Abstract
Climate and litter quality have been identified as major drivers of litter decomposition at large spatial scales. However, the role played by soil fauna remains largely unknown, despite its importance for litter fragmentation and microbial activity. We synthesized litterbag studies to quantify the effect sizes of soil fauna on litter decomposition rates at the global and biome scales, and to assess how climate, litter quality and soil fauna interact to determine such rates. Soil fauna consistently enhanced litter decomposition at both global and biome scales (average increment ~27%). However, climate and litter quality differently modulated the effects of soil fauna on decomposition rates between biomes, from climate-driven biomes to those where climate effects were mediated by changes in litter quality. Our results advocate for the inclusion of biome-specific soil fauna effects on litter decomposition as a mean to reduce the unexplained variation in large-scale decomposition models.
Keywords: carbon dynamics, climate, litter C:N ratio, litter decomposition, litter quality, meta-analysis, soil fauna
Introduction
In terrestrial ecosystems, more than 50% of net primary production is returned to the soil via the decomposition of leaf litter (Wardle et al. 2004). Therefore, the evaluation of factors controlling litter decomposition has major implications for present and future global carbon budgets (Aerts 2006). Litter decomposition is driven by multiple factors, including climate, litter quality, and soil biota, structure, and texture. Among these, climate, litter quality and soil organisms have been the most studied so far, and are thought to hierarchically control the decomposition of litter as follows: climate > litter quality > soil organisms (Lavelle et al. 1993; Hättenschwiler et al. 2005). Climate directly alters litter decay because of the sensitivity of soil biological processes to factors such as temperature or precipitation. In addition, climate also plays an indirect role on decomposition through its effects on litter quality and soil biota (Wardle et al. 2004). However, the relative contribution of each driver to the decomposition process is relatively lacking.
The effects of climate and litter quality upon litter decomposition rates have been previously evaluated at both regional and global scales (Swift et al. 1979; Cornwell et al. 2008). Recent models show that climate and litter quality explain about 60-70% of global litter decomposition rates (Parton et al. 2007). The extent to which soil biota might improve these estimates remains largely unknown. Specifically, soil fauna is necessary to adequately model the impacts of climate change on regional and global carbon dynamics (Wall et al. 2008). Furthermore, the influence of soil fauna on decomposition may be capital because, besides litter fragmentation, animals modify the structure and activity of microbial communities (Hättenschwiler et al. 2005), the ultimate actor in the decomposition process. Soil fauna are thought to possess a high degree of functional specialization (Heemsbergen et al. 2004), which is reinforced by the fact that their global diversity does not include many cosmopolitan groups, even at the family level (Wu et al. 2011). This contrasts with the functional redundancy commonly found in soil microbes at large spatial scales (Bradford & Fierer 2012). These findings are supported by the positive correlation between the diversity and abundance of microbes and litter decomposition rates commonly found at local scales (Strickland et al. 2009). However, the effects of soil fauna on such rates are typically idiosyncratic (Hooper et al. 2000).
The importance of soil fauna for litter decomposition has long been recognized (Swift et al. 1979; Seastedt 1984). Most empirical research on this topic has focused on soil fauna effects under different litter qualities (Hättenschwiler & Bracht-Jørgensen 2010; Makkonen et al. 2012) and climatic conditions (Moorhead & Reynolds 1991; Wall et al. 2008). A previous meta-analysis focusing on the methodological issues of litterbags addressed the contribution of microarthropods to litter decomposition using studies published until 2005 (Kampichler & Bruckner 2009), and thus did not include recent multisite comparisons of soil fauna, litter quality and climate effects on decomposition (Wall et al. 2008; Powers et al. 2009; Makkonen et al. 2012).
A general understanding of the role of soil fauna on litter decomposition and how this role is determined by climate and litter quality is hindered by: 1) the experimental designs employed to date, with most empirical studies addressing only one or two of these drivers, and 2) the low number of studies that jointly assess the effects of climate, soil fauna and litter quality on decomposition using multiple plant species and climatically contrasted sites. These logistic limitations may be overcome by conducting a literature synthesis explicitly addressing the complex interactions between these decomposition drivers at broader geographical scales. A global and biome-scale synthesis would also be fundamental to incorporate new research findings and summarize how the contribution of soil fauna to decomposition is modulated by litter quality and climate. We aimed to do so by conducting a quantitative meta-analysis to explore the effects of soil fauna on decomposition at the global scale, and to test how these effects vary between biomes. We also assess the relative importance of climate, litter quality and soil fauna for litter decomposition rates, and evaluate how climate and litter quality modulate the effect sizes of soil fauna on such rates. Finally, we identify research gaps and propose future directions that should advance the predictive capabilities of current global and biome-scale decomposition models by including the direct and indirect relationships between climate, litter quality and soil fauna.
Materials and methods
Data collection
We synthesized studies that evaluated the effects of soil fauna exclusion on litter decomposition. Searches were conducted using the ISI Web of Knowledge (http://apps.isiknowledge.com) on 5th November 2012, with no restriction on publication year. The search yielded 1371 references. In addition, we also screened previous reviews about the topic such as Seastedt (1984), Cepeda-Pizarro (1993) and Kampichler & Bruckner (2009). To be included in our database, studies had to quantitatively compare litter mass loss (using litterbags) in field experiments in both soil fauna excluded and soil fauna present treatments. The experimental treatment with less (fauna excluded) and more (fauna present) soil animals were set up using either contrasted litterbag mesh sizes (fine vs. coarse) or chemical agents (dose vs. control). See Appendix S1 for details on the term combinations used in the literature searching and the selection criteria followed to include studies in our review.
