Abstract
Purpose
Malignancy relapse remains a major obstacle for successful allogeneic hematopoietic cell transplantation (HCT). Chronic graft-versus-host disease (cGVHD) is associated with fewer relapses. However, when studying effects of cGVHD on relapse it is difficult to separate from acute GVHD effects as most cases of cGVHD occur within the first year post-transplant at the time when acute GVHD is still active.
Experimental design
The current study based on CIBMTR registry data investigated cGVHD and its association with the incidence of late relapse and survival in 7489 patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML) and myelodysplastic syndromes (MDS) who were leukemia-free at12 months after myeloablative allogeneic HCT.
Results
Forty-seven percent of the study population was diagnosed with cGVHD at 12 months after transplant. The protective effect of cGVHD on relapse was present only in patients with CML (RR: 0.47, 95% CI: 0.37-0.59, P <0.0001). cGVHD was significantly associated with higher risk of treatment related mortality, (RR: 2.43, 95% CI: 2.09-2.82, P <0.0001) and inferior overall survival (RR: 1.56, 95% CI: 1.41-1.73, P <0.0001) for all diseases. In patients with CML all organ sites and presentation types of cGVHD were equally associated with lower risk of late relapse.
Conclusions
These results indicate that clinically relevant anti-leukemia effects of cGVHD on late relapses are present only in CML but not in AML, ALL or MDS. Chronic GVHD in patients who are one year survivors after myeloablative allogeneic HCT is primarily associated with higher TRM and inferior survival.
Keywords: allogeneic hematopoietic cell transplantation, chronic graft-versus-host disease, relapse, leukemia
Introduction
Allogeneic hematopoietic cell transplantation (HCT) following myeloablative conditioning (MAC) regimen is a standard and curative treatment option for hematologic malignancies (1). The anti-leukemic activity of MAC and allogeneic HCT relies not only on the effects of high dose chemotherapy or irradiation given during the conditioning regimen, but also on the immune-mediated graft-versus leukemia (GVL) effect (2-4). The immune reactivity between donor T cells that is responsible for the GVL effect and the recipient is also associated with the major complications of allogeneic HCT, namely acute (aGVHD) and chronic graft-versus-host disease (cGVHD).
Chronic GVHD is a serious complication and is an important cause of morbidity and non-relapse mortality (NRM) in patients who survive 12 months post-transplantation (5). Chronic GVHD is associated with a lower risk of relapse (2, 4, 6). Despite the protective effect of cGVHD, adult patients with cGVHD experience leukemia late relapse (7). The reduction in relapse risk may be secondary to the immune-mediated graft versus tumor (GVT) effect associated with GVHD. This effect is most prominent during the first year after allogeneic HCT when the peak incidence of relapse occurs (7, 8). However, onsets of acute and chronic GVHD chronologically overlap and it is difficult to decipher their relative contributions to anti-leukemia effects because at the time both acute and chronic GVHD are likely active (9). Given this close time overlap between aGVHD and cGVHD, the current study sought to investigate antileukemic effects of cGVHD isolated from acute GVHD by assessing relapses and survival in allogeneic HCT recipients who were alive and relapse-free at one year following MAC transplant. In addition, we evaluated weather any specific cGVHD clinical characteristics or organ manifestations are more predominantly associated with the GVL effects of cGVHD.
Methods
Data source
The Center for International Blood and Marrow Transplant Research (CIBMTR) is a research affiliation of the International Bone Marrow Transplant Registry (IBMTR), Autologous Blood and Marrow Transplant Registry (ABMTR), and the National Marrow Donor Program (NMDP), that was established in 2004 and comprises a voluntary working group of more than 450 transplantation centers worldwide that contribute detailed data on consecutive allogeneic and autologous hematopoietic cell transplantation to a Statistical Center at the Medical College of Wisconsin in Milwaukee and the NMDP Coordinating Center in Minneapolis. Participating centers are required to report all transplants consecutively; compliance is monitored by on-site audits. Patients are followed longitudinally. Computerized checks for discrepancies, physicians’ review of submitted data, and on-site audits of participating centers ensure data quality. Observational studies conducted by the CIBMTR are performed in compliance with all applicable federal regulations pertaining to the protection of human research participants. Protected Health Information used in the performance of such research is collected and maintained in CIBMTR’s capacity as a Public Health Authority under the HIPAA Privacy Rule.
Patient selection
Between 1995-2004, 19,861 patients with acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), chronic myeloid leukemia (CML) and myelodysplastic syndromes (MDS) received allogeneic HCT. Patients who received reduced intensity conditioning regimen (n=2,620), died or relapsed (n=9,162) within the first year after allogeneic HCT were excluded from the analysis. Patients who received second allergenic HCT (n=146) within the first post-transplant year or syngeneic HCT were excluded as were patients who had relevant data missing from the CIBMTR database (n=444). The final study population consisted of a total of 7,489 patients that received MAC regimen who were alive and free of disease at one year after transplantation.
Study definitions and endpoints
Patients were considered to have early disease if they were in first remission (acute leukemia) or first chronic phase (CML) or MDS with refractory anemia or refractory anemia with ringed sideroblasts (RA, RARS); intermediate disease: second or later complete remission (acute leukemia), second or later chronic phase/accelerated phase (CML); advanced disease: relapse or primary induction failure (acute leukemia) or blast crisis (CML) or MDS with refractory anemia with excess blasts or excess blasts in transformation (RAEB, RAEB-t). The NMDP classification of HLA matching status that allows adequate adjustment for donor-recipient HLA compatibility while accounting for best available resolution of typing was used to categorize HLA matching status as well-matched, partially-matched, or mismatched. (10). Acute GVHD was grouped as none vs. grade I vs. grade II-IV and was graded according to IBMTR criteria based on the pattern of severity of abnormalities in skin, gastrointestinal tract, and liver (11) . cGVHD was diagnosed according to standard CIBMTR criteria, which included all patients with clinical criteria of cGVHD with or without positive histology, irrespective of time of onset of symptoms (9, 12). The NIH consensus criteria were not available at the time of data collection for this analysis (13).
The primary endpoint was leukemia relapse and was defined as time to onset of disease recurrence (by hematologic or cytogenetic criteria) with treatment-related mortality (TRM) as a competing risk. Secondary endpoints were overall survival (OS), TRM, and disease-free survival (DFS). Outcomes were assessed up to 5 years post-HCT. Treatment-related mortality was defined as death in continuous remission, and DFS was defined as either death or relapse with the time calculated from date of transplant. OS was calculated from date of transplant. Death from any cause was treated as an event and surviving patients were censored at the date of last contact.
