Abstract
Background and objective
Acute disseminated encephalomyelitis (ADEM) and relapsing remitting multiple sclerosis share overlapping clinical, radiologic, and laboratory features at onset. Because autoantibodies may contribute to the pathogenesis of both diseases, we sought to identify autoantibody biomarkers capable of distinguishing them.
Methods
We used custom antigen arrays to profile anti-myelin-peptide autoantibodies in sera derived from individuals with pediatric ADEM (n = 15), pediatric multiple sclerosis (n = 11), and adult multiple sclerosis (n = 15). Using isotype-specific secondary antibodies,we profiled both IgG and IgM reactivities. We used Statistical Analysis of Microarrays to confirm differences in autoantibody reactivity profiles between ADEM and multiple sclerosis samples. We used Prediction Analysis of Microarrays to generate and validate prediction algorithms based on the autoantibody reactivity profiles.
Results
ADEM was characterized by IgG autoantibodies targeting epitopes derived from myelin basic protein, proteolipid protein, myelin-associated oligodendrocyte basic glycoprotein, and alpha-B-crystallin. In contrast, multiple sclerosis was characterized by IgM autoantibodies targeting myelin basic protein, proteolipid protein, myelin-associated oligodendrocyte basic glycoprotein, and oligodendrocyte specific protein. We generated and validated prediction algorithms that distinguish ADEM serum (sensitivity 62–86%; specificity 56–79%) from multiple sclerosis serum (sensitivity 40–87%; specificity 62–86%) on the basis of combined IgG and IgM anti-myelin autoantibody reactivity to a small number of myelin peptides.
Conclusions
Combined profiles of serum IgG and IgM autoantibodies identify myelin antigens that may be useful for distinguishing multiple sclerosis from ADEM. Further studies are required to establish clinical utility. Further biological assays are required to delineate the pathogenic potential of these antibodies.
Keywords: pediatric, multiple sclerosis, acute disseminated encephalomyelitis, myelin, autoantibody, antigen, array, diagnosis, biomarker, immunoglobulin, IgG, IgM
Acute disseminated encephalomyelitis (ADEM) and relapsing remitting multiple sclerosis (MS) are distinct forms of autoimmune-mediated demyelination, yet they may share polysymptomatic neurological deficits, multifocal white matter lesions and laboratory findings. This can render initial prediction of monophasic outcome (typical of ADEM) versus prediction of relapsing-remitting MS, difficult and observation over time is essential to define outcome. An improved understanding of the biological factors that distinguish transient immune targeting of the CNS from chronic CNS-directed immunity has both diagnostic and potential therapeutic implications. Molecular biomarkers that can distinguish between MS and ADEM are therefore highly sought after.1–3
The etiology of MS is multi-factorial, with a role for both genetic and environmental factors. The natural history of MS involves episodic exacerbations overlying a chronic and progressive decline, though life-long immunomodulatory therapy can moderately attenuate the disease. The current diagnosis of MS is based on clinical and radiologic confirmation of relapsing inflammatory demyelination that occurs in multiple CNS regions separated in both space and time.4 In contrast, ADEM is a monophasic disorder in more than 90% of cases and affects primarily children. Many patients report recent upper respiratory illness, prompting consideration of an infectious trigger for the immune response. The criteria for diagnosing ADEM remain a subject of debate, though many clinicians apply the consensus criteria proposed by a 2007 international panel of experts who describe the disorder as a first episode of inflammatory demyelination with multi-focal CNS involvement and encephalopathy.5
In both ADEM and MS, autoantibodies to specific myelin proteins and lipids may contribute to disease pathogenesis6–9 and activate complement in some patients.10–12 We have developed antigen arrays for profiling autoantibodies in a variety of autoimmune diseases, including demyelinating disorders.13–15 Preliminary studies suggest that the two diseases have distinct autoantibody profiles,2, 16 including differences in immunoglobulin (Ig) subtype.1, 8, 9, 12, 17–19 Based on the available literature, we hypothesized that IgG and IgM autoantibodies in ADEM and MS patients might offer disease-specific profiles. In this study, we use antigen arrays to identify serum anti-myelin autoantibody profiles that can distinguish between ADEM and MS.
Materials and methods
Patient samples
Archived serum samples from patients with pediatric ADEM (n=15), pediatric MS (n=11), or adult MS (n=15) were available for analysis. Demographic data are summarized in Table 1. The samples were collected at several sites under protocols approved by the local Institutional Review Board. Informed consent was obtained from all subjects. Samples were stored at −80°C until used. Neurological history, relapse features, neurological examination, MRI, and CSF findings were reviewed when available. Each clinical diagnosis was validated using the data from the clinical intake form or available records. Patients diagnosed with pediatric or adult MS met the 2001 McDonald criteria for lesion dissemination in time and space at the time blood was drawn.20 Pediatric MS was defined as cases of MS with an onset of symptoms prior to 18 years of age. The definitions proposed by the International Pediatric MS Study Group were used to distinguish ADEM, and pediatric MS 9. None of the pediatric or adult MS patients were taking disease modifying therapies at the time of sample acquisition. Treatment data was not available for two pediatric MS patients. Patients were diagnosed with ADEM if they experienced a polysymptomatic inflammatory demyelinating event accompanied by encephalopathy, seizure or coma without subsequent evidence of further inflammatory disease. ADEM patients were drawn from four international sites located in Argentina, Canada, Germany, and the United Kingdom. Adult MS patients were diagnosed at a single center in the United States. Pediatric MS cases were drawn from the USA, Germany, Russia, and Canada. Samples were classified as acute, if collected within 90 days of an attack, and convalescent if >90 days after an attack.
Table I.
