Abstract
This review covers the spatial and temporal rules governing induction of hippocampal long-term potentiation (LTP) by theta-burst stimulation. Induction of LTP in field CA1 by high frequency stimulation bursts that resemble the burst discharges (complex-spikes) of hippocampal pyramidal neurons involves a multiple-step mechanism. A single burst is insufficient for LTP induction because it evokes both excitatory and inhibitory currents that partially cancel and limit postsynaptic depolarization. Bursts repeated at the frequency (~5 Hz) of the endogenous theta rhythm induce maximal LTP, primarily because this frequency disables feed-forward inhibition and allows sufficient postsynaptic depolarization to activate voltage-sensitive NMDA receptors. The disinhibitory process, referred to as “priming”, involves presynaptic GABA autoreceptors that inhibit GABA release. Activation of NMDA receptors allows a calcium flux into dendritic spines that serves as the proximal trigger for LTP. We include new data showing that theta-burst stimulation is more efficient than other forms of stimulation for LTP induction. In addityion, we demonstrate that associative interactions between synapses activated during theta-bursts are limited to major dendritic domains since such interactions occur within apical or basal dendritic trees but not between them. We review evidence that recordings of electrophysiological responses during theta burst stimulation can help to determine if experimental manipulations that affect LTP do so by affecting events antecedent to the induction process, such as NMDA receptor activation, or downstream signaling cascades that result from postsynaptic calcium fluxes. Finally, we argue that theta-burst LTP represents a minimal model for stable, non-decremental LTP that is more sensitive to a variety of experimental manipulations than is LTP induced by other stimulation paradigms.
Keywords: long-term potentiation, LTP, theta burst stimulation, NMDA, AMPA, GABA, hippocampus, CA1
Introduction
During the first decade after the discovery of long-term potentiation (LTP) was announced (Bliss and Gardner-Medwin, 1973; Bliss and Lomo, 1973), there was some skepticism that it could serve as a memory mechanism, given the conditions that seemed necessary to induce the effect. At the same time, it seemed to many that memory could only be studied neurobiologically in simple nervous systems in which stimulus and response could be traced directly. In this time period (the first decade after 1973), there were 49 papers published on LTP; in the same interval, Eric Kandel’s lab alone published 77 papers on mechanisms of learning in Aplysia. Of course, there were many factors that contributed to the lag in appreciation of LTP (and the decline in popularity of the invertebrate model system approach), but an important one was the question of whether or not LTP could be produced by physiological processes during learning.
The discovery that ‘theta-burst stimulation’ (TBS) was an effective stimulus for LTP (Larson et al., 1986) was significant for three reasons: first, it showed that patterns of neuronal firing (complex-spikes) that occurred spontaneously during behavior could, if appropriately timed, induce LTP; second, the optimal repetition rate corresponded to the frequency of the hippocampal theta rhythm, an EEG pattern previously related indirectly to memory storage processes; and third, patterned stimulation paradigms allowed the uncovering of multiple events that contribute to LTP induction.
We will begin with a historical review, then present some original data pertaining to a few unresolved issues, and conclude with a discussion of some theoretical and practical implications. LTP can be divided into two sets of processes which we will refer to as induction and expression. The induction events are transient and very brief (<1sec), involve both presynaptic and postsynaptic responses to unusual patterns of activation, and are modulated by local circuit mechanisms. Expression refers to the synapse-specific and enduring (> 1 hour) enhancement of synaptic transmission. In addition, persistence of expression requires mechanisms for stabilization or continued maintenance. This review wil focus on the mechanisms by which theta burst stimulation induces LTP and will not say much about the cellular events downstream from the induction events. The post-induction events are most likely common to LTP induced by TBS and other stimulation paradigms; i.e., they are not particular to theta-burst LTP.
The theta-burst paradigm mimics two characteristics of hippocampal physiology: complex-spike discharges of the pyramidal neurons (Ranck, Jr., 1973) and the rhythmic modulation of excitability of those cells during theta rhythm (Rudell et al., 1980). Hippocampal theta was originally described as the hippocampal “arousal rhythm” (Green and Arduini, 1954) since it was correlated with the neocortical desynchronization characteristic of awake, attentive states. Follow-up studies attempted to relate theta to specific psychological concepts such as attention, learning, memory encoding, etc.; however, Vanderwolf, in a very influential paper (Vanderwolf, 1969), argued effectively that theta should be considered as a hippocampal correlate of voluntary movement. This conclusion invites the question: why is the hippocampus concerned with movement? One explanation is that movement in space necessitates updating cognitive maps of the environment and the spatial activity of hippocampal place cells (O'Keefe and Nadel, 1978). A second explanation is that much of voluntary movement is exploratory in nature, involving the gathering of both spatial and non-spatial information relevant to goal-directed behaviors. Perhaps one of the most impressive examples of the coupling of behavior to neural activity in mammals occurs during exploratory sniffing in rodents: rhythmic respiration is coupled to theta rhythm throughout the ‘olfactory-hippocampal circuit’ (olfactory bulb, piriform cortex, entorhinal cortex, dentate gyrus, and hippocampus) both at the gross behavioral level and with synchronization of the behavioral rhythm with the EEG pattern on a cycle-by-cycle basis (Macrides, 1975; Macrides et al., 1982; Rojas-Líbano et al., 2014; Tsanov et al., 2014). This suggests that a stimulus sampling pattern organizes neuronal activity throughout a brain circuit that processes the information obtained by the sampling behavior. The synchronization of olfactory sampling with hippocampal theta may only be only the most direct and striking example of this: sniffing in rodents is also synchronized with active tactile sensation using the mystacial vibrissae (Deschenes et al., 2012; Welker, 1964), although the pathways for somatosensory information to reach the hippocampus are far less direct than those for olfaction. It is also intriguing that visual saccades in humans and non-human primates occur at frequencies in the theta range and may be coupled to hippocampal theta (Hoffman et al, 2013).
The efficacy of stimulation patterns mimicking complex-spike discharges was first tested in the in vivo dentate gyrus (Douglas and Goddard, 1975; Douglas, 1977) and eight-pulse, 400 Hz bursts for LTP induction became common in studies of this structure. In this system, each burst was thought to induce an increment of LTP; repetition served to only to summate these increments and little regard was placed on the repetition rate (Douglas, 1977). However, due to limited effectiveness, this pattern never caught on for studies of CA1 in hippocampal slices, where the most common induction pattern was a 1–2 sec-long 100 Hz train (tetanus). Combining the complex-spike pattern with a theta frequency repetition rate produced a robust, reliable, and stable LTP in the CA1 field of hippocampal slices (Larson et al., 1986). These experiments used a four-pulse burst at 100 Hz to mimic the complex-spike burst and repeated it ten times at five bursts per second to approximate the theta rhythm frequency. The significance of LTP induced by this pattern was not immediately apparent until it was found that burst repetition frequencies lower or higher that 5 Hz were significantly less effective for LTP induction. The frequency-dependence of burst-induced LTP is reproduced in Fig. 1(A, B).
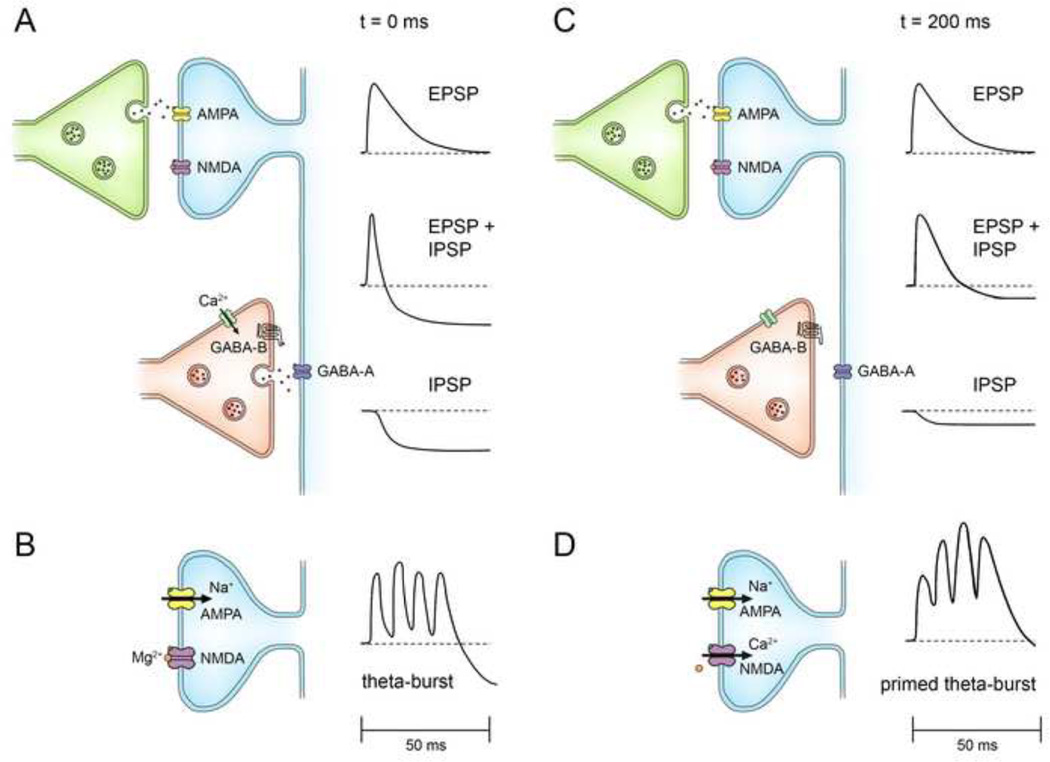
Fig. 1.
