Abstract
Background and Aims
Cytokine gene polymorphisms modify expression and their circulating protein levels reflect inflammatory response. Chronic inflammation plays key role in pathogenesis of colorectal neoplasia (CRN) associated with inflammatory bowel disease (IBD), but it is not clear if inflammation is a cause or effect of tumours in sporadic CRN. We therefore investigated association of cytokine gene polymorphisms and circulating cytokine levels on risk of CRN in North East Scotland, which has a high incidence of CRN.
Methods
We recruited two groups of subjects from a screening colonoscopy cohort, either pre-procedure or 3–24 months post-procedure. Participants with (CRN) were compared to participants with no evidence of CRN (controls). Blood-derived DNA was used to genotype polymorphisms in IL1B, IL1-RN, IL6, IL8, IL10, PTGS2 and TNFA genes. Circulating levels of high-sensitivity C-reactive protein (Hs-CRP) and 6 cytokines (IL-1beta, IL-4, IL-6, IL-8, IL-10 and TNF-alpha) were measured. In order to examine effect of CRN resection on marker levels, we used propensity score matching.
Results
There were 884 subjects eligible for analysis, including 388 CRN cases and 496 controls. Cases were older (mean age 64 vs. 62 yrs, p<0.01) and more likely to be male (67% vs. 55%, p<0.001). Controls were more likely to be regular users of NSAID (p<0.0001). Compared to homozygous carriage of respective common alleles, pro-inflammatory CC genotypes of IL1B-31 C>T [OR (95% CI) 1.68 (1.03–2.73)] and PTGS2-765 C>G [OR (95% CI) 2.97 (1.05–8.46)] were each associated with increased CRN risk. Conversely, carriage of the A allele of IL8-251 A>T was associated with lower CRN risk compared to the TT genotype [ORs (95% CI) 0.60 (0.41–0.86) for heterozygous, 0.88 (0.57–1.37) for homozygous, and 0.68 (0.48–0.95) for heterozygous and homozygous combined]. Compared to post-procedure cases, IL8, TNFα, and CRP levels were significantly higher in pre-procedure cases, but IL4 and IL10 protein levels were significantly lower.
Conclusions
Pro-inflammatory cytokine gene polymorphisms in IL1B-31 and PTGS2-765 increase the risk of developing CRN. Levels of pro-inflammatory IL8, TNFα and CRP markers are significantly higher whilst CRN is in-situ. Along with the NSAID findings, these data point to inflammation as an underlying pathogenetic mechanism in CRN.
Keywords: Colorectal neoplasia, cytokine, polymorphisms, screening
Introduction
Colorectal Cancer (CRC) is a leading cause of death and the third commonest cancer worldwide, with an estimated 1.23 million new cases diagnosed in 2008 (Ferlay et al. 2010). Chronic inflammation has been implicated as a cause of many types of cancer based on experimental, epidemiological and clinical evidence. Inflammation is a complex, highly orchestrated process involving many cell types and molecules, some of which drive the process, some modulate it and a number which do both at different points (Kushner, Rzewnicki 1994). Circulating levels of effector molecules and products, including acute phase proteins like C reactive protein (CRP), have the potential of being biomarkers for colorectal neoplasis (CRN) as they are for other chronic diseases such as diabetes (Liu et al. 2007)(Hu et al. 2004). Several studies have investigated the association of circulating inflammatory marker levels and CRN, with inconsistent findings. Some studies have found positive associations of CRN risk with certain inflammatory markers (Chan et al. 2011a) (Prizment et al. 2011) (Chiu et al. 2008) (Kim et al. 2008) (Tsilidis et al. 2008a) (Otani et al. 2006) whilst others showed opposite (Gunter et al. 2011) (Ognjanovic et al. 2010)or null associations (Tsilidis et al. 2008b) (Kim et al. 2008)(Chan et al. 2011a). Single nucleotide polymorphisms (SNPs) in the promoter regions of genes encoding these cytokines may influence changes in their expression and, potentially, cancer risk as a consequence. Many SNPs have been identified in the regulatory regions of cytokine genes, of which some have been associated with increased risk of different types of cancers, including gastrointestinal cancers. (Gunter et al. 2006, Landi et al. 2003, Camargo et al. 2006a, El-Omar et al. 2003) (Tsilidis et al. 2009)(Li, You & Wang 2011). It has also been shown that some of these SNPs influence the response to anti-inflammatory drugs which are potential chemopreventive agents against CRC (Ulrich et al. 2005, Sansbury et al. 2006, Macarthur et al. 2005). Accordingly, we have investigated these associations in a colorectal cancer screening population of Northeast Scotland, which has one of the highest incidences of CRC in the UK.
The aims of our study were first to investigate the associations of polymorphisms in the ILIB, IL1RN, IL6, IL8, IL10 and TNFA genes with CRN risk, second to examine the associations of circulating levels of Hs-CRP, IL1B, IL6, IL8, TNF α, IL4 and IL10 with prevalent CRN, third to evaluate the effect of removal of CRN on circulating cytokine levels and finally to evaluate the interaction of genotype and aspirin/NSAID use in CRN risk.
Materials and methods
Study population
Subjects enrolled in the present study were recruited from a cohort of individuals who participated in the National Bowel Cancer Screening Programme (BCSP). The BCSP is a biennial, guaiac FOBT (faecal occult blood test) screening programme offered to an unselected population aged between 50 to 74 years. All FOBT positive subjects are offered a colonoscopy as the first choice of screening. The Northeast region of Scotland was one of the first areas in the UK to participate in the BCSP pilot which started on 29th March 2000, conducted in the Northeast region of Scotland which includes rural, suburban and urban areas with a diverse socioeconomic mix of mainly Caucasian population. In the Scottish screening pilot, during the three rounds of screening a total of 931, 912 invitations were sent, and an evaluable result was obtained in 507, 345, giving an uptake of 54.4%. The proportion of those completing the test with a positive result requiring further investigation was 1.7%. The uptake for colonoscopy in those with a positive gFOBT result was 85.4%. Histopathological examination of biopsies, polypectomy specimens and resection specimens was carried out by selected specialist consultant gastrointestinal pathologists using an agreed pro forma for reporting biopsies and polypectomy specimens. In the screened subjects (507, 345), the adenoma detection rate was 0.48% and cancer was diagnosed in 0.13%. Subjects for the current study were identified from the Northeast of Scotland colorectal cancer screening database and approached to participate in the study.