Data extraction
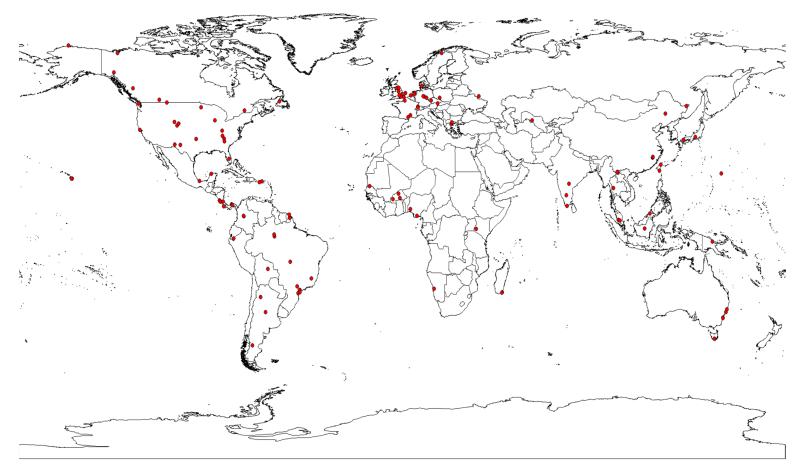
A total of 75 articles, representing 440 cases studies across 166 plant species, met our criteria (Table S1). Only 20% of these articles were reviewed in Kampichler & Bruckner (2009). As many articles included more than one location, a total of 129 globally distributed study sites were evaluated (Fig. 1, Map S1): 23% were from North America, 10% from Africa, 27% from Europe, 4% from Australia, 16% from Asia and 20% from South America. Over 83% of the case studies used litterbags of contrasting mesh sizes to exclude fauna, while ~17% employed chemical agents such as naphthalene or chlordane. When using graded mesh sizes, fine meshes ran from 10 to 2000 μm, and coarse meshes from 250 to 40000 μm. The study length varied from 30 to 1080 days, and in 75% of the case studies it lasted more than four months.
Figure 1.
Map showing the location of the 129 independent study sites used in our meta-analysis. Note that some of the 75 articles evaluated included more than one site. See Table S1 and Map S1 to identify the case studies evaluated in each site.
The information gathered from the papers was used to build three separate databases. Database 1 included the decay constant rates of the 440 case studies selected in both fauna excluded and present treatments. Mean, standard deviation and sample size values were extracted directly from tables or from graphs using the online tool provided by the German Astrophysical Virtual Observatory (http://dc.zah.uni-heidelberg.de/sdexter/). Information on methodological features such as type of soil fauna exclusion, mesh size of both fauna excluded and present litterbags, study length and number of harvests were also gathered for each study.
Database 2 contained the information on the traits defining the initial quality of the litter used in the different experiments. We collected data on litter Nitrogen (N) and Carbon (C) concentrations (%), and on specific leaf area (SLA; mm2 mg−1). Litter C:N ratios were calculated using these data. To fill the gaps we had in our trait database, values for litter C, N, C:N and SLA were obtained from the TRY database (Appendix S1), which contains trait data from a wide range of environments and data sources (Kattge et al. 2011). On a global scale, most of trait variation is represented by species identity (Kattge et al. 2011). Therefore, the use of a global trait dataset seems justified in the context of our global meta-analysis. The main data sources contributing to the TRY dataset we used were Niinemets (2001), Cornelissen et al. (2003), Wright et al. (2004), Kleyer et al (2008), Craine et al. (2009), Kattge et al. (2009), Poorter et al. (2009), Reich et al. (2009) and Yguel et al. (2011). In order to have a full trait matrix, some case studies had to be ruled out, rendering a final sample size of 335 case studies.
Database 3 contained the climate, spatial coordinates, elevation, and starting and ending dates of each study site. Using the spatial coordinates and the starting and ending dates of the study, we extracted the climate that occurred during the study period at each specific site from the CRU CL 2.0 dataset (Brohan et al. 2006). We selected the following climatic variables: total precipitation, monthly precipitation, minimum temperature, maximum temperature and mean temperature. We also calculated the Climate Decomposition Index (Moorhead et al. 1999, Appendix S1). Elevation was included in the analyses because of the important range found in this variable within our dataset (from 1 to 3400 m a.s.l.), which could encapsulate microclimatic features of the sites that are not properly captured by the climatic interpolations provided by the CRU database (Maestre et al. 2012). Elevation was obtained from Google Earth (http://www.google.com/earth/index.html). Based on its climate and spatial coordinates, each case study was assigned to a different biome as described in Appendix S1.
Analytical procedures
Does soil fauna exclusion increase or decrease litter decomposition at global and biome scales?
To answer this question, we calculated in each case study the response ratio of the decay rate (k) as a measure of effect size, lnRR(k) = (kExc/kAcc) where kExc and kAcc are the decay rates in the litterbags with fauna excluded and present, respectively. lnRR is a unit free index, which ranges from −∞ to +∞ and estimates the size of the impact and its direction (Hedges et al. 1999). Zero lnRR values means no difference in decomposition between fauna excluded and present litterbags, positive and negative values indicate faster and slower decomposition in fauna excluded litterbags, respectively.
To test whether average lnRR (k) values differed significantly from zero, we assessed whether the bias-corrected 95% bootstrap-confidence interval (CI) of LnRR (k) did not overlap zero based on 999 iterations (Rosenberg et al. 2000). We also tested whether effect sizes were homogeneous across case studies using the Qtotal statistic, which is based on a chi-squared test. A significant Qtotal indicates that the variance among effect sizes is greater than expected by sampling error, suggesting that effect sizes are not equal across studies and implying that other explanatory variables may affect the results. In this case, we either compared effect sizes between different categories of the parameters studied (e.g. physical vs. chemical fauna exclusion and biome) or calculated linear regressions between effect sizes and continuous variables (e.g. mesh size of both fauna excluded and present litterbags, study length and number of harvests). Bootstrapping was also used to generate CIs in the random-effects models. For categorical comparisons, we examined Prandom values associated to Qbetween, which describes the heterogeneity in effect sizes associated to differences between categories. We also tested whether the remaining within-group heterogeneity (Qwithin) was significant using a chi-squared test. The mean percentage of change in litter decomposition rates was estimated as (eR+ – 1) × 100, where R+ is the weighted mean response ratio across studies (Rosenberg et al. 2000). The approach to deal with the hierarchy of the dataset (case studies nested within studies) and publication bias is described in the Appendix S1. LnRR calculations and meta-analysis were conducted with MetaWin v2.1 (Rosenberg et al. 2000).
What is the relative importance of climate, litter quality and soil fauna for litter decomposition rates at global and biome scales?