Patient demographics.
| Adult MS |
Ped MS |
ADEM |
||||
|---|---|---|---|---|---|---|
| Cohort #1 (n= 7) |
Cohort #2 (n= 8) |
Cohort #1 (n= 10) |
Cohort #2 (n= 11) |
Cohort #1 (n= 7) |
Cohort #2 (n= 8) |
|
| Median age, years (range) | 41(29,62) | 40(18,74) | 14(6,15) | 14(6,15) | 5(2,15) | 4(1,10) |
| Female, % | 71.4% | 75% | 50% | 45.5% | 42.9% | 62.5% |
| Patients within 3 months of acute dernyelinating event, % | 14.3% | 0% | 80% | 81.8% | 85.7% | 88% |
| Median EDSS at blood draw (range) | 1(0,2.5) | 2(0,4) | 1(0,3.5) | 1(0,3.5) | Not specified | Not specified |
| Median disease duration at time of blood draw, months (range) | 16(1,101) | 120(36,516) | 10(1,36) | 12(1,36) | 1(0,2) | 0(0,4) |
| Median total duration of clinical follow-up, months (range) | 16(1,101) | 120(36,516) | 17.5(4,50) | 15(4,50) | 9(5,66) | 31(19,61) |
ADEM: acute disseminated encep halo myelitis; EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; Ped MS: pediatric MS.
Antigen arrays
We generated antigen arrays that contained overlapping peptide sequences derived from several myelin proteins, each selected based on previously published reports of possible antigenicity in demyelinating disorders: myelin basic protein (MBP)12, 17, 21, proteolipid protein (PLP)21, 22 myelin-associated oligodendrocyte basic protein (MOBP) 23, myelin oligodendrocyte glycoprotein (MOG)2, 12, 2’,3’-cyclic nucleotide 3’-phosphodiesterase (CNPase)24, oligodendrocyte specific protein (OSP)25, and the heat shock protein alpha-B-crystallin (abCrys)15. Whole-protein human IgG, IgA, and IgM were included as positive controls, and influenza virus, mouse, bovine, and chicken proteins as negative controls. The full set of peptides and proteins are listed in Supplemental Table 2. In total, each array contained 125 unique peptide sequences, with each putative antigen printed twice. Thus, each serum sample was screened against a total of 264 features (Supplemental Table 1). The antigen arrays were custom ordered from JPT Peptide Technologies, Inc (Berlin, Germany), which uses short molecular strands to fasten and orient each individual peptide and protein to the glass array, a technique that ensures a high degree of uniformity in protein orientation and concentration.26 Using these custom arrays, we profiled autoantibodies in patient sera as previously described.13 Briefly, we blocked the arrays overnight at 4 °C in PBS with 0.5% Tween-20 and 3% FCS, probed them for 1.5 hours at 4 °C with 300uL of 1:150 dilutions of the patient sera, and labeled them for 1 hour at 4 °C with 300uL of a 1:1000 dilution of Cy3-conjugated goat anti-human IgG (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA) and a 1:1500 dilution of Cy5-conjugated goat anti-human IgM (Jackson ImmunoResearch Laboratories, Inc. West Grove, PA, USA). Representative array results are shown in Supplemental Figure 1. The probed arrays were scanned with a GenePix 4000B scanner (MDS Analytical Technologies, Sunnyvale, CA, USA). Antibody reactivity was quantified in fluorescence units (FU) by using GenePix Pro 5.0 software. Mean values for antibody reactivity to each peptide or protein were calculated by averaging the median pixel intensity (median intensity of the feature minus the median intensity of background) from each feature, as measured at two distinct locations on each array. We used these antigen arrays to probe the serum of 41 patients in two array experiments on two separate occasions. From these data sets, we empirically selected non-overlapping patient cohorts (Supplemental Table 3) to generate and validate disease-specific prediction algorithms as described below.
Data analysis
