A refined spindle pole body (SPB) affinity purification method reveals that the telomere-associated protein Ndj1 also localizes to yeast SPBs, protects them from premature separation, and therefore regulates both SPB cohesion and telomere clustering during meiosis.
Abstract
Yeast centrosomes (called spindle pole bodies [SPBs]) remain cohesive for hours during meiotic G2 when recombination takes place. In contrast, SPBs separate within minutes after duplication in vegetative cells. We report here that Ndj1, a previously known meiosis-specific telomere-associated protein, is required for protecting SPB cohesion. Ndj1 localizes to the SPB but dissociates from it ∼16 min before SPB separation. Without Ndj1, meiotic SPBs lost cohesion prematurely, whereas overproduction of Ndj1 delayed SPB separation. When produced ectopically in vegetative cells, Ndj1 caused SPB separation defects and cell lethality. Localization of Ndj1 to the SPB depended on the SUN domain protein Mps3, and removal of the N terminus of Mps3 allowed SPB separation and suppressed the lethality of NDJ1-expressing vegetative cells. Finally, we show that Ndj1 forms oligomeric complexes with Mps3, and that the Polo-like kinase Cdc5 regulates Ndj1 protein stability and SPB separation. These findings reveal the underlying mechanism that coordinates yeast centrosome dynamics with meiotic telomere movement and cell cycle progression.
Introduction
During cell division, the centrosome, acting as the primary microtubule-organizing center, duplicates in coordination with DNA replication. Duplicated centrosomes are tethered during the G2 phase of the cell cycle. At G2-to-M transition, centrosomes separate to permit spindle assembly. Coordination of centrosome cohesion and separation with chromosome segregation is critical for maintaining genome integrity (Nigg and Stearns, 2011; Mardin and Schiebel, 2012).
The yeast centrosome, often referred to as the spindle pole body (SPB), is functionally equivalent to, and shares structural components with, the animal centrosome. The yeast SPB is embedded in the nuclear envelope and forms a layered structure (Byers and Goetsch, 1975). An appendage called the half-bridge connects to the central plaque of the SPB and is required for SPB duplication. During the late G1 phase of the cell cycle, the half-bridge elongates. Then, at the distal end of the elongated half-bridge, a satellite material is deposited to initiate SPB duplication. The satellite further expands to become a new mature SPB (Jaspersen and Winey, 2004). Duplicated SPBs are now tethered by the complete bridge and form a side-by-side configuration (Fig. 1 A), which we term SPB cohesion. Cleavage of the SPB bridge likely permits SPB separation and spindle assembly (Li et al., 2006; Anderson et al., 2007), but the site of half-bridge cleavage remains to be elucidated.
Figure 1.
Identification of Ndj1 as an SPB-associated protein. (A) Schematic diagram showing a pair of side-by-side SPBs during yeast meiosis. ONM, outer nuclear membrane; INM, inner nuclear membrane; OP, outer plaque; CP, central plaque; IP, inner plaque. (B) Comparison of SPB dynamics in vegetative and meiotic yeast cells. (C) A silver-staining gel showing the enrichment of SPB components after affinity purification of Spc97-TAP. Strain HY3674 was used. (D) List of SPB proteins identified by protein mass spectrometry of Spc97-TAP samples. Note that Ndj1 is meiosis specific. The extended list of peptides recovered by mass spectrometry of Spc97-TAP samples is available in Fig. S1. (E and F) Protein affinity purification of Ndj1-TAP (HY3813) and Mps3-TAP (HY3848) from meiotic yeast cells. Arrows point to the same protein bands identified by silver staining (left) and immunoblotting (right). Anti-GFP antibody was used to probe Mps3-GFP, and anti-HA antibody was used to probe Ndj1-3HA. Both antibodies also recognize Ndj1-TAP and Mps3-TAP. Representative proteins identified by protein mass spectrometry are listed in the tables below. (G) Localization of Ndj1 during meiosis. Yeast cells (HY3859) were induced to undergo meiosis, and time-lapse fluorescence microscopy was performed to localize Ndj1-GFP (green) and Spc42-RFP (red). Projected images of eight z sections are shown. Time zero is defined as the point of SPB separation. Arrows point to the Ndj1-GFP focus at the SPB. The graph below shows the relative intensity of the Ndj1-GFP focus at the SPB before SPB separation. The representative cell shown is from a single time-lapse experiment (n > 50). The SPB area was defined by the Spc42-RFP signal. Bar, 2 µm. (H) A timeline of Ndj1 localization, disassociation, and SPB separation during meiosis I. Duration of Ndj1 localization at SPB is not drawn to scale.
Among the 18 known SPB proteins, only four—Cdc31 (homologue of centrin), Kar1, Mps3 (the SUN domain protein in budding yeast), and Sfi1 (homologue of human hSfi1)—are subunits of the half-bridge (Jaspersen and Winey, 2004). Cdc31 and Sfi1 form fibrous filaments that span the full bridge and potentially connect duplicated SPBs (Kilmartin, 2003; Li et al., 2006). Supporting this idea, recent studies have revealed that phosphorylation of Sfi1 is critical for both SPB duplication and separation in vegetative yeast cells (Avena et al., 2014; Elserafy et al., 2014). Kar1 interacts with Cdc31 and Mps3 but has no obvious homologues in higher eukaryotes (Vallen et al., 1994; Spang et al., 1995; Jaspersen et al., 2002). The SUN-domain protein Mps3 is concentrated at the half-bridge and is necessary for the insertion of newly duplicated SPBs into the nuclear envelope (Jaspersen et al., 2002, 2006; Nishikawa et al., 2003; Friederichs et al., 2011). An emerging theme shows that SUN-domain proteins, which are integral membrane proteins of the inner nuclear envelope, bind to the KASH-domain proteins located at the outer nuclear envelope (Hiraoka and Dernburg, 2009; Tapley and Starr, 2013). Two KASH-like proteins are found in budding yeast: Mps2 (Jaspersen et al., 2006), which is only present at the SPB; and its paralogue Csm4 (Kosaka et al., 2008; Wanat et al., 2008), which is meiosis specific and localizes broadly to the yeast nuclear envelope but not to the SPB. The SUN-KASH protein pair connects the inner and outer nuclear envelopes and transmits cytoskeleton forces across the nuclear envelope, mediating nuclear migration and telomere movement (Hiraoka and Dernburg, 2009; Tapley and Starr, 2013). Whether Mps3 is required for mediating SPB cohesion is unclear, and if so, how Mps3 regulates SPB separation is unknown.
Here we investigate the novel factors that are associated with the yeast SPB and that regulate SPB cohesion in meiotic yeast cells. We hypothesize that meiosis-specific proteins directly interact with the half-bridge components to maintain SPB cohesion. During meiosis, the SPB is duplicated in coordination with DNA replication (Moens and Rapport, 1971), but duplicated SPBs remain tethered for hours during the extended G2 phase, also called prophase I, when meiotic recombination takes place (Padmore et al., 1991; Okaz et al., 2012; see diagrams in Fig. 1 B). This is in great contrast to SPB dynamics in vegetative yeast cells, where duplicated SPBs separate within minutes after duplication (Lim et al., 1996). Previous studies have shown that the delay of SPB separation during meiotic prophase I is due to reduced activity of the B-type cyclin-Cdk1 (Clb-Cdk1) at this stage of the cell cycle (Miller et al., 2012; Okaz et al., 2012) and the inhibition mediated by the Aurora kinase Ipl1 in yeast (Shirk et al., 2011; Kim et al., 2013; Newnham et al., 2013). However, it is currently unclear whether meiosis-specific factors contribute to keep SPBs cohesive, which is critical for preventing premature spindle assembly and chromosome missegregation during meiosis.
Using a refined SPB affinity purification method, we have identified meiosis-specific proteins that are copurified with the yeast SPB. One of them, Ndj1, which binds to Mps3, is a known telomere-associated protein (Conrad et al., 1997). Here, we show that there are two separable pools of Ndj1, one of which localizes to the SPB, the other to the telomeres. The SPB-associated Ndj1 protects SPBs from premature separation, which ensures that meiotic recombination takes place before spindle assembly. The fact that Ndj1 regulates both SPB cohesion and telomere clustering underscores the importance of the coordination of cell cycle events during meiotic cell progression.
Results
Identification of Ndj1 as an SPB-associated protein
We hypothesized that meiosis-specific proteins regulate SPB cohesion during the extended prophase I. To identify proteins bound to the SPB, we generated a functional SPC97-TAP allele, which served as the only copy of SPC97 in the experimental cells. Spc97 is a subunit of the γ-tubulin ring complex, which is required for nucleating microtubules and localizes to the surface of the SPB (Knop et al., 1997). By protein affinity purification (Rock et al., 2013), we enriched the yeast SPB from cells induced to undergo synchronous meiosis (Fig. 1 C). The enriched SPB components were determined by mass spectrometry–based protein identification (Fig. 1 D). As a positive control, SPBs were isolated from vegetative yeast cells by Spc97-TAP affinity purification (Fig. 1 D). Protein mass spectrometry revealed that our enriched SPB samples contained all known SPB subunits, with peptide coverage ranging from 20% to 88% for the meiotic sample and 12% to 97% for the mitotic sample (Fig. 1 D). In addition, we recovered SPB proteins belonging to the meiotic plaque, as well as other SPB-associated proteins that were copurified with Spc97-TAP (Fig. S1). One of them, Ndj1, a meiosis-specific telomere-associated protein, showed 37% peptide coverage by protein mass spectrometry (Fig. 1 D). We therefore propose that Ndj1 associates with the yeast SPB.
