Abstract
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) is a reliable, rapid, and reproducible technique for measuring and evaluating changes in gene expression. To facilitate gene expression studies and obtain more accurate RT-qPCR data, normalization relative to stable reference genes is required. In this study, expression profiles of seven candidate reference genes, including β-actin (Actin), elongation factor 1 α (EF1A), glyceralde hyde-3-phosphate dehydro-genase (GAPDH), cyclophilins A (CypA), vacuolar-type H+-ATPase (ATPase), 28S ribosomal RNA (28S), and 18S ribosomal RNA (18S) from Hippodamia convergens were investigated. H. convergens is an abundant predatory species in the New World, and has been widely used as a biological control agent against sap-sucking insect pests, primarily aphids. A total of four analytical methods, geNorm, Normfinder, BestKeeper, and the ΔCt method, were employed to evaluate the performance of these seven genes as endogenous controls under diverse experimental conditions. Additionally, RefFinder, a comprehensive evaluation platform integrating the four above mentioned algorithms, ranked the overall stability of these candidate genes. A suite of reference genes were specifically recommended for each experimental condition. Among them, 28S, EF1A, and CypA were the best reference genes across different development stages; GAPDH, 28S, and CypA were most stable in different tissues. GAPDH and CypA were most stable in female and male adults and photoperiod conditions, 28S and EF1A were most stable under a range of temperatures, Actin and CypA were most stable under dietary RNAi condition. This work establishes a standardized RT-qPCR analysis in H. convergens. Additionally, this study lays a foundation for functional genomics research in H. convergens and sheds light on the ecological risk assessment of RNAi-based biopesticides on this non-target biological control agent.
Introduction
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR) is a rapid, reliable, and reproducible method measuring gene expression during different biological processes [1]. Although RT-qPCR is considered as a major breakthrough in PCR technology, limitations exist, including variation in RNA extraction, reverse transcription, cDNA concentration, normalization, and PCR efficiency [2, 3, 4]. A commonly used technique in RT-qPCR to normalize the gene expression data is to simultaneously measure the expression of an internal reference gene in the same sample [1]. Reference genes, constitutively expressed to maintain cellular function, are the conventional choice for a standardized reference [1], although it is impractical to find a single reference gene expressed at constant levels under all biotic and abiotic conditions [5, 6].
The convergent lady beetle, Hippodamia convergens (Coleoptera: Coccinellidae), is a common lady beetle species in the New World. Although H. convergens larvae and adults are polyphagous, they prey primarily on aphids, including cotton, pea, melon, cabbage, potato, green peach, and corn leaf aphids [7]. As an effective biological control agent, H. convergens can reduce aphid densities in greenhouses [8, 9]. When aphids are limited, however, H. convergens feeds on thrips, small insect larvae and eggs, mites, and honeydew secreted by aphids and other sap-sucking insects, as well as plant based foods, such as pollen, nectar, and petals [10]. Nevertheless, convergent lady beetles must consume preys to reproduce [11].
The advent of the Genomics Era provides unprecedented opportunity to develop novel biopesticides with new modes of action to complement existing biological control agents. Most recently, RNA interference (RNAi)-based transgenic technology has been developed and offers a new approach for the management of insect pests [12–14]. For example, Baum and colleagues (2007) developed transgenetic corn plants that resisted the western corn rootworm, Diabrotica virgifera virgifera. By reducing translation of vacuolar H+-ATPase subunit A in the pest, the plant increased pest mortality and larval stunting and experienced less root damage as a result [12]. Although technical difficulties and regulatory hurdles still exist [15], the commercialization of a new generation of genetically modified crops is likely [16]. One major ecological concern regarding the biosafety of RNAi-based biopesticides and transgenic crops on the ecosystems is their potential effects on non-target organisms, especially biological control agents which play an important role in current pest management practices [17, 18]. Given the nature of RNAi mechanisms, the non-target effects will likely be through the modulation of gene expressions in non-target organisms [19]. Therefore, RT-qPCR will be a major research tool to evaluate potential non-target effects of this new biotechnology. Despite the demonstrated necessity for systematic validation of reference genes in RT-qPCR studies, normalization procedures for biological control agents have not yet received attention. These natural enemies are a major group of non-target organisms that will be exposed to the RNAi-based biopesticides and transgenic crops in the field.
The objective of this study is to evaluate and select appropriate reference genes with stable expression across various biotic and abiotic conditions in H. convergens. Seven candidate reference genes including β-actin (Actin), elongation factor 1 α (EF1A), glyceralde hyde-3-phosphate dehydro-genase (GAPDH), cyclophilins A (CypA), vacuolar-type H + -ATPase (ATPase), 28S ribosomal RNA (28S), and 18S ribosomal RNA (18S) from H. convergens were tested. The stability of these candidate genes was investigated under three biotic (developmental stage, tissue type, and sex) and three abiotic conditions (temperature, photoperiod, and dietary RNAi). As a result, different sets of reference genes were recommended based on the respective experimental conditions.
Materials and Methods
Insects
Hippodamia convergens (Coleoptera: Coccinellidae) was purchased from a commercial vender in California (High Sierra Ladybugs, http://www.highsierraladybugs.com). Hippodamia convergens larvae and adults were maintained in a growth chamber and provisioned with pea aphids, Acyrthosiphon pisum, at 23 ± 1°C temperature, 12L: 12D photoperiod, and 50% relative humidity. A. pisum clones were kindly provided by Dr. John Obrycki (University of Kentucky), and aphids were maintained at 20–28°C on seedlings of fava beans, Vicia faba (Fabales, Fabaceae) in a greenhouse.
Treatments
Biotic factors
The different developmental stages including eggs, four larval instars (collected at the first day of each instar), pupae, and adults. Tissues, including head, midgut, and carcass (body with its head and viscera removed) were dissected from the fourth instars. For the sex treatment, one adult female and male were collected separately and placed in 1.5 ml centrifuge tubes, respectively.