We assessed the relationships among climate, plant traits, soil fauna and litter decomposition rates (k) using structural equation modeling (SEM). SEM has seldom been used in a meta-analytical context, but its flexibility renders it highly useful for this aim (see Grace et al. 2007 and Eldridge et al. 2011 for examples). This technique starts with the development of an a priori model that features variables and hypothesized causal relationships among them in a path diagram. Two groups of models were tested: global and biome-specific SEMs. Our a priori global SEM predicted a direct effect of plant traits, climate, elevation and soil fauna on k, which describes the litter decay rate (Fig. S1). This model also predicts an indirect effect of climate and elevation on k, which is modulated by plant traits (Aerts 2006). We introduced the latitude and longitude of each site in the global model to account for the spatial autocorrelation of our data (some case studies were nested within studies). Elevation was introduced in all the models as an endogenous variable related to microclimate. Regarding plant traits, the C:N ratio and SLA were introduced in all the models as indicators of litter quality important for decomposition (Cornwell et al. 2008; Zhang et al. 2008). Soil fauna was modeled as a binary variable describing the fauna excluded and present treatments. The structure of the data was therefore nested as a consequence of the inclusion of the soil fauna variable in the models. The effect of this nested structure was tested by running our a priori global model (Fig. S1) without the soil fauna variable and using the litter decomposition rate from the fauna excluded treatment as the response variable (Fig. S2). Path coefficients were very similar between both models (Figs. S1 and S2), suggesting that the nested structure of our data did not affect the outcome of the model described in Fig. S1. Because of the high dimensionality of our dataset, data reduction was conducted prior to analysis on the spatial and climatic variables (Appendix S1). The structure of the biome SEM was identical, excepting for the absence of the spatial variables.
Using the variables either measured or derived as described above, we constructed our models and tested their fit. From the seven biomes assessed in the previous meta-analysis, the coniferous forests and the tropical dry forests were ruled out because of their small sample size (n = 10 and 15, respectively). Path coefficients were estimated with bootstrapping using the bias-corrected percentile method, as some of our data (e.g. the first component of the principal component analysis conducted with climatic variables [hereafter Comp1]) were not normal even after transformations, and this technique is better suited in these cases than maximum likelihood estimation. Overall goodness-of-fit of the models were tested against the dataset (see Appendix S1). In order to increase the degrees of freedom, any path with a coefficient <0.10 were removed from the model when not significant. Statistical analyses were performed with SPSS version 14.0 and AMOS (SPSS Inc., Chicago, IL, USA).
Do the effects of soil fauna exclusion on litter decomposition depend on climate and litter quality at global and biome scales?
To answer this question, we assessed the relationships between climate, litter quality and the soil fauna effect size on litter decomposition (lnRR (k)) using SEM. The structure of this SEM was very similar to that described above, but we used lnRR (k) instead of k as the response variable, and did not include soil fauna as a predictor variable. Therefore, this SEM does not have a nested structure, and thus the sample size was equal to the case studies evaluated. Global and biome-specific SEMs were also tested as described above.
Results
Soil fauna effects on litter decomposition rates at global and biome scales
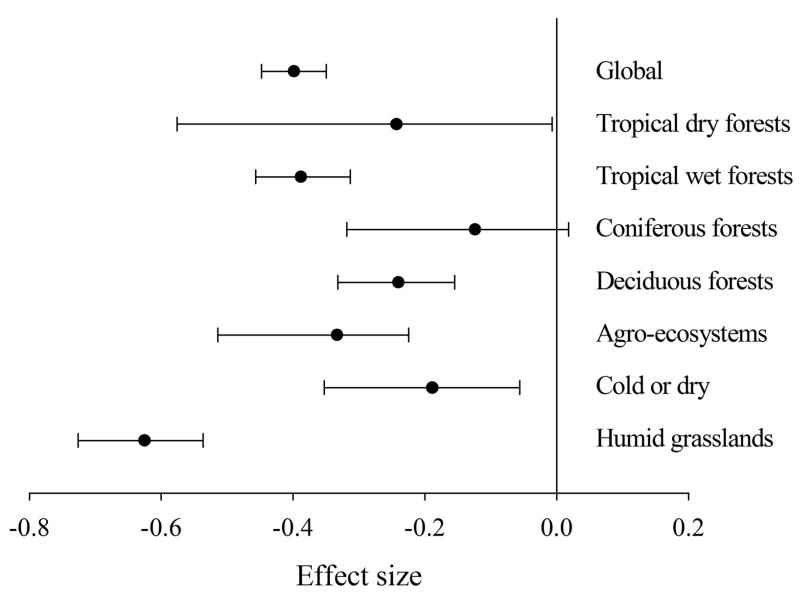
Averaged across all studies, there was considerable variability in the effect sizes (Qtotal = 777.64, d.f. = 426, P < 0.0001). Mean effect sizes differed significantly between the biomes examined (Qbetween = 86.85, d.f. = 6, Prandom = 0.001), but only in magnitude, as a positive effect size of soil fauna on litter decomposition rates was observed in all cases (Fig. 2). Fauna exclusion significantly reduced litter decomposition rates by 35% at the global scale, by 22% and 32% in the tropical dry and wet forests, respectively, by 13% in the coniferous forests, by 21% in the deciduous forests, by 30% in the agro-ecosystems, by 18% in the cold or dry systems and by 47% in the humid grasslands. The mean effect size within biomes was also heterogeneous (Qwithin = 690.79, d.f. = 420, P < 0.0001).
Figure 2.
Mean effect size of soil fauna exclusion on litter decomposition rates (lnRR (k)) at the global scale (n = 440), tropical dry forests (n = 15), tropical wet forests (n = 115), coniferous forests (n = 10), deciduous forests (n = 92), agro-ecosystems (n = 27), cold or dry (n = 42) and humid grasslands (n = 139). The bars around the means are bias-corrected 95%-bootstrap confidence intervals. Negative mean effect sizes indicate slower litter decomposition in the litterbags without soil fauna.
Litter decomposition responses did not change between types of fauna exclusion (Qbetween = 3.626, Prandom = 0.153). Both physical and chemical exclusion significantly reduced litter decomposition rates (ln RR (k) = −0.41 and −0.33, d.f. = 351 and 75, 95% CI = −0.47 to −0.36 and −0.42 to −0.24, respectively). The mesh size did not influence the observed effect sizes (slope = 0.0001 and < 0.0001 for fauna excluded and present litterbags, P = 0.233 and 0.794, respectively). Thus, the high variability of mesh sizes present in our database did not systematically affect the role of soil fauna on litter mass loss. The study length and the number of harvests conducted had a weak but significant influence on effect size (slope = 0.0006 and 0.0118, P = 0.001 and 0.021, respectively). However, the number of harvests was correlated with the study length (r = 0.301, P < 0.001), indicating that longer-term studies, which tend to conduct more harvests, show a lower decrease in litter decomposition rates with fauna exclusion. The comparison of effect sizes between the full and the reduced databases (Table S2) suggested the absence of pseudo-replication in our data, as the mean effect sizes were similar and the bias-corrected 95% bootstrap CI overlapped between both databases.