Statistical Analysis
Variables related to patient, disease, and transplant characteristics were summarized using descriptive statistics. Cumulative incidence for relapse was calculated treating TRM as a competing risk (14). Patient-related, disease-related, and treatment-related variables were included in the multivariate analyses using a stepwise forward selection technique and P ≤ 0.01 was the criterion for inclusion in final models. Patient- and transplant-related variables tested in the models included recipient age, sex, donor type, graft type (bone marrow or peripheral blood), donor-recipient gender mismatch, donor parity, donor-recipient cytomegalovirus (CMV) serology, use of TBI, use of ATG or alemtuzumab, GVHD prophylaxis, presence and grade of prior aGVHD, and year of transplantation. Disease-related variables were diagnosis and disease status pre-transplant. Chronic GVHD-specific variables included platelet count at diagnosis (<100 ×109/L and ≥100 ×109/L), serum bilirubin at diagnosis (<1 mg/dl, 1-2 mg/dl and >2 mg/dl), type of onset of cGVHD (progressive, interrupted, de novo), Karnofsky Performance Scale/Lansky score (KPS/L) at diagnosis, severity of cGVHD at one year post-transplant (mild vs. moderate vs. severe), and organ involvement of cGVHD at one-year post-transplant. In the multivariate analysis, all the endpoints were analyzed using the Cox proportional hazards model and all variables were tested for affirmation of the proportional hazards assumption. Variables that did not satisfy the proportional hazards assumption were adjusted for by stratification. A stepwise model building procedure was used to develop models for each outcome with a threshold of 0.05 for both entry and retention in the model. Interaction between the main variable and adjusted variables were tested, and no significant interactions were identified at p<0.01.
Results
Patients
The study included 7489 patients who were alive and disease-free one year after HCT. Patient and donor characteristics are summarized in Table 1. The median age at HCT was 32 years for AML, 17 years for ALL, 36 years for CML, and 37 years for MDS. At one year post-HCT 44%, 41%, 54%, and 54% of the AML, ALL, CML, MDS patients, respectively, had developed cGVHD. Chronic GVHD characteristics are summarized in Table 1.
Table 1. Patient, donor and GVHD characteristics.
| Patient and donor characteristics | ||||
|---|---|---|---|---|
| AML | ALL | CML | MDS | |
| Characteristics | N (%) | N (%) | N (%) | N (%) |
| Number of Patients (n=7489) | 2541 | 1798 | 2498 | 652 |
| Median recipient age at HCT, y (range) | 32 (<1-74) | 17 (<1-59) | 36 (1-66) | 37 (<1-65) |
| Age of recipient, years | ||||
| < 2 | 58 (2 ) | 58 (3 ) | 1 (<1) | 30 (5 ) |
| 2-17 | 541 (21) | 886 (49) | 233 (9 ) | 122 (19) |
| 18-29 | 561 (22) | 431 (24) | 551 (22) | 96 (15) |
| 30-39 | 512 (20) | 230 (13) | 768 (31) | 121 (19) |
| 40-49 | 565 (22) | 136 (8 ) | 673 (27) | 144 (22) |
| 50-59 | 281 (11) | 57 (3 ) | 259 (10) | 129 (20) |
| 60+ | 23 (<1) | 0 | 13 (<1) | 10 (2 ) |
| Gender | ||||
| Male | 1346 (53) | 1121 (62) | 1469 (59) | 359 (55) |
| Female | 1195(47) | 677(38) | 1029(41) | 293(45) |
| Disease status at HCT | ||||
| Early | 1518 (60) | 804 (45) | 2110 (84) | 229 (35) |
| Intermediate | 612 (24) | 862 (48) | 349 (14) | 6 ( 1) |
| Advanced | 411 (16) | 132 ( 7) | 39 ( 2) | 417 (64) |
| Graft source | ||||
| Bone Marrow | 1611 (63) | 1383 (77) | 1928 (77) | 434 (67) |
| Peripheral Blood | 930 (37) | 415 (23) | 570 (23) | 218 (33) |
| HLA match | ||||
| HLA-identical sibling | 1503 (59) | 810 (45) | 1284 (51) | 264 (40) |
| Well-matched unrelated | 445 (18) | 352 (20) | 434 (17) | 185 (28) |
| Partially matched unrelated | 368 (14) | 385 (21) | 486 (19) | 132 (20) |
| Mismatched unrelated | 225 ( 9) | 251 (14) | 294 (12) | 71 (11) |
| Donor-recipient gender match | ||||
| Male -> Male | 796 (31) | 675 (38) | 947 (38) | 246 (38) |
| Male -> Female | 640 (25) | 375 (21) | 551 (22) | 155 (24) |
| Female -> Male | 550 (22) | 446 (25) | 522 (21) | 113 (17) |
| Female -> Female | 555 (22) | 302 (17) | 478 (19) | 138 (21) |
| Donor-recipient CMV status | ||||
| Negative -> Negative | 727 (29) | 673 (37) | 791 (32) | 219 (34) |
| Negative -> Positive | 526 (21) | 316 (18) | 410 (16) | 139 (21) |
| Positive -> Negative | 313 (12) | 267 (15) | 328 (13) | 81 (12) |
| Positive -> Positive | 872 (34) | 483 (27) | 869 (35) | 189 (29) |
| Unknown | 103 ( 4) | 59 ( 3) | 100 ( 4) | 24 ( 4) |
| Median donor age, years (range) | 33 (<1-72) | 30 (<1-73) | 36 (<1-73) | 36 (<1-71) |
| HLA-identical sibling donor age, years, median (range) |
32 (<1-72) | 18 (<1-73) | 36 (<1-73) | 36 (<1-71) |
| HLA-identical sibling donor age, years | ||||
| < 10 | 135 (9 ) | 187 (23) | 32 (2 ) | 28 (11) |
| 10-17 | 181 (12) | 206 (25) | 86 (7 ) | 20 (8 ) |
| 18-29 | 360 (24) | 203 (25) | 269 (21) | 53 (20) |
| 30-39 | 330 (22) | 108 (13) | 388 (30) | 52 (20) |
