Dow, A Duerr, H Eliya, S Ellington, J Eron, S Farr, Y Owens Ferguson, S Fiscus, V Flax, A Fokar, S Galvin, L Guay, C Heilig, I Hoffman, E Hooten, M Hosseinipour, M Hudgens, S Hurst, L Hyde, D Jamieson, G Joaki (deceased), D Jones, E Jordan-Bell, Z Kacheche, E Kamanga, G Kamanga, C Kampani, P Kamthunzi, D Kamwendo, C Kanyama, A Kashuba, D Kathyola, D Kayira, P Kazembe, C King, R Knight, A Kourtis, R Krysiak, J Kumwenda, H Lee, E Loeliger, D Long, M Luhanga, V Madhlopa, M Majawa, A Maida, C Marcus, F Martinson, N Thoofer, C Matiki (deceased), D Mayers, I Mayuni, M McDonough, J Meme, C Merry, K Mita, C Mkomawanthu, G Mndala, I Mndala, A Moses, A Msika, W Msungama, B Mtimuni, J Muita, N Mumba, B Musis, C Mwansambo, G Mwapasa, J Nkhoma, M Parker, R Pendame, E Piwoz, B Raines, Z Ramdas, J Rublein, M Ryan, I Sanne, C Sellers, D Shugars, D Sichali, W Snowden, A Soko, A Spensley, J-M Steens, G Tegha, M Tembo, R Thomas, H-C Tien, B Tohill, C van der Horst, E Waalberg, E Widen, J Wiener, C Wilfert, P Wiyo, I Zgambo and C Zimba. The authors thank T Mixson, S Ramachandran, M Than, Y Khudyakov and G Xia for facilitating the serologic and molecular HBV studies. Finally and most especially, we are grateful to all the women and infants who participated in the BAN study.
Financial support: The BAN study was supported by grants from the Prevention Research Centers Special Interest Project of the Centers for Disease Control and Prevention [SIP 13-01 U48-CCU409660-09, SIP 26-04 U48-DP000059-01, and SIP 22-09 U48-DP001944-01]; the National Institute of Allergy and Infectious Diseases; the University of North Carolina (UNC) Center for AIDS Research [P30-AI50410]; University College Dublin (UCD) Ad Astra Fellowship; and the NIH Fogarty AIDS International Training and Research Program [DHHS/NIH/FIC 2-D43 Tw01039-06 and R24 Tw00798; the American Recovery and Reinvestment Act]. The antiretrovirals used in the BAN study were donated by Abbott Laboratories, GlaxoSmithKline, Boehringer Ingelheim, Roche Pharmaceuticals, and Bristol-Myers Squibb. The Call to Action PMTCT program was supported by the Elizabeth Glaser Pediatric AIDS Foundation, the United Nations Children’s Fund, the World Food Program, the Malawi Ministry of Health and Population, Johnson & Johnson, and the U.S. Agency for International Development.
Footnotes
Conflict of interest
The authors who have taken part in this study declared that they do not have anything to disclose regarding funding or conflict of interest with respect to this manuscript.
Disclaimer
The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Meeting information
Chasela C., Wall P, Teshale E, et al. Prevalence of hepatitis B virus (HBV) and occult HBV infections among pregnant women coinfected with human immunodeficiency virus type-1 (HIV-1) in Malawi: the BAN Study. In: Proceedings of the International AIDS Society Conference on HIV Pathogenesis, Treatment, and Prevention, Cape Town, South Africa, July 19–22, 2009:208 (Abstract No. WEPEB237).
