Abstract
Dietary nitrate (NO3−) supplementation via beetroot juice has been shown to increase the exercise capacity of younger and older adults. The purpose of this study was to investigate the effects of acute NO3− ingestion on the submaximal constant work rate exercise capacity of COPD patients. Fifteen patients were assigned in a randomized, single-blind, crossover design to receive one of two treatments (beetroot juice then placebo or placebo then beetroot juice). Submaximal constant work rate exercise time at 75% of the patient’s maximal work capacity was the primary outcome. Secondary outcomes included plasma NO3− and nitrite (NO2−) levels, blood pressure, heart rate, oxygen consumption (VO2), dynamic hyperinflation, dyspnea and leg discomfort. Relative to placebo, beetroot ingestion increased plasma NO3− by 938% and NO2− by 379%. Median (+ interquartile range) exercise time was significantly longer (p = 0.031) following the ingestion of beetroot versus placebo (375.0 + 257.0 vs. 346.2 + 148.0 sec., respectively). Compared to placebo, beetroot ingestion significantly reduced iso-time (p = 0.001) and end exercise (p = 0.008) diastolic blood pressures by 6.4 and 5.6 mmHg, respectively. Resting systolic blood pressure was significantly reduced (p = 0.019) by 8.2 mmHg for the beetroot versus the placebo trial. No other variables were significantly different between the beetroot and placebo trials. These results indicate that acute dietary NO3− supplementation can elevate plasma NO3− and NO2− concentrations, improve exercise performance, and reduce blood pressure in COPD patients.
Keywords: Chronic obstructive pulmonary disease, Nitrate, Nitrite, Nitric Oxide, Exercise, Blood pressure
1.0 INTRODUCTION
Chronic obstructive pulmonary disease (COPD) is a progressive lung disease affecting the airways and/or lung parenchyma of primarily older adults that results in a mostly irreversible airway obstruction (31, 43). Pathophysiological consequences of COPD include skeletal muscle deconditioning, ventilatory and gas exchange impairments resulting in tissue hypoxia, and psychological disturbances all of which result in a shortness of breath (dyspnea) and a poor exercise tolerance. Because of these problems, COPD patients engage in lower levels of physical activity as compared to age-matched controls (38, 49, 50). This reduction in activity leads to further cardiovascular and musculoskeletal system deconditioning and increases in activity-related dyspnea thus provoking further decreases in physical activity. Unless interrupted, this spiral of dyspnea, inactivity, and deconditioning ultimately leads to the inability of COPD patients to perform basic activities of daily living. This loss of physical function has the potential to further increase the risk of morbidity and mortality as well as healthcare costs. Exercise (5, 6, 45) and optimal pharmacological management (28, 33) have both been shown to improve the exercise capacity and physical function of COPD patients.
Recently, dietary nitrate (NO3−) has been shown to be a nutraceutical that can improve exercise capacity in young healthy individuals (3, 9, 20). Dietary NO3− supplementation has been shown to exert its effects as a result of its conversion to NO2− and then to NO. NO is recognized as an endogenous effector molecule that has a role in a variety of physiological functions including vasoregulation, vascular homeostasis, neurotransmission, cellular metabolism and immune function (24, 55). Following NO3− consumption, it is absorbed in the stomach and small intestines. While approximately 75% of the NO3− is excreted by the kidneys, the remainder is taken up by the salivary glands and concentrated in the saliva (47). Salivary NO3− is then reduced to NO2− via facultative bacteria found in the oral cavity (39). Once swallowed and within the acidic environment of the stomach, NO2− is then converted to NO (4, 25). This NO3−→NO2−-→NO pathway has been proposed as a complementary pathway to the L-arginine nitric oxide synthase (NOS) system as a source of NO (24). Given the L-arginine NOS system is oxygen dependent, whereas activity of the NO3−→NO2−-→NO pathway increases as oxygen tensions decrease, the latter pathway can be viewed as a back-up system for NO production during hypoxic conditions, which can occur in COPD patients and during exercise.
Vegetables are a primary source of NO3− in the human diet. In particular, green leafy vegetable and beetroots have a high NO3− concentration. Nitrate supplementation via beetroot juice has been shown to reduce oxygen consumption (VO2) during submaximal exercise and increase the time to exhaustion during high intensity exercise in young healthy subjects (3, 23). Vanhatalo et al. recently found that in young healthy subjects NO3− supplementation via beetroot juice reduced muscle metabolic perturbations during hypoxic exercise and restored exercise tolerance and oxidative function to values observed in normoxia (52). These results suggest that stimulating the NO3−→NO2−-→NO pathway via ingestion of dietary NO3− may have important therapeutic applications for improving muscle energetics and functional capacity during hypoxic conditions such as exercise in patients with cardiovascular, pulmonary and/or sleep disorders.
Kelly et al. hypothesized that NO3− supplementation may also provide beneficial effects for older adults because of the age associated decline in NO signaling (18). This NO defect is a result of reduced availability of L-arginine or the cofactor tetrahydrobiopterin, reduced endothelial NOS activity and/or increased superoxide production (44, 48). Kelly et al. reported that dietary supplementation of NO3− over a 2.5 day period significantly increased plasma NO2− levels and decreased resting blood pressure and the mean response time of VO2 in healthy older adults (18). These results suggest that dietary NO3− intake has the potential to improve the exercise capacity of older adults.
Given COPD patients experience greater degrees of tissue hypoxia, have a decreased exercise capacity, are often older and the fact that dietary NO3− has been shown to improve exercise performance, it was the purpose of this investigation to examine the effects of acute NO3− supplementation, via beetroot juice consumption, on submaximal constant work rate exercise in patients with COPD. If NO3− supplementation can increase exercise tolerance in COPD patients by reducing the oxygen cost of exercise at a given work rate, this molecule could potentially serve as a therapeutic agent allowing these patients to retain physical function and remain active thus delaying or even preventing the loss of physical function and independence.
2.0 METHODS
2.1 Design and Overview
This investigation was a single-blind, placebo-controlled, cross over study with submaximal constant work rate exercise time as the primary outcome. Patients completed four visits as part of the study protocol. During visit 1, patients completed baseline pulmonary function testing, health status questionnaires, had a brief medical examination and completed an incremental exercise test on an electronically braked cycle ergometer to determine their maximal exercise work rate. Visit 2 was performed approximately one week later and consisted of additional pulmonary function and lung volume testing, as well as a familiarization submaximal constant work rate exercise test on an electronically braked cycle ergometer at 75% of patients’ maximal work rate. This type of exercise test has been used in previous trials with COPD patients examining the effects of pharmaceutical agents on exercise performance and is designed to exhaust the patient between four and ten minutes (33, 37, 42). Upon successful completion of visits 1 and 2, patients were randomized into the single-blind, cross-over treatment segment of the study. In this part of the study, patients were randomly assigned to one of two treatments, beetroot juice (visit 3) and placebo (visit 4) or placebo (visit 3) and beetroot juice (visit 4). Visits 3 and 4 were separated by at least a seven day wash-out period. All visits were performed at a similar time in the morning. While patients were informed that the purpose of the study was to examine the effects of dietary NO3− on exercise performance, they were not informed by the study staff as to which beverage was high in NO3−. Investigators collecting study related data and supplying the beverages to the patients were not aware of the beverage supplied to the patient. Additionally, all patients were asked not to inform the study staff as to which beverage they had consumed when they returned for follow-up testing on visits 3 and 4. A schematic of the study visits is shown in Figure 1.
Figure 1.