Study objectives and design
This was a case control study. The definition of a “Case” was any subject with a histological diagnosis of an adenomatous polyp or colorectal carcinoma identified at screening colonoscopy. The “Control” was any subject who had other diagnosis including haemorrhoids, diverticulosis, hyperplasic polyps or who had a completely normal colonoscopy. As inflammatory bowel disease (IBD) is associated with elevated inflammatory markers, which in turn could cause a bias during analysis, any subject with a diagnosis of IBD was excluded from the analyses.
Between 2008 and 2010, a total of 905 subjects were recruited, of which 21 subjects were excluded due to diagnosis of IBD. Among the 884 remaining subjects, there were 388 CRN Cases and 496 Controls. Of the 496 controls, 299 subjects had a completely normal colonoscopy and 197 had other diagnoses including diverticular disease (n=163) and hyperplastic polyps (n=44) with some overlap. Cases were further classified as High Risk CRN (n=288) if they had a histological diagnosis of Cancer or High Grade Dysplasia (HGD) or TuboVillous Adenoma (TVA) or ≥3 adenomas or adenoma ≥ 1cm diameter and Low Risk CRN (n=100) if they had <3 adenomas all < 1cm diameter.
To investigate the association of the presence or absence of CRN with contemporaneous circulating levels of inflammatory markers, two groups of subjects were recruited (Fig 1). The “pre-procedure” group consisted of subjects in whom the inflammatory markers were measured just prior to screening colonoscopy (i.e., when they still had the adenoma or cancer in-situ) and the “post-procedure” group consisted of subjects in whom the inflammatory markers were measured after removal of the neoplastic lesion, within 3 to 24 months after screening colonoscopy. In the pre-procedure group, a total of 561 subjects were prospectively enrolled, of which 242 were determined to be cases and 319 were controls. In the post-procedure group, a total of 323 subjects were enrolled, including 146 previously detected cases and 177 controls (Fig 1). In order to compare pre- to post-procedure inflammatory marker levels among cases, we used propensity score matching to match pre-procedure to post-procedure subjects using their covariate characteristics. The propensity score method generates balanced subsamples in a synthetic experimental design, thus allowing comparison of pre- to post-procedure levels, even though measured on different individuals.
Fig 1.
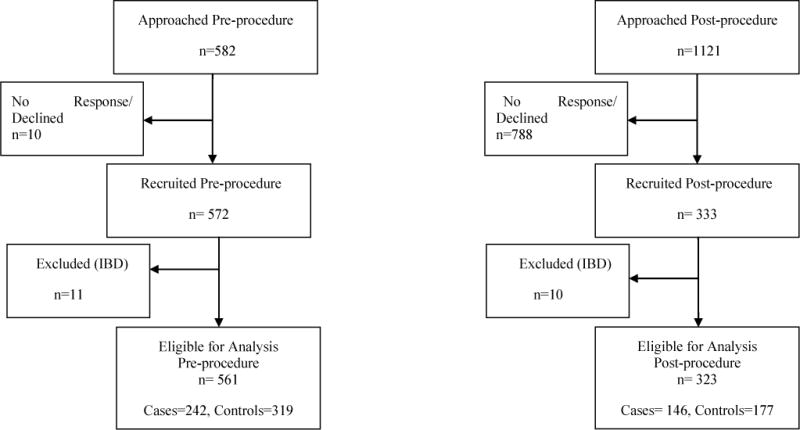
Study design flow chart showing recruitment process for both the pre-procedure and post-procedure subjects
Demographic life style data
At the time of recruitment, a research team member completed the clinical assessment record for every participant and collected data regarding the age, gender, self-reported height and weight, central abdominal girth, medical conditions and medication use, gastrointestinal symptoms, smoking status (never/ever) , alcohol use (any/none), occupation and history of CRC in first degree relatives. Daily use of aspirin and statins for >1yr or >5 yrs was recorded and analysed. Subjects were considered as regular users of other NSAID, if they consumed NSAID daily for > 1 month. Dietary intake for each participant was assessed by a previously validated (Masson et al. 2003) Scottish Collaborative Group (SCG) semi-quantitative Food Frequency Questionnaire (FFQ) version 6.5, consisting of a list of 170 food items.
Laboratory methods
Blood Inflammatory markers data
Fasting whole blood was collected at the time of recruitment for all subjects, with serum and plasma isolated by centrifugation and stored at −80°C. Serum Hs-CRP measurement was done using the CardioPhase® hsCRP assay (Dade Behring Inc., Newark, USA) on the BN Systems, which measures Hs-CRP concentrations by means of particle enhanced immunonephelometry. The limit of detection of the assay was 0.175mg/L for sample dilution of 1:20 and the coefficient of variation was <10% for concentrations between 0.5mg/L and 62mg/L (BN Pro-spec System instruction manual).
Plasma was used at 1:4 dilution to measure 6 cytokine levels (IL6, IL8, IL1B, TNF-α, IL4 and IL10) using the BD™ Cytometric Bead Array (CBA) Human soluble Protein Flex set system. For both the CRP and cytokine measurements, all the samples were blinded and randomly distributed in analysis plates.
Genotyping data
Candidate SNPs were selected for evaluation on the basis of functional data indicating effects on expression and minimum 5% minor allele frequency in Caucasians. Genomic DNA was purified from 6 mls of whole blood using the Invitrogen Gene Catcher™ gDNA Kit according to the manufacturer’s protocol. Genotyping for allele specific detection of SNPs was done using the TaqMan® SNP Genotyping assays which use the 5′ nuclease assay chemistry for amplifying and detecting specific SNP alleles in purified genomic DNA samples. The IL1B-31C>T, IL10-592 A>C, IL10-1082 A>G and TNFα-308 A>G have previously been successfully genotyped by our group and results published (Macarthur et al. 2005). Taqman assays for these SNPs were available off the shelf from Applied Biosystems, CA; USA. For the COX2 -765 G>C (rs20417) & IL6-174 C>G (rs1800795) SNPs, Applied Biosystems did not have an off the shelf assays and custom assays were designed (Applied Biosystems custom design service; see supplementary data, Table 6a). For the penta-allelic IL1RN VNTR polymorphisms, DNA was amplified using primers flanking the 86-bp tandem repeats polymorphic region in intron 2. The primers were 5′ CTCAGCAACACTCCTAT 3′ (Forward) and 5′TCCTGGTCTGCAGGTAA 3′ (Reverse) (Tarlow et al. 1993). The PCR was performed at 95°C for 5mins (35cycles), 94°C for 1min, 60°C for 1min, 72°C for 1min and the final step at 72°C for 10mins. PCR products were electrophoresed in 2% agarose gel and visualised under ultra violet light after ethidium bromide staining. IL1RN alleles were coded as allele 1, four repeats, product size 450bp; allele 2, two repeats, product size 270bp; allele 3, five repeats, product size 530bp; allele 4 , three repeats, product size 360 bp and allele 5, six repeats, product size 620 bp.
