Introduction
Cell viability and metabolism depend on cytoplasmic water and solute content, and organisms have evolved mechanisms to sense changes in cell water content, solute concentrations, cell volume, and/or turgor. This Perspective addresses the response to osmotic challenge in land plants and describes their special dependence on cellular water status for growth and development. Understanding how plants cope with water limitation may allow us to mitigate the agricultural effects of drought, a critical limitation on global crop productivity that is likely to increase in severity as the climate changes (Long and Ort, 2010). The signaling pathways by which plants respond to osmotic challenge are intriguing from an evolutionary standpoint: some aspects of these pathways resemble those of fungal or mammalian cells, some are similar to prokaryotic mechanisms, and yet others are unique to plants (as described below and in Hamann, 2012). In addition to the importance of osmotic homeostasis in land plants, we will discuss some of the specific context and language of plant stress biology, and describe what is known (and not known) about the molecular pathways by which plants sense and respond to osmotic challenges.
The roles of water in plant biology
Plants can only take up water from the soil in which they are growing, and are unable to relocate to find more favorable water conditions. The majority of the water extracted from the soil by a plant’s roots travels rapidly up the plant through the vascular system, eventually to be lost via regulated pores in the leaf epidermis called stomata. This vertical movement of water within the plant mediates the root-to-shoot movement of nutrients, hormones, and developmental signals. Importantly, open stomata are also the conduits through which gas is exchanged between the leaf and the atmosphere during photosynthesis. Thus, plants must continuously balance the advantages of efficient photosynthesis and solute transport with the drawbacks of water loss through open stomata; how well they accomplish this task contributes to their water use efficiency, a key factor in drought response (Long and Ort, 2010).
At the cellular level, water is critical for maintaining and controlling an important driving force in plant cells called turgor pressure. Much of a plant cell’s water—and therefore most of its volume—is accounted for by the vacuole (a large intracellular organelle found in plants, fungi, and animals). In plants, the vacuole often takes up >80% of the cell volume, and one of its many functions is to maintain turgor via active transport of ions in and out of the vacuolar space (Hedrich, 2012). The plant cell plasma membrane is surrounded by a strong but elastic cell wall comprised of cellulose microfibrils embedded in a gel of pectin, other carbohydrates, and protein. Turgor pressure is the pressure exerted by the protoplast (all portions of the cell enclosed by the plasma membrane) against the cell wall. The amount of turgor pressure in a particular cell is determined by several factors, including the osmotic difference that is generated by vacuolar and cytoplasmic solute accumulation and subsequent water uptake, and the elasticity of the cell wall (see Verslues et al., 2006, for additional explanation). The turgor pressure of a plant cell can be as high as the pressure of a car tire; high turgor pressure is critical to give plants their rigidity and structure, drive cell expansion, mediate the opening and closing of stomata, and allow movements like the closing of a Venus flytrap (Pritchard, 2001).
Terminology and concepts in plant osmosensing
Strictly defined, the term “osmosensing” refers to the direct perception of the osmolarity of the external or internal environment of a cell. This term is often used by plant researchers, but it is entirely possible that mechanical stimuli caused by change in membrane tension, cell wall damage, or the disruption of plasma membrane–cell wall connections are relevant factors in osmosensing, in addition to or instead of altered extracellular osmolarity per se (see Fig. 1). For the purposes of this Perspective, “osmosensing” is used to include both the direct perception of osmotic imbalance across a membrane as well as the perception of indirect effects of osmotic imbalance on the membrane, cell wall, or membrane–cell wall system. We anticipate that as the molecular mechanism(s) for the perception of osmotic challenge becomes clearer, so will the terms used to describe these processes.