Timing rules for theta-burst LTP. A. CA1 pyramidal neuron with apical and basal dendritic trees that receive excitatory synaptic connections from CA3 via Schaffer collaterals and commissural inputs to both apical and basal dendritic trees. Bursts of high frequency stimulation (S) are used to simulate complex-spike discharges in the afferent neurons. Timing rules are established by varying inter-burst frequency and spatial location of the activated synapses. B. At apical dendritic synapses, bursts repeated at 5 Hz produce optimal LTP. Histogram plots the magnitude of LTP induced by 5–20 bursts repeated at the frequencies indicated on the abscissa. Figure modified from (Larson et al., 1986). A similar frequency dependence is seen at basal dendritic synapses (Capocchi et al., 1992). C. A burst to one apical dendritic input (S1) does not itself produce LTP at those synapses, but primes the cell so that a second burst to a separate input (S2) 200 msec later triggers LTP at only the synapses activated by the second burst. D. A burst to an apical dendritic input (S1) can prime LTP induction at a basal dendritic input (S2). E. A burst to a basal dendritic input (S1) can prime LTP induction at an apical dendritic input (S2). Results depicted in panels C–D–E are from (Larson and Lynch, 1986).
Multiple-Step Induction Mechanism and Peculiarity of the Theta Frequency
What accounts for this frequency-dependence? Many mechanisms contribute. The first clue was that either a single burst or multiple bursts repeated at long intervals (>2 sec) were almost always ineffective (Larson and Lynch, 1986; Rose and Dunwiddie, 1986). However, a burst to one input could “prime” the circuit so that a second burst to a separate input 200 msec later would reliably induce LTP, but only on the synapses activated by the second burst (Fig. 1C). A single burst activates a feed-forward inhibitory postsynaptic potential (IPSP) that truncates the excitatory postsynaptic potentials (EPSPs) evoked by that burst or a subsequent burst within 100–150 msec. The key to the theta frequency optimum is that the feed-forward inhibition becomes suppressed after its own activation and does not recover for about a second (Davies et al., 1990; McCarren and Alger, 1985). Because of this disinhibition, a second burst at 200 msec after the first burst evokes maximal postsynaptic depolarization in the pyramidal neuron. Bursts that occur too early are shunted by the already-present IPSP; bursts that are delayed too long encounter a recovering IPSP. We initially referred to the IPSP suppression as "priming" the circuit and showed that it could be effectuated by a single pulse (no burst required) and that it was spatially widespread since priming of apical synapses could be induced by basal dendritic stimulation and vice-versa (Fig. 1D, E) (Larson and Lynch, 1986). The IPSP suppression was verified (Pacelli et al., 1989; Pacelli et al., 1991) and shown to be mediated by metabotropic GABAB autoreceptors that result in diminished GABA release (Davies et al, 1991; Mott and Lewis, 1991; Mott and Lewis, 1992). The enhanced burst-evoked depolarization of "primed" bursts allows measurable NMDA receptor-dependent currents to be activated (Larson and Lynch, 1988). There is evidence that the IPSP suppression is restricted to the feed-forward inhibitory circuit and absent in the feedback (recurrent) circuit, but this needs to be more firmly established (Arai et al., 1995). Interestingly, this may explain how theta rhythm is sustained if the GABAergic inhibitory interneurons (‘theta’ cells) are mainly the feedback rather than feed-forward interneurons. The main contributors to LTP induction by TBS are diagrammed in Fig. 2.
Fig. 2.
Multiple-step induction mechanism for theta-burst LTP in field CA1 of hippocampus. A. In the “unprimed state (t=0 msec), afferent stimulation activates a monosynaptic glutamatergic (excitatory) axo-spinous synapse and a disynaptic GABAergic (inhibitory) axo-dendritic synapse on the same pyramidal neuron. Glutamate activates postsynaptic AMPA receptors to depolarize the pyramidal neuron (EPSP) while co-expressed NMDA receptors are blocked by Mg2+. GABA activates postsynaptic GABAA receptors that hyperpolarize the neuron (IPSP). The IPSP curtails the depolarization evoked by the EPSP (EPSP+IPSP). The GAB A released at the inhibitory synapse also activates a slow, metabotropic GABAB receptor on the presynaptic inhibitory terminal. B. A high frequency burst (theta-burst) under these conditions results in poor temporal summation of the depolarization due to the feed-forward inhibition. C. In the “primed” state (t = 200 msec), There is little change in the synaptic action at the excitatory synapse, but the action of the presynaptic GABAB receptor produces a use-dependent suppression of GABA release. The diminished IPSP causes the EPSP to have a prolonged depolarizing effect (EPSP+IPSP). D. A high frequency burst in the primed state (primed theta-burst) shows enhanced temporal summation of excitation; the enhanced depolarization allows relief of the Mg2+ channel block and a calcium influx through the NMDA receptor.
Other factors that contribute to the pattern of dendritic depolarization and synaptic NMDA receptor activation are agents that enhance AMPA receptor activity by enhancing glutamate release or by allosteric modulation of AMPA receptors (ampakines): the latter drugs magnify the depolarization without changing the temporal pattern of burst response enhancement. Consequently, ampakines magnify the LTP induced by suboptimal burst repetitions (i.e., after 5 bursts) but do not alter the maximal LTP expressed (Arai and Lynch, 1992). In contrast, an agent such as forskolin that antagonizes the calcium-dependent AHP between theta bursts elevates the LTP "ceiling" without changing the fractional increment produced by suboptimal bursts (Arai and Lynch, 1992), possibly by extending the duration of NMD A receptor currents.
A four-pulse burst was used in these experiments because it is within the common range of hippocampal complex-spikes and because three-pulse bursts are rarely sufficient - see also (Arai et al., 2004; Diamond et al., 1988). We have used 100 Hz as the intra-burst frequency because it allows resolution of responses to the individual pulses. Other variations on pulse number and intra-burst frequency have not been studied systematically. It is likely that intra-burst frequency effects such as presynaptic facilitation and depletion (Pan and Zucker, 2009) interact with pulse number to determine the synaptic cleft glutamate concentration available to interact with postsynaptic NMDA receptors.
The theta frequency optimum has been questioned by Grover and colleagues who tested the efficacy of four-pulse bursts repeated 20 times at frequencies from 0.05 to 10.0 Hz, with maximal LTP induced at 2–3 Hz (Grover et al., 2009), making the interesting point that the lower frequency maximum may correspond more closely to the delta waves that are present in the hippocampal EEG during slow-wave sleep rather than the theta waves of behavioral arousal and REM sleep. It is an intriguing possibility to consider that the frequency-plasticity relationship may be altered by changes in ‘circuit kinetics’, perhaps dictated by brainstem modulatory systems, associated with different behavioral states. On the other hand, the frequency-plasticity relationship may also be distorted during regular repetition of bursts to a single input. A pair of appropriately timed bursts is much more likely to occur naturally during behavior than a series of 10–20 bursts repeated at regular intervals. Studies using the primed-burst paradigm (homosynaptic priming) or the two-input paradigm showed significant LTP maxima at 140 msec (~7 Hz) (Diamond et al., 1988) and 100–200 msec (5–10 Hz), respectively (Larson, 1987). Since a single (primed) burst is sufficient to induce a small increment of stable LTP that is additive (Larson and Lynch, 1986), this frequency-dependence seems more likely to reflect the conditions in vivo than those that obtain when many bursts are repeated in a long series. In chronic single-unit recording studies, Otto and Eichenbaum observed that complex-spike burst discharges of CA1 pyramidal cells were often preceded by spike or burst activity at theta periodicity during odor discrimination or spatial learning (Otto et al., 1991), validating the occurrence of theta-burst activity in freely-behaving animals.
