Abstract
Deregulated tyrosine kinase signaling alters cellular homeostasis to drive cancer progression. The emergence of a non-receptor tyrosine kinase, ACK1 as an oncogenic kinase, has uncovered novel mechanisms by which tyrosine kinase signaling promotes cancer progression. While early studies focused on ACK1 (also known as activated Cdc42-associated kinase 1 or TNK2) as a cytosolic effecter of activated transmembrane receptor tyrosine kinases (RTKs), wherein it shuttles between the cytosol and the nucleus to rapidly transduce extracellular signals from the RTKs to the intracellular effectors, recent data unfold a new aspect of its functionality as an epigenetic regulator. ACK1 interacts with the Estrogen Receptor (ER)/histone demethylase KDM3A (JHDM2a) complex, modifies KDM3A by tyrosine phosphorylation to regulate transcriptional outcome at HOXA1 locus to promote the growth of tamoxifen-resistant breast cancer. It is also well established that ACK1 regulates the activity of Androgen Receptor (AR) by tyrosine phosphorylation to fuel the growth of hormone-refractory prostate cancers. Further, recent explosion in genomic sequencing has revealed recurrent ACK1 gene amplification and somatic mutations in a variety of human malignancies, providing a molecular basis for its role in neoplastic transformation. In this review, we will discuss the various facets of ACK1 signaling, including its newly uncovered epigenetic regulator function, which enables cells to bypass the blockade to major survival pathways to promote resistance to standard cancer treatments. Not surprisingly, cancer cells appear to acquire an `addiction’ to ACK1 mediated survival, particularly under stress conditions, such as growth factor deprivation or genotoxic insults or hormone deprivation. With the accelerated development of potent and selective ACK1 inhibitors, targeted treatment for cancers harboring aberrant ACK1 activity may soon become a clinical reality.
Keywords: ACK1, TNK2, AKT, AR, KDM3A, ATM, WWOX, HOXA1, prostate cancer, breast cancer, tyrosine kinase, small molecule inhibitors, tamoxifen
Introduction
Oncogenomics and oncoproteomics has not only lead to unearthing of biomarkers specific to each cancer type and disease stage, but has also expedited our understanding of the molecular alterations/characteristic of each individual tumor. These approaches have heightened the significance of many understudied signaling proteins that promote tumor progression in less than favorable environments. A signaling protein that appears to be recurrently activated in cancer cells is the ACK1 tyrosine kinase.1, 2 Although originally identified as a non-receptor tyrosine kinase that specifically binds to the GTP-bound form of Cdc42 and inhibits its GTPase activity,3 its distinct signaling in cancer cells has rekindled interest in this structurally unique kinase- as a molecular entity for targeted therapeutics.
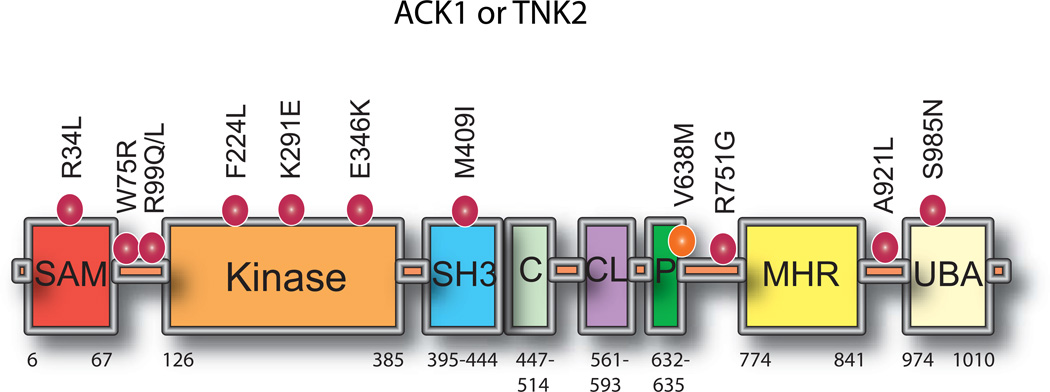
ACK1 gene encodes a large protein of 1038 amino acids (140 kDa) that is post-translationally modified by multiple tyrosine phosphorylations that regulate its kinase activation.4, 5 The multi-domain structure of ACK1 sets it apart from other non-receptor tyrosine kinases and includes at least 8 distinct domains; the Sterile alpha motif (SAM), kinase or catalytic domain, SH3 domain, GTPase binding domain (also known as Cdc42-binding domain or CRIB domain), Clathrin interacting region, PPXY motif or WW domain interacting region, a MIG6 homology region (MHR), also known as EGFR binding domain and an ubiquitin-association (UBA) domain (Figure 1). It is the only kinase known to possess an SH3 domain carboxy-terminal to the kinase domain.6 A most recognizable outcome of this multi-domain structure appears to be to not only facilitate ACK1 localization to different cellular compartments, but to also promote its association with disparate proteins fostering its functional diversity.1
Figure 1. A schematic representation of ACK1 domain architecture.
Different structural domains present in ACK1 are shown. SAM, Sterile alpha motif; Kinase, tyrosine kinase domain; SH3, Src homology domain 3; C, Cdc42 binding domain; CL, Clathrin interacting domain; P, PPXY motif or WW domain interacting region; MHR, Mig6 homology region; UBA, Ubiquitin association domain. Various mutations identified in ACK1 have been shown on top.
Being an intermediary kinase that bridges the receptor tyrosine kinases (RTKs) and effecter proteins, it became quickly apparent that the ACK1 was providing a niche for intra-cellular communication. Crystal structure analysis and extensive biochemical studies now provide a glimpse into the carefully orchestrated process of ACK1 activation in normal as well as cancer cells. Within ACK1, an insertion of a 10 amino acid sequence that is rich in proline residues has been identified upstream of the kinase recognition segment of the MHR. It has been suggested that this proline rich sequence interacts with the SH3 domain facilitating orientation of the MHR for inhibitory interactions with the C-terminal region of the kinase domain, stabilizing an auto-inhibited state of the ACK1 kinase.7–9 Activation of ACK1 is achieved when ligand activated RTKs interact with the MHR of ACK1 overcoming an auto-inhibitory interaction of the MHR with its own kinase domain.7, 9, 10 In addition, another step may be involved in accomplishing the complete ACK1 activation. Recently, it has been demonstrated that the amino-terminal SAM domain facilitates membrane localization and symmetric dimerization leading to trans-phosphorylation and activation.11 Thus, ACK1 may be switching to different modes of kinase activation, to adapt rapidly to cellular requirements.
