Abstract
Background
Physical cues of cellular environment affect cell fate and differentiation. For example, an environment with high stiffness drives mesenchymal stem cells (MSCs) to undergo osteogenic differentiation, while low stiffness leads to lipogenic differentiation. Such effects could be independent of chemical/biochemical inducers.
Scope of review
Stiffness and/or topography of cellular environment can control MSC differentiation and fate determination. In addition, physical factors such as tension, resulted from profound cytoskeleton reorganization during MSC differentiation, affect the gene expression essential for the differentiation. Although physical cues control MSC lineage specification probably by reorganizing and tuning cytoskeleton, the full mechanism is largely unclear. It also remains elusive how physical signals are sensed by cells and transformed into biochemical and biological signals. More importantly, it becomes pivotal to define explicitly the physical cue(s) essential for cell differentiation and fate decision. With a focus on MSC, we present herein current understanding of the interplay between i) physical cue and factors and ii) MSC differentiation and fate determination.
Major conclusions
Biophysical cues can initiate or strengthen the biochemical signaling for MSC fate determination and differentiation. Physical properties of cellular environment direct the structural adaptation and functional coupling of the cells to their environment.
General significance
These observations not only open a simple avenue to engineer cell fate in vitro, but also start to reveal the physical elements that regulate and determine cell fate.
Keywords: mesenchymal stem cell, topography, stiffness, cytoskeleton, physical cue
Physical Cues Are Important for the Lineage Specification of MSCs
MSCs were found to undergo osteogenic differentiation in vitro with supplements such as dexamethasone and β-glycerophosphate to the culture medium [1]. Later, MSCs were found to commit lipogenic, chondrogenic, and osteogenic differentiation in vitro induced by chemicals [2]. Dexamethasone, isobutylmethylxanthine, insulin, and indomethacin induce adipogenic differentiation; transforming growth factor β3 prompts chondrogenic differentiation; while dexamethasone, β-glycerol phosphate, and ascorbate drive osteogenic differentiation [3–11]. Hence, chemical inducers play a major role in MSC lineage specification. It was unknown whether mechanical/physical cues could induce stem cell differentiation, though the extracellular matrix (ECM) properties were found to regulate cell shape, cell survival, cell differentiation, and cytoskeletal mechanics [12–14]. Also, the chemically induced MSC differentiation involves the changes in cellular physical status such as stiffness and adhesiveness, and inhibition of these physical status changes impedes or reverses MSC differentiation [15].
ECM-controlled cell spreading can determine human MSC differentiation and fate through RhoA and Rho-associated protein kinase (Rock) signaling [16]. Osteogenic differentiation of MSCs requires extensive cell spreading and high RhoA activity; while adipogenic differentiation of MSCs needs limited cell spreading and low RhoA signaling [17, 18]. MSC differentiation and fate can also be determined by the plasticity/stiffness and geometric cue of ECM microenvironment [19–21]. The MSCs spread on the ECMs with osteoid-like rigidity become bone, with intermediate stiffness commit to muscular lineage, and with brain-like softness undergo neuronal differentiation. MSC form robust stress fibers and focal adhesions in response to rigid ECM microenvironment and fewer stress fibers and focal adhesions to soft microenvironment [19]. The rigidity of 3-dimensional (3D) ECM microenvironment can also regulate MSC lineage specification through altering integrin-ECM binding and ECM ligand distribution in microenvironment [22]. Thus, it is likely that microenvironment-induced reorganization of cellular/cytoskeletal force controls the differentiation and fate determination of MSCs.
Geometrical cue, mechanical cue, and biochemical cue: applications of hydrogel and elastomeric micropost
Cell-compatible hydrogels are natural, semi-synthetic, or synthesized polymeric materials that are engineered to resemble the extracellular environment of the body’s tissues [23]. Changeable chemical composition and pliable physical properties of hydrogel make it an ideal in vitro model to simplify the study of complex biological conditions and events like MSC lineage specification. Modulation of the crosslinker quantity can selectively vary the physical properties of hydrogel such as stiffness and porosity without affecting the chemical composition of the gel.
For example, collagen-coated polyacrylamide (PAAm) gel induces the differentiation of MSCs and epidermal stem cells, and the stiffness or elastic modulus of PAAm gel regulates the fate commitment of these stem cells [21]. But the PAAm gels with different stiffnesses differ not only in gel porosity or topography but also in collagen-anchorage density. At constant stiffness, the concentration and distance of collagens that are either cross-linked to PAAm gel or embedded in polyethylene glycol (PEG) gel affect epidermal stem cell differentiation, suggesting that the stem cells exert mechanical force on surrounding ECM and gauge the mechanical feedback of the ECM for cell-fate decision [21]. This result, together with the observation that polydimethylsiloxane (PDMS) gels of different stiffnesses don’t affect the differentiation and fate commitment of MSCs and epidermal stem cells [21], also exclude that stiffness is essential for stem cell differentiation. However, a recent study showed that varying porosity without altering stiffness of PAAm gel does not significantly change protein tethering, substrate deformations, or the osteogenic and adipogenic differentiation of human adipose- and marrow-derived MSCs [24]. Even with varied protein tethering, MSC lineage specification, surface–protein unfolding, or underlying substrate deformations remains affected. MSC differentiation is also unaffected even without protein tethering. Hence, based on this study, environmental stiffness can regulate MSC differentiation in protein tethering- and environment porosity-independent manner.