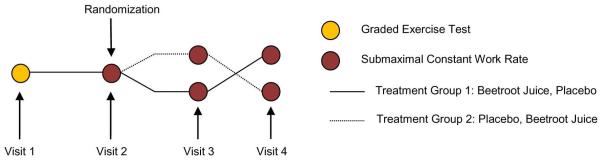
Illustrates the schematic of trial visits
2.2 Patients
The study sample consisted of fifteen COPD patients; eleven white males, one African-American male and three white females. Subjects were recruited from previous exercise interventions with COPD patients as well as newspaper adverts. All subjects who agreed to participate signed an informed consent form approved by the Institutional Review Board at Wake Forest University. Exclusion criteria and screening methods are listed in Table 1.
Table 1.
Exclusion criteria and screening methods
| CRITERIA | EXCLUSIONS | SCREENING METHODS |
|---|---|---|
| Health |
|
Medical history, Physical exam, Graded Exercise Test, Personal Physician |
| COPD | FEV1/FVC > 70%, FEV1 < 20% of predicted | Pulmonary function tests, History |
| Smoking History | Present Smoker | Questionnaire |
| Ability to comply with exercise interventions |
Inability to perform exercise due to physical disability |
History/physical exam |
| Inability to complete study |
Unwilling and/or unable to participate in all aspects of the study, i.e., randomization, exercise and pulmonary function testing, consumption of study beverages and blood draw |
History and questionnaire |
| Pharmacological | On nitroglycerine or NO3−preparations used with angina, phosphodiesterase type 5 (PDE5) inhibitors, including sildenafil (Viagra®), medications to alter stomach pH (antacids, proton pump inhibitors). |
Questionnaire |
2.3 Study Procedures
2.3.1 Visit 1
After signing the informed consent, patients completed pulmonary function tests to determine if they qualified to participate. These tests were conducted according to the American Thoracic Society/European Respiratory Society standards (30). Those who did qualify then completed a medical history questionnaire and a physical examination. Patients then performed an incremental exercise test on a Velotron Rehab electronically braked cycle ergometer (RacerMate Inc.) at a pedal rate between 50 and 70 rpm. The exercise test began with three minutes of quiet breathing followed by one minute of pedaling at five watts (W). The work rate was then increased by 10 or 15 W each minute thereafter until volitional exhaustion. Patient’s maximum work rate was defined as the highest work rate (watts) maintained for ≥ 30 seconds. Heart rate, arterial oxygen saturation levels and ratings of perceived dyspnea and leg discomfort were obtained at rest and each minute of the test. Blood pressure was assessed at rest and every two minutes thereafter. Oxygen consumption was monitored continuously throughout the test. Testing instructions and encouragement were standardized throughout all aspects of the testing for each patient. At the conclusion of Visit 1, patients were given a food log and instructed to record all foods and beverages consumed in the 24 hours prior to visit 2. The food log also contained a list of foods high in NO3− which patients were instructed to avoid 48 hours prior to subsequent visits. The food list was also used as a reference to replicate a similar dietary pattern before subsequent visits. To prevent the elimination of NO3− reducing oral bacteria, patients were instructed to avoid the use of mouthwash for 48 hours prior to and on the day of subsequent visits. Subjects were also instructed to maintain their normal medication routines.
2.3.2 Visit 2
Upon arrival for visit 2, patients completed lung volume and diffusion capacity testing according to the American Thoracic Society/European Respiratory Society standards (26, 53). A submaximal constant work rate exercise test was then performed. The exercise test began with three minutes of quiet breathing followed by three minutes of pedaling at five W, after which the work rate was increased to 75% of the patient’s maximal work rate. If this constant work rate was not maintained for at least four minutes, a 10% decrease in work rate was applied for visits 3 and 4. Conversely, if patients were able to maintain 75% of their maximum work rate for more than 10 minutes, a 10% increase in work rate was applied for visits 3 and 4. Throughout the test (rest, 5 W pedaling and 75% of max work rate), the patient’s VO2 was monitored continuously and recorded as 30 second averages. During the second minutes of the rest and the 5 W exercise period, blood pressure and arterial O2 saturation were measured. During the final minute of the 5 W exercise period, the inspiratory capacity was measured. During minute two and every subsequent third minute thereafter of the constant work rate exercise test, blood pressure and arterial O2 saturation were measured. During minute three and every subsequent third minute thereafter of the constant work rate exercise test, ratings of dyspnea and leg discomfort were measured. Following completion of the exercise test, patients were randomly assigned to one of two treatments - beetroot juice (visit 3) then placebo (visit 4) or placebo (visit 3) then beetroot juice (visit 4). At the conclusion of visit 2, patients received either the beetroot or placebo beverage from a member of the study staff. Patients were then given another food log and instructed on a dietary pattern to follow for the 48 hours prior to visit 3. Lastly, patients were instructed to ingest their beetroot or placebo beverage two and a half hours prior to their scheduled visit 3. The beetroot juice was administered in the form of two 70 ml bottles of commercially available beetroot juice (Beet It Stamina Shot, James White Drinks), each containing 3.79 millimoles of NO3−. Therefore, patients who received the beetroot beverage consumed a total of 7.58 millimoles of NO3− prior to study visit 3. The placebo beverage was administered in the form of one 163 ml can of commercially available prune juice (Sunsweet) which contained less than 0.01 millimoles of NO3−. The prune juice was selected as the placebo due to the fact that it had minimal levels of NO3−, had a similar consistency and similar levels of fiber and carbohydrates. The beetroot juice contained 1 gram of fiber, 32 grams of sugar and a total of 32 grams of carbohydrates. The prune juice contained 1 gram of fiber, 16 grams of sugar and a total of 28 grams of carbohydrates. Patients were again reminded to avoid foods high in NO3− and the use of mouthwash for 48 hours prior to visit 3.
2.3.3 Visit 3
Upon arrival for visit 3, one 4 mL venous blood sample was obtained to determine plasma NO3− and NO2− levels. A submaximal constant work rate exercise test was then performed at either 75% of the patient’s maximal work rate as determined from visit one or at an adjusted rate based on the results of visit two. The exercise test followed the same procedures described for visit 2. At the end of testing, patients were provided with their beverage for visit 4, a food log, and were again reminded to avoid foods high in NO3− and the use of mouthwash for 48 hours prior to visit 4.
2.3.4 Visit 4
All study procedures followed during study visit 3 were followed during study visit 4.
2.4 Study Outcomes
2.4.1 Primary Outcome
The primary outcome for this investigation was exercise time (in seconds) measured during the submaximal constant work rate exercise tests performed during visits 3 and 4. Exercise time was recorded as the time in seconds from the start of the 75% of max work rate to the point of symptom limitations. Standardized encouragement was provided throughout all tests.
2.4.2 Secondary Outcomes
Secondary outcomes included plasma NO3− and NO2− levels, VO2, rate of perceived dyspnea and leg discomfort, systolic and diastolic blood pressure, heart rate and dynamic hyperinflation. The evaluation of secondary outcomes was done at three time points during the constant work rate tests of visits 3 and 4. These were at rest, iso-time exercise and at the end of exercise. Iso-time exercise was defined as the last minute of the shortest exercise time during either visit 3 or 4. Values were then matched with those obtained at the same time from the subsequent longer duration exercise test. End of exercise blood pressure, inspiratory capacity and arterial oxygen saturation levels were measured as the last set of data collected during each of the tests. Oxygen consumption values were the final complete 30 second values collected. Heart rate, dyspnea and leg discomfort were obtained at the end of exercise.