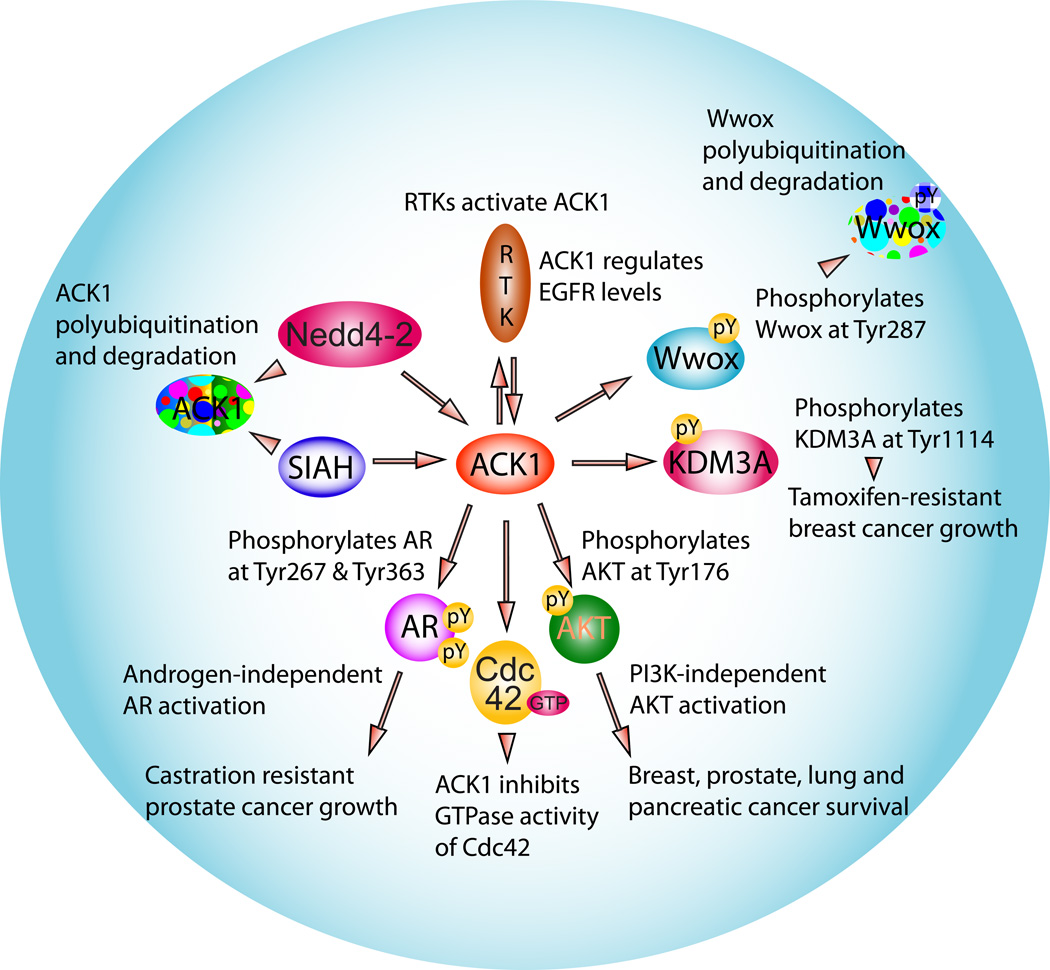
Multiple ligands such as EGF, GAS6, heregulin, IGF and insulin cause a rapid albeit temporal activation of the ACK1, suggesting that ACK1 acts as a major integrator of RTK signaling.4, 5, 12, 13 Interestingly, investigation into the consequences of EGFR mediated ACK1 activation not only revealed rapid Tyr-phosphorylation (and activation) of ACK1, followed by the loss of ACK1 levels, but also that the EGFR protein itself was significantly downregulated.12 These data indicate that not only EGFR mediated ACK1 activation may play a role in maintaining ACK1 homeostasis, but ACK1 in turn could also regulate EGFR levels in ligand-activated cells, thus switching off EGFR-ACK1 signaling. Accordingly, suppression of the ACK1 expression by siRNA resulted in inhibition of EGF-induced degradation of EGFR.14 Although, EGFR-ACK1 cross-talk provides clues to the delicate balance that exist within cells, whether other proteins could compensate for the loss of ACK1 function to regulate EGFR levels remain to be seen. At the molecular level, the loss of UBA domain of ACK1 blocked the ligand-dependent degradation of EGFR, suggesting that ACK1 may regulate both EGFR and its own degradation via its UBA domain. Consistent with these findings, some of the key players required to turn off ACK1 expression in cells are the ubiquitin ligases. In particular, ACK1 has been shown to interact with the WW2 and WW3 domains of E3 ubiquitin ligase Nedd4-2, via a conserved PPXY-containing region, resulting in ACK1 polyubiquitination and degradation.15 Interestingly, ACK1 interacts with the WW domain of tumor suppressor WWOX using the same region to cause its polyubiquitination and degradation.4, 15 It is plausible that in cancer cells, ACK1 interaction with WWOX may obstruct interaction with the E3 ubiquitin ligase Nedd4-2, and thus limit ACK1 degradation, stabilizing the ACK1 protein levels.
Recently, ACK1 was shown to interact with sequestosome 1 (p62/SQSTM1), an autophagy receptor, through an UBA domain in EGF dependent manner, indicating that ACK1 diverted activated EGFR into a degradative pathway by its association with p62/SQSTM1, NBR1 and Atg16L.16 A second mechanism that has recently been uncovered involves ACK1 interaction with Seven in absentia homolog (SIAH) ubiquitin ligases, SIAH1 and SIAH2 via a conserved SIAH-binding motif located in the far carboxy-terminus of ACK1.17 Interestingly, the association of ACK1 with SIAHs was independent of its kinase activity. These include ER-dependent signaling; interestingly, EGFR and ER signaling cascades are often regulated antagonistically.17 Moreover, SIAHs can restrict oncogenesis.18 Together, these data indicates that apart from EGFR activation, other pathways could also induce proteasomal degradation of ACK1 and play role in tumor initiation.