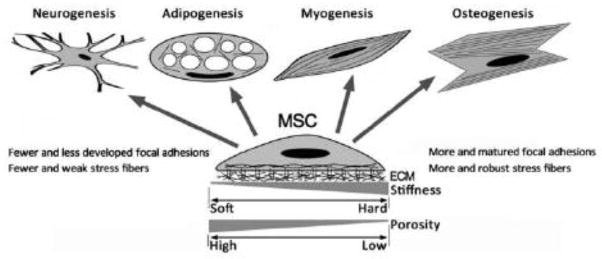
Despite of various advantages of hydrogels, hydrogel manipulation could alter surface chemistry, backbone flexibility, and binding property of gel-immobilized ligands. Micromolded elastomeric micropost array is an alternative approach to understand how cells sense changes in microenvironmental rigidity, which is controlled by hexagonally spaced PDMS microposts with different heights [25]. Elastomeric micropost array can decouple microenvironmental rigidity from adhesive and surface properties and correlate subcellular traction forces with focal adhesions. Bone lineage commitment is reflected by higher traction force and more focal adhesions of MSCs during differentiation, while fat formation is manifested by lower traction force and fewer or less developed focal adhesions [26].
The geometric cue that triggers cell spreading is more important for MSC differentiation than the size of cell spreading area [15]. MSCs grown in a confined region that 1) is elongated and spindle-shaped or with sharp edges and 2) favors focal adhesion formation and cytoskeleton organization will commit to osteogenic lineage, while MSCs grown in a relatively rounded region will become adipocytic. Moreover, sharp geometrically patterned edges generate high stress concentration and high density of focal adhesions [15, 27].
Other mechanical factors also play roles in MSC differentiation. Under microgravity, stem cells tend to differentiate into adipocytes with the activation of lipogenic factors such as PPARγ2, while osteogenic differentiation is reduced [28]. Shear stress can also induce osteogenic differentiation [29]. Strain inhibits adipogenesis [30] but stimulates osteogenesis [31]. Cyclic compression can cause MSCs undergo chondrogenesis [32].
Together, 1) a microenvironment with geometry, stiffness, and ECM ligand that favor the development of stress fibers and/or focal adhesions preferentially induces the osteogenic differentiation of MSC and 2) a microenvironment that inhibits focal adhesion or stress fiber favors adipogenic differentiation. Although cytoskeleton reorganization and cytoskeleton-adhesion receptor connection are important in MSC differentiation and lineage specification [14, 16, 19, 21], how the changes in stress fiber and focal adhesion alter MSC fate remains unclear. It is likely that soft ECM microenvironments cannot stabilize integrin-ECM binding, leading to integrin internalization [33]. Subsequently, focal adhesions could not form easily, stress could not be concentrated, and cells would not sense and transduce sufficient mechanical signals. Stiff ECM microenvironments make integrin-ECM binding relatively stable and stronger and allow more robust formation of focal adhesions and better tethering of stress fibers to the ECM-bound integrins at the plasma membrane [33, 34].
Cytoskeletal tension and MSC lineage specification: application of cytoskeleton regulators
Cytochalasin D disrupts actin cytoskeleton by inhibiting actin polymerization and was used in several studies for the differentiation of different stem cells [35, 36]. Disruption of actin cytoskeleton leads to adipogenic differentiation of MSCs and embryonic stem cells, accompanied by a decrease of Young’s modulus of the cells during the course of differentiation. Young’s modulus, also known as elastic modulus, is used to describe the stiffness or elasticity of materials. Actin cytoskeleton disruption in bone marrow stromal cells, which represent a population of multipotent MSCs, results in neuronal differentiation [37]. Therefore, with disrupted actin cytoskeleton, MSCs tend to differentiate into soft tissues such as fat and nerve, supporting that actin cytoskeleton organization can control MSC lineage specification.
Blebbistatin inhibits non-muscle myosin II [38] and promotes adipogenic differentiation of MSCs [39].Non-muscle myosin II is required for actin fiber bundling and contractile force generation. Without myosin II, cytoskeletal tensile force and cellular contractility are diminished [40]. The contractility is important for cells to sense the stiffness of microenvironment, re-program the gene expression profile of MSC, and exert forces to deform ECM [19]. Rock inhibitor Y27632 enhances adipogenic and reduces osteogenic differentiation of MSCs [41], by inhibiting stress fiber and focal adhesion formations. Besides cytoskeleton-regulatory reagents, virus-mediated cellular delivery of RhoA, which promotes stress fiber and focal adhesion formations [42], and miRNA-mediated alteration of focal adhesion kinase level also regulate MSC differentiation [16, 43–45]. Again, the studies from cellular aspect support the notion that focal adhesions and stress fibers regulate MSC differentiation and fate determination.
Although disassembly of microtubules with nocodazole promotes bone morphogenesis and osteoblast differentiation [46], the mechanism is unclear. According to the tensegrity model [47], tension is accumulated in actin fibers while microtubules balance the tension so that the cell won’t collapse. It is likely that, during osteogenic differentiation, the compression within microtubules is also increased to balance the elevated inward cellular tension from actin fibers.
Cell shape and MSC lineage specification
MSC differentiation can be controlled by the shape of MSCs [15, 48–50] (Figure 3) and results in the cells with different shapes to form functionally different tissues [16]. For example, osteogenic differentiation leads to a flattened or spreaded cell shape while adipogenic differentiation a rounded or non-spread cell shape. The MSCs being adapted to a cell shape that increases actomyosin contractility promote osteogenesis [15], consistent with the finding that myosin inhibition reduces osteogenesis. Cell shape manipulation also alters Rac1 activity [49], which promotes the formation of actin meshwork and antagonizes actomyosin contractility; while Rac1 inhibition promotes osteoblastic differentiation [51]. In addition to osteogenic and adipogenic differentiation, cell shape also modulates myocardial [52], neuronal [53], and myogenic [52, 54, 55] differentiation. Cell shape also contributes to the maintenance of differentiated cells. Chondrocytes, for example, gradually lose their cartilage phenotype when flattened [56].
Figure 3. Physical parameters of ECM regulate MSC fate and differentiation.