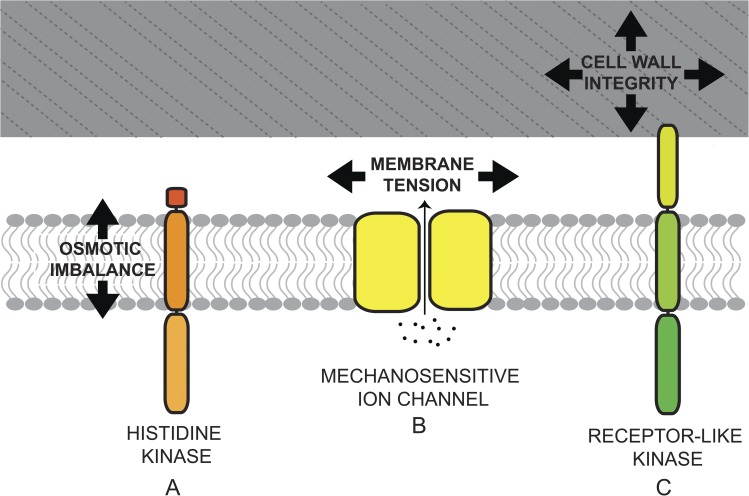
Figure 1.
Potential osmosensing mechanisms in plant cells. (A) Histidine kinases could be activated by osmotic imbalance across the plasma membrane, initiating a signal transduction pathway similar to the high osmolarity glycerol response 1 pathway in Saccharomyces cerevisiae. (B) Ion channels that are opened in response to planar membrane tension or membrane bending could respond to both protoplast swelling or shrinking. The release of ions in response to hypoosmotic swelling would be similar to the MscS/MscL “osmotic release valve” paradigm in Escherichia coli. In addition, the entry of Ca2+ through an opened MS ion channel could have downstream signaling properties. (C) Cell wall integrity-monitoring mechanisms could involve receptor-like kinases with extracellular domains capable of sensing the movement or disruption of cell wall components. The cell wall is presented as a gray box. The plasma membrane is represented as a bilayer of gray lipid molecules.
Plant water status is typically described in terms of “water potential,” a measure of the free energy status of water relative to pure water at a reference state. Water potential is derived from the chemical potential of water but is expressed in units of pressure, which makes it particularly useful in plant biology as a unified measure of soil and plant water status. Factors that decrease the free energy of water (such as increased concentration of dissolved solutes or adhesion to hydrophilic surfaces such as soil particles) make the water potential more negative, whereas factors that increase the free energy (such as increased turgor pressure) make the water potential more positive. Additional details of water potential can be found in Boyer and Kramer (1995) and Verslues et al. (2006). The measurement of water potentials allows for simple predictions regarding the direction of water fluxes and changes in turgor (Boyer and Kramer, 1995). Water potentials of 0 to −0.3 megapascals (MPa) are typical of well-watered plants, whereas water potentials of −1.5 to −2.0 MPa represent severe stress and permanent loss of turgor for many plants including most crop species and the model flowering plant Arabidopsis thaliana. However, drought-adapted plants such as those found in deserts can grow at much lower water potentials, and both inter- and intra-species variation are emerging as important tools in understanding plant osmosensing mechanisms and adaptation to dry environments.
The term “hyperosmotic stress” is infrequently used in plant biology. This is because water limitation is rarely caused by the presence of high levels of extracellular solutes but is more often created by the unavailability of water (quantified as a decrease in water potential). The downstream effects of water limitation on plants that are commonly assayed include: the accumulation of compatible solutes such as proline, the induction of protective proteins such as dehydrins, the production of the stress hormone abscisic acid (ABA), and increased sensitivity to ABA treatment in physiological assays (Verslues and Juenger, 2011; Christmann et al., 2013). An illustration of the relevance of distinguishing between hyperosmotic stress and low water availability comes from simple treatments with extracellular solutions. Treating plants with a solution of low molecular weight solutes such as NaCl will plasmolyze cells (a shrinking of the protoplast leading to its separation from the cell wall), whereas soil drying or treatment with large polymers that cannot penetrate the cell wall (such as PEG) will cause cytorrhysis (shrinking of both protoplast and cell wall). These two types of treatment elicit very different downstream responses and may in fact be perceived by different mechanisms. For example, root growth rate is much higher after cytorrhytic than plasmolytic treatments of the same MPa (Verslues et al., 2006). Furthermore, cytorrhysis and plasmolysis can have different effects on plasma membrane–cell wall connections (perhaps analogous to the effects of osmotic stress on focal adhesion complexes in mammalian cells; see below for further discussion), which in turn elicit different downstream signaling events.