ACK1 in neuronal signaling
ACK1 expression is detectable at fairly high levels in the developing and adult brain, with the highest expression seen in the hippocampus, neocortex, and cerebellum.19 These expression studies lead to uncovering the role for ACK1 in synaptic function; ACK1 mRNA levels were significantly upregulated by increased neural activity. ACK1 kinase was also shown to be expressed in proliferative areas and in migratory pathways in the developing brain.20 The mechanistic details of ACK1 signaling in brain became recently available wherein in response to neurotrophins, ACK1 was shown to interact with Trk receptors leading to its activation, which in turn activated AKT.21 Overall, these data indicate that ACK1 is an important player in neurotrophin signaling, neuronal extension and branching, however, the pathological significance of ACK1 signaling in brain was not entirely clear, till a Belgian-Italian family was identified where all the 3 siblings presented with autosomal recessive, infantile onset epilepsy and intellectual disability.22 The exome sequencing identified a homozygous missense variant, V638M in the ACK1 (Figure 1). Unlike WT ACK1 protein, the EGF ligand treatment of V638M variant protein did not result in its degradation, primarily because of its failure to associate with E3 ubiquitin ligases, NEDD4-1 and NEDD4-2. These data indicate that V638M is a gain of function mechanism of upregulation of ACK1 levels. Further, these data open up the possibility of ACK1’s role in intellectual disability and the onset of epilepsy, which would need further examination.
Oncogenic activation of ACK1
The oncogenic properties of ACK1 kinase first became evident when activated ACK1 was shown to promote anchorage-independent growth and tumor growth in vivo.4 Since this discovery, significant new information has become available, which has established that aberrant ACK1 activation leading to its oncogenicity may occur by at least three distinct mechanisms, (a) deregulated RTK activation feeding into ACK1 (b) gene amplification and (c) somatic missense mutations.1
An intriguing aspect of ACK1 activation is its tight temporal activation; stimulation of cells treated with growth factors not only exhibited rapid activation of their respective RTKs, but also led to ACK1 activation, as seen by Tyr-phosphorylation, in a time dependent manner.4, 5, 12 The precision with which the ligand mediated ACK1 activation occurred provided two major clues; first, RTKs directly activate ACK1 and, the second, the specificity of the ligand/RTK mediated ACK1 activation is cell type dependent. While multiple RTKs can potentially interact with ACK1 resulting in its activation, there are distinct differences in the kinetics of when and how long ACK1 remains activated in cell types of different origins.4, 5, 12, 23
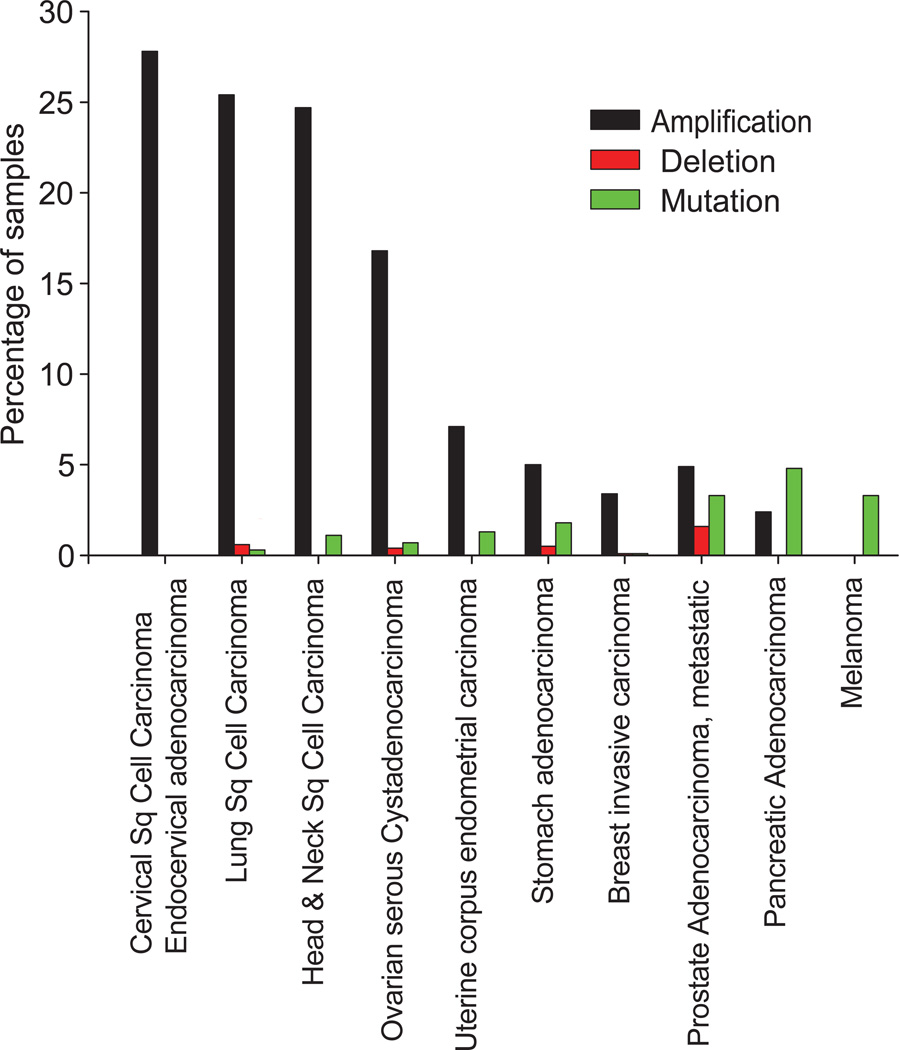
ACK1 gene is located on human chromosome 3q29. Advances in genome sequencing has revealed that ACK1 gene amplification, consequently causing mRNA overexpression as another mechanism of RTK-independent ACK1 activation in a variety of cancer cells (Figure 2). Notably, ACK1 gene amplification has been observed in cervical, ovarian, lung squamous, head & neck squamous cell carcinomas, breast, prostate and gastric cancers (cBioPortal; www.cbioportal.org).24–26
Figure 2. ACK1 gene alterations in human cancers.