Previous work indicates that Ndj1 binds to Mps3, a major component of the half-bridge (Conrad et al., 2007). To investigate their interaction, we generated NDJ1-TAP and MPS3-TAP alleles, which served as the only functional copy for each, and performed reciprocal affinity purification. Using immunoblotting, we found that Mps3, tagged with GFP, was copurified with Ndj1-TAP; and Ndj1, tagged with 3×HA, was copurified with Mps3-TAP (Fig. 1, E and F). These results confirm that Ndj1 and Mps3 are physically associated. Furthermore, by protein mass spectrometry of affinity-purified samples, we found that Mps3 was the major peptide copurified with Ndj1-TAP (Fig. 1 E), whereas Ndj1 was the predominant peptide copurified with Mps3-TAP (Fig. 1 F). The SPB protein, Spc72 (9% peptide coverage), was also recovered from the Ndj1-TAP sample (Fig. 1 F). These findings suggest that Ndj1 binds to Mps3, and perhaps through Mps3, Ndj1 associates with the SPB.
To localize Ndj1 in meiotic cells, we generated an NDJ1-GFP allele, which served as the only functional copy in the whole yeast genome, and performed time-lapse fluorescence microscopy (Fig. 1 G and Fig. S2 A). The majority of Ndj1-GFP signal was localized to the periphery of the yeast nucleus (Fig. 1 G) and showed colocalization with Mps3-RFP (see Fig. 2). These findings support the notion that Ndj1 localizes to the yeast telomeres, which are attached to the nuclear periphery at prophase I (Conrad et al., 2007). Importantly, Ndj1 formed a bright focus that overlapped with that of the SPB core component, Spc42, which was tagged with red fluorescent protein (RFP; Fig. 1 G, arrowheads). As determined by fluorescence microscopy, the intensity of the Ndj1-GFP focus at the SPB reduced more than fivefold immediately before SPB separation, a landmark of the onset of metaphase I (Fig. 1 G). On average, Ndj1 was removed from the SPB 16 minutes (n = 23) before SPB separation (Fig. 1 H). Ndj1-GFP was not observed in metaphase I cells (Fig. 1 G and Fig. S2 A), in contrast to Mps3-RFP, which remained at the nuclear periphery during the entire course of meiosis I (Fig. 2 A). We therefore conclude that in addition to telomeres, Ndj1 localizes to the yeast SPB but disappears from the SPB and the cell right before SPB separation.
Figure 2.
Localization of Ndj1 to SPB depends on Mps3. (A) Colocalization of Ndj1 and Mps3 during yeast meiosis. Time-lapse live-cell microscopy was performed as in Fig. 1 G. Strain HY3881 was used. Projected images of eight z sections are shown. Ndj1 was tagged with GFP (green), Mps3 with RFP (red). Time 0 is defined as the point of SPB separation. (B) Immunoblot showing the depletion of Mps3 protein in PCLB2-MPS3 cells (HY3911) during meiosis. Note that in wild-type cells (HY3871), Mps3 peaks around 4 h after induction of meiosis, then appears to be modified and degraded during meiosis. (C) Localization of Ndj1 in PCLB2-MPS3 cells (HY3911). Live-cell microscopy was performed as in A. SPB was marked by Spc42-RFP (red). Note that Ndj1 (green) fails to form a focus at the SPB. Quantification of Ndj1 localization to the SPB is shown to the right. The data shown are from a single representative experiment out of four repeats. For the experiment shown, n = 200. (D and E) Localization of Mps3-GFP in wild-type (HY4418) and ndj1Δ (HY4419) cells during meiosis. Time-lapse microscopy was performed as in A. Mps3 is tagged with GFP (green), Tub4 with RFP (red). Time 0 is defined as the point of SPB separation. Note that Mps3 localizes to the SPB in both strains (arrows). (F) Ndj1 localization in csm4Δ cells. Strains HY4086 (wild-type) and HY4852 (csm4Δ) were used. Live-cell microscopy was performed as in A, and three continuous z sections are shown. Ndj1-GFP, green; Tub4-RFP, red. (G) Csm4 localization in meiotic cells (HY4383). A GFP-CSM4 allele was used to localize Csm4 (green) in meiotic cells. Note that Csm4 does not form a focus at the SPB, as determined by Tub4-RFP (red). Bars, 2 µm.
Localization of Ndj1 to SPB depends on Mps3 but not on Csm4
Because Ndj1 localization to the yeast telomere depends on Mps3 (Conrad et al., 2007), we asked whether localization of Ndj1 to the SPB also depends on Mps3. To deplete Mps3 in yeast meiosis, we generated the PCLB2-MPS3 allele, in which the expression of MPS3 was under the control of the promoter from CLB2, the expression of which is mitosis specific. PCLB2-MPS3 cells were fully functional during vegetative growth, but were defective during meiosis and produced dead spores (unpublished data). Using immunoblotting, we found that the Mps3 protein was beyond detection in mutant cells 2 h after induction of meiosis (Fig. 2 B). In the absence of Mps3, Ndj1 no longer formed foci that localized to the SPB or to the nuclear periphery; instead, the Ndj1-GFP signal became diffused throughout the yeast nucleus (Fig. 2 C). However, Mps3 remained at the SPB and localized to the nuclear periphery in ndj1Δ cells during yeast meiosis (Fig. 2, D and E). These findings demonstrate that Mps3 is required for Ndj1 localization to both the SPB and the nuclear envelope, but not vice versa.
Csm4 interacts with Mps3 and Ndj1 at the yeast telomeres and is necessary for telomere movement in yeast meiosis (Kosaka et al., 2008; Wanat et al., 2008). To exclude the possibility that localization of Ndj1 to the SPB depends on telomere movement, we determined Ndj1 localization in csm4Δ cells by time-lapse fluorescence microscopy (Fig. 2 F). In the absence of Csm4, Ndj1 remained at the SPB, forming a distinctive focus that overlapped with that of Tub4, the γ-tubulin in budding yeast (Fig. 2 F). Therefore, we conclude that Csm4 is dispensable for Ndj1 localization to the SPB. Of note, the rest of the Ndj1-GFP signal that localized to the nuclear periphery often clustered at prophase I in wild-type cells when telomeres formed the bouquet configuration. In contrast, Ndj1 foci were more evenly distributed along the nuclear periphery in csm4Δ cells (Fig. 2 F), which confirms the important role of Csm4 in bouquet formation. To observe Csm4 localization during yeast meiosis, we generated a GFP-CSM4 allele and found that the Csm4 foci did not colocalize with the SPB marker Tub4 (Fig. 2 G). Finally, we determined whether the telomere-associated protein Rap1, which was recovered by mass spectrometry in affinity-purified Ndj1-TAP samples (Fig. 1 E), localized to the SPB during meiosis (Fig. S2). Using live-cell microscopy, we found that overall Rap1 foci were not colocalized with the Tub4 focus (Fig. S2 B); only ∼4% cells showed weak Rap1 signal in the vicinity of the SPB marker Tub4-RFP (Fig. S2 C). In contrast, Ndj1 formed a distinctive focus at the SPB in almost all the meiotic cells examined (Fig. S2 C). On the basis of these observations, we conclude that there are two separable pools of Ndj1: one binds to the SPB, the other to the telomeres, which associate with Csm4 and Rap1.
Ndj1 is removed from the SPB and degraded after prophase I
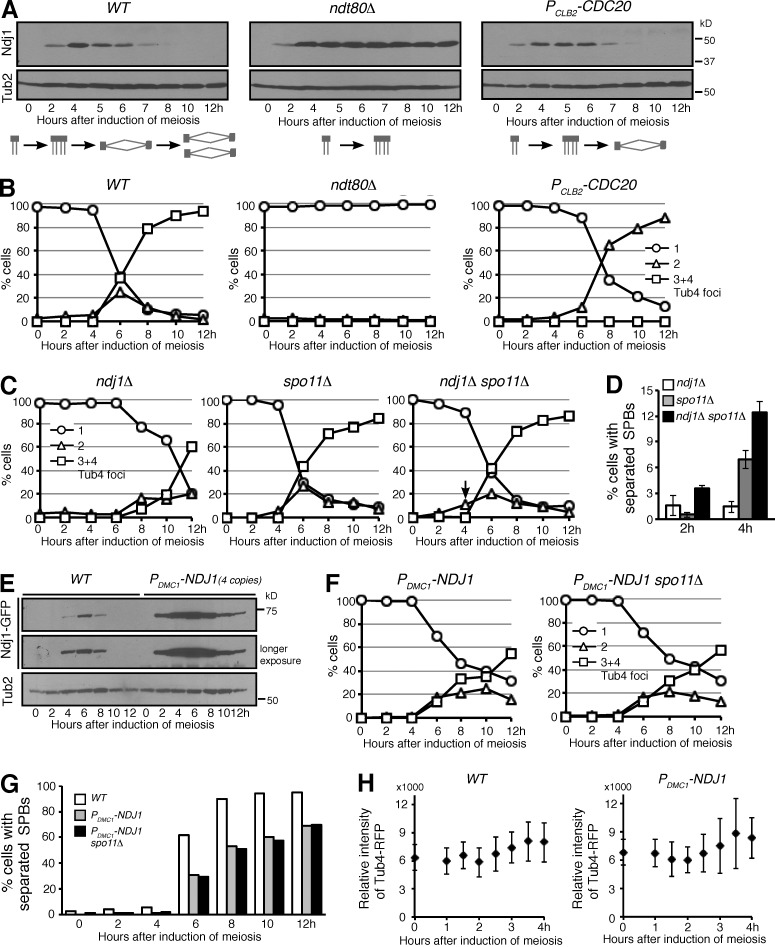
The fact that Ndj1 disappeared from the SPB right before SPB separation suggests that Ndj1 is an unstable protein (Figs. 1 G and 2 A). To determine the protein level of Ndj1, we induced yeast cells to undergo synchronous meiosis, collected representative time-point samples, and performed immunoblotting. Ndj1 was produced immediately after induction of meiosis and peaked around 4 h after (Fig. 3 A). Ndj1 was beyond detection by 8 h after induction (Fig. 3 A); by then the majority of the yeast cells had completed nuclear divisions (Fig. 3 B). To pinpoint the timing of Ndj1 degradation, we staged yeast cells at prophase I by way of ndt80Δ, of which the wild-type gene encodes a transcription factor required for the activation of mid and late meiotic genes (Xu et al., 1995), and at metaphase I by way of PCLB2-CDC20, which depletes the Cdc20 protein in meiotic cells (Lee and Amon, 2003). We found that the protein level of Ndj1 remained constant in ndt80Δ cells, but Ndj1 was degraded in arrested metaphase I cells (Fig. 3 A), which indicates that Ndj1 degradation requires the activation of Ndt80, which corresponds to the cell’s exit from prophase I. In ndt80Δ cells, duplicated SPBs remained cohesive, forming a single Tub4 focus observed by fluorescence microscopy; in contrast, at metaphase I, SPBs separated to form a bipolar spindle (Fig. 3 B). These findings are consistent with the idea that degradation of Ndj1 takes place after prophase I and before spindle assembly.