The relative importance of climate, litter quality and soil fauna for litter decomposition rates
Our SEM model was able to explain ~40% of the variance in litter decomposition rates at the global scale, and between 19% and 65% of this variance at the biome scale (Table 1). Climatic features (elevation and the Comp1) and litter quality (C:N ratio and SLA) accounted for most of the variance in litter decomposition rates, both in the global and the biome scale models. Soil fauna exerted a significant direct effect (r = 0.14) on litter decomposition rates in the global scale model. Soil fauna were also significantly related with litter decomposition rates in the humid grasslands, agro-ecosystems and tropical wet forests models, where it represented the largest contribution to the variance explained by the SEM conducted (Table 1).
Table 1.
Direct effects of elevation, climate, litter C:N, litter SLA and soil fauna on litter decomposition (rates) in the global and biomes scale structural equation models (SEMs). Climate represents the Component 1 of a Principal Component Analysis (PCA) conducted with the climatic variables, except in the deciduous forests model, where the first two components from this PCA were used. R2 = amount of variance explained in the litter decomposition rates (k).
| SEM | Elevation | Climate | Litter C:N | Litter SLA | Soil fauna | R2 |
|---|---|---|---|---|---|---|
| Global | −0.36*** | 0.09* | −0.26*** | 0.17*** | 0.14*** | 0.38 |
| Tropical wet forests | −0.23* | 0.12 | −0.11 | 0.17 | 0.27*** | 0.19 |
| Deciduous forests | −0.04 | 0.15* | −0.06 | 0.41**** | 0.13 | 0.24 |
| Agro-ecosystems | 0.33*** | 0.58*** | 0.18 | 0.12 | 0.20* | 0.65 |
| Cold or dry | −0.17 | 0.56*** | −0.09 | 0.20** | 0.05 | 0.59 |
| Humid grasslands | −0.44*** | −0.08 | −0.21*** | 0.36*** | 0.17*** | 0.62 |
Climate and litter quality modulation of the effects of soil fauna on litter decomposition
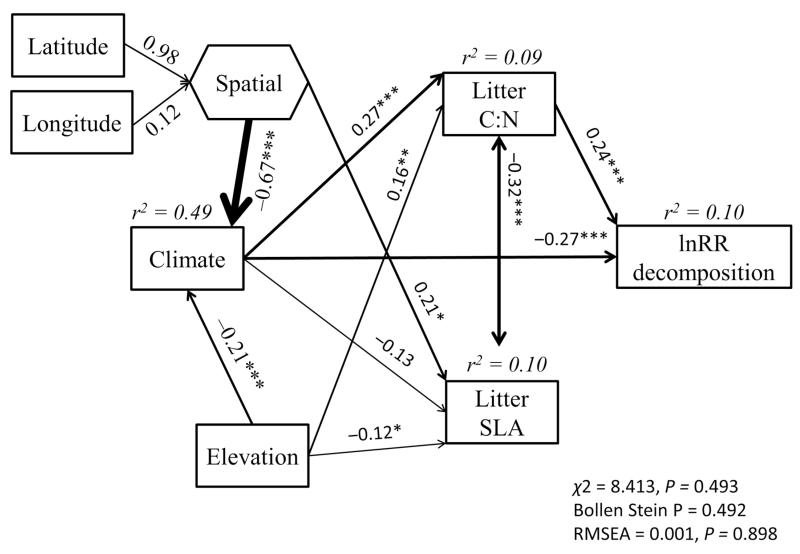
Climate (r = −0.27) and litter C:N ratio (r = 0.24) modulated the effect sizes of soil fauna exclusion on litter decomposition rates (Fig. 3) at the global scale. Climate effects were highly determined by the spatial component, and negatively affected lnRR (k). These results indicate a stronger positive effect of soil fauna on litter decomposition rates with higher total precipitation and higher minimum temperature. On the other hand, an increase in litter C:N promoted faster decomposition when soil fauna were excluded. Microclimatic variation related to elevation did not affect lnRR (k).
Figure 3.
Global-scale structural equation model depicting the direct and indirect influences of elevation, spatial coordinates, climate and plant litter traits on the effects of soil fauna exclusion on litter decomposition rates (lnRR (k)). Boxes indicate measured variables entered in the model. The hexagon indicates a composite variable (‘Spatial’) used to pool the effects of latitude and longitude. The variable ‘Climate’ indicates the Component 1 of a Principal Component Analysis conducted with mean total precipitation, monthly precipitation, minimum temperature and the climate decomposition index. These variables are highly related to ‘Climate’ (Spearman’s ρ > 0.84, P < 0.001 in all cases). The path widths are scaled proportionally to the path coefficient. Overall goodness-of-fit tests are shown in the bottom of the figure. *** = P < 0.001; ** = P < 0.01; * = P < 0.05; n = 335.