| 40-49 | 297 (20) | 66 (8 ) | 326 (25) | 65 (25) |
| 50-59 | 140 (9 ) | 29 (4 ) | 124 (10) | 33 (13) |
| 60+ | 41 (3 ) | 4 (<1) | 31 (2 ) | 8 (3 ) |
| Missing | 19 (1 ) | 7 (<1) | 28 (2 ) | 5 (2 ) |
| Unrelated donor age, years, median (range) | 35 (19-60) | 35 (19-59) | 35 (18-65) | 35 (19-60) |
| Unrelated donor age, years | ||||
| 18-29 | 315 (30) | 284 (29) | 365 (30) | 104 (27) |
| 30-39 | 404 (39) | 367 (37) | 434 (36) | 151 (39) |
| 40-49 | 233 (22) | 234 (24) | 287 (24) | 97 (25) |
| 50-59 | 52 (5 ) | 57 (6 ) | 78 (6 ) | 20 (5 ) |
| 60+ | 2 (<1) | 0 | 2 (<1) | 1 (<1) |
| Missing | 32 (3 ) | 46 (5 ) | 48 (4 ) | 15 (4 ) |
| Donor parity prior to transplant | ||||
| Male donor | 1436 (57) | 1050 (58) | 1498 (60) | 401 (62) |
| Female, no pregnancy | 420 (17) | 380 (21) | 324 (13) | 78 (12) |
| 1 or more pregnancies | 473 (19) | 266 (15) | 483 (19) | 128 (20) |
| Missing | 212 ( 8) | 102 ( 6) | 193 ( 8) | 45 ( 7) |
| GVHD prophylaxis | ||||
| Ex vivo T-cell depletion | 246 (10) | 218 (12) | 204 ( 8) | 81 (12) |
| Cyclosporine ± methotrexate ± other | 1900 (75) | 1362 (76) | 2008 (80) | 472 (72) |
| Tacrolimus ± methotrexate ± other | 395 (16) | 218 (12) | 286 (11) | 99 (15) |
| ATG and/or alemtuzumab used | ||||
| None | 2131 (84) | 1399 (78) | 2088 (84) | 519 (80) |
| Yes | 312 (12) | 332 (18) | 318 (13) | 113 (17) |
| Missing | 98 ( 4) | 67 ( 4) | 92 ( 4) | 20 ( 3) |
| Total body irradiation use in the conditioning regimen | ||||
| No | 1314 (52) | 228 (13) | 1268 (51) | 379 (58) |
| Yes | 1227 (48) | 1570 (87) | 1230 (49) | 273 (42) |
| Year of transplant | ||||
| 1995-1999 | 1332 (52) | 1011 (56) | 1724 (69) | 351 (54) |
| 2000-2004 | 1209 (48) | 787 (44) | 774 (31) | 301 (46) |
| Prior aGVHD grade | ||||
| 0-I | 1537 (60) | 864 (48) | 1334 (53) | 317 (49) |
| II-IV | 1004 (40) | 934 (52) | 1164 (47) | 335 (51) |
| Median follow-up of survivors, months (range) | 80 (12 -179) | 80 (12 - 175) | 93 (12 -172) | 80 (12 - 168) |
| Chronic GVHD characteristics | ||||
|---|---|---|---|---|
| AML | ALL | CML | MDS | |
| cGVHD present at one-year after HCT | 1117/2541 (44%) |
742/1798 (41%) |
1344/2498 (54%) |
350/652 (54%) |
| Time from HCT to cGVHD months, (range) | 5 (1-11) | 5 (1-11) | 5 (1-11) | 5 (1-11) |
| Severity of cGVHD | ||||
| Mild | 485 (43) | 265 (36) | 425 (32) | 136 (39) |
| Moderate | 243 (22) | 159 (21) | 307 (23) | 67 (19) |
| Severe | 70 ( 6) | 48 ( 6) | 77 ( 6) | 14 ( 4) |
| Missing | 319 (29) | 270 (36) | 535 (40) | 133 (38) |
| Onset of cGVHD | ||||
| Progressive | 366 (33) | 306 (41) | 509 (38) | 130 (37) |
| Interrupted | 277 (25) | 204 (27) | 339 (25) | 96 (27) |
| De novo | 392 (35) | 179 (24) | 422 (31) | 91 (26) |
| Missing | 82 ( 7) | 53 ( 7) | 74 ( 6) | 33 ( 9) |
| Organ involvement | ||||
| Skin | 597 (53) | 445 (60) | 733 (55) | 189 (54) |
| Eyes | 331 (30) | 170 (23) | 401 (30) | 105 (30) |
| Mouth | 560 (50) | 331 (45) | 685 (51) | 173 (49) |
| Lung | 93 ( 8) | 53 ( 7) | 109 ( 8) | 34 (10) |
| GI | 217 (19) | 177 (24) | 273 (20) | 89 (25) |
| GU | 33 ( 3) | 12 ( 2) | 29 ( 2) | 13 ( 4) |
| Liver | 425 (38) | 213 (29) | 517 (38) | 122 (35) |
| Musculoskeletal | 59 ( 5) | 54 ( 7) | 110 ( 8) | 21 ( 6) |
| Hematologic | 109 (10) | 95 (13) | 138 (10) | 45 (13) |
| Number of organs involved in cGVHD | ||||
| 1 | 259 (23) | 195 (26) | 304 (23) | 82 (23) |
| 2 | 258 (23) | 177 (24) | 342 (25) | 74 (21) |
| 3 | 212 (19) | 127 (17) | 251 (19) | 59 (17) |
| 4 | 138 (12) | 76 (10) | 149 (11) | 49 (14) |
| 5 | 35 ( 3) | 21 ( 3) | 46 ( 3) | 15 ( 4) |
| 6 | 5 (<1) | 2 (<1) | 9 ( 1) | 2 ( 1) |
| Missing | 210 (19) | 144 (19) | 243 (18) | 69 (20) |
| KPS at diagnosis of cGVHD, n (%) | ||||
| <80 | 215 (19) | 154 (21) | 367 (27) | 90 (26) |
| 80-100 | 704 (63) | 453 (61) | 789 (59) | 173 (49) |
| Missing | 198 (18) | 135 (18) | 188 (14) | 87 (25) |
| Platelet count at diagnosis cGVHD ×109/L, n (%) | ||||
| <100 | 294 (26) | 214 (29) | 450 (33) | 95 (27) |
| ≥ 100 | 639 (57) | 409 (55) | 682 (51) | 183 (52) |
| Missing | 184 (16) | 119 (16) | 212 (16) | 72 (21) |
| Serum bilirubin at diagnosis of cGVHD mg/dL, n (%) | ||||
| < 1 | 665 (60) | 445 (60) | 638 (47) | 191 (55) |
| 1-2 | 152 (14) | 86 (12) | 311 (23) | 66 (19) |
| > 2 | 159 (14) | 117 (16) | 220 (16) | 37 (11) |
| Missing | 141 (13) | 94 (13) | 175 (13) | 56 (16) |
Relapse
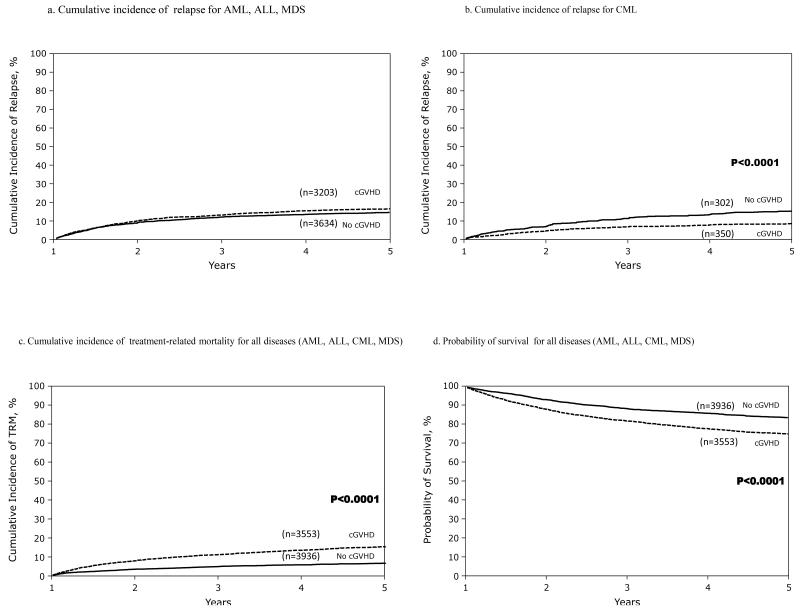
The cumulative incidence of relapse after the first post HCT year for each disease is shown in Table 2. In multivariate analysis, a protective effect of cGVHD was seen only in patients with CML (RR: 0.47, 95% CI: 0.37-0.59, P <0.0001) (Figure 1 a, b). Other factors protective against late relapse were aGVHD in the first 29 days after HCT, GVHD prophylaxis other than T-cell depletion, well-matched or partially-matched unrelated donor (URD) (as compared to HLA-identical sibling), and early disease status at transplant (Table 3, supplemental table 1).