Blood samples were collected in a 4 mL lithium heparin vial from the patient’s antecubital vein to determine plasma NO3− and NO2− levels during visits 3 and 4. The blood samples were centrifuged at 5000 rpm for two minutes, the plasma was removed and immediately frozen on dry ice in aliquots with 0.4 mL of plasma, and stored in a −80° C freezer for subsequent analysis. NO3− and NO2− levels were determined by nitric oxide analyzer ENO-20 (EICOM, CA), designed specifically for measuring nitrite and nitrate, used routinely for plasma samples and based on the colorimetric Greiss assay. Standard curves were obtained for all measurements and used for quantitative measurements. All pulmonary function and lung volume testing was performed using a Medical Graphics Corporation Elite series body plethysmograph. Oxygen consumption data was collected using a Medical Graphics Corporation Ultima series cardiorespiratory gas exchange system. Dynamic hyperinflation was determined from changes in the inspiratory capacity of patients between 5 W pedaling and the end of exercise values. Inspiratory capacity was measured according to the methods of O’Donnell et al (35). Perceived dyspnea and leg discomfort were measured with the Borg 10 point scale (8). Blood pressure was measured manually using the auscultatory method with an appropriately sized blood pressure cuff and the same aneroid sphygmomanometer and stethoscope throughout all testing. Patients were sitting on the bike at all times with their feet resting on the pedals. Only a single measurement was made at each time point throughout the exercise tests. Blood pressure was measured in the upper arm with the stethoscope over the brachial artery by the same experienced examiner. The subject’s wore loose clothing so that the cuff could be placed directly against the skin and the arm was supported at the level of the heart by the examiner. In most instances, blood pressure was measured in the right arm of the subject unless blood had been drawn from that arm for the nitrate and nitrite determinations. Arterial O2 saturation was obtained using a Nonin 7500 digital pulse oximeter.
2.5 Statistical Analyses
Data were analyzed using SPSS version 22.0. Normalcy of data were first assessed by visual inspection of normal quantile plots. Suspected deviations from normalcy were subsequently tested using a Shapiro Wilk test. For variables found not to be normally distributed, a Wilcoxon signed rank test was used to test for differences between the placebo and the beetroot juice trials (constant work rate exercise time, nitrite levels, iso-time VO2, leg discomfort and end of exercise inspiratory capacity). For all other variables, dependent t-tests were used to test for differences between the placebo and beetroot juice trials. All tests were two-sided and significance was set at p < 0.05. All normally distributed outcome variables are reported as means and 95% confidence intervals (CI). Non-normally distributed variables are reported as medians and interquartile ranges (IQR).
3.0 RESULTS
3.1 Baseline descriptive statistics (mean ± SD) for the fifteen patients are presented in Table 2.
Table 2.
Descriptive statistics for patients.
| Mean ± SD | |
|---|---|
| Age (yr) | 69.6 ± 8.5 |
| Height (cm) | 173.2 ± 7.1 |
| Weight (kg) | 88.0 ± 18.4 |
| BMI (kg/m2) | 29.2 ± 5.5 |
| FEV1 (l) | 1.8 ± 0.4 |
| FEV1 (% predicted) | 61.8 ± 17.2 |
| FEV1/FVC (%) | 55.4 ± 10.3 |
| TLC (l) | 6.5 ± 1.1 |
| RV (l) | 3.2 ± 1.0 |
| RV/TLC (%) | 46.0 ± 8.8 |
| VO2 max (ml*kg−1*min−1) | 13.9 ± 3.5 |
| Maximum Work Rate (watts) | 92.6 ± 31.6 |
BMI – body mass index. VO2 max – maximum volume of oxygen uptake. FEV1 – forced expiratory volume in one second. FVC – forced vital capacity. TLC – total lung capacity. RV – residual volume. All values are mean ± SD.
3.2 Ingestion of beetroot juice resulted in significantly greater levels of plasma NO3− (p < 0.001) and NO2− (p = 0.001) as compared to placebo ingestion. Figure 2 shows the mean ± SEM and individual patient NO3− levels for both the beetroot juice and placebo trials. Plasma NO3− levels were elevated by 938% when comparing the beetroot juice trial to the placebo trial. Figure 3 shows the median, IQR and 10th and 90th percentile levels for NO2− levels for both the beetroot juice and placebo trials. Plasma NO2− levels were elevated by 379% when comparing the beetroot juice trial to the placebo trial.
Figure 2.
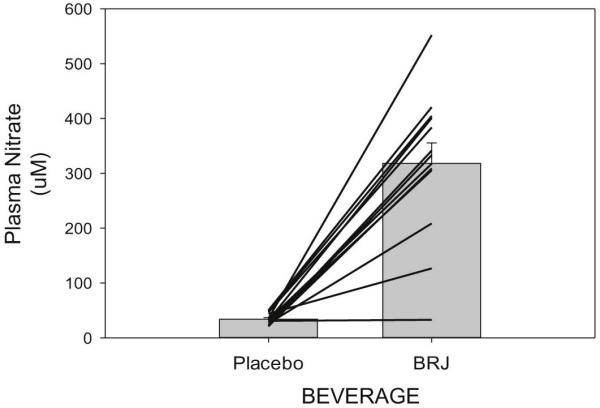
Illustrates plasma nitrate concentrations expressed as uM of plasma. Both individual and mean ± SEM concentrations are shown for the beetroot juice (BRJ) and placebo trials.
Figure 3.
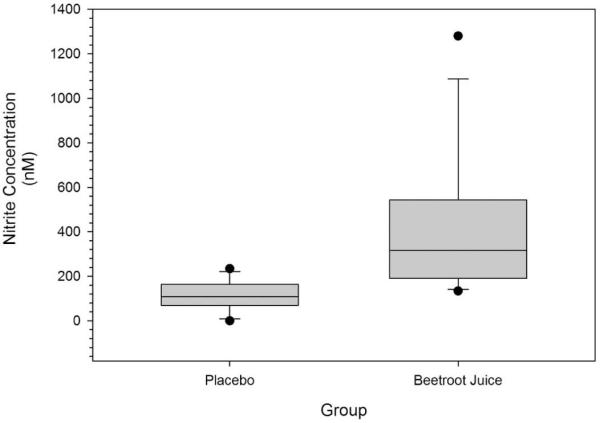
Illustrates plasma nitrite concentrations expressed as nM of plasma for both the beetroot juice and placebo trials. Boxes represent the interquartile ranges with the line within each representing the median value. The error bars project to the 10th and 90th percentiles with the circles at each end representing the smallest and largest values..
3.3 Figure 4 show the median, IQR and 10th and 90th percentile ranges for the submaximal constant work rate exercise times for both the beetroot and placebo juice trials. Median submaximal constant work rate exercise time was 28.8 sec longer (p = 0.031) following NO3− ingestion.
Figure 4.
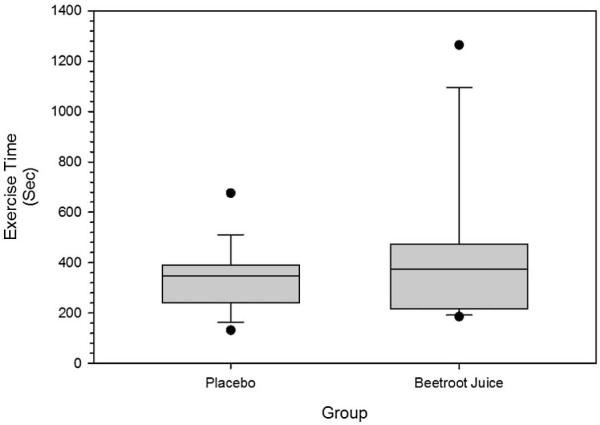
Illustrates submaximal constant work rate exercise time in seconds for both the beetroot juice and placebo trials. Boxes represent the interquartile ranges with the line within each representing the median value. The error bars project to the 10th and 90th percentiles with the circles at each end representing the smallest and largest values.
To test for an order effect, we also compared the submaximal constant work rate exercise times obtained during visit 3 against those from visit 4. There were no significant differences (p = 0.609) in median exercise times when comparing visit 3 and visit 4. Median and IQR exercise times for visit 3 and 4 were 350.0 + 175.0 and 346.2 + 232.0 seconds, respectively.