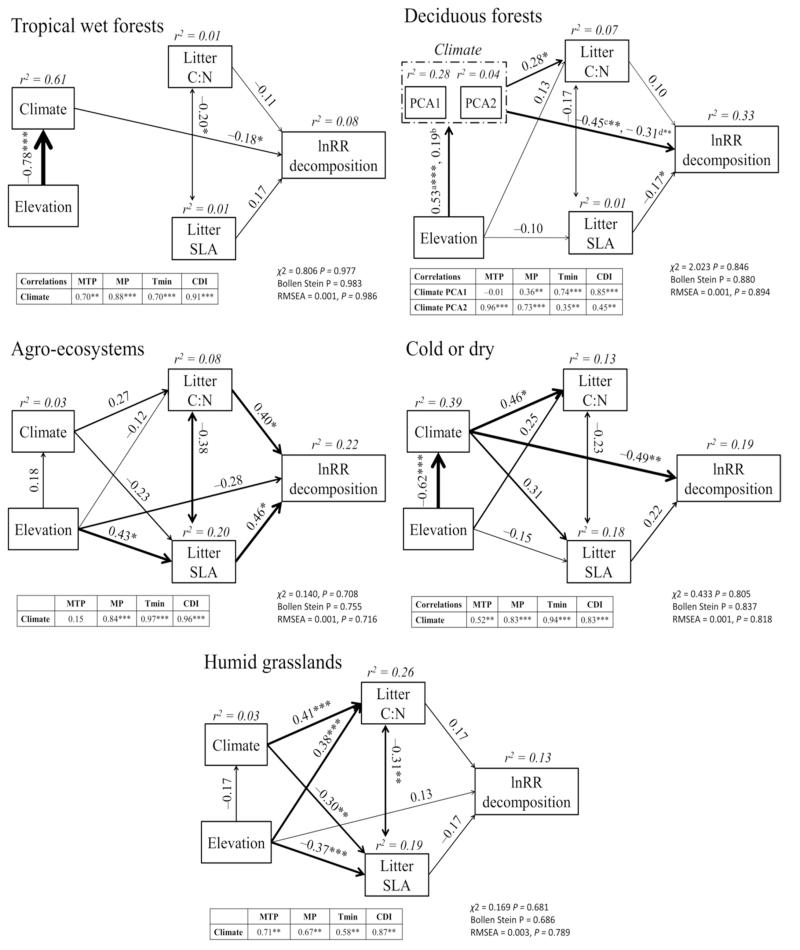
The greatest contributor to lnRR (k) varied between biomes (Fig. 4), with climate being the most important predictor in the cold or dry and deciduous forests, litter quality in the humid grasslands and agro-ecosystems, and both predictors in the tropical wet forests. Interestingly, the percentage of the total effects of climate on lnRR (k) indirectly determined by litter quality varied considerably across and between biomes (Table 2). Whereas litter quality modulated 20% of climate effects at the global scale, that fraction varied at the biome scale from less than 7% in the tropical wet forests, deciduous forests and cold or dry systems to 72% in the humid grasslands. The biomes with a strong effect of climate also showed larger positive effects of soil fauna on litter decomposition as precipitation and minimum temperature increased. We also found a negative relationship between litter quality (e.g. low C:N ratio and/or high SLA) and soil fauna exclusion in tropical wet forests and cold or dry biomes. This link was the opposite in the rest of biomes, where lower litter quality was associated with weaker effects of soil fauna exclusion on decomposition, and hence with a smaller role played by soil fauna on the decomposition process.
Figure 4.
Biome-scale structural equation models depicting the direct and indirect influences of elevation, climate and plant litter traits on the effects of soil fauna exclusion on litter decomposition rates (lnRR (k)). n = 96, 83, 23, 30 and 78 in the tropical wet forests, deciduous forests, agro-ecosystems, cold or dry and humid grasslands, respectively. The variable ‘Climate’ indicates the Component 1 of a Principal Component Analysis (PCA) conducted with mean total precipitation (MTP), monthly precipitation (MP), minimum temperature (MT) and the climate decomposition index (CDI). Note that two climate components of the PCA (PCA1 and PCA2) were included in the deciduous forests model because both had eigenvalues > 1 (Appendix S1). In this model, a and b denote path coefficients from Elevation to PCA1 and PCA2, respectively, and c and d from PCA1 and PCA2 to lnRR decomposition, respectively. Rest of caption as in Figure 3.
Table 2.
Standardized total, direct, indirect and absolute total effects of climate on the soil fauna effects on litter decomposition (lnRR (k)) in the global and biome scale structural equation models (SEMs). The proportion of the total absolute effects of climate on lnRR(k) modulated by plant litter traits (C:N ratio and SLA) in each SEM is also shown.
| SEMs | Total effect | Direct effect | Indirect effect | Absolute total effects | Proportion modulated by plant traits |
|---|---|---|---|---|---|
| Global | −0.20 | −0.27 | 0.07 | 0.34 | 20 |
| Tropical wet forests | −0.18 | −0.18 | 0 | 0.18 | 0 |
| Deciduous forests | −0.40 | −0.38 | −0.03 | 0.40 | 6 |
| Agro-ecosystems | −0.06 | −0.07 | 0.01 | 0.09 | 13 |
| Cold or dry | −0.47 | −0.49 | 0.02 | 0.51 | 4 |
| Humid grasslands | 0.08 | −0.05 | 0.13 | 0.18 | 72 |
Discussion
Soil fauna have a consistent positive effect over litter decomposition rates at both global and biome scales
Our analysis provides solid evidence that soil fauna promote litter decomposition rates at global and biome scales. Even though some heterogeneity was found among regions, the seven biomes evaluated showed a consistent positive effect of soil fauna on litter mass loss, indicating prevalent global- and regional-scale patterns. The 95% confidence intervals only overlapped zero in the coniferous forest, but this result was probably influenced by the small sample size found in this biome. Differences found in the magnitude of the soil fauna effects between biomes support previous findings (Wall et al. 2008), with higher effects in the temperate humid grasslands and tropical wet forests and weaker effects in biomes such as coniferous forests and cold or dry systems, where biological activity is more constrained by temperature and/or moisture.
Soil fauna exclusion decreased mass loss independently of the exclusion technique (physical vs. chemical) and the mesh size of the litterbags. This result allows for an overall comparison between studies using different exclusion techniques, which is contrary to the pattern found by Kampichler & Bruckner (2009). We can also argue that confounding factors found in coarse litterbags, such as leaching (Anderson 1973), and in fine litterbags, such as microclimatic alteration (Irmler 2000), do not determine the general outcome of soil fauna effects on litter decomposition. Even if we cannot disentangle the contribution of soil fauna to mass loss as a consequence of litter fragmentation (Peterson & Luxton 1982) or the stimulation of microbial biomass (Seastedt 1984), our results indicate that the positive effect of soil fauna on decomposition is robust across different soil trophic groups excluded by either body size or biocide sensitivity. However, the variability of mesh sizes used prevented us to elucidate which soil fauna group contributed the most to the pattern found, an issue that deserves further attention by future studies.
Interactions between climate, litter quality, soil fauna and litter decomposition rates
Our models (Table 1) identified climate (elevation and the Comp1) and litter quality as the major controls on litter decomposition at global and biome scales, supporting previous findings (Swift et al. 1979; Parton et al. 2007; Cornwell et al. 2008). Interestingly, soil fauna played a significant role, independently from that of climate and litter quality, determining litter decomposition rates at the global scale. This pattern was consistent in three of the five biomes evaluated, with soil fauna representing the major decomposition driver in the tropical wet forests. In general, the relative importance of soil fauna was similar to that of litter quality. It must be noted that the importance of soil fauna could be even higher if continuous data, such as community composition, were introduced in the models instead of the binary variable used here. Our results suggest that soil fauna exert a greater influence on litter decomposition than that previously assessed in a global experiment (Wall et al. 2008), where soil fauna provided a modest 7% improvement in decomposition variance explained with respect to the climatic predictors. Even though our results are not directly comparable to those of Wall et al. (2008) because of methodological differences, they indicate that soil fauna can explain a fraction of the variability in decomposition beyond that explained by climate and litter quality, and that the contribution of soil fauna can equal that of litter quality in certain biomes.