Theta-burst LTP exhibits three other canonical features of LTP: cooperativity (associativity), saturation, and stability. The requirement for adequate postsynaptic depolarization to activate voltage-sensitive NMDA receptors was discussed above; normally, this is provided by simultaneous stimulation of many afferents (cooperativity). How realistic is this? It is comforting perhaps that detectable and stable LTP is induced by a single appropriately-primed four-pulse burst (Larson and Lynch, 1986; Rose and Dunwiddie, 1986). In situ, some of the necessary depolarization may be provided by the theta rhythm itself: experiments show that single bursts in vivo can be effective when timed to occur at the peak depolarization of endogenous theta waves and are ineffective when evoked at the trough (Holscher et al., 1997; Hyman et al., 2003; McCartney et al., 2004; Orr et al., 2001; Pavlides et al., 1988). Thus, LTP induction may not require unrealistically massive synchronization of many bursting inputs to a cell. We will return to the spatial limits of within-burst spatial summation in a later section. With regard to saturation, LTP resulting from a single episode of TBS seems to reach a maximum after about 10 bursts (Larson et al., 1986); however, this limit may be exceeded with widely-spaced episodes (Kramar et al., 2012), suggesting that synapses may differ in LTP threshold, with later episodes recruiting less susceptible synapses (Lynch et al., 2013). Finally, theta-burst LTP in slice preparations typically is non-decremental after the first 15 minutes. This also appears to be true in vivo, where the potentiation is maintained at a stable level for weeks (Staubli and Lynch, 1987).
‘Economy’ of Theta-Burst Stimulation
It seems evident from the frequency-dependence of patterned burst-induced LTP that long continuous trains (tetanic stimulation) would be less efficient than TBS in the sense that each stimulus pulse would contribute less to the LTP effect. However, this has received little systematic study. One study of field CA1 in rat hippocampal slices (Hernandez et al., 2005) reported no statistically-significant differences between the amounts of LTP induced by tetani and theta-bursts containing the same total stimulation pulses. The minimal TBS stimulus consisted of ten theta bursts in that study, so the submaximal stimulation range was not investigated. Furthermore, the pulse number-LTP magnitude curves for both TBS and tetanic stimulation were fairly flat, suggesting that a ceiling effect may have obtained with the minimal conditions used.
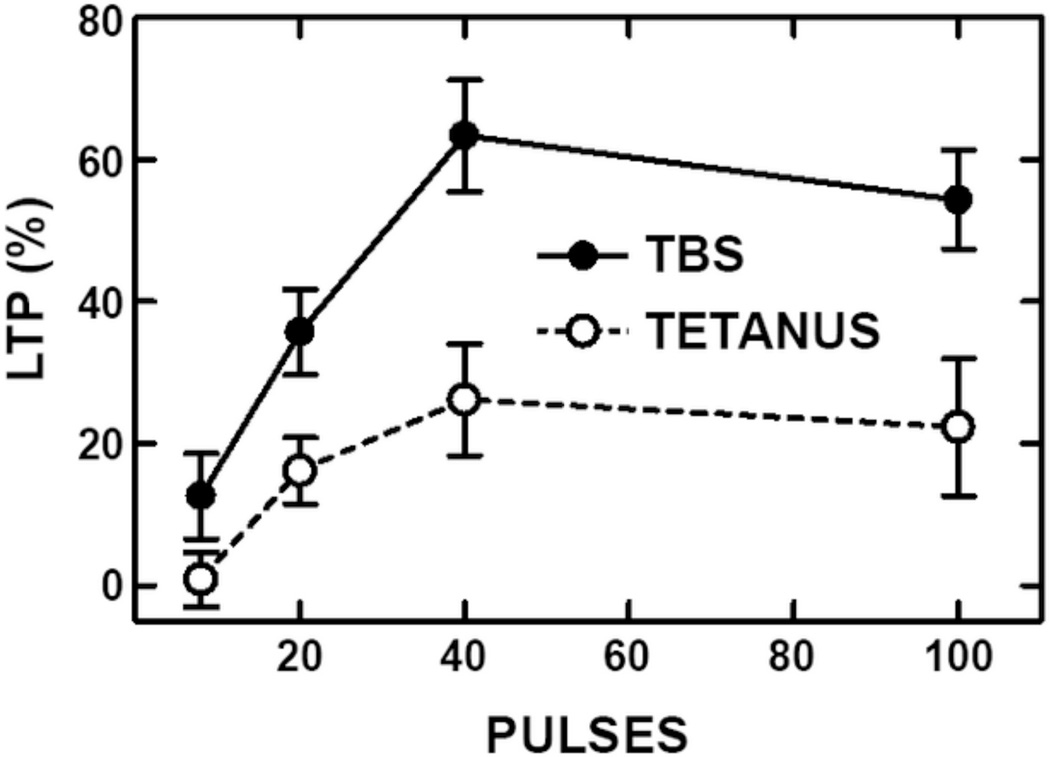
We re-examined this issue using a two-input paradigm in field CA1 of male adult (2–3 months) mouse (CD-I) hippocampal slices. Slices were prepared as described (Larson et al., 1999) and maintained at the interface between perfused (1 mL/min) aCSF (in mM: NaCl 124, KC1 3, KH2P04 1.2, NaHC03 26, MgSO4 2.5, CaC12 3.4, D-glucose 10, Na-L-ascorbate 2) and an atmosphere of 95%O2/5% CO2, at 34±l°C. Stimulation electrodes (bipolar twisted nichrome wires) were placed in stratum radiatum of CA1a and CA1c and field potentials recorded via a glass microelectrode (2M NaCl) in s. radiatum of CA1b. Test pulse intensity was set to evoke a 2.0 mV field EPSP using a stimulus pulse duration of 0.1 msec. Each slice tested was given a theta-burst stimulus on one pathway and a tetanus (continuous 100 Hz train) consisting of the same total number of stimulus pulses on the other pathway (in random order and with position counterbalanced) with at least 30 minutes delay between LTP induction episodes. Pulse duration was increased to 0.2 msec during the induction stimuli. The degree of LTP induced was quantified at 60 minutes post-stimulation for each pathway. The total number of induction pulses used in different slices was 8 (2 theta-bursts or a 80 msec long tetanus), 20 (5 theta-bursts or a 200 msec tetanus), 40 (10 theta-bursts or a 400 msec tetanus), or 100 (25 theta-bursts or a 1 sec tetanus). As shown in Fig. 3, no significant LTP was induced by the shortest tetanus, although significant LTP was induced by the same number of pulses (8) given as two theta-bursts (however, due to large variances, the direct comparison of TBS to tetanus was not significant). For greater numbers of pulses, theta-bursts yielded more LTP than the same number of pulses given as a single tetanus. A two-way ANOVA on the percent change persisting at 60 minutes post LTP stimulation showed significant main effects of stimulus pattern (F1,124 = 25.65, p<.0001) and pulse number (F3,124 = 11.39, p<.0001) but no significant interaction between the two independent variables (F3,124 = 1–37, p>25). These results confirm that TBS is indeed more economical for LTP induction than un-patterned stimulation. In fact, the 100-pulse tetanus yielded somewhat less LTP than a theta-burst pattern of only 20 pulses. More is not necessarily better! A possible explanation for the weaker effect of tetanic stimulation is that depletion of glutamate available for release occurs during continuous trains, thus reducing NMDA receptor activation.
Fig. 3.
Theta-burst stimulation is more efficient than continuous high frequency stimulation for LTP induction in hippocampal field CA1. LTP at apical dendritic synapses was measured as the percent increase in field EPSP slope 60 minutes after LTP induction by stimulation of Schaffer-commissural fibers with either a continuous, 100 Hz train consisting of 8–100 pulses (TETANUS) or the same total number of pulses given as bursts of four repeated at 200 msec intervals (TBS). Significantly greater LTP was evoked by TBS with 20 pulses (5 bursts, p<.05), 40 pulses (10 bursts, p<01), and 100 pulses (25 bursts, p<.01) than tetani consisting of the corresponding number of pulses. Results are from unpublished data by J. Larson and J. Crouch.
Spatial Limits of Associative Theta-Burst LTP
Classic “associative LTP” experiments in the CA1 stratum radiatum (apical dendritic layer) showed that a single stimulus train of intensity or duration too weak to induce LTP on its own can do so if simultaneously paired with a stronger train to a separate input (Barrionuevo and Brown, 1983; Gustafsson and Wigstrom, 1986; Kelso and Brown, 1986; Kelso et al., 1986). Further studies provided evidence that these associative interactions are most effective when the two inputs terminate in spatially-overlapping regions of the dendritic tree (Hardie and Spruston, 2009; White et al., 1988; White et al., 1990) or individual branches (Govindarajan et al., 2011), although there is one report that that strong stimulation of a basal dendritic (s. oriens) input can facilitate LTP in a simultaneously-stimulated apical dendritic input (Gustafsson and Wigstrom, 1986).