Figure 3.
Ndj1 regulates SPB separation during yeast meiosis. Yeast cells were induced to undergo synchronous meiosis; aliquots were withdrawn at the indicated times for immunoblotting (A and E) and for microscopy (B, C, F, G, and H). (A) Ndj1 protein level in wild-type (WT, HY3937), ndt80Δ (HY3973), and PCLB2-CDC20 (HY4031) cells. Ndj1 was tagged with 3×HA and probed with an anti-HA antibody. The level of Tub2, β-tubulin in yeast, serves as a loading control. Schematic diagrams at the bottom show SPB dynamics in these cells. (B) Quantification of SPB separation in WT (HY1635), ndt80Δ (HY4115), and PCLB2-CDC20 (HY4113) cells. Tub4-RFP was used as the SPB marker. Before their separation, duplicated SPBs were observed as a single Tub4-RFP focus. The graphs shown are from a representative time-lapse experiment out of three repeats. (C and D) Quantification of SPB separation in ndj1Δ (HY3945), spo11Δ (HY4133), and ndj1Δ spo11Δ (HY4204) cells. SPBs were marked by Tub4-RFP as in B. The arrow indicates premature SPB separation. The p-values for strains spo11Δ and ndj1Δ spo11Δ are <0.05 at both 2 h and 4 h. The graphs shown in C are from a representative time-lapse experiment out of three repeats. Error bars indicate standard deviation. (E) Overproduction of Ndj1 in yeast meiosis (HY4860). Four copies of PDMC1-GFP-NDJ1 were inserted into the yeast genome to overexpress NDJ1. Ndj1 was tagged with GFP and probed with an anti-GFP antibody. The level of Tub2 serves as a loading control. (F and G) Quantification of SPB separation in WT (HY1635), PDMC1-NDJ1 (HY4860), and PDMC1-NDJ1 spo11Δ (HY4861) cells. SPBs were marked by Tub4-RFP as in B. Note that overexpression of NDJ1 delays SPB separation. The p-values for WT and PDMC1-NDJ1 are <0.01 6 h after induction of meiosis. The data shown are from a representative time-lapse experiment out of three repeats. (H) Fluorescence-based assay of SPB duplication in WT (HY1635) and PDMC1-NDJ1 (HY4860) cells during meiosis. The intensity of Tub4-RFP from single optical sections was determined and plotted over time. The mean intensity of Tub4-RFP between WT and PDMC1-NDJ1 is not significantly different as determined by Student’s t tests (P > 0.05).
Ndj1 inhibits SPB separation during yeast meiosis
Because removal of Ndj1 from SPBs coincides with SPB separation, we hypothesized that meiotic SPBs would separate precociously in the absence of Ndj1. To test this hypothesis, we determined SPB dynamics in ndj1Δ cells (Fig. 3 C). Deletion of NDJ1 delays cell cycle progression and can lead to recombination defects (Conrad et al., 1997; Wu and Burgess, 2006). We therefore used spo11Δ to abolish meiotic recombination (Fig. 3 C). Noticeably, 12% of the cells showed separated SPBs in the ndj1Δ spo11Δ double mutant by 4 h after induction of meiosis, compared to only 6% of the cells in the spo11Δ control (Fig. 3 D). This finding suggests that meiotic SPBs separate prematurely in the absence of Ndj1.
If Ndj1 protects SPB cohesion, we predicted that overproduction of Ndj1 would delay SPB separation. Using the promoter from the meiosis-specific gene DMC1, we constructed PDMC1-NDJ1 and inserted four copies of this construct into the yeast genome to overexpress NDJ1 (Fig. 3 E). Similarly, we used spo11Δ to abolish meiotic recombination in our assay of SPB cohesion. More than 90% of wild-type NDJ1 cells separated duplicated SPBs by 8 h after induction of meiosis; in contrast, only about half of PDMC1-NDJ1 cells did so (Fig. 3, B, C, F, and G). Because overproduced Ndj1 was subjected to degradation (Fig. 3 E), SPB separation was delayed but not completely suppressed in PDMC1-NDJ1 cells (Fig. 3, F and G). Based on these observations, we propose that Ndj1 inhibits SPB separation during yeast meiosis.
To determine whether overproduced Ndj1 exerted an adverse effect on SPB duplication, we observed the intensity of the SPB marker Tub4, fused with RFP, using time-lapse fluorescence microscopy. As shown in Fig. 3 H, the intensity of Tub4-RFP increased ∼1.4-fold 3.5 h after induction of meiosis, demonstrating that SPB duplication was on time. This observation is further supported by our findings in vegetative yeast cells (see below). Therefore, we conclude that Ndj1, even when overproduced, doesn’t impair SPB duplication, but delays SPB separation.
Ipl1 regulates Ndj1 localization, but Cdc5 controls Ndj1 protein stability
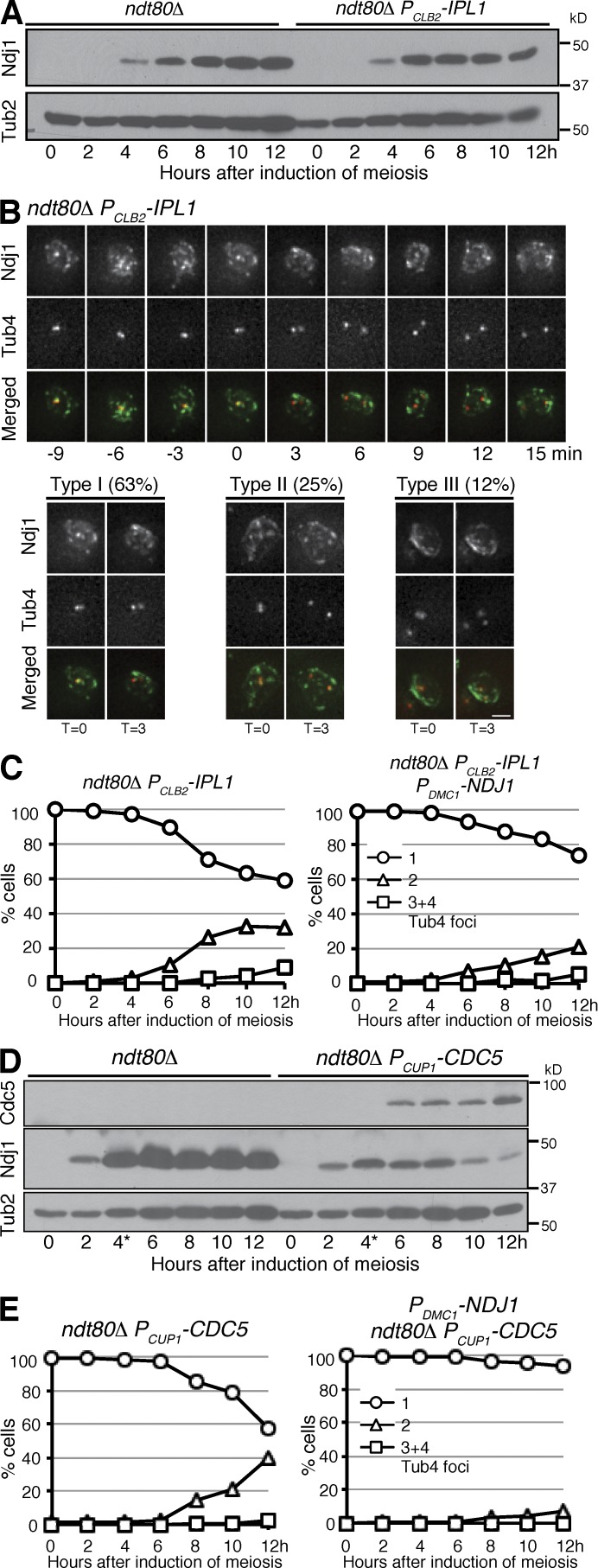
We and others have shown previously that the Aurora kinase Ipl1 in yeast regulates SPB separation during yeast meiosis (Shirk et al., 2011; Kim et al., 2013; Newnham et al., 2013). In Ipl1-depleted meiotic cells that are arrested at G2 by ndt80Δ, more than half of the cells separated SPBs (Fig. 4), because Ipl1 regulates microtubule-based force that is necessary for SPB separation (Kim et al., 2013). To determine whether removal of Ndj1 is sufficient for SPB separation, we generated the ndj1Δ ndt80Δ double mutant (Fig. S3 A). About 3% of these cells displayed separated SPBs 10 h after the induction of meiosis (Fig. S3 A), which indicates that in the absence of the SPB separating force, removal of Ndj1 is not sufficient for SPB separation and spindle assembly.