A percentage of patient population of each tumor type exhibiting gene amplification, homozygous deletion or somatic mutations are shown. Data is derived from cBioPortal for Cancer Genomic, Memorial Sloan-Kettering Cancer Center (www.cbioportal.org).
Exome sequencing has also revealed recurrent somatic mutations in the ACK1 gene. 71 distinct missense substitutions and 10 nonsense mutations were identified within the various domains of the ACK1 gene (COSMIC and cBioPortal databases). Of these, 4 missense mutations, R34L, R99Q, E346K and M409I (Figure 1) have been analyzed in detail because of their location within distinct domains of ACK1.12 The ACK1-E346K mutation identified in ovarian cancer, exhibits significant increase in ACK1 autoactivation.9, 12 R34L, located in SAM domain, R99Q and M409I, located within the SH3 domain, too display significant ACK1 autoactivation.9 Overall, unlike ACK1 amplification that occurs in almost a quarter of the cervical, ovarian, and lung cancer patients, missense substitutions in ACK1 occurs with a significantly lower frequency (Figure 2), suggesting that gene amplification underlies the etiology of deregulated ACK1 activation in majority of the cancers.
Modulation of AKT activation: Anti-apoptotic and pro-proliferative functions of ACK1
ACK1 can transduce extracellular signals to cytosolic and nuclear effecters, distinguishing it from many of the other non-receptor tyrosine kinases (Figure 3). The cellular function and role of the multiple ACK1-interacting proteins has been reviewed earlier.1 Here, we will discuss in detail some of effecters of ACK1 signaling e.g. AKT, AR, ER, and KDM3A that play a crucial role in prostate and breast cancers.
Figure 3. ACK1 interactome.
ACK1 kinase network where it functions as a conveyer of signals from a diverse group of activated receptor tyrosine kinases to transmit cell survival, growth and proliferative signals via modification of multiple downstream effectors by unique tyrosine phosphorylation events.
AKT activation is critically dependent on its phosphorylation. A predominant mode of activation is the RTK/PI3K signaling pathway which facilitates membrane recruitment of AKT, as a consequence of the binding of the Pleckstrin homology domain of AKT to phosphatidylinositol 3,4,5 trisphosphate (PIP3), followed by phosphorylation at two sites, Thr308 and Ser473, leading to its kinase activation.27–29 Recently, a novel mechanism of AKT activation has been uncovered that is PIP3 and PI3K-independent and requires interaction with a non-receptor tyrosine kinase. Serendipitously, we discovered that ACK1 directly interacts with the oncogene AKT and phosphorylates it at an evolutionary conserved tyrosine residue at the 176 position. Tyr176-phosphorylated AKT localized to the plasma membrane due to its preferential binding to the phospholipid, phosphatidic acid (PA), and was further phosphorylated at Ser473 and Thr308 sites leading to its activation.12 Subsequently AKT translocated to the nucleus to suppress expression of pro-apoptotic and cell cycle arrest genes, promoting mitotic progression.2, 12, 30 Significantly, somatic autoactivating mutation in ACK1 e.g. E346K, also catalyzes robust AKT Tyr176-phosphorylation and activation, suggesting that cancer cells could bypass blockade to RTK/PI3K-dependent AKT activation by utilizing alternate survival pathways, such as those mediated by the oncogenic ACK1.
In addition to AKT activation, ACK1 may also overcome cell death by interacting with other survival pathways. One such example has recently been uncovered in Drosophila wherein ACK1 was shown to possess potent anti-apoptotic activity that is dependent on its interaction with two effecter proteins, Drk, the Drosophila homolog of GRB2 and yorkie, a transcriptional co-activator that is downstream of the Salvador-Hippo-Warts pathway, promoting transcription of proliferative and anti-apoptotic genes.31
Androgen Receptor (AR) regulation: ACK1 signaling in prostate cancer
AR is a crucial regulator of prostate cancer growth and plays paramount role in the progression of cancer to the highly metastatic disease, often termed as castration-resistant prostate cancer (CRPC).32–34 AR upon binding to its cognate ligand, androgen, translocates to the nucleus and regulates transcriptional activation of a large number of genes. This androgen-dependent transcriptional activity has been the cornerstone of the androgen deprivation therapy (ADT), a preferred treatment to negate AR transcriptional co-activator activity for prostate cancer. While chemical treatment with anti-androgens or surgical treatment by orchiectomy provides immediate palliative benefits, these ADTs are ineffective long term, as the recalcitrant disease recurs within 2–3 years. In spite of `castrate’ levels of androgens, prostate cancer cells still accomplished AR activation and were able to drive disease to CRPC stage. Consequently, resistance to ADT has become one of the most perplexing problems in prostate cancer therapy.32, 35, 36 The role of ACK1 in AR regulation first became clear when treatment of prostate cancer derived cell lines with ligands such as EGF, heregulin and Gas6 resulted in not only robust ACK1 activation but surprisingly lead to Tyr-phosphorylation of AR.5 ACK1 bound AR and directly phosphorylated AR at two distinct sites, Tyr-267 and Tyr-363, both located within the transactivation domain, facilitating androgen-independent transcriptional activation of AR. The significance of ACK1/AR signaling nexus became obvious in xenograft tumor studies; castrated mice injected with cells expressing activated ACK1 not only formed androgen-independent tumors, but also exhibited compromised tumor growth when mutant AR (Y267F mutation) was co-expressed with activated ACK1.5
Over the last few years a critical role for ACK1 in prostate cancer pathogenesis is further underscored by several observations; namely, ACK1 mRNA is not only upregulated in prostate cancers, but activated ACK1 expression also correlates positively with the progression to the malignant CRPC stage.5, 13, 24 Indeed, 10 out 13 CRPC tumors exhibited 5- to >100-fold ACK1 overexpression.24 Notably, alterations in ACK1 is associated with median disease free state of only 1.3 months compared to 110 months for PC patients without the ACK1 alteration (cBioPortal). One of the most compelling evidence came from transgenic mice studies wherein Probasin-ACK1 transgenic mice that express activated ACK1 specifically in prostates developed prostatic intraepithelial neoplasia (mPINs) and rare carcinomas.12, 37 The prostates of ACK1 transgenic mice exhibited significant increase in not only AR Tyr267-phosphorylation but also in AKT Tyr176-phosphorylation leading to AKT substrate FOXO3a Ser318/321-phosphorylation, suggesting that both biochemical events may be needed for the neoplastic transformation of prostates.12 ACK1 mediated AKT Tyr176-phosphorylation and activation may be more proximal stage initiating processes in neoplastic progression that serve as an alternative to PTEN loss which has been well studied in the mouse models of prostate cancer,38 while, AR Tyr267-phosphorylation paving the way for the progression of disease.
