Abstract
Anhedonia—a psychopathologic trait indicative of diminished interest, pleasure, and enjoyment—has been linked to use of and addiction to several substances, including tobacco. We hypothesized that anhedonic drug users develop an imbalance in the relative reward value of drug versus nondrug reinforcers, which could maintain drug use behavior. To test this hypothesis, we examined whether anhedonia predicted the tendency to choose an immediate drug reward (i.e., smoking) over a less immediate nondrug reward (i.e., money) in a laboratory study of non–treatment-seeking adult cigarette smokers. Participants (N = 275, ≥ 10 cigarettes/day) attended a baseline visit that involved anhedonia assessment followed by 2 counterbalanced experimental visits: (a) after 16-hr smoking abstinence and (b) nonabstinent. At both experimental visits, participants completed self-report measures of mood state followed by a behavioral smoking task, which measured 2 aspects of the relative reward value of smoking versus money: (1) latency to initiate smoking when delaying smoking was monetarily rewarded and (2) willingness to purchase individual cigarettes. Results indicated that higher anhedonia predicted quicker smoking initiation and more cigarettes purchased. These relations were partially mediated by low positive and high negative mood states assessed immediately prior to the smoking task. Abstinence amplified the extent to which anhedonia predicted cigarette consumption among those who responded to the abstinence manipulation, but not the entire sample. Anhedonia may bias motivation toward smoking over alternative reinforcers, perhaps by giving rise to poor acute mood states. An imbalance in the reward value assigned to drug versus nondrug reinforcers may link anhedonia-related psychopathology to drug use.
Keywords: anhedonia, addiction, smoking, reward, mood, psychopathology
There are several critical barriers to understanding how psychopathological symptoms and syndromes influence drug use etiology. For one, there are high levels of co-occurrence across many forms of psychopathology (Kessler, Chiu, Demler, Merikangas, & Walters, 2005), which complicates identification of the precise psychopathological source of drug use vulnerability. Also, within particular disorders, there is a high degree of symptomatic heterogeneity, such that different symptoms within a particular syndrome often only loosely cluster together and have distinct etiologies (Krueger & Bezdjian, 2009). Hence, itis possible that only certain symptoms within a psychopathological syndrome directly impact drug use vulnerability, whereas others have limited influence on drug use motivation. These issues underscore the need to apply approaches that depart from traditional syndrome-based models of addiction–psychopathology comorbidity.
Anhedonia as a Promising Target for Drug Use Research
A promising means for addressing the problems of cross-syndromes co-occurrence and within-syndrome heterogeneity is to identify transdiagnostic traits that are shared across multiple syndromes yet also differentiate different subtypes within particular syndromes (Krueger & Bezdjian, 2009). Anhedonia is one such trait. Anhedonia was originally conceptualized as consummatory anhedonia, or the incapacity to experience pleasure in response to pleasant stimuli (Snaith, 1993). Anhedonia has since been considered as a multilevel continuous construct—a common higher order broad dimension indicative of diminished appetitive functioning that is composed of several related lower order dimensions (Gard, Gard, Kring, & John, 2006), including consummatory anhedonia, global anhedonia—reduced life enjoyment and happiness (Carleton et al., 2013)—and anticipatory anhedonia—diminished subjective desire, interest, and anticipation of pleasant events (Gard et al., 2006). Anhedonia has sometimes been considered as a state-like symptom that is acutely elevated in the context of an active psychiatric episode or in response to stress (Berenbaum & Connelly, 1993; Bogdan & Pizzagalli, 2006). Anhedonia has also been conceptualized as a trait-like dimension (Lyons et al., 1995; Meehl, 2001), which is reflected in the personality-oriented measurement strategies employed (questionnaires instructing respondents to agree/disagree with characteristics self-statements, e.g., “I enjoy taking a deep breath of fresh air when I walk outside”; Gard et al., 2006) and the stability of anhedonia levels over time (Franken, Rassin, & Muris, 2007). Integrative perspectives posit that anhedonia is a trait-like dimension that is stable yet malleable (Loas, 1996), which is empirically distinct from negative affect and other emotional constructs (Clark & Watson, 1991; Loas, 1996; e.g., Shafer, 2006).
Anhedonia is a cardinal symptom in a Diagnostic and Statistical Manual of Mental Disorders (DSM)-defined major depressive episode (American Psychiatric Association, 2013). However, anhedonia is not elevated in a significant portion of major depression cases (Zimmerman, McGlinchey, Young, & Chelminski, 2006) and is a key marker that distinguishes between empirically derived symptom subtypes or subdimensions of depression (Clark & Watson, 1991; Dichter, 2010). In addition to its role in depression, anhedonia has also been linked to other psychopathologies comorbid with drug use, including psychosis (Cohen, Najolia, Brown, & Minor, 2011), borderline personality disorder (Bandelow, Schmahl, Falkai, & Wedekind, 2010), social anxiety (Watson & Naragon-Gainey, 2010), attention-deficit/hyperactivity disorder (Meinzer, Pettit, Leventhal, & Hill, 2012), and posttraumatic stress disorder (Kashdan, Elhai, & Frueh, 2006). Hence, anhedonia is a promising transdiagnostic trait for research on the psychopathological determinants of drug use.
Although an emerging literature indicates that anhedonia is associated with use of and addiction to a variety of substances (Hatzigiakoumis, Martinotti, Giannantonio, & Janiri, 2011), a consistent evidence base links anhedonia to cigarette smoking. Research illustrates positive associations between anhedonia and several markers of tobacco addiction, including cigarette craving (Cook, Spring, McChargue, & Hedeker, 2004), nicotine withdrawal (Leventhal, Ramsey, Brown, LaChance, & Kahler, 2008), and risk of smoking relapse following a quit attempt (Cook, Spring, McChargue, & Doran, 2010; Leventhal et al., 2008; Niaura et al., 2001; Zvolensky, Stewart, Vujanovic, Gavric, & Steeves, 2009). Tobacco addiction shares prototypical characteristics common to many addictions (e.g., tolerance, withdrawal, compulsive use, relapse, mediated by dopaminergic neurotransmission, rapid reinstatement, impaired control over use; Hughes, 2006). Therefore, identifying the psychological processes underlying anhedonia’s influence on the motivation to smoke may advance knowledge regarding the affective underpinnings of tobacco use as well as shed light on the relation between psychopathology and addictive disorders more broadly.
An Imbalance in the Relative Value of Drug Versus Nondrug Rewards as a Mechanism Linking Anhedonia and Drug Use Motivation
In theory, pleasure is generated from nonpharmacological rewards via a processing mechanism whereby individuals psychologically perceive stimulus inputs, the reward potency (i.e., motivational salience) of stimuli are processed, and higher perceptions of reward potency correspond with proportionally greater subjective pleasure (Bozarth, 1994). Individuals with elevated anhedonia are not entirely incapable of experiencing positive emotions and do not necessarily lack a desire for enjoyment in most cases (Gard et al., 2006). Rather, they may be deficient in certain elements of reward processing and may therefore require a higher threshold of psychological reward stimulation and more potent reinforcers to experience strong emotional effects in response to nonpharmacological rewards (Schlaepfer et al., 2008; Wise, 2008). Hence, common nondrug reinforcers that are not of extreme potency/ salience are likely to be assigned a relatively low incentive value for anhedonic individuals. In contrast to nonpharmacological rewards, drug rewards bypass the stimulus perception input processing mechanism and directly (i.e., pharmacologically) stimulate the neural circuitry involved in pleasure perception and reward salience. Hence, drug rewards that do not require certain elements of reward processing are likely to engender strong effects regardless of anhedonia. Furthermore, drug rewards are by nature more potent reinforcers because of their pharmacological activity and immediate psychoactive effects; hence, they are likely to generate stimulation that surpasses the elevated threshold for hedonic response in anhedonic individuals.
Consistent with this notion, the neural correlates of anhedonia are posited to involve attenuated mesolimbic activity and reduced sensitivity to the effects of nondrug reward stimulus inputs on phasic mesolimbic dopamine release, which putatively relates to the tendency for anhedonic individuals to experience low hedonic responses to nondrug rewards and attribute low incentive values to them (Nutt et al., 2007). This pattern of deficient response to nondrug rewards has relevance for drug rewards, as chronic low levels of mesolimbic dopaminergic transmission due to muted reward responses in anhedonia could result in up-regulation of dopamine receptors, which in turn could heighten sensitivity to the acute rewarding effects of exogenous substances that directly stimulate mesocorticolimbic circuitry (Tremblay, Naranjo, Cardenas, Herrmann, & Busto, 2002). Indeed, individuals with higher anhedonia experience greater acute subjective rewarding effects following administration of nicotine and other drugs of abuse (Cook, Spring, & McChargue, 2007; Tremblay et al., 2002, 2005) than individuals with lower anhedonia. Hence, drug rewards may be appraised with a disproportionately high incentive value in anhedonic individuals.
We speculate that with repeated drug use experiences, anhedonic individuals develop an imbalance between the relative reward value of drug and nondrug reinforcers because of differences in the potency of drug (vs. nondrug) rewards, which may be heightened in anhedonic individuals, as reviewed above. Such a reward imbalance may bias anhedonic individuals’ behavior toward the pursuit and consumption of substances that generate more potent and immediate reward and away from less potent and less immediate alternative reinforcers that occur along with drug abstinence, such as money saved and improved health.