Table 2. Incidence of late relapse and death.
| Cumulative incidence of late relapse (up to 5 years post HCT) according to diagnosis among patients who were disease free at one year after HCT | |||||
|---|---|---|---|---|---|
| AML | ALL | CML | MDS | Total | |
| No relapse | 2170 (85.4%) | 1472 (81.87%) | 2176 (87.11%) | 582 (89.26%) | 6400 (85%) |
| Relapse | 371 (14.6%) | 326 (18.13%) | 322 (12.89%) | 70 (10.74%) | 1089 (15%) |
| Total | 2541 | 1798 | 2498 | 652 | 7489 |
| Cumulative incidence of late relapse according to diagnosis among patients who were disease free and had no cGVHD at one year after HCT | ||||
|---|---|---|---|---|
| Time after HCT |
Probability (95% CI) at different time points after HCT |
|||
| AML n=1424 |
ALL n=1056 |
CML n=1154 |
MDS n=302 |
|
|
|
||||
| 2 years | 9 (7-10) | 12 (10-14) | 8 (6-10) | 5 (3-8) |
| 3 years | 11 (10-13) | 15 (13-18) | 12 (11-15) | 8 (5-12) |
| 5 years | 15 (13-17) | 18 (16-21) | 17 (14-19) | 10 (7-14) |
| Cumulative incidence of late relapse according to diagnosis among patients who were disease free and had cGVHD at one year after HCT | ||||
|---|---|---|---|---|
| Time after HCT |
Probability (95% CI) at different time points after HCT |
|||
| AML n=1117 |
ALL n=742 |
CML n=1344 |
MDS n=350 |
|
|
|
||||
| 2 years | 7 (6-9) | 13 (11-15) | 4 (3-5) | 5 (3-8) |
| 3 years | 11 (9-13) | 15 (13-18) | 6 (5-8) | 7 (5-11) |
| 5 years | 13 (11-15) | 18 (16-21) | 8 (6-9) | 10 (7-13) |
| Cumulative incidence of death (up to 5 years post HCT) according to diagnosis among patients who were disease free at one year after HCT | |||||
|---|---|---|---|---|---|
| AML | ALL | CML | MDS | Total | |
| Alive | 1922 (75.6%) | 1354 (75.31%) | 2018 (80.78%) | 474 (72.7%) | 5768 (77%) |
| Dead | 619 (24.3%) | 444 (24.6%) | 480 (19.2%) | 178 (27.3%) | 1721 (23%) |
| Total | 2541 | 1798 | 2498 | 652 | 7489 |
Figure 1.
a-b. Cumulative incidence of late relapse according to prior history of chronic GVHD among patients who are disease free at 1 year after HCT
c-d.Cumulative incidence of treatment-related mortality and overall survival according to prior history of chronic GVHD among patients who are disease free at 1 year after HCT
Table 3. Multivariate analysis of late relapses up to 5 years after HCT.
| N | RR | 95% CI | p-value | ||
|---|---|---|---|---|---|
| AML Patients | |||||
| No cGVHD | 1424 | 1.00 | |||
| cGVHD | 1117 | 0.93 | 0.75 | 1.14 | 0.4809 |
| ALL patients | |||||
| No cGVHD | 1056 | 1.00 | |||
| cGVHD | 742 | 1.09 | 0.87 | 1.37 | 0.4423 |
| CML patients | |||||
| No cGVHD | 1154 | 1.00 | |||
| cGVHD | 1344 | 0.47 | 0.37 | 0.59 | <0.0001 |
| MDS patients | |||||
| No cGVHD | 302 | 1.00 | |||
| cGVHD | 350 | 1.12 | 0.70 | 1.79 | 0.6391 |
| Acute GVHD | <0.0001 | ||||
| none | 4051 | 1.00 | |||
| aGVHD in first 29 days after HCT | 2446 | 0.74 | 0.63 | 0.85 | <0.0001 |
| aGVHD after day 29 after HCT | 986 | 1.15 | 0.96 | 1.37 | 0.1223 |
| Disease Status at transplant | <.0001 | ||||
| Early | 4661 | 1.00 | |||
| Intermediate | 1823 | 1.57 | 1.35 | 1.83 | <0.0001 |
| Advanced | 999 | 2.57 | 2.13 | 3.10 | <0.0001 |
| HLA Status | 0.0200 | ||||
| Sibling Donor | 1861 | 1.00 | |||
| Well Matched Unrelated | 1412 | 0.83 | 0.69 | 0.99 | 0.0370 |
| Partially matched Unrelated | 1369 | 0.78 | 0.65 | 0.93 | 0.0049 |
| Poorly matched Unrelated | 841 | 0.85 | 0.69 | 1.04 | 0.1143 |
| GVHD prophylaxis | 0.0043 | ||||
| T-cell depletion | 748 | 1.00 | |||
| Cyclosporine±methotrexate±other | 5738 | 0.75 | 0.62 | 0.91 | 0.0032 |
| Tacrolimus±methotrexate±other | 997 | 0.68 | 0.53 | 0.88 | 0.0026 |
Factors tested, but not listed in table: Recipient age, sex, graft type, donor-recipient gender mismatch, donor parity, donor-recipient CMV serology, use of TBI, use of ATG, aGVHD grade, year of transplant, platelet count at cGVHD diagnosis, serum bilirubin at cGVHD diagnosis, type of cGVHD onset, KPS/L at cGVHD diagnosis, severity of cGVHD at 1 year post-transplant, organ involvement of cGVHD at 1 year post-transplant
Transplant-related mortality and overall survival
Multivariate analysis of risk factors associated with TRM and OS are outlined in Tables 4 and 5, respectively. The presence of cGVHD was associated with a higher risk of TRM for all diseases (RR: 2.43, 95% CI: 2.09-2.82, P <0.0001) (Figure 1c, Table 4). Other factors associated with a higher risk of TRM included aGVHD, advanced disease status at HCT, well-matched, partially matched, or poorly matched URD (as compared to HLA-identical sibling donor), use of total body irradiation (TBI) in conditioning, female donor to male recipient (versus male donor for male recipient), and use of peripheral blood stem cells (PBSC) (versus bone marrow) as the graft source (supplemental table 1).
