Abstract
Background: previously, frailty indices were constructed using mostly subjective health measures. The reporting error in this type of measure can have implications on the robustness of frailty findings.
Objective: to examine whether frailty assessment differs when we construct frailty indices using solely self-reported or test-based health measures.
Design: secondary analysis of data from The Irish LongituDinal study on Ageing (TILDA).
Subjects and methods: 4,961 Irish residents (mean age: 61.9 ± 8.4; 54.2% women) over the age of 50 years who underwent a health assessment were included in this analysis. We constructed three frailty indices using 33 self-reported health measures (SRFI), 33 test-based health measures (TBFI) and all 66 measures combined (CFI). The 2-year follow-up outcomes examined were all-cause mortality, disability, hospitalisation and falls.
Results: all three indices had a right-skewed distribution, an upper limit to frailty, a non-linear increase with age, and had a dose–response relationship with adverse outcomes. Levels of frailty were lower when self-reported items were used (SRFI: 0.12 ± 0.09; TBFI: 0.17 ± 0.15; CFI: 0.14 ± 0.13). Men had slightly higher frailty index scores than women when test-based measures were used (men: 0.17 ± 0.09; women: 0.16 ± 0.10). CFI had the strongest prediction for risk of adverse outcomes (ROC: 0.64–0.81), and age was not a significant predictor when it was included in the regression model.
Conclusions: except for sex differences, characteristics of frailty are similar regardless of whether self-reported or test-based measures are used exclusively to construct a frailty index. Where available, self-reported and test-based measures should be combined when trying to identify levels of frailty.
Keywords: frailty, frailty index, test-based health measures, self-reported health measures, older people
Introduction
Global populations are rapidly ageing, a pressing challenge for healthcare systems around the world. In Ireland, ∼13% of the population is 65 years or older, and this number is expected to double by 2040 [1]. Healthcare spending is also expected to rise from 5.8 to 6.7% of GDP by 2035 [2]. On the frontier of the challenges and opportunities of ageing is how we understand and respond to frailty in older adults.
As people age, they are more likely to experience health problems, but not everyone of the same age has the same risk for poor health. The concept of frailty captures this differential vulnerability to adverse outcomes among people of the same age: while many older adults are relatively fit, older adults who accumulate multiple health and social problems are frail [3]. Frail older adults are more vulnerable to a range of adverse health outcomes, which can make routine care less effective, more dangerous and more costly if this vulnerability is not identified and managed [4]. Even so, researchers, clinicians and policymakers have yet to agree on the best way to measure frailty [5].
A systematic review of frailty assessment tools concluded that the frailty index (FI) seems to be the most suitable instrument to capture frailty [6]. The properties of the FI have been investigated in databases from around the world and showed that this method is a robust measure of health, predicts mortality and other adverse outcomes, and has consistent characteristics among different populations [3]. Often, the FI is constructed using mostly subjective health measures (e.g. self-reported co-morbidities and mobility limitations). Given that cultural attitudes, variations in health literacy and diagnosis rates could affect health assessments [7–10], this could have implications for estimates about frailty. Although some early work from the Canadian Study of Health and Aging did not show persuasive differences between estimates based on self-report and objective test data from clinical assessments [11], more robust testing of these initial findings has not been undertaken. The current study examined whether frailty assessment differs when we construct FIs using solely self-reported or test-based health measures. For comparison, we have also evaluated a measure that combined the items.
Methods
Sample
This is a secondary analysis of the first (2010) and second (2012) waves of The Irish LongituDinal study on Ageing (TILDA). TILDA is a nationally representative study of over 8,500 community-dwelling residents in Ireland aged 50+ and their spouses/partners, independent of age. TILDA is unique among longitudinal studies internationally in the breadth of physical, mental health and cognitive measures and especially for the depth and quality of objective measures. Participants first completed a face-to-face computer-assisted interview in their own homes and then were invited to attend a health centre for a comprehensive health assessment carried out by qualified and trained research nurses [12]. For those who could not travel to a centre, part of the health assessments was conducted in their own home. Of the 8,175 participants aged 50+, 5,897 underwent an assessment at Wave 1 (85.4% in the health assessment centres and 15.6% in their own home). For the purpose of this project, we excluded from the analysis spouses/partners below the age of 50, those who completed the health assessment at Wave 1 in their own home and those for whom sufficient data were unavailable to identify their frailty level (see below). Ethical approval for TILDA was obtained from the Trinity College Dublin Research Ethics Committee, and all participants provided written informed consent.