Abiotic factors
To examine the influence of temperature, third instars were exposed to 10, 22, and 30°C for 3 h. For photoperiod, third instars were exposed to 16:8, 12:12, and 8:16 h light: dark period for 2 d. For the dietary RNAi treatment, neonate first instars were fed with 15% sucrose solution containing chemically synthesized dsRNAs for ATPase, which causes mortality in H. convergens larvae (ATPase-dsRNA) (HP Pan unpublished data), water and β-glucuronidase dsRNA (GUS-dsRNA) as controls. At the start of the experiment, H. convergens neonate larvae (< 24 h old) were kept individually in a petri dish. Each neonate was provisioned with a 2 μl droplet containing 1 μl of dsRNA (8μg /μl) and 1 μl of 30% sucrose solution on a daily basis. In 2 d, 16 μg of dsRNA were supplied to each H. convergens neonate. At day 3, five individuals in each treatment were collected as one sample for the subsequent RT-qPCR analysis.
For the developmental stage, a total of 15 eggs were collected as one biological replicate, while one pupa was collected individually as one replicate. For the remaining developmental stages, and all other biotic and abiotic conditions, five individuals were collected for each treatment. Each experiment was repeated three times. All collected samples were snap frozen in liquid nitrogen and stored at -80°C in 1.5 ml centrifuge tubes for the subsequent total RNA extraction
RNA extraction and cDNA synthesis
Total RNA was extracted using TRIzol reagent (Invitrogen, Carlsbad, CA) following the manufacturer’s instruction. The whole body homogenates were centrifuged at 12000 g for 15 min and then supernatant was transferred to a new 1.5 ml microcentrifuge tube. A volume of 200 μl chloroform was added to the supernatant and then the mixture was incubated at room temperature for 10 min and then centrifuged at 4°C, 12000 g for 15 min. After that, the supernatant was transferred to a new 1.5 ml microcentrifuge tube, 400 μl Isopropyl alcohol was added to it, and the mixture precipitated at room temperature for 10 min. Then, the supernatant was discarded after the mixture was centrifuged at 4°C, 12000 g for 8 min, and then 1 ml 75% alcohol was added and centrifuged at 4°C, 7500 g for 5 min to wash the pellet. Finally, the pellet was air dried for 8 min and then dissolved in 30–80 μl ddH2O. DNase treated total RNA was denatured at 75°C for 5 min and immediately chilled on ice. The concentration of RNA was quantified with a NanoDrop 2000c spectrophotometer with the result for eggs (530.1 ± 28.2 ng/μl), first instars (549.3 ± 35.6 ng/μl), second instars (1377.7 ± 164.7 ng/μl), third instars (2387.9 ± 369.6 ng/μl), fouth instars (1376.5 ± 332.0 ng/μl), pupae (733.8 ± 226.1 ng/μl), adults (914.9 ± 377.5 ng/μl), heads (1038.2 ± 78.9 ng/μl), carcasses (3147.9 ± 372.7 ng/μl), and guts (2591.7 ± 1620.0 ng/μl). First-strand cDNA was synthesized from 2.0 μg of total RNA using the M-MLV reverse transcription kit (Invitrogen, Carlsbad, CA) using a random N primer according the manufacturer’s recommendations. The cDNA was diluted 10-fold for the subsequent RT-qPCR studies.
Reference gene selection and primer design
Seven reference genes were selected (Table 1). Primers for 18S and 28S were designed based on the sequences obtained from NCBI (accession number: EU164617 and EU164644, respectively). For the remaining five reference genes, degenerate primers were designed using CODEHOP (http://blocks.fhcrc.org/codehop.html) according to the conserved amino acid residues among Coleopteran (S1 Table). PCR amplifications were performed in 50 μl reactions containing 10 μl 5×PCR Buffer (Mg2+ Plus), 1 μl dNTP mix (10 mM of each nucleotide), 5 μl of each primer (10 μM each), and 0.25 μl of Go Taq (5u /μl) (Promega). The PCR parameters were as follows: one cycle of 94°C for 3 min; 35 cycles of 94°C for 30 s, 59°C for 45 s and 72°C for 1 min; a final cycle of 72°C for 10 min. Amplicons of the expected sizes were purified, cloned into the pCR4-TOPO vector (Invitrogen, Carlsbad, CA), and sent out for sequencing (S2 Table). After the identity of these reference genes was confirmed, primers for the subsequent RT-qPCR analysis were designed online, https://www.idtdna.com/Primerquest/Home/Index.
Table 1. Primers used for RT-qPCR.
| Gene | Primer sequences (5’-3’) | Length (bp) | Efficiently (%) | R2 | Linear regression equation |
|---|---|---|---|---|---|
| EF1A | F: AGTGGAAGACGGAGGGGTTT | 123 | 103.0 | 0.9995 | y = -3.2539x+24.008 |
| R: ATGGTTCAAGGGATGGGCAA | |||||
| GAPDH | F: GCCAAGGTGATCCATGACAA | 80 | 103.2 | 0.9994 | y = -3.2473x+26.272 |
| R: GTCTTCTGAGTGGCAGTTGTAG | |||||
| Actin | F: CTCCAGAATCCAACACGATACC | 125 | 105.0 | 0.9995 | y = -3.2074x+21.506 |
| R: CAGGGAGAAGATGACCCAAATC | |||||
| CypA | F: TGAAGAACTGCGATCCGTTG | 112 | 102.1 | 0.9882 | y = -3.2723x+25.387 |
| R:TCCATCTACGGCAGCAAATTC | |||||
| 18S | F: TGCATGGCCGTTCTTAGTT | 103 | 101.5 | 0.9991 | y = -3.2864x+11.052 |
| R: GGGCCTTTGAGGATGTCTAAT | |||||
| 28S | F: CTTAGAGTCGGGTTGCTTGAG | 100 | 96.9 | 0.9995 | y = -3.3985x+12.095 |
| R: CTCACGGTACTTGTTCGCTATC | |||||
| ATPase | F: AGAAGCTCGCCCAACGTAAGCATT | 82 | 100.9 | 0.9999 | y = -3.3016x+18.029 |
| R: AAATCGTCCAGTGCCCTCGTGTACTT |
Reverse transcriptase-quantitative polymerase chain reaction (RT-qPCR)
PCR reaction (20 μl) contained 7 μl of ddH2O, 10 μl of 2×SYBR Green MasterMix (BioRad), 1 μl of each specific primer (10 μM), and 1 μl of the first-strand cDNA template. The RT-qPCR program included an initial denaturation for 3 min at 95°C followed by 40 cycles of denaturation at 95°C for 10 s, annealing for 30 s at 55°C, and extension for 30 s at 72°C. For melting curve analysis, a dissociation step cycle (55°C for 10 s, and then 0.5°C for 10 s until 95°C) was added. Reactions were set up in 96-well format Microseal PCR plates (Bio-Rad) in triplicates, and carried out in a MyiQ single Color Real-Time PCR Detection System (BioRad). Existence of a single peak in melting curve analysis was used to confirm gene-specific amplification and rule out non-specific amplification and primer-dimer generation. A standard curve for each primer pair was constructed with serial dilutions of cDNA (1, 1/5, 1/25, 1/125, 1/625, and 1/3125). PCR amplification efficiency (E) was calculated according to the equation: E = (10[-1/slope] -1)×100.