Figure 4.
Regulation of Ndj1 localization and protein stability during meiosis. (A) Immunoblot showing the Ndj1 protein level in ndt80Δ and ndt80Δ PCLB2-IPL1 (HY4506) cells. The level of Tub2 serves as a loading control. (B) Ndj1 localization in ndt80Δ PCLB2-IPL1 cells during meiosis. Time-lapse microscopy was performed as in Fig. 1 G. Red, Tub4-RFP; green, Ndj1-GFP. Bar, 2 µm. (C) SPB separation in ndt80Δ PCLB2-IPL1 and ndt80Δ PCLB2-IPL1 PDMC1-NDJ1 (HY4654) cells. SPBs were marked by Tub4-RFP. Note the delayed SPB separation in the presence of four copies of PDMC1-NDJ1 as shown in Fig. 3 E. The graphs shown are from a representative time-lapse experiment out of three repeats. (D) Immunoblot showing the Ndj1 protein level in ndt80Δ and ndt80Δ PCUP1-CDC5 (HY4074) cells. To induce CDC5 expression, 60 mM CuSO4 was added to the culture media 4 h (indicated by the asterisk) after induction of meiosis. (E) SPB separation in ndt80Δ PCUP1-CDC5 and ndt80Δ PCUP1-CDC5 PDMC1-NDJ1 (HY4803) cells during meiosis. The graphs shown are from a representative time-lapse experiment out of two repeats.
To determine whether Ipl1 regulates Ndj1 dynamics before SPB separation in staged prophase I cells, we examined Ndj1 protein stability and localization in ndt80Δ PCLB2-IPL1 cells (Fig. 4, A and B). Using immunoblotting, we found that the protein level of Ndj1 remained constant at prophase I with or without Ipl1 (Fig. 4 A), and Ndj1 localized to the SPBs before their separation in Ipl1-depleted cells just as in wild-type cells (Fig. 4 B), which suggests that Ipl1 is not necessary for maintaining Ndj1 protein stability. However, in ndt80Δ PCLB2-IPL1 cells with separating SPBs, 63% of them lost the Ndj1 signal from both the SPBs, and in about 25% the Ndj1 signal was detected only at one of the two SPBs (Fig. 4 B). In both cases, the Ndj1 signal located at the nuclear periphery remained (Fig. 4 B, bottom). These observations provide further evidence that removal of Ndj1 from the SPB correlates with SPB separation in yeast meiosis. In addition, we used the PDMC1-NDJ1 allele to overproduce Ndj1 in nd80Δ PCLB2-IPL1 cells and found that overproduction of Ndj1 significantly delayed SPB separation and spindle assembly (Figs. 3 E, 4 C, and S3 B). These observations demonstrate that Ipl1 regulates Ndj1 localization, perhaps indirectly, to the meiotic SPB but is not necessary for maintaining Ndj1 protein stability.
Activation of NDT80 leads to Ndj1 degradation, which corresponds to SPB separation at the beginning of metaphase I (Fig. 3, A and B). One key target of Ndt80-mediated transcriptional activation is CDC5, which encodes the Polo-like kinase in yeast (Chu and Herskowitz, 1998; Sourirajan and Lichten, 2008). Ectopic expression of CDC5 promotes SPB separation at prophase I (Fig. 4; Newnham et al., 2013). The fact that the timing of Cdc5 production correlates with that of Ndj1 degradation (Fig. 3 A) led us to hypothesize that Cdc5 regulates Ndj1 protein stability and SPB separation. To test this hypothesis, we generated a PCUP1-CDC5 allele to ectopically express Cdc5 in ndt80Δ cells (Fig. 4 D). Upon the addition of CuSO4, PCUP1-CDC5 was expressed and Cdc5 protein was produced (Fig. 4 D). In the presence of Cdc5, the protein level of Ndj1 was dramatically reduced in cells that lacked Ndt80 (Fig. 4 D), and Ipl1 was no longer concentrated at the parallel microtubules at the SPB (Shirk et al., 2011; Kim et al., 2013), instead forming an amorphous structure surrounding duplicated SPBs (Fig. S3 C). Consequently, ∼50% of ndt80Δ PCUP1-CDC5 cells separated their SPBs (Fig. 4 E), which indicates that Cdc5 is sufficient for Ndj1 degradation and SPB separation. Crucially, overexpression of NDJ1 by the PDMC1-NDJ1 allele suppressed SPB separation in ndt80Δ PCUP1-CDC5 cells (Fig. 4 E, right). These observations support the idea that Ndj1 protects SPB cohesion at prophase I, and lead us to conclude that Cdc5 is a critical factor that regulates Ndj1 protein stability in yeast meiosis.
Ectopic expression of NDJ1 inhibits SPB separation in vegetative yeast cells
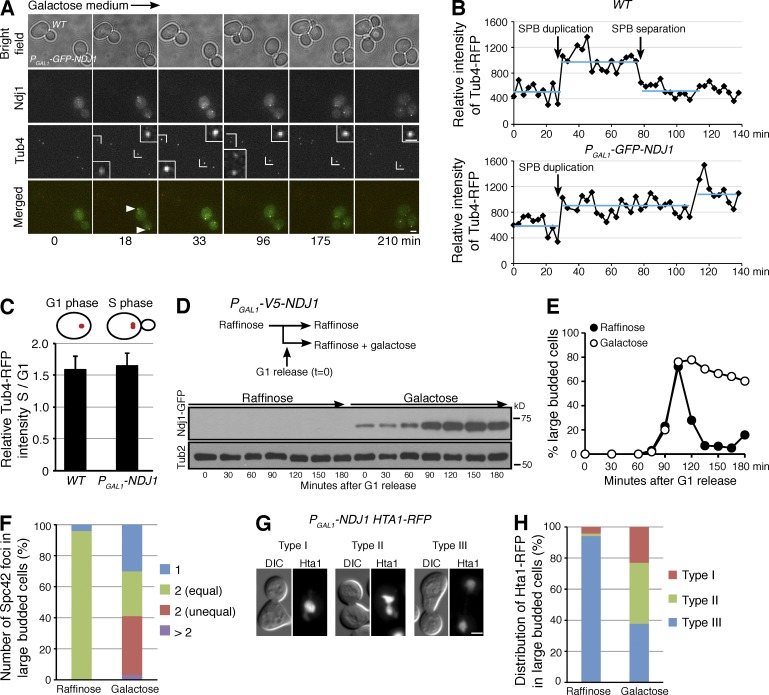
Having shown that Ndj1 inhibits SPB separation in meiotic cells, we reasoned that Ndj1, if ectopically expressed, would suppress SPB separation in vegetative yeast cells. We constructed PGAL1-NDJ1 to produce Ndj1 in these cells (Fig. 5). Upon the addition of galactose to the culture medium, we found that Ndj1 was produced and highly enriched at the yeast SPB (Fig. 5 A and see Fig. 6). Line-scanning of fluorescence intensity showed that the major focus of Ndj1-GFP primarily colocalized with that of Tub4-RFP (Fig. S4 A). Using time-lapse microscopy, we observed SPB dynamics in vegetative cells (Fig. 5, A and B). In wild-type cells, the Tub4 signal increased about twofold upon the emergence of a small bud (Fig. 5, B and C), which indicates SPB duplication. The Tub4 intensity then decreased when SPBs separated (Fig. 5 B). PGAL1-NDJ1 cells appeared competent in SPB duplication because the Tub4 signal increased, just as in wild-type cells, when the small bud emerged at S phase (Fig. 5 C), but, as in the typical mutant cell shown in Fig. 5 A, the Tub4 intensity never decreased; rather, it increased again 100 min after the first round of duplication, which suggests that duplicated SPBs failed to separate when Ndj1 was present. These findings are consistent with our observation that Ndj1 protects SPB cohesion in meiosis.
Figure 5.
Ectopic expression of NDJ1 in vegetative yeast cells. (A and B) PGAL1-NDJ1 produces Ndj1 in vegetative yeast cells. Time-lapse (3-min interval) microscopy shows Ndj1 and Tub4 localization. WT (HY3799) and PGAL1-NDJ1 (HY4128) cells were mixed and incubated in the same flask with 3% galactose. Two cells, one from WT, the other from PGAL1-NDJ1, were scoped from the same viewing field over time. Note that Ndj1-GFP (green) is enriched at the SPB (Tub4-RFP, red; arrows). Time in minutes is arbitrarily defined, and representative images are shown. Insets show 4× magnification of the Tub4-RFP signal. Fluorescence intensity of Tub4-RFP in the insets was determined and plotted in B. The cells shown are from a representative time-lapse experiment, n > 15. Bar, 2 µm. (C) The ratio of Tub4-RFP intensity from G1 phase (no bud) cells to those from S phase (small-budded) cells. Error bars indicate standard deviation. n = 10. (D) Protein level of Ndj1 in PGAL1-NDJ1 cells. Yeast cells grown in the raffinose medium were arrested at G1 with alpha factor; addition of 3% galactose induced the expression of PGAL1-NDJ1. (E and F) Cell aliquots were withdrawn at the indicated times upon removal of the alpha factor and prepared for budding index (E) and SPB separation (F). Spc42 was tagged with RFP and used as an SPB marker. The data shown are from a representative experiment out of four repeats. (G and H) Distribution of Hta1-RFP in PGAL1-NDJ1 cells (HY4249-A). Three categories were observed: type I, one Hta1-RFP mass; type II, separated Hta1-RFP masses with a bridge; type III, two separated Hta1-RFP masses. The data shown are from a representative experiment out of three repeats. Bars, 2 µm.