All the studied polymorphisms except for the IL1RN VNTR were confirmed by direct sequencing of PCR products of each genotype. The primers used for PCR and subsequent sequencing are shown in supplementary data, Table 6b. For the COX2 -765 G>C, the PCR products were sequenced following cloning into a PCR vector and using M13 sequence primers.
To ensure reproducibility of genotyping methods, multiple blinded quality control samples were embedded among the case-control samples and for all genotypes tested, the quality control samples indicated a reproducibility rate of 100% (data not shown). Genotyping success rate was >98% for all the studied genes except for TNF-308 G>A (94.4%). Among the subjects that failed to be genotyped for TNF -308 G>A, there were no significant differences in distribution between cases (n=27) and controls (n=28). The genotype frequencies in the Control group for all the selected SNPs were in Hardy-Weinberg equilibrium.
Statistical analyses
Statistical analyses were performed using SAS Version 9.3 (SAS Inc., Cary, North Carolina, USA) software. Baseline characteristics, SNPs, and serum inflammatory markers concentrations were evaluated by CRN status (i.e, any vs. none, high-risk vs. low risk vs. none, and pre-procedure vs. post-procedure) using Wilcoxon rank-sum test for continuous variables and Chi-square test for categorical variables and are shown as medians (interquartile ranges) and frequencies (percentages), respectively. In all analyses of the inflammatory marker SNPs, the homozygote of the more frequent allele was used as the referent category. For linear trend testing, the referent category was the more frequent allele that was scored on an ordinal scale as 0, the heterozygote was scored as 1, and the rare homozygote was scored as 2 (co-dominant model). For the dominant model, homozygous and heterozygous carriers of the less frequent allele were combined and scored as 1. Inflammatory markers were divided into two categories using medians, on the basis of the distribution among the controls. Logistic regression models were used to estimate the odds ratios (OR) and corresponding 95% confidence intervals (CIs) to compare cytokine genes polymorphism/median cytokines levels in cases vs. controls, separately for the pre-procedure and post-procedure subjects. To examine whether the effect of aspirin/NSAID use on CRN risk varies across strata of genotypes or cytokine levels, we assessed the interaction of aspirin/NSAID use, and genotype or cytokine levels by including cross product terms between aspirin/NSAID use, and genotype or cytokine levels. Polytomous logistic regression models were employed to estimate the OR and 95% CIs for elevated cytokine levels in low risk CRN and high risk CRN as compared to controls. To examine effect of CRN removal, logistic regression was first used to calculate a propensity score, based on age, gender, body mass index, alcohol consumption, exercise, smoking, diverticular disease, exercise and aspirin use. To create the propensity-matched data set, 1:1 Greedy matching was used, restricted to pre-procedure and post-procedure subjects within the area of propensity score overlap. As recommended by Austin 2011, a caliper of 0.2 standard deviation of the logit of the propensity score was used to match the two groups. We assessed variable balance post-matching using the standardized difference method. To estimate the effect of CRN removal (i.e., polypectomy) on cytokine levels, median log marker levels pre-procedure and post-procedure were compared using quantile regression models. This comparison was further validated in a subset of 23 subjects for whom both pre- and post-procedure blood samples were available.
Ethical approval
Ethical approval for the study was granted by the North of Scotland Research ethics committee. All participants gave written informed consent.
Results
In unadjusted analyses, cases were older than controls and more likely to be male and to consume alcohol, but less likely to report using NSAID for more than a month (Table 1).
Table 1.
Selected characteristics of CRN Cases and Controls
| Characteristic | Controls n=496 |
Cases n=388 |
Unadjusted p value* |
|---|---|---|---|
| Age (yrs) median(IQ range) | 62 (56,69) | 64 (59,70) | <0.01 |
| Male N (%) | 274 (55.2) | 260 (67.0) | <0.001 |
| BMI median(IQ range) | 28.2 (25.2,31.7) | 28.0 (25.0,31.3) | 0.69 |
| Abdominal girth >96cm N (%) | 284 (57.7) | 246 (63.4) | 0.09 |
| Aspirin use > 1 yr N (%) | 150 (30.3) | 112 (28.9) | 0.64 |
| Aspirin use > 5 yrs N (%) | 86 (17.4) | 65 (16.8) | 0.81 |
| NSAID use > 1 month N (%) | 58 (11.7) | 10 (2.6) | <0.0001 |
| Statin use > 1 yr N (%) | 142 (28.8) | 126 (32.7) | 0.21 |
| Statin use > 5 yrs N (%) | 71 (14.3) | 61 (15.7) | 0.57 |
| Family history N (%) | 75 (15.2) | 72 (18.6) | 0.18 |
| Ever smoking N (%) | 252 (50.9) | 217 (55.9) | 0.14 |
| Alcohol any N (%) | 375 (75.8) | 336 (86.6) | <0.0001 |
| Exercise N (%) | 230 (56.2) | 160 (53.5) | 0.47 |
From Chi-square test for categorical variables and Kruskal-Wallis test for continuous variable
Inflammatory gene polymorphisms and CRN risk
Genotype distributions and ORs for associations with CRN are shown in Table 2. Individuals with the CC genotype of IL1B-31C>T had significant excess risk of CRN [OR 1.68 (95% CI 1.03–2.73)]. Also, the proinflammatory CC genotype of PTGS2–765 C>G significantly increased the risk of CRN [OR 2.97 (95% CI 1.05–8.46)]. Conversely, the AT genotype or having at least one A allele of IL8–251 A>T was associated with lower risk of CRN compared to the TT genotype [ORs (95% CI) 0.68 (0.48–0.95) and 0.60 (0.41–0.86), respectively], but we did not detect any significant gene-dose response.
Table 2.