Frailty indices
The frailty level was identified in Wave 1 participants using the FI approach [13]. We first screened all items from personal interviews and health assessments to select the deficits that were included in the FIs. In general, a deficit can be any symptom, sign, disease, disability or laboratory abnormality that is associated with age and adverse outcomes, present in at least 1% of the population, covers several organ systems and has no >5% missing data [14]. A minimum of 30 deficits are required to construct a valid FI. We constructed FIs using 33 self-reported health measures (self-reported FI (SRFI); e.g. self-reported hypertension, mobility problems) and 33 test-based health measures (test-based FI (TBFI); e.g. measured blood pressure, walking speed). The two indices were independently constructed by members of our research team who did not try to match the content area of the variables. We also constructed a third index combining all 66 measures (combined FI (CFI)). Activities of daily living (ADL) and instrumental activities of daily living (IADL) limitations, hospitalisation and falls were not included in the FIs, because they were the outcome measures in our analysis. See Supplementary data, Appendix A available in Age and Ageing online for full list of measures. The included measures were binary, ordinal or continuous variables and were coded on a 0–1 interval (e.g. 0, 0.5, 1; 0 meaning no deficit, 1 meaning the deficit is fully represented). Continuous variables with no published cut points were categorised using percentiles; participants scoring in the lowest 5th percentile received the full deficit, whereas those scoring between the 20th and 5th percentile received a half deficit. The participant's FI score was calculated by dividing the number of recorded deficits by the total number of measures. For example, a participant with a deficit count of 20/33 from the self-reported measures would have an SRFI score of 0.61. Participants missing >20% of the variables (>6 variables in SRFI and TBFI; >13 variables in CFI) were excluded from analysis. SRFI scores were calculated for all participants; however, 66 participants (1.3%) were excluded from the TBFI and CFI calculations. For comparison with other frailty prevalence estimates, the continuous FIs were dichotomised at 0.25 [15].
Outcome measures
Wave 2 consisted of an in-home interview only. From this wave, outcome data were all-cause mortality, disability (ADL, IADL), hospitalisation and falls (recurrent, non-accidental). ADL and IADL disability was defined as experiencing limitations with at least one ADL (dressing, walking across a room, bathing, eating a meal, getting out of bed or using a toilet) or IADL (preparing a meal, household chores, grocery shopping, making phone calls, taking medications or managing money), respectively. Hospitalisation was identified as any overnight hospital visit in the past 12 months. Recurrent fallers were identified as those participants experiencing two or more falls since the last interview (approximately the past 24 months), and non-accidental falls (or unexplained falls) were defined as those falls without obvious or apparent cause.
Statistical analysis
Descriptive data, including histograms and relationships of each FI with age, were used to describe the properties of the three indices. We compared FI scores by sex, age, education and living status. Predictive validity was tested using receiver operating characteristic (ROC) curves and evaluated the area under the curve (AUC). The associations between SRFI and TBFI with outcomes were evaluated independently and within the same model using logistic regression analysis adjusted for age and sex. Last, to understand whether the combination of all variables was better at predicting the outcomes than either the SRFI or TBFI on its own, we tested the association between our outcome measures and CFI. For each predictive model (ROC and regression analysis), we excluded the participants who did not report the specific outcome at Wave 1. For example, when we examined whether FIs can predict new falls at Wave 2, we excluded all participants who reported falls at Wave 1. We also repeated analysis for the whole sample to examine whether findings were different. Analyses were conducted using SPSS (version 18, SPSS Inc.) and Stata version 12. All reported confidence intervals are within 95%, and statistical significance level was set at a P value of 0.05.