We investigated associative interactions between basal and apical dendritic synapses using TBS in field CA1 of hippocampal slices from adult male CD-I mice, prepared and maintained as described above. Two stimulating electrodes were used: one was designated as the test electrode (S1; weak) and positioned either in stratum radiatum (apical dendrite) or stratum oriens (basal dendrite) of CA1c; the other electrode was designated the conditioning electrode (S2; strong) and was positioned either in s. radiatum or s. oriens of CA1a. The recording electrode was positioned in CA1b in the same lamina as the test electrode. Stimulus intensity for the S1 (weak) electrode was always set to evoke a fEPSP about 1 mV in amplitude (typically about 20–25% of the maximal population spike-free fEPSP) while the S2 (strong) intensity was always set to evoke a population spike. In the first set of experiments (see Fig. 4A–D), S1 was positioned in s. radiatum. After establishing a baseline, two episodes of five theta-bursts (4 pulses at 100 Hz with 200 msec between the bursts) separated by 5 sec. were given to the test electrode (S1) alone. Recording was continued for 30 minutes to monitor the LTP induced. The conditioning (S2) stimulation electrode was then placed in s. oriens and S1 responses monitored for a 10 minute baseline before both test (S1) and conditioning (S2) electrodes were given the same theta-bursts simultaneously. After an additional 30 minutes of recording, the conditioning (S2) electrode was moved to s. radiatum, the combined theta-burst stimulation (S1+S2) repeated, and LTP monitored on S1 for an additional 30 minutes. Stimulus pulse duration was never increased during TBS. In the second set of experiments (see Fig. 4E–H), S1 was located in s. oriens and S2 was first in s. radiatum and subsequently in s. oriens.
Fig. 4.
Associative LTP induced by theta-burst stimulation is restricted to major dendritic domains. A. A test electrode (S1) placed in the s. radiatum of field CA1 was used to study the spatial rules governing associative LTP induced by theta-burst stimulation. Stimulus intensity was set to evoke a field EPSP too weak (~1 mV in amplitude) to induce LTP when stimulated with TBS alone (see text for details). Graph shows field EPSP amplitude measured at 20 sec. intervals for 15 min before and 35 min. after TBS (each point is the mean ± s.e.m. of 4 consecutive trials) in seven different slices, each from a different mouse. B. The same test input (S1) was then given TBS simultaneous with TBS to a strong input (S2) to the basal dendrites. The graph shows the S1 response amplitude (as in A) before and after TBS to S1 and S2 (n=7). C. The test input was then given TBS simultaneous with stimulation of a strong input to the apical dendritic field (S2). This resulted in robust LTP to the S1 input (n=6, one slice rejected from analysis due to instability). D. Histograms show field EPSP amplitude measured 30 minutes after TBS, relative to the pre-TBS baseline, in the three stimulation conditions. LTP was significantly greater after the apical-apical pairing than in the other two conditions (**, p<.01). E–F. As in A–D, except that the test input (S1) was to the basal dendrites (n=6 experiments in slices from different mice). Weak stimulation to the basal dendritic input did not induce LTP when stimulated alone (E) or in combination with an apical dendritic input (F), but did show robust LTP after combined stimulation of a strong (S2) basal dendritic input (G). Histograms (H) show that significantly greater LTP was induced after combined basal-basal stimulation than in the other two conditions (**, p<.01). One slice in (E) was rejected from analysis due to instability. In both (C) and (G), the physical location of weak (S1) and strong (S2) electrodes were in subfields CA1c and CA1a, respectively (recording electrode in CA1b), and paired-pulse tests confirmed that the activated fibers were independent. In all other cases, the stimulation electrodes were in CA1c (i.e., the subfield closest to CA3). Results are from unpublished experiments by E. Munkácsy, N. Bartolotti, and J. Larson.
Conditioning stimulation of an apical dendritic input effectively facilitated LTP in the apical dendritic test input. In contrast, no associative LTP was induced in the apical test input when the conditioning stimulation was a basal dendritic input. Likewise, the basal dendritic test input was facilitated by a basal conditioning stimulus, but not by an apical input.
These results suggest that associative interactions during LTP induction by theta-bursts are limited to dendritic compartments. Weak and strong synaptic inputs can cooperate for LTP induction within the same dendritic tree but not between dendrites separated by the soma. The conventional interpretation of such interactions is that they require spatial summation of depolarization to allow activation of synaptic NMDA receptors. This suggests that the spatial summation can occur within apical or basal dendritic trees but not between them. One possibility is that shunting inhibition at the soma limits the spread of depolarization from apical to basal dendrites and vice-versa. While feed-forward IPSPs are suppressed during theta-bursts, these may be preferentially located in the dendrites (Alger and Nicoll, 1982); somatic feedback IPSPs, GABAB-dependent postsynaptic IPSPs, and calcium-activated after-hyperpolarizations appear to be intact during theta-bursts. The only experiment in which associative interactions between apical and basal dendritic synapses were found was conducted with GABAA receptors blocked pharmacologically (Gustafsson and Wigstrom, 1986). Alternatively, if associative LTP depends on diffusion and summation of chemical signals, this process also appears to be compartment specific (Govindarajan et al., 2011). In any case, it appears that the conditions of our experiment allow effective summation of synaptic signals within branches of the apical or basal dendritic trees, but not between the apical and basal compartments.
A Within-Burst Timing Effect
The possible role of asynchrony within theta-bursts was studied using a three-input paradigm in field CA1 of hippocampal slices (Larson and Lynch, 1989). The paradigm and results are summarized in Figure 5. Three separate apical dendritic inputs were stimulated using a stimulus intensity too low for any one input to be potentiated by TBS independently. For LTP induction, each input was stimulated with a simultaneous “priming” pulse followed by a 4-pulse burst to each input in a staggered but partially overlapping sequence. The spatial position of the inputs was counterbalanced vis a vis the timing pattern in different slices. The pulse-burst pattern was repeated ten times at five-second intervals. The degree of LTP induced was significantly related to the position of the input in the temporal sequence, with the synapses stimulated first in the sequence showing the most potentiation and the last-stimulated input being potentiated the least. One explanation for this timing effect is that the later arriving bursts can maintain depolarization longer at the synapses activated first and sustain NMDA channel activity at those synapses (Larson and Lynch, 1989). There is some similarity between this within-burst rule and the rules that govern spike timing-dependent plasticity (Feldman, 2012). The functional significance of the within-burst timing rule was investigated in network models; this work indicated that it contributes to encoding and recall of cues consisting of temporal sequences (Granger et al., 1994).
Fig. 5.
Within-burst timing and LTP. Hippocampal slices were prepared with a recoding electrode and three stimulating electrodes in s. radiatum of CA1. After a baseline period of recording responses to all three inputs, patterned stimulation was applied as depicted at left. All three inputs received a priming pulse followed by asynchronous bursts. The first pulse in the burst (4 pulses, 100 Hz) to S1 occurred 180 msec after the priming pulse, the first pulse to S2 coincided with the third pulse in the S1 burst, and the first pulse in the S3 burst coincided with the third pulse in the S2 burst. The pattern was repeated ten times at 5 sec. intervals. The LTP induced at all three sets of synapses were statistically different from each other (p<.05). Modified from (Larson and Lynch, 1989).
Theta-Burst Responses as Diagnostics
Measuring postsynaptic responses to theta-bursts can be used to determine if experimental manipulations such as drugs or mutations affect LTP by interfering with the electrophysiological responses of the circuit to TBS or, alternatively, affect downstream signaling cascades. The activation of NMDA receptors by theta patterned stimulation was first demonstrated using a two-input paradigm (Larson and Lynch, 1988), but is also evident in the standard TBS paradigm, as shown in Figure 6. Mouse hippocampal slices were prepared as described above with two stimulation electrodes and an extracellular recording electrode in s. radiatum of field CA1. Slices were first stimulated with 10 theta-bursts on one pathway and synaptic transmission was monitored for 60 min. post-TBS. Slices were then perfused with the competitive NMDA receptor antagonist, D-AP5 (50 µM), and stimulated with an identical TBS on the second pathway. Synaptic transmission was again monitored for one hour post-TBS. Field potentials evoked by the theta bursts were recorded and measured (see Fig. 6 legend for details). The NMDA antagonist, while blocking LTP, had very little effect on the response to the first (“unprimed”) burst, but reduced the envelope of depolarization evoked by several of the succeeding bursts. With repeated theta-bursts, synaptic facilitation and depletion probably contribute to the inverted U shape of the burst response enhancement curves.
Fig. 6.