Genotype distributions and genotypic risks associated with CRN
| Genotype | Controls (n=496) N (%) |
Cases# (n=388) N (%) |
OR (95%)CI* |
|---|---|---|---|
| IL1B-31C>T | |||
| TT | 226 (45.6) | 173 (44.6) | 1 |
| CT | 221 (44.6) | 152 (39.2) | 0.78 (0.56 – 1.09) |
| CC | 49 (9.8) | 63 (16.2) | 1.68 (1.03 – 2.73) |
| CT+CC* TT | 0.94 (0.69 – 1.28) | ||
| P Trend = 0.28 | |||
| IL1RN VNTR | |||
| 1,1 | 229 (46.5) | 189 (48.8) | 1 |
| 1,2 | 217 (44.1) | 159 (41.1) | 0.97 (0.70 – 1.34) |
| 2,2 | 46 (9.4) | 39 (10.1) | 0.93 (0.54 – 1.62) |
| 2,2 + 1,2 * 1,1 | 0.96 (0.71 – 1.31) | ||
| P Trend = 0.78 | |||
| IL6-174 C>G | |||
| GG | 172 (34.8) | 140 (36.1) | 1 |
| GC | 245 (49.5) | 184 (47.4) | 0.93 (0.66 – 1.32) |
| CC | 78 (15.8) | 64 (16.5) | 1.01 (0.64 – 1.59) |
| GC+CC* GG | 0.95 (0.69 – 1.32) | ||
| P Trend= 0.94 | |||
| IL8-251 A>T | |||
| TT | 133 (27.1) | 113 (29.1) | 1 |
| AT | 249 (50.7) | 183 (47.2) | 0.60 (0.41 – 0.86) |
| AA | 109 (22.2) | 92 (23.7) | 0.88 (0.57 – 1.37) |
| AT +AA * TT | 0.68 (0.48 – 0.95) | ||
| P Trend = 0.42 | |||
| IL10-1082 A>G | |||
| GG | 130 (26.3) | 92 (23.8) | 1 |
| GA | 261 (52.8) | 210 (54.3) | 1.25 (0.86 – 1.83) |
| AA | 103 (20.9) | 58 (21.9) | 1.24 (0.78 – 1.97) |
| GA +AA * GG | 1.25 (0.87 – 1.80) | ||
| P Trend = 0.34 | |||
| IL10-592 A>C | |||
| CC | 311 (62.7) | 241 (62.3) | 1 |
| AC | 168 (33.9) | 134 (34.6) | 1.09 (0.79 – 1.51) |
| AA | 17 (3.4) | 12 (3.1) | 1.31 (0.53 – 2.56) |
| AC+AA * CC | 1.11 (0.80 – 1.52) | ||
| P Trend = 0.47 | |||
| PTGS2-765 C>G | |||
| GG | 363 (73.5) | 270 (69.8) | 1 |
| GC | 122 (24.7) | 105 (27.1) | 1.20 (0.84 – 1.71) |
| CC | 9 (1.8) | 12 (3.1) | 2.97 (1.05 – 8.46) |
| GC+CC * GG | 1.29 (0.92 – 1.82) | ||
| P Trend = 0.06 | |||
| TNFα-308 A>G | |||
| GG | 309 (62.4) | 253 (65.2) | 1 |
| GA | 167 (33.7) | 117 (30.2) | 0.98 (0.70 – 1.37) |
| AA | 19 (3.8) | 18 (4.6) | 1.18 (0.53 – 2.60) |
| GA+AA * GG | 1.00 (0.73 – 1.38) | ||
| P Trend = 0.88 |
Includes adenoma ± cancer
OR from logistic regression adjusted for the time of recruitment (i.e., pre- or post-procedure), age, gender, body mass index, alcohol consumption, exercise, smoking, diverticular disease, exercise and aspirin use
None of the cytokine polymorphisms significantly predicted CRN grade (data not shown).
Inflammatory marker levels and CRN risk
Associations between CRN and inflammatory marker levels are shown separately for the pre-procedure and post-procedure subjects (Tables 3 and 4, respectively). Within the pre-procedure subjects, none of the markers significantly predicted CRN overall, but CRP values above the median were significantly associated with low grade polyps [OR (95% CI) 2.95 (1.22–7.15)] (Table 3). Within the post-procedure group, none of the markers significantly predicted high risk, low risk or total CRN (Table 4).
Table 3.
Inflammatory marker levels association with CRN* among pre-procedure subjects
| Marker | Controls | Cases | All CRN cases n=242 |
High risk CRN n=192 |
Low risk CRN n=50 |
|---|---|---|---|---|---|
| Median (IQ range) | Median (IQ range) | $#OR (95%)CI | $*OR (95%)CI | $*OR (95%)CI | |
| IL1B | |||||
| ≤ 3.33 | 3.33 (2.66,4.02) | 3.39 (2.44,4.10) | 1 | 1 | 1 |
| > 3.33 | 0.82 (0.52 – 1.28) | 0.85 (0.52–1.36) | 0.72 (0.31–1.36) | ||
| IL4 | |||||
| ≤ 3.52 | 3.52 (2.41,4.32) | 3.77 (1.85,4.29) | 1 | 1 | 1 |
| > 3.52 | 1.29 (0.84 – 1.99) | 1.42 (0.90–2.26) | 0.87 (0.38–1.97) | ||
| IL6 | |||||
| ≤ 3.32 | 3.32 (2.98,3.72) | 3.28 (2.96,3.70) | 1 | 1 | 1 |
| > 3.32 | 0.94 (0.60 – 1.46) | 0.92 (0.57–1.48) | 1.00 (0.44–2.26) | ||
| IL8 | |||||
| ≤ 4.21 | 4.21 (3.77,4.63) | 4.30 (3.82,4.76) | 1 | 1 | 1 |
| > 4.21 | 1.19 (0.77 – 1.82) | 1.25 (0.79–1.98) | 0.77 (0.44–2.13) | ||
| IL10 | |||||
| ≤ 3.51 | 3.51 (2.89,3.89) | 3.66 (2.98,3.89) | 1 | 1 | 1 |
| > 3.51 | 1.20 (0.77 – 1.86) | 1.29 (0.80–2.07) | 0.90 (0.39–2.05) | ||
| TNF | |||||
| ≤ 3.53 | 3.53 (2.79,3.86) | 3.53 (2.65,3.97) | 1 | 1 | 1 |
| > 3.53 | 1.00 (0.65 – 1.55) | 1.09 (0.69–1.73) | 0.73 (0.32–1.65)ne | ||
| CRP | |||||
| ≤ 1.88 | 1.88 (1.14,2.67) | 1.84 (1.18,2.52) | 1 | 1 | 1 |
| > 1.88 | 1.02 (0.66 – 1.58) | 0.79 (0.49–1.27) | 2.95 (1.22–7.15) |
Includes adenoma ± cancer
OR from logistic regression adjusted for the time of recruitment (i.e., pre- or post-procedure), age, gender, body mass index, alcohol consumption, exercise, smoking, diverticular disease, exercise and aspirin use
OR from polytomous logistic regression adjusted for the time of recruitment (i.e., pre- or post-procedure), age, gender, body mass index, alcohol consumption, exercise, smoking, diverticular disease, exercise and aspirin use
Controls is the reference group
Table 4.