References
- 1.Kourtis AP, Bulterys M, Hu DJ, Jamieson DJ. HIV-HBV coinfection – a global challenge. N Engl J Med. 2012;366:1749–1752. doi: 10.1056/NEJMp1201796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barth RE, Huijgen Q, Taljaard J, Hoepelman AI. Hepatitis B/C and HIV in sub-Saharan Africa: an association between highly prevalent infectious diseases. A systematic review and meta-analysis Int J Infect Dis. 2010;14:e1024–e1031. doi: 10.1016/j.ijid.2010.06.013. [DOI] [PubMed] [Google Scholar]
- 3.Andersson MI, Maponga TG, Ijaz S, Theron G, Preiser W, Tedder RS. High HBV viral loads in HIV-infected pregnant women at a tertiary hospital, South Africa. J Acquir Immune Defic Syndr. 2012;60:e111–e112. doi: 10.1097/QAI.0b013e31825aeee7. [DOI] [PubMed] [Google Scholar]
- 4.Burk RD, Hwang LY, Ho GY, Shafritz DA, Beasley RP. Outcome of perinatal hepatitis B virus exposure is dependent on maternal virus load. J Infect Dis. 1994;170:1418–1423. doi: 10.1093/infdis/170.6.1418. [DOI] [PubMed] [Google Scholar]
- 5.Greenfield C, Osidiana V, Karayiannis P, et al. Perinatal transmission of hepatitis B virus in Kenya: its relation to the presence of serum HBV-DNA and anti-HBe in the mother. J Med Virol. 1986;19:135–142. doi: 10.1002/jmv.1890190205. [DOI] [PubMed] [Google Scholar]
- 6.Edmunds WJ, Medley GF, Nokes DJ, O’Callaghan CJ, Whittle HC, Hall AJ. Epidemiological patterns of hepatitis B virus (HBV) in highly endemic areas. Epidemiol Infect. 1996;117:313–325. doi: 10.1017/s0950268800001497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vardas E, Mathai M, Blaauw D, McAnerney J, Coppin A, Sim J. Preimmunization epidemiology of hepatitis B virus infection in South African children. J Med Virol. 1999;58:111–115. [PubMed] [Google Scholar]
- 8.Davis LG, Weber DJ, Lemon SM. Horizontal transmission of hepatitis B virus. Lancet. 1989;1:889–893. doi: 10.1016/s0140-6736(89)92876-6. [DOI] [PubMed] [Google Scholar]
- 9.Hill JB, Sheffield JS, Kim MJ, Alexander JM, Sercely B, Wendel GD. Risk of hepatitis B transmission in breast-fed infants of chronic hepatitis B carriers. Obstet Gynecol. 2002;99:1049–1052. doi: 10.1016/s0029-7844(02)02000-8. [DOI] [PubMed] [Google Scholar]
- 10.Louise Heiberg I, Hogh B. Horizontal transmission of hepatitis B virus-why discuss when we can vaccinate? J Infect Dis. 2012;206:464–465. doi: 10.1093/infdis/jis294. [DOI] [PubMed] [Google Scholar]
- 11.Beasley RP, Stevens CE, Shiao IS, Meng HC. Evidence against breast-feeding as a mechanism for vertical transmission of hepatitis B. Lancet. 1975;2:740–741. doi: 10.1016/s0140-6736(75)90724-2. [DOI] [PubMed] [Google Scholar]
- 12.Shi Z, Yang Y, Wang H, et al. Breastfeeding of newborns by mothers carrying hepatitis B virus: a meta-analysis and systematic review. Arch Pediatr Adolesc Med. 2011;165:837–846. doi: 10.1001/archpediatrics.2011.72. [DOI] [PubMed] [Google Scholar]
- 13.Botha JF, Ritchie MJ, Dusheiko GM, Mouton HW, Kew MC. Hepatitis B virus carrier state in black children in Ovamboland: role of perinatal and horizontal infection. Lancet. 1984;1:1210–1212. doi: 10.1016/s0140-6736(84)91694-5. [DOI] [PubMed] [Google Scholar]
- 14.Whittle H, Inskip H, Bradley AK, et al. The pattern of childhood hepatitis B infection in two Gambian villages. J Infect Dis. 1990;161:1112–1115. doi: 10.1093/infdis/161.6.1112. [DOI] [PubMed] [Google Scholar]
- 15.Menendez C, Sanchez-Tapias JM, Kahigwa E, et al. Prevalence and mother-to-infant transmission of hepatitis viruses B, C, and E in Southern Tanzania. J Med Virol. 1999;58:215–220. doi: 10.1002/(sici)1096-9071(199907)58:3<215::aid-jmv5>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 16.Edmunds WJ, Medley GF, Nokes DJ, Hall AJ, Whittle HC. The influence of age on the development of the hepatitis B carrier state. Proc Biol Sci. 1993;253:197–201. doi: 10.1098/rspb.1993.0102. [DOI] [PubMed] [Google Scholar]