Conditions under which plants experience osmotic challenges
Plants experience water stress when the water content, and therefore the water potential, of the soil decreases. In addition, plants growing in hot dry air can become dehydrated, even when the soil is relatively wet, if water cannot be taken up rapidly enough by the roots to compensate for the water lost through stomata. Many plants regularly lose turgor during the day when the humidity of the environment is low relative to that inside the leaf, and stomates are open to support photosynthesis. They then rehydrate during the night when environmental humidity is high and stomates are closed. In the laboratory, drought/water stress is imposed by withholding water from potted plants or by transferring plants to media with defined water potential (Verslues et al., 2006).
Hypoosmotic stress may occur transiently when dry soil is rapidly rewetted, and chronically upon repeated soil flooding. In the laboratory, hypoosmotic stress is typically reproduced by treating isolated cells with solutions of distilled water after equilibration in high levels of NaCl or mannitol. The phenomenon of water soaking, the accumulation of intercellular water during pathogenic invasion, promotes pathogen survival and may impose hypoosmotic stress on surrounding cells (Beattie, 2011). In addition, some stages of plant development necessarily involve osmotic challenges, including the desiccation of seeds and pollen and their subsequent rehydration and resumption of metabolic activity. It is also possible, though not yet demonstrated, that the cell wall weakening associated with cell expansion during normal plant growth may have some of the same effects as hypoosmotic stress.
Molecular mechanisms of osmosensing
It is not fully known how plants initially sense any of the osmotic challenges described above. Three general models for osmosensing in plant cells are illustrated in Fig. 1. Below, we further describe the molecular mechanisms thought to underlie each of these molecular models and summarize the supporting experimental evidence.
(1) Osmotic imbalance across the plasma membrane.
In theory, a protein embedded in the plasma membrane could directly sense changes in osmolarity outside the cell, perhaps via an osmotically regulated conformational change in an extracellular domain (Fig. 1 A; Parsegian et al., 1995). However, there is currently little experimental support for this model in any eukaryotic system (interested readers are directed to the accompanying discussion of prokaryotic osmosensors in the Perspective by Wood). Several osmosensors involved in hyperosmotic signaling through the Saccharomyces cerevisiae high osmolarity glycerol response (HOG)1 pathway, including the histidine kinase Synthetic Lethal of N-end rule 1 (SLN1), were originally proposed to function in this manner, but recent data suggest that one or more of the other mechanisms listed below may instead be involved (Saito and Posas, 2012). Plant homologues of SLN1 have been reported to serve as regulators of water stress responses (Tran et al., 2007; Wohlbach et al., 2008). However, other data have raised questions as to whether these kinases could be the main plant osmosensing system, as loss-of-function alleles have limited effects on key phenotypes such as the accumulation of ABA and osmoregulatory solutes (Kumar et al., 2013). Alternatively, a novel calcium channel, REDUCED HYPEROSMOLARITY-INDUCED [Ca2+]i INCREASE (OSCA)1, has recently been reported to be activated by hyperosmolarity and to be genetically required for several stress-related phenotypes (Hou et al., 2014; Yuan et al., 2014); additional physiological experiments are needed to fully understand the role of OSCA1 and related channels in drought response.
(2) Increased plasma membrane tension.
It is easy to imagine how hypoosmotic shock leads to increased plasma membrane tension; dehydration may have the same effect in plant cells because the plant plasma membrane and cell wall are physically linked at multiple locations. Upon plasmolysis, most of the protoplast separates from the cell wall, but thin strands of plasma membrane (Hechtian strands) remain connected. As a result, both shrinking and swelling of the protoplast could increase plasma membrane tension.
Mechanosensitive (MS) channels are capable of sensing membrane tension and responding to it by facilitating the flux of osmolytes across the membrane (Fig. 1 B). Multiple families of MS ion channels have been identified in plant genomes, and numerous MS ion channel activities have been detected in plant membranes (Monshausen and Haswell, 2013). The MID1-COMPLEMENTING ACTIVITY (MCA) proteins, a family of putative MS ion channels, promote Ca2+ influx in response to hypoosmotic shock and mechanical stimulus in several plant species (Kurusu et al., 2013). Although no defect in the response to hypoosmotic shock or water deficit has been reported in mca mutants, Arabidopsis MCA1 is required for a normal response to cell wall damage and for efficient root growth into a hard agar medium (Hamann, 2012; Kurusu et al., 2013). Members of two other MS ion channel families, the MscS-Like (MSL) channels and the Two-Pore K+ (TPK) channels, function in organelles (see below). An osmosensing function for plasma membrane–localized MSL or TPK channels has yet to be thoroughly investigated.