Figure 6.
Suppression of PGAL1-NDJ1 lethality in vegetative cells. (A and B) Ndj1 colocalization with Mps3 in vegetative yeast cells. Yeast strains PGAL1-GFP-NDJ1 MPS3-RFP (HY4179) and PGAL1-GFP-NDJ1 RAP1-RFP (HY4217) were grown in dextrose medium, then transferred to galactose medium 2 h before microscopy. Arrows point to the Ndj1 (green) focus that overlaps with Mps3-RFP (red) but not with Rap1-RFP (red). Quantification of Ndj1-GFP focus formation is shown in B. Bar, 2 µm. (C) Localization of Ndj1 to SPB depends on the N terminus of Mps3 in vegetative cells. Yeast strains 3HA-MPS3(Δ1-64) TUB4-RFP (HY4149) and 3HA-MPS3(Δ1-64) PGAL1-GFP-NDJ1 TUB4-RFP (HY4150) were prepared for microscopy as in A. Cells from the above strains were mixed before microscopy. The two cells shown were from the same microscopy field. Note that in the absence of the N terminus of Mps3, GFP-Ndj1 (green) no longer formed a focus at the SPB marked by Tub4-RFP (red). (D and E) Ectopic expression of NDJ1 is lethal in vegetative cells. Yeast cells (HY3799, HY4128, HY4149, HY4150, HY4917, and HY4933) were grown in dextrose medium, serially diluted, and spotted on dextrose and galactose plates. Note that both removal of the N terminus of Mps3 and pom152Δ suppressed the lethality caused by ectopic expression of Ndj1. (F) Quantification of Ndj1 localization to the SPB in selected yeast strains. The SPB was marked by Tub4-RFP. Strains HY4128, HY4150, and HY4947 were used.
To further elucidate how Ndj1 inhibits SPB separation, we staged haploid yeast cells at the G1 phase with alpha factor, induced NDJ1 expression, and then released these cells from G1 arrest by removing the alpha factor to allow cell division (Fig. 5, D–F). In the presence of galactose, Ndj1 was readily produced, and its protein level increased over time (Fig. 5 D). With Ndj1, yeast cells appeared competent to complete DNA synthesis and SPB duplication on the basis of FACS analysis of DNA content and increased Spc42 intensity (Fig. S5, A and B). But in a typical experiment with robust Ndj1 expression shown in Fig. 5 E, >60% of Ndj1-expressing cells were arrested at the large-budded state, of which >12% never separated the Spc42 focus, and ∼20% showed two unequal SPBs (Fig. 5 F and Fig. S5 C). Consequently, we observed that the majority of PGAL1-NDJ1 cells either failed to commit to nuclear division or formed massive chromosome bridges at anaphase, as shown by the distribution of the histone H2A signal (Fig. 5, G and H). Together, these findings support the idea that Ndj1, when produced in vegetative cells, inhibits SPB separation. Because Ndj1 interacts with Mps3 (see the following paragraph), one caveat is that the possibility that ectopically expressed Ndj1 causes defective SPB assembly is currently not excluded.
Ndj1 function at the SPB depends on the N terminus of Mps3
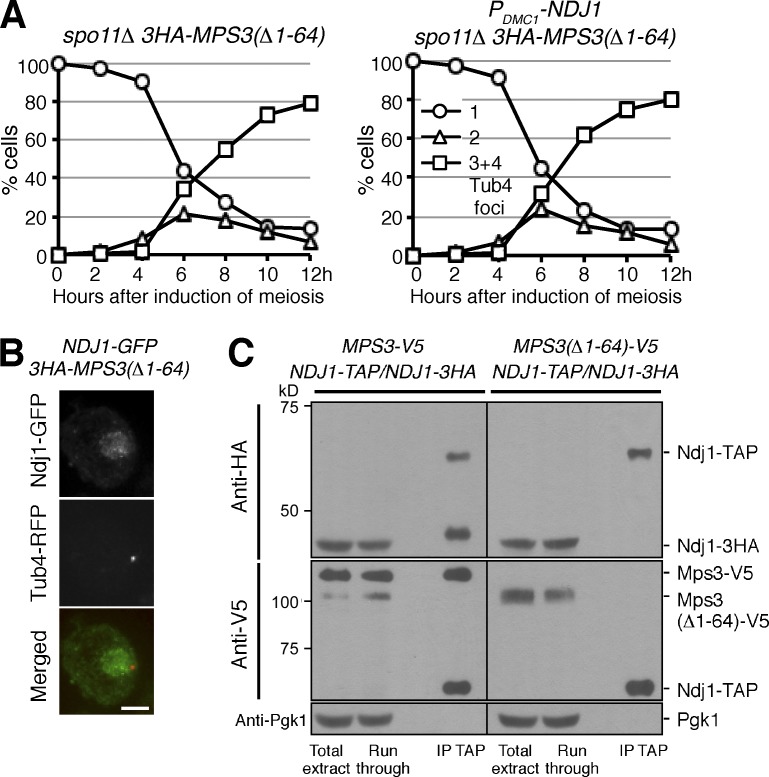
Because binding of Ndj1 to the SPB depends on Mps3 (Fig. 2 C and Fig. 6), we hypothesized that Ndj1 regulates SPB dynamics through Mps3. When Ndj1 was ectopically expressed in vegetative cells, the major Ndj1-GFP focus overlapped with Mps3-RFP but not with Rap1-RFP (Fig. 6, A and B), confirming that, like Mps3, Ndj1 is concentrated at the SPB. Using protein affinity purification and immunoblotting, we determined that the ectopically expressed Ndj1 physically interacted with Mps3 (Fig. S4 B). To disrupt the Ndj1 and Mps3 interaction, we used the N-terminal deletion allele of MPS3, which remains functional in vegetative yeast cells (Conrad et al., 2007; Fig. 6, C and D). Removal of the first 64 amino acids of Mps3 (MPS3(Δ1-64)) led to the loss of its interaction with Ndj1 (see Fig. 7). Consequently, Ndj1 was no longer concentrated at the SPB; instead, the Ndj1-GFP signal became diffused in the nucleus, as shown in Fig. 6 C. Ectopic expression of NDJ1 by the GAL1 promoter was lethal, but the MPS3(Δ1-64) allele suppressed this lethality (Fig. 6, C and D). In addition, we found that pom152Δ, which suppresses the mps3 lethal phenotype at the SPB (Chen et al., 2014), also suppressed PGAL1-NDJ1 (Fig. 6 E). In the absence of Pom152, Ndj1 remained concentrated at the SPB (Fig. 6 F). Together, these observations demonstrate that Ndj1-mediated delay of SPB separation and cell lethality in vegetative cells both depend on Mps3.
Figure 7.
Mps3 is the target of Ndj1 at the SPB during yeast meiosis. (A) Removal of the N terminus of Mps3 abolishes the Ndj1 activity at the SPB. Yeast cells (HY4864 and HY4865) were induced to undergo synchronous meiosis, and SPB separation was determined using fluorescence microscopy. Tub4-RFP was used as the SPB marker as in Fig. 3 B. The graphs shown are from a representative time-lapse experiment out of three repeats. (B) Localization of Ndj1 to SPB depends on the N terminus of Mps3 during yeast meiosis. Strain HY4865 was used. Ndj1-GFP, green; Tub4-RFP, red. Quantification of Ndj1 localization to SPB is shown in Fig. S2 C. Bar, 2 µm. (C) Ndj1 binds to the N terminus of Mps3. Yeast strains MPS3-V5/MPS3-V5 NDJ1-TAP/NDJ1-TAP (HY4393) and MPS3(Δ1-64)-V5/MPS3(Δ1-64)-V5 NDJ1-TAP/NDJ1-3HA (HY4412) were induced to undergo synchronous meiosis. Cells were collected 4.5 h after meiosis and prepared for TAP affinity purification. Note that Ndj1-3HA is copurified with Ndj1-TAP only in the presence of the full length of Mps3. The level of Pgk1 serves as a negative control.
To determine whether the Ndj1 and Mps3 interaction is also required for delaying SPB separation in meiosis, we overexpressed NDJ1 in yeast cells that lacked the N terminus of Mps3 (Fig. 7 A). To make our SPB cohesion assay more effective, we used spo11Δ to abolish the recombination checkpoint (Figs. 3 F and 7 A). In the absence of the N terminus of Mps3, cells with overproduced Ndj1 separated their SPBs on time (Fig. 7 A), and Ndj1 failed to localize to the SPB in these cells (Fig. 7 B and Fig. S2 C). These findings are in contrast to those from wild-type cells with overexpressed Ndj1 where SPBs delayed separation (Fig. 3, F and G), and further demonstrate that Ndj1-mediated inhibition of SPB separation depends on Mps3.