Inflammatory marker levels association with CRN* among post-procedure subjects
| Marker | Controls | Cases | All CRN cases n=146 |
High risk CRN n=96 |
Low risk CRN n=50 |
|---|---|---|---|---|---|
| Median (IQ range) | Median (IQ range) | $#OR (95%)CI | $*OR (95%)CI | $*OR (95%)CI | |
| IL1B | |||||
| ≤ 3.48 | 3.48 (1.21,4.01) | 3.32 (0.00,4.03) | 1 | 1 | 1 |
| > 3.48 | 0.96 (0.60 – 1.52) | 1.07 (0.63–1.83) | 0.80 (0.41–1.56) | ||
| IL4 | |||||
| ≤ 3.47 | 3.47 (0.00,4.00) | 3.68 (0.00,4.28) | 1 | 1 | 1 |
| > 3.47 | 1.32 (0.83 – 2.08) | 1.54 (0.91–2.62) | 1.03 (0.54–1.98) | ||
| IL6@ | |||||
| ≤ 3.11 | 3.11 (0.00,3.58) | 3.20 (2.76,3.62) | 1 | 1 | 1 |
| > 3.11 | 1.23 (0.78 – 1.95) | 1.46 (0.86–2.48) | 0.91 (0.47–1.73) | ||
| IL8 | |||||
| ≤ 3.81 | 3.81 (3.12,4.25) | 3.88 (3.45,4.34) | 1 | 1 | 1 |
| > 3.81 | 1.35 (0.85 – 2.14) | 1.45 (0.85–2.47) | 1.17 (0.61–2.25) | ||
| IL10 | |||||
| ≤ 3.71 | 3.71 (3.00,4.14) | 3.69 (2.60,4.26) | 1 | 1 | 1 |
| > 3.71 | 1.07 (0.66 – 1.74) | 0.97 (0.56–1.69) | 1.37 (0.68–2.75) | ||
| TNF | |||||
| ≤ 3.07 | 3.07 (0.00,3.72) | 3.11 (0.00,3.75) | 1 | 1 | 1 |
| > 3.07 | 1.06 (0.67 – 1.68) | 1.15 (0.68–1.95) | 0.95 (0.50–1.82)ne | ||
| CRP | |||||
| ≤ 1.37 | 1.37 (0.75,2.09) | 1.27 (0.81,2.10) | 1 | 1 | 1 |
| > 1.37 | 0.97 (0.60 – 1.56) | 0.89 (0.52–1.53) | 1.16 (0.59–2.28) |
Includes adenoma ± cancer
OR from logistic regression adjusted for the time of recruitment (i.e., pre- or post-procedure), age, gender, body mass index, alcohol consumption, exercise, smoking, diverticular disease, exercise and aspirin use
OR from polytomous logistic regression adjusted for the time of recruitment (i.e., pre- or post-procedure), age, gender, body mass index, alcohol consumption, exercise, smoking, diverticular disease, exercise and aspirin use
Controls is the reference group
the model did not include an interaction between NSAID/aspirin use and IL6
Of note, no significant interactions were detected between aspirin/NSAID use and cytokine polymorphisms. Similarly among pre-procedure subjects, no interaction between aspirin/NSAID use and cytokine levels was detected. However, among post-procedure subjects we detected an interaction between IL6 levels and aspirin/NSAID use, such that compared to individuals with low IL6 levels, participants with high IL6 levels were at higher risk of CRN (OR: 2.01, 95% CI: 1.13–3.58), among aspirin/NSAID non-users, in contrary to aspirin/NSAID users who were at no risk of CRN (OR: 0.61, 95% CI: 0.28–1.31), (data not shown).
The variables used for propensity score prediction were well balanced between pre- and post-procedure matched groups such that all standardized differences were less than 0.1. Comparing levels of the inflammatory markers, IL8, TNFα, and CRP levels were significantly higher pre-procedure vs. post-procedure, while IL4 was significantly lower (Table 5). In a sensitivity analysis of 23 patients who had both pre- and post-procedure samples, results were of similar magnitude as well as in the same direction, although with limited statistical power most comparisons were not significant (Table 5).
Table 5.
Differences between pre-procedure and post-procedure inflammatory marker levels
| Log marker | Propensity score matched set | Subset of patients with pre- and post-procedure samples | ||
|---|---|---|---|---|
| Median difference1 | Pvalue2 | Median difference1 | Pvalue2 | |
| IL-1β | −0.19 | 0.24 | −0.23 | 0.20 |
| IL-4 | −0.17 | 0.34 | −0.10 | 0.74 |
| IL-6 | 0.05 | 0.37 | 0.03 | 0.84 |
| IL-8 | 0.30 | <0.001 | 0.30 | 0.20 |
| IL-10 | −0.20 | 0.06 | 0.12 | 0.52 |
| TNFα | 0.30 | 0.03 | 0.20 | 0.49 |
| CRP | 0.64 | <0.0001 | 0.33 | 0.11 |
Comparing pre-procedure to post-procedure inflammatory marker level
Quantile regression
Discussion
More than half of the deaths due to colorectal cancer occur in developed countries (Ferlay et al. 2010) where screening colonoscopy in the last decade has significantly reduced CRC mortality. A meta-analysis of randomised controlled trials (RCT) found that screened patients had a 16% reduction in the risk of mortality due to CRC (Hewitson et al. 2007). Whilst this is encouraging, participation in screening still remains poor and hence the ultimate aim would be to develop preventive strategies against the development of this deadly condition. Chemoprevention of both CRC and its precursor lesions, adenomas, through long term use of aspirin and other NSAIDs is shown to be effective in both hereditary and sporadic CRN. (Burn et al. 2011)(Rothwell et al. 2011). Though such results are encouraging, both non-specific COX inhibitors, such as aspirin, and COX-2-specific inhibitors have significant gastrointestinal and cardiovascular toxicity that needs to be balanced against their demonstrated benefits (Menter, Schilsky & DuBois 2010). To overcome this, identification of suitable “at risk” candidates for chemoprevention through use of modern molecular profiling methods evaluating risk versus response pathways are essential. These methods when combined with standard clinical risk profiles will help in understanding how best to use aspirin/NSAIDs for cancer prevention by identifying patients most likely to benefit and/or excluding those individuals at highest risk of significant toxicity. (Menter, Schilsky & DuBois 2010). Aspirin/NSAIDs act through attenuation of inflammation, which has been proposed to play a key role in the pathogenesis of CRN and is also the target for preventive strategies (Trinchieri 2011). Hence, we have investigated the associations of inflammatory marker levels and genetic polymorphisms with the risk of CRN.