We used Significance Analysis of Microarrays (SAM) software (version 3.09, http://www-stat.stanford.edu/~tibs/SAM/) and the statistical package R (version 3.10) to identify antigens whose autoantibody reactivity differed between patient groups, with statistical accuracy presented as false discovery rate (“q”). We arranged the results of the SAM analysis into relationships by using the hierarchical clustering software Cluster® (http://rana.lbl.gov/EisenSoftware.htm), and then displayed the results of the Cluster analysis as autoantigen heatmaps by using TreeView® software (http://rana.lbl.gov/EisenSoftware.htm).
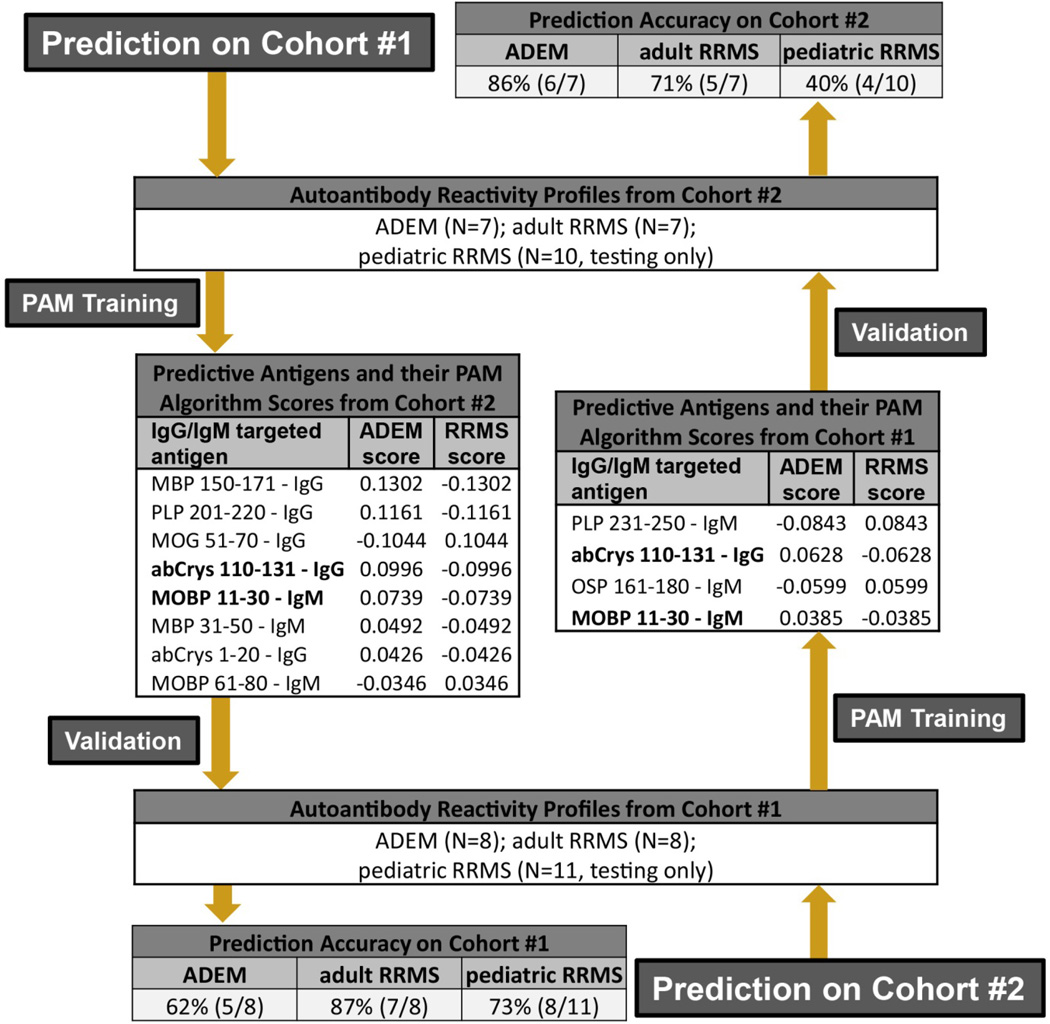
To identify a subset of serum autoantibody specificities that can correctly determine whether the tested serum sample is from a patient with ADEM or from a patient with MS (i.e. “diagnostic class prediction”), we used Prediction Analysis of Microarrays (PAM) software (version 2.1 http://www-stat.stanford.edu/~tibs/PAM/), as previously described.27 Briefly, after completing two large array experiments (Supplemental Table 3), we divided our ADEM and adult MS patients into two non-overlapping cohorts (Figure 2 and Supplemental Table 3). This allowed us to create completely independent “training” and “validation” groups such that our validation cohorts never contained patients from our training cohorts. Moreover, to replicate real-world interassay variability, our training and validation cohorts were drawn from array experiments performed on separate dates. To allow for a larger validation cohort, we analyzed the pediatric MS samples only in the validation experiments. The allotment of patients to Cohort 1 and Cohort 2 was determined empirically before beginning the analysis. We next applied the PAM algorithm to the autoantibody profiles of the “training” sample set, allowing PAM to establish mean levels of autoantibody reactivity to each protein or peptide in each group of patients with a given diagnosis (i.e. class). We narrowed the hierarchical list of antigens generated by PAM to the final list of classifying antigens by using the cross-validation results (Supplemental Figure 2) to select the detection threshold that offered the highest sensitivity and specificity while using the smallest number of classifying antigens. We then applied this final algorithm to our independent patient cohorts to determine whether our PAM analysis of autoantibody reactivity to this subset of differentiating antigens could enable “class prediction” (ADEM vs MS and ADEM vs Ped MS) (Figure 2). To validate further, we used the same process in reverse, training with the original validation cohorts and validating with the original training cohorts (Figure 2 and Supplemental Table 3).
Figure 2. Performance of serum autoantibody profiles in the differential diagnosis of ADEM and MS.
We used PAM to generate and validate prediction algorithms based on autoantibody reactivity profiles. For prediction on Cohort 1 (starting top left), we generated an algorithm (antigens and scores as listed) by ‘training’ PAM using the reactivity profiles from Cohort 2. We validated this algorithm using the autoantibody reactivity profiles from Cohort 1. The prediction accuracy of the algorithm is listed at the bottom left. To further test the validity of our autoantibody prediction paradigm, we reversed the order of training and validation (starting bottom right) such that the training analysis was done using reactivity profiles from Cohort 1 and validated using serum samples from Cohort 2. Shown in bold are the IgG-targeted antigens that differentiated between ADEM and MS in both prediction trials. There were no overlapping patients in Cohorts 1 & 2. SN, sensitivity; SP, specificity; MOBP, myelin-associated oligodendrocyte basic protein; abCrys, alpha-B-crystallin; PLP, proteolipid protein; OSP, oligodendrocyte specific protein; MBP, myelin basic protein; CNPase, 2’,3’ cyclic nucleotide phosphodiesterase, MOG, myelin oligodendrocyte glycoprotein.