Table 4.
Multivariate analyses of risk factors for late transplant-related mortality
| number | RR | 95% Conf Interval | p | ||
|---|---|---|---|---|---|
| Prior chronic GVHD | |||||
| No prior diagnosis of cGVHD | 3936 | 1.00 | |||
| Prior cGVHD | 3547 | 2.43 | 2.09 | 2.82 | <0.0001 |
| Acute GVHD History | <0.0001 | ||||
| None | 4051 | 1.00 | |||
| aGVHD in first 29 days post HCT | 2446 | 1.40 | 1.21 | 1.63 | <0.0001 |
| aGVHD after day 29 post HCT | 986 | 1.58 | 1.32 | 1.91 | <0.0001 |
| Disease Status at HCT | <0.0001 | ||||
| Early | 4661 | 1.00 | |||
| Intermediate | 1823 | 0.93 | 0.78 | 1.10 | 0.3908 |
| Late | 999 | 1.43 | 1.20 | 1.69 | <0.0001 |
| HLA match | <0.0001 | ||||
| Sibling Donor | 1861 | 1.00 | |||
| Well Matched Unrelated | 1412 | 1.39 | 1.15 | 1.67 | 0.0006 |
| Partially matched Unrelated | 1369 | 1.36 | 1.13 | 1.63 | 0.0013 |
| Poorly matched Unrelated | 841 | 1.56 | 1.25 | 1.93 | <0.0001 |
| TBI in the conditioning regimen | |||||
| no | 3186 | 1.00 | |||
| yes | 4292 | 1.47 | 1.27 | 1.70 | <0.0001 |
| Donor-recipient gender match | <0.0001 | ||||
| male -> male | 2662 | 1.00 | |||
| male -> female | 1720 | 0.63 | 0.77 | 1.11 | 0.4146 |
| female -> male | 1629 | 1.55 | 1.32 | 1.83 | <0.0001 |
| female -> female | 1472 | 1.11 | 0.93 | 1.33 | 0.2623 |
| Graft source | |||||
| Bone marrow | 5354 | 1.00 | |||
| Mobilized blood stem cells | 2129 | 1.48 | 1.26 | 1.73 | <0.0001 |
Factors tested, but not listed in table: Recipient age, sex, donor parity, donor-recipient CMV serology, use of ATG, GVHD prophylaxis, aGVHD grade, year of transplant, disease, platelet count at cGVHD diagnosis, serum bilirubin at cGVHD diagnosis, type of cGVHD onset, KPS/L at cGVHD diagnosis, severity of cGVHD at 1 year post-transplant, organ involvement of cGVHD at 1 year post-transplant
Table 5.
Multivariate analyses of risk factors for overall mortality among patients who were alive and disease free at one year after HCT
| number | RR | Odds Ratio | p-value | ||
|---|---|---|---|---|---|
| Presence of cGVHD | |||||
| No cGVHD | 3936 | 1.00 | |||
| cGVHD | 3547 | 1.56 | 1.41 | 1.73 | <0.0001 |
| Acute GVHD History | <0.0001 | ||||
| none | 4051 | 1.00 | |||
| aGVHD in first 29 days | 2446 | 1.11 | 0.93 | 1.24 | 0.0716 |
| aGVHD after day 29 | 986 | 1.46 | 1.28 | 1.67 | <0.0001 |
| Disease | <0.0001 | ||||
| AML | 2541 | 1.00 | |||
| ALL | 1798 | 1.26 | 1.10 | 1.45 | 0.0008 |
| CML | 2498 | 0.73 | 0.64 | 0.83 | <0.0001 |
| MDS | 652 | 0.84 | 0.70 | 1.00 | 0.0521 |
| Disease Status at HCT | <0.0001 | ||||
| Early | 4661 | 1.00 | |||
| Intermediate | 1823 | 1.25 | 1.10 | 1.41 | <0.0001 |
| Advanced | 999 | 1.83 | 1.59 | 2.11 | 0.0005 |
| HLA match | 0.0191 | ||||
| Sibling Donor | 1861 | 1.00 | |||
| Well Matched Unrelated | 1412 | 1.18 | 1.03 | 1.36 | 0.0199 |
| Partially matched Unrelated | 1369 | 1.12 | 0.92 | 1.29 | 0.1148 |
| Poorly matched Unrelated | 841 | 1.26 | 1.07 | 1.49 | 0.0049 |
| TBI in the conditioning regimen | |||||
| no | 3186 | 1.00 | |||
| yes | 4292 | 1.20 | 1.07 | 1.34 | 0.0015 |
| Sex match | <0.0001 | ||||
| Donor male/recipient male | 2662 | 1.00 | |||
| Donor male/recipient female | 1720 | 0.94 | 0.83 | 1.08 | 0.3982 |
| Donor female/recipient male | 1629 | 1.43 | 1.27 | 1.62 | <0.0001 |
| Donor female/recipient female | 1472 | 1.03 | 0.90 | 1.18 | 0.7114 |
| Graft Source | |||||
| Bone marrow | 5354 | 1.00 | |||
| Mobilized blood stem cells | 2129 | 1.24 | 1.10 | 1.40 | 0.0004 |
Factors tested, but not listed in table: Recipient age, sex, donor parity, donor-recipient CMV serology, use of ATG, GVHD prophylaxis, aGVHD grade, year of transplant, platelet count at cGVHD diagnosis, serum bilirubin at cGVHD diagnosis, type of cGVHD onset, KPS/L at cGVHD diagnosis, severity of cGVHD at 1 year post-transplant, organ involvement of cGVHD at 1 year post-transplant
The presence of cGVHD was associated with a higher risk of overall mortality for all diseases (RR: 1.56, 95% CI: 1.41-1.73, P <0.0001) (Figure 1d, Table 5). Patients undergoing HCT for CML and MDS had a lower risk of overall mortality as compared to AML, whereas patients undergoing HCT for ALL had the highest risk of overall mortality. Other factors associated with a higher overall mortality risk were aGVHD after the first 29 days of HCT, intermediate and advanced disease status at HCT, transplant from a well matched URD or a poorly matched URD (as compared to HLA-identical sibling donor), use of TBI, transplant from a female donor to a male recipient (as compared to male donor to a male recipient), and use of PBSC as the graft type (supplemental table 1).