Expression stability analysis of candidate reference genes
The stability of each candidate reference gene was evaluated by algorithms geNorm [1], NormFinder [20], BestKeeper [21], and the ΔCt method [22]. Finally, we compared and ranked the tested candidates based on the comprehensive web-based analysis tool RefFinder (http://www.leonxie.com/referencegene.php).
Results
Primer specificity and efficiency
Seven candidate reference genes were visualized as a single amplicon on a 1.5% agarose gel. Furthermore, gene-specific amplification of these genes was confirmed by a single peak in melting-curve analysis (S1 Fig). The correlation coefficient (R2) and PCR efficiency for each standard curve were shown in Table 1.
C t values of candidate reference genes
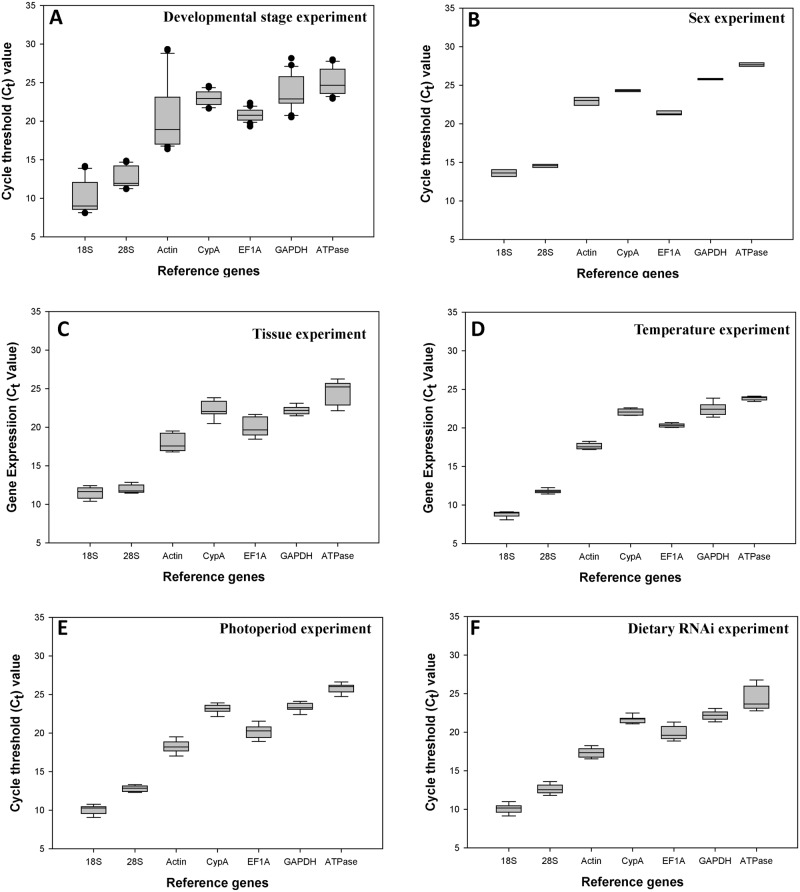
For the developmental stage experiment, the C t values ranged from 10 to 25. 18S and 28S showed average C t values below 15 cycles. Actin was less expressed in the egg stage with the C t values above 28. 18S and ATPase were the most and the least expressed reference gene, respectively (Fig 1A).
Fig 1. Expression profiles of the seven candidate reference genes in all six experiments in Hippodamia convergens.
The dot indicates the maximum or minimum value of replicated samples, while whiskers indicate the standard error of the mean.
For the sex experiment, the C t values ranged from 13 to 28. 18S and 28S showed average C t values below 15 cycles. All of the seven candidate reference genes were equally expressed in both female and male adults. 18S and ATPase were the most and the least expressed reference gene, respectively (Fig 1B).
For the tissue experiment, the C t values ranged from 11 to 24. 18S and 28S showed average C t values below 12 cycles. Actin showed average C t values below 18. 18S and ATPase were the most and the least expressed reference gene, respectively (Fig 1C).
For the temperature experiment, the C t values ranged from 9 to 24. 18S and 28S showed average C t values below 12 cycles. Actin showed average C t values below 18. EF1A, GAPDH, and CypA showed average C t values below 23. 18S and ATPase were the most and the least expressed reference gene, respectively (Fig 1D).
For the photoperiod experiment, the C t values ranged from 10 to 26. 18S, 28S, and Actin showed average C t values below 18 cycles. EF1A showed the C t values around 20, GAPDH and CypA were around 23. 18S and ATPase were the most and the least expressed reference gene, respectively (Fig 1E).
For the dietary RNAi experiment, the C t values ranged from 10 to 24. 18S, 28S, Actin, and EF1A showed average C t values below 20 cycles, GAPDH and CypA were around 22. The expression of ATPase was significantly depressed in the ATPase-dsRNA supplied samples compared to the water and GUS-dsRNA fed controls. 18S and ATPase were the most and the least expressed reference gene, respectively (Fig 1F).
Stability of candidate reference genes under biotic conditions
GeNorm bases its ranking on the geometric mean of the standard deviation (SD) of each transformed gene set of pair combinations (M-value). The lower the M-value is, the higher the ranking. Under the impact of developmental stage, CypA and EF1A were co-ranked as the most stable genes. The overall order based on geNorm from the most stable to least stable reference gene was: CypA = EF1A, 28S, ATPase, 18S, GAPDH, Actin (Table 2). For the tissue experiment, 28S and GAPDH were co-ranked as the most stable genes. The overall order based on geNorm from the most stable to least stable reference gene was: 28S = GAPDH, CypA, EF1A, ATPase, 18S, Actin (Table 2). For the sex experiment, CypA and GAPDH were co-ranked as the most stable genes. The overall order based on geNorm from the most stable to least stable reference gene was: CypA = GAPDH, 28S, EF1A, ATPase, 18S, Actin (Table 2).