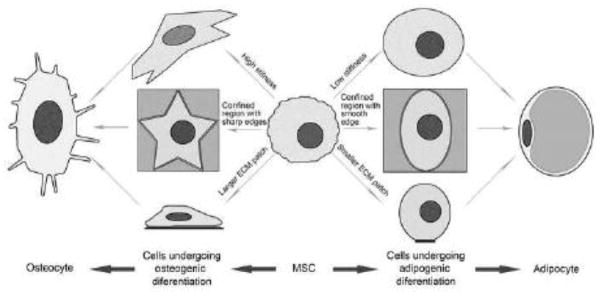
MSCs positioned on ECM substratum with high stiffness, a confined region with sharp edges, or large size may undergo osteogenic differentiation; while MSCs positioned on ECM substratum with low stiffness, a confined region with smooth edges, or small size may undergo adipogenic differentiation.
Cell shape and intracellular tension are correlated. On the one hand, intracellular tension increases when cell spreading area is increased [25, 57]. The highest force tends to be localized to protrusions or corners when either non-stem cells [57] or MSCs[15] are cultured on a confined region. On the other hand, cytoskeletal tension regulates cell shape and focal adhesion formation [58]. Since cell shape could directly reflect the physical environment of cells, the geometry of MSC niche is likely to play roles in MSC differentiation by, in parts, modulating cytoskeletal tension.
Physical cue-initiated signaling to specify MSC lineage
Cells on firm substrate tend to develop mature focal adhesions with extensive cell spreading, while cells on flexible substrate develop a relatively dynamic focal complex with limited cell spreading [59]. Focal adhesion formation requires stable binding of clustered integrins to ECM, while blocking integrins with antibodies or integrin binding to soft ECM makes the formation of stable and strong integrin-ECM bonds difficult [33, 60]. In addition, stiff ECM induces more phosphorylation/activation of non-muscular myosin light chain (MLC) and subsequently increases intracellular stress or cytoskeleton tension [61], by forming more focal adhesions and stress fibers [47]. We predict that the cell adhesion strengthening process can also modulate MSC lineage specification by building up cytoskeleton tension. Tissue stiffness stabilizes nuclearskeleton protein lamin-A, which in turn upregulates the expression of stress fiber-relevant genes through serum response factor (SRF) and hippo signaling factor YAP1[62]. Lamin-A silencing enhances MSC differentiation on soft matrix to fat, while increases in Lamin-A levels enhance MSC differentiation on stiff matrix to bone. The retinoic acid (RA) pathway transduces the signal of matrix stiffness to nucleus to regulate lamin-A transcription [62].
Signaling associated with osteogenic differentiation of MSCs includes RhoA/Rock/MLC, FAK, Ras/MAPK-ERK, NF-kB, and bone morphogenetic protein (BMP) II [17, 44, 63, 64]. The activities of lipogenic enzymes such as glycerophosphate dehydrogenase and fatty acid synthetase are increased in the adipogenic differentiation of 3T3-F442A cells [65]. In nucleus, lamin-A physically stabilizes the nuclear lamina and chromatin against stress and impedes nuclear remodeling under stress [62]. Other mechanical cues such as cyclic compression also trigger osteogenic differentiation [19, 66], reinforce cellular tensional structures [67], and thereby likely shares similar signaling mechanisms. However, why calcium deposition is linked to cytoskeleton reorganization remains unclear.
Adipogenic differentiation seems correlated with increased integrin activation and internalization and increased caveolae-mediated endocytosis of BMP receptor [33]. In a soft ECM environment, integrins are more easily internalized so that the formation of stable integrin-ECM bonds is inhibited. Consequently, it is difficult to assemble focal adhesions, form stress fibers, and then mount intracellular tension. In addition, BMP internalization makes the MSCs less likely to differentiate into bone cells. Cell shape, controlled by micro-patterned geometry, seems uncorrelated with adipogenic differentiation [68], and focal adhesions are inessential for adipogenic differentiation. In soft ECM, insulin receptor expression and signaling are enhanced [69], which strengthens the effects of insulin-induced lipogenesis. PPARγ is required for adipogenesis [70]. Mechanical loading downregulates PPARγ [71], and PPARγ agonist reduces focal adhesion formation [72]. Thus, soft ECM likely activates PPARγ, given that mechanical loading and PPARγ activity are inversely correlated.
Integrin signaling, triggered by cell-matrix adhesion, apparently modulates MSC lineage specification, although specific and coordinated roles of individual integrins remain to be determined. Integrin α2-mediated activation of Rock, FAK, and ERK promotes the osteogenic differentiation of human bone marrow-derived MSCs in stiffer ECM [73]. During adipogenic differentiation of mouse 3T3-L1 preadipocytes, integrin expression is differentially regulated, with a gradual decrease in the level of integrin α5 and an increase in α6. Overexpression of integrin α5 increases proliferation and decreases adipogenic differentiation, while overexpression of integrin α6 does not affect differentiation [74]. A functional-blocking antibody of α5β1 integrin reduces both formation of bone nodules and expressions of osteogenic genes [75]. Blocking integrin αvβ1 increases adipogenesis and decrease osteogenesis of mouse bone marrow-derived MSCs [76].
Summary
Extracellular environments in tissues and organs have distinct physical properties, which directly regulate cell behaviors and fates. How biochemical and biophysical factors of microenvironment affect MSC lineage specification remains to be fully elucidated. A relatively better understood example is that tissue stiffness scales with the collagen content extracellularly, the actin cytoskeleton tension intracellularly, and the lamin A level intranuclearly. This directional signaling flow changes the gene expression profile of MSCs and then the cell fate.