Results
ADEM and MS serum have distinct anti-myelin autoantibody profiles
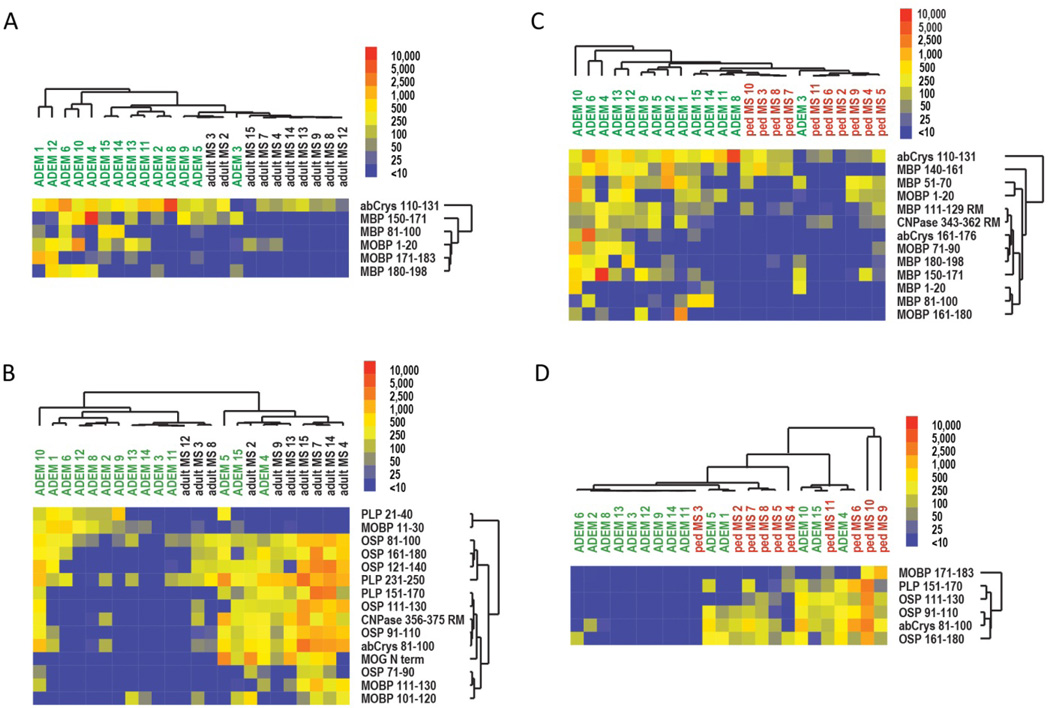
To compare serum autoantibody profiles in ADEM and MS, we used antigen arrays to profile IgG and IgM autoantibodies in sera from individuals with pediatric ADEM, pediatric MS, or adult MS. We performed two large independent array experiments (Supplemental Table 3). SAM analysis of the autoantibody profiles comparing ADEM vs adult MS and ADEM vs pediatric MS showed that several antigens were differentially targeted by autoantibodies in ADEM sera compared to those in MS sera (Figure 1; Supplemental Figure 3). SAM analysis comparing the adult and pediatric MS cohorts did not yield any significant differences (data not shown). In Cohort 1, six antigens were differentially targeted by IgG antibodies (q<8.8%) and 15 by IgM antibodies (q<23.1%) in ADEM sera compared to adult MS sera (Figures 1A & 1B). The IgG-targeted antigens comprised 3 MBP peptides (amino-acid sequences 81–100; 150–171; 180–198), 2 MOBP peptides (1–20; 171–183), and 1 abCrys peptide (110–131). The IgM-targeted antigens comprised 6 OSP peptides (81–100; 91–110; 111–130; 121–140; 161–180), 3 MOBP peptides (11–30; 101–120; 111–130), 3 PLP peptides (21–40; 151–-170; 231–250), MOG peptide (N-terminus), 1 abCrys peptide (81–100), and 1 CNPase peptide (356–375). Analysis of the antibody profiles obtained from a second, confirmatory experiment (Cohort 2) yielded similar results (Supplemental Figure 3). Thirteen antigens (q<16.7%) were differentially targeted by IgG antibodies in ADEM sera compared to pediatric MS sera: 7 MBP peptides (1–20; 51–70; 81–100; 111–129; 140–161; 150–171; 180–198), 3 MOBP peptides (1–20; 71–90; 161–180), 2 abCrys peptides (110–131; 161–176), and 1 CNPase peptide (343–362) (Supplemental Figure 3A). Six antigens (q<33.3%) were differentially targeted by IgM antibodies: 3 OSP peptides (91–110; 111–130; and 161–180), 1 PLP peptide (151–170), 1 MOBP peptide (171–183), and 1 abCrys peptide (81–100) (Supplemental Figure 3B).
Figure 1. Differences in IgG and IgM autoantibody reactivity differentiate between ADEM and MS.
SAM-generated heatmaps highlight differences between IgM- and IgG-specific autoantibody reactivity between serum samples from patients with pediatric ADEM, adult MS, or pediatric MS. Individual patients are listed above the heat map and the individual antigens are listed to the right of the heat map. Autoantibody reactivities are conveyed with blue, yellow, and red hues representing low, medium, and high reactivity respectively. Differences in serum IgG (A) and IgM (B) reactivity between patients with ADEM and patients with adult MS. Differences in serum IgG (C) and IgM (D) reactivity between patients with ADEM and patients with pediatric MS. Q values for individual SAM analyses are 8.8% in A, 23.1% in B, 16.7% in C, and 33.3% in D. MOBP, myelin-associated oligodendrocyte basic protein; abCrys, alpha-B-crystallin; PLP, proteolipid protein; OSP, oligodendrocyte specific protein; MBP, myelin basic protein; CNPase, 2’,3’ cyclic nucleotide phosphodiesterase.
Together, these findings suggest that ADEM and MS have distinct profiles of autoantibody reactivity to myelin peptides. Notably, we found that nearly all of the differentiating IgG-targeted antigens showed higher autoantibody reactivity among ADEM patients (Figures 1A & 1C and Supplemental Figure 3A), whereas nearly all of the differentiating IgM-targeted antigens showed higher autoantibody reactivity among MS patients (Figures 1B & 1D and Supplemental Figure 3B). We had noted a similar pattern in our preliminary studies in which we found higher myelin-specific IgG reactivity among ADEM patients (Supplemental Figures 4A & 4D) compared to MS. Analysis of these reactivity profiles using PAM yielded prediction algorithms (Supplemental Figures 4B & 4E) with greater sensitivity for ADEM than MS patients (Supplemental Figures 4C & 4F). These preliminary results (Supplemental Figure 4A–F), together with previous observations of elevated IgM levels in the CSF and serum of patients with acquired demyelinating syndromes,8, 9, 12, 18, 28, 29 provided our original impetus for profiling IgM antibodies in addition to IgG antibodies in ADEM and MS with the goal of achieving greater sensitivity for MS autoantibody reactivity profiles.
Distinct autoantibody profiles can be used to distinguish ADEM from MS
We used the prediction software PAM to determine whether the autoantibody profiles we identified could be used to reliably classify a patient’s clinical diagnosis as ADEM or MS. Using samples from 7 adult MS patients and 7 ADEM patients as the “training” cohort, PAM generated a prediction algorithm, comprising 5 IgG-targeted antigens (MBP 150–171; PLP 201–220; MOG 51–70; abCrys 1–20; abCrys 110–131) and 3 IgM-targeted antigens (MOBP 11–30; MBP 31–50; MOBP 61–80) (Figure 2). We then validated this algorithm using an independent set of 27 patient samples from Cohort 1. Our algorithm correctly classified 5/8 (62%) ADEM patients, 7/8 (87%) adult MS patients, and 8/11 (73%) pediatric MS patients (Figure 2, Prediction on Cohort 1). The sensitivity (SN) and specificity (SP) for each patient group in this analysis were as follows: ADEM SN 62%, SP 79%; adult MS SN 87%, SP 62%; pediatric MS SN 73%, SP 62%.