Inflammatory gene polymorphisms and CRN risk
Although the IL1B-31 CC genotype has previously been shown to increase the risk of gastric cancer (El-Omar et al. 2000b), its association with CRN has been inconsistent. In a previous study by our group, there was no association of IL1B-31C>T genotype with CRC risk in a Scottish population (Macarthur et al. 2005b). In contrast, a sigmoidoscopy-based case-control study found that heterozygosity at the IL1B-31C>T locus was associated with increased risk of colorectal adenoma [OR 1.8 (95% CI) 1.8 (1.2–2.9)] (Gunter et al. 2006). However, the authors were cautious in their interpretation of the latter study because the genotypes were not distributed in accordance with Hardy-Weinberg equilibrium and the possibility of false-positive reporting. Nevertheless, since that study and ours were done in screened populations in whom colorectal neoplasia is mostly detected at early stages (i.e., adenoma), these combined findings suggest that IL1B may be involved in the initiation of the neoplastic process.
Homozygosity of the less common C allele of the proinflammatory PTGS2–765 C>G SNP significantly increased the risk of CRN, reinforcing previous evidence implicating other PTGS2 polymorphisms. In a recent systematic review by Pereira et al, the PTGS2-899G>C and -1329G>A polymorphisms increased risk for CRC [ORs (95% CI) 1.35 (1.01–1.81) and 1.36 (1.11–1.66), respectively). They also found in a pooled analysis that the 429T>C polymorphism was associated with increased risk of adenoma suggesting involvement in early carcinogenesis, although they acknowledged the inherent limitations of findings from a systematic review (Pereira, Medeiros & Dinis-Ribeiro 2009).
Contrary to previous evidence, the A allele of IL8–251 A>T SNP was associated with significantly lower risk of CRN, although small numbers in these subgroup analyses suggest cautious interpretation. Conversely, a French study involving 1023 CRC cases and 1121 matched controls found that IL8–251 AA genotype increased CRC risk (Kury et al. 2008) despite previous null (Gunter et al. 2006, Theodoropoulos et al. 2006b) and opposite direction findings (Landi et al. 2003).
We did not find any significant associations of CRN with the IL1RN VNTR polymorphism. Despite its well established association with gastric cancer, not many studies have investigated associations with CRC. To our knowledge, only one study limited to 125 CRC cases and 134 controls has reported that IL1RN VNTR polymorphism increases the risk of CRC (Viet et al. 2005).
We did not find any significant association for the IL6–174 C>G polymorphism. The IL6–174 C>G polymorphism has been previously associated with CRC, with a large study involving 1579 CRC cases and 1977 Controls showing that the G allele decreased risk (Slattery et al. 2007) and a smaller study showing that the C allele increased risk (Landi et al. 2003). However, subsequent attempts at replication had null findings (Cacev et al. 2010, Tsilidis et al. 2009b).
For the IL10–1082 A>G and IL10–592 A>C SNPs, we did not see any statistically significant associations, in agreement with some previous reports (Gunter et al. 2006, Macarthur et al. 2005b). However, another study found the homozygous GG genotype at IL10–1082 was associated with a lower risk of CRC than homozygous AA [OR (95%CI) 0.58 (0.35–0.95)] (Tsilidis et al. 2009a).
The role of TNFα-308 A>G SNP has been widely studied in relation to gastric cancer, with a statistically significant increased risk associated with the AA genotype (Gorouhi et al. 2008). Whilst many studies have investigated its association with CRC, so far no study, including ours, has shown any significant association.
As replication is essential to establish the credibility of genotype-phenotype association studies (NCI-NHGRI Working Group on Replication in Association Studies et al. 2007), we appreciate that all of the above findings need further evaluation in studies involving different populations and larger sample sizes before any firm conclusions can be made. It is also possible that our null findings for certain SNPs may be due to the lack of adequate statistical power. Another important aspect of genetic association studies regarding inflammation is that the inflammatory process is complex, involving multiple cytokines controlled by the genotype. It would be prudent to examine the effects of combinations of these polymorphisms on CRN risk.
Inflammatory marker levels and CRN risk
There is inconsistent finding in the literature for the association of inflammatory markers and CRN. The results from two prospective studies suggested that elevated IL-6 was associated with an increased risk of CRC (Heikkila, Ebrahim & Lawlor 2008). In the CLUE II cohort, Tsilidis et al found that pre-diagnostic plasma C-reactive protein concentration was not associated with risk of colorectal adenoma. The OR of colorectal adenoma among those in the highest fourth (>2.95 mg/l) of C-reactive protein concentration compared with the lowest fourth (<0.65 mg/l) was 0.61 (95% CI, 0.29–1.25; p for trend = 0.25)(Tsilidis et al. 2008). In another study, there were no associations between serum levels of the inflammatory markers CRP and IL-6 and colorectal adenoma (Ognjanovic et al. 2010).
We found elevated CRP levels prior to resection (pre-procedure) of low-risk, but not high-risk, CRN. We found no associations after polypectomy (post-procedure) for low-risk, high-risk or total CRN. It has been suggested that some of the divergent results across studies of CRP may be related to sex differences (Chan et al. 2011b). Our positive results should be viewed with caution in light of a recent study among women in the Nurses’ Health Study that found no association of colorectal cancer with CRP or IL-6 level (Chan et al. 2011b), consistent with several other studies which have not observed any significant associations of CRP and cancer (Siemes et al. 2006) (Heikkilä et al. 2009) (Allin, Bojesen & Nordestgaard 2009) (Ito et al. 2005) (Allin et al. 2010). Furthermore, a nested case-control study of incident colorectal adenoma in the Prostate, Lung, Colorectal, and Ovarian Cancer Screening Trial found a 1-unit increase in log CRP level was associated with a 15% reduction in risk of developing colorectal adenoma [OR (95% CI) 0.85 (0.75–0.98), P-trend 0.01)]. (Gunter et al. 2011).
