Ion channels are embedded in the lipid bilayer of cell membranes; therefore, it is not surprising that their functions can be modulated by membrane phospholipids and their metabolites. Over the last decade, a number of ion channels—including multiple types of potassium channels, voltage-gated calcium channels, and transient receptor potential channels—have been shown to be modulated by phospholipids. This lipid modulation of ion channels has been implicated in the regulation of such neuronal functions as excitability and synaptic transmission. In a recent article published in The Journal of General Physiology, the research team of Vincent Jacquemond demonstrated that phosphoinositides modulate skeletal muscle calcium signaling (Berthier et al., 2015). Surprisingly, their results indicate that phosphoinositides can enhance calcium release from the SR without affecting currents through voltage-gated calcium channels.
Excitation–contraction (EC) coupling is the process whereby membrane depolarization in muscle is converted into a transient increase in the cytoplasmic free calcium concentration (Melzer et al., 1995), with the magnitude of the calcium signal determining the force of muscle contraction. In both skeletal and cardiac muscle, EC coupling relies on the close interaction of two calcium channels: a voltage-gated calcium channel (CaV1) in the plasma membrane and T-tubules, and a ryanodine receptor calcium release channel (RyR) in the SR. In cardiac muscle cells, CaV1.2 calcium channels open during the action potential; the resulting calcium influx contributes to the myoplasmic calcium signal and activates the type 2 RyR (RyR2) calcium release channel to trigger further release of calcium from the SR. Thus, in cardiac muscle, the magnitude of the calcium transient is directly related to that of the L-type calcium current. Consequently, modulation of the voltage-gated calcium channel CaV1.2 is an efficient mechanism to regulate cardiac contraction.
In adult skeletal muscle, on the other hand, L-type calcium currents contribute little if anything to the myoplasmic calcium signal that controls contraction. Rather, the CaV1.1 isoform acts solely as a voltage sensor (Ríos et al., 1992) that activates the type 1 RyR (RyR1) through conformational coupling. Voltage-dependent activation of RyR1 relies on the cytoplasmic II–III loop of the CaV1.1 α1S subunit (Grabner et al., 1999) and on the auxiliary β1a subunit (Schredelseker et al., 2005). Opening of the CaV1.1 channel pore is slow compared with voltage-dependent activation of the RyR1 and requires substantially higher membrane depolarization; moreover, the resulting calcium currents are small and functionally insignificant. Because L-type calcium currents do not contribute to cytoplasmic calcium transients in skeletal muscle under physiological conditions, CaV1.1 L-type calcium currents are an ineffective target for the modulation of skeletal muscle EC coupling. Nevertheless, modulation of the voltage-sensing function of CaV1.1 is likely to affect RyR1 calcium release and consequently contractile force.
Berthier et al. (2015), researchers from Lyon, France, showed that skeletal muscle EC coupling is sensitive to modulation by phosphoinositides. Notably, they demonstrated this in fully differentiated muscle fibers in real time. To do so, they had to tackle several experimental challenges: transfecting mature skeletal muscle cells in situ, controlling the phosphoinositide composition of the T-tubule membrane, and measuring EC coupling in real time. They used the voltage-sensitive phosphoinositide phosphatases Ciona intestinalis voltage-sensing phosphatase (VSP) and the eGFP-tagged Danio rerio VSP (Dr-VSP), which upon strong membrane depolarization specifically dephosphorylate PIP2 (Okamura et al., 2009), to rapidly change the phosphoinositide composition in muscle fiber membranes. Activation of VSPs requires substantially higher depolarization than does activation of EC coupling, allowing Berthier et al. (2015) to analyze depolarization-induced calcium release with and without simultaneous VSP activation. Transfection of VSP expression plasmids into flexor digitorum brevis and interosseous muscles in vivo was accomplished by microinjection followed by electroporation, allowing the researchers to use what probably is the best available model system for studying mature mammalian skeletal muscle function. 7–10 d later, they isolated individual muscle fibers and analyzed them in a system that enabled them to combine voltage-clamp analysis with fluorescence imaging. This sophisticated setup, which has been used and refined in the Jacquemond laboratory for many years, provides maximal experimental control over the composition of the intracellular and extracellular compartments (i.e., for the application of fluorescent calcium indicators or drugs, respectively) and over the membrane potential (i.e., to activate EC coupling, VSPs, or both). Moreover, this approach yields a large number of quantitative data from electrophysiology (including current properties and movement of gating charges of both native voltage-gated ion channels and of the heterologously expressed VSPs) and from fluorescence imaging (i.e., the localization and redistribution of eGFP- or mRFP-tagged probes, and changes in the concentration of cytoplasmic free calcium ions).