Conversely, when we performed this prediction in reverse, by using samples from Cohort 1 as the training group, we generated a classification algorithm comprising 1 IgG-specific (abCrys 110–131) antigen and 3 IgM-specific (PLP 231–250; OSP 161–180; MOBP 11–30) antigens (q<1.6) (Figure 2). We then validated this algorithm using an independent set of 25 patient samples from Cohort #2. This algorithm correctly classified 6/7 (86%) ADEM patients, 5/7 (71%) adult MS patients, and 4/10 (40%) pediatric MS patients (Figure 2, Prediction on Cohort #2). The sensitivity and specificity for each patient group in this prediction analysis were as follows: ADEM SN 86%, SP 56%; adult MS SN 71%, SP 86%; pediatric MS SN 40%, SP 86%. A comparison of the prediction algorithms derived from Cohort 1 and Cohort 2 suggests that IgG and IgM antibodies targeting portions of PLP, abCrys, and MOBP provide the greatest diagnostic value in our sample (Figure 2; Supplemental Table 4).
In total, our SAM and PAM analyses from Cohorts 1 and 2 resulted in 8 independently generated lists of antigens (6 lists from SAM analyses and 2 lists from PAM training analyses). Several of these antigens appeared in more than one statistical analysis (Supplemental Table 4). Two antigens appeared in both prediction matrices: IgM-targeted MOBP 11–30 and IgG-targeted abCrys 110–131. Both of these antigens had higher antibody reactivity with ADEM sera than MS sera.
Discussion
Using antigen arrays, we identified distinct profiles of anti-myelin autoantibody reactivity in a trial population of ADEM and MS patients. We used these profiles to generate prediction algorithms based on a small number of antigens capable of distinguishing our ADEM and MS patients with reasonable accuracy and reproducibility within this trial population. Several myelin autoantigens were validated in multiple analyses and should be considered for use in future studies seeking to develop diagnostic tools for classifying acquired demyelinating syndromes. This represents, to our knowledge, the first use of autoantibody array technology to distinguish between clinically distinct demyelinating syndromes.
Our results indicate that MS is characterized by the presence of serum autoantibodies of the IgM isotype targeting PLP, MOBP, and OSP, whereas ADEM is characterized by serum autoantibodies of the IgG isotype targeting MBP and MOBP. Because IgM is a non-class-switched isotype, our findings indicate that the autoantibody response to MBP in MS has not undergone the class-switching that is typically observed in antigen-driven immune responses, consistent with previous findings that many of the autoantibodies observed in MS are of the IgM isotype.8, 9, 12, 18, 19, 28 In contrast, that ADEM is characterized by class-switched IgG responses to MBP and MOBP supports the notion that the immune response is primed during the prodromal illness that often precedes the onset of demyelination in ADEM. These findings are consistent with recent work demonstrating that levels of clonal IgG are higher in the serum of ADEM patients than in that of MS patients.3 Several of us (BB, AB, DP, KR) have previously described higher levels of IgG reactivity in monophasic ADEM patients that disappear in the months following event resolution, yet persist in a small subset of MS patients.30, 31 In the present study, both ADEM and pediatric MS patients had blood collected within the same proximity to a demyelinating event (3 months or less), yet only the ADEM patients demonstrated the tendency toward elevated IgG reactivity. This suggests that the higher IgG reactivity observed in our ADEM cohorts is not simply an artifact of disease acuity.
Identifying biologically relevant antigens is a critical early step in developing autoantibody-based biomarker tests. In the current study, several myelin antigens were implicated in multiple analyses (Supplemental Table 4). Of the MBP peptides that were selectively targeted in our arrays, MBP 81–100 has the greatest precedence given the well-characterized encephalitogenic potential.32 The presence of peptides outside this region (MBP peptides 51–70 and 150–171) is consistent with previous reports.18, 29 The presence of diverse antibody reactivity in these regions may be related to the complementary roles that T-cells and B-cells, which function synergistically to recognize a variety of epitopes from both intact and damaged proteins.33 While T-cells recognize only linear epitopes, B-cells and their antibodies recognize both linear and conformational epitopes, which do not always overlap precisely with T-cell targeted regions.34 It is also possible that the sera of ADEM patients (and EAE mice) more accurately reflect the autoantibodies present in the CSF3 when compared to MS patients.29 This distinction may provide advantages when using serum autoantigen arrays for discriminating between these disorders.
IgM-targeted MOBP 11–30 and IgG-targeted abCrys 110–131 were validated in two independently generated prediction algorithms. Both of these antigens reacted more strongly with antibodies in ADEM sera than with those in MS sera. Although MBP has been previously implicated in ADEM,1, 21, 35 this is, to our knowledge, the first report of selective targeting of MOBP in ADEM sera. Here we see MOBP peptides differentially targeted by both IgG (1–20; Figure 1A) and IgM (11–30; Figure 1B) isotype antibodies in ADEM sera, with the latter playing a prominent role in our prediction algorithms (Figure 2). Reactivity to abCrys also differed between the ADEM and MS groups. Recently, we demonstrated that the heat-shock protein abCrys may bind immunoglobulins rather than the other way around.36 It is possible that this activity could be responsible for the binding observed at the abCrys features on the array, and thus, abCrys may not represent an “autoantigen” biomarker but rather represents a feature of heat shock protein binding.