3.4 Rest, iso-time exercise and end of exercise values for heart rate, oxygen consumption, systolic and diastolic blood pressures and arterial oxygen saturation levels during the placebo and beetroot juice trials are shown in Table 3. Heart rates were not significantly different between the beetroot and placebo trials at rest, iso-time exercise or end exercise. Oxygen consumption values were not significantly different between the beetroot and placebo trials at rest, iso-time or end exercise. Resting systolic blood pressure was significantly lower during the beetroot trial versus the placebo trial. There was a trend (p = 0.065) for resting diastolic blood pressure values to be lower during the beetroot trial as compared to the placebo trial. Diastolic blood pressures at iso-time exercise and end exercise were significantly lower following beetroot ingestion as compared to placebo ingestion. Arterial O2 saturation levels were not different between the beetroot juice and the placebo trials at baseline, iso-time or at the end of exercise.
Table 3.
Secondary outcome variables at baseline, Iso-time exercise and the end of exercise during the beetroot and placebo trials
| Beetroot | Placebo | P | |
|---|---|---|---|
| Heart Rate, bpm | |||
| Rest | 70 [62, 78] | 69 [61, 77] | 0.707 |
| Iso-time | 110 [97, 123] | 112 [99, 124] | 0.300 |
| End Exercise | 117 [100, 137] | 123[103, 142] | 0.475 |
| Oxygen Consumption, ml*kg−1*min−1 | |||
| Rest | 4.1 [3.3, 4.8] | 4.0 [3.4, 4.6] | 0.925 |
| *Iso-time | 14.1 + 5.4 | 13.4 + 5.5 | 0.099 |
| End Exercise | 14.1 [11.8, 16.4] | 14.4[12.4, 16.4] | 0.436 |
| Systolic Blood Pressure, mmHg | |||
| Rest | 124.3 [115.2, 133.4] | 132.5[121.7, 143.4] | 0.019 |
| Iso-time | 160.1 [147.8, 172.5] | 167.1 151.7, 182.4] | 0.137 |
| End Exercise | 164.0 [147.5, 180.5] | 170.4 [153.9, 186.9] | 0.296 |
| Diastolic Blood Pressure, mmHg | |||
| Rest | 77.2 [71.8, 82.6] | 80.9 [75.3, 86.6] | 0.065 |
| Iso-time | 79.9 [72.8, 86.9] | 86.3 [79.6, 92.9] | 0.001 |
| End Exercise | 80.0 [72.9, 87.1] | 85.6 [79.7, 91.5] | 0.008 |
| Arterial O2 Saturation, % | |||
| Rest | 95.4 [94.4, 96.5] | 95.4 [94.2, 96.7] | 1.0 |
| Iso-time | 95.1 [93.9, 96.2] | 95.1[94.0, 96.2] | 0.895 |
| End Exercise | 95.2 [93.9, 96.5] | 95.2 [94.1, 96.3] | 1.0 |
Non-normally distributed variables. Normally distributed va ues are presented as means and [95% confidence intervals]. Non-normally distributed variables are presented as medians and interquartile ranges.
The percent decrease in inspiratory capacity, as a measure of dynamic hyperinflation, was not significantly different when comparing the beetroot juice trial to the placebo trial at iso-time exercise (15.1 [8.7, 21.4] vs. 16.5 [10.3, 22.7] % (mean [95% CI]), respectively, p = 0.704) or at the end of exercise (18.5 + 11.0 vs. 19.0 + 18.5 % (median + IQR), respectively, p = 0.889). Dyspnea ratings were not significantly different when comparing the beetroot juice trial to the placebo trial at iso-time exercise (5.0 [4.1, 5.9] vs. 5.2 [4.2, 6.2], respectively, p = 0.582) or at the end of exercise (5.9 [4.7, 7.2] vs. 5.6 [4.6, 6.6], respectively, p = 0.454). Perceived leg discomfort ratings were not significantly different when comparing the beetroot juice trial to the placebo trial at iso-exercise time (5.0 + 3.0 vs. 5.0 + 3.0 (median + IQR), respectively, p = 0.601) or at the end of exercise (6.6 [5.3, 7.9] vs. 6.2 [5.0, 7.4], respectively, p = 0.305).
4.0 DISCUSSION
The results of this investigation showed that acute ingestion of NO3−-rich beetroot juice as compared to a placebo drink 1) increased plasma NO3− and NO2− concentrations, 2) extended exercise time during submaximal constant work rate cycle ergometer exercise, 3) reduced resting systolic blood pressure, and 4) reduced iso-time and end of exercise diastolic blood pressure in older adults with COPD. To our knowledge, this is the first study to demonstrate beneficial effects of dietary NO3− supplementation on exercise performance and blood pressure in patients with COPD. These findings are similar to those of previous studies in both young and old healthy subjects where NO3− was shown to positively influence exercise outcomes and reduce blood pressure (2, 18-20, 51, 57). Nitrate supplementation was not found to significantly affect heart rate, VO2, inspiratory capacity, arterial O2 saturation levels or perceptions of dyspnea and leg discomfort.
4.1 Effects of Dietary Nitrate Supplementation on Plasma Nitrite Levels
Following beetroot juice supplementation, plasma NO2− levels increased by 379% relative to those following placebo ingestion. These findings are consistent with previous studies reporting significant elevations in plasma NO2− concentration after supplementation with dietary NO3− in both young (2, 52, 57) and older adults (18, 29). Surprisingly, plasma NO2− concentrations during the placebo trial were similar to those in young healthy subjects (57). Previous research suggests that in the absence of supplementation, lower NO2− concentrations can be expected in older adults due to the increased oxidative stress associated with aging (46). Other mechanisms suggested for the blunted NO2− values reported in older adults include changes in oral bacteria colonization and increased gastric pH (40).
4.2 Effects of Dietary Nitrate Supplementation on Exercise Capacity
The finding of a significant improvement in constant work rate exercise time may be of importance to COPD patients, since these patients often present with significant reductions in their exercise capacity. These reductions in exercise capacity can lead to losses of physical function and a subsequent inability to perform activities of daily living. Presently, the use of exercise training, pharmacotherapy, and/or surgery are the only interventions that have been shown to positively affect exercise tolerance in these patients. Our results add dietary NO3− to this list of interventions that may improve the exercise capacity of these patients. Considering poor exercise capacity is a common condition affecting a large number of COPD patients, any intervention that improves exercise performance should be considered in the management of these patients.
We chose to use a submaximal constant work rate exercise test at 75% of the patient’s maximal work rate, as it has been shown to be reproducible and highly responsive to therapeutic interventions such as drug therapies and exercise rehabilitation in individuals with COPD (37, 41, 42). We found the median exercise time to be almost 29 seconds longer when patients had ingested the NO3− rich beetroot juice as compared to the placebo. It should be noted that the individual patient exercise responses during the beetroot juice and placebo trials were extremely variable; two patients had large improvements, eleven showed slight improvements, and two patients showed a slight decrease in exercise time. Puenta-Maestu et al. reported that the minimum clinically important difference for exercise time during a constant work rate exercise test at 75% of COPD patient’s maximal power output to be approximately 101 seconds or 33% of the patient’s baseline value (42). Five of the fifteen patients had an improvement in exercise time that exceeded the minimum clinically important difference of 101 seconds when comparing the beetroot and the placebo trails. In a large scale study investigating the effect of the bronchodilator tiotropium bromide, O’Donnell and colleagues found that tiotropium use increased post-dose constant work rate exercise endurance time by 40 seconds following an acute exposure to the drug (33). Our finding of a 29 second increase in endurance time with a single dose of beetroot juice compares favorably to that which was seen with acute bronchodilator use.