We did not observe any significant associations of circulating levels of IL6, IL8, TNFα, IL1β, IL4 or IL10 with CRN risk in case-control analyses, for both the pre-procedure and post-procedure groups.
In the small number of patients with both pre-procedure and post-procedure samples, CRP levels decreased following polypectomy. One possible reason for this could be that the presence of CRN creates a pro-inflammatory milieu that results in an increase in the circulating levels of the inflammatory markers. Moreover, higher levels of pre-diagnostic CRP are associated with poor prognosis post-operatively.
By use of the propensity-matched data set, we also observed significant differences in circulating marker levels between pre-procedure and post-procedure cases. Specifically, levels of the pro-inflammatory markers CRP, IL8 and TNFα were significantly higher when the CRN was in-situ (i.e., pre-procedure) than after removal (i.e., post-procedure), whereas the anti-inflammatory markers IL4 and IL10 were significantly lower. Even though these are significant findings, one should be cautious in interpreting and drawing conclusions as the pre- procedure and post-procedure measurements were from different subjects. But in light of these interesting observations, levels of inflammatory markers should be measured in a larger group of CRN patients before and after removal of their neoplastic lesions.
Our study suggests that the presence of CRN may increase levels of inflammatory markers, providing some insight into the possible sequence of events in the inflammation-cancer relationship. Replication of these findings and extension in larger studies could further define the role of inflammation in the pathogenesis of CRN.
Acknowledgments
The authors thank Dr Bernie Croal and Mr. William Mutch for technical assistance with the hs-CRP assays.
Grant support: Umesh Basavaraju was supported by a clinical fellowship awarded by the charity CRANES (Cancer Research Aberdeen and Northeast Scotland). This work was supported by funding CRANES and Friends of Anchor.
Abbreviations used in this paper
- HS-CRP
highly sensitive C-reactive protein
- CRC
colorectal cancer
- IL
interleukin
- OR
odds ratio
- CI
confidence interval
- FOBT
fecal occult blood test
- BCSP
bowel cancer screening programme
- NSAID
non-steroidal anti-inflammatory drugs
- FFQ
food frequency questionnaire
- IBD
inflammatory bowel disease
- HGD
high grade dysplasia
- TVA
tubovillous adenoma
- IL
interleukin
- TNF
tissue necrosis factor
- CBA
cytokine bead array
- SNP
single neucleotide polymorphism
- VNTR
variable number tandem repeat
Footnotes
Author contributions: Study conception, design and funding: UB, GLH, EEO
Provision of study materials: UB, EEO, Experimental work: UB, AJP, SB, GLH, Data analysis and interpretation: UB, FMS, CR, Manuscript writing: UB, FMS, CR, Final approval of manuscript: All authors
Disclosure of potential conflicts of interest: None
References
- Allin KH, Bojesen SE, Nordestgaard BG. Baseline C-Reactive Protein Is Associated With Incident Cancer and Survival in Patients With Cancer. Journal of Clinical Oncology. 2009;27(13):2217–2224. doi: 10.1200/JCO.2008.19.8440. [DOI] [PubMed] [Google Scholar]
- Allin KH, Nordestgaard BG, Zacho J, Tybjærg-Hansen A, Bojesen SE. C-Reactive Protein and the Risk of Cancer: A Mendelian Randomization Study. Journal of the National Cancer Institute. 2010;102(3):202–206. doi: 10.1093/jnci/djp459. [DOI] [PubMed] [Google Scholar]
- Austin PC. Optimal caliper widths for propensity-score matching when estimating differences in means and differences in proportions in observational studies. Pharm Stat. 2011;10(2):150–61. doi: 10.1002/pst.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burn J, Gerdes AM, Macrae F, Mecklin JP, Moeslein G, Olschwang S, Eccles D, Evans DG, Maher ER, Bertario L, Bisgaard ML, Dunlop MG, Ho JW, Hodgson SV, Lindblom A, Lubinski J, Morrison PJ, Murday V, Ramesar R, Side L, Scott RJ, Thomas HJ, Vasen HF, Barker G, Crawford G, Elliott F, Movahedi M, Pylvanainen K, Wijnen JT, Fodde R, Lynch HT, Mathers JC, Bishop DT, on behalf of the CAPP2 Investigators Long-term effect of aspirin on cancer risk in carriers of hereditary colorectal cancer: an analysis from the CAPP2 randomised controlled trial. Lancet. 2011;378(9809):2801–2807. doi: 10.1016/S0140-6736(11)61049-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory markers are associated with risk of colorectal cancer and chemopreventive response to anti-inflammatory drugs. Gastroenterology. 2011a;140(3):799–808. doi: 10.1053/j.gastro.2010.11.041. quiz e11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan AT, Ogino S, Giovannucci EL, Fuchs CS. Inflammatory Markers Are Associated With Risk of Colorectal Cancer and Chemopreventive Response to Anti-Inflammatory Drugs. Gastroenterology. 2011b;140(3):799–808. doi: 10.1053/j.gastro.2010.11.041. e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu H, Lin J, Chen TH, Lee Y, Chiu Y, Liang J, Shun C, Wu M. Elevation of C-reactive protein level is associated with synchronous and advanced colorectal neoplasm in men. American Journal of Gastroenterology. 2008;103(9):2317–2325. doi: 10.1111/j.1572-0241.2008.01952.x. [DOI] [PubMed] [Google Scholar]
- Gunter MJ, Cross AJ, Huang WY, Stanczyk FZ, Purdue M, Xue X, Schoen R, Limburg PJ, Schatzkin A, Sinha R, Hayes RB. A prospective evaluation of C-reactive protein levels and colorectal adenoma development. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2011;20(3):537–544. doi: 10.1158/1055-9965.EPI-10-1099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkilä Katriina, Harris Ross, Lowe Gordon, Rumley Ann, Yarnell John, Gallacher John, Ben-Shlomo Yoav, Ebrahim Shah, Lawlor Debbie. Associations of circulating C-reactive protein and interleukin-6 with cancer risk: findings from two prospective cohorts and a meta-analysis. Cancer Causes and Control. 2009;20(1):15–26. doi: 10.1007/s10552-008-9212-z. [DOI] [PubMed] [Google Scholar]