Activation of NMDA receptors during theta-burst stimulation. A. The NMDA receptor antagonist, D-AP5 (50 µM) blocks LTP induction by TBS consisting of ten bursts (4 pulses, 100 Hz) repeated at 5 Hz. LTP was measured as the percent increase in field EPSP slope 60 minutes after TBS in the absence of D-AP5 (CTRL) or 60 minutes after TBS given in the presence of the drug (AP5). Data are means + S.E.M. (n=7). B. Field EPSP waveforms evoked by the first two bursts in the TBS under control conditions (CTRL) and in the presence of the antagonist (AP5). The second burst response is larger than the first in both conditions. C. Measurements of the amplitude of the first response within the first burst (left panel) and the total area under the response to the first burst (right panel) were not significantly affected by AP5. D. The areas of bursts 2–10 were calculated and expressed as a percentage of the area of the first burst in both the AP5 condition and the control condition. The NMDA receptor antagonist significantly reduced the area of responses to second through fifth bursts of the TBS (**, p<.01). Unpublished data from J. Larson and J. Crouch.
Other conditions in which alterations of LTP are associated with altered burst responses include suppressed LTP in transgenic mouse models for Alzheimer’s disease (Larson et al., 1999), suppressed LTP by diazepam (del Cerro et al., 1992), reduced LTP after neonatal lesions of contralateral hippocampus (van Praag et al., 1998), reduced LTP after inhibition of matrix metalloproteinases (Meighan et al., 2007), enhanced LTP in mice overexpressing brain protease nexin-1 (Luthi et al., 1997), facilitated LTP by an Ml muscarinic antagonist (Boddeke et al., 1992), and enhanced LTP after environmental enrichment (Malik and Chattarji, 2012), among others. Recording and analyzing the burst response pattern can help to provide a first-pass evaluation of whether an experimental manipulation affects events before or after the NMDA receptor-mediated influx of calcium that serves as the essential trigger for LTP induction.
Sensitivity of Theta-Burst LTP to various experimental manipulations
There is considerable overlap between the downstream molecular events triggered by TBS and other high-frequency tetanic stimulation methods; however, there are many conditions in which theta-burst LTP is more sensitive to experimental manipulations than is the LTP induced by long trains of 100 Hz stimulation. For example, theta-burst LTP is more vulnerable to aging (Moore et al., 1993; Rex et al., 2005) and experimentally-induced stress (Diamond et al., 1989; Diamond et al., 1992; Diamond et al., 1994) than is tetanus-induced LTP and appears to be more sensitive to manipulations that affect BDNF (Chen et al, 1999; Chen et al, 2010; Kang et al, 1997; Pang et al, 2004; Patterson et al, 2001; Skucas et al., 2011), serotonin (Corradetti et al., 1992), endocannabinoid (Pan et al., 2011), or adenosine receptor-dependent (Costenla et al., 1999) signaling mechanisms. Experiments on a number of mutant mouse models show selective or larger disruptions of LTP induced by theta-bursts than of LTP induced by tetanic stimulation (Evers et al., 2002; Gong et al, 2009; Jedlicka et al, 2009; Luthi et al, 1997; Shalin et al, 2006). Many of these experimental conditions also affect learning and/or memory processes, suggesting that theta-burst LTP may be a better model for the memory effects than tetanus-induced LTP.
Few of the manipulations described above have been resolved into effects on events antecedent or consequent to the LTP induction triggering event. The preponderance of evidence suggests that theta-burst LTP represents a minimalist model for stable (non-decremental) LTP. If so, then we might expect that theta-burst LTP would be more sensitive to perturbations of events antecedent to LTP induction. Theta-burst stimulation exploits the endogenous circuit properties to maximize NMDA receptor activation with the least amount of afferent stimulation. Under some circumstances, long trains may simply be less efficient for triggering LTP (Fig. 3). However, this inefficiency may be overcome by high intensity trains or multiple repetitions at short (<1 minute) intervals. Some paradigms may even engage mechanisms in addition to those minimally essential for stable LTP. For example, trains of 200 Hz stimulation are reported to activate voltage-dependent calcium channels as well as synaptic NMDA receptor-mediated calcium fluxes (Grover and Teyler, 1990). Clearly, manipulations that alter the circuit dynamics exploited by theta-bursts, such as blockade of inhibition, have a major effect on the sensitivity of theta-burst LTP as compared to tetanus-induced LTP (Pacelli et al, 1989).
If we consider the events consequent to the minimal trigger for LTP - NMDA receptor-mediated calcium flux into the dendritic spine - the situation is more complex. The biochemical mechanisms for insertion of AMPA receptors (LTP expression) and cytoskeletal reorganization essential for LTP stabilization must be common to all stimulation paradigms that produce a stable effect. Under the mild induction conditions of TBS, these mechanisms may be more sensitive to disruption by experimental manipulations. On the other hand, more intense or protracted stimulation patterns may engage additional pathways that would be sensitive to disruptions that have little effect on theta-burst LTP (Smith et al, 2009).
Conclusions
The forty-odd years of LTP research have been filled with debates, even arguments, about its cellular mechanism and significance for learning and memory. We have reviewed here one aspect of this history: the relationship between naturally-occurring cell activity patterns and LTP induction. The traditions that have influenced attitudes about synaptic plasticity have been argued elsewhere (Larson et al., 1991) and will not be elaborated here except to say that one approach considers all manifestations of plasticity to be potential participants in brain substrates of experience, or at least worthy of study in their own right, whereas a second approach emphasizes the plastic changes that are triggered by neuronal activity patterns that actually occur during learning events. Theta-burst LTP emerged from the latter tradition. The former tradition has its own merits; indeed, the discovery of LTP arose from an exploration of the frequency-dependence of synaptic transmission (Andersen, 1991).
Most of the work reviewed here has used the field CA1 of hippocampus as a model system. However, theta-bursts induce LTP in other cortical networks, including the dentate gyrus (Greenstein et al., 1988; Yeckel and Berger, 1998), subiculum (Commins et al., 1999), perirhinal cortex (Cousens and Otto, 1998), piriform cortex (Jung et al., 1990; Kanter and Haberly, 1990), entorhinal cortex (de Curtis and Llinas, 1993; Yun et al., 2002), as well as visual (Heynen and Bear, 2001; Kirkwood et al., 1995), somatosensory (Castro-Alamancos and Connors, 1996; Hardingham et al., 2003), auditory (Hogsden et al., 2011), anterior cingulate (Liu and Zhuo, 2014), prefrontal (Maroun and Richter-Levin, 2003; Vickery et al., 1997), and insular (Liu et al., 2013) neocortices. Whether or not theta-burst LTP in these areas reflects resonance with local circuit operation and the same cellular mechanisms as in CA1 is not clear. Neocortical neurons show intrinsic rhythmogenesis (Silva et al., 1991) and circuit resonance (Castro-Alamancos, 2013) in the theta frequency range. Furthermore, there is now a large literature of studies using transcranial magnetic stimulation protocols based on theta burst stimulation for therapeutic purposes in neurological disease in which long-term enhancement of cortical function is indicated (Huang et al., 2005; Nettekoven et al., 2014).
We have presented a model for the means by which theta burst stimulation leads to NMDA receptor activation for LTP induction, tested the efficiency of theta-burst LTP, described the limitations of spatial summation of synaptic activity during theta bursts, and discussed the special sensitivity of theta-burst LTP to a variety of experimental manipulations. We argued that theta-burst LTP represents a minimal model for the LTP process. The discussion has emphasized cellular mechanisms and has omitted detailed consideration of the role of theta and bursting in learning and memory. A few studies have reported parallel effects of experimental manipulations on spontaneous theta rhythm in vivo and theta-burst LTP (Maren et al., 1994; Staubli and Xu, 1995). An alternative approach, showing that theta-burst stimulation in vivo produces synaptic potentiation as its behavioral significance is learned (Roman et al., 1987) was instramental in the development of the theta-burst LTP paradigm.
Highlights.
A model for induction of LTP by theta-burst stimulation is presented.
Theta-burst stimulation is shown to be more efficient than tetanic stimulation.
Associative synaptic interactions during theta bursts are limited to major dendritic compartments.
Theta-burst LTP is more sensitive to many experimental conditions than is tetanus-induced LTP.