Total precipitation and minimum temperature were the major drivers of the effects of soil fauna on litter decomposition rates at the global scale, as such effects were more positive at the warmer and wetter sites (Fig. 3). In addition, the litter C:N ratio was also a strong predictor at the global scale. However, and contrary to previous findings (Bradford et al. 2002), litter of lower quality (higher C:N ratio) promoted weaker positive effects of soil fauna on decomposition. We hypothesize two plausible explanations to deal with this inconsistency: 1) different study lengths may change the effects of the litter quality–soil fauna relationship on decomposition (Smith & Bradford 2003), and 2) litter quality is a function of the chemical composition of the litter, but also depends on how the soil organisms perceive that litter based on past exposure to similar substrates (Strickland et al. 2009). Overall, our results show how climate and litter quality, besides controlling litter decomposition rates at the global scale (Parton et al. 2007; Cornwell et al. 2008; Zhang et al. 2008), also drive the effects of soil fauna on this key ecosystem process.
One of the most striking findings of our study is that the relative importance of climate and litter quality as drivers of soil fauna effects on decomposition rates varied considerably between biomes (Fig. 4 and Table 2). These changes promote an interesting pattern, where climate exerts an important direct effect in some biomes, while affecting soil fauna effects on decomposition through litter quality alteration in other biomes. Therefore, ongoing climate change may differently influence the way soil fauna affect litter decomposition at the biome scale, and this specificity should be included in large-scale decomposition models. Direct effects will be especially important in climate-driven biomes such as tropical wet forests, deciduous forests and cold or dry systems, where soil fauna effects on decomposition increase with increases in precipitation and minimum temperature. In cold biomes, where climate change effects on litter decomposition are likely to be especially important (Aerts 2006), warmer and wetter climates will likely promote a faster decomposition and release of the old and large carbon pools found in these biomes (Hobbie 1996) through a higher soil fauna effect. On the other hand, litter-mediated effects of climate change will be key in temperate humid grasslands, where changes in litter quality with climate represent more than 70% of the total effects of climate on soil fauna effect sizes. In this biome, changes in litter quality, triggered by either changes in plant community composition and/or phenotypic responses to temperature and moisture (Murphy et al. 2002), may be more important in determining the role of soil fauna on litter decomposition than the direct effects of climate change.
Research gaps and guidelines for future studies
Despite increasing research efforts devoted to understand the role of climate, litter quality and soil organisms on litter decomposition rates and carbon cycling (Swift et al. 1979; Lavelle et al. 1993; Cornwell et al. 2008; Wall et al. 2008), certain biomes such as agro-ecosystems, cold or dry, tropical dry forests and coniferous forests deserve further attention. Specifically, coniferous forests, in the form of high latitude boreal forests, represent one of the largest soil carbon pools on Earth, with ~182 Gt of C stored (Amundson 2001), and hence constitute a key biome for future studies evaluating the effects of climate change, litter quality and soil organisms on litter decomposition and carbon release.
The potential for using litterbags in future decomposition studies is still high, but some methodological issues should be assessed in order to deal with the common pitfalls of this technique. We suggest that a new generation of custom-made field microcosms could be used to replace the traditional litterbags. A good example was recently applied in Makkonen et al. (2012), where PVC cylinders were used to prevent litter compaction, physical loss and microclimate alteration. Even though we found very similar results for studies using either physical or chemical soil fauna exclusion, biocides such as naphthalene should be avoided because they may have unknown non-target effects on other biota throughout the food chain (Blair et al. 1989). The use of 13C labelled litter may help to establish a biome-specific correction factor to assess the ‘true’ soil fauna effect in decomposition studies, and to rule out the confounding effects of litter fractionation in deep soil layers by soil animals. Important insights could be obtained from future experiments that rigorously include the role of soil fauna on litter decomposition at global and regional scales, and that aim to answer two key questions: 1) do regional patterns of soil faunal diversity differ with climate and litter quality?, and 2) how do these patterns affect litter decomposition dynamics under a climate change scenario? To advance our understanding of the decomposition process, we also propose the design of multisite and multispecies observational studies, where soil fauna will be directly extracted from the incubated litter. Instead of using initial litter chemistry, changes in litter quality should be analysed throughout the study to include the litter chemistry complexity during decomposition (Wickings et al. 2012).
Concluding remarks
Here we advance the first global- and biome-scale synthesis on the relative importance of climate, litter quality and soil fauna for litter decomposition rates, and on how climate and litter quality modulate the effects of soil fauna on such rates. Our review demonstrates, using a comprehensive gradient of soil faunal communities, climates and litter qualities, that soil fauna consistently promote litter decomposition across and within biomes. This positive effect was independent from the direct influence of climate and litter quality on decomposition, and had a similar magnitude than the effect of litter quality. It also highlights that the modulation of soil fauna effects on decomposition by climate and litter quality varies with the biome considered, from climate-driven biomes such as tropical wet forests, deciduous forests and cold or dry, to biomes where climate effects are mediated by changes in litter quality, such as temperate humid grasslands. Biome-scale decomposition models should incorporate these regional contingencies to improve their predictive capacity, and to adequately assess the impacts of climate change on regional and global carbon dynamics (O’Halloran et al. 2013). We still know remarkably little about how shifts in soil fauna community composition affect litter decomposition, as there is a lack of data on functional species roles (Aerts 2006). However, while this information becomes available, biome-specific whole soil fauna contributions and interactions with climate and litter quality can be used to reduce the unexplained residual variation in large-scale decomposition models (ca. 25%, Del Grosso et al. 2005).
Supplementary Material
Acknowledgements
We thank Ernie Marx (Colorado State University) for his help extracting the climate data from the CRU dataset, and Santiago Soliveres and Rubén Milla for helpful comments. We appreciate the use of data from the TRY initiative on plant traits (http://www.try-db.org). The TRY initiative and database is hosted, developed and maintained by J. Kattge and G. Bönisch (Max-Planck-Institute for Biogeochemistry, Germany). TRY is/has been funded by DIVERSITAS, IGBP, the Global Land Project, the UK-NERC, FRB, and GIS. PGP was funded by a Fulbright postdoctoral contract from the Spanish Ministerio de Educación for this research at Colorado State University, and by a European Commission’s FP7 Marie Curie IEF grant (DECOMFORECO-2011-299214). FTM is supported by the by the European Research Council under the European Community’s Seventh Framework Programme (FP7/2007-2013)/ERC Grant agreement 242658 (BIOCOM). DHW acknowledges support from National Science Foundation grant 0344834.
