Rasband studies clustering of axonal voltage-gated sodium channels.
Rasband studies clustering of axonal voltage-gated sodium channels.
Growing up, Matthew Rasband wanted to be just like his father, whom he characterizes as a Renaissance man: a lover of classical music, motorcycles, and numerous intellectual pursuits, who particularly relished his work as a professor of physics. That’s why the younger Rasband enrolled as a physics major in college. But sometimes one’s road leads to unexpected destinations.

Matthew Rasband
PHOTO COURTESY OF BAYLOR COLLEGE OF MEDICINE
Today, Rasband’s lab at Baylor College of Medicine in Houston, Texas, is driving our understanding of how neurons specifically cluster voltage-gated sodium channels at the sites where excitatory signals are integrated (the axon initial segment, or AIS) and propagated (at nodes of Ranvier). Through their efforts, his group has helped map out the inter- (1) and intracellular (2–4) interactions that affect both sodium channel clustering and neuronal biology (5) in health and disease. We called Rasband to learn about what we can expect to see on the road ahead.
“I discovered there wasn’t anything I couldn’t do… with enough effort and time.”
RENAISSANCE MAN
Why did you change course from physics to biology?
I did my undergraduate degree at Brigham Young University, where my father was on the faculty in the physics department. I admire my father immensely. He’s a very cool dude, but as a child it always seemed to me that he spoke a language that was a mystery to me. I wanted to be part of that world so I thought, “What better thing to do than to follow in my father’s footsteps?”
That’s one reason I majored in physics. I was particularly good in quantum mechanics, but at the end of my junior year I decided that I wanted to take a class from my father, who only taught graduate-level mathematical physics. These classes were the most difficult courses I’d ever taken. I’d get up at four o’clock in the morning and spend eight hours working to understand what was going on. I did fine in the course, but after the first semester my father told me, “Son, I don’t think theoretical physics is the career for you.”
It wasn’t that I was stupid, it was just that I did not view the world in terms of equations. He knew that I had a deep abiding interest in biology so he encouraged me to consider a career in biophysics. He helped arrange for me to do an honors thesis on medical imaging at The University of Utah, and that experience convinced me to pursue a doctorate in biophysics.
Having said that, I’m really glad that I pursued an undergraduate degree in physics, because after taking those classes from my father, I discovered there wasn’t anything I couldn’t do or couldn’t figure out with enough effort and time. I learned how to think and to ask questions, which has been very valuable. This is the advice I gave my children, and my son is now planning to major in physics, too.
Are you a Renaissance man like your father?
[Laughs] Well, I enjoy and have even studied music, but I don’t have much time for it these days. I’m an avid triathlete and I like to ride motorcycles. In fact, a neuroscience colleague and I recently rode our motorcycles through Death Valley, over to Boulder Dam, and around Lake Mead. I’ve also motorcycled through Spain, Greece, Mexico, and other places.
A FORK IN THE ROAD
When were you first introduced to neuroscience?
I decided to attend the University of Rochester for my PhD because they were very strong in imaging. But as soon as I arrived, my faculty advisors told me that even though I thought I knew what I wanted to do, they expected me to pick one rotation in a laboratory completely unrelated to that. I should pick it just because I thought it was cool.
I took a membrane biophysics class taught by Peter Shrager. He was a great teacher, and the topic just spoke to me. I asked Peter if I could do a rotation in his lab, and I’ve never looked back.
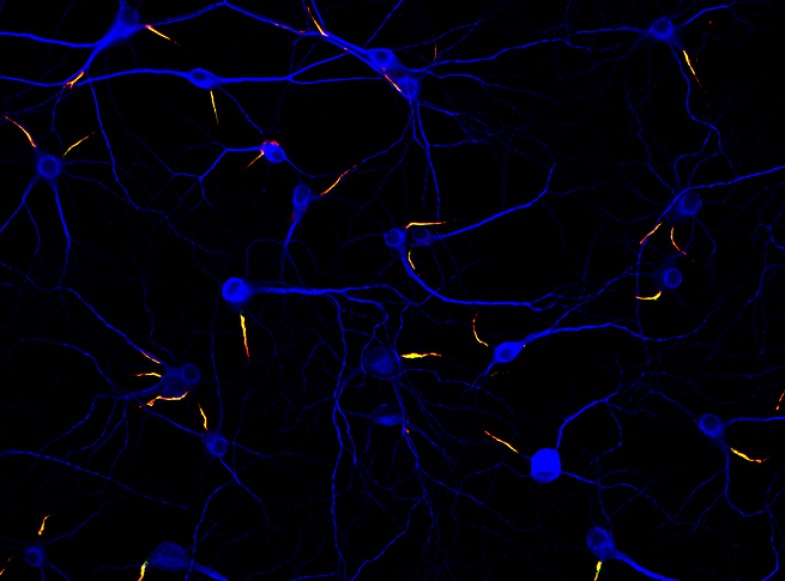
Neurons (blue) in culture can form their axon initial segments (yellow) without the help of other cells.
IMAGE COURTESY OF KRISTIAN L. HEDSTROM
What was known about the formation of nodes of Ranvier at this time?