Results
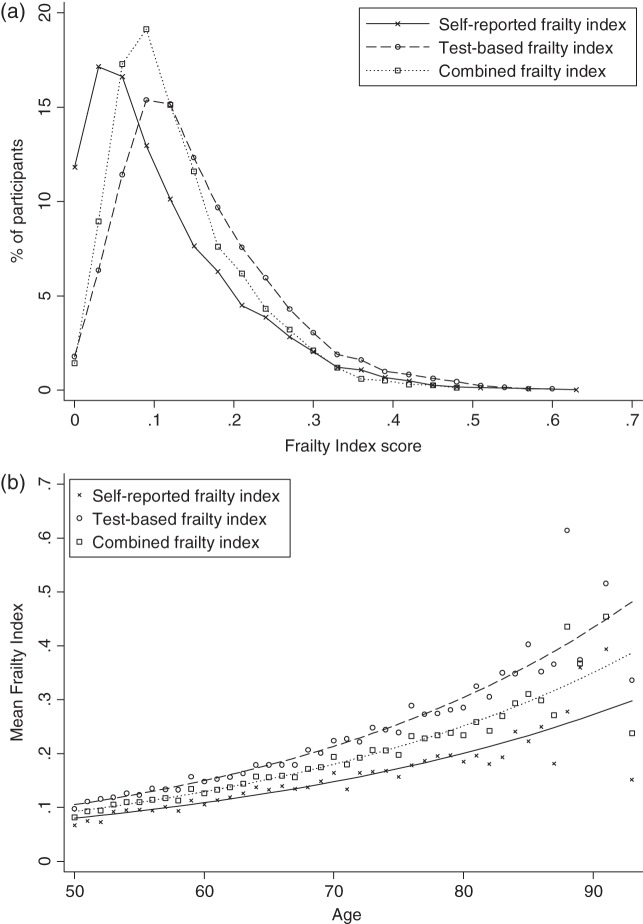
A total of 4,961 participants (mean age: 61.9 ± 8.4; 54.2% women) were included in this analysis. Among these participants, 93.5% completed Wave 2. All FIs had similar right-skewed distributions (Figure 1a) . The mean index score was higher for the TBFI (0.17 ± 0.15) compared with the SRFI (0.12 ± 0.09) (Table 1). The SRFI (r = 0.837, P < 0.001) and TBFI (r = 0.833, P < 0.001) were highly correlated with CFI, whereas the relationship between SRFI and TBFI was weaker (r = 0.395, P < 0.001). Age was positively associated with frailty; for all indices, the relationship could be reasonably represented by a non-linear (exponential) function (Figure 1b). FI scores increased ∼3.3% for the SRFI, 3.5% for the TBFI and 3.4% for the CFI for each additional year of age, on a log scale. SRFI and CFI were higher in women than in men (P < 0.01), whereas TBFI was higher in men (P = 0.03) (Table 1). Each index could discriminate across age, education and living status groups (P < 0.001) except the SRFI, which was similar among those with secondary and third/higher education (P = 0.34).
Figure 1.
(a) Distribution of scores on each frailty index. (b) Relationship of frailty scores with age by type of frailty index.
Table 1.