Table 2. Stability of reference gene expression under biotic conditions.
| Biotic conditions | Reference gene | geNorm | Normfider | BestKeeper | ΔCt | ||||
|---|---|---|---|---|---|---|---|---|---|
| Stability | Rank | Stability | Rank | Stability | Rank | Stability | Rank | ||
| Stage | 28S | 0.92 | 2 | 0.57 | 2 | 1.13 | 3 | 1.48 | 1 |
| CypA | 0.77 | 1 | 1.15 | 4 | 0.77 | 2 | 1.68 | 3 | |
| EF1A | 0.77 | 1 | 1.24 | 5 | 0.70 | 1 | 1.78 | 4 | |
| ATPase | 1.01 | 3 | 0.39 | 1 | 1.47 | 4 | 1.49 | 2 | |
| GAPDH | 1.35 | 5 | 0.91 | 3 | 1.92 | 6 | 1.84 | 5 | |
| 18S | 1.15 | 4 | 1.33 | 6 | 1.82 | 5 | 1.85 | 6 | |
| Actin | 1.93 | 6 | 3.26 | 7 | 3.35 | 7 | 3.37 | 7 | |
| Tissue | 28S | 0.28 | 1 | 0.14 | 1 | 0.44 | 2 | 0.88 | 2 |
| CypA | 0.49 | 2 | 0.46 | 2 | 0.82 | 4 | 0.95 | 3 | |
| EF1A | 0.54 | 3 | 0.71 | 3 | 0.97 | 6 | 1.07 | 4 | |
| ATPase | 0.79 | 4 | 1.33 | 5 | 1.29 | 7 | 1.48 | 6 | |
| GAPDH | 0.28 | 1 | 0.14 | 1 | 0.41 | 1 | 0.87 | 1 | |
| 18S | 1.00 | 5 | 1.15 | 4 | 0.57 | 3 | 1.43 | 5 | |
| Actin | 1.19 | 6 | 1.54 | 6 | 0.90 | 5 | 1.67 | 7 | |
| Sex | 28S | 0.28 | 2 | 0.14 | 2 | 0.18 | 3 | 0.38 | 2 |
| CypA | 0.24 | 1 | 0.28 | 3 | 0.15 | 2 | 0.44 | 3 | |
| EF1A | 0.33 | 3 | 0.38 | 4 | 0.26 | 5 | 0.50 | 4 | |
| ATPase | 0.35 | 4 | 0.41 | 5 | 0.22 | 4 | 0.51 | 5 | |
| GAPDH | 0.24 | 1 | 0.08 | 1 | 0.09 | 1 | 0.37 | 1 | |
| 18S | 0.43 | 5 | 0.48 | 6 | 0.38 | 6 | 0.57 | 6 | |
| Actin | 0.48 | 6 | 0.53 | 7 | 0.44 | 7 | 0.60 | 7 | |
A low stability value suggests a more stable gene by NormFinder. Under the impact of developmental stage, ATPase was the most stable gene. The overall order based on NormFinder from the most stable to least stable reference gene was: ATPase, 28S, GAPDH, CypA, EF1A, 18S, Actin (Table 2). For the tissue experiment, 28S and GAPDH were co-ranked as the most stable genes. The overall order based on NormFinder from the most stable to least stable reference gene was: 28S = GAPDH, CypA, EF1A, 18S, ATPase, Actin (Table 2). For the sex experiment, GAPDH was the most stable gene. The overall order based on NormFinder from the most stable to least stable reference gene was: GAPDH, 28S, CypA, EF1A, ATPase, 18S, Actin (Table 2).
The stability of a gene is inversely proportional to the BestKeeper computed standard deviation (SD) value. Under the impact of developmental stage, EF1A was the most stable gene. The overall order based on BestKeeper from the most stable to least stable reference gene was: EF1A, CypA, 28S, ATPase, 18S, GAPDH, Actin (Table 2). For the tissue experiment, GAPDH was the most stable gene. The overall order based on BestKeeper from the most stable to least stable reference gene was: GAPDH, 28S, 18S, CypA, Actin, EF1A, ATPase (Table 2). For the sex experiment, GAPDH was the most stable gene. The overall order based on BestKeeper from the most stable to least stable reference gene was: GAPDH, CypA, 28S, ATPase, EF1A, 18S, Actin (Table 2).
The ΔC t method relies on relative pair-wise comparisons. Using raw C t values, the average SD of each gene set is inversely proportional to its stability. Under the impact of developmental stage, 28S was the most stable gene. The overall order based on ΔC t method from the most stable to least stable reference gene was: 28S, ATPase, CypA, EF1A, GAPDH, 18S, Actin (Table 2). For the tissue experiment, GAPDH was the most stable gene. The overall order based on ΔC t method from the most stable to least stable reference gene was: GAPDH, 28S, CypA, EF1A, 18S, ATPase, Actin (Table 2). For the sex experiment, GAPDH was the most stable gene. The overall order based on ΔC t method from the most stable to least stable reference gene was: GAPDH, 28S, CypA, EF1A, ATPase, 18S, Actin (Table 2).