The reason for the highly variable responses among our patients is unclear. It should be noted, however, that studies examining the exercise responses to NO3− supplementation have produced variable results. In studies involving healthy, recreationally active young adults, NO3− supplementation has been shown to improve exercise performance during moderate to high intensity exercise (9, 57). Interestingly, investigations using more highly trained, but still relatively young, subjects have not shown improvements in exercise performance following NO3− supplementation (7, 11, 12, 56).
The one patient in this investigation that exhibited the greatest increase in exercise time following consumption of beetroot juice showed an increase of 902 seconds. While this improvement is of a much greater magnitude than that of the other patients in this study and may be considered an outlier, we chose not to remove it from our analysis for several reasons. First, the improvement in exercise time was not a data error. We observed the increase in exercise time in this patient, and, while we were blinded to which beverage the patient had received, we distinctly remember being impressed with the length of time this patient exercised. It was this patient’s fourth visit, and we examined both the familiarization and third visit exercise times and they were within the 4 to 10 minute window we had targeted. Additionally, this patient’s VO2 was similar among the three exercise trials. As such we did not feel that we had underestimated this patient’s 75% work load. A second reason for not removing the data point is that this improvement is lower than values reported in the literature for a pharmacological intervention. Maltais and colleagues reported that following 42 days of treatment with the long acting bronchodilator tiotropium bromide, two patients increased their exercise times during a constant work rate test at 75% of maximum work capacity by more than 3,000 seconds (27).
Although the precise mechanism to account for the improved exercise time following ingestion of beetroot juice has yet to be identified, there are several biologically plausible. First, within the lung, nitrites have been shown to reduce pulmonary artery pressure during hypoxia (16). Given pulmonary hypertension is a common complication of COPD and may contribute to exercise intolerance (10), a reduction in pulmonary artery pressure may improve exercise capacity. Second, nitrate ingestion has been linked to improved blood flow and oxygen delivery in healthy older adults and those with peripheral artery disease (18, 19). A final mechanism may be related to the fact that it has been reported that NO3− supplementation in mice can increase force production and calcium handling in type II fibers (15). Since COPD patients have been reported to have an increased percentage of type II fibers (14), nitrate supplementation may exert is beneficial effect on those with the greatest percent of type two fibers.
We are aware of only two other studies that have investigated the use of dietary NO3− on exercise performance in an older population. Kenjale and colleagues examined the effects of NO3− supplementation via beetroot juice on the exercise capacity of older adults with peripheral arterial disease (19). These investigators found that NO3− supplementation did increase plasma NO2− levels, and the change in NO2− levels was positively associated with improvements in walking time during a graded exercise test. Kelly et al. examined the effects of dietary NO3− supplementation on the O2 uptake kinetics and distance walked in six minutes in a group of healthy, older adults (18). These investigators found that NO3− supplementation decreased the VO2 mean response time in the transition from standing rest to moderate intensity walking. It did not, however, result in differences in the distance walked in six minutes. In contrast to the results of Kelly et al., our results and those of Kenjale et al. suggest that NO3− supplementation in the form of beetroot juice will increase exercise performance in older adults. There are several possible reasons for these differences. First, Kelly et al’s. lack of differences in exercise performance between the NO3− and placebo trial may be related to the exercise test used. The six minute walk test is generally considered to be a submaximal test of a patient’s functional capacity (1) and may be less responsive to an intervention as compared to a constant work rate test (37). In studies examining changes in six minute walk distance following an intervention of either a bronchodilator drug (37) or dietary NO3− (18) with older adults, changes in walk distance between the interventional agent and placebo were only about two percent. A second reason may be related to the exercise intensities used in the various studies. In our investigation and that of Kenjale et al., improvements in exercise capacity with NO3− supplementation were found during near maximal intensity levels. A final reason for the differences may be related to differences in the fiber type distribution among healthy older adults versus those with COPD or PAD. Both COPD and PAD patients have been reported to have a greater percentage of type II fibers, whereas healthy older adults have a greater percentage of type I fibers. As previously mentioned, NO3− supplementation has been reported to increase force production and calcium handling in type II fibers (15). This coupled with the fact that our patients and those of Kenjale et al. were exercising at near maximal levels where type II fiber recruitment is greater may help explain these differences.
4.3 Effects of Nitrate Supplementation on the O2 cost of Submaximal Constant Work Rate Exercise
Recent investigations with young healthy adults have shown that the consumption of dietary NO3− results in a significant reduction in the O2 cost of moderate and high intensity exercise. The underlying mechanism responsible for this effect has been ascribed to an increase in the efficiency of oxidative phosphorylation (13, 22) or a reduced energy cost of muscular contraction (2). While the mechanism responsible for the lower O2 cost has not been clearly defined, it is thought to be linked to the NO3−→NO2−→NO pathway (17). Lansley and colleagues demonstrated that NO3− consumption resulted in a reduced O2 cost of walking and moderate and severe intensity running in young, healthy, physically active males (20). Wylie et al. also demonstrated a reduced O2 cost of moderate intensity exercise in young healthy physically active males (57). The reduced O2 cost of exercise following NO3− supplementation has been reported in other studies with young healthy adults (3, 51). We did not find significant differences in resting, iso-time or end of exercise VO2 when comparing the beetroot juice and placebo trials. Thus, it does not appear that a more efficient aerobic metabolism during the exercise bout may have played a role in the improved exercise time found during the beetroot juice trial. Our results are similar to those of Kelly et al. who reported that dietary NO3− supplementation had no effect on the O2 cost of moderate intensity walking in older adults (18). In contrast Kenjale et al. reported that the O2 cost of exercise in peripheral arterial disease patients was significantly lower following NO3− ingestion (19). Kenhjale reported that these differences were noted during the initial stages of a graded exercise test (19). Reasons for these differences may be due to the intensity at which the exercise measurements are made and/or in the pathophysiology of older adults versus those with a chronic disease such as COPD or peripheral arterial disease.
4.4 Effects of Nitrate Supplementation on Blood Pressure
Dietary NO3− have been proposed as a promising therapy for the treatment of arterial dysfunction associated with aging (46). The NO3−→NO2−→NO pathway can increase NO bioavailability resulting in smooth muscle relaxation via the synthesis of cyclic guanosine monophosphate. Significant reductions in systolic and diastolic blood pressure have been reported in young healthy volunteers, and this reduction has been directly linked to an increase in NO availability following NO3− ingestion (21, 54). Findings from our investigation show a significant reduction in resting systolic (−8 mmHg) blood pressure and a trend for a decrease in resting diastolic (−3 mmHg) blood pressure following ingestion of beetroot juice, as compared to the placebo. In addition, iso-time and end of exercise diastolic blood pressures were significantly reduced (−6 and −5 mmHg, respectively) during the beetroot juice trial. These findings are very similar to those of a recent study in which NO3− supplementation increased plasma NO2− levels and reduced resting systolic and diastolic blood pressures in healthy older adults (18). Kenjale et al. reported that in patients with peripheral arterial disease diastolic blood pressure was significantly reduced three hours post NO3− ingestion and this reduction was maintained during the submaximal work rates of a graded exercise test (19). Our study provides evidence that dietary NO3− supplementation can reduce systolic and diastolic blood pressure in COPD patients in a similar fashion as that seen in young and older healthy adults and older adults with peripheral arterial disease.