Descriptive characteristics of the three frailty indices
| Self-reported frailty index (SRFI) | Test-based frailty index (TBFI) | Combined frailty index (CFI) | |
|---|---|---|---|
| Whole sample | |||
| Meana | 0.12 ± 0.09 | 0.17 ± 0.15 | 0.14 ± 0.13 |
| Median | 0.09 | 0.15 | 0.13 |
| Range | 0–0.64 | 0–0.70 | 0–0.51 |
| 99th percentile | 0.42 | 0.48 | 0.40 |
| % Frail (>0.25 FI) | 10.7 | 16.9 | 10.8 |
| Sex (mean ± SD) | |||
| Male | 0.11 ± 0.09b | 0.17 ± 0.09b | 0.14 ± 0.08b |
| Female | 0.13 ± 0.10 | 0.16 ± 0.10 | 0.15 ± 0.08 |
| Age (mean ± SD) | |||
| 50–64 | 0.10 ± 0.08b | 0.14 ± 0.07b | 0.12 ± 0.06b |
| 65–74 | 0.15 ± 0.10b | 0.21 ± 0.09b | 0.18 ± 0.08b |
| 75+ | 0.19 ± 0.11 | 0.29 ± 0.12 | 0.24 ± 0.09 |
| Education (mean ± SD) | |||
| Primary/none | 0.15 ± 0.11b | 0.23 ± 0.11b | 0.19 ± 0.09b |
| Secondary | 0.11 ± 0.09 | 0.16 ± 0.09b | 0.14 ± 0.08b |
| Third/higher | 0.11 ± 0.09 | 0.14 ± 0.08 | 0.12 ± 0.07 |
| Live alone (mean ± SD) | |||
| No | 0.11 ± 0.09b | 0.16 ± 0.09b | 0.14 ± 0.08b |
| Yes | 0.14 ± 0.11 | 0.20 ± 0.12 | 0.17 ± 0.09 |
aSignificantly different between frailty indices.
bSignificantly different between group(s) (within frailty indices).
Fifty-two participants died at Wave 2. Among those who did not report the other outcome measures at Wave 1, 2.4% developed new ADL disability, 3.1% developed new IADL disability, 11.1% were hospitalised, 5.7% were identified as new recurrent fallers and 3.3% reported new non-accidental falls at Wave 2. When we compared the ability of the three indices to predict the incident outcome measures, CFI had higher AUCs and odds ratios than did the other two indices. The SRFI had higher AUCs and odds ratios than did the TBFI for all outcomes, save mortality (Table 2). In the regression models that included the CFI, age was no longer a significant predictor for any of the outcome measures, and sex was only significant for hospitalisation and mortality. When the SRFI and TBFI were included in the same logistic regression model, each independently predicted ADL and IADL disability and non-accidental falls; the SRFI had a higher odds ratio (e.g. odds ratio (95% confidence interval) for IADL disability: SRFI 1.081 (1.063–1.098); TRFI 1.052 (1.033–1.072)). Even so, only the TBFI significantly predicted mortality, and only the SRFI significantly predicted hospitalisation and recurrent falls (Table 2). When we repeated the analyses using the whole sample, absolute values in the prediction models were slightly different; however, the findings about the FI differences were similar (Supplementary data, Appendix B available in Age and Ageing online).
Table 2.
Frailty indices in relation to outcome measures
| Mortality (N = 4,961) | ADL disability (N = 4,341) | IADL disability (N = 4,451) | Hospitalisation (N = 4,098) | Recurrent falls (N = 3,715) | Non-accidental falls (N = 3,712) | |
|---|---|---|---|---|---|---|