Overall, it appears that CRPC tumors may have hijacked the ACK1 oncogenic activity to phosphorylate AR causing transcriptional activation of AR-regulatory genes in a low androgen environment, however, could Tyr267-phosphorylated AR regulate transcription of the genes that are not regulated by androgen-bound AR? A large scale chromatin immunoprecipitation (ChIP) followed by hybridization to genomic chip revealed that indeed such a program may be operational in CRPC tumors. Tyr267-phosphorylated AR initiated a distinct androgen-independent transcription program in prostate cancer cells.37 Interestingly, many of the genes that are regulated by pTyr267-AR were the members of the p53-dependent DNA damage signaling pathway e.g. p300, MDM2, and ATM (ataxia telangiectasia mutated). Being a critical upstream regulator of the DNA damage signaling and checkpoint pathways that maintain genetic integrity and facilitate DNA repair in response to DNA damage, ATM expression was studied in greater detail. pTyr267-AR not only bound upstream of the ATM gene transcription start site facilitating increased ATM mRNA expression, but the primary human CRPCs with up-regulated activated ACK1 and pTyr267-AR also exhibited significant increase in ATM expression.37 Overall, these findings underscore a dominant role for ACK1 in hormone refractory prostate cancer.
ACK1 as an epigenetic modulator in breast cancer
The regulation of AR by ACK1 kinase has opened doors for an obvious question; can ACK1 be relevant in other hormonally regulated cancers? Estrogen receptor (ER) is an important nuclear hormone-receptor for the normal physiology of mammary cells.39 The ER protein binds to its cognate ligand estrogen (E2) with high affinity to form a functionally active complex that differentially interacts with co-regulator proteins to modify histones within the chromatin to modulate target gene expression.40 Similar to prostate cancer cells, the breast cancer cells are also critically dependent on estrogen for their growth, which has led to the successful use of a selective estrogen receptor modulator (SERM), tamoxifen in the treatment of ER-positive breast cancer.41, 42 Although a large number of the ER-positive breast tumors are sensitive to tamoxifen therapy, within 15–60 months, tumors become refractory to tamoxifen.43, 44 Since ER is expressed and functional in tamoxifen-resistant breast cancers, paradoxically, it may be the estrogen independent or ER-dependent signaling that promulgates tamoxifen resistance. The potential role of ACK1 in ER signaling to facilitate the growth and survival of breast cancers seemed plausible, specially based on its robust interaction with another related nuclear hormone receptor- the androgen receptor. Moreover, not only does heregulin mediated HER2 activation cause robust activation of ACK1 in breast cancer cell lines,12 but overexpression of ACK1 in human breast cancer cell lines followed by injection into immunocompromised mice induced tumor development.24 Further, activated ACK1 (and pTyr176-AKT) expression correlated with breast cancer progression and the Kaplan-Meir analyses revealed that the breast cancer patients with high expression of activated ACK1 (and AKT Tyr176-phosphorylation) are at a higher risk for cancer-related deaths.12
Although ACK1 has emerged to be an important player in breast, the precise mechanistic details of its modus operandi have become clear only recently. ACK1 phosphorylated the ER co-activator, KDM3A, also known as JHDM2A or Jmjd1a, a H3K9 demethylase at an evolutionary conserved tyrosine 1114 site in a heregulin dependent manner.45 Accordingly, ACK1 activation resulted in significant decrease in dimethyl-H3K9 epigenetic marks, facilitating transcriptional upregulation of a critical ER-regulated mammary tumor oncogene, HOXA1, even in the presence of tamoxifen.45 Overall, these data indicate that by its ability to regulate the epigenetic activity of an ER co-activator, ACK1 modulated the expression of ER target genes in the absence of E2, conferring tamoxifen-resistance.45 Since, two-thirds of breast cancers are ER positive and are initially expected to be responsive to SERMs, ACK1 inhibitors may be of crucial importance to counter tamoxifen resistance, particularly in those breast tumors that aberrantly activate ACK1.
Buchwald et al. have demonstrated that ACK1 levels are E2/ER dependent in ER positive breast cancer cells.17 Taken together, it opens up a new mechanism of ACK1 role in breast cancer; when E2/ER signaling is inhibited by Tamoxifen, loss of SIAH expression could lead to increased ACK1 levels, driving Tamoxifen resistance. Mechanistically, ACK1 can promote drug resistance in breast cancer by directly activating a second target, the serine threonine kinase AKT.12 Ligand bound HER2/ErbB-2 or EGFR activates ACK1 which in turn phosphorylates and activates AKT at tyrosine 176 that is not hindered by PI3K inhibition or knockdown of PI3K expression by RNA interference.12 Thus, a subset of drug-resistant breast cancer cells may be resensitized to undergo apoptosis by treatment with the ACK1 inhibitors. Overall, RTK/ACK1 signaling nexus explains alternate modes of AKT activation in those tumors that display amplification/activation of RTKs and non-receptor tyrosine kinases and therefore uncovers a new arsenal in form of ACK1 inhibitors.