Ndj1 binds to the N terminus of Mps3 and forms oligomeric complexes with Mps3
To confirm that Ndj1 binds to the N terminus of Mps3, we performed affinity purification of Ndj1-TAP in wild-type MPS3 and MPS3(Δ1-64) cells (Fig. 7 C). We also introduced an NDJ1-3HA allele in these cells (Fig. 7 C). Using Ndj1-TAP affinity purification and immunoblotting, we found that the full-length Mps3, which was tagged with the V5 epitope, physically bound to Ndj1-TAP (Fig. 7 C, middle left). In addition, we found that Ndj1-3HA was copurified with Ndj1-TAP (Fig. 7 C, top left). Crucially, the Ndj1–Ndj1 protein interaction depends on the full length of Mps3, because removal of the N terminus of Mps3 abolished both the Ndj1–Mps3 interaction and the Ndj1–Ndj1 interaction (Fig. 7 C, top right). Therefore, Ndj1 must interact with the N terminus of Mps3. Furthermore, we have revealed that Ndj1 forms oligomeric complexes with Mps3 during yeast meiosis.
Discussion
Interaction of Ndj1 with Mps3 at the meiotic SPB
In this report, we have shown that Ndj1 regulates SPB cohesion during the extended G2/prophase I in budding yeast meiosis. Ndj1 localizes to the SPB and inhibits premature SPB separation. Ndj1 has been previously known as a telomere-associated protein and regulates telomere movement (Conrad et al., 2008). But the following observations suggest that the mode of Ndj1 action at the SPB is separable from its function at the telomeres: (1) Ndj1 localization to the SPB, and Ndj1-mediated protection of SPB cohesion, are independent of Csm4 and Rap1, both of which interact with the telomeric Ndj1; (2) ectopic expression of Ndj1 in vegetative yeast cells inhibits SPB separation, and in these cells, Ndj1 enriches at the SPB but fails to colocalize with Rap1. However, the interaction of Ndj1 with Mps3 takes place both at the SPB and telomeres, which suggests that a similar theme is involved in regulating SPB dynamics and telomere movement. We speculate that Ndj1 differentially interacts with the putative SUN-KASH pairs: Mps3-Mps2 at the SPB and Mps3-Csm4 at the telomeres, for regulating SPB dynamics and telomere movement, respectively. Our observation of Mps3 forming oligomeric complexes with Ndj1 lends support to the notion that SUN domain proteins form oligomers (Sosa et al., 2012; Wang et al., 2012). The fact that Ndj1–Mps3 interaction takes place simultaneously at the SPB and telomeres during prophase I demonstrates that centrosome dynamics in budding yeast are intrinsically coupled with telomere movement, thus coordinating meiotic recombination and chromosome segregation.
Regulation of half-bridge disassembly and SPB separation during yeast meiosis
In G2-arrested yeast cells during meiosis, SPBs are linked by the half-bridge and form a side-by-side configuration. Depletion of Ipl1, the Aurora kinase in yeast, can cause premature SPB separation in these cells (Shirk et al., 2011; Kim et al., 2013). One idea is that Ipl1 inhibits microtubule-based motility (Kim et al., 2013). Upon the activation of the motor protein, for example kinesin-5 (Chee and Haase, 2010), SPBs are pushed apart by an “outward” force (Winey and Bloom, 2012). However, overexpression of NDJ1 in these cells delays SPB separation (this study), which suggests that microtubule-based force is necessary but not sufficient to separate SPBs. Our observation supports a model for the stepwise regulation of SPB separation: duplicated SPBs are tethered by the half-bridge, and disassembly of the half-bridge then permits SPB separation and spindle assembly (Li et al., 2006; Anderson et al., 2007; Chee and Haase, 2010).
Ndj1 binds to Mps3, a major component of the half-bridge that tethers duplicated SPBs. Removal of Ndj1 from the SPB takes place before SPB separation at the G2–to–metaphase I transition, demonstrating that Ndj1 is at the right place and time to regulate half-bridge disassembly. Deletion of the N terminus of Mps3 suppresses the delayed SPB separation phenotype caused by the overexpression of NDJ1 in both meiosis and mitosis. We propose that Ndj1 inhibits Mps3 protein modification, which is necessary for half-bridge disassembly (Fig. 8 A). In addition, posttranslational modifications of the C terminus of Sfi1, another key subunit of the half-bridge, also appear to be a prerequisite for SPB separation (Anderson et al., 2007; Avena et al., 2014; Elserafy et al., 2014). We therefore speculate that either Mps3 or Sfi1, or both, are the site of half-bridge cleavage, which permits SPB separation and spindle assembly.
Figure 8.
Model for Ndj1 action at the SPB (A) and pathways of SPB separation (B) during yeast meiosis. Broken line ovals represent Mps3-interacting proteins located at the outer nuclear membrane.
In vegetative yeast cells, which lack a distinctive G2 phase, SPBs separate within minutes after duplication, because the Clb-Cdk1 activity is present at S phase in these cells (Lim et al., 1996). We favor the explanation that the unequal separation of SPBs we observed in vegetative cells with ectopically expressed Ndj1 is due to the high activity of Clb-Cdk1 that promotes spindle assembly in cells with tethered SPBs. In contrast, during the meiotic G2/prophase I, duplicated SPBs remain cohesive for hours when recombination takes place (Miller et al., 2012; Okaz et al., 2012), because the expression of the B-type cyclins, including Clb1, -3, and -4, depends on Ndt80 (Chu and Herskowitz, 1998), whose activation leads to yeast cells exiting prophase I and, concomitantly, SPB separation. In this context, the cell cycle stages of yeast meiosis I, but not mitosis, resemble those of the mitotic cycle in mammalian cells.
We have revealed that degradation of Ndj1 is mediated by the Polo-like kinase Cdc5, because ectopic expression of Cdc5 in G2-arrested cells is sufficient for Ndj1 degradation. Ndj1 appears to be a phosphorylated protein in yeast meiosis, but the putative Ndj1 phosphorylation sites we identified do not fit well with the Cdc5 consensus sequence, and mutating them had little effect on Ndj1 protein stability (unpublished data), which suggests that Cdc5 acts indirectly on Ndj1 stability. This reasoning is supported by the observation that in the absence of Cdc5, the Ndj1 protein level decreased, and SPBs separated during meiosis (unpublished data). In the absence of Ndj1, SPBs remain cohesive at prophase I, indicating that Ndj1 regulates SPB cohesion but is not itself an intrinsic part of the half-bridge that tethers SPBs. However, in prophase I cells with ectopically expressed Cdc5, Ipl1 was no longer clustered around the SPB (this study), which indicates that Cdc5 not only regulates Ndj1 protein stability but also regulates Ipl1 and/or microtubule-based force that is necessary to separate SPBs (Fig. 8 B). Our finding that overproduction of Ndj1 inhibits SPB separation in either Ipl1-depleted or ectopically expressed Cdc5 cells that are arrested at prophase I supports the idea that redundant pathways, mediated by Cdc5 and Clb-Cdk1 (Haase et al., 2001; Crasta et al., 2008; Avena et al., 2014; Elserafy et al., 2014), perhaps lead to the dissolution of the SPB half-bridge and subsequent SPB separation (Fig. 8 B).
Coordination of SPB dynamics with telomere movement
Because Ndj1 plays a dual role at the SPB and telomeres, this protein is well positioned to coordinate SPB dynamics with telomere motility during yeast meiosis. Degradation of Ndj1 leads to the removal of the inhibitory signal that keeps SPBs cohesive; it also disrupts the linkage between the telomeres and the nuclear envelope (Conrad et al., 2008; Kosaka et al., 2008; Wanat et al., 2008). Therefore, separation of duplicated SPBs and the subsequent formation of a bipolar spindle can be coupled with the detachment of meiotic telomeres from the nuclear envelope as cells exit prophase I.
Deletion of the NDJ1 gene in budding yeast leads to erroneous homologue exchange and chromosome missegregation (Conrad et al., 1997; Wu and Burgess, 2006; Conrad et al., 2008), both of which are attributed to the essential role of Ndj1 in mediating telomere motility. In light of the new function of Ndj1 at the SPB described in this report, we propose that in the absence of Ndj1, premature SPB separation can also lead to recombination defects and chromosome missegregation. This study provides a framework for future studies to distinguish between these two Ndj1 mechanisms that are required for maintaining genome integrity.
Materials and methods
Yeast strains, plasmids, and culture methods
Yeast strains used in this study are listed in Table S1. Strains for meiotic experiments were diploid derivatives of SK1. Haploid derivatives of S288C were used in mitotic experiments. A PCR-based approach (Longtine et al., 1998) was used to generate ndj1Δ, csm4Δ, PCLB2-MPS3, PCUP1-MPS3(Δ1-64), PCLB2-MPS3(Δ1-64), and PCUP1-CDC5 alleles. Positive yeast transformants were confirmed by colony PCR. The PCLB2-CDC5, PCLB2-CDC20, and PCUP1-CLB3 alleles have been described previously (Lee and Amon, 2003; Miller et al., 2012). In brief, the promoters (∼1 kb) from CLB2 and CUP1 were amplified and used to replace the endogenous promoters of CDC5, CDC20, MPS3, and CLB3 by PCR-based yeast transformation. A similar PCR-based approach was used to tag the C termini of Tub1, Tub4, Spc42, Spc97, Spc72, Ndj1, Mps3, Rap1, and Hta1 with 3×HA, tandem affinity purification (TAP), GFP, and RFP at their endogenous gene loci, and tagged alleles are the only functional copies in the yeast genome.
Plasmids used in this study are listed in Table S2. To ectopically express NDJ1 in vegetative yeast cells, we constructed plasmids pHG302 and pHG335, which contain PGAL1-GFP-NDJ1 and PGAL1-V5-NDJ1, respectively. The backbone of these plasmids was derived from pRS305, and PGAL1-NDJ1 was cloned into the SacI and SalI sites. The GAL1 promoter (670 bp) was used to drive the expression of the full length of the NDJ1 open reading frame. Overexpression of NDJ1 in meiosis was achieved by inserting two copies each of plasmids pHG286 and pHG389 into the yeast genome. To construct PDMC1-GFP-NDJ1, the GAL1 promoter was replaced with the DMC1 promoter, which was expressed only in meiosis. Replacement of the GAL1 promoter with that of CUP1 allowed NDJ1 expression in the presence of Cu2+ (pHG274).