C. Altered cell wall integrity.
The cell wall–plasma membrane interface is a critical site for detecting stimuli generated by changing water status. The Hechtian strands described above are reminiscent of focal adhesions of mammalian cells and, like focal adhesions, can be disrupted by treatment with peptides that harbor the amino acid motif RGD (Canut et al., 1998). This has led to the hypothesis that focal adhesion–like complexes may exist in plants and serve to sense alterations in plasma membrane–cell wall attachments caused by dehydration. Although direct orthologues of mammalian proteins such as integrins have not been found in plants, molecular modeling has uncovered an Arabidopsis protein with an integrin-like structure that may play a role in osmoregulation (Knepper et al., 2011).
Alternatively, plant osmosensing might bear similarities to cell wall monitoring mechanisms in yeast (Hamann, 2012; Monshausen and Haswell, 2013). An osmosensor could bind directly to cell wall proteins, or mechanical change or cell wall damage could cause the release of specific molecules that are perceived by the osmosensor (Fig. 1 C). More than 600 receptor-like kinases are encoded by the Arabidopsis genome, and it is thought that some of these have drought signaling functions (Marshall et al., 2012). For example, THESEUS and FERONIA have lectin-like extracellular domains that may monitor cell wall status by binding directly to cell wall–derived carbohydrates or glycoproteins (Cheung and Wu, 2011). Another family of receptor-like kinases implicated in cell wall integrity is the Wall-Associated Kinase family (Kohorn and Kohorn, 2012). Genetic and biochemical studies support a model wherein Wall-Associated Kinases bind pectin, a component of the plant cell wall, and subsequently serve to control cell expansion through an unknown signal transduction pathway.
Osmotic imbalance across organelle membranes.
Vacuoles and plastids take up a substantial portion of plant cell volume, and the osmotic status of these compartments must be coordinated with that of the cytoplasm. Thus, the plasma membrane–based mechanisms described above are likely to be accompanied by an analogous osmosensing system in vacuolar and organellar membranes. However, little is known about these processes. The disruption of two plastid MSL channels constitutively activates low water potential responses, including ABA production and sensitivity, proline biosynthesis, and solute accumulation (Wilson et al., 2014). Vacuole membrane–localized members of the TPK family were recently shown to be modulated by membrane tension and might serve a similar role (Maathuis, 2011). Thus, although MS channels have traditionally been associated with protection from extracellular hypoosmotic shock, they are also important in coordinating the osmotic status of intracellular compartments and have an as yet undefined connection to physiological mechanisms important to low water potential response.
Connecting perception to response: Osmotic stress signaling pathways
Downstream osmotic stress signaling pathways have received a great deal more attention than the initial perception of water deficit. Interestingly, stress-responsive solute accumulation in plant cells may share some mechanisms with solute accumulation in bacterial cells (see accompanying Perspective by Wood). Other responses, such as the induction of protective proteins and stress-regulated gene expression, appear to involve plant-specific mechanisms, some of which depend on stress-induced increases in ABA levels (Yoshida et al., 2014).
Many studies have characterized the extensive changes in intracellular calcium, transcriptional regulation, epigenetic modification, and posttranslational protein modification (especially phosphorylation) that occur in plants in response to hyperosmolarity, water limitation, and ABA (Verslues and Juenger, 2011; Christmann et al., 2013). In addition, the molecular mechanisms of ABA perception are now known in detail (Cutler et al., 2010). Although some of the genes induced or proteins modified in response to water limitation have clear roles in signaling, the synthesis or import of specific solutes and ions, or protection against reactive oxygen species, the functions of many other stress-induced genes are less clear. A great deal of transcriptomic and proteomic data is being generated, but these data can be challenging to interpret because of the difficulty in distinguishing ABA-dependent signaling events from events that are directly related to osmosensing or mechanosensing. For example, several members of the SnRK2 family of plant-specific kinases are key players in ABA signaling and can be activated by exogenous ABA in the absence of osmotic stress. However, other SnRK2s cannot be activated by ABA but can still be rapidly activated by osmotic stress (Boudsocq et al., 2007). How activation of these latter SnRK2s is connected to osmotic stress is not known. Even the key downstream responses—ABA accumulation, the accumulation of protective solutes, and the induction of protective proteins—have not been definitively linked to signaling chains that lead all the way back to the initial perception of water limitation.