ACK1 signaling in the pathogenesis of pancreatic and lung cancers
The rapid activation of ACK1 in a variety of pancreatic, ovarian and lung cancer derived cells suggests cancer cells are primed to utilize this pathway for survival.12, 30 Conforming to this regulatory paradigm, AKT is also frequently activated in pancreatic cancer which has been shown to be highly correlated to HER-2/neu overexpression.46 Moreover, many of the pancreatic cell lines and tumors expressing activated AKT were not mutated for the tumor suppressor, PTEN.47, 48 We reported that PanIN and pancreatic adenocarcinomas exhibit significantly higher levels of activated ACK1 (phosphorylated at Tyr284) and Tyr176-phosphorylated AKT.30 Conversely, pancreatic cancer cell lines treated with ACK1 inhibitors, displayed inhibition of cell growth and apoptosis, indicating that AKT activation in the absence of PTEN mutations may be driven by RTK/ACK1 signaling axis.2, 30
TNK2/ACK1 as an effecter of mutant CSF3R in chronic neutrophilic leukemia
A tyrosine kinase-specific small interfering RNAs screen for genetic drivers of leukemia cells with activating mutations in the gene encoding the receptor for colony-stimulating factor 3 (CSF3R) revealed that the mutations segregate within two distinct regions of CSF3R and lead to preferential downstream kinase signaling through either the ACK1 or JAK kinases. These cells also displayed differential sensitivity to kinase inhibitors.49 A patient with chronic neutrophilic leukemia (CNL) or atypical CML carrying a JAK-activating CSF3R mutation had marked clinical improvement after the administration of the JAK1/2 inhibitor ruxolitinib, however, the effectiveness of ACK1 inhibitors in this patient population remains to be seen.
ACK1 small molecule inhibitors
Realizing the significance of ACK1 in cancer pathogenesis, as a quick route to clinic, previously characterized kinase inhibitors were reassessed for their inhibitory activities against ACK1. In addition, new ACK1-specific kinase inhibitors were developed after screening small molecule compound libraries. Detailed information about discovery and characterization of multiple ACK1 inhibitors has recently been reviewed.50 Two inhibitors, Dasatinib (BMS-354825 or Sprycel) and AIM-100 have primarily been assessed for inhibition of ACK1 signaling in vitro and in vivo. Dasatinib not only inhibited ACK1 phosphorylation but also its substrate AR Tyr-phosphorylation and KDM3A Tyr-phosphorylation.45, 51 However, because of the multi-target activity of Dasatinib against many tyrosine kinases, it is difficult to interpret whether the cellular toxicity is specifically due to inhibition of ACK1; thus limiting its use in vivo to target ACK1 activated tumors. A critical step in the progression of localized indolent cancer to the more aggressive and metastatic stage is the ability to invade into neighboring tissues and enter the blood stream. Recent reports reveal that Bosutinib, a small molecule kinase inhibitor that targets Src also inhibits ACK1 dependent migration and invasion of the KRAS mutant non-small cell lung cancer.52
In contrast to Dasatinib and Bosutinib, AIM-100 has emerged to be specific and the best-studied ACK1 inhibitor.13, 30, 37, 45, 53 AIM-100 prevented AKT Tyr176-phosphorylation and AR Tyr267-phosphorylation and function, in breast and CRPC cells in which ACK1 is activated due to RTK activation or when ACK1 is auto-activated by somatic mutation (e.g. E346K-ACK1).13, 30, 37 In spite of its ability to inhibit cancer cell proliferation, due to its limited solubility in aqueous environment, AIM-100 has not progressed further as a prospective therapeutic agent.
Future Perspective
The cross talk of tyrosine kinases with the epigenetic machinery has opened a new chapter into how activated kinases are directly modulating gene expression programs within cells. Past research has focused on the cytosolic function of ACK1; however, with the recent findings that ACK1 interacts with a histone demethylase, it opens hitherto unknown aspect of ACK1 functionality in the nucleus. Importantly, the epigenetic alterations are reversible, thus targeting ACK1 may be critical to reverse epigenetic changes and thus pro-proliferative gene expression programs. Future studies into the epigenetic regulatory roles of ACK1 in the pathophysiology of cancers, the availability of mouse tumors models in an ACK1 deficient background and the development of selective and potent inhibitors will allow us to precisely target individual tumors to have better success with ACK1 inhibitors for treatment of a variety of cancers.
Acknowledgements
This work was supported in part by Department of Defense (W81XWH-12-1-0248) to K.M. and by the National Cancer Institute, NIH (1R01CA135328), Department of Defense (W81XWH-14-1-0002 and W81XWH-14-1-0003) and Career Development Award by Moffitt Lung Cancer SPORE to N.P.M.
Footnotes
Conflict of interest
The authors are named as inventors on a US patent application no. 8,557,516, titled ‘AKT Tyrosine 176 Phosphorylation as Cancer Biomarker’.
References
- 1.Mahajan K, Mahajan NP. Shepherding AKT and androgen receptor by Ack1 tyrosine kinase. Journal of cellular physiology. 2010;224:327–333. doi: 10.1002/jcp.22162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mahajan K, Mahajan NP. PI3K-independent AKT activation in cancers: a treasure trove for novel therapeutics. Journal of cellular physiology. 2012;227:3178–3184. doi: 10.1002/jcp.24065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Manser E, Leung T, Salihuddin H, Tan L, Lim L. A non-receptor tyrosine kinase that inhibits the GTPase activity of p21cdc42. Nature. 1993;363:364–367. doi: 10.1038/363364a0. [DOI] [PubMed] [Google Scholar]
- 4.Mahajan NP, Whang YE, Mohler JL, Earp HS. Activated tyrosine kinase Ack1 promotes prostate tumorigenesis: role of Ack1 in polyubiquitination of tumor suppressor Wwox. Cancer research. 2005;65:10514–10523. doi: 10.1158/0008-5472.CAN-05-1127. [DOI] [PubMed] [Google Scholar]