For meiotic experiments, yeast cells were grown in yeast extract, peptone, potassium acetate (YPA) to OD600 ∼1.5–2.0 and then transferred to 2% potassium acetate to induce synchronous meiosis as described previously (Jin et al., 2009). Yeast samples were withdrawn at the indicated times for analysis of SPB separation by fluorescence microscopy, and protein stability was assessing using Western blots. Tetrads from selected diploid yeast strains were dissected, and their spore viability is shown in Table S3.
For mitotic experiments, yeast cells were grown in synthetic complete (SC) medium with 2% raffinose. To induce the expression of the GAL1 promoter, 3% galactose was then added to the culture medium. We used the alpha factor (10 µg/ml) to arrest yeast cells at the G1 phase (Fig. 5 D). To induce PGAL1-NDJ1 expression in these cells, 3% galactose was added to the yeast culture 30 min before the alpha factor was removed from the culture medium. We used 50 µM of CuSO4 to induce the expression of the CUP1 promoter.
Protein affinity purification and mass spectrometry
We used a previously described protocol for protein TAP affinity purification (Niepel et al., 2005). In brief, frozen yeast cells were ground in the presence of liquid nitrogen, and ∼10 g of cells were thawed into 15 ml of extraction buffer as described previously. We used epoxy-activated M-270 Dynabeads (Invitrogen), cross-linked to rabbit IgG, for TAP purification.
For mass spectrometry–based protein identification, purified TAP samples were subjected to tryptic digestion. An externally calibrated Thermo LTQ Orbitrap Velos was used for mass spectrometry. Three technical replicates of each sample were run to allow for statistical comparison. The raw files were analyzed with the Proteome Discoverer (version 1.4) software package.
Live-cell fluorescence microscopy
Yeast live-cell microscopy was carried out on a DeltaVision imaging system (Applied Precision) with a 60× objective lens (NA 1.40) on an inverted microscope (IX-71; Olympus). We used agarose pads filled with 2% potassium acetate for meiotic experiments as described previously (Li et al., 2011). To induce the expression of PGAL1-GFP-NDJ1 in vegetative yeast cells, we prepared agarose pads with the SC medium plus 2% raffinose and 3% galactose. The microscope stage was enclosed in an environmental chamber, with the acquisition temperature set at 30°C. For time-lapse microscopy, optical sections were set at 1 µm thickness with seven z sections for meiotic cells, and 0.5 µm thickness with nine z sections for mitotic cells. For single-time-point microscopy, optical sections were set at 0.3 µm thickness, and at least 15 z sections for mitotic cells and 20 z sections for meiotic cells were acquired. Images were acquired with a CoolSNAP HQ2 CCD camera (Photometrics) and deconvolved with SoftWoRx (Applied Precision), and projections or single optical sections were used for display.
Quantification of fluorescent signal intensity
We used the SoftWoRx measurement tools to determine fluorescence intensity in single optical sections. In brief, we defined a 7 × 7 pixel area that covered the Spc42-RFP, Tub4-RFP, or Ndj1 focus at the SPB. The mean background intensity was subtracted from the region of interest to yield the net intensity of Spc42, Tub4, or Ndj1. Calculated Spc42, Tub4, or Ndj1 measurements were plotted in Figs. 1 G, 3 H, 5 (B and C), and S5 B.
Immunoblotting
Protein extraction and immunoblotting were performed as described previously (Jin et al., 2009). For the mitotic samples (Fig. 5 D), yeast cells were precipitated in the presence of NaOH. HA-tagged proteins (Ndj1-3HA, 2HA-Cdc5, and Mps3-3HA) were detected by an anti-HA mouse monoclonal antibody (1:5,000 dilution, 16B12; Santa Cruz Biotechnology, Inc.). Ndj1-GFP was detected by an anti-GFP mouse monoclonal antibody (1:10,000 dilution, JL-8; Takara Bio Inc.). Ndj1-V5 was detected by an anti-V5 mouse monoclonal antibody (1:10,000 dilution, R960; Invitrogen). A β-tubulin antibody (1:10,000) was used to detect Tub2 for a loading control (Jin et al., 2009).
Flow cytometry analysis of genome content
Fluorescence-activated cell sorting of yeast cells was performed as described previously (Jin et al., 2009). Yeast cells were withdrawn at the indicated times, fixed in 70% ethanol, treated with RNAase and proteinase K, stained with propidium iodide (P4170; Sigma-Aldrich), and sorted using a cell-sorting system (FACSAria; BD).
Serial dilution assay of cell viability
Yeast cells were grown to early log phase at 30°C. 10-fold dilutions of cells were spotted onto SC plates with 2% dextrose and SC plates with 2% galactose, then incubated at 30°C for 2–3 d.
Online supplemental material
Fig. S1 shows the extended list of proteins copurified with Spc97-TAP. Fig. S2 shows the localization of Ndj1 and Rap1 in yeast meiosis. Fig. S3 shows SPB separation and Ipl1 localization in arrested prophase I cells. Fig. S4 shows the localization of Ndj1 to SPB in vegetative cells. Fig. S5 shows FACS analysis of genome content and SPB duplication and separation using fluorescence microscopy. Table S1 shows yeast strains. Table S2 shows plasmids used. Table S3 shows spore viability of selected strains. Online supplemental material is available at http://www.jcb.org/cgi/content/full/jcb.201408118/DC1.
Supplementary Material
Acknowledgments
We thank Y. Wang and R. Abblett for comments and discussions, M. Winey and T. Davis for suggestions on methods of SPB purification, and A. Amon and E. Unal for providing yeast strains. An anonymous reviewer suggested the Pom152 experiment. The Translational Medicine Laboratory at the Florida State University assisted with protein mass spectrometry, and Jen Kennedy assisted with text editing.
This work is supported by the National Science Foundation (MCB-1121771).
The authors declare no competing financial interests.
Footnotes
Abbreviations used in this paper:
- RFP
- red fluorescent protein
- SC
- synthetic complete
- SPB
- spindle pole body
- TAP
- tandem affinity purification
References
- Anderson V.E., Prudden J., Prochnik S., Giddings T.H. Jr, and Hardwick K.G.. 2007. Novel sfi1 alleles uncover additional functions for Sfi1p in bipolar spindle assembly and function. Mol. Biol. Cell. 18:2047–2056 10.1091/mbc.E06-10-0918 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Avena J.S., Burns S., Yu Z., Ebmeier C.C., Old W.M., Jaspersen S.L., and Winey M.. 2014. Licensing of yeast centrosome duplication requires phosphoregulation of sfi1. PLoS Genet. 10:e1004666 10.1371/journal.pgen.1004666 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers B., and Goetsch L.. 1975. Behavior of spindles and spindle plaques in the cell cycle and conjugation of Saccharomyces cerevisiae. J. Bacteriol. 124:511–523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee M.K., and Haase S.B.. 2010. B-cyclin/CDKs regulate mitotic spindle assembly by phosphorylating kinesins-5 in budding yeast. PLoS Genet. 6:e1000935 10.1371/journal.pgen.1000935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Smoyer C.J., Slaughter B.D., Unruh J.R., and Jaspersen S.L.. 2014. The SUN protein Mps3 controls Ndc1 distribution and function on the nuclear membrane. J. Cell Biol. 204:523–539 10.1083/jcb.201307043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu S., and Herskowitz I.. 1998. Gametogenesis in yeast is regulated by a transcriptional cascade dependent on Ndt80. Mol. Cell. 1:685–696 10.1016/S1097-2765(00)80068-4 [DOI] [PubMed] [Google Scholar]
- Conrad M.N., Dominguez A.M., and Dresser M.E.. 1997. Ndj1p, a meiotic telomere protein required for normal chromosome synapsis and segregation in yeast. Science. 276:1252–1255 10.1126/science.276.5316.1252 [DOI] [PubMed] [Google Scholar]
- Conrad M.N., Lee C.Y., Wilkerson J.L., and Dresser M.E.. 2007. MPS3 mediates meiotic bouquet formation in Saccharomyces cerevisiae. Proc. Natl. Acad. Sci. USA. 104:8863–8868 10.1073/pnas.0606165104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conrad M.N., Lee C.Y., Chao G., Shinohara M., Kosaka H., Shinohara A., Conchello J.A., and Dresser M.E.. 2008. Rapid telomere movement in meiotic prophase is promoted by NDJ1, MPS3, and CSM4 and is modulated by recombination. Cell. 133:1175–1187 10.1016/j.cell.2008.04.047 [DOI] [PubMed] [Google Scholar]
- Crasta K., Lim H.H., Giddings T.H. Jr, Winey M., and Surana U.. 2008. Inactivation of Cdh1 by synergistic action of Cdk1 and polo kinase is necessary for proper assembly of the mitotic spindle. Nat. Cell Biol. 10:665–675 10.1038/ncb1729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elserafy M., Šarić M., Neuner A., Lin T.C., Zhang W., Seybold C., Sivashanmugam L., and Schiebel E.. 2014. Molecular mechanisms that restrict yeast centrosome duplication to one event per cell cycle. Curr. Biol. 24:1456–1466 10.1016/j.cub.2014.05.032 [DOI] [PubMed] [Google Scholar]