Anti-MOG autoantibodies have been previously associated with ADEM and, to a lesser extent, MS 18, 29, 30, 37–40. The relative paucity of MOG peptide reactivity among the patient profiles presented here is best explained by the conformation-specific nature of autoantibody reactivity to MOG epitopes1, 37, 41, which is not preserved by the linear peptides employed on our array. In fact, Supplemental Figure 4 demonstrates increased reactivity to the whole MOG protein (cbMOG) among ADEM patients without strong reactivity to the MOG peptides on the same array, all of which is in keeping with previous studies. It is plausible that this conformational specificity may be relevant to other myelin proteins as well.19 Similarly, post-translational modification (e.g. acetylation) can also play an important role in antigen specificity42. Although our array performed well with only linear epitopes, the inclusion of conformational myelin epitopes with or without post-translational modifications, might benefit the development of future prediction arrays.
The strengths of our study include our inclusion of patients with both pediatric and adult MS, our use of an innovative prediction tool, and our generation of diagnostic prediction algorithms using relatively few antigens with reasonably accurate results. Our primary study limitation was our modest sample size. It is interesting to note that while our adult MS cohort differed in temporal proximity to an acute demyelinating event compared with our pediatric ADEM and MS cohorts, we found no significant differences between our pediatric and adult MS cohorts on SAM analyses, suggesting that neither the age of the MS patient nor the proximity to an acute event had a significant impact on autoantibody profiles in our cohorts.
Our findings support previous work2, 3, 18 suggesting that serum autoantibody arrays may eventually provide tools capable of reliably distinguishing a diverse set of inflammatory brain diseases. Follow-up studies using antibody arrays in demyelinating disorders might proceed in several potentially complementary directions. Correlation of clinical outcomes with autoantibody profiles might be applied to the study of prognostic tools perhaps capable of predicting risk of relapse following a first demyelinating event. The delineation of biologically distinct subgroups of autoimmune demyelinating disorders, particularly if augmented with emerging biomarkers such as IL-1743, 44, which associates with a pathological phenotype in a mouse model of MS that is induced specifically with MOG-antigen45. Further development and validation of autoantibody arrays may eventually produce tools capable of reliably distinguishing a diverse set of inflammatory brain diseases.
The goal of this study was to test the feasibility of using myelin autoantibody array platforms to differentiate ADEM and RRMS as distinct clinical entities. Our favorable results in a small cohort of individuals suggest these array platforms may be suitable for further development as diagnostic assays. We acknowledge, however, that clinical exam findings and MRI already set a high bar for diagnostic sensitivity and specificity in distinguishing these two disorders.46, 47 In this context, the autoantibody prediction paradigm outlined here might also be applied toward the study of the pathophysiologic origins of ADEM and MS. While a deep exploration of ADEM and MS pathophysiology was not within the intended scope of this project, our observations implicate differential IgG and IgM targeting of several myelin peptides as potentially distinct features of these disorders. Further validation and exploration of these findings in future investigations could expand our understanding of the antigenic triggers of ADEM and MS, perhaps eventually shedding insight on the very different natural histories associated with these two disorders.
Supplementary Material
Acknowledgements
KVH was supported by funding from NIH R25-NS070698, the NIH Loan Repayment Program, the Child Neurology Foundation, and the Lucile Packard Foundation Sprague/McHugh Multiple Sclerosis Fund.
BB is supported by grants from the Canadian Multiple Sclerosis Scientific Research Foundation and previously by the Wadsworth Foundation.
ABO is supported by grants from the Canadian Multiple Sclerosis Scientific Research Foundation.
KCO was supported by the Nancy Davis Foundation for Multiple Sclerosis and a Career Transition Fellowship from the National Multiple Sclerosis Society.
DAH was supported by funding from the US National Institutes of Health grants U19-AI070352 (NIAID), R01-NS024247 (NINDS), P01-AI039671 (NINDS), and P01-NS038037 (NINDS).
LS was supported by R01 NS55997 (NINDS).
WHR was supported by funding NIH NHLBI Proteomics Center contract N01-HV-00242 and a VA Merit Award.
Footnotes
Potential conflicts of interest:
KVH: None to declare.
BHT: None to declare.
BAK: None to declare.
BB: Dr. Banwell has received speaker’s honoraria and/or has served on pediatric advisory boards for Biogen-Idec, Novartis, Merk-Serono and Teva Neuroscience.
ABO: Dr. Bar-Or has received honoraria and/or research support from Amplimmune, Aventis, Biogen Idec, Bayhill Therapeutics, Berlex, Diogenix, Eli-Lilly, GlaxoSmithKline, Merck Serono, Novartis, Ono Pharma, Receptos, Roche/Genentech and Teva Neuroscience
TC: Dr. Chitnis has acted as an advisor/consultant/advisory board member or speaker for Biogen-Idec, Merck-Serono, Novartis, sanofi-aventis and Teva. She has received research support from Merck-Serono.
ST: Dr. Tenembaum has received speaker�s honoraria and/or has served on pediatric advisory boards for Biogen-Idec, Merck Serono, Genzyme, and Teva Neuroscience.
DP: Dr. Pohl has received speaker’s honoraria and/or has served on pediatric advisory boards for Bayer-Schering, Biogen-Idec, Merck-Serono, and Teva Neuroscience.
RD: None to declare
KCO: None to declare
DAH: None to declare
LS: None to declare.
WHR: None to declare.