| ROC analysis (AUC (95% CI)) | ||||||
| SRFI | 0.648 (0.566–0.730)* | 0.771 (0.726–0.815)* | 0.777 (0.735–0.819)* | 0.631 (0.603–0.659)* | 0.676 (0.637–0.715)* | 0.703 (0.652–0.753)* |
| TBFI | 0.729 (0.660–0.798)* | 0.728 (0.678–0.778)* | 0.721 (0.679–0.767)* | 0.599 (0.572–0.627)* | 0.607 (0.567–0.648)* | 0.645 (0.597–0.694)* |
| CFI | 0.721 (0.653–0.790)* | 0.796 (0.752–0.839)* | 0.809 (0.773–0.845)* | 0.640 (0.613–0.667)* | 0.670 (0.631–0.709)* | 0.715 (0.668–0.762)* |
| Logistic Regression Model 1: self-reported frailty index (SRFI), age and sex (OR (95% CI)) | ||||||
| Age (per 1 year) | 1.054 (1.021–1.088)* | 1.041 (1.017–1.066)* | 1.035 (1.014–1.056)* | 1.021 (1.009–1.034)* | 1.007 (0.990–1.024) | 1.016 (0.994–1.038) |
| Sex: Female | 0.539 (0.307–0.946)* | 0.925 (0.616–1.390) | 1.385 (0.965–1.989) | 0.752 (0.615–0.919)* | 0.858 (0.645–1.142) | 1.288 (0.880–1.886) |
| SRFI (per 0.01 score) | 1.036 (1.011–1.061)* | 1.083 (1.063–1.102)* | 1.093 (1.075–1.110)* | 1.043 (1.032–1.053)* | 1.066 (1.052–1.080)* | 1.071 (1.054–1.089)* |
| Logistic Regression Model 2: test-based frailty index (TBFI), age and sex (OR (95% CI)) | ||||||
| Age (per 1 year) | 1.023 (0.986–1.061) | 1.023 (0.996–1.051) | 1.017 (0.994–1.041) | 1.025 (1.011–1.039)* | 1.010 (0.990–1.029) | 1.010 (0.986–1.036) |
| Sex: Female | 0.562 (0.320–0.985)* | 1.109 (0.743–1.654) | 1.563 (1.095–2.230)* | 0.835 (0.686–1.017) | 1.002 (0.758–1.325) | 1.506 (1.035–2.191)* |
| TBFI (per 0.01 score) | 1.059 (1.031–1.088)* | 1.066 (1.045–1.087)* | 1.071 (1.052–1.090)* | 1.020 (1.008–1.031)* | 1.032 (1.016–1.048)* | 1.045 (1.026–1.065)* |
| Logistic Regression Model 3: SRFI, TBFI, age and sex (OR (95% CI)) | ||||||
| Age (per 1 year) | 1.019 (0.982–1.057) | 1.009 (0.982–1.037) | 1.002 (0.979–1.026) | 1.017 (1.003–1.031)* | 0.999 (0.979–1.018) | 1.000 (0.975–1.025) |
| Sex: Female | 0.537 (0.305–0.944)* | 0.915 (0.608–1.378) | 1.423 (0.989–2.048) | 0.756 (0.618–0.924)* | 0.864 (0.649–1.150) | 1.301 (0.888–1.905) |
| SRFI (per 0.01 score) | 1.022 (0.996–1.048) | 1.071 (1.052–1.091)* | 1.081 (1.063–1.098)* | 1.041 (1.030–1.052)* | 1.062 (1.047–1.077)* | 1.064 (1.045–1.083)* |
| TBFI (per 0.01 score) | 1.052 (1.023–1.082)* | 1.049 (1.027–1.071)* | 1.052 (1.033–1.072)* | 1.008 (0.996–1.020) | 1.014 (0.997–1.031) | 1.026 (1.005–1.047)* |
| Logistic Regression Model 4: combined frailty index (CFI), age and sex (OR (95% CI)) | ||||||
| Age (per 1 year) | 1.026 (0.990–1.063) | 1.004 (0.978–1.031) | 0.996 (0.974–1.019) | 1.011 (0.997–1.024) | 0.989 (0.970–1.008) | 0.992 (0.968–1.016) |
| Sex: Female | 0.527 (0.300–0.926)* | 0.927 (0.616–1.395) | 1.433 (0.996–2.061) | 0.783 (0.642–0.955)* | 0.905 (0.681–1.202) | 1.344 (0.919–1.965) |
| CFI (per 0.01 score) | 1.072 (1.040–1.106)* | 1.125 (1.098–1.152)* | 1.140 (1.115–1.165)* | 1.052 (1.038–1.066)* | 1.082 (1.062–1.101)* | 1.094 (1.070–1.119)* |
*P < 0.05.