The Current Study
The primary goal of this study was to test, within a sample of non–treatment-seeking daily cigarette smokers, the hypothesis that anhedonia would predict an imbalance in the reward value of drug relative to nondrug reinforcers. To this end, we examined whether individual differences in trait anhedonia would predict behavior on a laboratory task that required participants to choose between a drug reward with immediate effects (i.e., smoking) and a nondrug reward with delayed effects (i.e., money). This task measured two aspects of behavior indicative of the relative reward value of smoking: (1) latency to initiating the opportunity to smoke when delaying smoking is monetarily rewarded and (2) willingness to purchase individual cigarettes once the opportunity to smoke becomes available. We hypothesized that anhedonia would predict quicker smoking initiation and greater cigarette consumption.
One secondary goal was to investigate the role of mood state in the relation between anhedonia and smoking. Because an important determinant of positive mood states is the emotional response to nondrug rewards, anhedonic individuals may experience less frequent and robust positive mood states. Deficient positive mood states may be an important mechanism mediating trait anhedonia’s impact on drug use motivation because momentary low positive mood states may acutely enhance motivation to consume mood-altering substances to counteract deficient positive mood. By contrast, anhedonia is less strongly associated with negative mood states (Franken et al., 2007). We therefore hypothesized that the relation between trait anhedonia and the relative reward value of smoking would be mediated by low acute positive mood and not negative mood. It is also possible that characteristic mood state patterns may impact trait anhedonia, or anhedonia may be subsumed by mood state and merely reflects an epiphenomenon with regards to smoking motivation. Thus, we also explored whether anhedonia predicted smoking motivation over and above covariance with mood state.
Another secondary aim was to test the effects of acute drug abstinence on anhedonia–smoking relations. Presmoking abnormalities in brain reward circuitry that underlie anhedonia may sensitize one’s neural circuitry nicotine-induced neuroadaptations, which could lead to greater alterations in reward processing upon nicotine withdrawal (D’Souza & Markou, 2010). Accordingly, we hypothesized that the relation between trait anhedonia and the relative reward value of smoking would be stronger when participants were abstinent versus nonabstinent.
Method
Participants
Participants were recruited via announcements of opportunities to participate in a study on personality and smoking. To enhance generalization to the population of moderate-to-heavy adult smokers, inclusion criteria were (a) age ≥18 years old; (b) regular cigarette smoking for ≥2 years; (c) currently smoking ≥10 cigarettes/day; and (d) fluency in English. Exclusion criteria were (a) current DSM–IV dependence on substances other than nicotine in the past 30 days (to prevent modulation of responses due to withdrawal from other substances); (b) current DSM–IV mood disorder, psychotic symptoms, or use of psychiatric medications (to prevent cognitive or behavioral impairment that might interfere with completing the behavioral smoking task or modulation of tobacco abstinence effects by psychiatric medication); (c) breath carbon monoxide (CO) levels <10 ppm at intake (to exclude individuals who may be overreporting their smoking level); (d) use of noncigarette tobacco or nicotine products; and (e) currently pregnant. Participants were compensated $200 after completing the study. Individuals who met inclusion criteria (N = 502) following a preliminary telephone screen were invited for an in-person baseline screening and assessment session. Of these, 150 were ineligible because of low baseline CO (n = 95), current psychiatric disorder or use of psychiatric medications (n = 32), or other criteria (n = 23). Of the 352 eligible participants, 75 dropped out after study entry (there were no significant differences in dropouts vs. completers on anhedonia) and two twice failed to meet abstinence criteria at the abstinent session (see below), leaving a final sample of 275. The protocol was approved by the University of Southern California Institutional Review Board.
Procedure
Following a baseline visit that involved screening for study eligibility and completion of anhedonia and other baseline measures, participants attended two experimental visits (one 16-hr smoking abstinent and one nonabstinent) that began at 12 p.m. and were conducted within 2 to 14 days of each other; abstinence condition order was counterbalanced across participants. Participants were instructed to smoke normally before the nonabstinent session and smoked a cigarette in the laboratory at the outset of the nonabstinent session to standardize recency of smoking across participants. Participants were instructed not to smoke after 8 p.m. the day before the abstinent session, and abstinence was verified with a breath CO <10 ppm following from prior work and published recommendations (Leventhal, Waters, Moolchan, Heishman, & Pickworth, 2010; Society for Research on Nicotine and Tobacco, 2002). Those failing to meet the abstinence criterion could return later that week for a second attempt to complete their abstinent session (n = 15). Those with CO ≥10 ppm on their second attempt were discontinued (n = 2). Subsequently, participants completed measures of affect, nicotine withdrawal, and smoking urge (began at 12:15 p.m.) and then the behavioral procedure to measure of the reward value of smoking (began 1 p.m.; described below), followed by a rest period of no assessment or smoking (began 2–2:50 p.m. depending on choices made during the delay portion of the preceding task), and dismissal (4:10 p.m.).
Baseline Session Measures
The Structured Clinical Interview for DSM–IV Nonpatient Edition (First, Spitzer, Gibbon, & Williams, 2002) mood disorder, psychotic screen, and substance use disorder modules were used to assess psychiatric eligibility. To describe the sample, we administered measures of demographics and smoking history (e.g., age started smoking, cigarettes/day), the 10-item Alcohol Use Disorder Identification Test (Saunders, Aasland, Babor, de la Fuente, & Grant, 1993), the 20-item Drug Abuse Screening Test (Skinner, 1982), and the 10-item Anxious Arousal subscale of the 30-item short form of the Mood and Anxiety Symptom Questionnaire (MASQ-30; Wardenaar et al., 2010). To include as planned covariates, we also administered the six-item Fagerström Test of Nicotine Dependence (FTND; Heatherton, Kozlowski, Frecker, & Fagerström, 1991) and the 20-item Center for Epidemiologic Studies Depression scale (CES–D; Radloff, 1977).
Anhedonia Measures
Three measures were used to assess each of three common facets of anhedonia (i.e., global, anticipatory, and consummatory). For each measure, responses were coded such that higher scores reflect greater anhedonia.
Subjective Happiness Scale (SHS; Lyubomirsky & Lepper, 1999)
The SHS is a four-item scale that assesses trait global happiness and enjoyment from life (i.e., global anhedonia). Participants rate items on 7-point scales (e.g., “Some people enjoy life regardless of what is going on… . To what extent does this describe you?” 1 = not at all, 7 = a great deal) and a mean composite score is calculated across the items. Responses on the SHS exhibit good internal consistency, stability, convergent validity, and discriminant validity from composite depressive symptom indexes and negative emotionality (Lyubomirsky & Lepper, 1999; Neff, Rude, & Kirkpatrick, 2007).
Temporal Experience of Pleasure Scale (TEPS; Gard et al., 2006)
The TEPS assesses trait dispositions in the subjective experience of pleasure in response to normally pleasant situations (i.e., consummatory) as well as the interest, desire, and anticipation of pleasant events (i.e., anticipatory). Respondents rate 18 self-statements on a 6-point scale (1 = very false for me, 6 = very true for me). Two subscales are computed based on the average response per item within each scale—Consummatory (eight items; e.g., “A hot cup of coffee or tea on a cold morning is very satisfying to me”) and Anticipatory (10 items; e.g., “When I hear about a new movie starring my favorite actor, I can’t wait to see it”). TEPS subscales have exhibited adequate internal consistency, stability, and convergent validity with other relevant measures, as well as adequate discriminant validity from each other, negative emotionality, and composite measures of depressive symptoms (Favrod, Ernst, Giuliani, & Bonsack, 2009; Gard et al., 2006; Gard, Kring, Gard, Horan, & Green, 2007).
Composite anhedonia index
To capture the common broad construct shared among each of the three facets of anhedonia, we created a composite index based on the mean score of the SHS, TEPS–Anticipatory, and TEPS–Consummatory scales. Principal components analysis using oblique rotation illustrated that a single latent dimension accounted for 59% variance across the three scales (eigenvalues of 1.76, 0.89, 0.34), with prominent loadings from each indicator on that dimension (SHS = .53, TEPS–Consummatory = .82; TEPS–Anticipatory = .90), which is consistent with the notion that global, anticipatory, and consummatory anhedonia tap a common overarching construct (Gard et al., 2006).1
Experimental Session Measures
Manipulation checks
To assess the robustness of the abstinence manipulation, we administered (a) a breath CO assessment; (b) an 11-item version of the Minnesota Nicotine Withdrawal Scale (Hughes & Hatsukami, 1986), which measured symptoms experienced “so far today” on 6-point scales, yielding a mean composite (0–5 range); and (3) the Brief Questionnaire of Smoking Urges (Cox, Tiffany, & Christen, 2001), which assessed urge “right now,” yielding a mean score per item composite score (0–5 range).
Profile of Mood States (POMS; McNair, Lorr, & Droppleman, 1971)
The POMS is a well-validated affect scale commonly used to assess tobacco abstinence effects (Gilbert et al., 1999, 2002), and served as the sole measure of mood state. This study used a 72-item version in which participants rated affect adjectives based on how they were feeling “right now” (0 = not at all, 4 = extremely). Following prior work (Guadagnoli & Mor, 1989; Smith, Greenberg, & Seltzer, 2012), we calculated a positive mood composite based on the mean of the Elation, Vigor, and Friendless subscales as well as a negative mood composite based on the mean of the Anger, Anxiety, Confusion, Depression, and Fatigue subscales.