References
- 1.O'Connor KC, McLaughlin KA, De Jager PL, et al. Self-antigen tetramers discriminate between myelin autoantibodies to native or denatured protein. Nat Med. 2007;13:211–217. doi: 10.1038/nm1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lalive PH, Hausler MG, Maurey H, et al. Highly reactive anti-myelin oligodendrocyte glycoprotein antibodies differentiate demyelinating diseases from viral encephalitis in children. Mult Scler. 2011;17:297–302. doi: 10.1177/1352458510389220. [DOI] [PubMed] [Google Scholar]
- 3.Franciotta D, Columba-Cabezas S, Andreoni L, et al. Oligoclonal IgG band patterns in inflammatory demyelinating human and mouse diseases. J Neuroimmunol. 2008;200:125–128. doi: 10.1016/j.jneuroim.2008.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Polman CH, Reingold SC, Banwell B, et al. Diagnostic criteria for multiple sclerosis: 2010 revisions to the McDonald criteria. Ann Neurol. 2011;69:292–302. doi: 10.1002/ana.22366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Tenembaum S, Chitnis T, Ness J, Hahn JS. Acute disseminated encephalomyelitis. Neurology. 2007;68:S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 6.Weber MS, Hemmer B, Cepok S. The role of antibodies in multiple sclerosis. Biochim Biophys Acta. 2011;1812:239–245. doi: 10.1016/j.bbadis.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 7.Wootla B, Denic A, Keegan BM, et al. Evidence for the role of B cells and immunoglobulins in the pathogenesis of multiple sclerosis. Neurol Res Int. 2011;2011:780712. doi: 10.1155/2011/780712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Villar LM, Sadaba MC, Roldan E, et al. Intrathecal synthesis of oligoclonal IgM against myelin lipids predicts an aggressive disease course in MS. J Clin Invest. 2005;115:187–194. doi: 10.1172/JCI22833. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Perini P, Ranzato F, Calabrese M, Battistin L, Gallo P. Intrathecal IgM production at clinical onset correlates with a more severe disease course in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2006;77:953–955. doi: 10.1136/jnnp.2005.086116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram G, Hakobyan S, Robertson NP, Morgan BP. Complement in multiple sclerosis: its role in disease and potential as a biomarker. Clin Exp Immunol. 2009;155:128–139. doi: 10.1111/j.1365-2249.2008.03830.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Horstman LL, Jy W, Ahn YS, et al. Complement in neurobiology. Front Biosci. 2012;17:2921–2960. doi: 10.2741/3890. [DOI] [PubMed] [Google Scholar]
- 12.Egg R, Reindl M, Deisenhammer F, Linington C, Berger T. Anti-MOG and anti-MBP antibody subclasses in multiple sclerosis. Mult Scler. 2001;7:285–289. doi: 10.1177/135245850100700503. [DOI] [PubMed] [Google Scholar]
- 13.Robinson WH, DiGennaro C, Hueber W, et al. Autoantigen microarrays for multiplex characterization of autoantibody responses. Nat Med. 2002;8:295–301. doi: 10.1038/nm0302-295. [DOI] [PubMed] [Google Scholar]
- 14.Robinson WH, Fontoura P, Lee BJ, et al. Protein microarrays guide tolerizing DNA vaccine treatment of autoimmune encephalomyelitis. Nat Biotechnol. 2003;21:1033–1039. doi: 10.1038/nbt859. [DOI] [PubMed] [Google Scholar]
- 15.Ousman SS, Tomooka BH, van Noort JM, et al. Protective and therapeutic role for alphaB-crystallin in autoimmune demyelination. Nature. 2007;448:474–479. doi: 10.1038/nature05935. [DOI] [PubMed] [Google Scholar]
- 16.Di Pauli F, Mader S, Rostasy K, et al. Temporal dynamics of anti-MOG antibodies in CNS demyelinating diseases. Clin Immunol. 2011;138:247–254. doi: 10.1016/j.clim.2010.11.013. [DOI] [PubMed] [Google Scholar]
- 17.O'Connor KC, Chitnis T, Griffin DE, et al. Myelin basic protein-reactive autoantibodies in the serum and cerebrospinal fluid of multiple sclerosis patients are characterized by low-affinity interactions. J Neuroimmunol. 2003;136:140–148. doi: 10.1016/s0165-5728(03)00002-x. [DOI] [PubMed] [Google Scholar]
- 18.Quintana FJ, Farez MF, Viglietta V, et al. Antigen microarrays identify unique serum autoantibody signatures in clinical and pathologic subtypes of multiple sclerosis. Proc Natl Acad Sci U S A. 2008;105:18889–18894. doi: 10.1073/pnas.0806310105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hedegaard CJ, Chen N, Sellebjerg F, et al. Autoantibodies to myelin basic protein (MBP) in healthy individuals and in patients with multiple sclerosis: a role in regulating cytokine responses to MBP. Immunology. 2009;128:e451–e461. doi: 10.1111/j.1365-2567.2008.02999.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McDonald WI, Compston A, Edan G, et al. Recommended diagnostic criteria for multiple sclerosis: guidelines from the International Panel on the diagnosis of multiple sclerosis. Ann Neurol. 2001;50:121–127. doi: 10.1002/ana.1032. [DOI] [PubMed] [Google Scholar]
- 21.Hafler DA, Benjamin DS, Burks J, Weiner HL. Myelin basic protein and proteolipid protein reactivity of brain- and cerebrospinal fluid-derived T cell clones in multiple sclerosis and postinfectious encephalomyelitis. J Immunol. 1987;139:68–72. [PubMed] [Google Scholar]
- 22.Banwell B, Reder AT, Krupp L, et al. Safety and tolerability of interferon beta-1b in pediatric multiple sclerosis. Neurology. 2006;66:472–476. doi: 10.1212/01.wnl.0000198257.52512.1a. [DOI] [PubMed] [Google Scholar]
- 23.Kaushansky N, Eisenstein M, Zilkha-Falb R, Ben-Nun A. The myelin-associated oligodendrocytic basic protein (MOBP) as a relevant primary target autoantigen in multiple sclerosis. Autoimmun Rev. 2010;9:233–236. doi: 10.1016/j.autrev.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 24.Lovato L, Cianti R, Gini B, et al. Transketolase and 2',3'-cyclic-nucleotide 3'-phosphodiesterase type I isoforms are specifically recognized by IgG autoantibodies in multiple sclerosis patients. Mol Cell Proteomics. 2008;7:2337–2349. doi: 10.1074/mcp.M700277-MCP200. [DOI] [PubMed] [Google Scholar]