Discussion
TILDA provides a unique opportunity to compare frailty assessment methods using self-reported and test-based health measures in a larger sample. Here, we found that most characteristics of frailty are similar whether exclusively self-reported or test-based measures are used to construct an FI: a right-skewed density distribution, an upper limit <0.7, a non-linear increase with age at 3–3.5% per year rate and dose–response relationships with adverse health outcomes. Even so, levels of frailty were lower when only self-reported items were included in the index. When self-report and test-based measures were combined and used to predict outcomes, this index had the strongest prediction and age was no longer a significant predictor.
Our findings should be interpreted with caution. The TILDA sample includes only community-dwelling participants and our analysis excluded people who could not travel to one of the TILDA centres to participate in the health assessments. Due to this, our findings may not be generalisable to institutionalised, hospitalised and the frailest community-dwelling people. This is a common issue for longitudinal studies with test-based measures and limits the ability of using these types of measures to identify frailty. One of the advantages of the FI is that it can be constructed using only self-reported measures, and as this study showed, the findings are not very different. Using test-based measures to identify frailty could be considerably more time consuming than using self-reported measures. Furthermore, the participants (n = 66) excluded from analysis due to missing test-based FI data tended to be older (mean age: 67.7 ± 11.6; 53% women) and frailer (SRFI: 0.23 ± 0.17) than those included. An additional limitation is that while most of the test-based measures were continuous variables, these were subsequently transformed into categorical variables using either published cut points or cut points based on their distribution. Using different cut points could have an impact on the results. To examine this issue, we repeated our analysis using different distribution cut points for the TBFI variables (e.g. <20% percentile = 1 or <5% percentile = 1). We found that although the absolute frailty scores changed, the overall conclusions about the FIs comparisons remained similar. A previous study from our group showed that variables included in an FI can be coded either as dichotomous or ordinal, with negligible impact on the performance of the index in predicting mortality [16].
In the present study, the prevalence of frailty was ∼11% when the SRFI was used (mean FI: 0.12 ± 0.09). In our previous study, using data from the Survey of Health, Ageing and Retirement in Europe (SHARE), the prevalence of frailty in a representative sample of Irish people aged 50+ was 15%, and the mean FI was 0.14 [15]. The lower scores observed in this study could be related to the exclusion of the frailest people, due to their inability to participate in the health assessments and preventing construction of the TBFI. If we did not exclude this subgroup from the analysis, the prevalence of frailty based on the SRFI would have been 14.7%, and the mean FI would be 0.13, findings that are very similar to our previous SHARE study. The 6% higher prevalence of frailty in the TBFI compared with the SRFI could be related to participants underestimating health problems, or simply reflecting an increased prevalence using a subclinical definition. These results however show some significant discrepancies between self-reported and objective measures. For example, 58% of men and 49% of women with objective evidence of hypertension were undiagnosed and 85% of older adults with objective evidence of anxiety symptoms did not report a physician diagnosis [17]. This discrepancy was related to the education and wealth status of participants [10]. In contrast, the TBFI may overestimate some health problems, perhaps reflecting poor performance of participants on the test day, which might not be a consistent health problem (e.g. white coat syndrome).
The common frailty characteristics among the three FIs employed here are similar to the characteristics observed in previous studies using the FI and other frailty scales [18]. Population-based studies using different FIs have generally shown non-linear increases in frailty with age [11, 18–20]. Also, this study showed that women have higher frailty scores than men when the SRFI and CFI were used but not when TBFI was used (women were lower). The sex differences of the SRFI and CFI are in agreement with previous research that described the phenomenon that women have poorer health but longer life expectancy, known as the male–female health survival paradox [21]. This health survival paradox has been identified in studies of different FIs [11, 18–20, 22–25], and its existence underscores the notion that the previous FIs—constructed mostly with self-reported measures—were imperfect in their ability to measure frailty: if women are at lower risk of death, then they should have lower frailty scores. This is consistent with the findings of the TBFI in this study and may imply that test-based measures more accurately capture levels of frailty.