Behavioral task measure of the relative reward value of smoking (McKee, Krishnan-Sarin, Shi, Mase, & O’Malley, 2006)
This task yields objective behavioral measures of relative reward value of (a) initiating smoking versus delaying smoking for money and (b) self-administering cigarettes for money when given the opportunity to smoke. At the outset of this procedure, participants were given a tray containing eight cigarettes of their preferred brand, a lighter, and an ashtray. They were informed they could begin smoking at any point over the next 50 min but would receive $0.20 for each 5 min they delayed smoking (monetary value based on piloting among smokers from the same population). Hence, participants could receive a maximum of $2 for delaying smoking for the entire 50-min period. The delay period ended when the participant indicated they wished to smoke or at the end of 50 min if the participant chose not to smoke. Then participants began the self-administration period during which they were informed that they could smoke as much or little as they wished over the next 60 min, they had a $1.60 credit, and each cigarette lit would cost $0.20 (monetary value based on prior piloting). To limit the possibility that smoking choices during the session would be affected by the impending opportunity to smoke after the session ended, participants were reminded that they would not have another opportunity to smoke again until the end of the visit (4:10 p.m.). No additional measures were collected during the smoking task to avoid any disruption of natural smoking patterns. The two primary outcomes are latency to smoking initiation during the delay period (range 0–50 min) and number of cigarettes smoked during the self-administration period (range 0–8). In support of the validity of this task, prior work illustrates that tobacco deprivation, stress, and cessation medications modulate outcomes in expected directions (Leeman, O’Malley, White, & McKee, 2010; McKee et al., 2011; McKee, Weinberger, Shi, Tetrault, & Coppola, 2012).
Data Analyses
Design
Anhedonia was measured once for each participant at the baseline session. Two separate smoking task outcomes (i.e., latency to smoke, number of cigarettes purchased) were measured at two points: once at the abstinent session and once at the nonabstinent session. This study used a mixed design with the between-participants continuous variable of anhedonia fully crossed with the within-participant categorical variable of abstinence (abstinent vs. nonabstinent) as predictors of smoking task outcomes assessed at the abstinent and nonabstinent sessions.
Primary analytic approach
We used generalized estimating equations (GEE; Zeger, Liang, & Albert, 1988), an extension of the general linear model that accounts for nonindependent observations, because of the repeated measurement of study outcomes at both abstinent and nonabstinent visits. To assess the robustness of the abstinence manipulation, we initially tested a set of GEE models that included the within-participant abstinence variable (abstinent vs. nonabstinent) as the sole predictor, with separate GEEs for each outcome. To examine the relation of anhedonia to each smoking task outcome averaged across abstinent and nonabstinent conditions, we tested a set of GEE main effects models that included the between-participants continuous anhedonia composite index as the primary predictor after controlling for the within-participant abstinence variable. We then added the Anhedonia × Abstinence interaction term to each model to examine whether anhedonia’s relation to smoking task outcomes differed as a function of abstinence. All models were retested after adjusting for the planned covariate of overall depressive symptom severity (CES–D total score) to examine whether relations were specific to interest/ pleasure/enjoyment deficits or explained by the possibility that anhedonia is a proxy for psychiatric or emotional disturbance more broadly.2 We also adjusted for the planned covariates of nicotine dependence (FTND) and gender, as these are important clinical characteristics that may be linked with both smoking motivation and anhedonia (Gard et al., 2006; Leventhal, Kahler, Ray, & Zimmerman, 2009; Leventhal et al., 2007). Additional baseline variables that were significantly correlated with anhedonia were also included as covariates in adjusted models (MASQ-30 Anxious Arousal subscale and cigarettes/day).
Mediation analyses
Mediational paths were analyzed by computing the product of the coefficients from two GEE models: (1) baseline session anhedonia → experimental session mood state and (2) experimental session mood state (time-varying predictor with two data points for each participant, i.e., abstinent and non-abstinent) → experimental smoking task outcome. Separate models were tested for each smoking outcome. The product of the coefficients from these models indicated the strength of the indirect (“mediated”) effect. Significance was determined using the PRODCLIN approach involving estimation of asymmetric confidence intervals (CIs) around the mediational effect (MacKinnon, Fritz, Williams, & Lockwood, 2007). Separate analyses were performed for POMS positive and negative mood composites. Remaining direct effects were reported as the effect of anhedonia when included in the model with the mediator.
GEE analyses were conducted in SAS using PROC GENMOD specifying a continuous distribution (SAS Institute, 2003). Results for all analyses are reported as standardized parameter estimates (β + 95% CIs). Alpha for all analyses was set to .05.
Results
Preliminary Analyses
Sample characteristics
As illustrated in Table 1, the sample was demographically heterogeneous and consisted of, on average, moderate-to-heavy smokers with medium levels of tobacco dependence who had been smoking for many years and reported little alcohol and drug use problems. Approximately half of the sample reported Black race/ethnicity, which is representative of the area near the study site. Scores on the MASQ-30 Anxious Arousal subscale and CES–D (Tables 1 and 2) showed mild levels of emotional symptoms on average, with 15.5% and 22.5% surpassing screening cutoffs for anxiety (18+; Schulte-van Maaren et al., 2012) and depressive (16+; Radloff, 1977) symptoms, respectively.
Table 1.
Sample Baseline Characteristics
| Demographic | Value |
|---|---|
| Mean (SD) age (years) | 44.2 (10.6) |
| Male, % | 69 |
| Race/ethnicity, % | |
| Black | 53 |
| White | 33 |
| Multiracial | 4 |
| Other | 3 |
| Hispanic | 7 |
| Mean (SD) annual household income ($) | 26,693 (20,190) |
| Mean (SD) smoking characteristics | |
| FTND score | 5.27 (1.96) |
| Cigarettes smoked per day | 16.7 (7.0) |
| Age started smoking regularly (years) | 19.4 (5.6) |
| Mean (SD) emotional symptoms and substance use | |
| CES–D | 10.85 (8.27) |
| MASQ-30 Anxious Arousal subscale | 13.0 (4.5) |
| AUDIT | 3.47 (4.97) |
| DAST | 2.08 (3.78) |
Note. N = 275. FTND = Fagerström Test of Nicotine Dependence; CES–D = Center for Epidemiologic Studies Depression scale; MASQ-30 = Mood and Anxiety Symptom Questionnaire 30-item short form; AUDIT = Alcohol Use Disorder Identification Test; DAST = Drug Abuse Screening Test.
Table 2.
Descriptive Statistics and Correlations of Anhedonia Measures to Each Other and Other Characteristics
| Intercorrelation |
|||||
|---|---|---|---|---|---|
| Variable | Mean (SD) | 1 | 2 | 3 | 4 |
| Anhedonia measure | |||||
| 1. Anhedonia composite | 2.65 (0.71)a | (.59) | |||
| 2. SHS | 2.72 (1.13)b | .72† | (.76) | ||
| 3. TEPS–Consummatory subscale | 2.64 (0.90)c | .74† | .14* | (.77) | |
| 4. TEPS–Anticipatory subscale | 2.57 (0.79)c | .82† | .33† | .63† | (.77) |
| Correlations of anhedonia measures to other characteristics | |||||
| Age | −.01 | .07 | .06 | −.12* | |
| Male (vs. female) | .14* | −13* | .17** | .04 | |
| Black (vs. other) race | −.10 | .08 | −.12 | −.17** | |
| Annual household income | −.04 | .06 | −.07 | −.07 | |
| FTND | −.02 | −.03 | −.004 | .01 | |
| Cigarettes smoked per day | .14* | .06 | .12* | .11 | |
| Age started smoking regularly | .02 | .09 | −05 | −.08 | |
| CES–D | .29† | .39† | .07 | .14* | |
| MASQ-30 Anxious Arousal subscale | .17** | .01 | .03 | .29† | |
| AUDIT | .01 | −.05 | .02 | .05 | |
| DAST | .06 | .01 | .05 | .06 | |
Note. N = 275. Higher scores reflect greater anhedonia. Values on the diagonal within parentheses represent Cronbach’s as. Anhedonia composite = mean of SHS, TEPS-Consummatory, and TEPS–Anticipatory scales. SHS = Subjective Happiness Scale; TEPS = Temporal Experience of Pleasure Scale; FTND = Fagerström Test of Nicotine Dependence score; CES–D = Center for Epidemiologic Studies Depression scale; MASQ-30 = Mood and Anxiety Symptom Questionnaire 30-item short form; AUDIT = Alcohol Use Disorder Identification Test; DAST = Drug Abuse Screening Test.
Possible range = 1.0–6.3.
Possible range = 1.0–70.
Possible range = 1.0–6.0.
p < .0001.
p < .05.
p < .01.
p < .001.
Anhedonia
As shown in Table 2, the current sample evidenced variability across the anhedonia continuum and reported slightly higher anhedonia, on average, in comparison to mean values reported in prior community adult samples (Gard et al., 2007; Gooding & Pflum, 2012; Lyubomirsky & Lepper, 1999; Strauss, Wilbur, Warren, August, & Gold, 2011). The individual anhedonia scales exhibited adequate internal consistency and were closely correlated with the anhedonia composite index. Correlations with external characteristics showed discriminant validity from depression, anxiety, and alcohol/drug use problems, and null or modest correlations with demographic and smoking characteristics (see Table 2). MASQ-30 Anxious Arousal subscale and cigarettes/day were the only factors significantly associated with the composite anhedonia index and were therefore included as additional covariates in adjusted analyses described below. No anhedonia measure was correlated with annual household income (ps ≥ .24), suggesting that the income did not confound key analyses due to differential subjective weighting of money values on the smoking task.