Acknowledgments
We thank Nancy Long Bartolotto and Joel Crouch for assistance with some of the experiments reported here. JL was supported by grants from the NIH (DC005793) and Department of Defense (USAMRMC 10917352). EM was supported by the UIC Honors College, UIC College of Liberal Arts and Sciences, the Glenn Foundation for Medical Research, and NIH (T32 AG021890).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger BE, Nicoll RA. Feed-forward dendritic inhibition in rat hippocampal pyramidal cells studied in vitro. J Physiol. 1982;328:105–123. doi: 10.1113/jphysiol.1982.sp014255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersen P. Foreword: LTP-an exciting and continuing saga. In: Baudry M, Davis JL, editors. In: Long-Term Potentiation: A Debate of Current Issues. Cambridge, MA: MIT Press; 1991. pp. xiii–xvii. Vol. [Google Scholar]
- Arai A, Lynch G. Factors regulating the magnitude of long-term potentiation induced by theta pattern stimulation. Brain Res. 1992;598:173–184. doi: 10.1016/0006-8993(92)90181-8. [DOI] [PubMed] [Google Scholar]
- Arai A, Silberg J, Lynch G. Differences in the refractory properties of two distinct inhibitory circuitries in field CA1 of the hippocampus. Brain Res. 1995;704:298–306. doi: 10.1016/0006-8993(95)01137-4. [DOI] [PubMed] [Google Scholar]
- Arai AC, Xia YF, Suzuki E. Modulation of AMPA receptor kinetics differentially influences synaptic plasticity in the hippocampus. Neuroscience. 2004;123:1011–1024. doi: 10.1016/j.neuroscience.2003.10.033. [DOI] [PubMed] [Google Scholar]
- Barrionuevo G, Brown TH. Associative long-term potentiation in hippocampal slices. Proc Natl Acad Sci USA. 1983;80:7347–7351. doi: 10.1073/pnas.80.23.7347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Gardner-Medwin AR. Long-lasting potentiation of synaptic transmission in the dentate area of the unanaestetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:357–374. doi: 10.1113/jphysiol.1973.sp010274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bliss TV, Lomo T. Long-lasting potentiation of synaptic transmission in the dentate area of the anaesthetized rabbit following stimulation of the perforant path. J Physiol. 1973;232:331–356. doi: 10.1113/jphysiol.1973.sp010273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boddeke EWGM, Enz A, Shapiro G. SDZ ENS 163, a selective muscarinic Ml receptor agonist, facilitates the induction of long-term potentiation in rat hippocampal slices. Eur J Pharmacol. 1992;222:21–25. doi: 10.1016/0014-2999(92)90457-f. [DOI] [PubMed] [Google Scholar]
- Capocchi G, Zampolini M, Larson J. Theta burst stimulation is optimal for induction of LTP at both apical and basal dendritic synapses on hippocampal CA1 neurons. Brain Res. 1992;591:332–336. doi: 10.1016/0006-8993(92)91715-q. [DOI] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Connors BW. Short-term synaptic enhancement and long-term potentiation in neocortex. Proc.Natl.Acad.Sci.U.S.A. 1996;93:1335–1339. doi: 10.1073/pnas.93.3.1335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA. The motor cortex: a network tuned to 7–14 Hz. Front Neural Circuits. 2013;7:21. doi: 10.3389/fncir.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen G, et al. Relative contribution of endogenous neurotrophins in hippocampal long-term potentiation. J.Neurosci. 1999;19:7983–7990. doi: 10.1523/JNEUROSCI.19-18-07983.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen LY, et al. Learning induces neurotrophin signaling at hippocampal synapses. Proc.Natl.Acad.Sci.U.S.A. 2010;107:7030–7035. doi: 10.1073/pnas.0912973107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Commins S, et al. The effects of single and multiple episodes of theta patterned or high frequency stimulation on synaptic transmission from hippocampal area CA1 to the subiculum in rats. Neurosci Lett. 1999;270:99–102. doi: 10.1016/s0304-3940(99)00486-3. [DOI] [PubMed] [Google Scholar]
- Corradetti R, et al. Serotonin blocks the long-term potentiation induced by primed burst stimulation in the CA1 region of rat hippocampal slices. Neuroscience. 1992;46:511–518. doi: 10.1016/0306-4522(92)90140-w. [DOI] [PubMed] [Google Scholar]
- Costenla AR, de Mendonca A, Ribeiro JA. Adenosine modulates synaptic plasticity in hippocampal slices from aged rats. Brain Res. 1999;851:228–234. doi: 10.1016/s0006-8993(99)02194-0. [DOI] [PubMed] [Google Scholar]
- Cousens G, Otto TA. Induction and transient suppression of long-term potentiation in the peri- and postrhinal cortices following theta-related stimulation of hippocampal field CM. Brain Res. 1998;780:95–101. doi: 10.1016/s0006-8993(97)01151-7. [DOI] [PubMed] [Google Scholar]
- Davies CH, Davies SN, CoUingridge GL. Paired-pulse depression of monosynaptic GABA-mediated inhibitory postsynaptic responses in rat hippocampus. J Physiol. 1990;424:513–531. doi: 10.1113/jphysiol.1990.sp018080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davies CH, et al. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- de Curtis M, Llinas RR. Entorhinal cortex long-term potentiation evoked by theta-patterned stimulation of associative fibers in the isolated in vitro guinea pig brain. Brain Res. 1993;600:327–330. doi: 10.1016/0006-8993(93)91391-5. [DOI] [PubMed] [Google Scholar]
- del Cerro S, Jung M, Lynch G. Benzodiazepines block long-term potentiation in slices of hippocampus and piriform cortex. Neuroscience. 1992;49:1–6. doi: 10.1016/0306-4522(92)90071-9. [DOI] [PubMed] [Google Scholar]
- Deschenes M, Moore J, Kleinfeld D. Sniffing and whisking in rodents. Curr Opin Neurobiol. 2012;22:243–250. doi: 10.1016/j.conb.2011.11.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, Dunwiddie TV, Rose GM. Characteristics of hippocampal primed burst potentiation in vitro and in the awake rat. J.Neurosci. 1988;8:4079–4088. doi: 10.1523/JNEUROSCI.08-11-04079.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diamond DM, et al. Adrenalectomy reduces the threshold for hippocampal primed burst potentiation in the anesthetized rat. Brain Res. 1989;492:356–360. doi: 10.1016/0006-8993(89)90919-0. [DOI] [PubMed] [Google Scholar]
- Diamond DM, et al. Inverted-U relationship between the level of peripheral corticosterone and the magnitude of hippocampal primed burst potentiation. Hippocampus. 1992;2:421–430. doi: 10.1002/hipo.450020409. [DOI] [PubMed] [Google Scholar]
- Diamond DM, Fleshner M, Rose GM. Psychological stress repeatedly blocks hippocampal primed burst potentiation in behaving rats. Behav.Brain Res. 1994;62:1–9. doi: 10.1016/0166-4328(94)90032-9. [DOI] [PubMed] [Google Scholar]
- Douglas RM, Goddard GV. Long-term potentiation of the perforant path-granule cell synapse in the rat hippocampus. Brain Res. 1975;86:205–215. doi: 10.1016/0006-8993(75)90697-6. [DOI] [PubMed] [Google Scholar]
- Douglas RM. Long lasting synaptic potentiation in the rat dentate gyrus following brief high frequency stimulation. Brain Res. 1977;126:361–365. doi: 10.1016/0006-8993(77)90733-8. [DOI] [PubMed] [Google Scholar]
- Evers MR, et al. Impairment of L-type Ca2+ channel-dependent forms of hippocampal synaptic plasticity in mice deficient in the extracellular matrix glycoprotein tenascin-C. J Neurosci. 2002;22:7177–7194. doi: 10.1523/JNEUROSCI.22-16-07177.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feldman Daniel E. The Spike-Timing Dependence of Plasticity. Neuron. 2012;75:556–571. doi: 10.1016/j.neuron.2012.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong N, et al. GABA transporter-1 activity modulates hippocampal theta oscillation and theta burst stimulation-induced long-term potentiation. J Neurosci. 2009;29:15836–15845. doi: 10.1523/JNEUROSCI.4643-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Govindarajan A, et al. The dendritic branch is the preferred integrative unit for protein synthesis-dependent LTP. Neuron. 2011;69:132–146. doi: 10.1016/j.neuron.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Granger R, et al. Non-Hebbian properties of long-term potentiation enable high-capacity encoding of temporal sequences. Proc.Natl.Acad.Sci.U.S.A. 1994;91:10104–10108. doi: 10.1073/pnas.91.21.10104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green JD, Arduini A. Hippocampal electrical activity in arousal. J.Neurophysiol. 1954;17:533–557. doi: 10.1152/jn.1954.17.6.533. [DOI] [PubMed] [Google Scholar]