- 25.Bronstein JM, Lallone RL, Seitz RS, Ellison GW, Myers LW. A humoral response to oligodendrocyte-specific protein in MS: a potential molecular mimic. Neurology. 1999;53:154–161. doi: 10.1212/wnl.53.1.154. [DOI] [PubMed] [Google Scholar]
- 26.Masch A, Zerweck J, Reimer U, Wenschuh H, Schutkowski M. Antibody signatures defined by high-content peptide microarray analysis. Methods Mol Biol. 2010;669:161–172. doi: 10.1007/978-1-60761-845-4_13. [DOI] [PubMed] [Google Scholar]
- 27.Tibshirani R, Hastie T, Narasimhan B, Chu G. Diagnosis of multiple cancer types by shrunken centroids of gene expression. Proc Natl Acad Sci U S A. 2002;99:6567–6572. doi: 10.1073/pnas.082099299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rauer S, Euler B, Reindl M, Berger T. Antimyelin antibodies and the risk of relapse in patients with a primary demyelinating event. J Neurol Neurosurg Psychiatry. 2006;77:739–742. doi: 10.1136/jnnp.2005.077784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quintana FJ, Farez MF, Izquierdo G, Lucas M, Cohen IR, Weiner HL. Antigen microarrays identify CNS-produced autoantibodies in RRMS. Neurology. 2012;78:532–539. doi: 10.1212/WNL.0b013e318247f9f3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Baranzini SE, Nickles D. Genetics of multiple sclerosis: swimming in an ocean of data. Current opinion in neurology. 2012;25:239–245. doi: 10.1097/WCO.0b013e3283533a93. [DOI] [PubMed] [Google Scholar]
- 31.Mikaeloff Y, Moreau T, Debouverie M, et al. Interferon-beta treatment in patients with childhood-onset multiple sclerosis. J Pediatr. 2001;139:443–446. doi: 10.1067/mpd.2001.117004. [DOI] [PubMed] [Google Scholar]
- 32.Steinman L, Waisman A, Altmann D. Major T-cell responses in multiple sclerosis. Molecular medicine today. 1995;1:79–83. doi: 10.1016/s1357-4310(95)92366-7. [DOI] [PubMed] [Google Scholar]
- 33.Pomes A. Relevant B cell epitopes in allergic disease. International archives of allergy and immunology. 2010;152:1–11. doi: 10.1159/000260078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Corcoran A, Mahon BP, Doyle S. B cell memory is directed toward conformational epitopes of parvovirus B19 capsid proteins and the unique region of VP1. The Journal of infectious diseases. 2004;189:1873–1880. doi: 10.1086/382963. [DOI] [PubMed] [Google Scholar]
- 35.Pohl-Koppe A, Burchett SK, Thiele EA, Hafler DA. Myelin basic protein reactive Th2 T cells are found in acute disseminated encephalomyelitis. J Neuroimmunol. 1998;91:19–27. doi: 10.1016/s0165-5728(98)00125-8. [DOI] [PubMed] [Google Scholar]
- 36.Rothbard JB, Zhao X, Sharpe O, et al. Chaperone activity of alpha B-crystallin is responsible for its incorrect assignment as an autoantigen in multiple sclerosis. J Immunol. 2011;186:4263–4268. doi: 10.4049/jimmunol.1003934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.McLaughlin KA, Chitnis T, Newcombe J, et al. Age-dependent B cell autoimmunity to a myelin surface antigen in pediatric multiple sclerosis. J Immunol. 2009;183:4067–4076. doi: 10.4049/jimmunol.0801888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Klawiter EC, Piccio L, Lyons JA, Mikesell R, O'Connor KC, Cross AH. Elevated intrathecal myelin oligodendrocyte glycoprotein antibodies in multiple sclerosis. Arch Neurol. 2010;67:1102–1108. doi: 10.1001/archneurol.2010.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.O'Connor KC, Appel H, Bregoli L, et al. Antibodies from inflamed central nervous system tissue recognize myelin oligodendrocyte glycoprotein. J Immunol. 2005;175:1974–1982. doi: 10.4049/jimmunol.175.3.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sadaka Y, Verhey LH, Shroff MM, et al. 2010 McDonald criteria for diagnosing pediatric multiple sclerosis. Ann Neurol. 2012;72:211–223. doi: 10.1002/ana.23575. [DOI] [PubMed] [Google Scholar]
- 41.Brilot F, Dale RC, Selter RC, et al. Antibodies to native myelin oligodendrocyte glycoprotein in children with inflammatory demyelinating central nervous system disease. Ann Neurol. 2009;66:833–842. doi: 10.1002/ana.21916. [DOI] [PubMed] [Google Scholar]
- 42.He XL, Radu C, Sidney J, Sette A, Ward ES, Garcia KC. Structural snapshot of aberrant antigen presentation linked to autoimmunity: the immunodominant epitope of MBP complexed with I-Au. Immunity. 2002;17:83–94. doi: 10.1016/s1074-7613(02)00340-0. [DOI] [PubMed] [Google Scholar]
- 43.Hueber W, Tomooka BH, Batliwalla F, et al. Blood autoantibody and cytokine profiles predict response to anti-tumor necrosis factor therapy in rheumatoid arthritis. Arthritis Res Ther. 2009;11:R76. doi: 10.1186/ar2706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Axtell RC, de Jong BA, Boniface K, et al. T helper type 1 and 17 cells determine efficacy of interferon-beta in multiple sclerosis and experimental encephalomyelitis. Nat Med. 2010;16:406–412. doi: 10.1038/nm.2110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Herges K, de Jong BA, Kolkowitz I, et al. Protective effect of an elastase inhibitor in a neuromyelitis optica-like disease driven by a peptide of myelin oligodendroglial glycoprotein. Mult Scler. 2012;18:398–408. doi: 10.1177/1352458512440060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simpson S, Jr, Taylor B, Blizzard L, et al. Higher 25-hydroxyvitamin D is associated with lower relapse risk in multiple sclerosis. Ann Neurol. 2010;68:193–203. doi: 10.1002/ana.22043. [DOI] [PubMed] [Google Scholar]
- 47.Waubant E, Hietpas J, Stewart T, et al. Interferon beta-1a in children with multiple sclerosis is well tolerated. Neuropediatrics. 2001;32:211–213. doi: 10.1055/s-2001-17370. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.