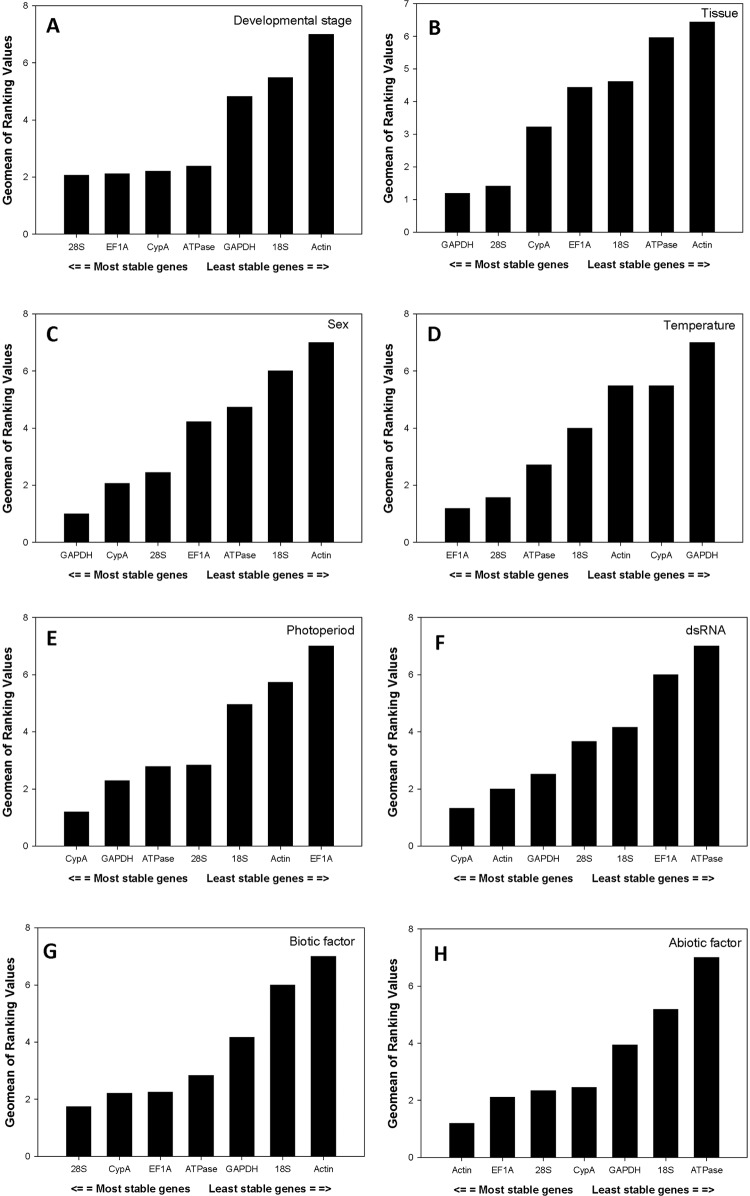
Under the impact of developmental stage, according to RefFinder, which integrates the above-mentioned four software tools to compare and rank the candidates, the comprehensive ranking of candidate reference gene from the most to the least stable was: 28S, EF1A, CypA, ATPase, GAPDH, 18S, Actin (Fig 2A). For the tissue experiment, the comprehensive ranking of candidate reference gene from the most to the least stable was: GAPDH, 28S, CypA, EF1A, 18S, ATPase, Actin (Fig 2B). For the sex experiment, the overall ranking from the most to the least stable reference gene in both adult females and males was: GAPDH, CypA, 28S, EF1A, ATPase, 18S, Actin (Fig 2C). To summarize, the overall ranking from the most to the least stable reference gene under the biotic condition was: 28S, CypA, EF1A, ATPase, GAPDH, 18S, Actin (Fig 2G).
Fig 2. Stability of candidate reference genes expression under different treatment according to their stability value by RefFinder.
A lower Geomean value indicates more stable expression.
Stability of candidate reference genes under abiotic conditions
Based on GeNorm, for the temperature experiment, 28S and EF1A were co-ranked as the most stable genes. The overall order based on geNorm from the most stable to least stable reference gene was: 28S = EF1A, ATPase, 18S, Actin, CypA, GAPDH (Table 3). For the photoperiod experiment, ATPase and GAPDH were co-ranked as the most stable genes. The overall order based on geNorm from the most stable to least stable reference gene was: ATPase = GAPDH, CypA, 28S, Actin, EF1A, 18S (Table 3). For the dietary RNAi experiment, Actin and GAPDH were co-ranked as the most stable genes. The overall order based on geNorm from the most stable to least stable reference gene was: Actin = GAPDH, CypA, 28S, 18S, EF1A, ATPase (Table 3).
Table 3. Stability of reference gene expression under abiotic conditions.
| Abiotic conditions | Reference gene | geNorm | Normfider | BestKeeper | ΔCt | ||||
|---|---|---|---|---|---|---|---|---|---|
| Stability | Rank | Stability | Rank | Stability | Rank | Stability | Rank | ||
| Temperature | 28S | 0.19 | 1 | 0.15 | 3 | 0.17 | 1 | 0.38 | 2 |
| CypA | 0.35 | 5 | 0.39 | 5 | 0.35 | 5 | 0.53 | 5 | |
| EF1A | 0.19 | 1 | 0.10 | 1 | 0.19 | 2 | 0.37 | 1 | |
| ATPase | 0.21 | 2 | 0.14 | 2 | 0.19 | 2 | 0.39 | 3 | |
| GAPDH | 0.50 | 6 | 0.83 | 7 | 0.59 | 6 | 0.87 | 7 | |
| 18S | 0.22 | 3 | 0.18 | 4 | 0.26 | 3 | 0.41 | 4 | |
| Actin | 0.30 | 4 | 0.44 | 6 | 0.33 | 4 | 0.55 | 6 | |
| Photoperiod | 28S | 1.44 | 3 | 1.50 | 4 | 0.47 | 1 | 2.57 | 4 |
| CypA | 1.30 | 2 | 0.66 | 2 | 1.77 | 5 | 2.35 | 2 | |
| EF1A | 2.25 | 5 | 3.51 | 6 | 1.53 | 4 | 3.73 | 6 | |
| ATPase | 1.15 | 1 | 0.78 | 3 | 1.50 | 3 | 2.39 | 3 | |
| GAPDH | 1.15 | 1 | 0.57 | 1 | 0.79 | 2 | 2.25 | 1 | |
| 18S | 3.04 | 6 | 1.50 | 7 | 5.11 | 7 | 4.99 | 7 | |
| Actin | 1.87 | 4 | 1.75 | 5 | 2.54 | 6 | 2.96 | 5 | |
| 28S | 0.43 | 5 | 0.48 | 6 | 0.38 | 6 | 0.57 | 6 | |
| CypA | 0.48 | 6 | 0.53 | 7 | 0.44 | 7 | 0.60 | 7 | |
| dsRNA | 28S | 0.45 | 3 | 0.42 | 3 | 0.46 | 5 | 0.65 | 3 |
| CypA | 0.28 | 2 | 0.21 | 1 | 0.35 | 1 | 0.60 | 1 | |
| EF1A | 0.53 | 5 | 0.52 | 6 | 0.72 | 6 | 0.75 | 6 | |
| ATPase | 0.76 | 6 | 1.29 | 7 | 1.27 | 7 | 1.35 | 7 | |
| GAPDH | 0.25 | 1 | 0.44 | 4 | 0.44 | 2 | 0.68 | 5 | |
| 18S | 0.48 | 4 | 0.48 | 5 | 0.45 | 3 | 0.68 | 4 | |
| Actin | 0.25 | 1 | 0.22 | 2 | 0.45 | 4 | 0.62 | 2 | |
Based on NormFinder, for the temperature experiment, EF1A was the most stable gene. The overall order based on NormFinder from the most stable to least stable reference gene was: EF1A, ATPase, 28S, 18S, CypA, Actin, GAPDH (Table 3). For the photoperiod experiment, GAPDH was the most stable gene. The overall order based on NormFinder from the most stable to least stable reference gene was: GAPDH, CypA, ATPase, 28S, Actin, EF1A, 18S (Table 3). For the dietary RNAi experiment, CypA was the most stable gene. The overall order based on NormFinder from the most stable to least stable reference gene was: CypA, Actin, 28S, GAPDH, 18S, EF1A, ATPase (Table 3).