Primary Analyses
Main effects of abstinence
As shown in Table 3, abstinence reduced CO levels and increased withdrawal symptoms, negative affect, and smoking urges, and reduced positive affect. The magnitudes of abstinence effects were medium to large in size, suggesting that the abstinence manipulation was effective. Abstinence also decreased latency to begin smoking during the delay portion of the smoking task by an average 16 min and increased smoking during the self-administration portion by an average of approximately one fourth of a cigarette (see Table 3).
Table 3.
Main Effects of Abstinence on Study Outcomes
| Nonabstinent |
Abstinent |
Abstinence effect |
||
|---|---|---|---|---|
| Variable | Mean (SD) | Mean (SD) | β (95% CI) | p |
| CO (ppm) | 27.8 (12.9) | 5.6 (2.1) | −.77 (−.82, −.72) | <.0001 |
| MNWSa | 1.06 (0.94) | 1.83 (1.10) | .35 (.29,.40) | <.0001 |
| QSUa | 1.01 (1.14) | 3.30 (1.06) | .72 (.67,.76) | <.0001 |
| POMS: Positive Mood Scaleb | 2.27 (0.88) | 1.85 (0.90) | −.23 (−.27, −.18) | <.0001 |
| POMS: Negative Mood Scaleb | 0.64 (0.61) | 0.81 (0.72) | .12 (.07,.17) | <.0001 |
| Smoking task | ||||
| Minutes delayed | 39.3 (17.8) | 23.3 (22.8) | −.34 (−.40, −.27) | <.0001 |
| Number of cigarettes smoked in postdelay Self-administration procedure | 1.25 (0.93) | 1.54 (0.94) | .17 (.11,.22) | <.0001 |
Note. N = 275. Standardized results of generalized estimating equations examining the within-participant effect of abstinence (abstinent vs. nonabstinent) on outcomes. CO = carbon monoxide; MNWS = Minnesota Nicotine Withdrawal Scale; QSU = Questionnaire of Smoking Urges; POMS = Profile of Mood States.
Possible range = 1.0–5.0.
Possible range = 1.0–4.0.
Main effects of anhedonia on smoking task outcomes
Averaged across abstinence condition, participants with higher anhedonia had shorter latencies to initiate smoking during the delay procedure (β = −.10, 95% CI [−.20, −.01], p = .03) and consumed more cigarettes during the self-administration procedure, (β = .13, 95% CI [.04, .22], p = .003). These findings were not changed after controlling for gender, CES–D, MASQ-30 Anxious Arousal subscale, cigarettes/day, and FTND (delay time: β = −.11, 95% CI [−.21, .01], p = .03; cigarettes smoked: β = .17, 95% CI [.07, .27], p = .001). In terms of raw outcome data, the adjusted models indicated that an increase in 1 standard deviation on the anhedonia composite was associated with a corresponding reduction of 2.27 min in delay time and a increase in 0.15 cigarettes smoked.3
Abstinence as a moderator of the relation of anhedonia to smoking task outcomes
The Anhedonia × Abstinence interaction term did not significantly predict latency to smoke (β = .03, p = .30) or cigarettes consumed (β = .04, p = .48). However, it is possible that Anhedonia × Abstinence interactions may be present only for those most affected by the abstinence manipulation. We therefore conducted post hoc analyses of Anhedonia × Abstinence interactions among the subset of individuals who exhibited changes in smoking behavior as a function of abstinence. In these analyses, there was no Anhedonia × Abstinence interaction effect on latency to smoke among individuals who exhibited an abstinence-induced reduction in latency to smoke of a least one reward increment (5 min, n = 134; β = .12, p = .20). In those who exhibited an abstinence-induced increase in cigarettes consumed of one or greater (n = 101), there was an Anhedonia × Abstinence interaction on cigarettes smoked (β = .23, p = .004). Here, the relation between anhedonia and cigarettes consumed was larger when abstinent (β = .49, p < .0001) than nonabstinent (β = .25, p = .01)4
Mediation of the main effect of anhedonia on smoking task outcomes via mood state
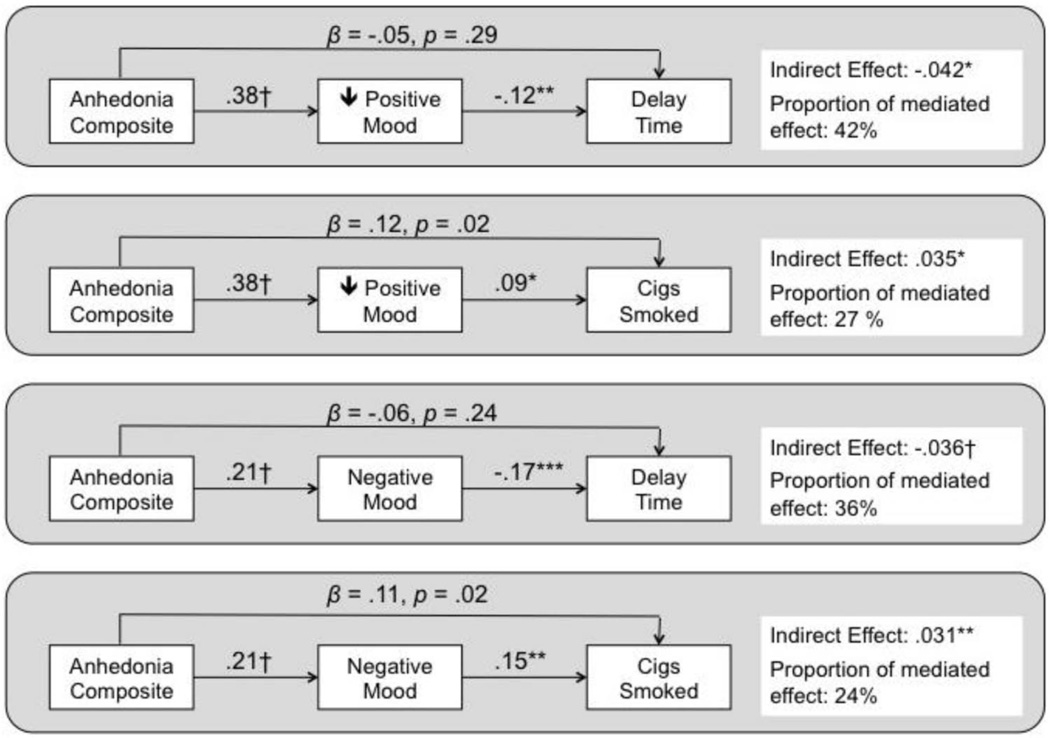
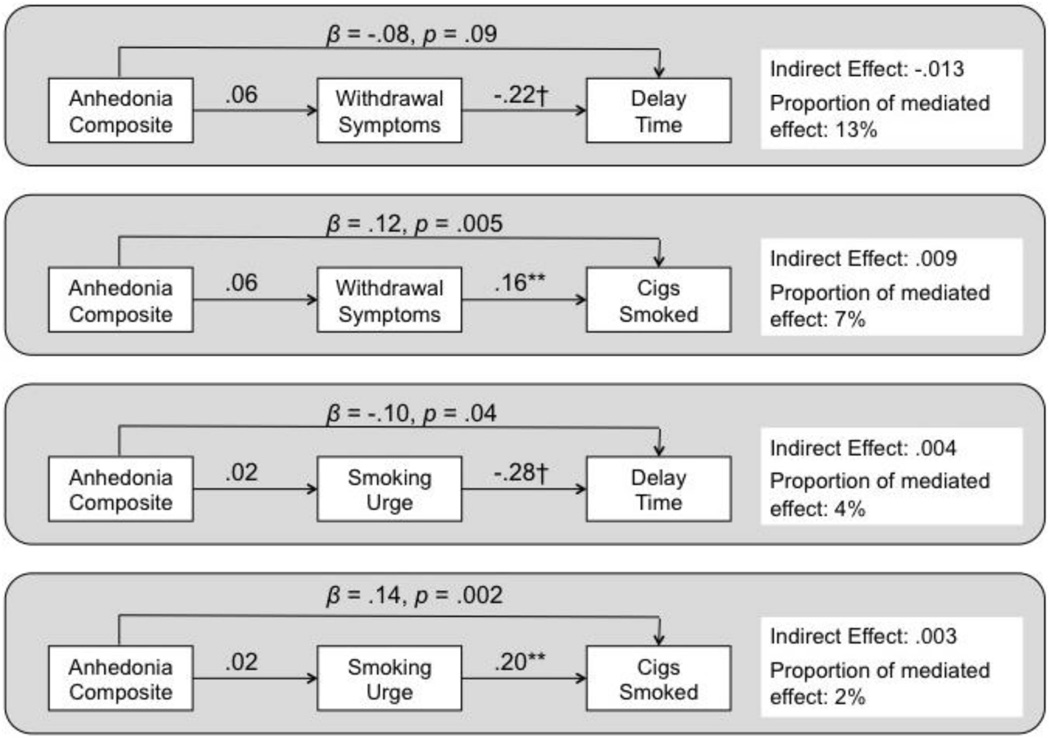
As illustrated in Figure 1, low positive and high negative mood states significantly mediated the main effects of the anhedonia on each smoking task outcome. Averaged across both conditions, greater baseline anhedonia predicted lower experimental session positive mood state, which in turn predicted shorter delay and greater smoking (top of Figure 2). Similarly, greater anhedonia predicted higher negative mood state, which in turn predicted shorter delay and greater smoking (bottom of Figure 2). For both mediators, there was a significant remaining direct effect of anhedonia over and above what was accounted for in the mediational pathway when predicting cigarettes consumed, but not latency to smoking initiation. Additional post hoc analyses of urge and nicotine withdrawal as mediators of the relation of anhedonia to smoking task outcomes yielded no significant mediation effects (see Figure 2).5
Figure 1.