Although each FI predicted health outcomes at 2 years, the combined FI increased the prediction precision. Whether this reflects an effect of examining both types of measures or the impact of using an FI with more items is not yet clear. Of note, when test-based measures, either on their own (TBFI) or in the combined FI, were included in the regression model, age was not a significant predictor of outcomes. This is unusual since very few frailty measures outperform age in predicting outcomes, especially in community-dwelling populations. Previous studies of hospitalised patients showed that adding frailty to a regression model resulted in age no longer being a significant independent predictor. There, however, the FI was based on a clinical comprehensive geriatric assessment [26, 27]. Together, these observations suggest systematic differences between self-reported and test-based health deficits.
Key points.
Previously, frailty indices were constructed using mostly subjective health measures.
Most characteristics of frailty are similar when self-reported or test-based measures are used to construct the FI.
Men have slightly higher FI scores than women when test-based measures are used to construct this index.
All frailty indices predicted health outcomes; the combined FI increased the prediction precision.
The combined FI outperformed age in predicting health outcomes.
Conflicts of interest
None declared.
Funding
This work was supported by the Ireland Canada University Foundation through a Dobbin Scholarship and by a Health Research Award from the Irish Health Research Board. TILDA is funded by the Irish Government, The Atlantic Philanthropies and Irish Life PLC. O.T. is supported by a Banting Postdoctoral Fellowship. B.K.K. has received funding from a Career Integration Grant from the European Commission's Marie Curie Actions (PCIG12-GA-2012-334041 Ageing Network). A.O.H. is funded by a Centre for Ageing Research and Development in Ireland (CARDI) Fellowship under the Ageing Research Leadership Programme (Reference: LP3). K.R. receives funding from the Canadian Institutes of Health Research and from the Dalhousie Medical Research Foundation as Kathryn Allen Weldon Professor of Alzheimer Research. The funding sources had no role in the design, methodology, data analysis or preparation of this manuscript.
Supplementary data
Supplementary data mentioned in the text are available to subscribers in Age and Ageing online.
Acknowledgements
The authors acknowledge the contribution of the participants in the study, members of the TILDA research team, study nurses, and administrators. Researchers interested in using TILDA data may access the data for free from the following sites: Irish Social Science Data Archive (ISSDA) at University College Dublin http://www.ucd.ie/issda/data/tilda/. Interuniversity Consortium for Political and Social Research (ICPSR) at the University of Michigan http://www.icpsr.umich.edu/icpsrweb/ICPSR/studies/34315.
References
- 1.Central Statistics Office. Population and Labour Force Projections. Dublin: Stationery Office, 2008. [Google Scholar]
- 2.European Commission/Economic Policy Committee. The 2009 Ageing Report: Economic and Budgetary Projections for the EU-27 Member States (2008–2060). http://europa.eu/epc/pdf/2009_ageing_report.pdf (June 2014, date last accessed) 2009.
- 3.Rockwood K, Mitnitski A. Frailty defined by deficit accumulation and geriatric medicine defined by frailty. Clin Geriatr Med 2011; 27: 17–26. [DOI] [PubMed] [Google Scholar]
- 4.Theou O, Rockwood K. Should frailty status always be considered when treating the elderly patient? Aging Health 2012; 8: 261–71. [Google Scholar]
- 5.Morley JE, Vellas B, van Kan GA, et al. Frailty consensus: a call to action. J Am Med Dir Assoc 2013; 14: 392–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Vries NM, Staal JB, van Ravensberg CD, Hobbelen JS, Olde Rikkert MG, Nijhuis-van der Sanden MW. Outcome instruments to measure frailty: a systematic review. Ageing Res Rev 2011; 10: 104–14. [DOI] [PubMed] [Google Scholar]