Compared to patients without cGVHD the presence of cGVHD was associated with worse DFS in patients with AML (RR: 1.40, 95% CI: 1.20-1.63, P <0.0001), ALL (RR: 1.46, 95% CI: 1.22-1.75, P <0.0001) and MDS (RR: 1.58, 95% CI: 1.16-2.14, P <0.0001) whereas the presence of cGVHD in CML did not significantly affect DFS (p=0.4).
Chronic GVHD characteristics and impact on relapse, TRM and survival
Since the presence of cGVHD reduced the risk of relapse only in CML patients compared to those without cGVHD, we evaluated cGVHD-related variables associated with protection against relapse in CML. All types of cGVHD (i.e., mild, moderate, or severe; progressive, interrupted, or de novo), and any site of cGVHD involvement (i.e., skin or liver) provided protection against late relapse when compared to no cGVHD (supplemental Table 2, CML patients with and without cGVHD). Pairwise comparisons performed to evaluate the impact of each subtype of cGVHD against each other (for example, the impact of presence of skin versus no skin cGVHD, or severe cGVHD versus moderate cGVHD or mild cGVHD) showed that none provided better protection. This finding was similar when the analysis on cGVHD specific variables was limited only to patients with cGVHD; in CML patients, any site or type of cGVHD provided protection against relapse, whereas in AML, ALL, and MDS patients, the presence of cGVHD at any site or type did not provide protection against relapse.
In patients with CML, cGVHD characteristics associated with higher TRM and lower OS were the presence of moderate or severe cGVHD, skin involvement, lower platelet count and KPS at cGVHD diagnosis. Moderate or severe cGVHD, lower platelet count and KPS were associated with lower DFS (supplemental table 3).
In patients with AML, ALL, and MDS, moderate or severe cGVHD, low KPS at cGVHD diagnosis, and liver or gastro-intestinal (GI) or hematologic involvement were associated with higher TRM. Higher cGVHD severity, and GI, liver and hematologic involvement were also associated with lower OS. Moderate or severe cGVHD, GI or GU or liver or hematologic involvement were associated with lower DFS (supplemental table 4).
Discussion
Allogeneic HCT is an effective immunotherapy for hematological malignancies which is mediated by GVL effect. Clinical evidence implicates acute and chronic GVHD in enhancing malignancy control and is best demonstrated in acute leukemia and CML patients after MAC (2, 4, 6, 7, 15). GVHD is also a major complication of allogeneic HCT substantially contributing to overall TRM but its prevention or treatment with systemic immunosuppression may have harmful effects on GVL. Currently there are no established clinical strategies to guide the intensity of systemic immunosuppressive therapy for GVHD based on presumed leukemia relapse risk. It would be useful to know more precisely in which setting GVHD is most beneficial for leukemia control versus those which predominantly increase TRM. Since occurrence and effects of acute and chronic GVHD overlap during the first year post-transplant, it is difficult to study antitumor effects attributed specifically to cGVHD in the proximity of acute GVHD and therapy related systemic immunosuppression. We focused here on assessing cGVHD effects isolated from acute GVHD by studying only patients who were alive and free of disease at one year post-HCT when acute GVHD effects were sufficiently distant.
This current study demonstrates the protective effect of cGVHD on late relapse only in patients with CML. A protective effect of cGVHD against late relapse was not seen in AML, ALL, or MDS. Data in the current study also confirm that the presence of cGVHD is associated with significantly higher TRM and worse OS across all diseases studied (9).
Several retrospective studies have demonstrated that cGVHD decreases relapses in AML, ALL, and especially in CML (2, 4, 6, 16). These studies evaluated anti-leukemia effects from time of transplant to relapse and did not account for possible confounding effects by acute GVHD. The lack of impact of cGVHD on late relapse in acute leukemia and MDS in this study may be due to differences in biology and susceptibility to immune responses in each disease.
Relapse of leukemia following allo-HCT could be due to failure of GVL or immune clonal escape of the leukemia cells including down regulation of MHC class I and II antigens, associated with a decreased ability to stimulate allo-geneic proliferative T cell responses, and decreased susceptibility to lysis by cytotoxic T lymphocytes or natural killer cells. Since GVL requires time to become fully established after immune reconstitution, the degree to which GVL can control leukemia after allo-HCT may depend on the growth kinetics of the leukemia. Thus, patients with CML compared to patients with acute leukemia may be more susceptible to a durable GVL effect because of their slower pace of proliferation.
The majority of leukemia relapses occur within the first year post-HCT. Similar risk of relapse has been observed using unrelated donor transplant or HLA-identical sibling donors in AML, ALL and CML (15). In the current study, late relapses occurred in all leukemia types and a beneficial cGVHD effect was detected only in those with CML. Differential susceptibility to GVL effects has been observed after administration of donor leukocyte infusion (DLI), which is more effective in relapsed CML than other hematologic malignancies (17-20).
Despite the expectation of protective effects of cGVHD in all diseases, the five- year incidence of relapse was only lower in CML. For this reason we analyzed weather any of the cGVHD specific clinical characteristics or organ manifestations, showed a more dominant association with relapse in CML patients, as such findings could lead towards a better understanding of GVL mechanisms dependent on cGVHD. We found that all sites and types of cGVHD involvement equally affected relapse, suggesting that CML patients with minimal cGVHD clinical manifestation may benefit from its antitumor effect.