When expressed in muscle fibers, Dr-VSP localized to the T-tubule system, and activation of either of the VSP constructs by depolarizing pulses to 100 mV or higher resulted in the depletion of PI(4,5)P2 within seconds. This depletion was independently demonstrated by the rapid translocation of the specific PI(4,5)P2-binding probe PLCδ1PH-mRFP from the T-tubules into the cytoplasm. The central finding of the study is that pulse protocols that result in depletion of PI(4,5)P2 led to a decay of the peak cytoplasmic calcium signals that was faster than that found without PI(4,5)2 depletion. The rate of calcium release was depressed by ∼30% in VSP-expressing fibers. Equally strong depolarizations in eGFP-transfected control muscle fibers, or depolarization to voltages that fully activated SR calcium release but not VSPs, did not show a similar rundown. The VSP effect on calcium signals was reversible, and its time course (in the 10-s range) as well as its voltage dependence was similar to values expected for VSP action based on previous work (Okamura et al., 2009; Kurokawa et al., 2012), and to the time course of the PLCδ1PH-mRFP translocation induced by activation of VSP. Therefore, it is plausible to conclude that the activation of VSP caused the reduction of the depolarization-induced calcium signals in skeletal muscle.
This work has several important implications. First, it is a remarkable technical accomplishment to experimentally control PI(4,5)P2 levels in the T-tubule system of differentiated muscle cells in real time. VSPs have been successfully used to manipulate phosphoinositides in various cell systems (Okamura et al., 2009). Putting them to use in differentiated muscle cells to deplete PI(4,5)P2 from the rather inaccessible T-tubule system raises the use of this technique to a new level of sophistication. Second, demonstrating the rapid translocation of VSP PLCδ1PH-mRFP from the T-tubules into the cytoplasm and its equally rapid reversibility provide valuable information about phosphoinositide metabolism in this unique muscle membrane system. It demonstrates that T-tubules contain physiologically relevant concentrations of PI(4,5)P2 and of the PI(5) kinase. To our knowledge, this has not previously been shown for the T-tubule system of skeletal muscle and has important implications for this membrane system’s role in signal transduction pathways that rely on phosphoinositide metabolites. Finally, the most important finding of the study is that skeletal muscle EC coupling is sensitive to PI(4,5)P2 signaling. Consequently, physiological PI(4,5)P2 concentrations in the T-tubules appear to be important for maintaining calcium signals and thus contractile force during repetitive strong activation of EC coupling. This result is in agreement with those of several earlier studies demonstrating effects of direct application of PI(4,5)P2 to chemically skinned muscle fibers and isolated SR membranes (Kobayashi et al., 1989; Ogawa and Harafuji, 1989; Ohizumi et al., 1999).
This identification of a role for phosphoinositide modulation of EC coupling raises several questions. Which phosphoinositide is the physiologically active compound? What is the source and what is the target of PI(4,5)P2 in skeletal muscle? What, if any, is the physiological signaling pathway and what is its functional importance in modulation of EC coupling? The experimental design itself enables a conclusive answer to the question of the physiologically active phosphoinositide. In native signaling pathways, PI(4,5)P2 is a substrate for several enzymes and can therefore yield a range of biologically active compounds including IP3, diacylglycerol, and arachidonic acid. This can make it difficult to dissect whether in response to activation of a given receptor depletion of PI(4,5)P2 or accumulation of one or more of its metabolites is responsible for the observed cellular response. In contrast, VSP specifically dephosphorylates the 5′ position of PI(3,4,5)P3 and PI(4,5)P2, and the 3′ position of PI(3,4)P2 (Kurokawa et al., 2012). Moreover, the observed translocation of the specific PI(4,5)P2 probe PLCδ1PH-mRFP further identifies PI(4,5)P2 as the VSP substrate. Therefore, the signaling mechanisms relevant for modulation of EC coupling after VSP activation and PI(3,4)P2 depletion is most likely either the dissociation of PI(4,5)P2 from a specific target protein in the T-tubule membrane or the loss of membrane targeting of a PI(4,5)P2-binding protein (e.g., a pleckstrin homology [PH] domain containing triad protein).