- 17.Hainaut P, Boyle P. Curbing the liver cancer epidemic in Africa. Lancet. 2008;371:367–368. doi: 10.1016/S0140-6736(08)60181-6. [DOI] [PubMed] [Google Scholar]
- 18.Lessells RJ, Cooke GS. Effect of the HIV epidemic on liver cancer in Africa. Lancet. 2008;371:1504. doi: 10.1016/S0140-6736(08)60652-2. [DOI] [PubMed] [Google Scholar]
- 19.Current status of routine immununization and polio eradication in the African region: challenges and recommendations. Regional Committee for Africa, sixtieth session; Malabo, Equatorial Guinea. 30 August–3 September; 2010. Available at: < http://www.afro.who.int/en/clusters-a-programmes/rin.html>. [Google Scholar]
- 20.Chasela CS, Hudgens MG, Jamieson DJ, et al. Maternal or infant antiretroviral drugs to reduce HIV-1 transmission. N Engl J Med. 2010;362:2271–2281. doi: 10.1056/NEJMoa0911486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.van der Horst C, Chasela C, Ahmed Y, et al. Modifications of a large HIV prevention clinical trial to fit changing realities: a case study of the Breastfeeding, Antiretroviral, and Nutrition (BAN) protocol in Lilongwe, Malawi. Contemp Clin Trials. 2009;30:24–33. doi: 10.1016/j.cct.2008.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jamieson DJ, Chasela CS, Hudgens MG, et al. Maternal and infant antiretroviral regimens to prevent postnatal HIV-1 transmission: 48-week follow-up of the BAN randomised controlled trial. Lancet. 2012;379:2449–2458. doi: 10.1016/S0140-6736(12)60321-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Forbi JC, Vaughan G, Purdy MA, et al. Epidemic history and evolutionary dynamics of hepatitis B virus infection in two remote communities in rural Nigeria. PLoS One. 2010;5:e11615. doi: 10.1371/journal.pone.0011615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Teshale EH, Ramachandran S, Xia GL, et al. Genotypic distribution of hepatitis B virus (HBV) among acute cases of HBV infection, selected United States counties, 1999–2005. Clin Infect Dis. 2011;53:751–756. doi: 10.1093/cid/cir495. [DOI] [PubMed] [Google Scholar]
- 25.Sloan RD, Ijaz S, Moore PL, Harrison TJ, Teo CG, Tedder RS. Antiviral resistance mutations potentiate hepatitis B virus immune evasion through disruption of its surface antigen a determinant. Antivir Ther. 2008;13:439–447. [PubMed] [Google Scholar]
- 26.Pirillo MF, Bassani L, Germinario EA, et al. Seroprevalence of hepatitis B and C viruses among HIV-infected pregnant women in Uganda and Rwanda. J Med Virol. 2007;79:1797–1801. doi: 10.1002/jmv.21007. [DOI] [PubMed] [Google Scholar]
- 27.Adesina O, Oladokun A, Akinyemi O, et al. Human immuno-deficiency virus and hepatitis B virus coinfection in pregnancy at the University College Hospital, Ibadan. Afr J Med Sci. 2010;39:305–310. [PubMed] [Google Scholar]
- 28.Candotti D, Danso K, Allain JP. Maternofetal transmission of hepatitis B virus genotype E in Ghana, west Africa. J Gen Virol. 2007;88:2686–2695. doi: 10.1099/vir.0.83102-0. [DOI] [PubMed] [Google Scholar]
- 29.Ilboudo D, Simpore J, Ouermi D, et al. Towards the complete eradication of mother-to-child HIV/HBV coinfection at Saint Camille Medical Centre in Burkina Faso, Africa. Braz J Infect Dis. 2010;14:219–224. doi: 10.1016/s1413-8670(10)70047-7. [DOI] [PubMed] [Google Scholar]
- 30.Kfutwah AK, Tejiokem MC, Njouom R. A low proportion of HBeAg among HBsAg-positive pregnant women with known HIV status could suggest low perinatal transmission of HBV in Cameroon. Virol J. 2012;9:62. doi: 10.1186/1743-422X-9-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rouet F, Chaix ML, Inwoley A, et al. HBV and HCV prevalence and viraemia in HIV-positive and HIV-negative pregnant women in Abidjan, Cote d’Ivoire: the ANRS 1236 study. J Med Virol. 2004;74:34–40. doi: 10.1002/jmv.20143. [DOI] [PubMed] [Google Scholar]