Results of mediational analyses examining the extent to which the predictive effects of the baseline anhedonia composite index score on experimental session smoking task outcomes are mediated by acute positive mood state and negative mood state at the experimental session. Values reflect standardized parameter estimates from generalized estimating equations for component paths as well as the estimated indirect (mediated) effect. Values for arrows from anhedonia to smoking task outcomes reflect remaining the direct effect over and above the mediated effect. Significance of component path or indirect effect: * p < .05; ** p < .01; *** p <.001; † p < .0001.
Figure 2.
Results of mediational analyses examining the extent to which the predictive effects of the baseline anhedonia composite index score on experimental session smoking task outcomes are mediated by acute urge and nicotine withdrawal symptom level at the experimental session. Values reflect standardized parameter estimates from generalized estimating equations for component paths as well as the estimated indirect (mediated) effect. Values for arrows from anhedonia to smoking task outcomes reflect remaining the direct effect over and above the mediated effect. Significance of component path or indirect effect: * p < .05; ** p < .01; *** p < .001; † p < .0001.
Discussion
This laboratory study found that anhedonia predicted behavior indicative of a heightened bias in the relative reward value assigned to smoking versus money. The current investigation extends prior work documenting a relation between anhedonia and drug use (Ameringer & Leventhal, 2010; Hatzigiakoumis et al., 2011) by identifying a mechanism that may maintain drug use behavior in anhedonic individuals—an imbalanced incentive value of drug versus nondrug rewards. Extant smoking cessation research illustrates that anhedonia predicts quicker lapse as well as greater odds of returning to regular smoking patterns (Cook et al., 2010; Leventhal et al., 2008; Zvolensky et al., 2009). Similarly, this study found that anhedonia predicted greater reward value for both initiating smoking faster and consuming more cigarettes. As in prior research (Leventhal, Piper, Japuntich, Baker, & Cook, in press; Leventhal et al., 2008), the relations identified here remained after statistically controlling for depression, nicotine dependence, cigarettes smoked per day, anxiety, and gender, suggesting specificity of anhedonia as a correlate of drug use motivation.
The predictive influence of anhedonia on the reward value of smoking was partially mediated via individual differences in high positive and low negative mood state at the time preceding the smoking task, averaged across abstinence condition. The pattern of effects for the component paths from anhedonia to mood are consistent with data illustrating that although anhedonia is associated with both mood states, it may be more robustly related to positive than negative mood (Leventhal et al., 2009). The component paths leading from mood to the reward value of smoking are consistent with theoretical notions and empirical evidence that although both low positive and high negative mood relate to drug use motivation, negative mood states are comparatively more influential and may reflect core features of drug use motivation (Baker, Piper, McCarthy, Majeskie, & Fiore, 2004; Leventhal et al., 2013). Hence, anhedonia’s impact on drug use motivation via mood state may be multifaceted, and perhaps treatments for anhedonic drug use may benefit from alleviating negative mood as well as boosting positive mood (e.g., MacPherson et al., 2010). Two other state factors (i.e., urge and a composite measure of nicotine withdrawal symptoms) often implicated in smoking motivation did not mediate anhedonia–smoking relations, and anhedonia retained a significant remaining direct effect on smoking task outcomes over and above these factors. In addition, there was a remaining direct effect of anhedonia over and above mood state on cigarette consumption, but not smoking latency, suggesting partial mediation via mood state on this outcome. Hence, anhedonia appears to provide incremental prediction over and above clinically important state indexes for explaining some aspects of smoking motivation.
Tobacco abstinence did not moderate the relation between anhedonia and the relative reward value of smoking in the overall sample. One issue to consider is that the abstinence manipulation may not have produced sufficient differentiation in smoking task performance by abstinence condition as a function of anhedonia. During the nonabstinent condition, almost 1 hr elapsed in between participants’ last cigarette and the onset of the smoking task, yet some withdrawal symptoms emerge within 30 min of abstinence (Hendricks, Ditre, Drobes, & Brandon, 2006), which opens the possibility that very early withdrawal in the nonabstinent condition may have obscured effects induced by abstinence status. It is also possible that 16 hr of abstinence was not a sufficient duration. Post hoc analyses in the subsample that modulated their smoking in response to the abstinence manipulation revealed that anhedonia was significantly related to increased cigarette purchases in both conditions; however, the association was significantly stronger in the abstinent condition. Hence, the findings altogether suggest that, relative to individuals with low anhedonia, those with high anhedonia assign disproportionately higher reward values to smoking versus money, and that there may be some amplification of this effect upon abstinence for certain individuals and outcomes.
The abstinence manipulation was limited in this study because only a single duration of abstinence was tested. Furthermore, abstinence was externally imposed by the study, leaving unclear whether similar findings would be demonstrated within the context of a self-imposed quit attempt (Perkins & Lerman, 2014). Although we included multiple measures of anhedonia, they tapped only three different kinds of anhedonia. A number of anhedonia conceptualizations that were not addressed in this study have been proposed, including notions that anhedonia reflects (a) abnormalities in encoding and retrieving emotional experiences rather than disturbances in in-the-moment emotional experience per se (Strauss & Gold, 2012), (b) a decision-making bias toward refraining from partaking in pleasant activities (Treadway & Zald, 2011), and (c) diminished tendency to modulate behavior as a function of previous reward (i.e., deficient reward learning; Pizzagalli, Jahn, & O’Shea, 2005). We excluded participants in an active mood disorder, psychosis, and substance dependence and those on psychiatric medications to increase the study’s internal validity, which leaves unclear the extent to which these relations might generalize across populations and to the very extreme end of the anhedonia continuum. Given that variation in anhedonia severity among individuals without an active mood disorder predicts risk of smoking relapse (Cook et al., 2010; Leventhal et al., 2008), the current methodology sheds light on a portion of the population in which anhedonia may be an important determinant of drug use. Furthermore, although this study excluded individuals with current DSM-defined major depression, 22.5% surpassed low-threshold screening cutoffs for the CES–D of “mild depression” (score ≥16). Because this CES–D cutoff reflects a lower threshold that is more prevalent in the population but has relatively lower concordance with major depression (Lee, Hasche, Choi, Proctor, & Morrow-Howell, 2013; Ritchey, La Gory, Fitzpatrick, & Mullis, 1990), it suggests that a sizable proportion of the sample had subthreshold yet clinically relevant symptoms.
This study examined only one transdiagnostic trait (i.e., anhedonia). It will be interesting for future work to investgiate several transdiagnostic traits implicated in smoking (e.g., distress tolerance, impulsivity, and anxiety sensitivity; Brown, Lejuez, Kahler, Strong, & Zvolensky, 2005; Doran, McChargue, & Cohen, 2007; Zvolensky et al., 2009) to compare the findings across alternative constructs and provide more comprehensive evidence of the utility of this paradigm for smoking–psychopathology comorbidity. It is unclear whether these findings would be similar if other nondrug reinforcers (e.g., food, sex, social reward) were available as the alternative choice to smoking. This is particularly relevant because the two reward types differed on two different dimensions: (1) pharmacological activity (pharmacological vs. nonpharmacological, which may be aligned with differences in potency) and (2) immediacy (smoking now vs. money to be spent later). Hence, we cannot determine the extent to which the findings reflect stronger valuation of smoking in anhedonic individuals because of smoking’s pharmacological effects or immediacy. However, a prior study found that anhedonia was associated with greater preference for larger delayed rewards over smaller immediate rewards (Lempert & Pizzagalli, 2010), which would have biased anhedonic individuals toward choosing money in the present study. Thus, preference for smoking over monetary reinforcement among anhedonic individuals in the present study might have been driven by differences in pharmacological effects rather than immediacy.
An additional caveat is that the current findings shed light on only how anhedonia modulates the relative reward value of drug versus nondrug reinforcers. Hence, the extent to which these findings reflect diminished incentive value attributed to nondrug rewards or heightened incentive value attributed to smoking is unclear. Nevertheless, the relative value of these two types of reinforcers may reflect the reality of the decisions that habitual drug users face every day. That is, drug users choose to either (a) use a drug and experience the immediate rewarding effects of drug intoxication or (b) abstain from using and experience the multitude of alternative less-immediate nondrug rewards associated with abstinence, such as enhanced social functioning, health, finances (i.e., money savings due to omitting drug purchases), and many other alternative rewards. If the imbalance between the reward value of drug and nondrug rewards could be reversed via treatment, the relation of anhedonia to drug use might be mitigated. Candidate interventions possibly capable of increasing the value of nondrug rewards include (a) behavioral activation, which aims to enhance one’s ability to access healthy reinforcers and recognize their mood-enhancing effects (MacPherson et al., 2010), and (b) positive psychotherapy, which aims to cultivate positive emotions and traits via various counseling techniques, such as increasing one’s ability to savor pleasure (Kahler et al., in press). Candidate interventions that may decrease the reward value of drugs might be those that can successfully mitigate a drug’s subjective mood-altering effects (e.g., vareniciline for smoking cessation; Sofuoglu, Herman, Mooney, & Waters, 2009).