In RGD peptide-conjugated 3D alginate gel system, the osteogenic differentiation of MSCs favors an environment with stiffness of ~11–30 Kpa[22]. The osteoid without mineralization, which is composed primarily of collagen, has a stiffness of ~27 Kpa [19] and provides appropriate mechanical cues for osteoblast differentiation, which in turn leads to osteoid mineralization during bone development or repair. Higher stiffness would inhibit osteogenesis in a 3D environment [22]. Bone has a Young’s modulus of ~10–20 Gpa [77], which results apparently from calcification after osteogenic differentiation. In contrast, adipose tissue has a Young’s modulus of ~3 Kpa [78], similar to the stiffness used in vitro to induce adipogenic differentiation of MSCs in a 3D environment [22].
In addition to being the crucial machinery that controls cell movement, morphology, division, and organelle transport, cytoskeleton is also a key determinant for stem cell differentiation and fate determination. Although cytoskeleton tension is critical in controlling MSC lineage specification [16, 17], why and how cytoskeleton tension regulates MSC lineage specification are not understood. Cytoskeleton tension probably couples microenvironmental mechanical or geometric signals [47] to nuclearskeleton reorganization. Indeed, lamin A becomes increased in response to extracellular tension, making the nucleus more resistant to deformation [62]. Stem cell differentiation driven by cytoskeleton and nuclearskeleton tension is probably adaptation process that directs cell destiny suitable for the environmental physical properties. Also possibly, when the biochemical signals for differentiation such as hormones and growth factors are at suboptimal concentrations, environmental physical cues serve as a double-check mechanism that promotes the differentiation of stem cells toward specific lineages.
Conclusion
Like biochemical signals, the biophysical cues of MSC environment contribute to MSC fate determination and differentiation, which involve multiple signaling pathways. The signaling triggered by biochemical and biophysical cues is probably indistinguishable from each other, especially for downstream signaling events. The biochemical signaling of MSC lineage specification induces profound cytoskeleton reorganization and subsequent changes in cellular biophysical properties such as tension and adhesiveness; while biophysical changes in cytoskeleton and nuclearskeleton during MSC fate commitment and differentiation also affect and alter biochemical signaling. Thus, biophysical cues and factors i) generate, strengthen, or sustain biochemical signals for MSC lineage specification, to fine-tune the differentiation processes, and ii) play crucial roles in establishing the tissue structures proper for biological functions. However, how the stimulations from environmental physical cues are converted to arrays of cellular signaling remains unclear, how biophysical changes from cytoskeleton reorganization result in expression profile switch in the lineage-specific genes is largely elusive, and how the crosstalk between biochemical and biophysical signals needs further investigation.
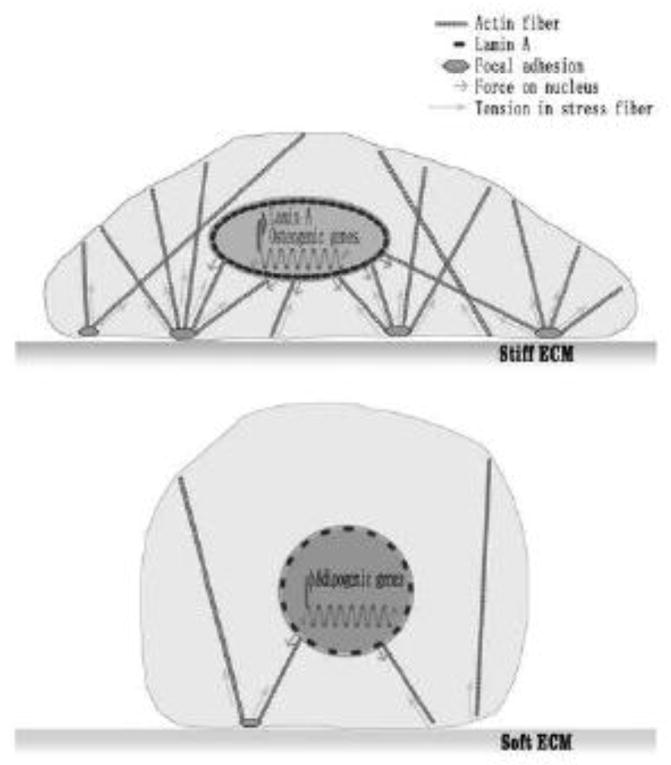
Figure 1. Environmental physical cues regulate MSC fate and differentiation.
MSC differentiation and lineage commitment can be controlled by physical cues such as the stiffness and topography of ECM environments, through the changes in actin cytoskeleton and cell adhesion structures.
Figure 2. Environmental physical cues affect gene expression by altering cytoskeletal and nuclearskeletal tension.
ECM with high stiffness increases intracellular tension, leading to the deformation of nucleus and upregulation of lamin A and osteogenic gene expression. ECM with low stiffness decreases intracellular tension, leading to the upregulation of adipogenic gene expression.
Highlights.
Environmental physical cues can determine MSC fate and differentiation.
Cytoskeleton and nuclear skeleton undergo reorganization during MSC differentiation.
MSC fate determination and differentiation are associated with changes in cellular biophysical properties.
Physical cues and chemical inducers specify MSC lineages by regulating cell adhesion molecules and Rho GTPases.
Acknowledgments
This work was supported by NIH grant CA096991, AHA grant 13GRNT17040028, and OCAST grant HR13-207 to XAZ. XAZ is an Oklahoma TSET Cancer Research Scholar. Owing to the page and reference number limitations, we could not cite all of relevant original research articles and thereby apologize to the authors whose publications are not cited.