Calcium signals in skeletal muscle EC coupling principally arise from SR calcium release through the RyR1. Indeed, earlier studies provide evidence that PI(4,5)P2 induces RyR-dependent calcium release from heavy SR fractions reconstituted in lipid bilayers (Chu and Stefani, 1991), making RyR1 a leading candidate for the target of PI(4,5)P2 action. However, ER/SR membranes do not contain significant amounts of PI(4,5)P2 (Hidalgo et al., 1986). Furthermore, it is rather unlikely that activation of VSP in the outer membrane (here the T-tubule membrane) would dephosphorylate PI(4,5)P2 in the adjacent SR membrane. In contrast, a facilitating action of PI(4,5)P2 on CaV1.1 is a likely scenario for PI(4,5)P2 modulation of EC coupling. In skeletal muscle, CaV1.1 controls the opening of RyR1, and other members of the L-type calcium channel family (CaV1.2, CaV1.3) have been shown previously to be modulated by PI(4,5)P2 (Suh et al., 2010; Hille et al., 2015). Berthier et al. (2015) tested this possibility and found no effect of PI(4,5)P2 depletion on L-type currents in their muscle fibers. It is, however, possible that PI(4,5)P2 binding could either specifically facilitate the voltage-sensing function of CaV1.1 for EC coupling or the molecular mechanism that transduces the activating signal from the CaV1.1 voltage sensor to the RyR1. Because CaV1.1 activation of EC coupling is much more sensitive than L-current activation, it is conceivable that the EC-coupling function of CaV1.1 could be modulated without changing its currents. Such a CaV1.1-dependent modulation of RyR1 channel gating would be reminiscent of the action of malignant hyperthermia mutations in CaV1.1 that facilitate gating of the RyR1 independently of their action on CaV1.1 current properties (Weiss et al., 2004; Pirone et al., 2010).
If the modulation of EC coupling originates from binding of PI(4,5)P2 to CaV1.1, one would expect to find a specific PI(4,5)P2-binding site on the cytoplasmic side of the CaV1.1 α1 subunit or in the auxiliary β1a subunit, which is also essential for EC coupling. In this context, Berthier et al. (2015) refer to a positively charged amino acid cluster in the C terminus of the neuronal CaV2.1 channel isoform that has been reported to bind phosphoinositides (Rousset et al., 2004). However, it is not known whether a corresponding phosphoinositide-binding site exists in the very dissimilar C terminus of the skeletal muscle CaV1.1 isoform. Recently, a phosphoinositide-sensitive polybasic membrane–binding motif has been described in the cytoplasmic loop (I–II linker) of all CaV1 channels (Kaur, G., A. Lieb, M. Sinnegger-Brauns, G.J. Obermair, B.E. Flucher, and J. Striessnig. 2012. Society of Neuroscience Meeting 2012. Abstr. 235.01). Considering its strategic position near the binding site of the β1a subunit, it is intriguing to speculate that CaV1.1 binding of PI(4,5)P2 to this sequence might stabilize the I–II linker and with it β1a, and thus facilitate the efficient transmission of the EC-coupling activation signal through the tripartite complex of CaV1.1α1, β1a, and the RyR1 (Fig. 1). Further experiments will be necessary to test this hypothesis.
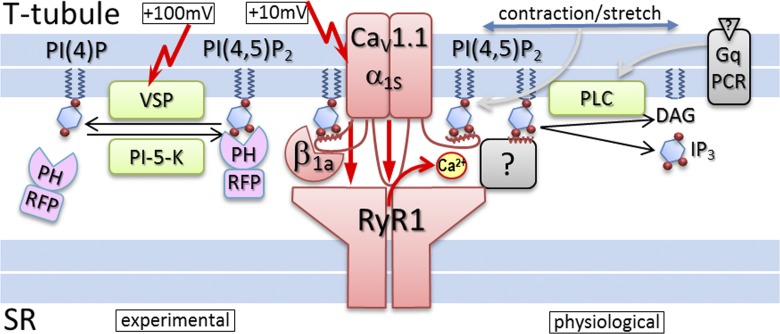
Figure 1.
PI(4,5)2 modulation of skeletal muscle EC coupling. In the center, the EC-coupling complex (red)—composed of the L-type calcium channel subunits CaV1.1 α1s and β1a in the T-tubule and the RyR1 calcium release channel in the SR—is displayed. The experimental tools used by Berthier et al. (2015) to deplete PI(4,5)P2 (VSP) and monitor PI(4,5)P2 depletion (PH-RFP, PLCδ1PH-mRFP) are shown on the left. Note that EC coupling is activated at membrane potentials of ≥10 mV, whereas activation of VSP requires repetitive depolarization to ≥100 mV. On the right, physiological signaling pathways that mediate PI(4,5)P2 depletion are shown. It is also possible that PI(4,5)P2 modulation of the EC-coupling complex is caused by the physical force of membrane stretch during contraction. PI(4,5)P2 might interact with binding sites on CaV1.1, like that in close proximity to the binding site for β1a, and thereby facilitate EC coupling. Alternatively, PI(4,5)P2 could interact with other triad proteins spanning the gap between the T-tubule membrane and the RyR1 to facilitate SR calcium release without modulating CaV1.1 calcium currents.