The current findings also shed light on the psychopathological determinants of drug use more broadly. Anhedonia is a transdiagnostic trait that is implicated in multiple forms of psychopathology (Hatzigiakoumis et al., 2011). Studies indicate that both schizophrenia and depression are associated with greater relative reward value for smoking compared with equally nicotine-dependent smokers without psychiatric illness (MacKillop & Tidey, 2011; Spring, Pingitore, & McChargue, 2003; Tidey, Rohsenow, Kaplan, Swift, & Adolfo, 2008). Hence, anhedonia might help to explain why multiple psychopathological syndromes increase risk of smoking and use of other substances. Given this fact, research of anhedonia and other transdiagnostic traits may be a valuable approach for understanding psychopathological comorbidity in drug use.
Acknowledgments
This research was supported by National Institutes of Health Grants R01-DA026831, K08-DA025041, and T32CA009492.
Footnotes
We chose the approach of taking the mean of the three indicators as opposed to using the principal components analysis-derived factor score because (a) weights on factor scores can be sample-specific, which can make replication difficult across samples, and (b) mean scores reflect equal weighting across each indicator, which is appropriate when a rationale for differential weighing across indicators is absent (DiStefan, Zhu, & Mîndrilǎ, 2009; Hammond, 1986). When the primary analyses were retested using the mean principal components analysis-derived factor scores as a composite index, the results were unchanged from the analyses using the mean composite (i.e., the statistical significance determination of each relation involving anhedonia were consistent across both indexes and effect sizes were close in value).
The CES–D has four anhedonia-relevant items, which could generate content overlap between the CES–D total score covariate in the primary predictor. We therefore ran exploratory analyses controlling for a modified score of the remaining 16 CES–D items, after removing the four anhedonia-relevant items, and found equivalent findings to the analyses involving the standard 20-item CES–D score (i.e., the statistical significance determinations of each relation involving anhedonia were consistent and effect sizes were close in value).
Because these variables were not normally distributed, we recoded each of these variables into binary outcomes—delay all 50 min versus delay less than 50 min during the delay period and smoke at least one cigarette versus smoke zero cigarettes during the self-administration period. We tested the primary main effects of these outcomes and found results consistent with the continuous outcomes in GEE analyses using a binary outcome distribution specification: delay all 50 (vs. <50) min: OR = 0.78, 95% CI [0.63, 0.97], p = .02; smoking ≥1 (vs. 0) cigarettes: OR = 1.58, 95% CI [1.21, 2.06], p = .0008.
Order effects might have masked potential Anhedonia × Abstinence interactions; hence, we ran additional analyses considering order. In post hoc GEE analyses, there were no significant effects of order (completing abstinent session first and nonabstinent session second or vice versa) or Order × Anhedonia interactions on the smoking task outcomes (ps > .38). However, there was a significant Order × Abstinence interactions on cigarettes consumed (but not latency to smoke), β = .22, p < .0001, such that the abstinence-induced increases in cigarettes consumed were larger in participants who completed the nondeprived session first. We therefore reran each GEE analysis after controlling for order and the Order × Abstinence interactions and found main effects for anhedonia on latency to smoke, β = −.10, p = .03, and cigarettes consumed, β = .13, p = .004, that were virtually identical to the results not controlling for order. Anhedonia × Abstinence interactions were not significant in models controlling for order (latency to smoke: β = .04, p = .25; cigarettes consumed: β = −.005, p = .89). We further examined the effects in only the first experimental session using a fully between-participants design and found that Anhedonia × Abstinence interactions were not significant (latency to smoke: β = .008, p = .88; cigarettes consumed: β = .03, p = .55).
Given results of the post hoc analyses demonstrating that abstinence moderated the relation of anhedonia and cigarettes smoked among the subset of participants exhibiting abstinence-induced increase in cigarettes consumed of one or greater (n = 101), we conducted follow-up mediational analyses by abstinence condition to explore moderated mediation. For each mediator (positive affect, negative affect, withdrawal, urge), the 95% CI surrounding the βindirect effects overlapped across abstinent and nonabstinent conditions, which suggests that the strength of the mediated effect was not substantially moderated by abstinence condition (i.e., no moderated mediation).
The authors report no disclosures or conflicts of interests related to this study.
Contributor Information
Adam M. Leventhal, Department of Preventive Medicine, University of Southern California Keck School of Medicine; Department of Psychology, University of Southern California
Michael Trujillo, Department of Preventive Medicine, University of Southern California Keck School of Medicine.
Katherine J. Ameringer, Department of Preventive Medicine, University of Southern California Keck School of Medicine
Jennifer W. Tidey, Center for Alcohol and Addiction Studies and the Department of Behavioral and Social Sciences, Brown University School of Public Health
Steve Sussman, Department of Preventive Medicine, University of Southern California Keck School of Medicine; Department of Psychology, University of Southern California.
Christopher W. Kahler, Center for Alcohol and Addiction Studies and the Department of Behavioral and Social Sciences, Brown University School of Public Health
References
- Ameringer KJ, Leventhal AM. Applying the tripartite model of anxiety and depression to cigarette smoking: An integrative review. Nicotine & Tobacco Research. 2010;12:1183–1194. doi: 10.1093/ntr/ntq174. [DOI] [PMC free article] [PubMed] [Google Scholar]
- American Psychiatric Association. Diagnostic and statistical manual of mental disorders. 4th ed. Washington, DC: Author; 2013. [Google Scholar]
- Baker TB, Piper ME, McCarthy DE, Majeskie MR, Fiore MC. Addiction motivation reformulated: An affective processing model of negative reinforcement. Psychological Review. 2004;111:33–51. doi: 10.1037/0033-295X.111.1.33. [DOI] [PubMed] [Google Scholar]
- Bandelow B, Schmahl C, Falkai P, Wedekind D. Borderline personality disorder: A dysregulation of the endogenous opioid system? Psychological Review. 2010;117:623–636. doi: 10.1037/a0018095. [DOI] [PubMed] [Google Scholar]
- Berenbaum H, Connelly J. The effect of stress on hedonic capacity. Journal of Abnormal Psychology. 1993;102:474–481. doi: 10.1037//0021-843x.102.3.474. [DOI] [PubMed] [Google Scholar]
- Bogdan R, Pizzagalli DA. Acute stress reduces reward responsiveness: Implications for depression. Biological Psychiatry. 2006;60:1147–1154. doi: 10.1016/j.biopsych.2006.03.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bozarth MA. Pleasure systems in the brain. In: Warburton DM, editor. Pleasure: The politics and the reality. New York: John Wiley & Sons; 1994. pp. 5–14. + refs. [Google Scholar]
- Brown RA, Lejuez CW, Kahler CW, Strong DR, Zvolensky MJ. Distress tolerance and early smoking lapse. Clinical Psychology Review. 2005;25:713–733. doi: 10.1016/j.cpr.2005.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carleton RN, Thibodeau MA, Teale MJ, Welch PG, Abrams MP, Robinson T, Asmundson GJ. The Center for Epidemiologic Studies Depression scale: A review with a theoretical and empirical examination of item content and factor structure. PLoS One. 2013;8:e58067. doi: 10.1371/journal.pone.0058067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark LA, Watson D. Tripartite model of anxiety and depression: Psychometric evidence and taxonomic implications. Journal of Abnormal Psychology. 1991;100:316–336. doi: 10.1037//0021-843x.100.3.316. [DOI] [PubMed] [Google Scholar]
- Cohen AS, Najolia GM, Brown LA, Minor KS. The state-trait disjunction of anhedonia in schizophrenia: Potential affective, cognitive and social-based mechanisms. Clinical Psychology Review. 2011;31:440–448. doi: 10.1016/j.cpr.2010.11.001. [DOI] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D. Influence of nicotine on positive affect in anhedonic smokers. Psychopharmacology. 2007;192:87–95. doi: 10.1007/s00213-006-0688-5. [DOI] [PubMed] [Google Scholar]
- Cook J, Spring B, McChargue D, Doran N. Effects of anhedonia on days to relapse among smokers with a history of depression: A brief report. Nicotine & Tobacco Research. 2010;12:978–982. doi: 10.1093/ntr/ntq118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook JW, Spring B, McChargue D, Hedeker D. Hedonic capacity, cigarette craving, and diminished positive mood. Nicotine & Tobacco Research. 2004;6:39–47. doi: 10.1080/14622200310001656849. [DOI] [PubMed] [Google Scholar]