Abbreviation
- 3D
3-dimesional
- BMP
bone morphogenetic protein
- ECM
extracellular matrix
- FN
fibronectin
- LN
laminin
- MLC
myosin light chain
- MSC
mesenchymal stem cell
- PAAm
polyarylamide
- PDMS
polydimethylsiloxane
- PEG
polyethylene glycol
- Rock
Rho-associated protein kinase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Schoeters GE, de Saint-Georges L, Van den Heuvel R, Vanderborght O. Mineralization of adult mouse bone marrow in vitro. Cell and tissue kinetics. 1988;21:363–374. doi: 10.1111/j.1365-2184.1988.tb00794.x. [DOI] [PubMed] [Google Scholar]
- 2.Jaiswal N, Haynesworth SE, Caplan AI, Bruder SP. Osteogenic differentiation of purified, culture-expanded human mesenchymal stem cells in vitro. Journal of cellular biochemistry. 1997;64:295–312. [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Sun L, Nicholson AC, Hajjar DP, Gotto AM, Jr, Han J. Adipogenic differentiating agents regulate expression of fatty acid binding protein and CD36 in the J744 macrophage cell line. Journal of lipid research. 2003;44:1877–1886. doi: 10.1194/jlr.M300084-JLR200. [DOI] [PubMed] [Google Scholar]
- 5.Leong DT, Abraham MC, Rath SN, Lim TC, Chew FT, Hutmacher DW. Investigating the effects of preinduction on human adipose-derived precursor cells in an athymic rat model. Differentiation; research in biological diversity. 2006;74:519–529. doi: 10.1111/j.1432-0436.2006.00092.x. [DOI] [PubMed] [Google Scholar]
- 6.Gupta A, Leong DT, Bai HF, Singh SB, Lim TC, Hutmacher DW. Osteo-maturation of adipose-derived stem cells required the combined action of vitamin D3, beta-glycerophosphate, and ascorbic acid. Biochemical and biophysical research communications. 2007;362:17–24. doi: 10.1016/j.bbrc.2007.07.112. [DOI] [PubMed] [Google Scholar]
- 7.Leong DT, Khor WM, Chew FT, Lim TC, Hutmacher DW. Characterization of osteogenically induced adipose tissue-derived precursor cells in 2-dimensional and 3-dimensional environments. Cells, tissues, organs. 2006;182:1–11. doi: 10.1159/000091713. [DOI] [PubMed] [Google Scholar]
- 8.Zuk PA, Zhu M, Mizuno H, Huang J, Futrell JW, Katz AJ, Benhaim P, Lorenz HP, Hedrick MH. Multilineage cells from human adipose tissue: implications for cell-based therapies. Tissue engineering. 2001;7:211–228. doi: 10.1089/107632701300062859. [DOI] [PubMed] [Google Scholar]
- 9.Hsu WK, Wang JC, Liu NQ, Krenek L, Zuk PA, Hedrick MH, Benhaim P, Lieberman JR. Stem cells from human fat as cellular delivery vehicles in an athymic rat posterolateral spine fusion model. The Journal of bone and joint surgery American volume. 2008;90:1043–1052. doi: 10.2106/JBJS.G.00292. [DOI] [PubMed] [Google Scholar]
- 10.Guilak F, Awad HA, Fermor B, Leddy HA, Gimble JM. Adipose-derived adult stem cells for cartilage tissue engineering. Biorheology. 2004;41:389–399. [PubMed] [Google Scholar]
- 11.Ashjian PH, Elbarbary AS, Edmonds B, DeUgarte D, Zhu M, Zuk PA, Lorenz HP, Benhaim P, Hedrick MH. In vitro differentiation of human processed lipoaspirate cells into early neural progenitors. Plastic and reconstructive surgery. 2003;111:1922–1931. doi: 10.1097/01.PRS.0000055043.62589.05. [DOI] [PubMed] [Google Scholar]
- 12.Chen CS, Mrksich M, Huang S, Whitesides GM, Ingber DE. Geometric control of cell life and death. Science. 1997;276:1425–1428. doi: 10.1126/science.276.5317.1425. [DOI] [PubMed] [Google Scholar]
- 13.Mooney D, Hansen L, Vacanti J, Langer R, Farmer S, Ingber D. Switching from differentiation to growth in hepatocytes: control by extracellular matrix. Journal of cellular physiology. 1992;151:497–505. doi: 10.1002/jcp.1041510308. [DOI] [PubMed] [Google Scholar]
- 14.Wang N, Ingber DE. Control of cytoskeletal mechanics by extracellular matrix, cell shape, and mechanical tension. Biophysical journal. 1994;66:2181–2189. doi: 10.1016/S0006-3495(94)81014-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kilian KA, Bugarija B, Lahn BT, Mrksich M. Geometric cues for directing the differentiation of mesenchymal stem cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:4872–4877. doi: 10.1073/pnas.0903269107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McBeath R, Pirone DM, Nelson CM, Bhadriraju K, Chen CS. Cell shape, cytoskeletal tension, and RhoA regulate stem cell lineage commitment. Developmental cell. 2004;6:483–495. doi: 10.1016/s1534-5807(04)00075-9. [DOI] [PubMed] [Google Scholar]
- 17.Arnsdorf EJ, Tummala P, Kwon RY, Jacobs CR. Mechanically induced osteogenic differentiation--the role of RhoA, ROCKII and cytoskeletal dynamics. Journal of cell science. 2009;122:546–553. doi: 10.1242/jcs.036293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Tay CY, Koh CG, Tan NS, Leong DT, Tan LP. Mechanoregulation of stem cell fate via micro-/nano-scale manipulation for regenerative medicine. Nanomedicine (Lond) 2013;8:623–638. doi: 10.2217/nnm.13.31. [DOI] [PubMed] [Google Scholar]
- 19.Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126:677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- 20.Rowlands AS, George PA, Cooper-White JJ. Directing osteogenic and myogenic differentiation of MSCs: interplay of stiffness and adhesive ligand presentation. American journal of physiology Cell physiology. 2008;295:C1037–1044. doi: 10.1152/ajpcell.67.2008. [DOI] [PubMed] [Google Scholar]