Based on BestKeeper, for the temperature experiment, 28S was the most stable gene. The overall order based on BestKeeper from the most stable to least stable reference gene was: 28S, EF1A = ATPase, 18S, Actin, CypA, GAPDH (Table 3). For the photoperiod experiment, 28S was the most stable gene. The overall order based on BestKeeper from the most stable to least stable reference gene was: 28S, GAPDH, ATPase, EF1A, CypA, Actin, 18S (Table 3). For the dietary RNAi experiment, CypA was the most stable gene. The overall order based on BestKeeper from the most stable to least stable reference gene was: CypA, GAPDH, 18S, Actin, 28S, EF1A, ATPase (Table 3).
Based on ΔC t method, for the temperature experiment, EF1A was the most stable gene. The overall order based on ΔC t method from the most stable to least stable reference gene was: EF1A, 28S, ATPase, 18S, CypA, Actin, GAPDH (Table 3). For the photoperiod experiment, GAPDH was the most stable gene. The overall order based on ΔC t method from the most stable to least stable reference gene was: GAPDH, CypA, ATPase, 28S, Actin, EF1A, 18S (Table 3). For the dietary RNAi experiment, CypA was the most stable gene. The overall order based on ΔC t method from the most stable to least stable reference gene was: CypA, Actin, 28S, 18S, GAPDH, EF1A, ATPase (Table 3).
Based on RefFinder, for the temperature experiment, the comprehensive ranking of candidate reference gene from the most to the least stable was: EF1A, 28S, ATPase, 18S, Actin, CypA, GAPDH (Fig 2D). For the photoperiod experiment, the overall ranking from the most to the least stable reference gene under the photoperiod stress was: CypA, GAPDH, ATPase, 28S, 18S, Actin, EF1A (Fig 2E). For the dietary RNAi experiment, the overall ranking from the most to the least stable reference gene under the dsRNA stress was: CypA, Actin, GAPDH, 28S, 18S, EF1A, ATPase (Fig 2F). To summarize, the overall ranking from the most to the least stable reference gene under the abiotic condition was: Actin, EF1A, 28S, CypA, GAPDH, 18S, ATPase (Fig 2H).
Quantitative analysis of candidate reference genes based on geNorm
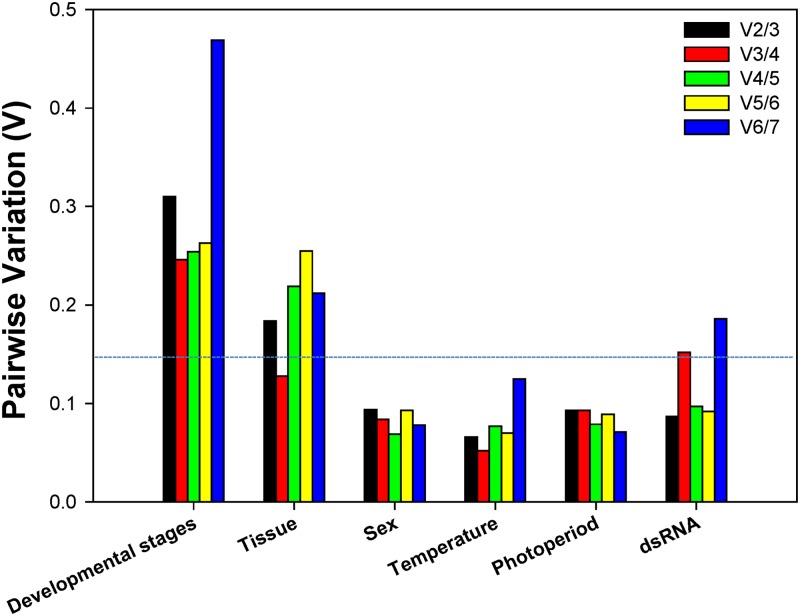
To decide the minimal number of reference genes mandatory for normalization, the V-value was computed by geNorm. Beginning with two genes, the software continuously adds another gene and recalculates the normalization factor ratio. If the added gene does not elevate the normalization factor ratio over the proposed 0.15 cut-off value, then the starting pair of genes is sufficient for the normalization. Otherwise, more genes should be incorporated. However, if the new ratio is above 0.15, then more genes should be included. For the developmental stage experiment, although none of the V-value was less than 0.15, the lowest value appeared at V3/4, suggesting that three reference genes were desirable for reliable normalization throughout developmental stages (Fig 3). For the tissue experiment, the first V-value < 0.15 emerged at V3/4, suggesting that three reference genes were required for reliable normalization in different tissue types (Fig 3). For the sex, temperature, photoperiod, and dietary RNAi experiments, the first V-value < 0.15 showed at V2/3, recommending that two reference genes were sufficient for reliable normalization (Fig 3).
Fig 3. Pairwise variation (V) analysis of the candidate reference genes.
The geNorm first calculates an expression stability value (M) for each gene and then compares the pair-wise variation (V) of this gene with the others. A threshold of V<0.15 was suggested for valid normalization. Starting with two genes, the software sequentially adds another gene and recalculates the normalization factor ratio. If the added gene does not increase the normalization factor ratio above the proposed 0.15 cut-off value, then the original pair of genes is enough for normalization. However, if the new ratio is above 0.15, then more genes should be included [1].