This study has several limitations. Chronic GVHD was not classified here per NIH consensus criteria in the CIBMTR database at the time and the data analysis used retrospective design. Specific treatment information for cGVHD is not ascertained and variability in treatment modalities for acute and chronic GVHD during the first year post-HCT may have affected the incidence of late relapse. However, any such effects should be balanced out by the very large number of patients in this study cohort. This study was selected to evaluate a specific question: impact of cGVHD on late relapse (after one year post HCT). To classify patients accurately and determine cut points for analysis, we evaluated time to onset of cGVHD. Most patients (>95%) developed cGVHD within one year of HCT. Hence, we chose this time point for evaluation. Patients who developed cGVHD after one year and were eligible to be included in the dataset were classified as non-cGVHD patients. This may also have affected the analysis. In addition, patients who developed cGVHD during the first year after allogeneic HCT and were successfully treated for their cGVHD may have been reported to the registry as not having active cGVHD. This would be quite unlikely, considering the usually slowly receding nature of cGVHD, and the standard procedure of 12 months registry data collection which requires reporting events during the whole previous observation period.
Data on the use of tyrosine kinase inhibitors (TKI) prior to allogeneic HCT was not available in patients with CML, however, during this study period of 1995-2004, only a minority of patients could have had prior TKI exposure.
This report findings only applies to cGVHD effects after MAC. The more recently expanded use of reduced intensity conditioning (RIC) or truly non-myeloablative conditioning regimens has shifted some of the burden of tumor cell kill after allogeneic HCT from the conditioning regimen to the immune-mediated GVL effects (18). Weisdorf et al recently investigated the effects of acute and chronic GVHD on late relapse after RIC and MAC conditionings regimens in patients with AML and MDS (21). Similar to this current study, in AML and MDS patients following MAC they found the risk of late relapse not significantly affected by cGVHD. However, following RIC regimens, in patients who had both acute and cGVHD late relapse rates were significantly lower. Baron et al also evaluated the GVL effects of cGVHD in AML patients that underwent RIC allo-HCT. In a landmark analysis of patients who were leukemia-free at 18 months after HCT, patients with cGVHD before the landmark day had a lower relapse rate than those without cGVHD (22). These data combined, demonstrate a differential effect of cGVHD on late relapse based on the type of conditioning used in allo-HCT.
In conclusion, this study suggest that cGVHD impact on late relapse of leukemia after MAC HCT is not clinically relevant in AML, ALL and MDS since the beneficial effects on late relapse are confined to patients with CML. The potentially positive impact of the GVL effects on survival after MAC HCT are blunted by a higher cGVHD-related mortality resulting with higher TRM and lower OS for all studied diseases. These data may have practical clinical implications as developing more aggressive strategies to prevent and treat cGVHD may be justified for hematological malignancy patients after HCT with MAC regimens. Future studies aiming to advance the understanding of the cGVHD role in controlling hematologic malignancy should be done prospectively in contemporary patient cohorts and incorporating high level of detail on cGVHD clinical data collection.
Supplementary Material
Translational relevance.
Clinical evidence implicates graft-versus-host disease (GVHD) in enhancing malignancy control. We focused here on assessing chronic GVHD effects in 7,489 patients who were alive and free of disease at one year post-transplant. The current study demonstrates the protective effect of chronic GVHD on late relapse only in patients with CML. A protective effect of chronic GVHD against late relapse was not seen in AML, ALL, or MDS. The presence of chronic GVHD was associated with significantly higher treatment-related mortality and worse overall survival across all diseases studied. As it is important to identify the setting in which chronic GVHD is most beneficial for leukemia control, these data provide support for focusing on developing better chronic GVHD therapies and prevention in order to improve survival of leukemia patients after myeloablative allogeneic HCT.
Acknowledgement
none
Grant support: The CIBMTR is supported by Public Health Service Grant/Cooperative Agreement U24-CA76518 from the National Cancer Institute (NCI), the National Heart, Lung and Blood Institute (NHLBI) and the National Institute of Allergy and Infectious Diseases (NIAID); a Grant/Cooperative Agreement 5U01HL069294 from NHLBI and NCI; a contract HHSH234200637015C with Health Resources and Services Administration (HRSA/DHHS); two Grants N00014-06-1-0704 and N00014-08-1-0058 from the Office of Naval Research; and grants from Allos, Inc.; Amgen, Inc.; Angioblast; Anonymous donation to the Medical College of Wisconsin; Ariad; Be the Match Foundation; Blue Cross and Blue Shield Association; Buchanan Family Foundation; CaridianBCT; Celgene Corporation; CellGenix, GmbH; Children’s Leukemia Research Association; Fresenius-Biotech North America, Inc.; Gamida Cell Teva Joint Venture Ltd.; Genentech, Inc.; Genzyme Corporation; GlaxoSmithKline; HistoGenetics, Inc.; Kiadis Pharma; The Leukemia & Lymphoma Society; The Medical College of Wisconsin; Merck & Co, Inc.; Millennium: The Takeda Oncology Co.; Milliman USA, Inc.; Miltenyi Biotec, Inc.; National Marrow Donor Program; Optum Healthcare Solutions, Inc.; Osiris Therapeutics, Inc.; Otsuka America Pharmaceutical, Inc.; RemedyMD; Sanofi; Seattle Genetics; Sigma-Tau Pharmaceuticals; Soligenix, Inc.; StemCyte, A Global Cord Blood Therapeutics Co.; Stemsoft Software, Inc.; Swedish Orphan Biovitrum; Tarix Pharmaceuticals; Teva Neuroscience, Inc.; THERAKOS, Inc.; and Wellpoint, Inc. The views expressed in this article do not reflect the official policy or position of the National Institute of Health, the Department of the Navy, the Department of Defense, or any other agency of the U.S. Government.
Footnotes
Author Contributions: MB, SZP, SRS, and MA did study design and manuscript preparation. AH, MH, and JPK did the statistical analysis. MB, MA, JPK, AH, MH, JHA, BJB, J-YYC, MSC, CSC, MEF, RPG, RH, LMI, DAJ, MHJ, TRK, SJL, EWP, SS, HCS, LFV, JRW, DJW, MMH, AUI, and SZP participated in interpretation of data and approval of final manuscript.
Conflicts of Interest: We declare that we have no conflicts of interest.