- 21.Trappmann B, Gautrot JE, Connelly JT, Strange DG, Li Y, Oyen ML, Cohen Stuart MA, Boehm H, Li B, Vogel V, Spatz JP, Watt FM, Huck WT. Extracellular-matrix tethering regulates stem-cell fate. Nature materials. 2012;11:642–649. doi: 10.1038/nmat3339. [DOI] [PubMed] [Google Scholar]
- 22.Huebsch N, Arany PR, Mao AS, Shvartsman D, Ali OA, Bencherif SA, Rivera-Feliciano J, Mooney DJ. Harnessing traction-mediated manipulation of the cell/matrix interface to control stem-cell fate. Nature materials. 2010;9:518–526. doi: 10.1038/nmat2732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carbonetto ST, Gruver MM, Turner DC. Nerve fiber growth on defined hydrogel substrates. Science. 1982;216:897–899. doi: 10.1126/science.7079743. [DOI] [PubMed] [Google Scholar]
- 24.Wen JH, Vincent LG, Fuhrmann A, Choi YS, Hribar KC, Taylor-Weiner H, Chen S, Engler AJ. Interplay of matrix stiffness and protein tethering in stem cell differentiation. Nature materials. 2014 doi: 10.1038/nmat4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tan JL, Tien J, Pirone DM, Gray DS, Bhadriraju K, Chen CS. Cells lying on a bed of microneedles: an approach to isolate mechanical force. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:1484–1489. doi: 10.1073/pnas.0235407100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fu J, Wang YK, Yang MT, Desai RA, Yu X, Liu Z, Chen CS. Mechanical regulation of cell function with geometrically modulated elastomeric substrates. Nature methods. 2010;7:733–736. doi: 10.1038/nmeth.1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim MC, Neal DM, Kamm RD, Asada HH. Dynamic modeling of cell migration and spreading behaviors on fibronectin coated planar substrates and micropatterned geometries. PLoS computational biology. 2013;9:e1002926. doi: 10.1371/journal.pcbi.1002926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zayzafoon M, Gathings WE, McDonald JM. Modeled microgravity inhibits osteogenic differentiation of human mesenchymal stem cells and increases adipogenesis. Endocrinology. 2004;145:2421–2432. doi: 10.1210/en.2003-1156. [DOI] [PubMed] [Google Scholar]
- 29.Yourek G, McCormick SM, Mao JJ, Reilly GC. Shear stress induces osteogenic differentiation of human mesenchymal stem cells. Regenerative medicine. 2010;5:713–724. doi: 10.2217/rme.10.60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Sen B, Xie Z, Case N, Ma M, Rubin C, Rubin J. Mechanical strain inhibits adipogenesis in mesenchymal stem cells by stimulating a durable beta-catenin signal. Endocrinology. 2008;149:6065–6075. doi: 10.1210/en.2008-0687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kearney EM, Farrell E, Prendergast PJ, Campbell VA. Tensile strain as a regulator of mesenchymal stem cell osteogenesis. Annals of biomedical engineering. 2010;38:1767–1779. doi: 10.1007/s10439-010-9979-4. [DOI] [PubMed] [Google Scholar]
- 32.Duty AO, Oest ME, Guldberg RE. Cyclic mechanical compression increases mineralization of cell-seeded polymer scaffolds in vivo. Journal of biomechanical engineering. 2007;129:531–539. doi: 10.1115/1.2746375. [DOI] [PubMed] [Google Scholar]
- 33.Du J, Chen X, Liang X, Zhang G, Xu J, He L, Zhan Q, Feng XQ, Chien S, Yang C. Integrin activation and internalization on soft ECM as a mechanism of induction of stem cell differentiation by ECM elasticity. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:9466–9471. doi: 10.1073/pnas.1106467108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Higuchi A, Ling QD, Chang Y, Hsu ST, Umezawa A. Physical cues of biomaterials guide stem cell differentiation fate. Chemical reviews. 2013;113:3297–3328. doi: 10.1021/cr300426x. [DOI] [PubMed] [Google Scholar]
- 35.Feng T, Szabo E, Dziak E, Opas M. Cytoskeletal disassembly and cell rounding promotes adipogenesis from ES cells. Stem cell reviews. 2010;6:74–85. doi: 10.1007/s12015-010-9115-8. [DOI] [PubMed] [Google Scholar]
- 36.Yourek G, Hussain MA, Mao JJ. Cytoskeletal changes of mesenchymal stem cells during differentiation. ASAIO journal. 2007;53:219–228. doi: 10.1097/MAT.0b013e31802deb2d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Neuhuber B, Gallo G, Howard L, Kostura L, Mackay A, Fischer I. Reevaluation of in vitro differentiation protocols for bone marrow stromal cells: disruption of actin cytoskeleton induces rapid morphological changes and mimics neuronal phenotype. Journal of neuroscience research. 2004;77:192–204. doi: 10.1002/jnr.20147. [DOI] [PubMed] [Google Scholar]
- 38.Kovacs M, Toth J, Hetenyi C, Malnasi-Csizmadia A, Sellers JR. Mechanism of blebbistatin inhibition of myosin II. The Journal of biological chemistry. 2004;279:35557–35563. doi: 10.1074/jbc.M405319200. [DOI] [PubMed] [Google Scholar]