- Friederichs J.M., Ghosh S., Smoyer C.J., McCroskey S., Miller B.D., Weaver K.J., Delventhal K.M., Unruh J., Slaughter B.D., and Jaspersen S.L.. 2011. The SUN protein Mps3 is required for spindle pole body insertion into the nuclear membrane and nuclear envelope homeostasis. PLoS Genet. 7:e1002365 10.1371/journal.pgen.1002365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haase S.B., Winey M., and Reed S.I.. 2001. Multi-step control of spindle pole body duplication by cyclin-dependent kinase. Nat. Cell Biol. 3:38–42 10.1038/35050543 [DOI] [PubMed] [Google Scholar]
- Hiraoka Y., and Dernburg A.F.. 2009. The SUN rises on meiotic chromosome dynamics. Dev. Cell. 17:598–605 10.1016/j.devcel.2009.10.014 [DOI] [PubMed] [Google Scholar]
- Jaspersen S.L., and Winey M.. 2004. The budding yeast spindle pole body: structure, duplication, and function. Annu. Rev. Cell Dev. Biol. 20:1–28 10.1146/annurev.cellbio.20.022003.114106 [DOI] [PubMed] [Google Scholar]
- Jaspersen S.L., Giddings T.H. Jr, and Winey M.. 2002. Mps3p is a novel component of the yeast spindle pole body that interacts with the yeast centrin homologue Cdc31p. J. Cell Biol. 159:945–956 10.1083/jcb.200208169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaspersen S.L., Martin A.E., Glazko G., Giddings T.H. Jr, Morgan G., Mushegian A., and Winey M.. 2006. The Sad1-UNC-84 homology domain in Mps3 interacts with Mps2 to connect the spindle pole body with the nuclear envelope. J. Cell Biol. 174:665–675 10.1083/jcb.200601062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin H., Guacci V., and Yu H.G.. 2009. Pds5 is required for homologue pairing and inhibits synapsis of sister chromatids during yeast meiosis. J. Cell Biol. 186:713–725 10.1083/jcb.200810107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilmartin J.V.2003. Sfi1p has conserved centrin-binding sites and an essential function in budding yeast spindle pole body duplication. J. Cell Biol. 162:1211–1221 10.1083/jcb.200307064 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Meyer R., Chuong H., and Dawson D.S.. 2013. Dual mechanisms prevent premature chromosome segregation during meiosis. Genes Dev. 27:2139–2146 10.1101/gad.227454.113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Knop M., Pereira G., Geissler S., Grein K., and Schiebel E.. 1997. The spindle pole body component Spc97p interacts with the γ-tubulin of Saccharomyces cerevisiae and functions in microtubule organization and spindle pole body duplication. EMBO J. 16:1550–1564 10.1093/emboj/16.7.1550 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kosaka H., Shinohara M., and Shinohara A.. 2008. Csm4-dependent telomere movement on nuclear envelope promotes meiotic recombination. PLoS Genet. 4:e1000196 10.1371/journal.pgen.1000196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.H., and Amon A.. 2003. Role of Polo-like kinase CDC5 in programming meiosis I chromosome segregation. Science. 300:482–486 10.1126/science.1081846 [DOI] [PubMed] [Google Scholar]
- Li S., Sandercock A.M., Conduit P., Robinson C.V., Williams R.L., and Kilmartin J.V.. 2006. Structural role of Sfi1p-centrin filaments in budding yeast spindle pole body duplication. J. Cell Biol. 173:867–877 10.1083/jcb.200603153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., Jin H., Hoang M.L., and Yu H.G.. 2011. Tracking chromosome dynamics in live yeast cells: coordinated movement of rDNA homologs and anaphase disassembly of the nucleolus during meiosis. Chromosome Res. 19:1013–1026 10.1007/s10577-011-9253-0 [DOI] [PubMed] [Google Scholar]
- Lim H.H., Goh P.Y., and Surana U.. 1996. Spindle pole body separation in Saccharomyces cerevisiae requires dephosphorylation of the tyrosine 19 residue of Cdc28. Mol. Cell. Biol. 16:6385–6397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Longtine M.S., McKenzie A. III, Demarini D.J., Shah N.G., Wach A., Brachat A., Philippsen P., and Pringle J.R.. 1998. Additional modules for versatile and economical PCR-based gene deletion and modification in Saccharomyces cerevisiae. Yeast. 14:953–961 [DOI] [PubMed] [Google Scholar]
- Mardin B.R., and Schiebel E.. 2012. Breaking the ties that bind: new advances in centrosome biology. J. Cell Biol. 197:11–18 10.1083/jcb.201108006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller M.P., Unal E., Brar G.A., and Amon A.. 2012. Meiosis I chromosome segregation is established through regulation of microtubule-kinetochore interactions. eLife. 1:e00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moens P.B., and Rapport E.. 1971. Spindles, spindle plaques, and meiosis in the yeast Saccharomyces cerevisiae (Hansen). J. Cell Biol. 50:344–361 10.1083/jcb.50.2.344 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newnham L., Jordan P.W., Carballo J.A., Newcombe S., and Hoffmann E.. 2013. Ipl1/Aurora kinase suppresses S-CDK-driven spindle formation during prophase I to ensure chromosome integrity during meiosis. PLoS ONE. 8:e83982 10.1371/journal.pone.0083982 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niepel M., Strambio-de-Castillia C., Fasolo J., Chait B.T., and Rout M.P.. 2005. The nuclear pore complex-associated protein, Mlp2p, binds to the yeast spindle pole body and promotes its efficient assembly. J. Cell Biol. 170:225–235 10.1083/jcb.200504140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nigg E.A., and Stearns T.. 2011. The centrosome cycle: Centriole biogenesis, duplication and inherent asymmetries. Nat. Cell Biol. 13:1154–1160 10.1038/ncb2345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishikawa S., Terazawa Y., Nakayama T., Hirata A., Makio T., and Endo T.. 2003. Nep98p is a component of the yeast spindle pole body and essential for nuclear division and fusion. J. Biol. Chem. 278:9938–9943 10.1074/jbc.M210934200 [DOI] [PubMed] [Google Scholar]
- Okaz E., Argüello-Miranda O., Bogdanova A., Vinod P.K., Lipp J.J., Markova Z., Zagoriy I., Novak B., and Zachariae W.. 2012. Meiotic prophase requires proteolysis of M phase regulators mediated by the meiosis-specific APC/CAma1. Cell. 151:603–618 10.1016/j.cell.2012.08.044 [DOI] [PubMed] [Google Scholar]
- Padmore R., Cao L., and Kleckner N.. 1991. Temporal comparison of recombination and synaptonemal complex formation during meiosis in S. cerevisiae. Cell. 66:1239–1256 10.1016/0092-8674(91)90046-2 [DOI] [PubMed] [Google Scholar]
- Rock J.M., Lim D., Stach L., Ogrodowicz R.W., Keck J.M., Jones M.H., Wong C.C., Yates J.R. III, Winey M., Smerdon S.J., et al. 2013. Activation of the yeast Hippo pathway by phosphorylation-dependent assembly of signaling complexes. Science. 340:871–875 10.1126/science.1235822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shirk K., Jin H., Giddings T.H. Jr, Winey M., and Yu H.G.. 2011. The Aurora kinase Ipl1 is necessary for spindle pole body cohesion during budding yeast meiosis. J. Cell Sci. 124:2891–2896 10.1242/jcs.086652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sosa B.A., Rothballer A., Kutay U., and Schwartz T.U.. 2012. LINC complexes form by binding of three KASH peptides to domain interfaces of trimeric SUN proteins. Cell. 149:1035–1047 10.1016/j.cell.2012.03.046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sourirajan A., and Lichten M.. 2008. Polo-like kinase Cdc5 drives exit from pachytene during budding yeast meiosis. Genes Dev. 22:2627–2632 10.1101/gad.1711408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spang A., Courtney I., Grein K., Matzner M., and Schiebel E.. 1995. The Cdc31p-binding protein Kar1p is a component of the half bridge of the yeast spindle pole body. J. Cell Biol. 128:863–877 10.1083/jcb.128.5.863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapley E.C., and Starr D.A.. 2013. Connecting the nucleus to the cytoskeleton by SUN-KASH bridges across the nuclear envelope. Curr. Opin. Cell Biol. 25:57–62 10.1016/j.ceb.2012.10.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallen E.A., Ho W., Winey M., and Rose M.D.. 1994. Genetic interactions between CDC31 and KAR1, two genes required for duplication of the microtubule organizing center in Saccharomyces cerevisiae. Genetics. 137:407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanat J.J., Kim K.P., Koszul R., Zanders S., Weiner B., Kleckner N., and Alani E.. 2008. Csm4, in collaboration with Ndj1, mediates telomere-led chromosome dynamics and recombination during yeast meiosis. PLoS Genet. 4:e1000188 10.1371/journal.pgen.1000188 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Shi Z., Jiao S., Chen C., Wang H., Liu G., Wang Q., Zhao Y., Greene M.I., and Zhou Z.. 2012. Structural insights into SUN-KASH complexes across the nuclear envelope. Cell Res. 22:1440–1452 10.1038/cr.2012.126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winey M., and Bloom K.. 2012. Mitotic spindle form and function. Genetics. 190:1197–1224 10.1534/genetics.111.128710 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu H.Y., and Burgess S.M.. 2006. Ndj1, a telomere-associated protein, promotes meiotic recombination in budding yeast. Mol. Cell. Biol. 26:3683–3694 10.1128/MCB.26.10.3683-3694.2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L., Ajimura M., Padmore R., Klein C., and Kleckner N.. 1995. NDT80, a meiosis-specific gene required for exit from pachytene in Saccharomyces cerevisiae. Mol. Cell. Biol. 15:6572–6581. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.