- 7.Bostock S, Steptoe A. Association between low functional health literacy and mortality in older adults: longitudinal cohort study. BMJ 2012; 344: e1602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Butler J, Burkhauser RV, Mitchell JM, Pincus TP. Measurement error in self-reported health variables. Rev Econ Statist 1987; 69: 644–50. [Google Scholar]
- 9.Johnston DW, Propper C, Shields MA. Comparing subjective and objective measures of health: evidence from hypertension for the income/health gradient. J Health Econ 2009; 28: 540–52. [DOI] [PubMed] [Google Scholar]
- 10.Mosca I, Bhuachalla BN, Kenny RA. Explaining significant differences in subjective and objective measures of cardiovascular health: evidence for the socioeconomic gradient in a population-based study. BMC Cardiovasc Disord 2013; 13: 64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mitnitski A, Song X, Skoog I, et al. Relative fitness and frailty of elderly men and women in developed countries and their relationship with mortality. J Am Geriatr Soc 2005; 53: 2184–9. [DOI] [PubMed] [Google Scholar]
- 12.Cronin H, O'Regan C, Kearney P, Finucane C, Kenny RA. Health and ageing: development of the TILDA health assessment. J Am Geriatr Soc 2013; 13 (s2): S269–78. [DOI] [PubMed] [Google Scholar]
- 13.Mitnitski A, Mogilner A, Rockwood K. Accumulation of deficits as a proxy measure of aging. Sci World J 2001; 1: 323–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Searle SD, Mitnitski A, Gahbauer EA, et al. A standard procedure for creating a frailty index. BMC Geriatr 2008; 8: 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Theou O, Brothers TD, Rockwood MR, Haardt D, Mitnitski A, Rockwood K. Exploring the relationship between national economic indicators and relative fitness and frailty in middle-aged and older Europeans. Age Ageing 2013; 42: 614–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Peña FG, Theou O, Wallace L, et al. Comparison of alternate scoring of variables on the performance of the frailty index. BMC Geriatr 2014; 14: 25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Barrett A, Savva G, Timonen V, Kenny RA, eds. Fifty Plus in Ireland 2011: First Results From the Irish Longitudinal Study on Ageing (TILDA). Dublin: Trinity College Dublin, 2011. [Google Scholar]
- 18.Theou O, Brothers TD, Peña FG, Mitnitski A, Rockwood K. Identifying common characteristics of frailty across seven scales. J Am Geriatr Soc 2014; 62: 901–6. [DOI] [PubMed] [Google Scholar]
- 19.Kulminski A, Yashin A, Ukrainsteva S, et al. Accumulation of health disorders as a systemic measure of aging: findings from the NLTCS data. Mech Ageing Dev 2006; 127: 840–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Romero-Ortuno R, Kenny RA. The frailty index in Europeans: association with age and mortality. Age Ageing 2012; 41: 684–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hubbard RE, Rockwood K. Frailty in older women. Maturitas 2011; 69: 203–7. [DOI] [PubMed] [Google Scholar]
- 22.Rockwood K, Mitnitski A, Song X, Steen B, Skoog I. Long-term risks of death and institutionalization of elderly people in relation to deficit accumulation at age 70. J Am Geriatr Soc 2006; 54: 975–9. [DOI] [PubMed] [Google Scholar]
- 23.Kulminski AM, Culminskaya IV, Ukraintseva SV, et al. Sex-specific health deterioration and mortality: the morbidity-mortality paradox over age and time. Exp Gerontol 2008; 42: 1052–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garcia-Gonzalez JJ, Garcia-Pena C, Franco-Marina F, Gutierrez-Robledo LM. A frailty index to predict the mortality risk in a population of senior Mexican adults. BMC Geriatr 2009; 9: 47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Goggins WB, Woo J, Sham A, Ho SC. Frailty index as a measure of biological age in a Chinese population. J Gerontol A Biol Sci Med Sci 2005; 60: 1046–51. [DOI] [PubMed] [Google Scholar]
- 26.Evans SJ, Sayers M, Mitnitski A, Rockwood K. The risk of adverse outcomes in hospitalized older patients in relation to a frailty index based on a comprehensive geriatric assessment. Age Ageing 2014; 43: 127–32. [DOI] [PubMed] [Google Scholar]
- 27.Singh I, Gallacher J, Davis K, Johansen A, Eeles E, Hubbard RE. Predictors of adverse outcomes on an acute geriatric rehabilitation ward. Age Ageing 2012; 41: 242–6. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.