When I started out as a graduate student, we were just beginning to get some of the tools—antibodies, for example—that would allow us to study nodes of Ranvier. Near the end of my graduate career, three different labs simultaneously discovered one of the key cell adhesion molecules that mediates the interaction between the myelin sheath and the axon at the perinode, the structure that flanks the nodes of Ranvier. Then, Ben Barres at Stanford University published a paper reporting that oligodendrocytes secreted a soluble factor that induced the clustering of sodium channels. On the other hand, my graduate work suggested sodium channel clustering in the central nervous system was a contact-dependent mechanism, mediated by the perinodal junction between glial and axonal membranes. Now, 20 years later, we’ve begun to map out the specific molecules that mediate these interactions and have discovered that in fact both models are likely right: there are multiple overlapping mechanisms that underlie the clustering of voltage-gated sodium channels.
What spurred your work on cytoskeletal regulation of sodium channel clustering?
It was Vann Bennett who first discovered that ankyrins are clustered at nodes and that they interact with the sodium channels and cell adhesion molecules present there. This immediately suggested a model for how sodium channels could be clustered; that interactions with glial proteins position cell adhesion molecules in the axonal membrane that bind to ankyrins, and the ankyrins then function as a kind of clustering machine to recruit voltage-gated sodium channels at high density. It was a simple model that turned out to be true—though we weren’t able to prove it unequivocally until just last year, when we set out to show that loss of ankyrin-G from the axons of sensory neurons prevents sodium channel clustering at nodes of Ranvier.
Surprisingly, and in contrast to basically every review that had ever been written, we found in this conditional knockout model that ankyrin-G was dispensable. We discovered there’s another ankyrin in axons, ankyrin-R, that, together with its binding partner, β1-spectrin, could compensate for loss of ankyrin-G and its binding partner, β4-spectrin. Only in mice lacking both ankyrin-G and ankyrin-R do sodium channels fail to cluster at nodes of Ranvier. We’ve further investigated the binding affinities and shown that there is a hierarchy of spectrins and ankyrins that can cluster sodium channels at nodes of Ranvier.
Is the function of the spectrins known?
I think spectrins participate in stabilizing the complex. However, this is really the last piece of the model that remains to be proven: how or whether spectrins link the entire complex to the underlying actin cytoskeleton.
THE TRIP AHEAD
Is sodium channel clustering managed differently at the AIS?
The mechanisms of clustering at initial segments and at nodes are different. For example, node of Ranvier formation is a complex process requiring multiple interactions between different cell types, whereas neurons cultured in isolation will spontaneously form an AIS without the help of any other cell. Nonetheless, most of the molecules that are found in axon initial segments are also found at nodes of Ranvier. Therefore, the AIS represents a simple model system where we can investigate intrinsic mechanisms that, on their own, are sufficient to cluster sodium channels.
“[Serendipitous discoveries] are oftentimes the most interesting and fun.”
We started investigating how the AIS is formed. Vann Bennett had previously proven that ankyrins are essential for sodium channel clustering at the AIS, and we showed that ankyrin-G is absolutely required; ankyrin-R cannot compensate for loss of ankyrin-G there. We also saw that if we removed ankyrin-G from a neuron that already had a mature AIS, the axon began to de-differentiate and turn into a dendrite. The idea that ankyrin-G was required to maintain neuronal polarity and identity was totally new.
Rasband stops to enjoy a vista while on a motorcycle tour.
PHOTO COURTESY OF MATTHEW RASBAND
This raised the question: If ankyrin-G is the key linchpin for initial segments, then how does it get there? We recently reported that the AIS has a distal cytoskeleton consisting of ankyrin-B with α2- and β2-spectrin. This distal cytoskeleton creates an intraaxonal boundary or barrier that limits ankyrin-G to the proximal axon.
What are the hot questions in the lab now?
Currently we’re focused mainly on spectrins because we found that loss of spectrins—particularly the brain’s lone α-spectrin—results in severe neuronal phenotypes.
We’d also like to understand how myelination takes place. Another interesting concept that I think needs to be investigated is plasticity. There’s evidence that the position of a node of Ranvier in the nervous system may not be set throughout life, but may change in response to altered neuronal activity. This could have implications for myelin diseases and injury.
Recently our study of brain plasticity led us to an entirely serendipitous discovery—these are oftentimes the most interesting and fun discoveries, because you weren’t expecting them. I had a student who was looking at the consequences of traumatic brain injury on AIS. She had labeled injured brains with a marker for microglia and noticed that about 8–10% of all microglia in the cortex specifically and precisely contacted neuronal AIS, but that under conditions of traumatic brain injury, this association goes away. We think these contacts are part of the healthy brain, and it will be fascinating to figure out what controls their formation and loss.
References
- 1.Susuki, K., et al. 2013. Neuron. 78:469–482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hedstrom, K.L., Ogawa Y., and Rasband M.N.. 2008. J. Cell Biol. 183:635–640. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Galiano, M.R., et al. 2012. Cell. 149:1125–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ho, T.S.-Y., et al. 2014. Nat. Neurosci. 17:1664–1672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baalman, K., et al. 2015. J. Neurosci. 35:2283–2292. [DOI] [PMC free article] [PubMed] [Google Scholar]