- Hu FB, Meigs JB, Li TY, Rifai N, Manson JE. Inflammatory Markers and Risk of Developing Type 2 Diabetes in Women. Diabetes. 2004;53(3):693–700. doi: 10.2337/diabetes.53.3.693. [DOI] [PubMed] [Google Scholar]
- Ito Y, Suzuki K, Tamakoshi K, Wakai K, Kojima M, Ozasa K, Watanabe Y, Kawado M, Hashimoto S, Suzuki S, Tokudome S, Toyoshima H, Hayakawa N, Kato K, Watanabe M, Ohta Y, Maruta M, Tamakoshi A, Mori M, Motohashi Y, Tsuji I, Nakamura Y, Iso H, Mikami H, Inaba Y, Hoshiyama Y, Suzuki H, Shimizu H, Kikuchi S, Koizumi A, Kawamura T, Miki T, Date C, Sakata K, Nose T, Yoshimura T, Shibata A, Okamoto N, Shio H, Ohno Y, Kitagawa T, Kukori T, Tajima K. Coloretal cancer and serum C-reactive protein levels: A case-control study nested in the JACC study. Journal of Epidemiology. 2005;15(SUPPL. 2):S185–S189. doi: 10.2188/jea.15.S185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S, Keku TO, Martin C, Galanko J, Woosley JT, Schroeder JC, Satia JA, Halabi S, Sandler RS. Circulating Levels of Inflammatory Cytokines and Risk of Colorectal Adenomas. Cancer Research. 2008;68(1):323–328. doi: 10.1158/0008-5472.CAN-07-2924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li M, You Q, Wang X. Association Between Polymorphism of Tumor Necrosis Factor Alpha-308 Gene Promoter and Colon Cancer in Chinese Population. Genetic testing and molecular biomarkers. 2011;15(11):743–747. doi: 10.1089/gtmb.2011.0068. [DOI] [PubMed] [Google Scholar]
- Liu S, Tinker L, Song Y, Rifai N, Bonds DE, Cook NR, Heiss G, Howard BV, Hotamisligil GS, Hu FB, Kuller LH, Manson JE. A prospective study of inflammatory cytokines and diabetes mellitus in a multiethnic cohort of postmenopausal women. Archives of Internal Medicine. 2007;167(15):1676–1685. doi: 10.1001/archinte.167.15.1676. [DOI] [PubMed] [Google Scholar]
- Macarthur M, Sharp L, Hold GL, Little J, El-Omar EM. The role of cytokine gene polymorphisms in colorectal cancer and their interaction with aspirin use in the northeast of Scotland. Cancer Epidemiology, Biomarkers & Prevention. 2005;14(7):1613–1618. doi: 10.1158/1055-9965.EPI-04-0878. [DOI] [PubMed] [Google Scholar]
- Menter DG, Schilsky RL, DuBois R. Cyclooxygenase-2 and cancer treatment: understanding the risk should be worth the reward. Clinical cancer research : an official journal of the American Association for Cancer Research. 2010;16(5):1384–1390. doi: 10.1158/1078-0432.CCR-09-0788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ognjanovic S, Yamamoto J, Saltzman B, Franke A, Ognjanovic M, Yokochi L, Vogt T, Decker R, Le Marchand L. Serum CRP and IL-6, genetic variants and risk of colorectal adenoma in a multiethnic population. Cancer causes & control : CCC. 2010;21(7):1131–1138. doi: 10.1007/s10552-010-9540-7. [DOI] [PubMed] [Google Scholar]
- Otani T, Iwasaki M, Sasazuki S, Inoue M, Tsugane S, Japan Public Health Center-Based Prospective Study Group Plasma C-reactive protein and risk of colorectal cancer in a nested case-control study: Japan Public Health Center-based prospective study. Cancer epidemiology, biomarkers & prevention : a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology. 2006;15(4):690–695. doi: 10.1158/1055-9965.EPI-05-0708. [DOI] [PubMed] [Google Scholar]
- Prizment AE, Anderson KE, Visvanathan K, Folsom AR. Association of Inflammatory Markers with Colorectal Cancer Incidence in the Atherosclerosis Risk in Communities Study. Cancer Epidemiology Biomarkers & Prevention. 2011;20(2):297–307. doi: 10.1158/1055-9965.EPI-10-1146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothwell PM, Fowkes FGR, Belch JF, Ogawa H, Warlow CP, Meade TW. Effect of daily aspirin on long-term risk of death due to cancer: Analysis of individual patient data from randomised trials. The Lancet. 2011;377(9759):31–41. doi: 10.1016/S0140-6736(10)62110-1. [DOI] [PubMed] [Google Scholar]
- Siemes C, Visser LE, Coebergh JW, Splinter TAW, Witteman JCM, Uitterlinden AG, Hofman A, Pols HAP, Stricker BHC. C-Reactive Protein Levels, Variation in the C-Reactive Protein Gene, and Cancer Risk: The Rotterdam Study. Journal of Clinical Oncology. 2006;24(33):5216–5222. doi: 10.1200/JCO.2006.07.1381. [DOI] [PubMed] [Google Scholar]
- Tarlow JK, Blakemore AI, Lennard A, Solari R, Hughes HN, Steinkasserer A, Duff GW. Polymorphism in human IL-1 receptor antagonist gene intron 2 is caused by variable numbers of an 86-bp tandem repeat. Human genetics. 1993;91(4):403–404. doi: 10.1007/BF00217368. [DOI] [PubMed] [Google Scholar]
- Trinchieri G. Innate inflammation and cancer: Is it time for cancer prevention? F1000 medicine reports. 2011;3:11. doi: 10.3410/M3-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsilidis KK, Branchini C, Guallar E, Helzlsouer KJ, Erlinger TP, Platz EA. C-reactive protein and colorectal cancer risk: a systematic review of prospective studies. International Journal of Cancer. 2008a;123(5):1133–1140. doi: 10.1002/ijc.23606. [DOI] [PubMed] [Google Scholar]
- Tsilidis KK, Erlinger TP, Rifai N, Hoffman S, Hoffman-Bolton J, Helzlsouer KJ, Platz EA. C-reactive protein and colorectal adenoma in the CLUE II cohort. Cancer Causes & Control. 2008b;19(6):559–567. doi: 10.1007/s10552-008-9117-x. [DOI] [PubMed] [Google Scholar]
- Tsilidis KK, Helzlsouer KJ, Smith MW, Grinberg V, Hoffman-Bolton J, Clipp SL, Visvanathan K, Platz EA. Association of common polymorphisms in IL10, and in other genes related to inflammatory response and obesity with colorectal cancer. Cancer causes & control : CCC. 2009;20(9):1739–1751. doi: 10.1007/s10552-009-9427-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