Discussion
RT-qPCR quantification requires a comprehensive normalization by reference genes to counteract confounding variation in experimental data. Generally considered to be expressed in all cell types of an organism at a constant level to maintain basic cellular functions, reference genes have been widely adopted as internal controls for various analyses involving gene quantification, including Western blot, Northern blot, and RT-qPCR. Each candidate reference gene, however, should be evaluated under specific experimental conditions for gene profiling to ensure a constant level of expression [23]. Our results demonstrate that the best suited reference genes can be different in response to different biotic and abiotic conditions. This is consistent with previous reports showing that reference genes are differentially expressed under specific experimental conditions in the sweet potato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae), diamondback moth, Plutella xylostella (Lepidoptera: Plutellidae), brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae), beet armyworm, Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae), oriental leafworm moth, Spodoptera litura (Lepidoptera: Noctuidae), oriental fruit fly, Bactrocera dorsalis (Diptera: Tephritidae), pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera, Aphidiae), and Colorado potato beetle, Leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae) [5, 6, 24–29]. Even under the same insecticide treatment, different classes of insecticides warranted different sets of reference genes to normalize target gene expression in B. tabaci, indicating the necessity for custom reference gene selection [30].
The large body of recent works clearly suggested that there are no "universal" reference genes that are stably expressed and applicable for all cell and tissue types and various experimental conditions [5, 6, 27–29]. For example, as a major component of the protein scaffold which supports the cell and determines its shape, Actin has been widely regarded as the universal reference gene and used extensively as the internal reference without any validation. For H. convergens, however, Actin was the least stable reference gene under different biotic conditions (developmental stage, tissue, and sex). Recently, Lin and Redies [31] compared histological expression profile of GAPDH and Actin in the developing chicken embryo. Neither GAPDH nor Actin was expressed in all cell types or tissues at high levels, and in some organs, these two reference genes exhibited partially complementary expression patterns. Specifically, Actin is highly expressed in the gizzard, but it was virtually non-existent in cardiac muscle cells.
There has been ongoing discussion about the optimal number of reference genes required for RT-qPCR analysis. Previously, gene expression studies have predominantly used a single endogenous control, however, this will profoundly influence the statistical outcome and may lead to inaccurate data interpretation [32] or it is simply insufficient to normalize the expression of target genes [33]. To avoid biased normalization, more and more researchers have moved away from a single endogenous control and started to embrace the idea of using multiple reference genes to analyze gene expression [5, 6, 27–29]. Determination of the optimal number of reference genes, however, has always being a trade-off between accuracy and practicality. A minimum of three most stably expressed reference genes is recommended by Thomas and colleagues [34]. In this study, two reference genes are sufficient to normalize the expression and provide a more conservative estimation of target gene expression under abiotic conditions, including temperature, photoperiod, and dietary RNAi. In contrast, three stable reference genes are generally required under biotic conditions, including developmental stage and tissue.
This is the first study to investigate candidate reference genes for gene expression analyses in the predatory species H. convergens. Based on a comprehensive analysis integrating five commonly used analytical methods to compare and rank the candidate reference genes under an array of biotic (developmental stage, tissue, and sex) and abiotic (temperature, photoperiod, and dietary RNAi) conditions, a suite of candidate reference genes were specifically recommended for each experimental condition. Among them, 28S, EF1A, and CypA were the best reference genes across different development stage; GAPDH, 28S, and CypA were most stable in different tissues. GAPDH and CypA were most stable in female and male adults and photoperiod conditions, 28S and EF1A were most stable under a range of temperatures, Actin and CypA were most stable under dietary RNAi condition. This work is the initial first step to establish a standardized RT-qPCR analysis in H. convergens. Additionally, this study lays a foundation for the functional genomics research in H. convergens, and sheds light on the ecological risk assessment of RNAi-based biopesticides on this non-target biological control agent.
Supporting Information
(TIFF)
(DOCX)
(DOCX)
Acknowledgments
The authors are grateful to anonymous reviewers and the editor for their constructive criticisms. Special thanks go to Dr. John Obrycki at the University of Kentucky provided editorial revision for this manuscript, Dr. Xun Zhu for his assistance with the data analysis. The information reported in this paper (No.14-08-064) is part of a project of the Kentucky Agricultural Experiment Station and is published with the approval of the Director.
Data Availability
All relevant data are within the paper and its Supporting Information files.
Funding Statement
This research was supported by a start-up fund from the University of Kentucky to XGZ, a grant from USDA BRAG grant (Award Agreement No.: 3048108827) to XGZ and BDS. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3: research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Bustin SA, Benes V, Garson JA, Hellemans J, Huggett J, Kubista M, et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem. 2009;55: 611–622. 10.1373/clinchem.2008.112797 [DOI] [PubMed] [Google Scholar]
- 3. Bustin SA, Beaulieu JF, Huggett J, Jaggi R, Kibenge FS, Olsvik PA, et al. MIQE précis: Practical implementation of minimum standard guidelines for fluorescence-based quantitative real-time PCR experiments. BMC Mol Biol. 2010;11: 74 10.1186/1471-2199-11-74 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Strube C, Buschbaum S, Wolken S, Schnieder T. Evaluation of reference genes for quantitative real-time PCR to investigate protein disulfide isomerase transcription pattern in the bovine lungworm Dictyocaulus viviparus . Gene. 2008;425: 36–43. 10.1016/j.gene.2008.08.001 [DOI] [PubMed] [Google Scholar]