Of course, other constituents of the functional EC-coupling protein complex could also be possible targets for PI(4,5)P2. Necessary conditions for carrying out this function would be that the responsible triad protein interacts (directly or indirectly) with the RyR1 and either partitions into the cytoplasmic leaflet of the T-tubule membrane or is anchored to the T-tubule membrane by a PH or a similar PI(4,5)P2-binding domain.
Finally, the question of the possible physiological upstream activators of PI(4,5)P2 depletion must be addressed. PI(4,5)P2 modulation of ion channels has been principally discussed in two different contexts (Hille et al., 2015). According to the regulation in time hypothesis, PI(4,5)P2 binding to channels could function as a mechanism to fine-tune calcium channel function in response to upstream signaling events like the activation of Gq protein–coupled receptors or other pathways that activate signaling phospholipases or PI5 kinase. According to the regulation in space hypothesis, PI(4,5)P2 interactions could stabilize normal channel function in the appropriate membrane compartment, whereas, in membrane compartments lacking PI(4,5)P2, channel function would be inhibited. In line with this second hypothesis, the results from Berthier et al. (2015) would indicate that the specific phosphoinositide composition of the T-tubule membrane is important for the optimal function of the EC-coupling apparatus. Furthermore, PI(4,5)P2 has been shown to mediate physical fore (i.e., membrane stretch) between the lipid bilayer and voltage-gated ion channels (Anishkin et al., 2014). Therefore, skeletal muscle contraction could by itself be the mechanism activating PI(4,5)P2 modulation of EC coupling. In this case, no need for endogenous PI(4,5)P2-modulating enzymes or upstream regulators would exist. On the other hand, if indeed active PI(4,5)P2 depletion functions as a physiological modulator of EC coupling, the receptor initiating this pathway and the essential enzymes remain to be identified. PLC and PI3K have been implicated in various signaling pathways controlling myoblast fusion, muscle differentiation, metabolism, and insulin-dependent regulation of glucose uptake. In light of the present findings, any of these signaling events could modulate SR calcium release in parallel by activating PI(4,5)P2 depletion.
Many previous studies have identified PI(4,5)P2 modulation of ion channels in heterologous expression systems. This reductionist approach facilitated the elucidation of the molecular mechanisms underlying PI(4,5)P2 action on these channels. However, in many cases, the physiological importance of channel modulation by phosphoinositides remained unclear. With their bold approach, Jacquemond and coworkers provide compelling functional evidence for modulation of EC coupling by PI(4,5)P2 under physiological conditions. Now the molecular details of the signaling mechanism and the upstream activators must be identified.
Acknowledgments
Current research in the author’s laboratory is funded by grants of the Austrian Science Fund (FWF): P23479, P27031, and W1101.
Elizabeth M. Adler served as editor.