- 39.Schiller ZA, Schiele NR, Sims JK, Lee K, Kuo CK. Adipogenesis of adipose-derived stem cells may be regulated via the cytoskeleton at physiological oxygen levels in vitro. Stem cell research & therapy. 2013;4:79. doi: 10.1186/scrt230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ma X, Kovacs M, Conti MA, Wang A, Zhang Y, Sellers JR, Adelstein RS. Nonmuscle myosin II exerts tension but does not translocate actin in vertebrate cytokinesis. Proceedings of the National Academy of Sciences of the United States of America. 2012;109:4509–4514. doi: 10.1073/pnas.1116268109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Eyckmans J, Lin GL, Chen CS. Adhesive and mechanical regulation of mesenchymal stem cell differentiation in human bone marrow and periosteum-derived progenitor cells. Biology open. 2012;1:1058–1068. doi: 10.1242/bio.20122162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Chrzanowska-Wodnicka M, Burridge K. Rho-stimulated contractility drives the formation of stress fibers and focal adhesions. The Journal of cell biology. 1996;133:1403–1415. doi: 10.1083/jcb.133.6.1403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Meyers VE, Zayzafoon M, Douglas JT, McDonald JM. RhoA and cytoskeletal disruption mediate reduced osteoblastogenesis and enhanced adipogenesis of human mesenchymal stem cells in modeled microgravity. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2005;20:1858–1866. doi: 10.1359/JBMR.050611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Salasznyk RM, Klees RF, Williams WA, Boskey A, Plopper GE. Focal adhesion kinase signaling pathways regulate the osteogenic differentiation of human mesenchymal stem cells. Experimental cell research. 2007;313:22–37. doi: 10.1016/j.yexcr.2006.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Salasznyk RM, Klees RF, Boskey A, Plopper GE. Activation of FAK is necessary for the osteogenic differentiation of human mesenchymal stem cells on laminin-5. Journal of cellular biochemistry. 2007;100:499–514. doi: 10.1002/jcb.21074. [DOI] [PubMed] [Google Scholar]
- 46.Zhao M, Ko SY, Liu JH, Chen D, Zhang J, Wang B, Harris SE, Oyajobi BO, Mundy GR. Inhibition of microtubule assembly in osteoblasts stimulates bone morphogenetic protein 2 expression and bone formation through transcription factor Gli2. Molecular and cellular biology. 2009;29:1291–1305. doi: 10.1128/MCB.01566-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Walcott S, Sun SX. A mechanical model of actin stress fiber formation and substrate elasticity sensing in adherent cells. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:7757–7762. doi: 10.1073/pnas.0912739107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dalby MJ, Gadegaard N, Tare R, Andar A, Riehle MO, Herzyk P, Wilkinson CD, Oreffo RO. The control of human mesenchymal cell differentiation using nanoscale symmetry and disorder. Nature materials. 2007;6:997–1003. doi: 10.1038/nmat2013. [DOI] [PubMed] [Google Scholar]
- 49.Gao L, McBeath R, Chen CS. Stem cell shape regulates a chondrogenic versus myogenic fate through Rac1 and N-cadherin. Stem cells. 2010;28:564–572. doi: 10.1002/stem.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Ruiz SA, Chen CS. Emergence of patterned stem cell differentiation within multicellular structures. Stem cells. 2008;26:2921–2927. doi: 10.1634/stemcells.2008-0432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Onishi M, Fujita Y, Yoshikawa H, Yamashita T. Inhibition of Rac1 promotes BMP-2-induced osteoblastic differentiation. Cell death & disease. 2013;4:e698. doi: 10.1038/cddis.2013.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tay CY, Yu H, Pal M, Leong WS, Tan NS, Ng KW, Leong DT, Tan LP. Micropatterned matrix directs differentiation of human mesenchymal stem cells towards myocardial lineage. Experimental cell research. 2010;316:1159–1168. doi: 10.1016/j.yexcr.2010.02.010. [DOI] [PubMed] [Google Scholar]
- 53.Lee J, Abdeen AA, Zhang D, Kilian KA. Directing stem cell fate on hydrogel substrates by controlling cell geometry, matrix mechanics and adhesion ligand composition. Biomaterials. 2013;34:8140–8148. doi: 10.1016/j.biomaterials.2013.07.074. [DOI] [PubMed] [Google Scholar]
- 54.Yu T, Chua CK, Tay CY, Wen F, Yu H, Chan JK, Chong MS, Leong DT, Tan LP. A generic micropatterning platform to direct human mesenchymal stem cells from different origins towards myogenic differentiation. Macromolecular bioscience. 2013;13:799–807. doi: 10.1002/mabi.201200481. [DOI] [PubMed] [Google Scholar]
- 55.Yu H, Tay CY, Pal M, Leong WS, Li H, Li H, Wen F, Leong DT, Tan LP. A bio-inspired platform to modulate myogenic differentiation of human mesenchymal stem cells through focal adhesion regulation. Advanced healthcare materials. 2013;2:442–449. doi: 10.1002/adhm.201200142. [DOI] [PubMed] [Google Scholar]
- 56.von der Mark K, Gauss V, von der Mark H, Muller P. Relationship between cell shape and type of collagen synthesised as chondrocytes lose their cartilage phenotype in culture. Nature. 1977;267:531–532. doi: 10.1038/267531a0. [DOI] [PubMed] [Google Scholar]
- 57.Wang N, Ostuni E, Whitesides GM, Ingber DE. Micropatterning tractional forces in living cells. Cell motility and the cytoskeleton. 2002;52:97–106. doi: 10.1002/cm.10037. [DOI] [PubMed] [Google Scholar]
- 58.Chen CS, Alonso JL, Ostuni E, Whitesides GM, Ingber DE. Cell shape provides global control of focal adhesion assembly. Biochemical and biophysical research communications. 2003;307:355–361. doi: 10.1016/s0006-291x(03)01165-3. [DOI] [PubMed] [Google Scholar]
- 59.Pelham RJ, Jr, Wang Y. Cell locomotion and focal adhesions are regulated by substrate flexibility. Proceedings of the National Academy of Sciences of the United States of America. 1997;94:13661–13665. doi: 10.1073/pnas.94.25.13661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Schneider GB, Zaharias R, Stanford C. Osteoblast integrin adhesion and signaling regulate mineralization. Journal of dental research. 2001;80:1540–1544. doi: 10.1177/00220345010800061201. [DOI] [PubMed] [Google Scholar]