4.5 Effects of Nitrate Supplementation on Dyspnea and Dynamic Hyperinflation
Studies have shown that dynamic hyperinflation is a significant contributor to dyspnea and exercise intolerance (35, 36). Given dyspnea is the most commonly reported exercise-limiting symptoms for COPD patients, it should be regarded as an important outcome when assessing the effect of interventions on the exercise capacity of COPD patients (34). Previous studies using long-acting anticholinergic bronchodilators have shown that reductions in dynamic lung hyperinflation can improve exercise performance in patients with COPD (32, 33). In the present study, no effects on dynamic lung hyperinflation (as measured by the percent drop in inspiratory capacity) were evident at either iso-time or end exercise. Furthermore, ratings of dyspnea and leg discomfort during iso-time and end exercise were not significantly different when comparing the beetroot juice and placebo trials. These findings suggest that supplementation of NO3−-rich beetroot juice does not affect dynamic lung hyperinflation in this population. Moreover, a reduction in dynamic hyperinflation or an improvement in the patient’s perceptions of breathing and leg discomfort do not appear to contribute to the improved exercise capacity in the present study.
4.6 Limitations
Our study does have several limitations. First, in addition to NO3−, beetroot juice contains several potentially metabolically active compounds that may have an effect on physiological function in COPD patients, both at rest and during exercise (e.g. polyphenols and/or quercetin). We did not use a beetroot juice placebo (i.e. NO3− depleted beetroot juice); therefore, the beneficial effects of beetroot juice supplementation cannot be attributed exclusively to its high NO3− content. However, previous studies that have used NO3− depleted beetroot juice have shown similar results which would suggest that it is the NO3− in beetroot juice that is responsible for these beneficial effects (20). Second, the study sample consisted of patients with mild or moderate disease severity. It is not known whether acute NO3− supplementation will improve the exercise capacity of patients with severe or very severe COPD. Third, cycling is not a typical activity of daily living for these patients; therefore, using a cycle ergometer for exercise testing may be a limitation. Fourth, resting blood pressure measurements were not made in duplicate after five minutes of resting in a seated position with the patient’s back supported. A single measurement was made after two minutes of rest while sitting on the bicycle ergometer. Finally, it is also important to note that the supplementation for this intervention was acute (a single dose consumed 2.5 hours prior to exercise). Chronic NO3− supplementation may evoke different physiological responses in these patients. Future studies should investigate the benefits of longer-term NO3− supplementation, in addition to the use of a NO3−-depleted beetroot juice placebo in this population.
4.7 Conclusions
In conclusion, acute dietary NO3− supplementation increased plasma NO3− and NO2− concentrations, extended the time-to-exhaustion during submaximal constant work rate exercise, reduced resting systolic blood pressure, and reduced exercise diastolic blood pressure in mild to moderately diseased COPD patients. COPD is associated with a number of functional and structural changes to the pulmonary and muscular systems that alter oxygen delivery and utilization resulting in greater amounts of tissue hypoxia and a decreased exercise tolerance. Given dietary NO3− has been shown to improve the physiological responses to exercise, some patients with COPD may benefit from the effects of dietary NO3− on exercise capacity.
Highlights.
Dietary nitrate consumption increases plasma nitrate and nitrite levels in chronic obstructive disease (COPD) patients.
Dietary nitrate consumption increases submaximal exercise capacity in COPD patients.
Dietary nitrate consumption decreases resting systolic blood pressure in COPD patients.
Dietary nitrate consumption decreases exercise diastolic blood pressure in COPD patients.
ACKNOWLEDGEMENTS
Funding: This work was supported by the Translational Science Center on the Reynolda Campus at Wake Forest University and National Institutes of Health Grant NR011186. These funding sources had no involvement in study design, data collection, data analysis and interpretation or in the decision to submit this article for publication.
Abbreviations
- COPD
Chronic Obstructive pulmonary disease
- VO2
Oxygen Consumption
- VO2 max
Maximal Oxygen Consumption
- W
Watts
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.American ST. ATS statement: guidelines for the six-minute walk test. American Journal of Respiratory and Critical Care Medicine. 2002;166:111–117. doi: 10.1164/ajrccm.166.1.at1102. [DOI] [PubMed] [Google Scholar]
- 2.Bailey SJ, Fulford J, Vanhatalo A, Winyard PG, Blackwell JR, DiMenna FJ, Wilkerson DP, Benjamin N, Jones AM. Dietary nitrate supplementation enhances muscle contractile efficiency during knee-extensor exercise in humans. Journal of Applied Physiology. 2010;109:135–148. doi: 10.1152/japplphysiol.00046.2010. [DOI] [PubMed] [Google Scholar]
- 3.Bailey SJ, Winyard P, Vanhatalo A, Blackwell JR, Dimenna FJ, Wilkerson DP, Tarr J, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of low-intensity exercise and enhances tolerance to high-intensity exercise in humans. Journal of Applied Physiology. 2009;107:1144–1155. doi: 10.1152/japplphysiol.00722.2009. [DOI] [PubMed] [Google Scholar]
- 4.Benjamin N, O’Driscoll F, Dougall H, Duncan C, Smith L, Golden M, McKenzie H. Stomach NO synthesis. Nature. 1994;368:502. doi: 10.1038/368502a0. [DOI] [PubMed] [Google Scholar]
- 5.Berry MJ, Rejeski WJ, Adair NE, Zaccaro D. Exercise rehabilitation and chronic obstructive pulmonary disease stage. American Journal of Respiratory and Critical Care Medicine. 1999;160:1248–1253. doi: 10.1164/ajrccm.160.4.9901014. [DOI] [PubMed] [Google Scholar]
- 6.Berry MJ, Rejeski WJ, Miller ME, Adair NE, Lang W, Foy CG, Katula JA. A lifestyle activity intervention in patients with chronic obstructive pulmonary disease. Respiratory Medicine. 2010;104:829–839. doi: 10.1016/j.rmed.2010.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bescos R, Ferrer-Roca V, Galilea PA, Roig A, Drobnic F, Sureda A, Martorell M, Cordova A, Tur JA, Pons A. Sodium nitrate supplementation does not enhance performance of endurance athletes. Medicine and Science in Sports and Exercise. 2012;44:2400–2409. doi: 10.1249/MSS.0b013e3182687e5c. [DOI] [PubMed] [Google Scholar]
- 8.Borg GA. Psychophysical bases of perceived exertion. Medicine Science in Sports and Exercise. 1982;14:377–381. [PubMed] [Google Scholar]
- 9.Breese BC, McNarry MA, Marwood S, Blackwell JR, Bailey SJ, Jones AM. Beetroot juice supplementation speeds O2 uptake kinetics and improves exercise tolerance during severe-intensity exercise initiated from an elevated metabolic rate. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2013;305:R1441–1450. doi: 10.1152/ajpregu.00295.2013. [DOI] [PubMed] [Google Scholar]