References
- 1.Passweg JR, Baldomero H, Gratwohl A, Bregni M, Cesaro S, Dreger P, et al. The EBMT activity survey: 1990-2010. Bone marrow transplantation. 2012;47:906–23. doi: 10.1038/bmt.2012.66. [DOI] [PubMed] [Google Scholar]
- 2.Weiden PL, Sullivan KM, Flournoy N, Storb R, Thomas ED. Antileukemic effect of chronic graft-versus-host disease: contribution to improved survival after allogeneic marrow transplantation. N Engl J Med. 1981;304:1529–33. doi: 10.1056/NEJM198106183042507. [DOI] [PubMed] [Google Scholar]
- 3.Weiden PL, Flournoy N, Thomas ED, Prentice R, Fefer A, Buckner CD, et al. Antileukemic effect of graft-versus-host disease in human recipients of allogeneic-marrow grafts. N Engl J Med. 1979;300:1068–73. doi: 10.1056/NEJM197905103001902. [DOI] [PubMed] [Google Scholar]
- 4.Horowitz MM, Gale RP, Sondel PM, Goldman JM, Kersey J, Kolb HJ, et al. Graft-versus-leukemia reactions after bone marrow transplantation. Blood. 1990;75:555–62. [PubMed] [Google Scholar]
- 5.Ferrara JL, Levine JE, Reddy P, Holler E. Graft-versus-host disease. Lancet. 2009;373:1550–61. doi: 10.1016/S0140-6736(09)60237-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sullivan KM, Weiden PL, Storb R, Witherspoon RP, Fefer A, Fisher L, et al. Influence of acute and chronic graft-versus-host disease on relapse and survival after bone marrow transplantation from HLA-identical siblings as treatment of acute and chronic leukemia. Blood. 1989;73:1720–8. [PubMed] [Google Scholar]
- 7.Pavletic SZ, Kumar S, Mohty M, de Lima M, Foran JM, Pasquini M, et al. NCI First International Workshop on the Biology, Prevention, and Treatment of Relapse after Allogeneic Hematopoietic Stem Cell Transplantation: report from the Committee on the Epidemiology and Natural History of Relapse following Allogeneic Cell Transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2010;16:871–90. doi: 10.1016/j.bbmt.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bierman PJ, Sweetenham JW, Loberiza FR, Jr., Taghipour G, Lazarus HM, Rizzo JD, et al. Syngeneic hematopoietic stem-cell transplantation for non-Hodgkin’s lymphoma: a comparison with allogeneic and autologous transplantation--The Lymphoma Working Committee of the International Bone Marrow Transplant Registry and the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2003;21:3744–53. doi: 10.1200/JCO.2003.08.054. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Klein JP, Barrett AJ, Ringden O, Antin JH, Cahn JY, et al. Severity of chronic graft-versus-host disease: association with treatment-related mortality and relapse. Blood. 2002;100:406–14. doi: 10.1182/blood.v100.2.406. [DOI] [PubMed] [Google Scholar]
- 10.Weisdorf D, Spellman S, Haagenson M, Horowitz M, Lee S, Anasetti C, et al. Classification of HLA-matching for retrospective analysis of unrelated donor transplantation: revised definitions to predict survival. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2008;14:748–58. doi: 10.1016/j.bbmt.2008.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bacigalupo A, Lamparelli T, Barisione G, Bruzzi P, Guidi S, Alessandrino PE, et al. Thymoglobulin prevents chronic graft-versus-host disease, chronic lung dysfunction, and late transplant-related mortality: long-term follow-up of a randomized trial in patients undergoing unrelated donor transplantation. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2006;12:560–5. doi: 10.1016/j.bbmt.2005.12.034. [DOI] [PubMed] [Google Scholar]
- 12.Shulman HM, Sullivan KM, Weiden PL, McDonald GB, Striker GE, Sale GE, et al. Chronic graft-versus-host syndrome in man. A long-term clinicopathologic study of 20 Seattle patients. Am J Med. 1980;69:204–17. doi: 10.1016/0002-9343(80)90380-0. [DOI] [PubMed] [Google Scholar]
- 13.Filipovich AH, Weisdorf D, Pavletic S, Socie G, Wingard JR, Lee SJ, et al. National Institutes of Health consensus development project on criteria for clinical trials in chronic graft-versus-host disease: I. Diagnosis and staging working group report. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2005;11:945–56. doi: 10.1016/j.bbmt.2005.09.004. [DOI] [PubMed] [Google Scholar]
- 14.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Stat Med. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 15.Ringden O, Pavletic SZ, Anasetti C, Barrett AJ, Wang T, Wang D, et al. The graft-versus-leukemia effect using matched unrelated donors is not superior to HLA-identical siblings for hematopoietic stem cell transplantation. Blood. 2009;113:3110–8. doi: 10.1182/blood-2008-07-163212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kolb HJ, Mittermuller J, Clemm C, Holler E, Ledderose G, Brehm G, et al. Donor leukocyte transfusions for treatment of recurrent chronic myelogenous leukemia in marrow transplant patients. Blood. 1990;76:2462–5. [PubMed] [Google Scholar]
- 17.Collins RH, Jr., Shpilberg O, Drobyski WR, Porter DL, Giralt S, Champlin R, et al. Donor leukocyte infusions in 140 patients with relapsed malignancy after allogeneic bone marrow transplantation. J Clin Oncol. 1997;15:433–44. doi: 10.1200/JCO.1997.15.2.433. [DOI] [PubMed] [Google Scholar]
- 18.Porter DL. Allogeneic immunotherapy to optimize the graft-versus-tumor effect: concepts and controversies. Hematology Am Soc Hematol Educ Program. 2011;2011:292–8. doi: 10.1182/asheducation-2011.1.292. [DOI] [PubMed] [Google Scholar]
- 19.Porter DL, Collins RH, Jr., Hardy C, Kernan NA, Drobyski WR, Giralt S, et al. Treatment of relapsed leukemia after unrelated donor marrow transplantation with unrelated donor leukocyte infusions. Blood. 2000;95:1214–21. [PubMed] [Google Scholar]
- 20.Simula MP, Marktel S, Fozza C, Kaeda J, Szydlo RM, Nadal E, et al. Response to donor lymphocyte infusions for chronic myeloid leukemia is dose-dependent: the importance of escalating the cell dose to maximize therapeutic efficacy. Leukemia. 2007;21:943–8. doi: 10.1038/sj.leu.2404641. [DOI] [PubMed] [Google Scholar]
- 21.Weisdorf D, Zhang MJ, Arora M, Horowitz MM, Rizzo JD, Eapen M. Graft-versus-host disease induced graft-versus-leukemia effect: greater impact on relapse and disease-free survival after reduced intensity conditioning. Biology of blood and marrow transplantation: journal of the American Society for Blood and Marrow Transplantation. 2012;18:1727–33. doi: 10.1016/j.bbmt.2012.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Baron F, Labopin M, Niederwieser D, Vigouroux S, Cornelissen JJ, Malm C, et al. Impact of graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation for acute myeloid leukemia: a report from the Acute Leukemia Working Party of the European group for blood and marrow transplantation. Leukemia. 2012;26:2462–8. doi: 10.1038/leu.2012.135. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.