Many of the mechanisms described above are also likely to be involved in hypoosmotic signaling, but more research is needed to establish the precise nature of the pathway and identify the critical components. Hypoosmotic stress administered to plant cells in suspension cultures elicits a rapid oxidative burst that depends on a phosphorylation cascade involving Mitogen-Activated Protein Kinase and/or Calcium-Dependent Protein Kinase activation (Cazale et al., 1999; Romeis et al., 2001). The pathway is proposed to induce anion efflux, which is the driving force of osmoregulation in guard cells, but the molecular mechanism has not been determined.
Summary and outlook
Despite our strong motivation to understand plant osmosensing and signaling, plant scientists still have a great deal of work to do. Key goals for future investigation include: (a) determining the molecular identity of the osmosensing apparatus, as detailed here and illustrated in Fig. 1; (b) characterizing the nature of the osmotic stress response signaling pathways upstream of the phytohormone ABA and distinguishing it from responses to other abiotic stresses; and (c) using a wider range of assays in genetic screens to include the full range of phenotypes relevant for plant adaptation to water-limited environments.
With regard to conclusively identifying plant osmosensors, the primary limitation at the moment is that no candidate osmosensors have been conclusively linked to stress signaling and key stress phenotypes. This bottleneck can be explained in part by redundancy; something as critical to plant life as monitoring and responding to water status probably involves multiple mechanisms. As described above, testing candidate genes that are homologues of known osmosensors in other systems is a start, but it will also be important to leverage the power of forward molecular genetic screens in Arabidopsis. For this to take place, phenotypic assays that are amenable to high throughput analysis and that either measure the primary stimulus of osmotic challenge or measure downstream responses directly regulated by osmosensors are needed. A promising recent approach taken by several laboratories is to directly screen for altered calcium fluxes in response to osmotic stress (for example, Yuan et al., 2014). Natural variation and genome-wide association analysis also provide new and promising ways to find key genes that may be difficult to discover by classic forward or reverse genetic screens (Verslues et al., 2014).
In addition, new tools that would allow one to measure osmolyte movement, ion flux, membrane tension, or turgor in the proper cell biological context and in a noninvasive manner would likely be transformative for the field. Finally, the cytoskeleton is intricately involved in force sensing and controlling cell shape in mammalian cells (see accompanying Perspective by Sachs and Sivaselvan) and is likely to have as-yet-undiscovered roles in the perception and signaling of osmotic stress in plants (in addition to controlling cell wall deposition and other properties). We anticipate that future work on all of these fronts from many laboratories will reveal the ways in which plant osmosensing is similar to osmosensing in other systems, and the ways in which it is unique to plants.
This Perspectives series includes articles by Andersen, Sachs and Sivaselvan, and Wood.
Acknowledgments
We thank Thorsten Hamann and members of the Haswell Lab for valuable comments on this manuscript.
Our research into plant osmosensing is supported by the National Science Foundation (NSF CAREER MCB-1253103 to E.S. Haswell), the National Institutes of Health (2R01GM084211 to Doug Rees, Rob Phillips, and E.S. Haswell), and by Academia Sinica and the Taiwan Ministry of Science and Technology (to P.E. Verslues). P.E. Verslues was partially supported by a Freiberg Visiting Professorship from Washington University in St. Louis during the writing of this Perspective.
The authors declare no competing financial interests.
Olaf S. Andersen served as guest editor.