References
- 1.Amundson R. The carbon budget in soils. Annu. Rev. Earth Planet. Sci. 2001;29:535–562. [Google Scholar]
- 2.Aerts J. The freezer defrosting: global warming and litter decomposition rates in cold biomes. J. Ecol. 2006;94:713–724. [Google Scholar]
- 3.Anderson JM. The breakdown and decomposition of sweet chestnut (Castanea sativa Mill.) and beech (Fagus sylvatica L.) leaf litter in two deciduous woodland soils. I. Breakdown, leaching and decomposition. Oecologia. 1973;12:251–274. doi: 10.1007/BF00347566. [DOI] [PubMed] [Google Scholar]
- 4.Blair JM, Crossley DA, Jr., Rider S. Effects of naphthalene on microbial activity and nitrogen pools in soil-litter microcosms. Soil. Biol. Biochem. 1989;21:507–510. [Google Scholar]
- 5.Bradford MA, Tordoff GM, Eggers T, Jones TH, Newington JE. Microbiota, fauna, and mesh size interactions in litter decomposition. Oikos. 2002;99:317–323. [Google Scholar]
- 6.Bradford MA, Fierer N. The biogeography of microbial communities and ecosystem processes: Implications for soil and ecosystem models. In: Wall DH, editor. Soil Ecology and Ecosystem Services. Oxford University Press; Oxford, UK: 2012. pp. 189–200. [Google Scholar]
- 7.Brohan P, Kennedy JJ, Harris I, Tett SFB, Jones PD. Uncertainty estimates in regional and global observed temperature changes: a new dataset from 1850. J. Geophys. Res. 2006;111:D12106. [Google Scholar]
- 8.Cepeda-Pizarro JG. Litter decomposition in deserts: an overview with an example from coastal arid Chile. Rev. Chil. Hist. Nat. 1993;66:323–336. [Google Scholar]
- 9.Cornelissen JHC, Cerabolini B, Castro-Díez P, Villar-Salvador P, Montserrat-Martí G, Puyravaud JP, et al. Functional traits of woody plants: correspondence of species rankings between field adults and laboratory-grown seedlings? J. Veg. Sci. 2003;14:311–322. [Google Scholar]
- 10.Cornwell WK, Cornelissen JHC, Amatangelo K, Dorrepaal E, Eviner VTO, Godoy O, et al. Plant species traits are the predominant control on litter decomposition rates within biomes worldwide. Ecol. Lett. 2008;11:1065–1071. doi: 10.1111/j.1461-0248.2008.01219.x. [DOI] [PubMed] [Google Scholar]
- 11.Craine JM, Elmore AJ, Aidar MPM, Bustamante M, Dawson TE, Hobbie EA, et al. Global patterns of foliar nitrogen isotopes and their relationships with climate, mycorrhizal fungi, foliar nutrient concentrations, and nitrogen availability. New Phytol. 2009;183:980–992. doi: 10.1111/j.1469-8137.2009.02917.x. [DOI] [PubMed] [Google Scholar]
- 12.De Deyn GB, Cornelissen H, Bardgett RD. Plant traits and soil carbon sequestration in contrasting biomes. Ecol. Lett. 2008;11:516–531. doi: 10.1111/j.1461-0248.2008.01164.x. [DOI] [PubMed] [Google Scholar]
- 13.Del Grosso SJ, Parton WJ, Mosier AR, Holland EA, Pendall E, Schimel DS, et al. Modeling soil CO2 emissions from ecosystems. Biogeochemistry. 2005;73:71–91. [Google Scholar]
- 14.Eldridge D, Bowker MA, Maestre FT, Roger E, Reynolds JF, Whitford WG. Impacts of shrub encroachment on ecosystem structure and functioning: towards a global synthesis. Ecol. Lett. 2011;14:709–722. doi: 10.1111/j.1461-0248.2011.01630.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Grace JB, Anderson TM, Smith MD, Seabloom E, Andelman SJ, Meche G, et al. Does species diversity limit productivity in natural grassland communities? Ecol. Lett. 2007;10:680–689. doi: 10.1111/j.1461-0248.2007.01058.x. [DOI] [PubMed] [Google Scholar]
- 16.Hättenschwiler S, Tiunov AV, Scheu S. Biodiversity and litter decomposition in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 2005;36:191–218. [Google Scholar]
- 17.Hättenschwiler S, Bracht-Jørgensen H. Carbon quality rather than stoichiometry controls litter decomposition in a tropical rain forest. J Ecol. 2010;98:754–763. [Google Scholar]
- 18.Hedges LV, Gurevitch J, Curtis P. The meta-analysis using response ratios in experimental ecology. Ecology. 1999;80:1150–1156. [Google Scholar]
- 19.Heemsbergen D, Berg MP, van Hall J, Faber JH, Verhoef HA. Biodiversity effects on soil processes explained by inter-specific functional trait dissimilarity. Science. 2004;306:1019–1020. doi: 10.1126/science.1101865. [DOI] [PubMed] [Google Scholar]
- 20.Hobbie SE. Temperature and plant species control over carbon and nitrogen cycling through litter and soil in Alaskan tundra. Ecol. Monogr. 1996;66:503–522. [Google Scholar]
- 21.Hooper DU, Bignell DE, Brown VK, Brussaard L, Dangerfield JM, Wall DH, et al. Interactions between above- and belowground biodiversity in terrestrial ecosystems: patterns, mechanisms, and feedbacks. BioScience. 2000;50:1049–1061. [Google Scholar]
- 22.Irmler U. Changes in the fauna and its contribution to mass loss and N release during leaf litter decomposition in two deciduous forests. Pedobiologia. 2000;44:105–118. [Google Scholar]
- 23.Kampichler C, Bruckner A. The role of microarthropods in terrestrial decomposition: a meta-analysis of 40 years of litterbag studies. Biol. Rev. 2009;84:375–389. doi: 10.1111/j.1469-185X.2009.00078.x. [DOI] [PubMed] [Google Scholar]
- 24.Kattge J, Díaz S, Lavorel S, Prentice IC, Leadley P, Bönisch G, et al. TRY – a global database of plant traits. Global Change Biol. 2011;17:2905–2935. [Google Scholar]
- 25.Kleyer M, Bakker RM, Knevel IC, Bakker JP, Thompson K, Sonnenschein M, et al. The LEDA Traitbase: a database of life history traits of the Northwest European flora. J. Ecol. 2008;96:1266–1274. [Google Scholar]