- 10.Butler J, Chomsky DB, Wilson JR. Pulmonary hypertension and exercise intolerance in patients with heart failure. Journal of the American College of Cardiology. 1999;34:1802–1806. doi: 10.1016/s0735-1097(99)00408-8. [DOI] [PubMed] [Google Scholar]
- 11.Cermak NM, Res P, Stinkens R, Lundberg JO, Gibala MJ, van Loon LJ. No improvement in endurance performance after a single dose of beetroot juice. International Journal of Sport Nutrition and Exercise Metabolism. 2012;22:470–478. doi: 10.1123/ijsnem.22.6.470. [DOI] [PubMed] [Google Scholar]
- 12.Christensen PM, Nyberg M, Bangsbo J. Influence of nitrate supplementation on VO(2) kinetics and endurance of elite cyclists. Scandinavian Journal of Medicine & Science in Sports. 2013;23:e21–31. doi: 10.1111/sms.12005. [DOI] [PubMed] [Google Scholar]
- 13.Clerc P, Rigoulet M, Leverve X, Fontaine E. Nitric oxide increases oxidative phosphorylation efficiency. Journal of Bioenergetics and Biomembranes. 2007;39:158–166. doi: 10.1007/s10863-007-9074-1. [DOI] [PubMed] [Google Scholar]
- 14.Gosker HR, Zeegers MP, Wouters EF, Schols AM. Muscle fibre type shifting in the vastus lateralis of patients with COPD is associated with disease severity: a systematic review and meta-analysis. Thorax. 2007;62:944–949. doi: 10.1136/thx.2007.078980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hernandez A, Schiffer TA, Ivarsson N, Cheng AJ, Bruton JD, Lundberg JO, Weitzberg E, Westerblad H. Dietary nitrate increases tetanic [Ca2+]i and contractile force in mouse fast-twitch muscle. The Journal of Physiology. 2012;590:3575–3583. doi: 10.1113/jphysiol.2012.232777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ingram TE, Pinder AG, Bailey DM, Fraser AG, James PE. Low-dose sodium nitrite vasodilates hypoxic human pulmonary vasculature by a means that is not dependent on a simultaneous elevation in plasma nitrite. American Journal of Physiology Heart and Circulatory Physiology. 2010;298:H331–339. doi: 10.1152/ajpheart.00583.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones AM, Vanhatalo A, Bailey SJ. Influence of dietary nitrate supplementation on exercise tolerance and performance. Nestle Nutrition Institute Workshop Series. 2013;75:27–40. doi: 10.1159/000345815. [DOI] [PubMed] [Google Scholar]
- 18.Kelly J, Fulford J, Vanhatalo A, Blackwell JR, French O, Bailey SJ, Gilchrist M, Winyard PG, Jones AM. Effects of short-term dietary nitrate supplementation on blood pressure, O2 uptake kinetics, and muscle and cognitive function in older adults. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2013;304:R73–83. doi: 10.1152/ajpregu.00406.2012. [DOI] [PubMed] [Google Scholar]
- 19.Kenjale AA, Ham KL, Stabler T, Robbins JL, Johnson JL, Vanbruggen M, Privette G, Yim E, Kraus WE, Allen JD. Dietary nitrate supplementation enhances exercise performance in peripheral arterial disease. Journal of Applied Physiology. 2011;110:1582–1591. doi: 10.1152/japplphysiol.00071.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lansley KE, Winyard PG, Fulford J, Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Gilchrist M, Benjamin N, Jones AM. Dietary nitrate supplementation reduces the O2 cost of walking and running: a placebo-controlled study. Journal of Applied Physiology. 2011;110:591–600. doi: 10.1152/japplphysiol.01070.2010. [DOI] [PubMed] [Google Scholar]
- 21.Larsen FJ, Ekblom B, Sahlin K, Lundberg JO, Weitzberg E. Effects of dietary nitrate on blood pressure in healthy volunteers. The New England Journal of Medicine. 2006;355:2792–2793. doi: 10.1056/NEJMc062800. [DOI] [PubMed] [Google Scholar]
- 22.Larsen FJ, Schiffer TA, Borniquel S, Sahlin K, Ekblom B, Lundberg JO, Weitzberg E. Dietary inorganic nitrate improves mitochondrial efficiency in humans. Cell Metabolism. 2011;13:149–159. doi: 10.1016/j.cmet.2011.01.004. [DOI] [PubMed] [Google Scholar]
- 23.Larsen FJ, Weitzberg E, Lundberg JO, Ekblom B. Effects of dietary nitrate on oxygen cost during exercise. Acta Physiology. 2007;191:59–66. doi: 10.1111/j.1748-1716.2007.01713.x. [DOI] [PubMed] [Google Scholar]
- 24.Lundberg JO, Weitzberg E, Gladwin MT. The nitrate-nitrite-nitric oxide pathway in physiology and therapeutics. Nature Reviews: Drug Discovery. 2008;7:156–167. doi: 10.1038/nrd2466. [DOI] [PubMed] [Google Scholar]
- 25.Lundberg JO, Weitzberg E, Lundberg JM, Alving K. Intragastric nitric oxide production in humans: measurements in expelled air. Gut. 1994;35:1543–1546. doi: 10.1136/gut.35.11.1543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Macintyre N, Crapo RO, Viegi G, Johnson DC, van der Grinten CP, Brusasco V, Burgos F, Casaburi R, Coates A, Enright P, Gustafsson P, Hankinson J, Jensen R, McKay R, Miller MR, Navajas D, Pedersen OF, Pellegrino R, Wanger J. Standardisation of the single-breath determination of carbon monoxide uptake in the lung. The European Respiratory Journal. 2005;26:720–735. doi: 10.1183/09031936.05.00034905. [DOI] [PubMed] [Google Scholar]
- 27.Maltais F, Hamilton A, Marciniuk D, Hernandez P, Sciurba FC, Richter K, Kesten S, O’Donnell D. Improvements in symptom-limited exercise performance over 8 h with once-daily tiotropium in patients with COPD. Chest. 2005;128:1168–1178. doi: 10.1378/chest.128.3.1168. [DOI] [PubMed] [Google Scholar]
- 28.Maltais F, Mahler DA, Pepin V, Nadreau E, Crater GD, Morris AN, Emmett AH, Ferro TJ. Effect of fluticasone propionate/salmeterol plus tiotropium versus tiotropium on walking endurance in COPD. The European Respiratory Journal. 2013;42:539–541. doi: 10.1183/09031936.00074113. [DOI] [PubMed] [Google Scholar]
- 29.Miller GD, Marsh AP, Dove RW, Beavers D, Presley T, Helms C, Bechtold E, King SB, Kim-Shapiro D. Plasma nitrate and nitrite are increased by a high-nitrate supplement but not by high-nitrate foods in older adults. Nutrition Research. 2012;32:160–168. doi: 10.1016/j.nutres.2012.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Miller MR, Hankinson J, Brusasco V, Burgos F, Casaburi R, Coates A, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Jensen R, Johnson DC, MacIntyre N, McKay R, Navajas D, Pedersen OF, Pellegrino R, Viegi G, Wanger J. Standardisation of spirometry. The EuropeanRespiratory Journal. 2005;26:319–338. doi: 10.1183/09031936.05.00034805. [DOI] [PubMed] [Google Scholar]
- 31.National Heart L. Blood Institute . Chronic Obstructive Pulmonary Disease. National Institutes of Health; Bethesda, MD: 2003. [Google Scholar]
- 32.O’Donnell DE, Casaburi R, Vincken W, Puente-Maestu L, Swales J, Lawrence D, Kramer B. Effect of indacaterol on exercise endurance and lung hyperinflation in COPD. Respiratory Medicine. 2011;105:1030–1036. doi: 10.1016/j.rmed.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 33.O’Donnell DE, Fluge T, Gerken F, Hamilton A, Webb K, Aguilaniu B, Make B, Magnussen H. Effects of tiotropium on lung hyperinflation, dyspnoea and exercise tolerance in COPD. The European Respiratory Journal. 2004;23:832–840. doi: 10.1183/09031936.04.00116004. [DOI] [PubMed] [Google Scholar]
- 34.O’Donnell DE, Lam M, Webb KA. Measurement of symptoms, lung hyperinflation, and endurance during exercise in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 1998;158:1557–1565. doi: 10.1164/ajrccm.158.5.9804004. [DOI] [PubMed] [Google Scholar]