- Greenstein YJ, Pavlides C, Winson J. Long-term potentiation in the dentate gyrus is preferentially induced at theta rhythm periodicity. Brain Res. 1988;438:331–334. doi: 10.1016/0006-8993(88)91358-3. [DOI] [PubMed] [Google Scholar]
- Grover LM, Teyler TJ. Two components of long-term potentiation induced by different patterns of afferent activation. Nature. 1990;347:477–479. doi: 10.1038/347477a0. [DOI] [PubMed] [Google Scholar]
- Grover LM, et al. LTP in hippocampal area CA1 is induced by burst stimulation over a broad frequency range centered around delta. Learn Mem. 2009;16:69–81. doi: 10.1101/lm.1179109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gustafsson B, Wigstrom H. Hippocampal long-lasting potentiation produced by pairing single volleys and brief conditioning tetani evoked in separate afferents. J.Neurosci. 1986;6:1575–1582. doi: 10.1523/JNEUROSCI.06-06-01575.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardie J, Spruston N. Synaptic depolarization is more effective than back-propagating action potentials during induction of associative long-term potentiation in hippocampal pyramidal neurons. J.Neurosci. 2009;29:3233–3241. doi: 10.1523/JNEUROSCI.6000-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hardingham N, et al. Neocortical long-term potentiation and experience-dependent synaptic plasticity require alpha-calcium/calmodulin-dependent protein kinase II autophosphorylation. J.Neurosci. 2003;23:4428–4436. doi: 10.1523/JNEUROSCI.23-11-04428.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hernandez RV, et al. Differences in the magnitude of long-term potentiation produced by theta burst and high frequency stimulation protocols matched in stimulus number. Brain Res.Brain Res.Protoc. 2005;15:6–13. doi: 10.1016/j.brainresprot.2005.02.003. [DOI] [PubMed] [Google Scholar]
- Heynen AJ, Bear MF. Long-term potentiation of thalamocortical transmission in the adult visual cortex in vivo. J.Neurosci. 2001;21:9801–9813. doi: 10.1523/JNEUROSCI.21-24-09801.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoffman KL, et al. Saccades during visual exploration align hippocampal 3–8 Hz rhythms in human and non-human primates. Frontiers in Systems Neuroscience. 2013;7 doi: 10.3389/fnsys.2013.00043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hogsden JL, Rosen LG, Dringenberg HC. Pharmacological and deprivation-induced reinstatement of juvenile-like long-term potentiation in the primary auditory cortex of adult rats. Neuroscience. 2011;186:208–219. doi: 10.1016/j.neuroscience.2011.04.002. [DOI] [PubMed] [Google Scholar]
- Holscher C, Anwyl R, Rowan MJ. Stimulation on the positive phase of hippocampal theta rhythm induces long-term potentiation that can Be depotentiated by stimulation on the negative phase in area CA1 in vivo. J.Neurosci. 1997;17:6470–6477. doi: 10.1523/JNEUROSCI.17-16-06470.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YZ, et al. Theta burst stimulation of the human motor cortex. Neuron. 2005;45:201–206. doi: 10.1016/j.neuron.2004.12.033. [DOI] [PubMed] [Google Scholar]
- Hyman JM, et al. Stimulation in hippocampal region CA1 in behaving rats yields long-term potentiation when delivered to the peak of theta and long-term depression when delivered to the trough. J.Neurosci. 2003;23:11725–11731. doi: 10.1523/JNEUROSCI.23-37-11725.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jedlicka P, et al. Impairment of in vivo theta-burst long-term potentiation and network excitability in the dentate gyrus of synaptopodin-deficient mice lacking the spine apparatus and the cisternal organelle. Hippocampus. 2009;19:130–140. doi: 10.1002/hipo.20489. [DOI] [PubMed] [Google Scholar]
- Jung MW, Larson J, Lynch G. Long-term potentiation of monosynaptic EPSPs in rat piriform cortex in vitro. Synapse. 1990;6:279–283. doi: 10.1002/syn.890060307. [DOI] [PubMed] [Google Scholar]
- Kang H, et al. Neurotrophins and time: different roles for TrkB signaling in hippocampal long-term potentiation. Neuron. 1997;19:653–664. doi: 10.1016/s0896-6273(00)80378-5. [DOI] [PubMed] [Google Scholar]
- Kanter ED, Haberly LB. NMDA-dependent induction of long-term potentiation in afferent and association fiber systems of piriform cortex in vitro. Brain Res. 1990;525:175–179. doi: 10.1016/0006-8993(90)91337-g. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Brown TH. Differential conditioning of associative synaptic enhancement in hippocampal brain slices. Science. 1986;232:85–87. doi: 10.1126/science.3952501. [DOI] [PubMed] [Google Scholar]
- Kelso SR, Ganong AH, Brown TH. Hebbian synapses in hippocampus. Proc Natl Acad Sci USA. 1986;83:5326–5330. doi: 10.1073/pnas.83.14.5326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirkwood A, Lee HK, Bear MF. Co-regulation of long-term potentiation and experience-dependent synaptic plasticity in visual cortex by age and experience. Nature. 1995;375:328–331. doi: 10.1038/375328a0. [DOI] [PubMed] [Google Scholar]
- Kramar EA, et al. Synaptic evidence for the efficacy of spaced learning. Proc Natl Acad Sci USA. 2012;109:5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larson J, Lynch G. Induction of synaptic potentiation in hippocampus by patterned stimulation involves two events. Science. 1986;232:985–988. doi: 10.1126/science.3704635. [DOI] [PubMed] [Google Scholar]
- Larson J, Wong D, Lynch G. Patterned stimulation at the theta frequency is optimal for the induction of hippocampal long-term potentiation. Brain Res. 1986;368:347–350. doi: 10.1016/0006-8993(86)90579-2. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Role of N-methyl-D-aspartate receptors in the induction of synaptic potentiation by burst stimulation patterned after the hippocampal theta-rhythm. Brain Res. 1988;441:111–118. doi: 10.1016/0006-8993(88)91388-1. [DOI] [PubMed] [Google Scholar]
- Larson J, Lynch G. Theta pattern stimulation and the induction of LTP: the sequence in which synapses are stimulated determines the degree to which they potentiate. Brain Res. 1989;489:49–58. doi: 10.1016/0006-8993(89)90007-3. [DOI] [PubMed] [Google Scholar]
- Larson J, Ambros-Ingerson J, Lynch G. Sites and mechanisms for expression of long-term potentiation. In: Baudry M, Davis JL, editors. In: Long-Term Potentiation: A Debate of Current Issues. Cambridge, MA: MIT Press; 1991. pp. 121–139. Vol. [Google Scholar]
- Larson J, et al. Alterations in synaptic transmission and long-term potentiation in hippocampal slices from young and aged PDAPP mice. Brain Res. 1999;840:23–35. doi: 10.1016/s0006-8993(99)01698-4. [DOI] [PubMed] [Google Scholar]
- Larson JR. Activity Patterns, Postsynaptic Events, and the Induction of Synaptic Plasticity in the Hippocampus. Irvine, Irvine, CA: University of California; 1987. p. 152. Vol. PhD, ed.^eds. [Google Scholar]
- Liu MG, et al. Long-term potentiation of synaptic transmission in the adult mouse insular cortex: multielectrode array recordings. J Neurophysiol. 2013;110:505–521. doi: 10.1152/jn.01104.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu MG, Zhuo M. No requirement of TRPV1 in long-term potentiation or long-term depression in the anterior cingulate cortex. Mol Brain. 2014;7:27. doi: 10.1186/1756-6606-7-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luthi A, et al. Endogenous serine protease inhibitor modulates epileptic activity and hippocampal long-term potentiation. J.Neurosci. 1997;17:4688–4699. doi: 10.1523/JNEUROSCI.17-12-04688.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch G, et al. Differences between synaptic plasticity thresholds result in new timing rules for maximizing long-term potentiation. Neuropharmacology. 2013;64:27–36. doi: 10.1016/j.neuropharm.2012.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F. Temporal relations between hippocampal slow waves and exploratory sniffing in hamsters. Behav.Biol. 1975;14:295–308. doi: 10.1016/s0091-6773(75)90419-8. [DOI] [PubMed] [Google Scholar]
- Macrides F, Eichenbaum HB, Forbes WB. Temporal relationship between sniffing and the limbic theta rhythm during odor discrimination reversal learning. J.Neurosci. 1982;2:1705–1717. doi: 10.1523/JNEUROSCI.02-12-01705.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malik R, Chattarji S. Enhanced intrinsic excitability and EPSP-spike coupling accompany enriched environment-induced facilitation of LTP in hippocampal CA1 pyramidal neurons. J Neurophysiol. 2012;107:1366–1378. doi: 10.1152/jn.01009.2011. [DOI] [PubMed] [Google Scholar]