Footnotes
Abbreviations used in this paper:
- ABA
- abscisic acid
- MCA
- MID1-COMPLEMENTING ACTIVITY
- Mpa
- megapascals
- MS
- mechanosensitive
- MSL
- MscS-Like
- TPK
- Two-Pore K+
References
- Beattie G.A.2011. Water relations in the interaction of foliar bacterial pathogens with plants. Annu. Rev. Phytopathol. 49:533–555 10.1146/annurev-phyto-073009-114436 [DOI] [PubMed] [Google Scholar]
- Boudsocq M., Droillard M.J., Barbier-Brygoo H., and Laurière C.. 2007. Different phosphorylation mechanisms are involved in the activation of sucrose non-fermenting 1 related protein kinases 2 by osmotic stresses and abscisic acid. Plant Mol. Biol. 63:491–503 10.1007/s11103-006-9103-1 [DOI] [PubMed] [Google Scholar]
- Boyer J.S., and Kramer P.J.. 1995. Measuring the Water Status of Plants and Soils. Academic Press, San Diego: 178 pp. [Google Scholar]
- Canut H., Carrasco A., Galaud J.P., Cassan C., Bouyssou H., Vita N., Ferrara P., and Pont-Lezica R.. 1998. High affinity RGD-binding sites at the plasma membrane of Arabidopsis thaliana links the cell wall. Plant J. 16:63–71 10.1046/j.1365-313x.1998.00276.x [DOI] [PubMed] [Google Scholar]
- Cazale A.C., Droillard M.J., Wilson C., Heberle-Bors E., Barbier-Brygoo H., and Lauriere C.. 1999. MAP kinase activation by hypoosmotic stress of tobacco cell suspensions: towards the oxidative burst response? Plant J. 19:297–307 10.1046/j.1365-313X.1999.00528.x [DOI] [PubMed] [Google Scholar]
- Cheung A.Y., and Wu H.M.. 2011. THESEUS 1, FERONIA and relatives: a family of cell wall-sensing receptor kinases? Curr. Opin. Plant Biol. 14:632–641 10.1016/j.pbi.2011.09.001 [DOI] [PubMed] [Google Scholar]
- Christmann A., Grill E., and Huang J.. 2013. Hydraulic signals in long-distance signaling. Curr. Opin. Plant Biol. 16:293–300 10.1016/j.pbi.2013.02.011 [DOI] [PubMed] [Google Scholar]
- Cutler S.R., Rodriguez P.L., Finkelstein R.R., and Abrams S.R.. 2010. Abscisic acid: Emergence of a core signaling network. Annu. Rev. Plant Biol. 61:651–679 10.1146/annurev-arplant-042809-112122 [DOI] [PubMed] [Google Scholar]
- Hamann T.2012. Plant cell wall integrity maintenance as an essential component of biotic stress response mechanisms. Front. Plant Sci. 3:77 10.3389/fpls.2012.00077 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedrich R.2012. Ion channels in plants. Physiol. Rev. 92:1777–1811 10.1152/physrev.00038.2011 [DOI] [PubMed] [Google Scholar]
- Hou C., Tian W., Kleist T., He K., Garcia V., Bai F., Hao Y., Luan S., and Li L.. 2014. DUF221 proteins are a family of osmosensitive calcium-permeable cation channels conserved across eukaryotes. Cell Res. 24:632–635 10.1038/cr.2014.14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knepper C., Savory E.A., and Day B.. 2011. Arabidopsis NDR1 is an integrin-like protein with a role in fluid loss and plasma membrane-cell wall adhesion. Plant Physiol. 156:286–300 10.1104/pp.110.169656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohorn B.D., and Kohorn S.L.. 2012. The cell wall-associated kinases, WAKs, as pectin receptors. Front. Plant Sci. 3:88 10.3389/fpls.2012.00088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar M.N., Jane W.N., and Verslues P.E.. 2013. Role of the putative osmosensor Arabidopsis histidine kinase1 in dehydration avoidance and low-water-potential response. Plant Physiol. 161:942–953 10.1104/pp.112.209791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurusu T., Kuchitsu K., Nakano M., Nakayama Y., and Iida H.. 2013. Plant mechanosensing and Ca2+ transport. Trends Plant Sci. 18:227–233 10.1016/j.tplants.2012.12.002 [DOI] [PubMed] [Google Scholar]
- Long S.P., and Ort D.R.. 2010. More than taking the heat: crops and global change. Curr. Opin. Plant Biol. 13:240–247 10.1016/j.pbi.2010.04.008 [DOI] [PubMed] [Google Scholar]