- 35.O’Donnell DE, Revill SM, Webb KA. Dynamic hyperinflation and exercise intolerance in chronic obstructive pulmonary disease. American Journal of Respiratory and Critical Care Medicine. 2001;164:770–777. doi: 10.1164/ajrccm.164.5.2012122. [DOI] [PubMed] [Google Scholar]
- 36.O’Donnell DE, Webb KA. Exertional breathlessness in patients with chronic airflow limitation. The role of lung hyperinflation. The American Review of Respiratory Disease. 1993;148:1351–1357. doi: 10.1164/ajrccm/148.5.1351. [DOI] [PubMed] [Google Scholar]
- 37.Oga T, Nishimura K, Tsukino M, Hajiro T, Ikeda A, Izumi T. The effects of oxitropium bromide on exercise performance in patients with stable chronic obstructive pulmonary disease. A comparison of three different exercise tests. American Journal of Respiratory and Critical Care Medicine. 2000;161:1897–1901. doi: 10.1164/ajrccm.161.6.9905045. [DOI] [PubMed] [Google Scholar]
- 38.Park SK, Richardson CR, Holleman RG, Larson JL. Physical activity in people with COPD, using the National Health and Nutrition Evaluation Survey dataset (2003-2006) Heart Lung. 2013;42:235–240. doi: 10.1016/j.hrtlng.2013.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Petersson J, Carlstrom M, Schreiber O, Phillipson M, Christoffersson G, Jagare A, Roos S, Jansson EA, Persson AE, Lundberg JO, Holm L. Gastroprotective and blood pressure lowering effects of dietary nitrate are abolished by an antiseptic mouthwash. Free Radical Biology & Medicine. 2009;46:1068–1075. doi: 10.1016/j.freeradbiomed.2009.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Presley TD, Morgan AR, Bechtold E, Clodfelter W, Dove RW, Jennings JM, Kraft RA, King SB, Laurienti PJ, Rejeski WJ, Burdette JH, Kim-Shapiro DB, Miller GD. Acute effect of a high nitrate diet on brain perfusion in older adults. Nitric Oxide: Biology and Chemistry. 2011;24:34–42. doi: 10.1016/j.niox.2010.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Puente-Maestu L, SantaCruz A, Vargas T, Martinez-Abad Y, Whipp BJ. Effects of training on the tolerance to high-intensity exercise in patients with severe COPD. Respiration. 2003;70:367–370. doi: 10.1159/000072899. [DOI] [PubMed] [Google Scholar]
- 42.Puente-Maestu L, Villar F, de Miguel J, Stringer WW, Sanz P, Sanz ML, de Pedro JG, Martinez-Abad Y. Clinical relevance of constant power exercise duration changes in COPD. The European Respiratory Journal. 2009;34:340–345. doi: 10.1183/09031936.00078308. [DOI] [PubMed] [Google Scholar]
- 43.Qaseem A, Wilt TJ, Weinberger SE, Hanania NA, Criner G, van der Molen T, Marciniuk DD, Denberg T, Schunemann H, Wedzicha W, MacDonald R, Shekelle P. Diagnosis and management of stable chronic obstructive pulmonary disease: a clinical practice guideline update from the American College of Physicians, American College of Chest Physicians, American Thoracic Society, and European Respiratory Society. Annals of Internal Medicine. 2011;155:179–191. doi: 10.7326/0003-4819-155-3-201108020-00008. [DOI] [PubMed] [Google Scholar]
- 44.Reckelhoff JF, Kellum JA, Blanchard EJ, Bacon EE, Wesley AJ, Kruckeberg WC. Changes in nitric oxide precursor, L-arginine, and metabolites, nitrate and nitrite, with aging. Life Sciences. 1994;55:1895–1902. doi: 10.1016/0024-3205(94)00521-4. [DOI] [PubMed] [Google Scholar]
- 45.Ries AL, Kaplan RM, Limberg TM, Prewitt LM. Effects of pulmonary rehabilitation on physiologic and psychosocial outcomes in patients with chronic obstructive pulmonary disease. Annals of Internal Medicine. 1995;122:823–832. doi: 10.7326/0003-4819-122-11-199506010-00003. [DOI] [PubMed] [Google Scholar]
- 46.Sindler AL, Devan AE, Fleenor BS, Seals DR. Inorganic nitrite supplementation for healthy arterial aging. Journal of Applied Physiology. 2014;116:463–477. doi: 10.1152/japplphysiol.01100.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Spiegelhalder B, Eisenbrand G, Preussmann R. Influence of dietary nitrate on nitrite content of human saliva: possible relevance to in vivo formation of N-nitroso compounds. Food and Cosmetics Toxicology. 1976;14:545–548. doi: 10.1016/s0015-6264(76)80005-3. [DOI] [PubMed] [Google Scholar]
- 48.Tiefenbacher CP. Tetrahydrobiopterin: a critical cofactor for eNOS and a strategy in the treatment of endothelial dysfunction? American Journal of Physiology Heart and Circulatory Physiology. 2001;280:H2484–2488. doi: 10.1152/ajpheart.2001.280.6.H2484. [DOI] [PubMed] [Google Scholar]
- 49.Troosters T, Sciurba F, Battaglia S, Langer D, Valluri SR, Martino L, Benzo R, Andre D, Weisman I, DeCramer M. Physical inactivity in patients with COPD, a controlled multi-center pilot-study. Respiratory Medicine. 2010;104:1005–1011. doi: 10.1016/j.rmed.2010.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Van Remoortel H, Hornikx M, Demeyer H, Langer D, Burtin C, DeCramer M, Gosselink R, Janssens W, Troosters T. Daily physical activity in subjects with newly diagnosed COPD. Thorax. 2013;68:962–963. doi: 10.1136/thoraxjnl-2013-203534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vanhatalo A, Bailey SJ, Blackwell JR, DiMenna FJ, Pavey TG, Wilkerson DP, Benjamin N, Winyard PG, Jones AM. Acute and chronic effects of dietary nitrate supplementation on blood pressure and the physiological responses to moderate-intensity and incremental exercise. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2010;299:R1121–1131. doi: 10.1152/ajpregu.00206.2010. [DOI] [PubMed] [Google Scholar]
- 52.Vanhatalo A, Fulford J, Bailey SJ, Blackwell JR, Winyard PG, Jones AM. Dietary nitrate reduces muscle metabolic perturbation and improves exercise tolerance in hypoxia. The Journal of Physiology. 2011;589:5517–5528. doi: 10.1113/jphysiol.2011.216341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Wanger J, Clausen JL, Coates A, Pedersen OF, Brusasco V, Burgos F, Casaburi R, Crapo R, Enright P, van der Grinten CP, Gustafsson P, Hankinson J, Jensen R, Johnson D, Macintyre N, McKay R, Miller MR, Navajas D, Pellegrino R, Viegi G. Standardisation of the measurement of lung volumes. The European Respiratory Journal. 2005;26:511–522. doi: 10.1183/09031936.05.00035005. [DOI] [PubMed] [Google Scholar]
- 54.Webb AJ, Patel N, Loukogeorgakis S, Okorie M, Aboud Z, Misra S, Rashid R, Miall P, Deanfield J, Benjamin N, MacAllister R, Hobbs AJ, Ahluwalia A. Acute blood pressure lowering, vasoprotective, and antiplatelet properties of dietary nitrate via bioconversion to nitrite. Hypertension. 2008;51:784–790. doi: 10.1161/HYPERTENSIONAHA.107.103523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Weitzberg E, Lundberg JO. Novel aspects of dietary nitrate and human health. Annual Review of Nutrition. 2013;33:129–159. doi: 10.1146/annurev-nutr-071812-161159. [DOI] [PubMed] [Google Scholar]
- 56.Wilkerson DP, Hayward GM, Bailey SJ, Vanhatalo A, Blackwell JR, Jones AM. Influence of acute dietary nitrate supplementation on 50 mile time trial performance in well-trained cyclists. European Journal of Applied Physiology. 2012;112:4127–4134. doi: 10.1007/s00421-012-2397-6. [DOI] [PubMed] [Google Scholar]
- 57.Wylie LJ, Kelly J, Bailey SJ, Blackwell JR, Skiba PF, Winyard PG, Jeukendrup AE, Vanhatalo A, Jones AM. Beetroot juice and exercise: pharmacodynamic and dose-response relationships. Journal of Applied Physiology. 2013;115:325–336. doi: 10.1152/japplphysiol.00372.2013. [DOI] [PubMed] [Google Scholar]