- Maren S, et al. Parallel augmentation of hippocampal long-term potentiation, theta rhythm, and contextual fear conditioning in water-deprived rats. Behavioral Neuroscience. 1994;108:44–56. doi: 10.1037//0735-7044.108.1.44. [DOI] [PubMed] [Google Scholar]
- Maroun M, Richter-Levin G. Exposure to acute stress blocks the induction of long-term potentiation of the amygdala-prefrontal cortex pathway in vivo. J.Neurosci. 2003;23:4406–4409. doi: 10.1523/JNEUROSCI.23-11-04406.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarren M, Alger BE. Use-dependent depression of IPSPs in rat hippocampal pyramidal cells in vitro. J Neurophysiol. 1985;53:557–571. doi: 10.1152/jn.1985.53.2.557. [DOI] [PubMed] [Google Scholar]
- McCartney H, et al. Theta reset produces optimal conditions for long-term potentiation. Hippocampus. 2004;14:684–687. doi: 10.1002/hipo.20019. [DOI] [PubMed] [Google Scholar]
- Meighan PC, et al. Effects of matrix metalloproteinase inhibition on short- and long-term plasticity of schaffer collateral/CA1 synapses. J Neurochem. 2007;102:2085–2096. doi: 10.1111/j.1471-4159.2007.04682.x. [DOI] [PubMed] [Google Scholar]
- Moore CI, Browning MD, Rose GM. Hippocampal plasticity induced by primed burst, but not long-term potentiation, stimulation is impaired in area CA1 of aged Fischer 344 rats. Hippocampus. 1993;3:57–66. doi: 10.1002/hipo.450030106. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 1991;252:1718–1720. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- Mott DD, Lewis DV. GABAB receptors mediate disinhibition and facilitate long-term potentiation in the dentate gyrus. Epilepsy Res.Suppl. 1992;7:119–134. [PubMed] [Google Scholar]
- Nettekoven C, et al. Dose-dependent effects of theta burst rTMS on cortical excitability and resting-state connectivity of the human motor system. J Neurosci. 2014;34:6849–6859. doi: 10.1523/JNEUROSCI.4993-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Keefe J, Nadel L. The Hippocampus as a Cognitive Map. Oxford University Press; New York: 1978. Vol. [Google Scholar]
- Orr G, et al. Hippocampal synaptic plasticity is modulated by theta rhythm in the fascia dentata of adult and aged freely behaving rats. Hippocampus. 2001;11:647–654. doi: 10.1002/hipo.1079. [DOI] [PubMed] [Google Scholar]
- Otto T, et al. Learning-related patterns of CA1 spike trains parallel stimulation parameters optimal for inducing hippocampal long-term potentiation. Hippocampus. 1991;1:181–192. doi: 10.1002/hipo.450010206. [DOI] [PubMed] [Google Scholar]
- Pacelli GJ, Su W, Kelso SR. Activity-induced depression of synaptic inhibition during LTP-inducing patterned stimulation. Brain Res. 1989;486:26–32. doi: 10.1016/0006-8993(89)91273-0. [DOI] [PubMed] [Google Scholar]
- Pacelli GJ, Su W, Kelso SR. Activity-induced decrease in early and late inhibitory synaptic conductances in hippocampus. Synapse. 1991;7:1–13. doi: 10.1002/syn.890070102. [DOI] [PubMed] [Google Scholar]
- Pan B, Zucker RS. A general model of synaptic transmission and short-term plasticity. Neuron. 2009;62:539–54. doi: 10.1016/j.neuron.2009.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan B, et al. Alterations of endocannabinoid signaling, synaptic plasticity, learning, and memory in monoacylglycerol lipase knock-out mice. J Neurosci. 2011;31:13420–13430. doi: 10.1523/JNEUROSCI.2075-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang PT, et al. Cleavage of proBDNF by tPA/plasmin is essential for long-term hippocampal plasticity. Science. 2004;306:487–491. doi: 10.1126/science.1100135. [DOI] [PubMed] [Google Scholar]
- Patterson SL, et al. Some forms of cAMP-mediated long-lasting potentiation are associated with release of BDNF and nuclear translocation of phospho-MAP kinase. Neuron. 2001;32:123–140. doi: 10.1016/s0896-6273(01)00443-3. [DOI] [PubMed] [Google Scholar]
- Pavlides C, et al. Long-term potentiation in the dentate gyrus is induced preferentially on the positive phase of theta-rhythm. Brain Res. 1988;439:383–387. doi: 10.1016/0006-8993(88)91499-0. [DOI] [PubMed] [Google Scholar]
- Rex CS, et al. Long-term potentiation is impaired in middle-aged rats: regional specificity and reversal by adenosine receptor antagonists. J.Neurosci. 2005;25:5956–5966. doi: 10.1523/JNEUROSCI.0880-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rojas-Libano D, et al. The olfactory bulb theta rhythm follows all frequencies of diaphragmatic respiration in the freely behaving rat. Frontiers in Behavioral Neuroscience. 2014;8 doi: 10.3389/fnbeh.2014.00214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roman F, Staubli U, Lynch G. Evidence for synaptic potentiation in a cortical network during learning. Brain Res. 1987;418:221–226. doi: 10.1016/0006-8993(87)90089-8. [DOI] [PubMed] [Google Scholar]
- Rose GM, Dunwiddie TV. Induction of hippocampal long-term potentiation using physiologically patterned stimulation. Neurosci.Lett. 1986;69:244–248. doi: 10.1016/0304-3940(86)90487-8. [DOI] [PubMed] [Google Scholar]
- Shalin SC, et al. Kinase suppressor of Rasl compartmentalizes hippocampal signal transduction and subserves synaptic plasticity and memory formation. Neuron. 2006;50:765–779. doi: 10.1016/j.neuron.2006.04.029. [DOI] [PubMed] [Google Scholar]
- Silva LR, Amitai Y, Connors BW. Intrinsic oscillations of neocortex generated by layer 5 pyramidal neurons. Science. 1991;251:432–435. doi: 10.1126/science.1824881. [DOI] [PubMed] [Google Scholar]
- Skucas VA, et al. Impairment of select forms of spatial memory and neurotrophin-dependent synaptic plasticity by deletion of glial aquaporin-4. Journal of Neuroscience. 2011;31:6392–6397. doi: 10.1523/JNEUROSCI.6249-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JP, et al. Stimulus pattern dependence of the Alzheimer's disease amyloid-beta 42 peptide's inhibition of long term potentiation in mouse hippocampal slices. Brain Res. 2009;1269:176–184. doi: 10.1016/j.brainres.2009.03.007. [DOI] [PubMed] [Google Scholar]
- Staubli U, Lynch G. Stable hippocampal long-term potentiation elicited by 'theta' pattern stimulation. Brain Res. 1987;435:227–234. doi: 10.1016/0006-8993(87)91605-2. [DOI] [PubMed] [Google Scholar]
- Staubli U, Xu FB. Effects of 5-HT3 receptor antagonism on hippocampal theta rhythm, memory, and LTP induction in the freely moving rat. J.Neurosci. 1995;15:2445–2452. doi: 10.1523/JNEUROSCI.15-03-02445.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsanov M, et al. Respiratory cycle entrainment of septal neurons mediates the fast coupling of sniffing rate and hippocampal theta rhythm. European Journal of Neuroscience. 2014;39:957–974. doi: 10.1111/ejn.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Praag H, et al. Unilateral hippocampal ablation at birth causes a reduction in contralateral LTP. Brain Res. 1998;795:170–178. doi: 10.1016/s0006-8993(98)00287-x. [DOI] [PubMed] [Google Scholar]
- Vanderwolf CH. Hippocampal electrical activity and voluntary movement in the rat. Electroencephalogr.Clin.Neurophysiol. 1969;26:407–418. doi: 10.1016/0013-4694(69)90092-3. [DOI] [PubMed] [Google Scholar]
- Vickery RM, Morris SH, Bindman LJ. Metabotropic glutamate receptors are involved in long-term potentiation in isolated slices of rat medial frontal cortex. J Neurophysiol. 1997;78:3039–3046. doi: 10.1152/jn.1997.78.6.3039. [DOI] [PubMed] [Google Scholar]
- Welker WI. Analysis of sniffing of the albino rat. Behaviour. 1964;22:223–244. [Google Scholar]
- White G, Levy WB, Steward O. Evidence that associative interactions between synapses during the induction of long-term potentiation occur within local dendritic domains. Proc.Natl.Acad.Sci.U.S.A. 1988;85:2368–2372. doi: 10.1073/pnas.85.7.2368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White G, Levy WB, Steward O. Spatial overlap between populations of synapses determines the extent of their associative interaction during the induction of long-term potentiation and depression. J.Neurophysiol. 1990;64:1186–1198. doi: 10.1152/jn.1990.64.4.1186. [DOI] [PubMed] [Google Scholar]
- Yeckel MF, Berger TW. Spatial distribution of potentiated synapses in hippocampus: dependence on cellular mechanisms and network properties. J Neurosci. 1998;18:438–450. doi: 10.1523/JNEUROSCI.18-01-00438.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun SH, Mook-Jung I, Jung MW. Variation in effective stimulus patterns for induction of long-term potentiation across different layers of rat entorhinal cortex. J.Neurosci. 2002;22 doi: 10.1523/JNEUROSCI.22-05-j0003.2002. RC214. [DOI] [PMC free article] [PubMed] [Google Scholar]