- 32.Barth RE, Huijgen Q, Tempelman HA, Mudrikova T, Wensing AM, Hoepelman AI. Presence of occult HBV, but near absence of active HBV and HCV infections in people infected with HIV in rural South Africa. J Med Virol. 2011;83:929–934. doi: 10.1002/jmv.22026. [DOI] [PubMed] [Google Scholar]
- 33.Firnhaber C, Viana R, Reyneke A, et al. Occult hepatitis B virus infection in patients with isolated core antibody and HIV co-infection in an urban clinic in Johannesburg, South Africa. Int J Infect Dis. 2009;13:488–492. doi: 10.1016/j.ijid.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lukhwareni A, Burnett RJ, Selabe SG, Mzileni MO, Mphahlele MJ. Increased detection of HBV DNA in HBsAg-positive and HBsAg-negative South African HIV/AIDS patients enrolling for highly active antiretroviral therapy at a Tertiary Hospital. J Med Virol. 2009;81:406–412. doi: 10.1002/jmv.21418. [DOI] [PubMed] [Google Scholar]
- 35.N’Dri-Yoman T, Anglaret X, Messou E, et al. Occult HBV infection in untreated HIV-infected adults in Cote d’Ivoire. Antivir Ther. 2010;15:1029–1034. doi: 10.3851/IMP1641. [DOI] [PubMed] [Google Scholar]
- 36.Mast EE, Margolis HS, Fiore AE, et al. A comprehensive immunization strategy to eliminate transmission of hepatitis B virus infection in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP) part 1: immunization of infants, children, and adolescents. MMWR. 2005;54:1–31. [PubMed] [Google Scholar]
- 37.Ngui SL, Andrews NJ, Underhill GS, Heptonstall J, Teo CG. Failed postnatal immunoprophylaxis for hepatitis B: characteristics of maternal hepatitis B virus as risk factors. Clin Infect Dis. 1998;27:100–106. doi: 10.1086/514610. [DOI] [PubMed] [Google Scholar]
- 38.Xu DZ, Yan YP, Choi BC, et al. Risk factors and mechanism of transplacental transmission of hepatitis B virus: a case-control study. J Med Virol. 2002;67:20–26. doi: 10.1002/jmv.2187. [DOI] [PubMed] [Google Scholar]
- 39.Zou H, Chen Y, Duan Z, Zhang H, Pan C. Virologic factors associated with failure to passive-active immunoprophylaxis in infants born to HBsAg-positive mothers. J Viral Hepat. 2012;19:e18–e25. doi: 10.1111/j.1365-2893.2011.01492.x. [DOI] [PubMed] [Google Scholar]
- 40.Martinson FE, Weigle KA, Royce RA, Weber DJ, Suchindran CM, Lemon SM. Risk factors for horizontal transmission of hepatitis B virus in a rural district in Ghana. Am J Epidemiol. 1998;147:478–487. doi: 10.1093/oxfordjournals.aje.a009474. [DOI] [PubMed] [Google Scholar]
- 41.Abdool Karim SS, Thejpal R, Coovadia HM. Household clustering and intra-household transmission patterns of hepatitis B virus infection in South Africa. Int J Epidemiol. 1991;20:495–503. doi: 10.1093/ije/20.2.495. [DOI] [PubMed] [Google Scholar]
- 42.Corbett AH. Antiretroviral Pharmacology in Breast Milk. In: Kourtis A, Bulterys M, editors. Human Immunodeficiency Virus type 1 (HIV-1) and Breastfeeding Science, Research Advances, and Policy Adv Exp Med Biol. Vol. 743. 2012. pp. 109–20. [DOI] [PubMed] [Google Scholar]
- 43.Kantelhardt VC, Schwarz A, Wend U, et al. Re-evaluation of anti-HBc nonreactive serum samples from patients with persistent hepatitis B infection by immune precipitation with labelled HBV core antigen. J Clin Virol. 2009;46:124–128. doi: 10.1016/j.jcv.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 44.WHO. Hepatitis B vaccines: WHO position paper-recommendations. Vaccine. 2010;28:589–590. doi: 10.1016/j.vaccine.2009.10.110. [DOI] [PubMed] [Google Scholar]
- 45.Kramvis A, Clements CJ. Implementing a birth dose of hepatitis B vaccine for home deliveries in Africa – too soon? Vaccine. 2010;28:6408–6410. doi: 10.1016/j.vaccine.2010.07.042. [DOI] [PubMed] [Google Scholar]