- 5.Mahajan NP, Liu Y, Majumder S, Warren MR, Parker CE, Mohler JL, et al. Activated Cdc42-associated kinase Ack1 promotes prostate cancer progression via androgen receptor tyrosine phosphorylation. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:8438–8443. doi: 10.1073/pnas.0700420104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Yokoyama N, Miller WT. Biochemical properties of the Cdc42-associated tyrosine kinase ACK1. Substrate specificity, authphosphorylation, and interaction with Hck. The Journal of biological chemistry. 2003;278:47713–47723. doi: 10.1074/jbc.M306716200. [DOI] [PubMed] [Google Scholar]
- 7.Gajiwala KS, Maegley K, Ferre R, He YA, Yu X. Ack1: activation and regulation by allostery. PLoS ONE. 2013;8:e53994. doi: 10.1371/journal.pone.0053994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Prieto-Echague V, Miller WT. Regulation of ack-family nonreceptor tyrosine kinases. J Signal Transduct. 2011;2011:742372. doi: 10.1155/2011/742372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prieto-Echague V, Gucwa A, Craddock BP, Brown DA, Miller WT. Cancer-associated mutations activate the nonreceptor tyrosine kinase Ack1. The Journal of biological chemistry. 2010;285:10605–10615. doi: 10.1074/jbc.M109.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lin Q, Wang J, Childress C, Yang W. The activation mechanism of ACK1 (activated Cdc42-associated tyrosine kinase 1) Biochem J. 2012;445:255–264. doi: 10.1042/BJ20111575. [DOI] [PubMed] [Google Scholar]
- 11.Prieto-Echague V, Gucwa A, Brown DA, Miller WT. Regulation of Ack1 localization and activity by the amino-terminal SAM domain. BMC Biochem. 2010;11:42. doi: 10.1186/1471-2091-11-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mahajan K, Coppola D, Challa S, Fang B, Chen YA, Zhu W, et al. Ack1 mediated AKT/PKB tyrosine 176 phosphorylation regulates its activation. PloS one. 2010;5:e9646. doi: 10.1371/journal.pone.0009646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mahajan K, Challa S, Coppola D, Lawrence H, Luo Y, Gevariya H, et al. Effect of Ack1 tyrosine kinase inhibitor on ligand-independent androgen receptor activity. The Prostate. 2010;70:1274–1285. doi: 10.1002/pros.21163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Shen F, Lin Q, Gu Y, Childress C, Yang W. Activated Cdc42-associated kinase 1 is a component of EGF receptor signaling complex and regulates EGF receptor degradation. Molecular biology of the cell. 2007;18:732–742. doi: 10.1091/mbc.E06-02-0142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chan W, Tian R, Lee YF, Sit ST, Lim L, Manser E. Down-regulation of active ACK1 is mediated by association with the E3 ubiquitin ligase Nedd4-2. The Journal of biological chemistry. 2009;284:8185–8194. doi: 10.1074/jbc.M806877200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones S, Cunningham DL, Rappoport JZ, Heath JK. The non-receptor tyrosine kinase Ack1 regulates the fate of activated EGFR by inducing trafficking to the p62/NBR1 pre-autophagosome. J Cell Sci. 2014;127:994–1006. doi: 10.1242/jcs.136895. [DOI] [PubMed] [Google Scholar]
- 17.Buchwald M, Pietschmann K, Brand P, Gunther A, Mahajan NP, Heinzel T, et al. SIAH ubiquitin ligases target the nonreceptor tyrosine kinase ACK1 for ubiquitinylation and proteasomal degradation. Oncogene. 2012 doi: 10.1038/onc.2012.515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kramer OH, Stauber RH, Bug G, Hartkamp J, Knauer SK. SIAH proteins: critical roles in leukemogenesis. Leukemia. 2013;27:792–802. doi: 10.1038/leu.2012.284. [DOI] [PubMed] [Google Scholar]
- 19.Urena JM, La Torre A, Martinez A, Lowenstein E, Franco N, Winsky-Sommerer R, et al. Expression, synaptic localization, and developmental regulation of Ack1/Pyk1, a cytoplasmic tyrosine kinase highly expressed in the developing and adult brain. The Journal of comparative neurology. 2005;490:119–132. doi: 10.1002/cne.20656. [DOI] [PubMed] [Google Scholar]
- 20.La Torre A, del Rio JA, Soriano E, Urena JM. Expression pattern of ACK1 tyrosine kinase during brain development in the mouse. Gene Expr Patterns. 2006;6:886–892. doi: 10.1016/j.modgep.2006.02.009. [DOI] [PubMed] [Google Scholar]
- 21.La Torre A, del Mar Masdeu M, Cotrufo T, Moubarak RS, del Rio JA, Comella JX, et al. A role for the tyrosine kinase ACK1 in neurotrophin signaling and neuronal extension and branching. Cell death & disease. 2013;4:e602. doi: 10.1038/cddis.2013.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Hitomi Y, Heinzen EL, Donatello S, Dahl HH, Damiano JA, McMahon JM, et al. Mutations in TNK2 in severe autosomal recessive infantile onset epilepsy. Annals of neurology. 2013;74:496–501. doi: 10.1002/ana.23934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Galisteo ML, Yang Y, Urena J, Schlessinger J. Activation of the nonreceptor protein tyrosine kinase Ack by multiple extracellular stimuli. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:9796–9801. doi: 10.1073/pnas.0603714103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Horst EH, Degenhardt YY, Strelow A, Slavin A, Chinn L, Orf J, et al. Metastatic properties and genomic amplification of the tyrosine kinase gene ACK1. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:15901–15906. doi: 10.1073/pnas.0508014102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mahajan K, Mahajan NP. ACK1/TNK2 tyrosine kinase:An emerging target for cancer therapeutics. AACR Education Book. 2014;2014:6. [Google Scholar]
- 26.Shinmura K, Kiyose S, Nagura K, Igarashi H, Inoue Y, Nakamura S, et al. TNK2 gene amplification is a novel predictor of a poor prognosis in patients with gastric cancer. Journal of surgical oncology. 2013 doi: 10.1002/jso.23482. [DOI] [PubMed] [Google Scholar]
- 27.Manning BD, Cantley LC. AKT/PKB signaling: navigating downstream. Cell. 2007;129:1261–1274. doi: 10.1016/j.cell.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Greer EL, Brunet A. FOXO transcription factors at the interface between longevity and tumor suppression. Oncogene. 2005;24:7410–7425. doi: 10.1038/sj.onc.1209086. [DOI] [PubMed] [Google Scholar]
- 29.Huang H, Tindall DJ. Dynamic FoxO transcription factors. Journal of cell science. 2007;120:2479–2487. doi: 10.1242/jcs.001222. [DOI] [PubMed] [Google Scholar]