- 61.Polte TR, Eichler GS, Wang N, Ingber DE. Extracellular matrix controls myosin light chain phosphorylation and cell contractility through modulation of cell shape and cytoskeletal prestress. American journal of physiology Cell physiology. 2004;286:C518–528. doi: 10.1152/ajpcell.00280.2003. [DOI] [PubMed] [Google Scholar]
- 62.Swift J, Ivanovska IL, Buxboim A, Harada T, Dingal PC, Pinter J, Pajerowski JD, Spinler KR, Shin JW, Tewari M, Rehfeldt F, Speicher DW, Discher DE. Nuclear lamin-A scales with tissue stiffness and enhances matrix-directed differentiation. Science. 2013;341:1240104. doi: 10.1126/science.1240104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hess K, Ushmorov A, Fiedler J, Brenner RE, Wirth T. TNFalpha promotes osteogenic differentiation of human mesenchymal stem cells by triggering the NF-kappaB signaling pathway. Bone. 2009;45:367–376. doi: 10.1016/j.bone.2009.04.252. [DOI] [PubMed] [Google Scholar]
- 64.Peng S, Zhou G, Luk KD, Cheung KM, Li Z, Lam WM, Zhou Z, Lu WW. Strontium promotes osteogenic differentiation of mesenchymal stem cells through the Ras/MAPK signaling pathway. Cellular physiology and biochemistry : international journal of experimental cellular physiology, biochemistry, and pharmacology. 2009;23:165–174. doi: 10.1159/000204105. [DOI] [PubMed] [Google Scholar]
- 65.Spiegelman BM, Ginty CA. Fibronectin modulation of cell shape and lipogenic gene expression in 3T3-adipocytes. Cell. 1983;35:657–666. doi: 10.1016/0092-8674(83)90098-3. [DOI] [PubMed] [Google Scholar]
- 66.Sumanasinghe RD, Bernacki SH, Loboa EG. Osteogenic differentiation of human mesenchymal stem cells in collagen matrices: effect of uniaxial cyclic tensile strain on bone morphogenetic protein (BMP-2) mRNA expression. Tissue engineering. 2006;12:3459–3465. doi: 10.1089/ten.2006.12.3459. [DOI] [PubMed] [Google Scholar]
- 67.Titushkin I, Cho M. Modulation of cellular mechanics during osteogenic differentiation of human mesenchymal stem cells. Biophysical journal. 2007;93:3693–3702. doi: 10.1529/biophysj.107.107797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Song W, Lu H, Kawazoe N, Chen G. Adipogenic differentiation of individual mesenchymal stem cell on different geometric micropatterns. Langmuir : the ACS journal of surfaces and colloids. 2011;27:6155–6162. doi: 10.1021/la200487w. [DOI] [PubMed] [Google Scholar]
- 69.Li Q, Hosaka T, Jambaldorj B, Nakaya Y, Funaki M. Extracellular matrix with the rigidity of adipose tissue helps 3T3-L1 adipocytes maintain insulin responsiveness. The journal of medical investigation : JMI. 2009;56:142–149. doi: 10.2152/jmi.56.142. [DOI] [PubMed] [Google Scholar]
- 70.Rosen ED, Hsu CH, Wang X, Sakai S, Freeman MW, Gonzalez FJ, Spiegelman BM. C/EBPalpha induces adipogenesis through PPARgamma: a unified pathway. Genes & development. 2002;16:22–26. doi: 10.1101/gad.948702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.David V, Martin A, Lafage-Proust MH, Malaval L, Peyroche S, Jones DB, Vico L, Guignandon A. Mechanical loading down-regulates peroxisome proliferator-activated receptor gamma in bone marrow stromal cells and favors osteoblastogenesis at the expense of adipogenesis. Endocrinology. 2007;148:2553–2562. doi: 10.1210/en.2006-1704. [DOI] [PubMed] [Google Scholar]
- 72.Chen Y, Wang SM, Wu JC, Huang SH. Effects of PPARgamma agonists on cell survival and focal adhesions in a Chinese thyroid carcinoma cell line. Journal of cellular biochemistry. 2006;98:1021–1035. doi: 10.1002/jcb.20839. [DOI] [PubMed] [Google Scholar]
- 73.Shih YR, Tseng KF, Lai HY, Lin CH, Lee OK. Matrix stiffness regulation of integrin-mediated mechanotransduction during osteogenic differentiation of human mesenchymal stem cells. Journal of bone and mineral research : the official journal of the American Society for Bone and Mineral Research. 2011;26:730–738. doi: 10.1002/jbmr.278. [DOI] [PubMed] [Google Scholar]
- 74.Liu J, DeYoung SM, Zhang M, Zhang M, Cheng A, Saltiel AR. Changes in integrin expression during adipocyte differentiation. Cell metabolism. 2005;2:165–177. doi: 10.1016/j.cmet.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 75.Moursi AM, Globus RK, Damsky CH. Interactions between integrin receptors and fibronectin are required for calvarial osteoblast differentiation in vitro. Journal of cell science. 1997;110(Pt 18):2187–2196. doi: 10.1242/jcs.110.18.2187. [DOI] [PubMed] [Google Scholar]
- 76.Chen Q, Shou P, Zhang L, Xu C, Zheng C, Han Y, Li W, Huang Y, Zhang X, Shao C, Roberts AI, Rabson AB, Ren G, Zhang Y, Wang Y, Denhardt DT, Shi Y. An osteopontin-integrin interaction plays a critical role in directing adipogenesis and osteogenesis by mesenchymal stem cells. Stem cells. 2014;32:327–337. doi: 10.1002/stem.1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Rho JY, Ashman RB, Turner CH. Young’s modulus of trabecular and cortical bone material: ultrasonic and microtensile measurements. Journal of biomechanics. 1993;26:111–119. doi: 10.1016/0021-9290(93)90042-d. [DOI] [PubMed] [Google Scholar]
- 78.Geerligs M, Peters GW, Ackermans PA, Oomens CW, Baaijens FP. Linear viscoelastic behavior of subcutaneous adipose tissue. Biorheology. 2008;45:677–688. [PubMed] [Google Scholar]