- 26.Lavelle P, Blanchart E, Martin A, Martin S. A hierarchical model for decomposition in terrestrial ecosystems: application to soils of the humid tropics. Biotropica. 1993;25:130–150. [Google Scholar]
- 27.Maestre FT, Quero JL, Gotelli NJ, Escudero A, Ochoa V, Delgado-Baquerizo M, et al. Plant species richness and ecosystem multifunctionality in global drylands. Science. 2012;335:214–218. doi: 10.1126/science.1215442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Makkonen M, Berg MP, Handa IT, Hättenschwiler S, van Ruijven J, van Bodegom PM, et al. Highly consistent effects of plant litter identity and functional traits on decomposition across a latitudinal gradient. Ecol. Lett. 2012;15:1033–1041. doi: 10.1111/j.1461-0248.2012.01826.x. [DOI] [PubMed] [Google Scholar]
- 29.Moorhead DL, Reynolds JF. A general model of litter decomposition in the northern Chihuahuan Desert. Ecol. Model. 1991;56:197–219. [Google Scholar]
- 30.Moorhead DL, Currie B, Rastetter E, Parton W, Harmon M. Climate and litter quality controls on decomposition: An analysis of modelling approaches. Global Biogeochem. Cy. 1999;13:575–589. [Google Scholar]
- 31.Murphy KL, Burke IC, Vinton MA, Lauenroth WK, Aguiar MR, Wedin DA, et al. Regional analysis of litter quality in the central grassland region of North America. J. Veg Sci. 2002;13:395–402. [Google Scholar]
- 32.Niinemets U. Global-scale climatic controls of leaf dry mass per area, density, and thickness in trees and shrubs. Ecology. 2001;82:453–469. [Google Scholar]
- 33.O’Halloran LR, Borer ET, Seabloom EW, MacDougall AS, Cleland EE, McCulley RL, et al. Regional contingencies in the relationship between aboveground biomass and litter in the world’s grasslands. PLoS ONE. 2013;8:e54988. doi: 10.1371/journal.pone.0054988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Parton W, Silver WL, Burke IC, Grassens L, Harmon ME, Currie WS, et al. Global-scale similarities in nitrogen release patterns during long-term decomposition. Science. 2007;315:361–364. doi: 10.1126/science.1134853. [DOI] [PubMed] [Google Scholar]
- 35.Petersen H, Luxton M. A comparative analysis of soil fauna populations and their role in decomposition process. Oikos. 1982;39:287–388. [Google Scholar]
- 36.Poorter H, Niinemets U, Poorter L, Wright IJ, Villar R. Causes and consequences of variation in leaf mass per area (LMA): a meta-analysis. New Phytol. 2009;182:565–588. doi: 10.1111/j.1469-8137.2009.02830.x. [DOI] [PubMed] [Google Scholar]
- 37.Powers JS, Montgomery RA, Adair EC, Brearley FQ, DeWalt SJ, Castanho CT, et al. Decomposition in tropical forests: a pan-tropical study of the effects of litter type, litter placement and mesofaunal exclusion across a precipitation gradient. J. Ecol. 2009;97:801–811. [Google Scholar]
- 38.Reich PB, Oleksyn J, Wright IJ. Leaf phosphorus influences the photosynthesis-nitrogen relation: a cross-biome analysis of 314 species. Oecologia. 2009;160:207–212. doi: 10.1007/s00442-009-1291-3. [DOI] [PubMed] [Google Scholar]
- 39.Rosenberg MS, Adams DC, Gurevitch J. Metawin: Statistical Software for Meta-Analysis. Sinauer Associates; Sunderland, USA: 2000. [Google Scholar]
- 40.Seastedt TR. The role of microarthropods in decomposition and mineralization processes. Annu. Rev. Entomol. 1984;29:25–46. [Google Scholar]
- 41.Smith VC, Bradford MA. Litter quality impacts on grassland litter decomposition are differently dependent on soil fauna across time. Appl. Soil Ecol. 2003;24:197–203. [Google Scholar]
- 42.Strickland MS, Osburn E, Lauber C, Fierer N, Bradford MA. Litter quality is in the eye of the beholder: decomposition rates as a function of inoculum characteristics. Funct. Ecol. 2009;23:627–636. [Google Scholar]
- 43.Swift MJ, Heal OW, Anderson JM. Decomposition in Terrestrial Ecosystems. University of California Press; Berkely, USA: 1979. [Google Scholar]
- 44.Wall DH, Bradford MA, John MGS, Trofymow JA, Behan-Pelletier V, Bignell DE, et al. Global decomposition experiment shows soil animal impacts on decomposition are climate-dependent. Global Change Biol. 2008;14:2661–2677. [Google Scholar]
- 45.Wardle DA, Bardgett RD, Klironomos JN, Setälä H, van der Putten WH, Wall DH. Ecological linkages between aboveground and belowground biota. Science. 2004;304:1629–1633. doi: 10.1126/science.1094875. [DOI] [PubMed] [Google Scholar]
- 46.Wickings K, Grandy AS, Reed SC, Cleveland CC. The origin of litter chemical complexity during decomposition. Ecol. Lett. 2012;15:1180–1188. doi: 10.1111/j.1461-0248.2012.01837.x. [DOI] [PubMed] [Google Scholar]
- 47.Wright IJ, Reich PB, Westoby M, Ackerly DD, Baruch Z, Bongers F, et al. The worldwide leaf economics spectrum. Nature. 2004;428:821–827. doi: 10.1038/nature02403. [DOI] [PubMed] [Google Scholar]
- 48.Wu T, Ayres E, Bardgett RD, Wall DH, Garey JR. Molecular study of worldwide distribution and diversity of soil animals. P. Natl. Acad. Sci. USA. 2011;108:17720–17725. doi: 10.1073/pnas.1103824108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Yguel B, Bailey R, Tosh ND, Vialatte A, Vasseur C, Vitrac X. Phytophagy on phylogenetically isolated trees: why hosts should escape their relatives. Ecol. Lett. 2011;14:1117–1124. doi: 10.1111/j.1461-0248.2011.01680.x. [DOI] [PubMed] [Google Scholar]
- 50.Zhang D, Hui D, Luo Y, Zhou G. Rates of litter decomposition in terrestrial ecosystems: global patterns and controlling factors. J. Plant Ecol. 2008;1:85–93. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.