- 5. Zhu X, Yuan M, Shakeel M, Zhang YJ, Wang SL, Wang X, et al. Selection and evaluation of reference genes for expression analysis using qRT-PCR in the beet armyworm Spodoptera exigua (Hübner) (Lepidoptera: Noctuidae). PLoS One. 2014;9: e84730 10.1371/journal.pone.0084730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Li RM, Xie W, Wang SL, Wu QJ, Yang NN, Yang X, et al. Reference gene selection for qRT-PCR analysis in the sweetpotato whitefly, Bemisia tabaci (Hemiptera: Aleyrodidae). PLoS One. 2013;8: e53006 10.1371/journal.pone.0053006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Obrycki JJ, Kring TJ. Predaceous Coccinellidae in biological control. Annu Rev Entomol. 1998;43: 295–321. [DOI] [PubMed] [Google Scholar]
- 8. Dreistadt SH, Flint ML. Melon aphid (Homoptera: Aphididae) control by inundative convergent lady beetle (Coleoptera: Coccinellidae) release on chrysanthemum. Environ Entomol. 1996;25: 688–697. [Google Scholar]
- 9. Raupp MJ, Hardin MR, Braxton SM, Bull BB. Augmentative releases for aphid control on landscape plants. J Arboricult. 1994;20: 241–249. [Google Scholar]
- 10. Hodek I, Honek A, van Emden HF. Ecology and behaviour of the ladybird beetles (Coccinellidae). John Wiley & Sons; 2012. [Google Scholar]
- 11. Haug GW. Rearing the Coccinellid Hippodamia convergens on frozen aphids. Ann Entomol Soc Am. 1938;31: 240–248. [Google Scholar]
- 12. Baum JA, Bogaert T, Clinton W, Heck GR, Feldmann P, Ilagan O, et al. Control of coleopteran insect pests through RNA interference. Nat Biotechnol. 2007;25: 1322–1326. [DOI] [PubMed] [Google Scholar]
- 13. Mao YB, Cai WJ, Wang JW, Hong GJ, Tao XY, Wang LJ, et al. Silencing a cotton bollworm P450 monooxygenase gene by plant-mediated RNAi impairs larval tolerance of gossypol. Nat Biotechnol. 2007;25: 1307–1313. [DOI] [PubMed] [Google Scholar]
- 14. Zha WJ, Peng XX, Chen RZ, Du B, Zhu LL, He GC. Knockdown of midgut genes by dsRNA-transgenic plant-mediated RNA interference in the hemipteran insect Nilaparvata lugens . PLoS One. 2011;6: e20504 10.1371/journal.pone.0020504 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Lundgren JG, Duan JJ. RNAi-based insecticidal crops: potential effects on non target species. BioScience. 2013;63: 657–665. [Google Scholar]
- 16. Kupferschmidt K. A lethal dose of RNA. Science. 2013;341: 732–733. 10.1126/science.341.6147.732 [DOI] [PubMed] [Google Scholar]
- 17. Romeis J, Bartsch D, Bigler F, Candolfi MP, Gielkens MM, Hartley SE, et al. Assessment of risk of insect-resistant transgenic crops to nontarget arthropods. Nat Biotechnol. 2008;26: 203–208. 10.1038/nbt1381 [DOI] [PubMed] [Google Scholar]
- 18. Romeis J, Meissle M, Bigler F. Transgenic crops expressing Bacillus thuringiensis toxins and biological control. Nat Biotechnol. 2006;24: 63–71. [DOI] [PubMed] [Google Scholar]
- 19. Berezikov E. Evolution of microRNA diversity and regulation in animals. Nat Rev Genet. 2011;12: 846–860. 10.1038/nrg3079 [DOI] [PubMed] [Google Scholar]
- 20. Andersen CL, Jensen JL, Ørntoft TF. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res. 2004;64: 5245–5250. [DOI] [PubMed] [Google Scholar]
- 21. Pfaffl MW, Tichopad A, Prgomet C, Neuvians TP. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper—Excel-based tool using pair-wise correlations. Biotechnol Lett. 2004;26: 509–515. [DOI] [PubMed] [Google Scholar]
- 22. Silver N, Best S, Jiang J, Thein SL. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol Biol. 2006;7: 33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Thellin O, Zorzi W, Lakaye B, De Borman B, Coumans B, Hennen G, et al. Housekeeping genes as internal standards: use and limits. J Biotechnol. 1999;75: 291–295. [DOI] [PubMed] [Google Scholar]
- 24. Lu YH, Yuan M, Gao XW, Kang TH, Zhan S, Wan H, et al. Identification and validation of reference genes for gene expression analysis using quantitative PCR in Spodoptera litura (Lepidoptera: Noctuidae). PLoS One. 2013;8: e68059 10.1371/journal.pone.0068059 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Shen GM, Jiang HB, Wang XN, Wang JJ. Evaluation of endogenous references for gene expression profiling in different tissues of the oriental fruit fly Bactrocera dorsalis (Diptera: Tephritidae). BMC Mol Biol. 2010;11: 76 10.1186/1471-2199-11-76 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Yang CX, Pan HP, Liu Y, Zhou XG. Selection of reference genes for expression analysis using quantitative real-time PCR in the pea aphid, Acyrthosiphon pisum (Harris) (Hemiptera, Aphidiae). PLoS One. 2014;9: e110454 10.1371/journal.pone.0110454 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Shi XQ, Guo WC, Wan PJ, Zhou LT, Ren XL, Ahmat T, et al. Validation of reference genes for expression analysis by quantitative real-time PCR in Leptinotarsa decemlineata (Say). BMC Res Notes. 2013;6: 93 10.1186/1756-0500-6-93 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Fu W, Xie W, Zhang Z, Wang SL, Wu QJ, Liu Y, et al. Exploring valid reference genes for quantitative real-time PCR analysis in Plutella xylostella (Lepidoptera: Plutellidae). Int J Biol Sci. 2014;9: 792–802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Yuan M, Lu YH, Zhu X, Wan H, Shakeel M, Zhan S, et al. Selection and evaluation of potential reference genes for gene expression analysis in the brown planthopper, Nilaparvata lugens (Hemiptera: Delphacidae) using reverse-transcription quantitative PCR. PLoS One. 2014;9: e86503 10.1371/journal.pone.0086503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Liang P, Guo YJ, Zhou XG, Gao XW. Expression profiling in Bemisia tabaci under insecticide treatment: indicating the necessity for custom reference gene selection. PLoS One. 2014;9: e87514 10.1371/journal.pone.0087514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lin JT, Redies C. Histological evidence: housekeeping genes beta-actin and GAPDH are of limited value for normalization of gene expression. Dev Genes Evol. 2012;222: 369–376. 10.1007/s00427-012-0420-x [DOI] [PubMed] [Google Scholar]
- 32. Ferguson BS, Nam H, Hopkins RG, Morrison RF. Impact of reference gene selection for target gene normalization on experimental outcome using real-time qRT-PCR in adipocytes. PLoS One. 2010;5: e15208 10.1371/journal.pone.0015208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Veazey KJ, Golding MC. Golding selection of stable reference genes for quantitative RT-PCR comparisons of mouse embryonic and extra-embryonic stem cells. PLoS One. 2011;6: e27592 10.1371/journal.pone.0027592 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Thomas KC, Zheng XF, Garces Suarez F, Raftery JM, Quinlan KGR, Yang N, et al. Evidence based selection of commonly used RT-qPCR reference genes for the analysis of mouse skeletal muscle. PLoS One. 2014;9: e88653 10.1371/journal.pone.0088653 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(TIFF)
(DOCX)
(DOCX)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files.