- 30.Mahajan K, Coppola D, Chen YA, Zhu W, Lawrence HR, Lawrence NJ, et al. Ack1 tyrosine kinase activation correlates with pancreatic cancer progression. The American journal of pathology. 2012;180:1386–1393. doi: 10.1016/j.ajpath.2011.12.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Schoenherr JA, Drennan JM, Martinez JS, Chikka MR, Hall MC, Chang HC, et al. Drosophila activated Cdc42 kinase has an anti-apoptotic function. PLoS genetics. 2012;8:e1002725. doi: 10.1371/journal.pgen.1002725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Burnstein KL. Regulation of androgen receptor levels: implications for prostate cancer progression and therapy. Journal of cellular biochemistry. 2005;95:657–669. doi: 10.1002/jcb.20460. [DOI] [PubMed] [Google Scholar]
- 33.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nature medicine. 2004;10:33–39. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 34.Lupien M, Brown M. Cistromics of hormone-dependent cancer. Endocrine-related cancer. 2009;16:381–389. doi: 10.1677/ERC-09-0038. [DOI] [PubMed] [Google Scholar]
- 35.Feldman BJ, Feldman D. The development of androgen-independent prostate cancer. Nature reviews. 2001;1:34–45. doi: 10.1038/35094009. [DOI] [PubMed] [Google Scholar]
- 36.Edwards J, Bartlett JM. The androgen receptor and signal-transduction pathways in hormone-refractory prostate cancer. Part 1: Modifications to the androgen receptor. BJU international. 2005;95:1320–1326. doi: 10.1111/j.1464-410X.2005.05526.x. [DOI] [PubMed] [Google Scholar]
- 37.Mahajan K, Coppola D, Rawal B, Chen YA, Lawrence HR, Engelman RW, et al. Ack1-mediated androgen receptor phosphorylation modulates radiation resistance in castration-resistant prostate cancer. The Journal of biological chemistry. 2012;287:22112–22122. doi: 10.1074/jbc.M112.357384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Blanco-Aparicio C, Renner O, Leal JF, Carnero A. PTEN, more than the AKT pathway. Carcinogenesis. 2007;28:1379–1386. doi: 10.1093/carcin/bgm052. [DOI] [PubMed] [Google Scholar]
- 39.Liang J, Shang Y. Estrogen and cancer. Annual review of physiology. 2013;75:225–240. doi: 10.1146/annurev-physiol-030212-183708. [DOI] [PubMed] [Google Scholar]
- 40.Green KA, Carroll JS. Oestrogen-receptor-mediated transcription and the influence of co-factors and chromatin state. Nat Rev Cancer. 2007;7:713–722. doi: 10.1038/nrc2211. [DOI] [PubMed] [Google Scholar]
- 41.Fisher B, Costantino JP, Wickerham DL, Redmond CK, Kavanah M, Cronin WM, et al. Tamoxifen for prevention of breast cancer: report of the National Surgical Adjuvant Breast and Bowel Project P-1 Study. J Natl Cancer Inst. 1998;90:1371–1388. doi: 10.1093/jnci/90.18.1371. [DOI] [PubMed] [Google Scholar]
- 42.Fisher B, Costantino JP, Wickerham DL, Cecchini RS, Cronin WM, Robidoux A, et al. Tamoxifen for the prevention of breast cancer: current status of the National Surgical Adjuvant Breast and Bowel Project P-1 study. J Natl Cancer Inst. 2005;97:1652–1662. doi: 10.1093/jnci/dji372. [DOI] [PubMed] [Google Scholar]
- 43.Howell A, DeFriend D, Robertson J, Blamey R, Walton P. Response to a specific antioestrogen (ICI 182780) in tamoxifen-resistant breast cancer. Lancet. 1995;345:29–30. doi: 10.1016/s0140-6736(95)91156-1. [DOI] [PubMed] [Google Scholar]
- 44.Clarke R, Leonessa F, Welch JN, Skaar TC. Cellular and molecular pharmacology of antiestrogen action and resistance. Pharmacological reviews. 2001;53:25–71. [PubMed] [Google Scholar]
- 45.Mahajan K, Lawrence HR, Lawrence NJ, Mahajan NP. ACK1 Tyrosine Kinase Interacts with Histone Demethylase KDM3A to Regulate the Mammary Tumor Oncogene HOXA1. The Journal of biological chemistry. 2014 doi: 10.1074/jbc.M114.584425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Schlieman MG, Fahy BN, Ramsamooj R, Beckett L, Bold RJ. Incidence, mechanism and prognostic value of activated AKT in pancreas cancer. British journal of cancer. 2003;89:2110–2115. doi: 10.1038/sj.bjc.6601396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Matsumoto J, Kaneda M, Tada M, Hamada J, Okushiba S, Kondo S, et al. Differential mechanisms of constitutive Akt/PKB activation and its influence on gene expression in pancreatic cancer cells. Jpn J Cancer Res. 2002;93:1317–1326. doi: 10.1111/j.1349-7006.2002.tb01240.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sakurada A, Suzuki A, Sato M, Yamakawa H, Orikasa K, Uyeno S, et al. Infrequent genetic alterations of the PTEN/MMAC1 gene in Japanese patients with primary cancers of the breast, lung, pancreas, kidney, and ovary. Jpn J Cancer Res. 1997;88:1025–1028. doi: 10.1111/j.1349-7006.1997.tb00324.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Maxson JE, Gotlib J, Pollyea DA, Fleischman AG, Agarwal A, Eide CA, et al. Oncogenic CSF3R mutations in chronic neutrophilic leukemia and atypical CML. N Engl J Med. 2013;368:1781–1790. doi: 10.1056/NEJMoa1214514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mahajan K, Mahajan NP. ACK1 tyrosine kinase: targeted inhibition to block cancer cell proliferation. Cancer letters. 2013;338:185–192. doi: 10.1016/j.canlet.2013.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Liu Y, Karaca M, Zhang Z, Gioeli D, Earp HS, Whang YE. Dasatinib inhibits site-specific tyrosine phosphorylation of androgen receptor by Ack1 and Src kinases. Oncogene. 2010;29:3208–3216. doi: 10.1038/onc.2010.103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tan DS, Haaland B, Gan JM, Tham SC, Sinha I, Tan EH, et al. Bosutinib inhibits migration and invasion via ACK1 in KRAS mutant non-small cell lung cancer. Mol Cancer. 2014;13:13. doi: 10.1186/1476-4598-13-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.DiMauro EF, Newcomb J, Nunes JJ, Bemis JE, Boucher C, Buchanan JL, et al. Discovery of 4-amino-5,6-biaryl-furo[2,3-d]pyrimidines as inhibitors of Lck: development of an expedient and divergent synthetic route and preliminary SAR. Bioorganic & medicinal chemistry letters. 2007;17:2305–2309. doi: 10.1016/j.bmcl.2007.01.057. [DOI] [PubMed] [Google Scholar]