References
- Anishkin A., Loukin S.H., Teng J., and Kung C.. 2014. Feeling the hidden mechanical forces in lipid bilayer is an original sense. Proc. Natl. Acad. Sci. USA. 111:7898–7905. 10.1073/pnas.1313364111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berthier C., Kutchukian C., Bouvard C., Okamura Y., and Jacquemond V.. 2015. Depression of voltage-activated Ca2+ release in skeletal muscle by activation of a voltage-sensing phosphatase. J. Gen. Physiol. 145:315–330. 10.1085/jgp.201411309 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A., and Stefani E.. 1991. Phosphatidylinositol 4,5-bisphosphate-induced Ca2+ release from skeletal muscle sarcoplasmic reticulum terminal cisternal membranes. Ca2+ flux and single channel studies. J. Biol. Chem. 266:7699–7705. [PubMed] [Google Scholar]
- Grabner M., Dirksen R.T., Suda N., and Beam K.G.. 1999. The II-III loop of the skeletal muscle dihydropyridine receptor is responsible for the bi-directional coupling with the ryanodine receptor. J. Biol. Chem. 274:21913–21919. 10.1074/jbc.274.31.21913 [DOI] [PubMed] [Google Scholar]
- Hidalgo C., Carrasco M.A., Magendzo K., and Jaimovich E.. 1986. Phosphorylation of phosphatidylinositol by transverse tubule vesicles and its possible role in excitation-contraction coupling. FEBS Lett. 202:69–73. 10.1016/0014-5793(86)80651-2 [DOI] [PubMed] [Google Scholar]
- Hille B., Dickson E.J., Kruse M., Vivas O., and Suh B.C.. 2015. Phosphoinositides regulate ion channels. Biochim. Biophys. Acta. 1851:844–856. 10.1016/j.bbalip.2014.09.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M., Muroyama A., and Ohizumi Y.. 1989. Phosphatidylinositol 4,5-bisphosphate enhances calcium release from sarcoplasmic reticulum of skeletal muscle. Biochem. Biophys. Res. Commun. 163:1487–1491. 10.1016/0006-291X(89)91147-9 [DOI] [PubMed] [Google Scholar]
- Kurokawa T., Takasuga S., Sakata S., Yamaguchi S., Horie S., Homma K.J., Sasaki T., and Okamura Y.. 2012. 3′ Phosphatase activity toward phosphatidylinositol 3,4-bisphosphate [PI(3,4)P2] by voltage-sensing phosphatase (VSP). Proc. Natl. Acad. Sci. USA. 109:10089–10094. 10.1073/pnas.1203799109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melzer W., Herrmann-Frank A., and Lüttgau H.C.. 1995. The role of Ca2+ ions in excitation-contraction coupling of skeletal muscle fibres. Biochim. Biophys. Acta. 1241:59–116. 10.1016/0304-4157(94)00014-5 [DOI] [PubMed] [Google Scholar]
- Ogawa Y., and Harafuji H.. 1989. Ca-release by phosphoinositides from sarcoplasmic reticulum of frog skeletal muscle. J. Biochem. 106:864–867. [DOI] [PubMed] [Google Scholar]
- Ohizumi Y., Hirata Y., Suzuki A., and Kobayashi M.. 1999. Two novel types of calcium release from skeletal sarcoplasmic reticulum by phosphatidylinositol 4,5-biphosphate. Can. J. Physiol. Pharmacol. 77:276–285. 10.1139/y99-017 [DOI] [PubMed] [Google Scholar]
- Okamura Y., Murata Y., and Iwasaki H.. 2009. Voltage-sensing phosphatase: actions and potentials. J. Physiol. 587:513–520. 10.1113/jphysiol.2008.163097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pirone A., Schredelseker J., Tuluc P., Gravino E., Fortunato G., Flucher B.E., Carsana A., Salvatore F., and Grabner M.. 2010. Identification and functional characterization of malignant hyperthermia mutation T1354S in the outer pore of the Cavα1S-subunit. Am. J. Physiol. Cell Physiol. 299:C1345–C1354. 10.1152/ajpcell.00008.2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ríos E., Pizarro G., and Stefani E.. 1992. Charge movement and the nature of signal transduction in skeletal muscle excitation-contraction coupling. Annu. Rev. Physiol. 54:109–133. 10.1146/annurev.ph.54.030192.000545 [DOI] [PubMed] [Google Scholar]
- Rousset M., Cens T., Gouin-Charnet A., Scamps F., and Charnet P.. 2004. Ca2+ and phosphatidylinositol 4,5-bisphosphate stabilize a Gβγ-sensitive state of CaV2 Ca2+ channels. J. Biol. Chem. 279:14619–14630. 10.1074/jbc.M313284200 [DOI] [PubMed] [Google Scholar]
- Schredelseker J., Di Biase V., Obermair G.J., Felder E.T., Flucher B.E., Franzini-Armstrong C., and Grabner M.. 2005. The beta 1a subunit is essential for the assembly of dihydropyridine-receptor arrays in skeletal muscle. Proc. Natl. Acad. Sci. USA. 102:17219–17224. 10.1073/pnas.0508710102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suh B.C., Leal K., and Hille B.. 2010. Modulation of high-voltage activated Ca2+ channels by membrane phosphatidylinositol 4,5-bisphosphate. Neuron. 67:224–238. 10.1016/j.neuron.2010.07.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss R.G., O’Connell K.M.S., Flucher B.E., Allen P.D., Grabner M., and Dirksen R.T.. 2004. Functional analysis of the R1086H malignant hyperthermia mutation in the DHPR reveals an unexpected influence of the III-IV loop on skeletal muscle EC coupling. Am. J. Physiol. Cell Physiol. 287:C1094–C1102. 10.1152/ajpcell.00173.2004 [DOI] [PubMed] [Google Scholar]