- Maathuis F.J.2011. Vacuolar two-pore K+ channels act as vacuolar osmosensors. New Phytol. 191:84–91 10.1111/j.1469-8137.2011.03664.x [DOI] [PubMed] [Google Scholar]
- Marshall A., Aalen R.B., Audenaert D., Beeckman T., Broadley M.R., Butenko M.A., Caño-Delgado A.I., de Vries S., Dresselhaus T., Felix G., et al. . 2012. Tackling drought stress: Receptor-like kinases present new approaches. Plant Cell. 24:2262–2278 10.1105/tpc.112.096677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen G.B., and Haswell E.S.. 2013. A force of nature: molecular mechanisms of mechanoperception in plants. J. Exp. Bot. 64:4663–4680 10.1093/jxb/ert204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsegian V.A., Rand R.P., and Rau D.C.. 1995. Macromolecules and water: Probing with osmotic stress. Methods Enzymol. 259:43–94 10.1016/0076-6879(95)59039-0 [DOI] [PubMed] [Google Scholar]
- Pritchard J.2001. Turgor pressure. Encyclopedia of the Life Sciences. John Wiley & Sons, Ltd., Hoboken, NJ: 1–3 10.1038/npg.els.0001687 [DOI] [Google Scholar]
- Romeis T., Ludwig A.A., Martin R., and Jones J.D.. 2001. Calcium-dependent protein kinases play an essential role in a plant defence response. EMBO J. 20:5556–5567 10.1093/emboj/20.20.5556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito H., and Posas F.. 2012. Response to hyperosmotic stress. Genetics. 192:289–318 10.1534/genetics.112.140863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tran L.S., Urao T., Qin F., Maruyama K., Kakimoto T., Shinozaki K., and Yamaguchi-Shinozaki K.. 2007. Functional analysis of AHK1/ATHK1 and cytokinin receptor histidine kinases in response to abscisic acid, drought, and salt stress in Arabidopsis. Proc. Natl. Acad. Sci. USA. 104:20623–20628 10.1073/pnas.0706547105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verslues P.E., and Juenger T.E.. 2011. Drought, metabolites, and Arabidopsis natural variation: a promising combination for understanding adaptation to water-limited environments. Curr. Opin. Plant Biol. 14:240–245 10.1016/j.pbi.2011.04.006 [DOI] [PubMed] [Google Scholar]
- Verslues P.E., Agarwal M., Katiyar-Agarwal S., Zhu J., and Zhu J.K.. 2006. Methods and concepts in quantifying resistance to drought, salt and freezing, abiotic stresses that affect plant water status. Plant J. 45:523–539 10.1111/j.1365-313X.2005.02593.x [DOI] [PubMed] [Google Scholar]
- Verslues P.E., Lasky J.R., Juenger T.E., Liu T.W., and Kumar M.N.. 2014. Genome-wide association mapping combined with reverse genetics identifies new effectors of low water potential-induced proline accumulation in Arabidopsis. Plant Physiol. 164:144–159 10.1104/pp.113.224014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson M.E., Basu M.R., Bhaskara G.B., Verslues P.E., and Haswell E.S.. 2014. Plastid osmotic stress activates cellular stress responses in Arabidopsis. Plant Physiol. 165:119–128 10.1104/pp.114.236620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wohlbach D.J., Quirino B.F., and Sussman M.R.. 2008. Analysis of the Arabidopsis histidine kinase ATHK1 reveals a connection between vegetative osmotic stress sensing and seed maturation. Plant Cell. 20:1101–1117 10.1105/tpc.107.055871 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T., Mogami J., and Yamaguchi-Shinozaki K.. 2014. ABA-dependent and ABA-independent signaling in response to osmotic stress in plants. Curr. Opin. Plant Biol. 21:133–139 10.1016/j.pbi.2014.07.009 [DOI] [PubMed] [Google Scholar]
- Yuan F., Yang H., Xue Y., Kong D., Ye R., Li C., Zhang J., Theprungsirikul L., Shrift T., Krichilsky B., et al. . 2014. OSCA1 mediates osmotic-stress-evoked Ca increases vital for osmosensing in Arabidopsis. Nature. 514:367–371 10.1038/nature13593 [DOI] [PubMed] [Google Scholar]