- Cox LS, Tiffany ST, Christen AG. Evaluation of the brief Questionnaire of Smoking Urges (QSU-brief) in laboratory and clinical settings. Nicotine & Tobacco Research. 2001;3:7–16. doi: 10.1080/14622200020032051. [DOI] [PubMed] [Google Scholar]
- Dichter G. Anhedonia in unipolar major depressive disorder: A review. The Open Psychiatry Journal. 2010;4:1–9. [Google Scholar]
- DiStefan C, Zhu M, Mîndrilǎ D. Understanding and using factor scores: Considerations for the applied researcher. Practical Assessment, Research, and Evaluation. 2009;14:1–11. [Google Scholar]
- Doran N, McChargue D, Cohen L. Impulsivity and the reinforcing value of cigarette smoking. Addictive Behaviors. 2007;32:90–98. doi: 10.1016/j.addbeh.2006.03.023. [DOI] [PubMed] [Google Scholar]
- D’Souza MS, Markou A. Neural substrates of psychostimulant withdrawal-induced anhedonia. Current Topics in Behavioral Neurosciences. 2010;3:119–178. doi: 10.1007/7854_2009_20. [DOI] [PubMed] [Google Scholar]
- Favrod J, Ernst F, Giuliani F, Bonsack C. Validation française de l’échelle d’ expérience temporelle du plaisir [Validation of the Temporal Experience of Pleasure Scale (TEPS) in a French-speaking environment] L’Encephale. 2009;35:241–248. doi: 10.1016/j.encep.2008.02.013. [DOI] [PubMed] [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JBW. Structured Clinical Interview for DSM–IV–TR Axis I Disorders, Research Version, Non-Patient Edition (SCID-I/NP) New York, NY: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- Franken IHA, Rassin E, Muris P. The assessment of anhedonia in clinical and non-clinical populations: Further validation of the Snaith-Hamilton Pleasure Scale (SHAPS) Journal of Affective Disorders. 2007;99:83–89. doi: 10.1016/j.jad.2006.08.020. [DOI] [PubMed] [Google Scholar]
- Gard DE, Gard MG, Kring AM, John OP. Anticipatory and consummatory component of the experience of pleasure: A scale development study. Personality and Individual Differences. 2006;40:1086–1102. [Google Scholar]
- Gard DE, Kring AM, Gard MG, Horan WP, Green MF. Anhedonia in schizophrenia: Distinctions between anticipatory and consummatory pleasure. Schizophrenia Research. 2007;93:253–260. doi: 10.1016/j.schres.2007.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Dibb WD, Plath LC, Hiyane S, Gehlbach BA. EEG, physiology, and task-related mood fail to resolve across 31 days of smoking abstinence: Relations to depressive traits, nicotine exposure, and dependence. Experimental and Clinical Psychopharmacology. 1999;7:427–443. doi: 10.1037//1064-1297.7.4.427. [DOI] [PubMed] [Google Scholar]
- Gilbert DG, McClernon FJ, Rabinovich NE, Plath LC, Masson CL, Anderson AE, Sly KF. Mood disturbance fails to resolve across 31 days of cigarette abstinence in women. Journal of Consulting and Clinical Psychology. 2002;70:142–152. doi: 10.1037//0022-006x.70.1.142. [DOI] [PubMed] [Google Scholar]
- Gooding DC, Pflum MJ. The nature of diminished pleasure in individuals at risk for or affected by schizophrenia. Psychiatry Research. 2012;198:172–173. doi: 10.1016/j.psychres.2011.07.029. author reply 174–175. [DOI] [PubMed] [Google Scholar]
- Guadagnoli E, Mor V. Measuring cancer patients’ affect: Revision and psychometric properties of the Profile of Mood States (POMS) Psychological Assessment. 1989;1:150–154. [Google Scholar]
- Hammond S. Some pitfalls in the use of factor scores. Personality and Individual Differences. 1986;7:401–407. [Google Scholar]
- Hatzigiakoumis DS, Martinotti G, Giannantonio MD, Janiri L. Anhedonia and substance dependence: Clinical correlates and treatment options. Frontiers in Psychiatry. 2011;2:10. doi: 10.3389/fpsyt.2011.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström Test for Nicotine Dependence: A revision of the Fagerström Tolerance Questionnaire. British Journal of Addiction. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Hendricks PS, Ditre JW, Drobes DJ, Brandon TH. The early time course of smoking withdrawal effects. Psychopharmacology. 2006;187:385–396. doi: 10.1007/s00213-006-0429-9. [DOI] [PubMed] [Google Scholar]
- Hughes JR. Should criteria for drug dependence differ across drugs? Addiction. 2006;101(Suppl. 1):134–141. doi: 10.1111/j.1360-0443.2006.01588.x. [DOI] [PubMed] [Google Scholar]
- Hughes JR, Hatsukami D. Signs and symptoms of tobacco withdrawal. Archives of General Psychiatry. 1986;43:289–294. doi: 10.1001/archpsyc.1986.01800030107013. [DOI] [PubMed] [Google Scholar]
- Kahler CW, Spillane NS, Day A, Clerkin E, Parks A, Leventhal AM, Brown RA. Positive psychotherapy for smoking cessation: Treatment development, feasibility and preliminary results. Journal of Positive Psychology. doi: 10.1080/17439760.2013.826716. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kashdan TB, Elhai JD, Frueh BC. Anhedonia and emotional numbing in combat veterans with PTSD. Behaviour Research and Therapy. 2006;44:457–467. doi: 10.1016/j.brat.2005.03.001. [DOI] [PubMed] [Google Scholar]
- Kessler RC, Chiu WT, Demler O, Merikangas KR, Walters EE. Prevalence, severity, and comorbidity of 12-month DSM–IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:617–627. doi: 10.1001/archpsyc.62.6.617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krueger RF, Bezdjian S. Enhancing research and treatment of mental disorders with dimensional concepts: Toward DSM-V and ICD-11. World Psychiatry. 2009;8:3–6. doi: 10.1002/j.2051-5545.2009.tb00197.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee MJ, Hasche LK, Choi S, Proctor EK, Morrow-Howell N. Comparison of major depressive disorder and subthreshold depression among older adults in community long-term care. Aging & Mental Health. 2013;17:461–469. doi: 10.1080/13607863.2012.747079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman RF, O’Malley SS, White MA, McKee SA. Nicotine and food deprivation decrease the ability to resist smoking. Psychopharmacology. 2010;212:25–32. doi: 10.1007/s00213-010-1902-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lempert KM, Pizzagalli DA. Delay discounting and future-directed thinking in anhedonic individuals. Journal of Behavior Therapy and Experimental Psychiatry. 2010;41:258–264. doi: 10.1016/j.jbtep.2010.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Greenberg JB, Trujillo MA, Ameringer KJ, Lisha NE, Pang RD, Monterosso J. Positive and negative affect as predictors of urge to smoke: Temporal factors and mediational pathways. Psychology of Addictive Behaviors. 2013;27:262–267. doi: 10.1037/a0031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Kahler CW, Ray LA, Zimmerman M. Refining the depression-nicotine dependence link: Patterns of depressive symptoms in psychiatric outpatients with current, past, and no history of nicotine dependence. Addictive Behaviors. 2009;34:297–303. doi: 10.1016/j.addbeh.2008.11.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Piper ME, Japuntich SJ, Baker TB, Cook JW. Anhedonia, depressed mood, and smoking cessation outcome. Journal of Consulting and Clinical Psychology. doi: 10.1037/a0035046. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Ramsey SE, Brown RA, LaChance HR, Kahler CW. Dimensions of depressive symptoms and smoking cessation. Nicotine & Tobacco Research. 2008;10:507–517. doi: 10.1080/14622200801901971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Boyd S, Moolchan ET, Lerman C, Pickworth WB. Gender differences in acute tobacco withdrawal: Effects on subjective, cognitive, and physiological measures. Experimental and Clinical Psychopharmacology. 2007;15:21–36. doi: 10.1037/1064-1297.15.1.21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leventhal AM, Waters AJ, Moolchan ET, Heishman SJ, Pickworth WB. A quantitative analysis of subjective, cognitive, and physiological manifestations of the acute tobacco abstinence syndrome. Addictive Behaviors. 2010;35:1120–1130. doi: 10.1016/j.addbeh.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loas G. Vulnerability to depression: A model centered on anhedonia. Journal of Affective Disorders. 1996;41:39–53. doi: 10.1016/0165-0327(96)00065-1. [DOI] [PubMed] [Google Scholar]
- Lyons MJ, Toomey R, Faraone SV, Kremen WS, Yeung AS, Tsuang MT. Correlates of psychosis proneness in relatives of schizophrenic patients. Journal of Abnormal Psychology. 1995;104:390–394. doi: 10.1037//0021-843x.104.2.390. [DOI] [PubMed] [Google Scholar]
- Lyubomirsky S, Lepper H. A measure of subjective happiness: Preliminary reliability and construct validation. Social Indicators Research. 1999;46:137–155. [Google Scholar]
- MacKillop J, Tidey JW. Cigarette demand and delayed reward discounting in nicotine-dependent individuals with schizophrenia and controls: An initial study. Psychopharmacology. 2011;216:91–99. doi: 10.1007/s00213-011-2185-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacKinnon DP, Fritz MS, Williams J, Lockwood CM. Distribution of the product confidence limits for the indirect effect: Program PRODCLIN. Behavior Research Methods. 2007;39:384–389. doi: 10.3758/bf03193007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacPherson L, Tull MT, Matusiewicz AK, Rodman S, Strong DR, Kahler CW, Lejuez CW. Randomized controlled trial of behavioral activation smoking cessation treatment for smokers with elevated depressive symptoms. Journal of Consulting and Clinical Psychology. 2010;78:55–61. doi: 10.1037/a0017939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Krishnan-Sarin S, Shi J, Mase T, O’Malley SS. Modeling the effect of alcohol on smoking lapse behavior. Psychopharmacology. 2006;189:201–210. doi: 10.1007/s00213-006-0551-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Sinha R, Weinberger AH, Sofuoglu M, Harrison EL, Lavery M, Wanzer J. Stress decreases the ability to resist smoking and potentiates smoking intensity and reward. Journal of Psychopharmacology. 2011;25:490–502. doi: 10.1177/0269881110376694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKee SA, Weinberger AH, Shi J, Tetrault J, Coppola S. Developing and validating a human laboratory model to screen medications for smoking cessation. Nicotine & Tobacco Research. 2012;14:1362–1371. doi: 10.1093/ntr/nts090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McNair D, Lorr M, Droppleman DL. Profile of Mood States. San Diego, CA: EdITS; 1971. [Google Scholar]
- Meehl PE. Primary and secondary hypohedonia. Journal of Abnormal Psychology. 2001;110:188–193. doi: 10.1037//0021-843x.110.1.188. [DOI] [PubMed] [Google Scholar]
- Meinzer MC, Pettit JW, Leventhal AM, Hill RM. Explaining the covariance between attention-deficit hyperactivity disorder symptoms and depressive symptoms: The role of hedonic responsivity. Journal of Clinical Psychology. 2012;68:1111–1121. doi: 10.1002/jclp.21884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neff K, Rude S, Kirkpatrick K. An examination of self-compassion in relation to positive psychological functioning and personality traits. Journal of Research in Personality. 2007;41:908–916. [Google Scholar]
- Niaura R, Britt DM, Shadel WG, Goldstein M, Abrams D, Brown R. Symptoms of depression and survival experience among three samples of smokers trying to quit. Psychology of Addictive Behaviors. 2001;15:13–17. doi: 10.1037/0893-164x.15.1.13. [DOI] [PubMed] [Google Scholar]
- Nutt D, Demyttenaere K, Janka Z, Aarre T, Bourin M, Canonico PL, Stahl S. The other face of depression, reduced positive affect: The role of catecholamines in causation and cure. Journal of Psychopharmacology. 2007;21:461–471. doi: 10.1177/0269881106069938. [DOI] [PubMed] [Google Scholar]
- Perkins KA, Lerman C. An efficient early phase 2 procedure to screen medications for efficacy in smoking cessation. Psychopharmacology. 2014;231:1–11. doi: 10.1007/s00213-013-3364-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pizzagalli DA, Jahn AL, O’Shea JP. Toward an objective characterization of an anhedonic phenotype: A signal-detection approach. Biological Psychiatry. 2005;57:319–327. doi: 10.1016/j.biopsych.2004.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Applied Psychological Measurement. 1977;1:385–401. [Google Scholar]
- Ritchey FJ, La Gory M, Fitzpatrick KM, Mullis J. A comparison of homeless, community-wide, and selected distressed samples on the CES-Depression scale. American Journal of Public Health. 1990;80:1384–1386. doi: 10.2105/ajph.80.11.1384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SAS Institute Inc. The SAS System for Windows (Version 8.2) Cary, NC: SAS Institute Inc; 2003. [Google Scholar]
- Saunders JB, Aasland OG, Babor TF, de la Fuente JR, Grant M. Development of the Alcohol Use Disorders Identification Test (AUDIT): WHO Collaborative Project on Early Detection of Persons With Harmful Alcohol Consumption–II. Addiction. 1993;88:791–804. doi: 10.1111/j.1360-0443.1993.tb02093.x. [DOI] [PubMed] [Google Scholar]
- Schlaepfer TE, Cohen MX, Frick C, Kosel M, Brodesser D, Axmacher N, Sturm V. Deep brain stimulation to reward circuitry alleviates anhedonia in refractory major depression. Neuropsychopharmacology. 2008;33:368–377. doi: 10.1038/sj.npp.1301408. [DOI] [PubMed] [Google Scholar]
- Schulte-van Maaren YW, Carlier IV, Zitman FG, van Hemert AM, de Waal MW, van Noorden MS, Giltay EJ. Reference values for generic instruments used in routine outcome monitoring: The Leiden Routine Outcome Monitoring Study. BMC Psychiatry. 2012;12:203. doi: 10.1186/1471-244X-12-203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shafer AB. Meta-analysis of the factor structures of four depression questionnaires: Beck, CES-D, Hamilton, and Zung. Journal of Clinical Psychology. 2006;62:123–146. doi: 10.1002/jclp.20213. [DOI] [PubMed] [Google Scholar]
- Skinner HA. The drug abuse screening test. Addictive Behaviors. 1982;7:363–371. doi: 10.1016/0306-4603(82)90005-3. [DOI] [PubMed] [Google Scholar]
- Smith LE, Greenberg JS, Seltzer MM. Social support and well-being at mid-life among mothers of adolescents and adults with autism spectrum disorders. Journal of Autism and Developmental Disorders. 2012;42:1818–1826. doi: 10.1007/s10803-011-1420-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snaith P. Anhedonia: A neglected symptom of psychopathology. Psychological Medicine. 1993;23:957–966. doi: 10.1017/s0033291700026428. [DOI] [PubMed] [Google Scholar]
- Society for Research on Nicotine and Tobacco. Biochemical verification of tobacco use and cessation. Nicotine & Tobacco Research. 2002;4:149–159. doi: 10.1080/14622200210123581. [DOI] [PubMed] [Google Scholar]
- Sofuoglu M, Herman AI, Mooney M, Waters AJ. Varenicline attenuates some of the subjective and physiological effects of intravenous nicotine in humans. Psychopharmacology. 2009;207:153–162. doi: 10.1007/s00213-009-1643-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spring B, Pingitore R, McChargue DE. Reward value of cigarette smoking for comparably heavy smoking schizophrenic, depressed, and nonpatient smokers. The American Journal of Psychiatry. 2003;160:316–322. doi: 10.1176/appi.ajp.160.2.316. [DOI] [PubMed] [Google Scholar]
- Strauss GP, Gold JM. A new perspective on anhedonia in schizophrenia. The American Journal of Psychiatry. 2012;169:364–373. doi: 10.1176/appi.ajp.2011.11030447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauss GP, Wilbur RC, Warren KR, August SM, Gold JM. Anticipatory vs. consummatory pleasure: What is the nature of hedonic deficits in schizophrenia? Psychiatry Research. 2011;187:36–41. doi: 10.1016/j.psychres.2011.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tidey JW, Rohsenow DJ, Kaplan GB, Swift RM, Adolfo AB. Effects of smoking abstinence, smoking cues and nicotine replacement in smokers with schizophrenia and controls. Nicotine & Tobacco Research. 2008;10:1047–1056. doi: 10.1080/14622200802097373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treadway MT, Zald DH. Reconsidering anhedonia in depression: Lessons from translational neuroscience. Neuroscience and Biobehavioral Reviews. 2011;35:537–555. doi: 10.1016/j.neubiorev.2010.06.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Cardenas L, Herrmann N, Busto UE. Probing brain reward system function in major depressive disorder: Altered response to dextroamphetamine. Archives of General Psychiatry. 2002;59:409–416. doi: 10.1001/archpsyc.59.5.409. [DOI] [PubMed] [Google Scholar]
- Tremblay LK, Naranjo CA, Graham SJ, Herrmann N, Mayberg HS, Hevenor S, Busto UE. Functional neuroanatomical substrates of altered reward processing in major depressive disorder revealed by a dopaminergic probe. Archives of General Psychiatry. 2005;62:1228–1236. doi: 10.1001/archpsyc.62.11.1228. [DOI] [PubMed] [Google Scholar]
- Wardenaar KJ, van Veen T, Giltay EJ, de Beurs E, Penninx BW, Zitman FG. Development and validation of a 30-item short adaptation of the Mood and Anxiety Symptoms Questionnaire (MASQ) Psychiatry Research. 2010;179:101–106. doi: 10.1016/j.psychres.2009.03.005. [DOI] [PubMed] [Google Scholar]
- Watson D, Naragon-Gainey K. On the specificity of positive emotional dysfunction in psychopathology: Evidence from the mood and anxiety disorders and schizophrenia/schizotypy. Clinical Psychology Review. 2010;30:839–848. doi: 10.1016/j.cpr.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Dopamine and reward: The anhedonia hypothesis 30 years on. Neurotoxicity Research. 2008;14:169–183. doi: 10.1007/BF03033808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger SL, Liang KY, Albert PS. Models for longitudinal data: A generalized estimating equation approach. Biometrics. 1988;44:1049–1060. [PubMed] [Google Scholar]
- Zimmerman M, McGlinchey JB, Young D, Chelminski I. Diagnosing major depressive disorder I: A psychometric evaluation of the DSM–IV symptom criteria. The Journal of Nervous and Mental Disease. 2006;194:158–163. doi: 10.1097/01.nmd.0000202239.20315.16. [DOI] [PubMed] [Google Scholar]
- Zvolensky MJ, Stewart SH, Vujanovic AA, Gavric D, Steeves D. Anxiety sensitivity and anxiety and depressive symptoms in the prediction of early smoking lapse and relapse during smoking cessation treatment. Nicotine & Tobacco Research. 2009;11:323–331. doi: 10.1093/ntr/ntn037. [DOI] [PMC free article] [PubMed] [Google Scholar]